Abstract
When making decisions, individuals must often compensate for cognitive limitations, particularly in the face of advanced age. Recent findings suggest that age-related variability in striatal activity may increase financial risk-taking mistakes in older adults. In two studies, we sought to further characterize neural contributions to optimal financial risk taking and to determine whether decision aids could improve financial risk taking. In Study 1, neuroimaging analyses revealed that individuals whose mesolimbic activation correlated with the expected value estimates of a rational actor made more optimal financial decisions. In Study 2, presentation of expected value information improved decision making in both younger and older adults, but the addition of a distracting secondary task had little impact on decision quality. Remarkably, provision of expected value information improved the performance of older adults to match that of younger adults at baseline. These findings are consistent with the notion that mesolimbic circuits play a critical role in optimal choice, and imply that providing simplified information about expected value may improve financial risk taking across the adult life span.
Keywords: aging, decision making, learning, memory, reward, fMRI
INTRODUCTION
Unlike the rational actors posited by traditional financial models of optimal choice (Huang and Litzenberger, 1988), humans (and other organisms) must rely upon limited cognitive capacities when making decisions (Simon, 1982). Furthermore, some cognitive capacities related to attention, memory and cognitive control decline with age (Park et al., 2002; Salthouse, 2004; Birren and Schaie, 2006). These limitations might bias human choice, with more extreme repercussions for older adults. Little research, however, has focused on whether aging exacerbates biases in financial decision making, which neuropsychological mechanisms underlie those biases, and how they might be minimized.
Although financial decision-making doubtlessly requires some explicit recall (which typically enlists attentional and declarative memory resources), it may also rely on implicit evaluative processes. Declarative memory, which supports explicit recall, has been primarily associated with activity in lateral prefrontal and medial temporal brain regions (Brewer et al., 1998; Wagner et al., 1998; Ranganath and D'Esposito, 2001; Paller and Wagner, 2002; Davachi, 2006; Blumenfeld and Ranganath, 2007), whereas valuation has been associated primarily with activity in mesolimbic dopamine projection regions, including the medial prefrontal cortex (MPFC) and connected ventral striatum, including the nucleus accumbens (NAcc; O'Doherty, 2004; Knutson and Bossaerts, 2007; Knutson et al., 2008).
Event-related functional magnetic resonance imaging (fMRI) studies have specifically implicated mesolimbic projection areas associated with valuation in both optimal and suboptimal financial risk taking (Kuhnen and Knutson, 2005). For example, while increased NAcc activity precedes optimal financial risk-seeking choices, excessive NAcc activity can foreshadow suboptimal risk-seeking ‘mistakes’ (which deviate from the choices of a risk-neutral Bayesian-updating actor).
Extensive research has linked age-related deficits in attention, memory and cognitive control to changes in lateral prefrontal and medial temporal cortical function (Cabeza et al., 2005; Hedden and Gabrieli, 2004), but remarkably little research has investigated the influence of aging on valuation and associated mesolimbic function (Samanez-Larkin and Carstensen, in press). Emerging findings suggest age-related declines in the structure of frontal and striatal circuits (Hicks and Birren, 1970; Rubin, 1999; Buckner, 2004; Head et al., 2005); however, it is not yet clear whether these structural declines contribute to functional deficits in decision making. Early evidence has implied preservation of mesolimbic function in older adults in simple value assessment tasks (Samanez-Larkin et al., 2007), while other studies have documented age-related declines in mesolimbic function during probabilistic learning tasks (Fera et al., 2005; Aizenstein et al., 2006; Mell et al., 2009; Samanez-Larkin et al., 2010).
Although prevalent stereotypes suggest that older adults avoid risk, in some situations older adults may seek risk, or simply make more errors than younger adults (Denburg et al., 2005; Henninger et al., in press; Mather, 2006; Mohr et al., 2009). For instance, in a recent study, we found that older adults made more risk-seeking financial mistakes in an investment task than younger adults (Samanez-Larkin et al., 2010). This bias did not extend to risk-aversion mistakes. Furthermore, increased variability in NAcc function could account for the observed age differences in investment mistakes. While these findings implicate ‘noisier’ striatal activity in suboptimal financial risk taking, they do not specify which associated psychological processes impair choice, or how the impairment could be minimized. If NAcc activation supports the representation of expected value (Knutson et al., 2005; Yacubian et al., 2006), and disruptions in NAcc function compromise financial risk taking (Kuhnen and Knutson, 2005; Samanez-Larkin et al., 2010), then interventions that provide expected value information might improve decision making.
Alternatively, deficits in cognitive control associated with lateral prefrontal activity (D'Esposito et al., 1995; Miller and Cohen, 2001; Koechlin et al., 2003; Badre and Wagner, 2004; Badre, 2008) may compromise financial risk taking. Specifically, age-related changes in prefrontal function have been associated with impairments in attention and memory (Gazzaley and D'Esposito, 2007). These age-related deficits in cognitive control may underlie age-related impairments in decision making (Brand and Markowitsch, 2010; however, see McCarrey et al., 2010). If disrupted lateral prefrontal function compromises financial risk taking, then interventions that interfere with attention and declarative memory might impair decision making.
In two studies, we sought to determine whether disruptions in mesolimbic function might account for financial mistakes, and to improve the financial risk taking of both younger and older investors. In Study 1, we tested whether individuals whose mesolimbic activity most closely tracked expected value also made more optimal risky financial choices by reanalyzing data from a recently published study (Samanez-Larkin et al., 2010). In Study 2, we examined whether increasing cognitive load or providing expected value information would alter the financial risk taking of healthy younger and older adults. Based on previous neuroimaging research, we speculated that individuals whose mesolimbic activation most closely tracked expected value would make more optimal choices in Study 1, and that provision of expected value information would improve the choices of both younger and older investors in Study 2.
METHODS
Study 1
Study 1 presents a new analysis of a recently published dataset (Samanez-Larkin et al., 2010). More detailed information on the subjects and procedures is presented in the prior publication. While previous analyses focused on age differences in NAcc activity, the goal of the present analysis was to further determine whether individual differences in rational choices correlated with the degree to which neural activation in the NAcc and MPFC tracked expected value.
Fifty-four subjects (mean age = 51.3 years, range = 21–85, 54% female) played an investment task while undergoing functional magnetic resonance imaging (fMRI). All subjects were recruited by a local survey research firm to socio-economically represent the population of the San Francisco Bay Area peninsula. Across the age range, subjects were matched on basic demographic variables (SES, income and ethnicity) by the recruitment agency. Subjects received a fixed compensation of $20 per hour, as well as a tenth of their total task earnings (or a deduction of a tenth of their total task losses), contingent on their performance.
BIAS task
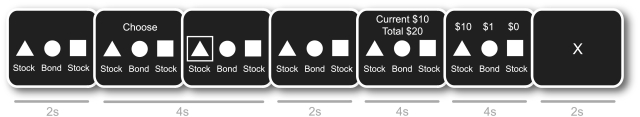
A modified version of the Behavioral Investment Allocation Strategy (BIAS) task (Kuhnen and Knutson, 2005) elicited both optimal and suboptimal financial choices from each subject. During the task, subjects completed 10 blocks of 10 trials for a total of 100 trials. During each trial (Figure 1), subjects first saw two stocks and a bond (2 s), selected an asset when prompted with the word ‘choose’, and then viewed their highlighted choice on the screen (4 s). After a brief delay (2 s) their earnings for that trial and total earnings were displayed (4 s), followed by a display of the outcomes of all assets on that trial (4 s), and finally, a fixation cross (fixation, 2 s).
Fig. 1.
Study 1 BIAS task design. The investment task used in Study 1 for functional neuroimaging. For brain imaging analyses, the rational actor’s expected value estimate was modeled during the anticipatory period prior to choice at the beginning of the trial. A response deadline for choice was set at 4 s.
At the beginning of each block, the computer randomly assigned one of the two stocks to be the ‘good’ stock and the other to be the ‘bad’ stock, without the subject’s knowledge. On average, outcomes of the good stock (i.e. +$10 with 50% probability, +$0 with 25% probability and −$10 with 25% probability) were better than outcomes of the bad stock (i.e. +$10 with 25% probability, +$0 with 25% probability and −$10 with 50% probability) for each trial. The bond paid $1 with 100% probability on each trial. Earnings were independently drawn from each distribution for each trial, and subjects were informed about the distributions before performing the task.
In the context of the BIAS task, the optimal strategy of a rational, risk-neutral agent is to pick a stock if he or she expects to receive a return that is at least as large as the bond return. Since the actual monetary amounts at stake in each trial were small (from −$1 to $1), we adopted risk neutrality as the baseline model of investor behavior—a model which assumes that individuals maximize expected return. Performance was assessed by comparing the choices of individual subjects to those made by a risk-neutral Bayesian-updating rational actor on each trial (for complete model details, see Kuhnen and Knutson, 2005). The model makes a discrete choice (i.e. chooses one asset) on each trial. Any deviation from the model by the subject (i.e. choosing either of the other two assets) on each trial was classified as a ‘mistake’.
fMRI acquisition and analyses
Images were acquired with a 1.5 T General Electric MRI scanner using a standard birdcage quadrature head coil. Twenty-four 4-mm-thick slices (in-plane resolution 3.75 × 3.75 mm, no gap) extended axially from the mid-pons to the top of the skull, providing adequate spatial resolution of subcortical regions of interest (e.g. midbrain, ventral striatum). Functional scans of the whole brain were acquired every 2 s (TR = 2 s) with a T2*-sensitive in-/out-spiral pulse sequence (TE = 40 ms, flip = 90°) designed to minimize signal dropout at the base of the brain (Glover and Law, 2001). High-resolution structural scans were subsequently acquired using a T1-weighted spoiled grass sequence (TR = 100 ms; TE = 7 ms, flip = 90°), facilitating subsequent localization and coregistration of functional data. Preprocessing and whole brain analyses were conducted using Analysis of Functional Neural Images (AFNI) software (Cox, 1996). For preprocessing, voxel time series were sinc interpolated to correct for non-simultaneous slice acquisition within each volume, corrected for three-dimensional motion, slightly spatially smoothed (FWHM = 4 mm), converted to percentage signal change and high-pass filtered.
Analyses of brain imaging data involved two steps. In the first analytic step (a within-subject analysis), preprocessed time series were submitted to a regression model that included a primary regressor of interest that indexed the rational actor’s current trial estimate of the expected value of a stock (i.e. the integrated value estimate later used in Study 2) during anticipation. Specifically, the raw expected value estimates for the individual stock that the subject subsequently chose on each trial were modeled during anticipation of choice in one single regressor. Trials where subjects chose bonds were not included in this regressor. The regression model also included covariate regressors of potential interest representing anticipation of stock vs bond choices, individual trial earnings at outcome (–$10, $0, $1, $10), and two separate regressors representing task phases (anticipation, outcome). The regression model also included covariate regressors of non-interest, which indexed cumulative earnings (current wealth earned during the task, updated at each outcome period), current trial uncertainty (updated at each market period), residual motion and trends across the scan session (i.e. baseline, linear and quadratic). Regressors of interest were convolved with a γ-variate function that modeled a canonical hemodynamic response prior to inclusion in regression models (Cohen, 1997). These statistical maps were coregistered with structural images for each individual and spatially normalized by warping to Talairach space.
The second analytic step (a between subject analysis) investigated whether individuals whose NAcc and MPFC activation closely tracked expected value also made fewer mistakes (or more choices that conformed to those of the rational actor). Across subjects, expected value coefficients derived from the first analysis were regressed against the proportion of rational stock choices and age across the whole brain. Thus, the second analysis specifically regressed coefficients representing the dynamic association of brain activity with the rational actor’s estimate of expected value for each subject against a summary measure of task performance for each subject (i.e. number of rational stock choices), controlling for age.
Voxelwise thresholds for statistical significance at the whole brain level were set at P < 0.005, uncorrected. AFNI’s AlphaSim (Cox, 1996) was used to estimate the minimum cluster size of 36 2.0-mm3 voxels for a P < 0.05 whole-brain corrected threshold. Small volume correction was applied to the NAcc at the same threshold (P < 0.005) but without the cluster criterion (which was too large to allow detection of activation in regions as small as the NAcc). In summary, this analysis examined whether individuals whose mesolimbic regions more closely tracked expected value also made fewer financial mistakes.
Methodological issues related to age differences
In all fMRI analyses, care was taken to minimize potential confounds associated with age differences in subject characteristics, brain morphology and hemodynamics (Samanez-Larkin and D'Esposito, 2008). Each individual was screened for dementia using the Mini-Mental State Exam and their structural and functional brain imaging data were inspected for abnormalities. Three individuals (not included in the 54 subjects described above) were excluded due to a structural abnormality (71-year-old male) or motion >4 mm in any dimension from one volume acquisition to the next (26- and 74-year-old male). Each individual’s brain was warped into Talairach space with reference to hand-placed anatomical landmarks.
Study 2
Subjects
A separate sample of 108 healthy subjects completed Study 2. Forty-nine younger adults between the ages of 20–35 years (mean age = 27.3, 35% female) and 59 older adults between the ages of 64–82 years (mean age = 70.6, 37% female) were recruited by a local survey research firm to socio-economically represent the population of the San Francisco Bay Area peninsula. Across age groups, subjects were matched on basic demographic variables (SES, income and ethnicity) by the recruitment agency prior to being scheduled for a laboratory visit. Across the sample, 62% percent of subjects were Caucasian, 13% Asian American, 11% Hispanic, 10% African American and 4% more than one race. Fifty-five percent of subjects were married, 40% single and 5% divorced. As displayed in Supplementary Table 1, for both trait affect and cognitive abilities, these age groups were similar to other between-group studies in the literature. Subjects received fixed compensation of $20 per hour, as well as a 10th of their total task earnings or a deduction of a 10th of their total task losses contingent on performance. Subjects in this study did not undergo fMRI.
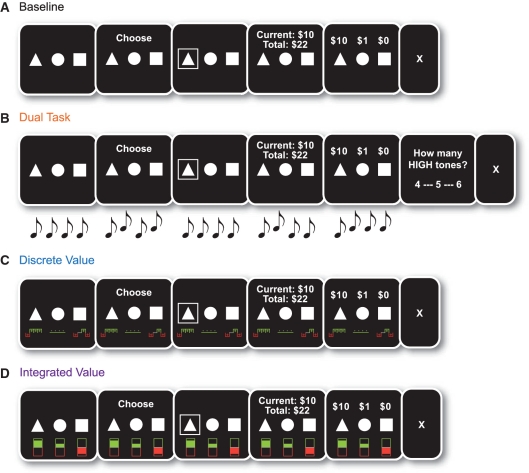
Baseline BIAS task
The same version of the BIAS task used in Study 1 was used in Study 2 as a baseline condition with two modifications. First, subjects completed five blocks of 10 trials each for a total of 50 trials (i.e. half of the trials included in Study 1). Second, the response deadline was removed for choices, such that all subject responses were self-paced. During each trial, subjects first saw two stocks and a bond (2 s), selected an asset when prompted with the word ‘choose’ (self-paced), and then viewed their highlighted choice on the screen (2 s). After a brief delay (2 s), the subjects’ earnings for that trial and total earnings were displayed (4 s), followed by the outcomes of all assets on that trial (4 s), and finally, a fixation cross (2 s; Figure 2, top row). After being led through extensive instructions by an experimenter, subjects played three blocks of practice baseline trials (totaling 30 trials) before playing the baseline task for actual cash. Although subjects viewed all probability distributions, the experimenter also explicitly stated that the stocks were risky and the bonds were riskless. For instance, an excerpt from the instructions reads: ‘once again, the three assets available to choose from are two stocks and a bond. The stocks are risky, because their earnings can be +$10, –$10 or $0. The bond is riskless, because it always pays $1’.
Fig. 2.
Study 2 modified BIAS task design. All four conditions of the modified investment task used in Study 2. (A) Baseline task, (B) dual-task condition, (C) discrete value condition and (D) integrated value condition. Although not depicted here, the asset icons (triangle, circle and square) were labeled with the words ‘stock’ or ‘bond’.
In all conditions, performance was assessed by comparing each subject’s choices to the choices of a risk-neutral rational actor on each trial. Choices that matched the model were characterized as ‘rational’ choices. Choices that deviated from the model were characterized as ‘irrational’ mistakes and classified into one of three different categories: risk-aversion (bond choice), risk-seeking (stock choice) or confusion (stock choice) mistakes. Risk-seeking mistakes occurred if subjects chose a risky option (i.e. a stock) when the riskless option (i.e. a bond) was the optimal investment. Risk-seeking mistakes tended to occur early within blocks when the rational actor lacked sufficient evidence to distinguish the good from the bad stock. Confusion mistakes occurred if subjects chose a risky option (i.e. a stock) when the other risky option (i.e. the other stock) was the optimal choice. Confusion mistakes tended to occur later within blocks when the rational actor had sufficient evidence to distinguish the good from the bad stock. Risk-aversion mistakes occurred if subjects chose the riskless option (i.e. the bond) when a risky option (i.e. a stock) was the optimal investment. Risk-aversion mistakes also tended to occur later within blocks when the rational actor had sufficient evidence to distinguish the good from the bad stock. The threshold for distinguishing between risk-seeking mistakes and confusion or risk-aversion mistakes occurred in the trial when the expected value of one stock exceeded the expected value of the bond. In Study 2, the rational actor chose the bond on 50% of trials. Thus, the maximum number of risk-seeking mistakes was 25 (out of 50 choices) in each condition and the maximum sum of confusion and risk-aversion mistakes was 25 (out of 50 choices). Analyses of individual mistake types appear in the Supplementary Results section.
Performance was examined using ANOVAs with rational choices (rational, irrational) and asset choices (stock, bond) as within-subject factors and age group (younger, older) as a between-subject factor. Follow-up t-tests are reported when main effects or interactions required further clarification. The analyses reported below focus on rational choices at baseline as well as on changes in rational choices in each manipulated condition with respect to the baseline condition. In addition to examining subjects’ investment choices, we also assessed their explicit knowledge of which assets had performed best at the end of each block (series of 10 trials). Means of rational choices on individual trials and asset knowledge at the end of blocks by age group are provided in Supplementary Tables 2 and 3, respectively.
Dual-task condition
In the dual-task manipulation, we examined whether adding an auditory task (i.e. dividing attention) previously shown to engage lateral prefrontal cortex and reduce explicit (declarative) memory (Foerde et al., 2006) would disrupt rational choices relative to baseline. Thus, a continuous series of high- and low-pitched tones were played during the display frame of each individual trial (Figure 2, second row). Subjects were asked to keep a running sum of the count of both high- and low-pitched tones during each trial. After viewing the assets, choosing, viewing the outcome and market results, subjects were asked either how many high- or how many low-pitched tones were played during that trial by choosing from one of three options. Tones were not played during the end-block question in which subjects indicated which stock performed best overall.
Discrete value condition
In the discrete value manipulation, we examined whether adding a decision aid that provided episodic value information would enhance rational choices relative to baseline. Thus, the outcomes of each individual previous trial within a block were presented to subjects as they made investment decisions. During each trial, a visual representation of the individual outcomes of all prior trials within that block appeared below each asset (Figure 2, third row). Specifically, a large green plus symbol indicated that a stock had won $10 in the past, whereas a large red minus symbol indicated that a stock had lost $10 in the past. An unsigned grey line indicated that a stock had yielded $0 in the past. A small green plus symbol (one-tenth the size of the green plus symbol corresponding to $10 under stocks) indicated that the bond had earned $1 on each previous trial. This discrete value display was updated on the first screen of the following trial. Discrete value displays were reset after each block of ten trials and did not appear during the end-block question in which subjects identified which stock seemed best overall.
Integrated value condition
In the integrated value manipulation, we examined whether adding a decision aid that provided integrated value information would enhance rational choices relative to baseline. In this integrated value condition, subjects saw a summary of the current expected value of each asset based on prior outcomes within a block as they made individual investment decisions. During each trial, a visual representation of the rational actor’s current value estimate appeared below each asset (Figure 2, bottom row). For each stock, the expected value was equal to the current probability of that stock being the ‘good’ stock multiplied by the expected value of the good stock (+$2.50) plus the current probability of that stock being the ‘bad’ stock multiplied by the expected value of the bad stock (–$2.50).
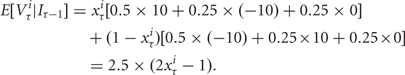 |
These estimates were updated on each trial according to Bayes’ rule. For bonds, the expected value on each trial was equal to $1 and never changed. Estimates were displayed on a bivariate ‘meter’ with an increasingly positive green bar indicating increasingly positive expected values and an increasingly negative red bar indicating increasingly negative expected values. This display of the integrated value of each asset was updated on the first screen of the following trial. The displays reset after each block of 10 trials and were not displayed during the end-block question in which subjects identified which of the stocks performed best overall.
An experimenter led subjects through a brief summary of the instructions, the probability distributions, and one sample trial of each condition before playing each of the three manipulated versions of the task. In the dual-task condition, subjects listened to sample high, low, and mixed high/low-tone series before viewing the sample trial, and were additionally asked to ‘try hard to focus on both counting tones and making wise investment decisions on every single trial’. In the decision aid conditions, subjects were informed that the additional information provided was only an aid and that they should always use their best judgment to make the final decision. Beyond explaining the information that the decision aids represented, subjects were not instructed to use the aids in any specific way. Complete task instructions can be obtained by contacting the authors.
While all subjects played the baseline condition first, the order of the subsequent manipulated blocks was counterbalanced between subjects. Outcomes were pseudorandomly generated for each condition. Specifically, multiple sets of 50 trials of outcomes were randomly drawn from the probability distributions, and four of these randomly generated series were selected for the four task conditions for all subjects. The four series of outcomes were selected such that the rational actor model earned $75 in each to control for difficulty across conditions. Outcomes earned by individual subjects, however, were determined by their individual choices and were not in any way manipulated or controlled. Subjects earned significantly less than the rational actor in all versions of the task (baseline: t107 = –11.98, P < 0.0001; dual-task: t107 = –10.89, P < 0.0001; discrete value: t107 = –10.16, P < 0.0001; integrated value: t107 = –11.89, P < 0.0001). Younger and older adults did not significantly differ in their actual earnings in the baseline, t106 = 0.30, P = 0.77, dual-task, t106 = –1.66, P = 0.10, discrete value, t106 = 1.32, P = 0.19, or integrated value, t106 = 0.02, P = 0.98, conditions.
RESULTS
Study 1
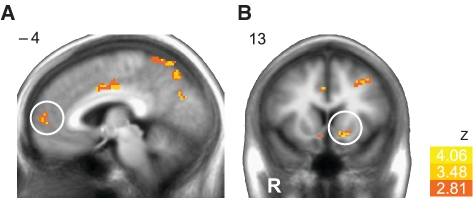
The key neuroimaging analysis examined whether individuals whose mesolimbic regions most closely tracked expected value also made more rational choices overall while investing. As predicted, whole brain regression revealed that individuals whose activation most closely tracked expected value (i.e. the rational actor’s integrated value computation) in mesolimbic regions (i.e. NAcc and MPFC) during anticipation made more rational choices overall. More specifically, this analysis revealed a correlation between coefficients representing the dynamic association of activity in mesolimbic regions with the rational actor’s estimate of expected value for each subject and a summary measure of task performance for each subject (i.e. number of rational stock choices) (Table 1 and Figure 3). This association implicates mesolimbic circuitry not only in the computation of expected value, but also in rational financial risk taking.
Table 1.
Whole brain individual difference analysis
| Region | BA | Z | Voxels | R | A | S |
|---|---|---|---|---|---|---|
| L Medial frontal gyrus | 9 | 3.633 | 86 | −13 | 49 | 20 |
| R Superior frontal gyrus | 9 | −3.841 | 44 | 35 | 45 | 30 |
| L Superior frontal gyrus | 8 | 3.794 | 125 | −23 | 29 | 44 |
| L Nucleus accumbens | 4.055 | 22 [SVC] | −15 | 14 | −8 | |
| L Insula | 13 | 3.896 | 93 | −39 | 1 | 18 |
| L Middle frontal gyrus | 6 | 3.622 | 51 | −31 | 1 | 40 |
| R Middle frontal gyrus | 9 | 3.805 | 47 | 51 | −1 | 34 |
| R Cingulate gyrus | 24 | 4.007 | 144 | 7 | −5 | 52 |
| R Precentral gyrus | 4 | 4.371 | 428 | 51 | −11 | 38 |
| L Cingulate gyrus | 24 | 3.852 | 135 | −5 | −11 | 38 |
| L Precentral gyrus | 6 | 4.177 | 264 | −59 | −15 | 42 |
| R Parahippocampal gyrus | 22 | 4.082 | 63 | 39 | −19 | −8 |
| R Inferior parietal lobule | 40 | 3.979 | 55 | 47 | −31 | 34 |
| L Superior temporal gyrus | 13 | 4.081 | 37 | −49 | −49 | 20 |
| R Superior parietal lobule | 7 | 3.541 | 41 | 25 | −51 | 50 |
| R Superior parietal lobule | 7 | 3.263 | 53 | 27 | −53 | 60 |
| L Middle temporal gyrus | 39 | 4.35 | 52 | −43 | −53 | 10 |
| L Precuneus | 7 | 4.308 | 112 | −9 | −61 | 58 |
| L Precuneus | 7 | 3.971 | 60 | −7 | −65 | 48 |
| L Cuneus | 18 | 4.123 | 45 | −3 | −75 | 28 |
BA: Brodmann area. Regions where coefficients representing the association between brain activation and the actor’s changing estimate of expected value over time were correlated (controlling for age) with individual differences in the number of rational stock choices.
Fig. 3.
Expected value signals in mesolimbic regions correlate with task performance. An individual difference analysis revealed that more accurate representation of the actor’s estimate of expected value in mesolimbic regions, the MPFC (A) and NAcc (B), at anticipation was positively correlated with rational choices in the investment task.
Beyond these regions of interest, activity in other areas associated with declarative memory (i.e. parahippocampal gyrus) and attention (i.e. superior parietal lobule) also showed a positive association between rational choices and the representation of expected value. The only brain region that showed a negative association between expected value and rational choices was a small region of the right dorsolateral prefrontal cortex (Table 1).
Study 2
Beyond comparison of age groups in a baseline condition, the key behavioral analyses focused on how manipulations of attention and value information might influence individuals’ rational choices. Based on the neuroimaging findings, we sought to determine whether manipulations of attention or value might influence both rational choices and the acquisition of explicit knowledge about which assets were best (Foerde et al., 2006). We predicted that the presentation of value information would increase rational choices in both younger and older adults.
Baseline condition
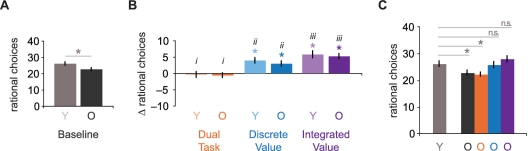
Analysis of choices in the baseline condition yielded a significant rational choice × group interaction, F1,106 = 5.824, P < 0.05, suggesting that performance differed between the two age groups. Follow-up tests confirmed that the older adults made fewer rational choices than the younger adults overall, t106 = –2.41, P < 0.05 (Figure 3A). Despite these differences in choice, older adults did not differ from younger adults in their explicit knowledge of which assets were best, since older adults did not make significantly more errors when explicitly identifying the correct stock at the end of a block, t106 = 0.64, P = 0.53.
Dual-task condition
In the dual-task (divided-attention) condition, secondary task performance (tone counting accuracy) was significantly above chance (33%) for both younger, t48 = 6.18, P < 0001; and older, t58 = 6.24, P < 0001, subjects. Although mean tone counting accuracy was numerically higher for younger (45.1%) than older adults (41.4%), the two groups did not significantly differ, t106 = –1.59, P = 0.12, suggesting a similar effect of the manipulation on attention. The dual-task condition effectively disrupted declarative memory contributions, as revealed by a significant decrease in explicit asset knowledge at the end of blocks in the dual-task condition relative to baseline in younger adults, t48 = –2.10, P < 0.05, as well as a trend toward decreased explicit asset knowledge in older adults, t58 = –1.82, P = 0.07. The two age groups did not differ, however, in this decrease in asset knowledge, t106 = –0.40, P = 0.69.
Relative to the baseline condition, a non-significant condition (baseline, dual-task) × rational choice interaction, F1,106 = 0.707, P = 0.40, revealed that the number of rational choices was similar in baseline and dual-task conditions, implying that the presence of the secondary task did not significantly influence rational choices. A non-significant interaction of condition, rational choices and age group, F1,106 = 0.03, P = 0.86, suggested that this lack of an effect of the secondary task on rational choices did not differ between younger and older adults. Follow-up tests confirmed that in the dual-task condition, the number of rational choices did not differ from baseline in younger adults, t48 = –0.42, P = 0.67; or older adults, t58 = –0.80, P = 0.43 (Figure 3B). These findings, together with the negative impact of dual-task inference on the acquisition of explicit knowledge, suggest that at least partially distinct forms of learning and memory may support investment choices and explicit asset knowledge.
Discrete value condition
A significant condition (baseline, discrete value) × rational choice interaction, F1,106 = 34.58, P < 0.001, revealed that relative to the baseline condition, the number of rational choices increased in the discrete value condition. A non-significant interaction of condition, rational choice, and age group, F1,106 = 0.68, P = 0.41, suggested that these improvements did not differ between younger and older adults. Follow-up tests confirmed that overall rational choices increased in both younger adults, t48 = 4.80, P < 0.0001; and older adults, t58 = 3.60, P < 0.001, with provision of discrete value information (Figure 3B).
The addition of discrete value information on individual trials also improved explicit asset knowledge at the end of blocks (even when decision aids were no longer visible). There was a significant increase in explicit asset knowledge in the discrete value condition in younger adults, t48 = 5.51, P < 0.0001; and older adults, t58 = 5.49, P < 0.0001. The two age groups did not differ in increased asset knowledge, t106 = 0.15, P = 0.88.
Integrated value condition
A significant condition (baseline, integrated value) × rational choice interaction, F1,106 = 67.27, P < 0.0001, revealed that relative to the baseline condition, the number of rational choices also increased in the integrated value condition. A non-significant interaction of condition, rational choice, and age group, F1,106 = 0.18, P = 0.67, suggested that these improvements did not differ between younger and older adults. Follow-up tests confirmed that overall rational choices increased in both younger adults, t48 = 5.42, P < 0.0001; and older adults, t58 = 6.19, P < 0.0001, with the provision of integrated value information (Figure 3B).
Adding integrated value information on individual trials also improved explicit asset knowledge at the end of blocks (even when decision aids were no longer visible). There was a significant increase in asset knowledge in the integrated value condition in both younger adults, t48 = 4.90, P < 0.0001, and in older adults, t58 = 5.91, P < 0.0001. The two age groups did not differ in increased asset knowledge, t106 = –0.49, P = 0.63.
Comparisons across conditions
All of the manipulations appeared to have similar effects on both age groups. Older adults, however, made fewer rational choices than younger adults at baseline and in every manipulated condition of the task (Supplementary Results section). One of the primary goals of this study was to improve the decision making of older adults. Relative to younger adults at baseline, older adults made significantly fewer rational choices at baseline and in the dual-task condition, t106 = –3.13, P < 0.01 (Figure 4C). Older adults, however, did not differ in rational choices from younger adults at baseline in either the discrete value, t106 = –0.27, P = 0.79; or integrated value, t106 = 1.08, P = 0.28, conditions (Figure 4C). The increase from baseline in rational choices was higher in the discrete value condition than the dual-task condition for both younger adults, t48 = 4.89, P < 0.0001; and older adults, t58 = 3.90, P < 0.0001. Furthermore, rational choices were even higher in the integrated value condition than in the discrete value condition for both younger adults, t48 = 2.39, P < 0.05; and older adults, t58 = 2.75, P < 0.01 (Figure 4B). Thus, providing value information (particularly in an integrated and simplified format) increased older adults’ rational choices to a level comparable to those of younger adults in the baseline condition.
Fig. 4.
Improving financial risk taking. (A) Older adults made fewer rational choices at baseline. (B) The addition of a secondary task did not disrupt performance relative to baseline (orange) for either younger (lighter bars) or older (darker bars) adults. However, the addition of discrete value information (blue) or integrated value information (purple) increased rational choices from baseline for both younger (lighter bars) and older adults (darker bars). For both age groups, the integrated value condition (iii) produced greater improvements than the discrete value condition (ii), which produced greater improvements than the dual-task condition (i). (C) Although older adults at baseline (dark grey O) and in the dual-task condition (orange O) made fewer rational choices than younger adults at baseline (light grey Y), older adults did not differ significantly in either the discrete value (blue O) or integrated value (purple O) conditions from young adults at baseline. Thus, presentation of information related to expected value matched the performance of older adults to that of younger adults at baseline. *P < 0.05; n.s.: not statistically significant.
DISCUSSION
In two studies of community members spanning a broad age range, we examined neural and behavioral evidence for individual differences in financial risk taking, and sought to identify interventions that could minimize those differences. Study 1 combined neuroimaging with an investment task to reveal that individuals whose mesolimbic activation (i.e. in the NAcc and MPFC) most closely tracked a rational actor’s expected value estimates also made the most rational risky choices. Study 2 demonstrated not only that older adults made more mistakes (or irrational choices) than younger adults (Samanez-Larkin et al., 2010), but also that task modifications related to expected value improved rational choice in both younger and older adults. Specifically, while attentional interference of declarative memory had little influence on rational choices in either group, provision of decision aids that provided value information increased rational choices in both groups, matching older adults’ rational choices to the level of younger adults at baseline. Together, these findings suggest that accurate neural representation of expected value supports rational financial risk taking, and suggest that providing expected value information can improve financial risk taking in both younger and older adults.
From a neural standpoint, these findings are consistent with the notion that optimal financial risk taking requires input from mesolimbic circuits. Remarkably, disrupting the acquisition of declarative memory by dividing attention in the dual-task condition did not compromise the rational choices of younger or older adults (although it did make younger adults more conservative in their choices; Supplementary Results section). Dividing attention did, however, reduce the accuracy of subjects’ explicit retrospective estimates of which stock was best. This dissociation between explicit report and implicit performance has previously been observed in studies in which attentional interference with explicit declarative learning occurs without influencing implicit probabilistic learning performance (Foerde et al., 2006). Neuroimaging research suggests that when lateral prefrontal resources are occupied, striatal systems may play a more prominent role in learning (Poldrack and Foerde, 2008). Although Study 2 did not assess neural activity, its behavioral findings conceptually extend the distinction between explicit and implicit processing to a group of older adults. Since a ‘functional lesion’ of declarative memory in the dual-task condition did not impair performance, these findings clearly contradict the notion that declarative memory is the primary or critical process required for rational choice in this investment task.
These findings also indicated that providing both discrete and integrated value information increased rational choices relative to baseline in both younger and older adults. Investment choices of both age groups, however, still only matched those of the rational actor less than 60% of the time, demonstrating room for further improvement (for comparison, 75% of choices of Stanford PhD students matched those of the rational actor in a previous study; Kuhnen and Knutson, 2005). In the discrete value condition, although each trial presented complete information about the prior history of outcomes, this temporally varying episodic representation of value may have misled subjects on individual trials. Previous researchers have reported choice anomalies in the context of discrete and sequentially updated value information, including illusory correlations, the gambler’s fallacy, and others (de Laplace, 1951; Tversky and Kahneman, 1971; Gilovich et al., 2002; Ayton and Fischer, 2004). Accordingly, providing integrated rather than discrete estimates of expected value further increased rational choices in both groups, suggesting that this simplified and integrated value information was more effective in improving decision making.
If integrated value information is dynamically computed by or acts through mesolimbic circuits to promote rational risk taking, then individuals whose mesolimbic activity best represents the expected value estimates of a rational actor should make the most rational choices, as seen in Study 1. Although subjects in Study 2 did not undergo neuroimaging, it is plausible that the value information they received either directly or indirectly provided a more accurate estimate of expected value to upstream neural systems that guide behavioral choice (e.g. the dorsal striatum and connected supplementary motor cortex) (O'Doherty, 2004; Knutson and Cooper, 2005).
While converging evidence across the two studies suggests that expected value information may commonly act through mesolimbic circuits to improve financial risk taking, it is also important to acknowledge that the findings of Study 2 do not provide direct verification of this underlying neural mechanism. Although NAcc and MPFC activation have been implicated in representing expected value, integrating value across different stimulus dimensions, and assigning value to appropriate actions (O'Doherty, 2004; Knutson and Cooper, 2005), external presentation of expected value information in Study 2 may have bypassed the need for mesolimbic recruitment. It is also possible that the presence of expected value information provided a concurrent complementary source of evidence via the declarative memory system. The improvements in both individual investment decisions and explicit asset knowledge in the value conditions provides some evidence for this possibility, suggesting that task performance can be based on either implicit or explicit knowledge. This is consistent with evidence from related experimental tasks which rely on experience-based learning from probabilistic feedback (Poldrack and Packard, 2003; Poldrack and Foerde, 2008; Shohamy et al., 2008; Filoteo et al., in press). The lack of a choice impairment in the dual-task condition indicates that explicit knowledge isn’t typically necessary, but the choice improvements in the presence of expected value information might suggest that explicit representations of value may supplement implicit representations under some conditions. Future neuroimaging research will be required to determine whether expected value information improves financial risk taking by modulating activity in mesolimbic or dorsolateral prefrontal circuits.
One important alternative explanation for performance differences between age groups or across conditions is that these manipulations invoked the use of different or even divergent strategies. Performance measures over time, however, suggest that subjects (either knowingly or unknowingly) approximated the strategies of the rational actor in all conditions. Specifically, subjects chose the bond early, followed by an increasing preference for the good stock over time (Supplementary Figure 1). Furthermore, in the presence of additional value information both younger and older subjects’ choices more closely matched those of the rational actor over time (Supplementary Figure 1).
Although the present analyses focused on ‘rational’ choices (or choices that converged with those of the rational actor), additional analyses of ‘mistakes’ (or choices that diverged from those of the rational actor; Supplementary Results section) revealed that age differences in performance across conditions were driven by age-related increases in mistakes when subjects chose stocks relatively early in blocks (i.e. made risk-seeking mistakes) (Supplementary Figure 2). Additionally, analyses of mistakes revealed that expected value information selectively reduced stock mistakes both early and later in blocks (Supplementary Results section). No age differences in mistakes were observed when subjects chose bonds (i.e. made risk-aversion mistakes), and presentation of expected value information did not influence risk-aversion mistakes.
While presentation of value information improved the decisions of older adults, age differences still persisted across conditions. This same pattern of findings has been documented in classic cognitive training studies (Baltes and Kliegl, 1992). Although age differences were not eliminated within any particular condition, the present findings suggest that appropriately tailored interventions can improve the decision making of older adults to the baseline performance of younger adults. In the case of financial risk taking, decision aids that provide simplified estimates of expected value may help because they mimic the output of neural mechanisms that represent expected value. Informational content alone is not sufficient, and style of presentation may also matter, since both younger and older adults improved more when presented with integrated value information rather than with discrete value information. Unfortunately, in the world of financial risk taking, expected value information often cannot be reliably computed or is not available to investors. Nonetheless, the present findings suggest that understanding how the brain processes value information may eventually inform the design of more targeted and effective behavioral interventions for investors of all ages.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Acknowledgments
We thank Daniel Yoo, Dennis Chan, Jessica Jones, Ian Bardenstein and Yasmin Saleem for assisting with data collection. This research is supported by funding from the Financial Industry Regulatory Authority Investor Education Foundation and US National Institute on Aging grants AG030778, AG024957 and AG017253. G.R.S.L. was supported by National Institute on Aging fellowship AG032804 and A.D.W. was supported by National Institute of Mental Health grant MH080309.
REFERENCES
- Aizenstein HJ, Butters MA, Clark KA, et al. Prefrontal and striatal activation in elderly subjects during concurrent implicit and explicit sequence learning. Neurobiology of Aging. 2006;27(5):741–51. doi: 10.1016/j.neurobiolaging.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Ayton P, Fischer I. The hot hand fallacy and the gambler’s fallacy: Two faces of subjective randomness? Memory and Cognition. 2004;32:1369–78. doi: 10.3758/bf03206327. [DOI] [PubMed] [Google Scholar]
- Badre D. Cognitive control, hierarchy, and the rostro-caudal organization of the frontal lobes. Trends in Cognitive Science. 2008;12(5):193–200. doi: 10.1016/j.tics.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Badre D, Wagner AD. Selection, integration, and conflict monitoring; assessing the nature and generality of prefrontal cognitive control mechanisms. Neuron. 2004;41:473–87. doi: 10.1016/s0896-6273(03)00851-1. [DOI] [PubMed] [Google Scholar]
- Baltes PB, Kliegl R. Further testing of limits of cognitive plasticity: Negative age differences in a mnemonic skill are robust. Developmental Psychology. 1992;28(1):121–5. [Google Scholar]
- Birren JE, Schaie KW. Handbook of the Psychology of Aging, 6th. San Diego: Academic Press; 2006. [Google Scholar]
- Blumenfeld RS, Ranganath C. Prefrontal cortex and long-term memory encoding: an integrative review of findings from neuropsychology and neuroimaging. Neuroscientist. 2007;13(3):280–91. doi: 10.1177/1073858407299290. [DOI] [PubMed] [Google Scholar]
- Brand M, Markowitsch HJ. Aging and decision-making: a neurocognitive perspective. Gerontology. 2010;56:319–24. doi: 10.1159/000248829. [DOI] [PubMed] [Google Scholar]
- Brewer JB, Zhao Z, Desmond JE, Glover GH, Gabrieli JD. Making memories: brain activity that predicts how well visual experience will be remembered. Science. 1998;281(5380):1185–7. doi: 10.1126/science.281.5380.1185. [DOI] [PubMed] [Google Scholar]
- Buckner RL. Memory and executive function in aging and AD: multiple factors that cause decline and reserve factors that compensate. Neuron. 2004;44(1):195–208. doi: 10.1016/j.neuron.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L, Park D. Cognitive Neuroscience of Aging: Linking Cognitive and Cerebral Aging. New York, NY: Oxford University Press; 2005. [Google Scholar]
- Cohen MS. Parametric analysis of fMRI data using linear systems methods. NeuroImage. 1997;6:93–103. doi: 10.1006/nimg.1997.0278. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Detre JA, Alsop DC, Shin RK, Atlas S, Grossman M. The neural basis of the central executive system of working memory. Nature. 1995;378(6554):279–81. doi: 10.1038/378279a0. [DOI] [PubMed] [Google Scholar]
- Davachi L. Item, context and relational episodic encoding in humans. Current Opinion in Neurobiology. 2006;16(6):693–700. doi: 10.1016/j.conb.2006.10.012. [DOI] [PubMed] [Google Scholar]
- de Laplace PSM. A Philosophical Essay on Probabilities. New York: Dover; 1951. [Google Scholar]
- Denburg NL, Tranel D, Bechara A. The ability to decide advantageously declines prematurely in some normal older persons. Neuropsychologia. 2005;43(7):1099–1106. doi: 10.1016/j.neuropsychologia.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Fera F, Weickert TW, Goldberg TE, et al. Neural mechanisms underlying probabilistic category learning in normal aging. Journal of Neuroscience. 2005;25(49):11340–8. doi: 10.1523/JNEUROSCI.2736-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filoteo JV, Lauritzen S, Maddox WT. Removing the frontal lobes: the effects of engaging executive functions on perceptual category learning. Psychological Science. 2010;21(3):415–23. doi: 10.1177/0956797610362646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foerde K, Knowlton BJ, Poldrack RA. Modulation of competing memory systems by distraction. Proceedings of the National Academy of Sciences of United States of America. 2006;103(31):11778–83. doi: 10.1073/pnas.0602659103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaley A, D'Esposito M. Top-down modulation and normal aging. Annals of the New York Academy of Science. 2007;1097:67–83. doi: 10.1196/annals.1379.010. [DOI] [PubMed] [Google Scholar]
- Gilovich T, Griffin D, Kahneman D. Heuristics and Biases: The Psychology of Intuitive Judgment. Cambridge: Cambridge University Press; 2002. [Google Scholar]
- Glover GH, Law CS. Spiral-in/out BOLD fMRI for increased SNR and reduced susceptibility artifacts. Magnetic Resonance Medicine. 2001;46:515–22. doi: 10.1002/mrm.1222. [DOI] [PubMed] [Google Scholar]
- Head D, Snyder AZ, Girton LE, Morris JC, Buckner RL. Frontal-hippocampal double dissociation between normal aging and Alzheimer's disease. Cerebral Cortex. 2005;15(6):732–9. doi: 10.1093/cercor/bhh174. [DOI] [PubMed] [Google Scholar]
- Hedden T, Gabrieli JD. Insights into the ageing mind: a view from cognitive neuroscience. Nature Reviews Neuroscience. 2004;5(2):87–96. doi: 10.1038/nrn1323. [DOI] [PubMed] [Google Scholar]
- Henninger DE, Madden DJ, Huettel SA. Processing speed and memory mediate age-related differences in decision making. Psychology and Aging. in press doi: 10.1037/a0019096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks LH, Birren JE. Aging, brain damage, and psychomotor slowing. Psychological Bulletin. 1970;74(6):377–96. doi: 10.1037/h0033064. [DOI] [PubMed] [Google Scholar]
- Huang C, Litzenberger RH. Foundations for Financial Economics. Upper Saddle River, NJ: Prentice Hall; 1988. [Google Scholar]
- Knutson B, Bossaerts P. Neural antecedents of financial decisions. Journal of Neuroscience. 2007;27(31):8174–7. doi: 10.1523/JNEUROSCI.1564-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Cooper JC. Functional magnetic resonance imaging of reward prediction. Current Opinion in Neurology. 2005;18(4):411–7. doi: 10.1097/01.wco.0000173463.24758.f6. [DOI] [PubMed] [Google Scholar]
- Knutson B, Delgado MR, Phillips PEM. Representation of subjective value in the striatum. In: Glimcher PW, Camerer CF, Fehr E, Poldrack RA, editors. Neuroeconomics: Decision Making and the Brain. London: Academic Press; 2008. pp. 389–406. [Google Scholar]
- Knutson B, Taylor J, Kaufman M, Peterson R, Glover G. Distributed neural representation of expected value. Journal of Neuroscience. 2005;25(19):4806–12. doi: 10.1523/JNEUROSCI.0642-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koechlin E, Ody C, Kouneiher F. The architecture of cognitive control in the human prefrontal cortex. Science. 2003;302(5648):1181–5. doi: 10.1126/science.1088545. [DOI] [PubMed] [Google Scholar]
- Kuhnen CM, Knutson B. The neural basis of financial risk taking. Neuron. 2005;47(5):763–70. doi: 10.1016/j.neuron.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Mather M. A review of decision making processes: weighing the risks and benefits of aging. In: Carstensen LL, Hartel CR, editors. When I'm 64. Washington, DC: The National Academies Press; 2006. pp. 145–73. [Google Scholar]
- McCarrey AC, Henry JD, Luszcz M. Potential mechanisms contributing to decision-making difficulties in late adulthood. Gerontology. 2010 doi: 10.1159/000275060. [E-pub ahead of print: January 12] [DOI] [PubMed] [Google Scholar]
- Mell T, Wartenburger I, Marschner A, Villringer A, Reischies FM, Heekeren HR. Altered function of ventral striatum during reward-based decision making in old age. Frontiers in Human Neuroscience. 2009;3(34):34. doi: 10.3389/neuro.09.034.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Mohr PN, Li S-C, Heekeren HR. Neuroeconomics and aging: neuromodulation of economic decision making in old age. Neuroscience and Biobehavioral Reviews. 2009;34:678–88. doi: 10.1016/j.neubiorev.2009.05.010. [DOI] [PubMed] [Google Scholar]
- O'Doherty JP. Reward representations and reward-related learning in the human brain: insights from neuroimaging. Current Opinion in Neurobiology. 2004;14(6):769–76. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Paller KA, Wagner AD. Observing the transformation of experience into memory. Trends in Cognitive Science. 2002;6(2):93–102. doi: 10.1016/s1364-6613(00)01845-3. [DOI] [PubMed] [Google Scholar]
- Park DC, Lautenschlager G, Hedden T, Davidson NS, Smith AD, Smith PK. Models of visuospatial and verbal memory across the adult life span. Psychology and Aging. 2002;17:299–320. [PubMed] [Google Scholar]
- Poldrack RA, Foerde K. Category learning and the memory systems debate. Neuroscience and Biobehavioral Reviews. 2008;32(2):197–205. doi: 10.1016/j.neubiorev.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Packard MG. Competition among multiple memory systems: converging evidence from animal and human brain studies. Neuropsychologia. 2003;41(3):245–251. doi: 10.1016/s0028-3932(02)00157-4. [DOI] [PubMed] [Google Scholar]
- Ranganath C, D'Esposito M. Medial temporal lobe activity associated with active maintenance of novel information. Neuron. 2001;31(5):865–73. doi: 10.1016/s0896-6273(01)00411-1. [DOI] [PubMed] [Google Scholar]
- Rubin DC. Frontal-striatal circuits in cognitive aging: evidence for caudate involvement. Aging, Neuropsychology, and Cognition. 1999;6(4):241–59. [Google Scholar]
- Salthouse TA. What and when of cognitive aging. Current Directions in Psychological Science. 2004;13(4):140–4. doi: 10.1177/0963721414535212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanez-Larkin GR, Carstensen LL. Socioemotional functioning and the aging brain. In: Decety J, Cacioppo JT, editors. The Handbook of Social Neuroscience. New York: Oxford University Press; in press. [Google Scholar]
- Samanez-Larkin GR, D'Esposito M. Group comparisons: imaging the aging brain. Social Cognitive and Affective Neuroscience. 2008;3(3):290–7. doi: 10.1093/scan/nsn029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanez-Larkin GR, Gibbs SEB, Khanna K, Nielsen L, Carstensen LL, Knutson B. Anticipation of monetary gain but not loss in healthy older adults. Nature Neuroscience. 2007;10(6):787–91. doi: 10.1038/nn1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanez-Larkin GR, Kuhnen CM, Yoo DJ, Knutson B. Variability in nucleus accumbens activity mediates age-related suboptimal financial risk taking. Journal of Neuroscience. 2010;30(4):1426–34. doi: 10.1523/JNEUROSCI.4902-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohamy D, Myers CE, Kalanithi J, Gluck MA. Basal ganglia and dopamine contributions to probabilistic category learning. Neuroscience & Biobehavioral Reviews. 2008;32(2):219–36. doi: 10.1016/j.neubiorev.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon HA. Models of Bounded Rationality. Cambridge, MA: MIT Press; 1982. [Google Scholar]
- Tversky A, Kahneman D. Belief in the law of small numbers. Psychological Bulletin. 1971;76:105–10. [Google Scholar]
- Wagner AD, Schacter DL, Rotte M, et al. Building memories: remembering and forgetting of verbal experiences as predicted by brain activity. Science. 1998;281(5380):1188–91. doi: 10.1126/science.281.5380.1188. [DOI] [PubMed] [Google Scholar]
- Yacubian J, Gläscher J, Schroeder K, Sommer T, Braus DF, Büchel C. Dissociable systems for gain- and loss-related value predictions and errors of prediction in the human brain. Journal of Neuroscience. 2006;26(37):9530–7. doi: 10.1523/JNEUROSCI.2915-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.