Abstract
People have present-biased preferences: they choose more impatiently when choosing between an immediate reward and a delayed reward, than when choosing between a delayed reward and a more delayed reward. Following McClure et al. [McClure, S.M., Laibson, D.I., Loewenstein, G., Cohen, J.D. (2004). Separate neural systems value immediate and delayed monetary rewards. Science, 306, 503.], we find that areas in the dopaminergic reward system show greater activation when a binary choice set includes both an immediate reward and a delayed reward in contrast to activation measured when the binary choice set contains only delayed rewards. The presence of an immediate reward in the choice set elevates activation of the ventral striatum, pregenual anterior cingulate cortex and anterior medial prefrontal cortex. These dopaminergic reward areas are also responsive to the identity of the recipient of the reward. Even an immediate reward does not activate these dopaminergic regions when the decision is being made for another person. Our results support the hypotheses that participants show less affective engagement (i) when they are making choices for themselves that only involve options in the future or (ii) when they are making choices for someone else. As hypothesized, we also find that behavioral choices reflect more patience when choosing for someone else.
Keywords: present-biased preferences, intertemporal discounting, fMRI, multiple systems hypothesis
Offered the choice between two monetary rewards, most people would prefer $10 today over $12 in a week, but preferences typically switch when people are offered $10 in a year or $12 in a year and a week (Ainslie, 1975). Examples like this have led some researchers to argue that people have dynamically inconsistent time preferences. Specifically, people prefer to be ‘impatient' when immediate gratification is an option, but tend to be patient when making tradeoffs between ‘future' rewards (Ainslie, 1975). In this study we examine whether such preference reversals also arise when actors make intertemporal decisions for another person.
Decision-making for another person is an important topic of study for two reasons. First, it sheds light on decision-making mechanisms. For example, if time delay is the only important characteristic of an intertemporal choice, then moving a choice from the ‘own’ domain to the ‘other-agent’ domain should not affect the outcome. However, changing the domain may matter if choices for oneself trigger different processes in the brain than choices for others. For instance, for a smoker, foregoing a cigarette is affectively different from advising someone else to quit.
Second, decision-making for another person has always been widespread—think of the leading example of parents and minors—and is becoming even more important. Delegation from one adult to another is commonplace in modern societies that are characterized by a high degree of division of labor: for example, politicians represent the interests of their constituents, asset managers make decisions for their investors, physicians make medical choices for their patients and attorneys negotiate for their clients. Moreover, delegated choices are likely to become even more important as people live longer and declining health—including cognitive impairment—force older adults to rely on their families and other agents (like trustees, fiduciaries and physicians) to make decisions on their behalf. Advanced medical directives are just one important example of this ongoing trend.
In this article, we present an fMRI-study on brain correlates of intertemporal choice for oneself or for another person. Our study design is motivated by the hypothesis that the degree to which a participant’s choice behavior for herself is either patient or impatient arises from an interplay of emotional and cognitive processes. It is not clear how many processing systems are engaged in this interplay, but some researchers have argued for two basic systems, which are also sometimes referred to as hot- and cool- or System 1 and 2 (Metcalfe and Mischel, 1999; Kahneman, 2003; Mischel and Ayduk, 2003; McClure et al., 2007a; Rustichini, 2008). In most of these dual-process theories, it is assumed that the cool (reasoning) system is a deliberative, slow, rule-governed and emotionally neutral system, which is the seat of self-control, while the hot (affective) system is fast and automatic, and prone to develop earlier in life (Kahneman, 2003; Metcalfe and MIschel, 1999). The activation of the hot system is suggested to occur automatically and to be imperfectly monitored by the cool system (Metcalfe and Mischel, 1999).
Thus, in the case of intertemporal decisions in which participants need to trade off smaller, immediate rewards with larger, later rewards, the hot system is assumed to typically prefer immediate rewards, which promise instant gratification, while the cool system may intervene to ‘argue’ for the delayed, but larger option, which is more rewarding in the long term (Mischel and Ayduk, 2003; Rustichini, 2008). When the intertemporal choice concerns two differently delayed rewards of which both are in the future, no immediate gratification is possible, and hence relatively little engagement of the hot system would be expected.
McClure et al. (2004) have investigated the neural basis of intertemporal preferences for oneself and found that people, offered the choice between a smaller, sooner and a larger, later monetary reward, showed different neural activation patterns depending on the date at which the earlier reward was made available. Only choice sets including an immediate reward were accompanied by strong activation in the dopaminergic reward system. However, all decisions generated roughly similar levels of activation in the lateral prefrontal and posterior parietal cortex, which are known for their involvement in higher order cognitive functions. The authors argue that this evidence supports the distinction between a hot and a cool system, where the activation in the dopaminergic reward system is the neural correlate of the hot (impatient) system, and the lateral prefrontal and parietal activation reflects the influence of the cool (patient) system. However, the interpretations proposed by McClure et al., have been disputed and the multiple systems hypothesis remains speculative (Kable and Glimcher, 2007; Kalenscher and Pennartz, 2008).
A crucial question arising from the findings of McClure et al. (2004) is whether the activation of separate neural systems when immediate or only delayed rewards are available is a robust phenomenon that is independent of who is the beneficiary of the rewards. As argued above, there are many situations in everyday-life when people are responsible for making decisions for others. There are reasons to assume that decision-making for another person—instead of for oneself—may have the property of generating less concern for immediate gratification. Hence, having a third-party decide may engender more patient decisions, particularly in the presence of immediately available rewards.
Recent evidence provides support for this conjecture. Ersner-Hershfield and colleagues (2009) have found a correlation between activation in the pregenual anterior cingulate gyrus and behavioral discounting measures when subjects make current-self-related judgments. No correlation has been found, however, if subjects had to make judgements about their future self, or current- or future-related judgments about other persons. These results may carry over to intertemporal choices with real consequences for oneself or another person, such that choices for a current self are different from choices made for a future self, a current other or a future other. Our study addresses this question.
Since behavioral studies on decision-making for oneself and for other persons have often elicited non-congruent choices (Borresen, 1987; Hsee and Weber, 2001; Stone et al., 2002), Beisswanger et al. (2003) have suggested that the degree of emotional involvement in a task might be a decisive factor. The more one is emotionally involved in a decision-making task when deciding for oneself, the larger are the behavioral differences in decision-making for oneself or for other persons. This is in line with the assumption that decisions for other persons cause less emotional involvement, hence making the difference larger between one’s emotional arousal for decisions concerning oneself and decisions concerning others. Hence, we hypothesize that there is less affective- and reward-related neural activation (McClure et al., 2004; 2007b) when choices including an immediate option are made for another person compared to choices made for oneself. We hypothesize that this will be the case because of less personal involvement (Moran et al., 2006) and thus no reward expectation (Knutson and Peterson, 2005) and less emotional involvement (Grezes et al., 2006) when making decisions for other persons.
Based on the findings by Beisswanger et al. (2003), we further hypothesize that these activation differences will be larger in impulsive participants—i.e. participants who are estimated to have relatively high short-run discount rates when making choices for themselves—as high short-run discount rates are assumed to correspond to high levels of emotional involvement (Metcalfe and Mischel 1999; McClure et al., 2004, 2007a and b). Behaviorally, we therefore expect the most impulsive participants to show reductions in impulsivity when their choices for other people are compared to their choices for themselves. We do not expect to observe a substantial difference between other- and own-choices for participants with moderate levels of discounting.
METHODS
To test our predictions, we used the experiment developed by McClure et al. (2004). Participants (28 total participants; 14 female) chose between a series of sooner-smaller and later-larger rewards for themselves (SELF-condition) as well as for another person (i.e. a stranger they would never meet, OTHER-condition). In both the SELF and OTHER conditions, there were some trials in which participants chose between an immediate and a delayed reward (today trials), and some trials in which participants chose between two rewards that were both available in the future at different delays (delay trials). The delay to the sooner-smaller reward was either zero (in today trials) or 2 or 4 weeks (in delay trials). The amount of additional delay between the sooner-smaller reward and the larger-later reward was either 2 or 4 weeks.
Choices were presented in two parts (one for SELF and one for OTHER), in an order balanced for receiver (SELF or OTHER). Only after having made their decisions for the 40 individually pseudo-randomized binary-choice trials in the first part, participants were informed about the rules of the second part (where the condition changed from SELF to OTHER or vice versa). At the end of the experiment, one binary-choice trial from each part of the experiment was randomly selected and the reward chosen by the participant in that binary-choice trial was paid to the actual decision-maker (in the case of a choice from the SELF-condition) or to another person (in the case of a choice from the OTHER-condition) at the appropriate delay time. All participants also received a flat reward of €5 for making choices for the other person, irrespective of what they chose. (See Supplementary Data for further details.)
For the analysis we performed a median split of our sample by how much participants discounted future rewards when choosing for themselves (using the choices from the SELF-condition). Specifically, we used a maximum likelihood logit estimator to estimate the parameters of the quasi-hyperbolic discount function (Laibson, 1997).1 The quasi-hyperbolic discount function parametrically captures the preference for immediate gratification. This discount function contains two critical parameters; β representing the special discount factor that uniformly down-weights all rewards that are not immediate rewards, and δ representing the general exponential discount factor that geometrically discounts all rewards. Thus, the value of a reward u received immediately is u. The discounted value vt of a reward u received at delay t > 0 is vt = βδtu.
We then split our sample into two groups, separating the strong discounters (with βs below the median) from the moderate discounters (with βs above the median; see Supplementary Table S3 in the Supplementary Data).
BEHAVIORAL RESULTS
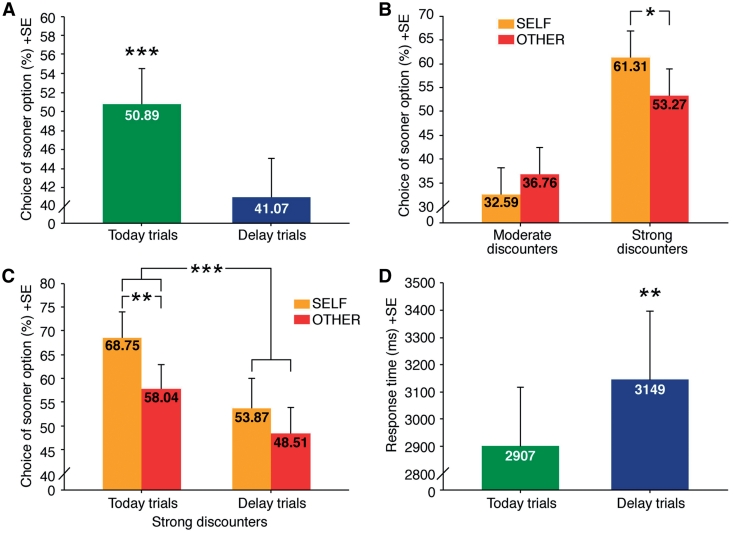
To test our behavioral hypotheses, we carried out a repeated-measurement analysis of variance (ANOVA) for ‘choice’ (percentage of times the sooner, but smaller option was chosen), including the two-level within-subject variables ‘receiver’ (SELF or OTHER) and ‘temporal distance’ (today trials, in which one of the two rewards was available immediately, or delay trials, in which both rewards were available in the future) as well as the between-subject variable type (moderate or strong discounters). The analysis yielded a main effect of ‘temporal distance', with the sooner option chosen significantly more often in today than in delay trials [F(1,26) = 34.93, P < 0.001; Figure 1A]. The comparison further revealed a main effect of the between-subject variable type [F(1,26) = 9.12, P = 0.005], which supports our division of participants into strong and weak discounters. There was no main effect of receiver [F(1,27) = 0.481, P = 0.494], but a significant interaction of type and receiver [F(1,26) = 5.61, P = 0.026]. Paired-sample t-tests revealed that only participants who strongly discounted future rewards chose the sooner option for themselves more often than for the other person [t(13) = −2.34, P = 0.036, Figure 1B). A further analysis showed that this difference was significant, though, only for today trials. This confirms our hypothesis by showing that strongly discounting participants became more patient in the OTHER-condition by choosing more frequently the larger, but later reward in today trials [t(13)=3.18, P = 0.007]. There were no such choice differences between SELF and OTHER in delay trials [t(13) = 1.45, P = 0.170, Figure 1C]. In other words, moving from the SELF to the OTHER task did not affect trials in which subjects were choosing between two delayed options.
Fig. 1.
(A) Choice of sooner option significantly differs for today and delay trials [F(1,27) = 33.62, P < 0.001]. (B) Strong discounters significantly more often chose the sooner reward for SELF than for OTHER [t(13) = −2.34, P = 0.036]. (C) Strong discounters chose the sooner reward in SELF significantly more often than in OTHER only in today trials [t(13) = 3.18, P = 0.007]. (D) Response time is significantly shorter in today trials [F(1,27) = 9.12, P = 0.005). *P < 0.05, **P < 0.01, ***P < 0.001; error bars represent standard errors (SE)].
Using the same independent variables as above, a repeated-measurement ANOVA of ‘response time’ confirmed a main effect of ‘temporal distance’, with participants choosing faster when an immediate reward was available [F(1,26) = 12.33, P = 0.002; Figure 1D). No interaction effect could be shown, i.e. this response time difference was found for both strong and moderate discounters.
IMAGING RESULTS
Single subject contrast images generated for every participant were entered into a second-level analysis on the basis of Bayesian statistics (Neumann, 2003). In this approach, posterior probability maps and maps of the effect size are calculated on the basis of the resulting least-squares estimates of parameters for the general linear model (GLM). The output of the Bayesian second-level analysis is a probability map showing the probability that the contrast is greater than zero. For visualization, a threshold of 99% was applied to the probability maps.
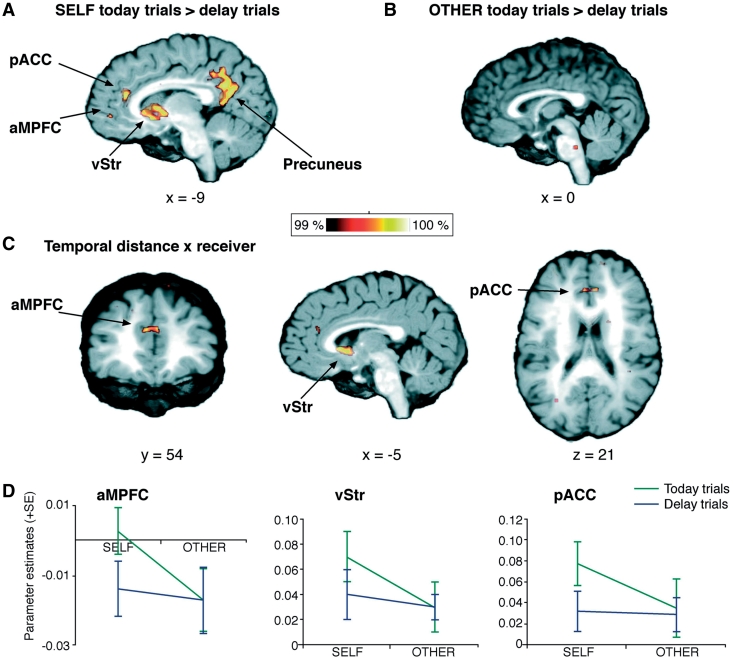
We investigated the hemodynamic response elicited by all trials that include an immediate option (today trials) in contrast to all trials without an immediate option (delay trials), separately for choices made for SELF and OTHER.
In SELF, we found higher hemodynamic activity for choices including an immediate reward within the pregenual anterior cingulate cortex (pACC, BA 32), ventral striatum, anterior medial prefrontal cortex (aMPFC), and anterior and posterior precuneus (Figure 2A).
Fig. 2.
(A) Brain regions that were activated by choices containing an immediate option compared to choices with only delayed options in SELF. (B) There were no such activation differences between today and delay trials in OTHER. (C) An interaction contrast of temporal distance (today vs delay trials) and receiver (SELF vs OTHER) showed activation differences within the anterior medial prefrontal cortex (aMPFC), ventral striatum (vStr) and pregenual anterior cingulate cortex (pACC). (For visualization, a threshold of 99% was applied to the probability maps.) (D) Parameter estimates indicate that these activation differences were mainly due to elevated activation in today trials in SELF, whereas in all other conditions activation in these brain areas was similarly low.
In OTHER, there was no higher hemodynamic activity for choices including an immediate option within any of these areas (Figure 2B).
To analyse the interaction of differences between today and delay trials with the differences between SELF and OTHER, we calculated an interaction contrast of ‘temporal distance’ (today vs delay trials) with receiver (SELF vs OTHER). We found activation differences in the pACC, aMPFC and ventral striatum (Figure 2C).
To test for whether today trials for SELF are really special and are showing higher activations than any other condition, these areas were subjected to a further post hoc analysis. Figure 2D shows the mean parameter estimates (i.e. parameters from the GLM) of the different conditions, indicating that the most elevated activation usually took place during choices for SELF in today trials.
The only significant differences in our regions of interest between the SELF and OTHER conditions (in either direction) arose from the interaction with immediacy. No activation differences in these regions were found when contrasting choices made for SELF with choices made for OTHER in general.
We also wanted to identify hemodynamic activity differences within these regions depending on how patiently or impatiently participants were choosing in the SELF-condition. Hence, we again carried out the aforementioned analyses of contrasting today trials with delay trials, this time for participants with high and low discount values separately.
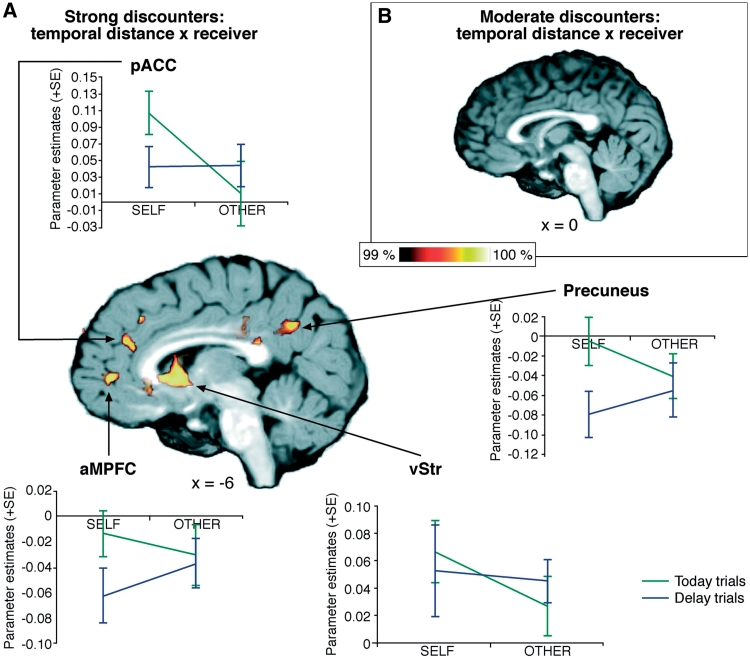
For participants who strongly discounted future rewards, we found higher hemodynamic activity for today trials compared to delay trials in SELF in the pACC, aMPFC, ventral striatum and anterior and posterior precuneus. However, when looking at the same contrast for more patient participants (who discounted future rewards only weakly) elevated activity within the network was not observed.
In the OTHER condition, high-discounting participants showed almost no elevated activation in the areas that were activated in the SELF condition, but only showed an elevated activity within the right MPFC.
An interaction contrast of temporal distance and receiver for participants who strongly discounted future rewards yielded the following activation areas: the pACC, aMPFC, ventral striatum and anterior precuneus. Most elevations took place only when choices for SELF were made in today trials (Figure 3A).
Fig. 3.
Contrast values and brain regions with activation differences in the interaction contrast (temporal distance × receiver) (A) for strong and (B) moderate discounters. (For visualization, a threshold of 99% was applied to the probability maps.)
An interaction contrast of ‘temporal distance’ and ‘receiver’ for moderate-discounting participants yielded no such neural activation differences (Figure 3B).
It is noteworthy that we performed another robustness check by implementing a different median split of subjects, using the parameter β based on choices for the OTHER condition. Based on this classification of impulsive and non-impulsive discounters (for other subjects) we then checked whether neural activation in the SELF condition differed when comparing the impulsive (low β) and non-impulsive (high β) participants. We found no significant differences. This implies that impulsiveness when choosing for OTHER does not predict brain activation when choices are made for oneself.
DISCUSSION
When analysing choice sets including an immediate reward for SELF, we found an activated network of brain areas thought to be engaged in emotion- and reward-related processes (McClure et al., 2004, 2007b). Specifically, the ventral striatum, pACC and aMPFC were found to be especially activated for choices in today trials made for oneself compared to either choices made for another person or to choices made in delay trials for oneself (Figure 3). From these findings we conclude that choices for SELF involving an immediate option differentially activate an affective brain network. This network is implicated in choices associated with the possibility of immediate gratification of one’s own needs.
With respect to the involvement of the ventral striatum in emotion-driven processes such as opting for immediate rewards, note that the ventral striatum has been found to have strong reciprocal connections to neurons in the midbrain dopamine system (Schultz et al., 1997; Breiter et al., 2001). The midbrain dopamine system is thought to play a role in reward-dependent learning (Schultz et al., 1997). Furthermore, findings of imaging studies suggest that the ventral striatum is also activated by reward anticipation (Knutson et al., 2001) and that this activation is higher for more immediate rewards compared to more delayed ones (McClure et al., 2004).
Like the ventral striatum, the ACC receives rich dopaminergic innervations, which indicates that it may be involved in reward-related processes (Gaspar et al., 1989; Schultz, 1998). Based on cytoarchitectural, lesion, electrophysiological and imaging studies, the ACC has been divided into subregions, with the ventral ones being responsible for the processing of emotions, such as happiness, sadness and fear (Bush et al., 2000; Vogt, 2005). Among other areas, the emotional part has connections to the nucleus accumbens of the ventral striatum and orbitofrontal cortex (Devinsky et al., 1995), both also found to be activated by reward-related stimuli (Knutson et al., 2001; McClure et al., 2004; Winstanley et al., 2004). The elevated activity associated with the interaction of SELF and today trials was located in the pregenual ACC, which is an area in the ventral part of the ACC, anterior to the genu of the corpus callosum. This part of the ACC has been found to be engaged in decisions involving gambles containing large gains (Rogers et al., 2004) and has also been associated with happy emotions (Vogt, 2005).
We also observe heightened aMPFC activation for today trials in the SELF condition. The MPFC is active in self-related judgments (Craik et al., 1999; Kelley et al., 2002; Ochsner et al., 2004), thought and attention (Gusnard and Raichle, 2001). It has also been found to be involved in the processing of externally and internally cued emotions (Lane et al., 1997, 1998). It has been suggested therefore that the MPFC might be engaged in identifying and evaluating positive emotions (Drevets and Raichle, 1998). In our study, this region showed more activation when participants chose a reward for themselves instead of for another person. The MPFC’s higher engagement also in today trials shows that participants were more self-focused when there was an immediate option, and possibly more engaged with their own happy emotions towards immediate rewards, perhaps evaluating how good exactly such immediate gratification would feel.
These findings are consistent with behavioral results by Sayette and colleagues (2008), who reported that participants in a low-craving state for cigarettes underestimated future craving, suggesting a cold-to-hot empathy gap: participants could not empathize with a future self and hence not correctly predict future states and preferences they would hold in these states. Differences in neural activation concerning choices for a present and a future self could be responsible for this empathy gap.
When looking at choices made for another person, we also found results in accordance with our hypothesis: There were no ROI differences between today and delay trials when choices were made for another person. Furthermore, there were no such neural activation differences in delay trials between choices made for oneself and choices made for another person. In sum, the choices made in today trials for OTHER seem not to be based on the same emotions and reward expectations that were engaged by SELF trials. Our behavioral hypotheses were also supported by our results. Participants who strongly discounted future rewards chose the immediate option more often in SELF than in OTHER, which is consistent with the findings in Beisswanger et al. (2003), who assume that behavioral differences in decision-making for SELF and OTHER are greater the greater the emotional involvement of the participants. Hence, while strong discounters act highly impatiently when choosing immediate rewards for themselves, they act more patiently when choosing for another person.
However, looking at the imaging results when comparing today with delay trials for OTHER in strongly discounting participants, we do not find neural activation that explains why the sooner, smaller reward was still preferred to the later, larger reward more often in today than in delay trials. The same is true for comparing today and delay trials of moderately discounting participants both in SELF and OTHER. Thus, although strongly discounting participants chose more patiently for the other person than for themselves, they still chose dynamically inconsistently for the other person, as did moderately discounting participants. One explanation is related to the manner in which decisions are taken in the two different conditions. The activation within the aMPFC and precuneus, which was found to be responsible for mostly self-related episodic memory retrieval and evaluation (Zysset et al., 2002; Addis et al., 2004), suggests that in SELF there was a new evaluation based on the question what was preferred ‘right now’ before ‘every choice', while in OTHER participants might have employed another more general strategy that did not rely on a repeated evaluation of what the other person might have preferred. This conjecture could not be evaluated with the present experiment, though, but it seems plausible and in keeping with our behavioral and neural findings, implying a need for additional research.
Addressing possible limitations of our study it is, first, important to consider the perception of ‘today’ in SELF and OTHER. While ‘today’ in SELF is clearly identified as the end of the experimental session, ‘today’ in OTHER was specified slightly differently for logistical reasons. In the experimental instructions for OTHER it was stated that the other person is ‘a participant in a subsequent experimental session’. The instructions go on to say that: ‘in each choice, you will have to choose between two amounts of money. Importantly, with every choice, you will simultaneously with the amount of money select a date, at which the other person will receive this amount: there are four possible payment dates: (i) today, (ii) in 2 weeks, (iii) in 4 weeks or (iv) in 6 weeks. If you choose the amount with the date “today”, the other person will receive the money immediately after her experimental session. If you choose an amount with the date (ii), (iii) or (iv), the other person will receive the money at the selected date’. In our view, this leaves the subject with the strong impression that the other subject will receive rewards today if that is what the original subject chooses. In fact, for 24 out of 28 participants the subsequent session for the OTHER subject took place later on the very same day. In four cases, however, it was not possible to do so, and in these cases the session for the OTHER subject took place on the next day. We can not rule out the possibility that there may have been some ambiguity about what subjects perceived when thinking about ‘today’ for themselves vs ‘today’ for the OTHER subject. Future work should clarify whether it makes a difference if a participant is explicitly told that the other person is participating on the same day (and in that case the experiment would be run so that this outcome is indeed guaranteed).
Another limitation is that our experimental design is not suited to resolve the issue of whether decision-making is driven by a unitary processing system or by multiple processing systems in the brain. Hare et al. (2009) investigate experimentally the appliance of self-control, though their work does not resolve this issue. Rustichini (2008) provides a review of the evidence on both sides of the debate.
To conclude, our main results imply that the processes underlying intertemporal choices that involve immediate rewards for oneself are different from processes underlying the evaluation of immediate rewards for others. Activations in emotion- and reward-related brain areas suggest that affective processes occur primarily when immediate gratification for oneself is possible. Making decisions for another person does not elicit activation in reward-related brain areas, explaining why in particular impulsive participants choose relatively patiently when making decisions for others.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Acknowledgments
The authors thank B. Johst for support in programming, E. Weig for research assistance in preparing the stimulus material and S. Liebig and H. Schmidt-Duderstedt for the figures. Furthermore, we thank J. Stevens, D. Taubinsky and S. Zysset for their comments and assistance in the data analysis.
Footnotes
1We use this quasi-hyperbolic model instead of other proposed models of time preference (Samuelson, 1937; Koopmans, 1960; Green et al., 1994), because the quasi-hyperbolic model has been found to fit experimental data well and is consistent with the two-systems approach (McClure et al., 2004). However, in order to check robustness, we have also estimated a hyperbolic function 1/(1+kt). A median split of subjects based on the hyperbolic function yields the same group assignments (into more and less impulsive subjects) as the quasi hyperbolic function, except for two subjects switching groups. Also note that the correlation between β in the quasi-hyperbolic model and k in the hyperbolic model is very high and significant (Spearman’s rho = −0.79, P < 0.0001).
REFERENCES
- Addis DR, McIntosh AR, Moscovitch M, Crawley AP, McAndrews MP. Characterizing spatial and temporal features of autobiographical memory retrieval networks: a partial least squares approach. NeuroImage. 2004;23:1460–71. doi: 10.1016/j.neuroimage.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Ainslie G. Specious reward: a behavioral theory of impulsiveness and impulse control. Psychological Bulletin. 1975;82:463–96. doi: 10.1037/h0076860. [DOI] [PubMed] [Google Scholar]
- Beisswanger AH, Stone ER, Hupp JM, Allgaier L. Risk taking in relationships: differences in deciding for oneself versus for a friend. Basic and Applied Social Psychology. 2003;25:121–35. [Google Scholar]
- Borresen CR. Decision making as a function of self and others. Perceptual and Motor Skills. 1987;64:1301–2. [Google Scholar]
- Breiter HC, Aharon I, Kahneman D, Dale A, Shizgal P. Functional imaging of neural responses to expectancy and experience of monetary gains and losses. Neuron. 2001;30:619–39. doi: 10.1016/s0896-6273(01)00303-8. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Science. 2000;4:215–22. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Craik F, Moroz T, Moscovitchm M, et al. In search of the self: a positron emission tomography study. Psychological Science. 1999;10:26–34. [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118:279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Raichle ME. Reciprocal suppression of regional cerebral blood flow during emotional versus higher cognitive processes: implications for interactions between emotion and cognition. Cognition and Emotion. 1998;12:353–85. [Google Scholar]
- Ersner-Hershfield H, Wimmer GE, Knutson B. Saving for the future self: neural measures of future self-continuity predict temporal discounting. Social Cognitive and Affective Neuroscience. 2009;4:85–92. doi: 10.1093/scan/nsn042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar P, Berger B, Febvret A, Vigny A, Henry JP. Catecholamine innervation of the human cerebral cortex as revealed by comparative immunohistochemistry of tyrosine hydrolaxe and dopamine-beta-hydrolaxe. The Journal of Comparative Neurology. 1989;279:249–71. doi: 10.1002/cne.902790208. [DOI] [PubMed] [Google Scholar]
- Green L, Fristoe N, Myerson J. Temporal discounting and preference reversals in choice between delayed outcomes. Psychonomic Bulletin & Review. 1994;1:383–89. doi: 10.3758/BF03213979. [DOI] [PubMed] [Google Scholar]
- Grezes J, Berthoz S, Passingham RE. Amygdala activation when one is the target of deceit: did he lie to you or to someone else? NeuroImage. 2006;30:601–8. doi: 10.1016/j.neuroimage.2005.09.038. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nature Review Neuroscience. 2001;2:685–94. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Hare T, Camerer C, Rangel A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science. 2009;324(5927):646–8. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- Hsee CK, Weber EU. A fundamental prediction error: Self-others discrepancies in risk preference. Journal of Experimental Psychology. 2001;126:45–53. [Google Scholar]
- Kable JW, Glimcher PW. The neural correlates of subjective value during intertemporal choice. Nature Neuroscience. 2007;10:1625–33. doi: 10.1038/nn2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahneman D. Maps of bounded rationality: psychology for behavioral economics. American Economy Review. 2003;93:1449–75. [Google Scholar]
- Kalenscher T, Pennartz CMA. Is a bird in the hand worth two in the future? The neuroeconomics of intertemporal decision-making. Progress in Neurobiology. 2008;84:284–315. doi: 10.1016/j.pneurobio.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Kelley WM, Macrae CN, Wyland. CL, et al. Finding the self? An event-related fMRI study. Journal of Cognition Neuroscience. 2002;14:785–94. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Adams CM, Varner JL, Hommer D. Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport. 2001;12:3683–7. doi: 10.1097/00001756-200112040-00016. [DOI] [PubMed] [Google Scholar]
- Knutson B, Peterson R. Neurally reconstructing expected utility. Games and Economic Behavior. 2005;52:305–15. [Google Scholar]
- Koopmans TC. Stationary ordinal utility and impatience. Econometrica. 1960;28:287–309. [Google Scholar]
- Laibson D. Golden eggs and hyperbolic discounting. Quarterly Journal of Economy. 1997;112:443–77. [Google Scholar]
- Lane RD, Reiman EM, Ahern GL, Schwartz GE, Davidson RJ. Neuroanatomical correlates of happiness, sadness, and disgust. American Journal of Psychiatry. 1997;154:926–33. doi: 10.1176/ajp.154.7.926. [DOI] [PubMed] [Google Scholar]
- Lane RD, Reiman EM, Axelrod B, et al. Neural correlates of levels of emotional awareness. Evidence of an interaction between emotion and attention in the anterior cingulate cortex. Journal of Cognitive Neuroscience. 1998;10:525–35. doi: 10.1162/089892998562924. [DOI] [PubMed] [Google Scholar]
- McClure SM, Botvinick MM, Yeung N, Greene J D, CohenJ D. In: Handbook of Emotion Regulation. Gross JJ, editor. New York: Guilford Press; 2007a. pp. 204–26. [Google Scholar]
- McClure SM, Ericson KM, Laibson DI, Loewenstein G, Cohen JD. Time discounting for primary rewards. Journal of Neuroscience. 2007b;27:5796–804. doi: 10.1523/JNEUROSCI.4246-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure SM, Laibson DI, Loewenstein G, Cohen JD. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306:503–7. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- Metcalfe J, Mischel W. A hot/cool-system analysis of delay of gratification: dynamics of willpower. Psychology Review. 1999;106:3–19. doi: 10.1037/0033-295x.106.1.3. [DOI] [PubMed] [Google Scholar]
- Mischel W, Ayduk O, Mendoza-Denton R. In: Time and Decision. Loewenstein G, Read D, Baumeister R, editors. New York: Russell Sage Foundation; 2003. [Google Scholar]
- Moran JM, Macrae CN, Heatherton TF, Wyland CL, Kelley WM. Neuroanatomical evidence for distinct cognitive and affective components of self. Journal of Cognitive Neuroscience. 2006;18:1586–94. doi: 10.1162/jocn.2006.18.9.1586. [DOI] [PubMed] [Google Scholar]
- Neumann J, Lohmann G. Bayesian second-level analysis of functional magnetic resonance images. NeuroImage. 2003;20:1346–55. doi: 10.1016/S1053-8119(03)00443-9. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Knierim K, Ludlow DH, et al. Reflecting upon feelings: an fMRI study of neural systems supporting the attribution of emotion to self and other. Journal of Cognitive Neuroscience. 2004;16:1746–72. doi: 10.1162/0898929042947829. [DOI] [PubMed] [Google Scholar]
- Rustichini A. Dual or unitary system? Two alternative models of decision making. Cognitive, Affective, & Behavioral Neuroscience. 2008;8(4):355–62. doi: 10.3758/CABN.8.4.355. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Ramnani N, Mackay C, et al. Distinct portions of anterior cingulate cortex and medial prefrontal cortex are activated by reward processing in separable phases of decision-making cognition. Biological Psychiatry. 2004;55:594–602. doi: 10.1016/j.biopsych.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Samuelson PA. A note on measurement of utility. The Review of Economic Studies. 1937;4:155–61. [Google Scholar]
- Sayette AA, Loewenstein G, Griffin KM, Black JJ. Exploring the cold-to-hot empathy gap in smokers. Psychological science. 2008;19(9):926–32. doi: 10.1111/j.1467-9280.2008.02178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. Journal of Neurophysiology. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–9. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Stone ER, Yates AJ, Caruthers AS. Risk taking in decision making for others versus the self. Journal of Applied Social Psychology. 2002;32:1797–824. [Google Scholar]
- Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nature Review Neuroscience. 2005;6:533–44. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DE, Cardinal RN, Robbins TW. Contrasting roles of basolateral amygdala and orbitofrontal cortex in impulsive choice. Journal of Neuroscience. 2004;24:4718–22. doi: 10.1523/JNEUROSCI.5606-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zysset S, Huber O, Ferstl E, von Cramon DY. The anterior frontomedian cortex and evaluative judgment: an fMRI study. NeuroImage. 2002;15:983–91. doi: 10.1006/nimg.2001.1008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.