Abstract
The regulation of PLD2 activation is poorly understood at present. Transient transfection of COS-7 with a mycPLD2 construct results in elevated levels of PLD2 enzymatic activity and tyrosyl phosphorylation. To investigate whether this phosphorylation affects PLD2 enzymatic activity, anti-myc immunoprecipitates were treated with recombinant protein tyrosine phosphatase PTP1B. Surprisingly, lipase activity and PY levels both increased over a range of PTP1B concentrations. These increases occurred in parallel to a measurable PTP1B-associated phosphatase activity. Inhibitor studies demonstrated that an EGF-receptor type kinase is involved in phosphorylation. In a COS-7 cell line created in the laboratory that stably expressed myc-PLD2, PTP1B induced a robust (>6-fold) augmentation of myc-PLD2 phosphotyrosine content. The addition of growth factor receptor-bound protein 2 (Grb2) to cell extracts also elevated PY levels of myc-PLD (>10-fold). Systematic co-immunoprecipitation-immunoblotting experiments pointed at a physical association between PLD2, Grb2 and PTP1B in both physiological conditions and in overexpressed cells. This is the first report of a demonstration of the mammalian isoform PLD2 existing in a ternary complex with a protein tyrosine phosphatase, PTP1b, and the docking protein Grb2 which greatly enhances tyrosyl phosphorylation of the lipase.
INTRODUCTION
Phospholipase D (PLD) plays major roles in the maintenance and remodeling of cellular membranes and generating of signal transduction molecules. It catalyzes the hydrolysis of cell membrane glycerophospholipids, preferentially phosphatidylcholine (PC), to form phosphatidic acid (PA) and a related base (i.e. choline). PA and its derivatives diacylglycerol (DAG) and lysophosphatidic acid (LPA) are important signaling molecules whereas choline is the precursor to the common neurotransmitter acetylcholine [1]. Two distinct isoforms of PLD, denoted PLD1 and PLD2, have been identified from several mammalian sources [2–11]. They share two phosphatidyltransferase “HKD” motifs, recognized by the consensus peptide sequence HxKx4D, that are crucial for lipase activity [2,12–14].
Regulation of total PLD activity in cell homogenates by tyrosyl phosphorylation has been previously documented [15–18]. In HL-60 granulocytes, the isoform PLD1 is tyrosyl phosphorylated in response to peroxides of vanadate [19]. In Swiss 3T3 fibroblasts, hydrogen peroxide induces PLD1 activation via protein tyrosine kinases [20]. In contrast, less is known about the role of tyrosyl phosphorylation in regulating PLD2. Although PLD2 can form a complex with the EGF receptor that leads to phosphorylation of Tyr11 [21], site-directed mutagenesis and pharmacological studies suggest that this phosphorylation is not essential for stimulating enzyme activity [21,22]. Serine and threonine phosphorylation by PKC is similarly dispensable [23]. More recently, others have show that PLD2 can be phosphorylated on tyrosine by Fyn and Fgr kinases in mast cells [24]. This phosphorylation is concomitant with PLD2 activation and cell degranulation [24].
Although a great body of data has been accumulated over the last 10 years on the complex mechanism of PLD1 regulation in mammalian cells, the regulation of PLD2 activation is poorly understood at present. In this report, we show that overexpressing human PLD2 tagged with an N-terminal myc peptide in COS-7 cells is tyrosyl phosphorylated by an EGF-R type kinase. The effect of the tyrosine protein phosphatase PTP1B, itself a well-characterized regulator of cell signaling [25–27] was also investigated. In contrast to expectations, both enzyme activity and PY content increased, suggesting that PLD2 is in a complex with a tyrosine kinase that can stimulate enzyme activity. We provide evidence for the first time of a physical association between PLD2, PTP1b and the docking protein Grb2 into a ternary complex and a strong activation of phosphorylation by the latter molecule.
MATERIALS AND METHODS
Materials
pcDNA3.1 plasmid, lipofectamine “plus” reagent, Opti-MEM reduced serum medium and SOC medium were from Invitrogen (Carlsbad, CA); D-MEM and COS-7 kidney fibroblasts [Cercopithecus aethiops (monkey, African green)] SV40-transformed, were from ATCC (Rockville, MD); XL1-Blue competent E. coli were from Stratagene (La Jolla, CA); QIAfilter plasmid maxi-prep kit was from Qiagen Corp. (Valencia, CA); 1,2-dioctanoyl-sn-glycero-3-phosphocholine (PC8) and anti-rabbit IgG (agarose beads) were from Sigma (St. Louis, MO); n-[1-3H] butanol (5 Ci/mmol) was from American Radiolabeled Chemicals (St. Louis, MO); 1,2-dioleoyl-sn-glycero-3-phosphobutanol (PBut) standard was from Avanti Polar Lipids (Alabaster, AL); LK6D silica gel 60 Å TLC plates were from Whatman (Clifton, NJ); Scintiverse II scintillation cocktail was from Fisher (Pittsburgh, PA); electrophoresis and Bradford protein assay chemicals were from Bio-Rad Laboratories (Richmond, CA); Immobilon PVDF membranes were from Millipore (Bedford, MA); enhanced chemiluminescence (ECL) Western blotting detection reagents were from Amersham Pharmacia Biotech (Piscataway, NJ); anti-PLD2 (N-terminal) affinity-purified polyclonal antibody was from BioSource (Camarillo, CA); c-myc (9E10) agarose-conjugated monoclonal, c-myc (A-14) polyclonal, anti-phosphotyrosine (PY-20 and PY-100 clones) and GFP plasmid (pCruz-GFP, 7.3 kb) were from Santa Cruz Biotech (Santa Cruz, CA); genistein, AG-18 and PTP1B were from Calbiochem (La Jolla, CA); recombinant Grb2 was from Upstate Biotechnology (Lake Placid, NY).
Transient transfection of COS-7 cells with pcDNA3.1-mycPLD2
The plasmid pcDNA3.1-mycPLD2 (8.8 kb) was originally described in [9]. It contains the complete ORF of human PLD2 (2.8 kb) and a 566 bp 3′-UTR, cloned from a B lymphocyte library and inserted in 5′-HindIII-3′-Not1 of the pcDNA3.1 multiple cloning site. The myc tag sequence (EQKLISEEDL) was synthesized by PCR at the 5′-end directly contiguous to the ATG start/Kozac consensus site of PLD2 cDNA. COS-7 cells were initially seeded at 1×105 cells/well in 6-well tissue culture plates, in 1.5 ml D-MEM containing 10% FBS (D-MEM/FBS) and nonessential amino acids. Cells were grown at 37 °C in a CO2 incubator until they were 70–80% confluent (~48 h). For transient transfection, the growth media was removed, washed twice with 2 ml of reduced-serum opti-MEM and 0.3 ml of this media was added to each well. Then, 0.3 ml of a lipid-DNA complex (2 μg pcDNA-mycPLD2 plasmid, 3 μl lipofectamine and 5 μl “plus” reagent in Opti-MEM, previously mixed in sterile glass test tubes) was added to the cells. After a two-hour incubation, cells were washed with D-MEM/FBS and were incubated in 0.6 ml of the same medium for 48 h to achieve the maximum level of PLD2 expression. In control experiments, co-transfection of pcDNA-mycPLD2 with a plasmid of similar size (7.3 kb) that contained the green fluorescent protein gene (pCruz-GFP) was used in the same experimental conditions to confirm the efficiency of transfection as >75%. After 48-hr transfection PLD2-expressing COS-7 cells were resuspended in 0.3-ml PLD Assay Buffer (5 mM HEPES, pH 7.8, 5 μg/ml aprotinin and pepstatin) and sonicated briefly.
Generation of COS-7 stable transformants expressing PLD2
A stable cell line that continuously expresses PLD2 in COS-7 cells was constructed as follows. COS-7 cells were grown in D-MEM media in 6-well tissue culture plates at 37 °C in a 5% CO2 incubator until they reached at least ~80% confluency. The growth media was removed, washed twice with 2 ml of reduced-serum opti-MEM and 0.3 ml of this media was added to each well. Then, 0.3 ml of a lipid-DNA complex (pcDNA3.1-mycPLD2 plasmid in a 0.75–2.5 μg DNA range, 3 μl lipofectamine and 5 μl “plus” reagent in Opti-MEM, previously mixed in sterile glass test tubes) was added to the cells. After a two-hour incubation, cells were washed with D-MEM/FBS and were incubated in 0.6 ml of the same medium for 48 hours. From previous experiments, this is the time at which transient transfection yields the maximum of PLD2 expression. Expression was confirmed by fluorescence of GFP in cells co-transfected with a pCruz-GFP vector. Several colonies were then started on fresh plates on an antibiotic cycle (taking advantage of the neomycin resistance gene existing into the pcDNA3.1 plasmid), to determine the minimum concentration of antibiotic needed to kill the untransfected cells. One group of plates received 400 μg/ml geneticin and a second group received 800 μg/ml. After one month of growth, these concentrations were changed to 800 μg/ml and 1200 μg/ml, respectively, to induce increased cell death and selection of surviving clones in continuous growth at 37 °C and 5% CO2. In about two additional weeks, suspected stable transfectants were trypsinized and transferred to fresh plates with D-MEM/FBS media containing the same antibiotic concentrations.
Confluent cells (>70%) were used for confirmation of protein expression (by immunoprecipitation and Western blotting) and in vitro enzymatic. For this, six-well plates containing stable hPLD2 COS7 cells were incubated for 1 hour with 0.25x tyrosine phosphatase inhibition cocktail-II (Sigma). Cells were rinsed, trypsinized, collected and spun briefly. Cell pellets were resuspended in sonication buffer (5 mM Hepes, pH 7.2) and total cell sonicates (100 μl per sample) were used for incubation with PTP1B or Grb2 for 30 min at 30 °C, with slight agitation and vortexing. Samples were used for immunoprecipitation with anti-myc agarose-conjugated antibodies. Immunocomplex beads were taken for either SDS/PAGE and Western blotting or to in vitro phospholipase enzyme assay. Four viable, fully characterized, clones were identified and used for further experiments.
Immunoprecipitation, Western blotting and measurement of in vitro PLD2 activity
Immunoprecipitation and immunoblotting were executed as before [28,29]. Immune complexes were then subjected to SDS-PAGE and immunoblotting with antibodies specific for myc, PLD2, phosphotyrosine, PTP1B and Grb2. Overexpressed mycPLD was immunoprecipitated with 9E10 monoclonal antibody as previously described [30]. Immune complexes were resuspended in a final volume of 40 μgl ice-cold PLD Assay Buffer. Measurement of lipase activity began with the addition of the following reagents (final concentrations): 24 mM PC8 phospholipid, 75 mM HEPES (pH 7.8), and 4.7 μCi [3H]Butanol in a liposome form, as indicated in [32]. Samples were incubated for 20 minutes at 30 °C with continuous shaking. Addition of 0.3 ml ice-cold chloroform/methanol (1:2) and 70 μl of 1% perchloric acid stopped the reactions [31]. Lipids were then isolated and resolved by thin layer chromatography. The amount of 3H-PBut that co-migrated with PBut standards was measured by scintillation spectrometry. Control reactions lacking PC8 were used to remove background counts.
Phosphatase/Grb2 treatment and phosphatase activity assays
COS-7 cell pellets were resuspended in 5 mM Hepes, pH 7.2 and sonicated briefly [(Sonic Dismembrator 70 (Fisher Scientific), medium setting, 2-five second cycles]. Sonicates were then supplemented with PTP1B essentially as described elsewhere [32]. Briefly, PTP1B was resuspended in a 160 μl total volume pre-chilled 25 mM Tris-HCl, pH 7.6, added to cell lysates and incubated for 30 minutes at 30 ûC. PTP1B stock was 0.5 μg/μl, 25 mU/μl (one unit hydrolyzing 1 μmol of phosphopeptide substrate/min at pH 7.2). In other experiments, different concentrations of recombinant Grb2 (see results) were incubated with cell sonicates under comparable conditions. Placing the samples on ice stopped the reactions. mycPLD2 was immediately immunoprecipitated with anti-myc antibodies and lipase activity was measured as described above. In some experiments, PLD2 was first immunoprecipated and then treated with PTP1B before measuring enzyme activity. The activity of PTP1B was confirmed in assays measuring the cleavage of p-nitrophenylphosphate (PNPP) into p-nitrophenol (PNP) exploiting the differences in absorbance peaks of PNPP and PNP (400 and 420 nm respectively).
Statistical Analysis
Data are presented as the mean ± SEM. The difference between means was assessed by the Single Factor Analysis of Variance (ANOVA) test. Probability of less than 0.05 (p<0.05) was considered to indicate a significant difference.
RESULTS
A tyrosyl-phosphorylated PLD2 protein in transiently transfected cells
pcDNA3.1-mycPLD2 was transfected into COS-7 cells to study the regulation of PLD2 activity by tyrosine phosphorylation. Two days later, PLD2 was immunoprecipitated using myc-specific antibodies from transfected, mock transfected and untransfected cells, and subjected to SDS-PAGE and immunoblotting with anti-PLD2 antibodies (Fig. 1A). An abundant ~103 kDa protein corresponding to PLD2 was present in transfected cells. COS-7 lysates were also immunoprecipitated using myc-specific antibodies and derived immunoblots were probed also with anti-myc (Fig. 1B).
Figure 1. Detection of PLD2 as constitutively phosphorylated on tyrosine.
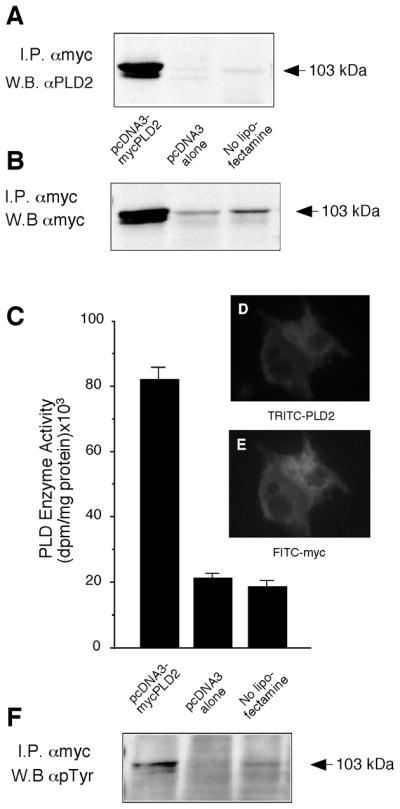
(A) COS-7 cells were co-transfected with pcDNA3.1-mycPLD2, with the empty vector (pcDNA3 alone) or with plain COS-7 cells (no lipofectamine) for 36 hours. After this, cells were harvested and whole sonicates were immunoprecipitated with anti-Myc (9E-10) monoclonal antibody and immunocomplexes were analyzed by SDS/PAGE and immuoblotting developed with anti-PLD2 antibodies. (B) Cells treated as in the previous panel were also immunprecipitated with anti-myc antibodies and Western blots were also probed with anti-myc. (C) anti-Myc immunoprecipitates were used for measuring enzymatic activity in PC8 liposomes and [3H]n-butanol. (D, E) Cellular co-localization of myc and PLD2 by immunofluorescence micrographs: COS-7 cells transfected with pcDNA3-mycPLD2 were immunolabeling of slides with PLD2-TRITC (D) and myc-FITC (E) antibodies. (F) Same blot as shown in panel B, derived from anti-Myc immunoprecipitates and Western blots, were stripped and developed with anti-phosphotyrosine antibodies. Results are representative of 4 experiments in duplicate and, for panel C, data represent mean ± SEM of 4 independent experiments.
The presence of mycPLD2 was also confirmed in these experimental conditions, and it far exceeded endogenous levels of PLD2. Since anti-myc immunoblotting of myc immunoprecipitates was essentially similar to anti-PLD2 immunoblotting of myc immunoprecipitates, we concluded that anti-myc and anti-PLD2 antibodies could be used indistinctly for the purpose of this study. In vitro enzyme assays of anti-myc immunoprecipitates with PC8 liposomes and [3H]n-butanol, confirmed the presence of PLD2 enzymatic activity (Fig. 1C). Lipase activity in transfected cells was ~4 times higher than that of negative controls. Levels of expressed mycPLD2 and associated enzymatic activity increased as a function of concentration of DNA used for transfection (not shown). Maximal protein expression and enzyme activity occurs 2–3 days after transfection that coincides with a peak of expression of GFP following co-transfection with pCruz-GFP (>75%).
Cellular localization of myc-PLD2 was confirmed by immunostaining with PLD2 and myc specific antibodies (Fig. 1D, E). Identical staining patterns seen with both antibodies indicated that the readily detected antigen detected with PLD2-specific antibodies was predominantly the overexpressed protein. Consistent with the prior observation that PLD2 forms a complex with EGFR [21], addition of 100 nM EGF to transfected cells increased the amount of mycPLD2 localized to the plasma membrane (not shown). Reprobing these blots with PY-specific antibodies showed that mycPLD2 was constitutively phosphorylated on tyrosine residues (Fig. 1F). This observation, coupled with the ability to detect lipase activity attributable to the transgene, indicated that transfected COS-7 cells provide a unique opportunity to study the role of PY in regulated PLD2 in vivo.
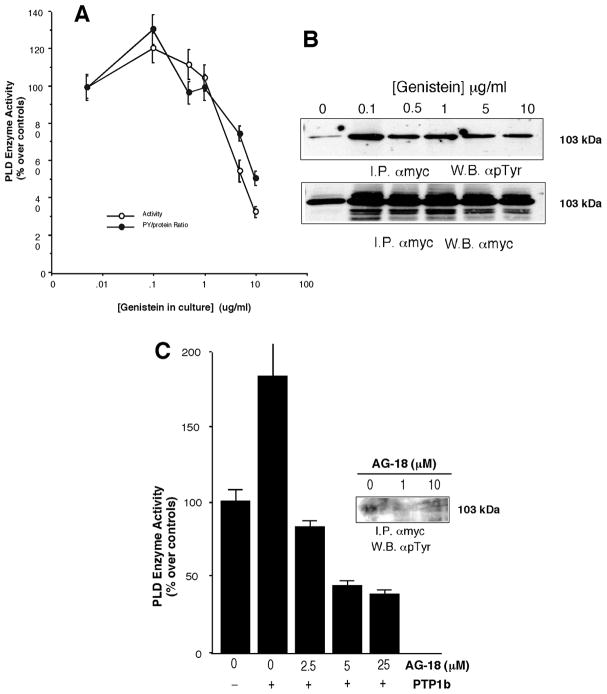
The type of kinase involved in phosphorylation was investigated. Culture cells were incubated with genistein for 48 hours. Concentrations of genistein between 0.1 and 10 mg/ml caused a decease in enzymatic activity (Fig. 2A). Genistein treatment in continuous culture also caused a decrease in tyrosine phosphorylation (Fig. 2B). Further experiments were carried out with a AG-18, specific inhibitor of the epidermal growth factor (EGF) receptor kinase [33]. In the in vitro data presented in (Fig. 2C), AG-18 was able to negate the effect of PPTP1B and decrease both phosphorylation and enzymatic activity. COS-7 cells treated with 100 nM EGF recruit mycPLD2 to the plasma membrane (data not shown). Thus, the EGF-R or an EGF-R type kinase is involved in the phosphorylation of PLD2. [33]
Figure 2. Effect of tyrosine kinase inhibitors on PLD2.
Transfected COS-7 cells were cultured continuously with genistein for 48 hours. Cells were harvested and cell lysates (~0.8 mg protein/ml) were immunoprecipitates with anti-myc antibodies that were used for measuring enzymatic activity (A) or immunoblotting (B). In (C) the effect of the EGF-R inhibitor AG-18 was assayed n vitro in the presence or assensu of PTP1B. Both activity and immunoblotting (inset) were measured.
Effect of protein tyrosine phosphatases on tyrosyl phosphorylated PLD2
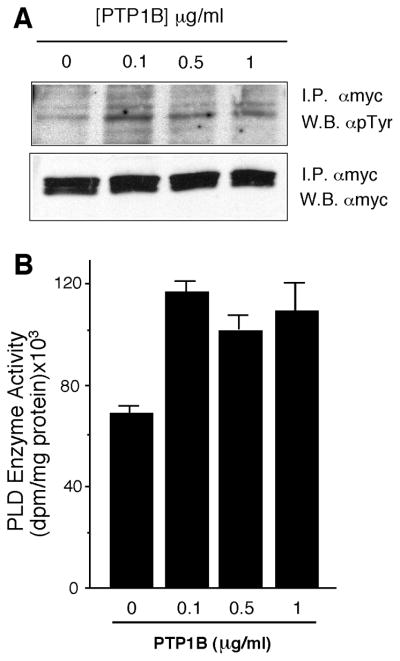
To study the effects of tyrosyl phosphorylation on PLD2 activity, we attempted to dephosphorylate PLD2 using the tyrosine-specific phosphatase PTP1B and then measure enzyme activity. mycPLD2 was immunoprecipitated from transfected and control cells using anti-myc antibodies and then treated with PTP1B. Surprisingly, PY levels increased with treatment 0.1 and 1 μg/ml PTP1B (Fig. 3A). Likewise, PLD2 activity present in these immunoprecipitates increased 150% following phosphatase treatment (Fig. 3B). Similar results occurred when cell extracts were treated with phosphatase before immunoprecipitation (not shown). Treatment with CD45 and Yersinia tyrosine phosphatases also elicited the increases in PY content and PLD2 activity although to lesser extents (data not shown). Therefore there is a positive correlation between the phosphorylation state of PLD2 and its activity and that a tyrosine protein kinase must be present in both cell lysates and in mycPLD2 immune complexes that can account for the increase in PY content.
Figure 3. Action of PTP1B on PLD2 tyrosine phosphorylation and activity.
Transfected COS-7 cells were harvested and cell lysates were immunoprecipitates with anti-myc antibodies. Immunocomplex beads were treated with the indicated concentrations of PTP1B and then processed for by SD/PAGE and Western blot with anti-PY antibodies or with anti-myc for confirmation of equal protein loading (A) (shown is the region corresponding to PLD2, 103 kDa) or for PLD2 activity measurement also in immunocomplexes (B). Data represent mean ± SEM of 3 independent experiments in duplicate.
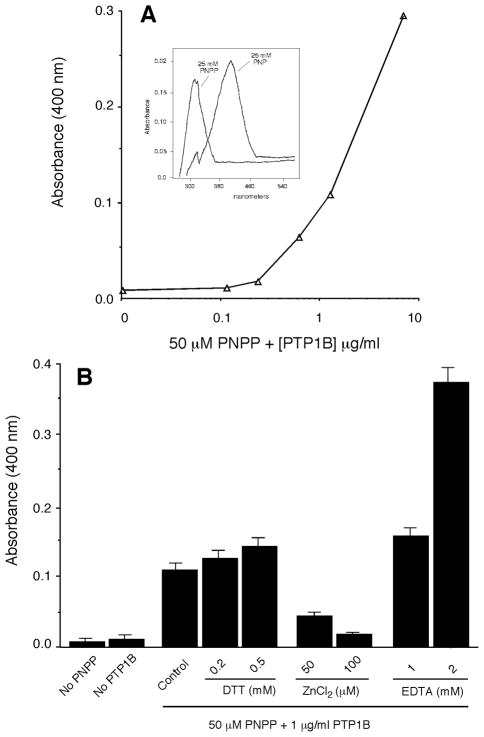
Because PY levels increased and not decreased after phosphatase treatment, we assayed PTP1B activity using PNPP as a substrate to determine if phosphatase activity was lost or altered under the experimental conditions. The results presented in Fig. 4A indicate that PTP1B was active in a concentration dependent manner in the buffers used above. Moreover, under these conditions, PTP1B exhibited its normal range of regulation: stimulation by millimolar concentrations of DTT and EDTA and inhibition by micromolar concentrations of ZnCl2 (Fig. 4B).
Figure 4. Confirmation of the presence of phosphatase activity.
(A) Increase in the detection of the phosphatase reaction product, PNP, over a range of exogenously added PTP1B. Insert: Absorption maxima for PNPP and PNP. (B) Effect of exogenously added PTP1B on the cleavage of PNPP and production of PNP in the same experimental conditions as those used in Figure 2. Also indicated is the effect of DTT, ZnCl2 and EDTA on phosphatase activity in. Data represent mean ± SEM of 3 independent experiments in duplicate.
Tyrosine phosphorylation of PLD2 in a stable COS-7 cell line
Being mindful of the artifacts of liposome-mediated transfection, we created in our laboratory four COS-7 cell lines, taking advantage of the neomycin-resistant gene in pcDNA3.1, that stably overexpressed PLD2. Characterization of a representative line is shown in Fig. 4. It expressed an abundant ~103 kDa protein and possesses 2.5–3 times more PLD2 activity than COS-7 control cells (Fig. 5A and B). As with the transient transfections, mycPLD2 could be pulled down and blotted with antibodies specific for either myc or PLD2 (Fig. 5C and D). Although enzyme levels were somewhat less than transiently transfected cells, lipase activity increased after treatment with PIP2 to levels that are comparable to transiently expressing cells (Fig. 5B). This was consistent with previous observations that, whereas PLD1 has a nearly absolute dependence on cofactors as PIP2 and Rho/Arf, PLD2 activity can be stimulated by PIP2 [9]. As before, treating cell line extracts with phosphatase increased both PY and activity levels. Fig. 6A shows that PTP1B increased PLD2 PY levels roughly ~6-fold over controls, consistent with the results in Fig. 2A but quantitatively larger as PTP1B was added to cell lysates in the stably transfected cells.
Figure 5. A stable cell line overexpressing PLD2 also shows constitutive tyrosyl phosphorylation of PLD2.
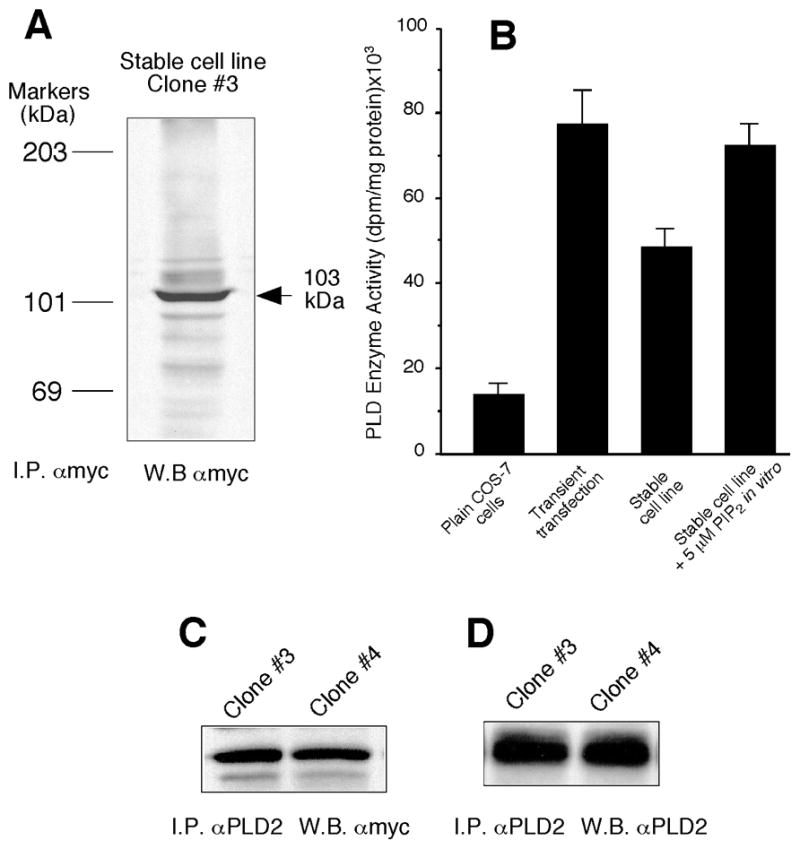
(A) Several clones of transformed COS-7 cells were grown on 6-well tissue culture plates (1×106 cells/well) in the continuous presence of 400 μg/ml geneticin. Shown in the figure is clone #3, out of 4 valid ones, that was harvested and sonicated briefly in lysis buffer, followed by immunoprecipitation with anti-Myc (9E-10) monoclonal antibodies. Immunocomplex beads were used for SDS/PAGE and subsequent Western blotting with anti-Myc antibodies. (B) Detection of in vitro PLD2 activity in anti-myc immunoprecipitates and its comparison to cells transiently transfected, and the effect of PIP2. (C, D) Confirmation of exchangeability of myc and PLD2 antibodies in the detection of overexpressed PLD2 in two similar clones of stable cells (clones #3 and #4). Shown are representative blots among a total of 4.
Figure 6. PTP1B increases tyrosine phosphorylation of PLD2 in a stable cell line.
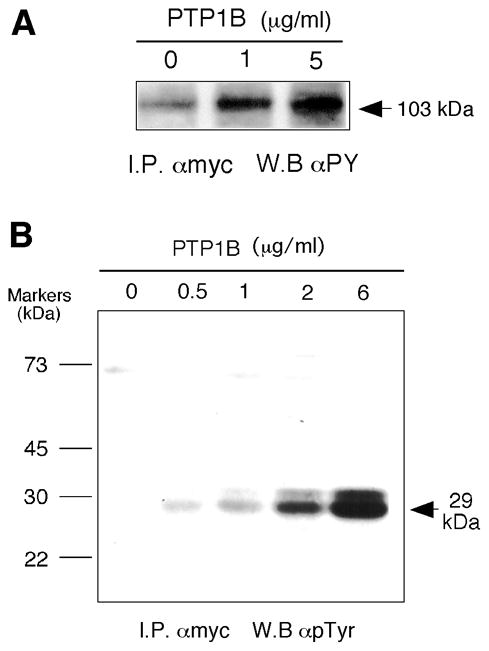
(A) Stable COS-7 cells were harvested and cell lysates (~0.8 mg protein/ml) were treated with the indicated concentrations of recombinant PTP1B, immunoprecipitated with anti-myc antibodies and used for SDS/Western blotting developed with anti-phosphotyrosine antibodies (clone PY100). (B) COS-7 cell lysates treated with the indicated concentrations of PTP1B were immunoprecipitated with anti-myc antibodies. Immunocomplex beads were run in SDS/PAGE gels and resulting blots were developed with anti-PY antibodies. The arrow marks the position a heavily Tyr-phosphorylated protein with an apparent Mw of 29 kDa.
Identifying a PLD2 activating protein (Grb2) and the existence of a ternary complex PLD2-PTP1b-Grb2 in overexpressed COS-7 cells
In the course of immunoblotting samples treated with PTP1B (Figs. 2A and 6A), we noted the presence of a low-molecular weight protein (~29 kDa) that was increasingly tyrosyl phosphorylated after PTP1B treatment in a dose dependent manner (Fig. 6B). A literature search revealed that proteins of this size that could play a role in tyrosine phosphorylation signaling included, among others, the Src homology-2 (SH2)-containing growth factor receptor-bound protein 2 (Grb2). Since Grb2 serves as an anchor between PTP1B and tyrosine protein kinases through the SH2 and SH3 domains [34], we investigated the possibility that Grb2 might have a role in regulating PLD2 phosphorylation.
Lysates of COS-7-mycPLD2 cells were supplemented with Grb2 to test whether Grb2 could stimulate PLD phosphorylation and activity. mycPLD2 was then immunoprecipitated and subjected to immunodetection with PY-specific antibodies. Addition of 1 and 10 μg/ml Grb2 induced a ~4- and ~10-fold increased PY levels of mycPLD compared to untreated controls creating a “hyper-phosphotyrosyl” isoform (Fig. 7A). Furthermore, Grb2 itself was also tyrosyl phosphorylated (Fig. 7B). Increases in PLD and Grb2 phosphorylation stimulated PLD2 (130%) although enzymatic activity did not increase proportionately to increases in PY.
Figure 7. Induction of a hyperphosphorylated PLD2 band by recombinant Grb2.
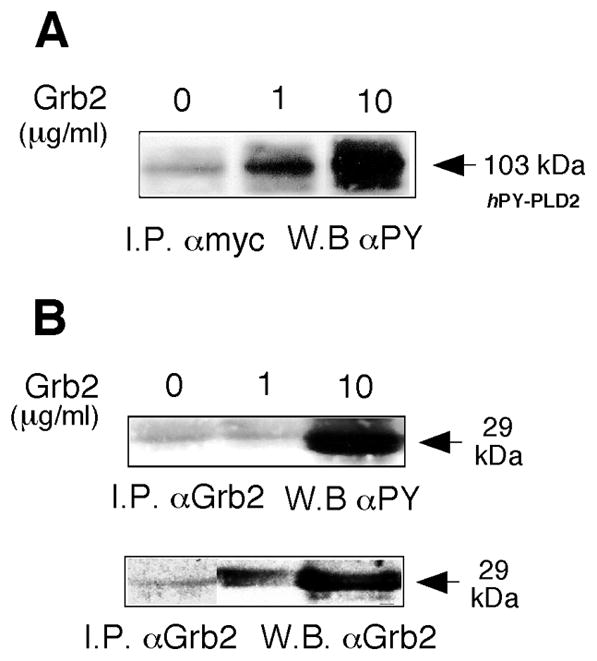
(A) COS-7 lysates were treated with the indicated concentrations of Grb2 and immunoprecipitated with anti-myc antibodies followed by Western blotting, probed with anti-phosphotyrosine antibodies. (B) Confirmation of Grb2 tyrosyl phosphorylation and of the presence of the Grb2 protein itself.
Finally, to investigate the possibility that Grb2 serves as a “docking” point for phosphatases, kinases and PLD2 itself, we used pull-down experiments looking for evidence of interactions between PLD2, PTP1B and Grb2. Figure 8A shows that PLD2 was immunoprecipitated with Grb2-specific antibodies; an interaction that was stimulated by addition of recombinant Grb2 to the lysates prior to immunoprecipitation. Conversely, Grb2 was pulled-down with myc-specific antibodies (Fig. 8B). Similarly, PTP1B and Grb2 were reciprocally pulled down with Grb2 and PTP1B specific antibodies, respectively (Fig. 9A). Lastly, Fig. 9B shows that PTP1B and PLD2 were reciprocally co-immunoprecipatable. Note that in all cases, immunoprecipitation of the three different molecules was observed in physiological conditions, i.e., in lanes derived from cells not transfected (plain COS-7 cells, broadening the implication of these results. Taken together, these observations indicated that PLD2, PTP1B and Grb2 form part of a ternary complex both in vivo and in cells overexpressing PLD2.
Figure 8. Co-immunoprecipitation of PLD2 and Grb2.

(A) Immunoprecipitation with anti-Grb2 antibodies and immunoblotting with anti-PLD2. Shown is the region of the immunoblots where PLD2 localizes (103 kDa). (B) Immunoprecipitation with anti-myc antibodies and immunoblotting with anti-Grb2. Shown is the region of the immunoblots where Grb2 localizes (29 kDa). The COS-7 “Control” in (A) refers to non-transfected cells. The “Grb2 positive control” in (B) refers to an extract of cells that are overexpressing Grb2. Shown are typical experiments among three total.
Figure 9. Co-immunoprecipitation of PTP1B and Grb2 and of PLD2 and PTP1B.
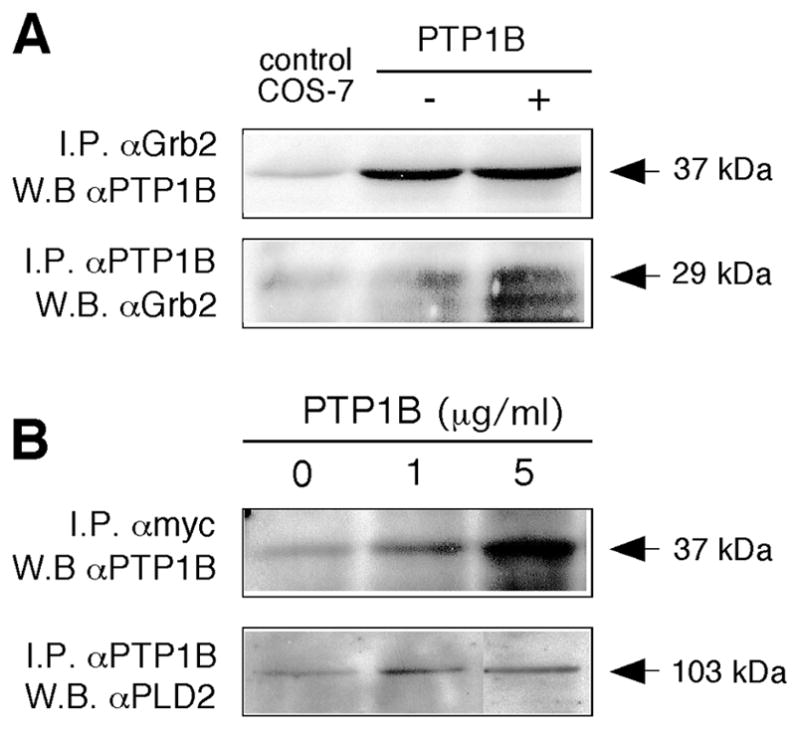
(A) Upper panel: Immunoprecipitation with anti-Grb2 antibodies and immunoblotting with anti-PTP1B. Shown in the is the region of the immunoblots where PTP1B localizes (37 kDa). “Control COS-7” refers to plain COS-7 cells. Lower panel: Immunoprecipitation with anti-PTP1B antibodies and immunoblotting with anti-Grb2. Shown is the region of the immunoblots where Grb2 localizes (29 kDa). (B) Upper panel: Immunoprecipitation with anti-myc antibodies and immunoblotting with anti-PTP1B. Shown is the region of the immunoblots where PTP1B localizes (37 kDa). Lower panel: Immunoprecipitation with anti-PTP1B antibodies and immunoblotting with anti-PLD2. Shown is the region of the immunoblots where PLD2 localizes (103 kDa) of an experiment typical among a total of three.
DISCUSSION
The data presented here shows first, that PLD2 can be transiently and stably overexpressed in COS-7 cells, that the expressed protein is constitutively active, and that it is tyrosyl-phosphorylated. Surprisingly, treatment of cell extracts or PLD2 immunoprecipitates with protein tyrosine phosphatases concomitantly increases levels of PY and PLD2 activity. We believe that this is the first report of a protein tyrosine phosphatase affecting mammalian PLD2 activity. There is precedent for a functional link between a protein phosphatase (PP2C) and PLD activity in Arabdidopsis where PA, the product of phospholipase Dα1 activity, interacts with the phosphatase that in turn regulates abscisic acid signaling responses [35]. However, the homology between mammalian PLD2 and plant PLD1 is low, except for the highly conserved HxKx4D sequence that are crucial for lipase activity.
Our observation that PTP1b treatment of cell lysates or PLD2 immunoprecipitates stimulates PLD2 tyrosyl-phosphorylation, indicates that a tyrosine protein kinase must be in a complex with PLD2. Based on the current literature, the likely candidates are: (a) EGFR, that is known to phosphorylate PLD2 [21]; or (b) Src family of kinases Fyn and Fgr, as recently demonstrated [24], regarding PLD2 phosphorylation, activation and mast cell degranulation. Moreover, Ahn et al. [36] have observed a physical interaction between the plectstrin homology domain of PLD2 and c-Src which provides a molecular basis for the interaction of PLD2 and its kinase. The data shown in this report indicate that an EGF-Receptor type kinase is involved in the phosphorylation of PLD2. Presumably, this kinase could in turn activate the PTP1b in a signaling complex. There are many examples where a protein tyrosine phosphatase regulates kinase function [37–46].
We provide here also evidence for the first time of a physical association between PLD2, PTP1b and the docking protein Grb2 into a ternary complex and a strong activation of phosphorylation by the latter molecule. As for the PLD2-Grb2 interaction, addition of Grb2 to extracts of cells expressing mycPLD2 leads to hyperphosphorylation of PLD2 and Grb2, with only a modest effect on enzyme activity. The disconnection between phosphorylation and enzyme activity has been observed previously [22, 23]. It seems likely that Grb2-stimulated hyperphosphorylation may be regulating interactions protein interactions PLD2. It is also likely that the pleckstrin homology domain and SH3 binding motifs will be important for PLD2 interactions with PTP1B, Grb2, and the unidentified protein tyrosine kinase. The C-terminus of PTP1B contains two proline-rich SH3 domains (Pro-X-X-Pro-X-Arg) that mediates with other proteins including Grb2, Crk and p130(Cas) [47]. PKCα is known to be involved. Chen and Exton [23] and Han et al. [48] have demonstrated recently that PLD2 is phosphorylated by PKCα and that both molecules physically interact with each other. Similar to our results with Grb2 addition, PKCα phosphorylation is not required activation of the lipase.
The biological significance of the data presented here is that for the first time evidence is presented that PLD2 is part of a ternary complex with a tyrosine phosphatase and a docking protein that can make the regulation of PLD2 as complex as that of PLD1. Possibly, a fourth signaling protein (a EGF-R type kinase) might be included in this complex as inhibition data indicates. The demonstration that the adapter protein Grb2 can greatly change the phosphorylation state of PLD2 suggest the potential for many undiscovered protein interactions with known signaling proteins used by growth factors and mitogens. The relevance of these novel findings is also heightened given that evidence of a ternary complex is observed in physiological conditions.
Acknowledgments
We thank the technical assistance of Dr. Trycia Frye and Jason Lehman. This work has been supported by the National Institutes of Health grant HL056653 and American Heart Association (National Program) 0250417N (J.G.-C.).
ABBREVIATIONS
- PLD
phospholipase D
- PA
phosphatidic acid
- PBut
phosphatidylbutanol
- PC8
1,2-dioctanoyl-sn-glycero-3-phosphocholine
- PTP1B
protein tyrosine phosphatase-1B
- PNPP
p-nitrophenylphosphate
- PNP
p-nitrophenol
- FITC
fluorescein iso-thiocyanate
- TRITC
tetramethyl rhodamine iso-thiocyanate
- GFP
green fluorescence protein
- Grb2
growth factor receptor-bound protein 2
- AG-18
specific inhibitor of the epidermal growth factor (EGF) receptor kinase
References
- 1.Klein J, Chalifa V, Liscovitch M, Loffelholz K. Role of phospholipase D activation in nervous system physiology and pathophysiology. J Neurochem. 1995;65:1445–1455. doi: 10.1046/j.1471-4159.1995.65041445.x. [DOI] [PubMed] [Google Scholar]
- 2.Hammond SM, Altshuller YM, Sung TC, Rudge SA, Rose K, Engebrecht J, Morris AJ, Frohman MA. Human ADP-ribosylation factor-activated phosphatidylcholine-specific phospholipase D defines a new and highly conserved gene family. J Biol Chem. 1995;270:29640–29643. doi: 10.1074/jbc.270.50.29640. [DOI] [PubMed] [Google Scholar]
- 3.Hammond SM, Jenco JM, Nakashima S, Cadwallader K, Gu Q, Cook S, Nozawa Y, Prestwich GD, Frohman MA, Morris AJ. Characterization of two alternately spliced forms of phospholipase D1. Activation of the purified enzymes by phosphatidylinositol 4,5-bisphosphate, ADP-ribosylation factor, and Rho family monomeric GTP-binding proteins and protein kinase C-alpha. J Biol Chem. 1997;272:3860–3868. doi: 10.1074/jbc.272.6.3860. [DOI] [PubMed] [Google Scholar]
- 4.Park SK, Provost JJ, Bae CD, Ho WT, Exton JH. Cloning and characterization of phospholipase D from rat brain. J Biol Chem. 1997;272:29263–29271. doi: 10.1074/jbc.272.46.29263. [DOI] [PubMed] [Google Scholar]
- 5.Katayama K, Kodaki T, Nagamachi Y, Yamashita S. Cloning, differential regulation and tissue distribution of alternatively spliced isoforms of ADP-ribosylation-factor-dependent phospholipase D from rat liver. Biochem J. 1998;329(Pt 3):647–652. doi: 10.1042/bj3290647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colley WC, Altshuller YM, Sue-Ling CK, Copeland NG, Gilbert DJ, Jenkins NA, Branch KD, Tsirka SE, Bollag RJ, Bollag WB, Frohman MA. Cloning and expression analysis of murine phospholipase D1. Biochem J. 1997;326(Pt 3):745–753. doi: 10.1042/bj3260745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colley WC, Sung TC, Roll R, Jenco J, Hammond SM, Altshuller Y, Bar-Sagi D, Morris AJ, Frohman MA. Phospholipase D2, a distinct phospholipase D isoform with novel regulatory properties that provokes cytoskeletal reorganization. Curr Biol. 1997;7:191–201. doi: 10.1016/s0960-9822(97)70090-3. [DOI] [PubMed] [Google Scholar]
- 8.Kodaki T, Yamashita S. Cloning, expression, and characterization of a novel phospholipase D complementary DNA from rat brain. J Biol Chem. 1997;272:11408–1413. doi: 10.1074/jbc.272.17.11408. [DOI] [PubMed] [Google Scholar]
- 9.Lopez I, Arnold RS, Lambeth JD. Cloning and initial characterization of a human phospholipase D2 (hPLD2). ADP-ribosylation factor regulates hPLD2. J Biol Chem. 1998;273:12846–12852. doi: 10.1074/jbc.273.21.12846. [DOI] [PubMed] [Google Scholar]
- 10.Steed PM, Clark KL, Boyar WC, Lasala DJ. Characterization of human PLD2 and the analysis of PLD isoform splice variants. Faseb J. 1998;12:1309–1317. doi: 10.1096/fasebj.12.13.1309. [DOI] [PubMed] [Google Scholar]
- 11.Morris AJ, Engebrecht J, Frohman MA. Structure and regulation of phospholipase D. Trends Pharmacol Sci. 1996;17:182–185. doi: 10.1016/0165-6147(96)10016-x. [DOI] [PubMed] [Google Scholar]
- 12.Waksman M, Eli Y, Liscovitch M, Gerst JE. Identification and characterization of a gene encoding phospholipase D activity in yeast. J Biol Chem. 1996;271:2361–2364. doi: 10.1074/jbc.271.5.2361. [DOI] [PubMed] [Google Scholar]
- 13.Sung TC, Roper RL, Zhang Y, Rudge SA, Temel R, Hammond SM, Morris AJ, Moss B, Engebrecht J, Frohman MA. Mutagenesis of phospholipase D defines a superfamily including a trans-Golgi viral protein required for poxvirus pathogenicity. Embo J. 1997;16:4519–4530. doi: 10.1093/emboj/16.15.4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xie Z, Ho WT, Exton JH. Association of the N- and C-terminal domains of phospholipase D. Contribution of the conserved HKD motifs to the interaction and the requirement of the association for Ser/Thr phosphorylation of the enzyme. J Biol Chem. 2000;275:24962–24969. doi: 10.1074/jbc.M909745199. [DOI] [PubMed] [Google Scholar]
- 15.Gomez-Cambronero J. Immunoprecipitation of a phospholipase D activity with antiphosphotyrosine antibodies. J Interferon Cytokine Res. 1995;15:877–885. doi: 10.1089/jir.1995.15.877. [DOI] [PubMed] [Google Scholar]
- 16.Natarajan V, Scribner WM, Vepa S. Regulation of phospholipase D by tyrosine kinases. Chem Phys Lipids. 1996;80:103–116. doi: 10.1016/0009-3084(96)02548-0. [DOI] [PubMed] [Google Scholar]
- 17.Exton JH. Regulation of phospholipase D. Biochim Biophys Acta. 1999;1439:121–133. doi: 10.1016/s1388-1981(99)00089-x. [DOI] [PubMed] [Google Scholar]
- 18.Liscovitch M, Czarny M, Fiucci G, Tang X. Phospholipase D: molecular and cell biology of a novel gene family. Biochem J. 2000;345(Pt 3):401–415. [PMC free article] [PubMed] [Google Scholar]
- 19.Marcil J, Harbour D, Naccache PH, Bourgoin S. Human phospholipase D1 can be tyrosine-phosphorylated in HL-60 granulocytes. J Biol Chem. 1997;272:20660–20664. doi: 10.1074/jbc.272.33.20660. [DOI] [PubMed] [Google Scholar]
- 20.Min DS, Kim EG, Exton JH. Involvement of tyrosine phosphorylation and protein kinase C in the activation of phospholipase D by H2O2 in Swiss 3T3 fibroblasts. J Biol Chem. 1998;273:29986–29994. doi: 10.1074/jbc.273.45.29986. [DOI] [PubMed] [Google Scholar]
- 21.Slaaby R, Jensen T, Hansen HS, Frohman MA, Seedorf K. PLD2 complexes with the EGF receptor and undergoes tyrosine phosphorylation at a single site upon agonist stimulation. J Biol Chem. 1998;273:33722–33727. doi: 10.1074/jbc.273.50.33722. [DOI] [PubMed] [Google Scholar]
- 22.Mehta S, Maglio J, Kobayashi MS, Sipple AM, Horwitz J. Activation of phospholipase D is not mediated by direct phosphorylation on tyrosine residues. Biochim Biophys Acta. 2003;1631:246–254. doi: 10.1016/s1388-1981(03)00023-4. [DOI] [PubMed] [Google Scholar]
- 23.Chen JS, Exton JH. Regulation of phospholipase D2 activity by protein kinase C alpha. J Biol Chem. 2004;279:22076–22083. doi: 10.1074/jbc.M311033200. [DOI] [PubMed] [Google Scholar]
- 24.Choi WS, Hiragun T, Lee JH, Kim YM, Kim HP, Chahdi A, Her E, Han JW, Beaven MA. Activation of RBL-2H3 mast cells is dependent on tyrosine phosphorylation of phospholipase D2 by Fyn and Fgr. Mol Cell Biol. 2004;24:6980–6992. doi: 10.1128/MCB.24.16.6980-6992.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Byon JC, Kusari AB, Kusari J. Protein-tyrosine phosphatase-1B acts as a negative regulator of insulin signal transduction. Mol Cell Biochem. 1998;182:101–108. [PubMed] [Google Scholar]
- 26.Mahadev K, Zilbering A, Zhu L, Goldstein BJ. Insulin-stimulated hydrogen peroxide reversibly inhibits protein-tyrosine phosphatase 1b in vivo and enhances the early insulin action cascade. J Biol Chem. 2001;276:21938–21942. doi: 10.1074/jbc.C100109200. [DOI] [PubMed] [Google Scholar]
- 27.Chan AC, Desai DM, Weiss A. The role of protein tyrosine kinases and protein tyrosine phosphatases in T cell antigen receptor signal transduction. Annu Rev Immunol. 1994;12:555–592. doi: 10.1146/annurev.iy.12.040194.003011. [DOI] [PubMed] [Google Scholar]
- 28.Joseph DE, Paul CC, Baumann MA, Gomez-Cambronero J. S6 kinase p90rsk in granulocyte-macrophage colony-stimulating factor-stimulated proliferative and mature hematopoietic cells. J Biol Chem. 1996;271:13088–13093. doi: 10.1074/jbc.271.22.13088. [DOI] [PubMed] [Google Scholar]
- 29.Gomez-Cambronero J. Rapamycin inhibits GM-CSF-induced neutrophil migration. FEBS Lett. 2003;550:94–100. doi: 10.1016/s0014-5793(03)00828-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gomez-Cambronero J, Horwitz J, Sha’afi RI. Measurements of phospholipases A2, C, and D (PLA2, PLC, and PLD). In vitro microassays, analysis of enzyme isoforms, and intact-cell assays. Methods Mol Biol. 2003;218:155–176. doi: 10.1385/1-59259-356-9:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Horn JM, Lehman JA, Alter G, Horwitz J, Gomez-Cambronero J. Presence of a phospholipase D (PLD) distinct from PLD1 or PLD2 in human neutrophils: immunobiochemical characterization and initial purification. Biochim Biophys Acta. 2001;1530:97–110. doi: 10.1016/s1388-1981(00)00172-4. [DOI] [PubMed] [Google Scholar]
- 32.Lehman JA, Calvo V, Gomez-Cambronero J. Mechanism of ribosomal p70S6 kinase activation by granulocyte macrophage colony-stimulating factor in neutrophils: cooperation of a MEK-related, THR421/SER424 kinase and a rapamycin-sensitive, m-TOR-related THR389 kinase. J Biol Chem. 2003;278:28130–28138. doi: 10.1074/jbc.M300376200. [DOI] [PubMed] [Google Scholar]
- 33.Dvir A, Milner Y, Chomsky O, Gilon C, Gazit A, Levitzki A. The inhibition of EGF-dependent proliferation of keratinocytes by tyrphostin tyrosine kinase blockers. J Cell Biol. 1991;113:857–865. doi: 10.1083/jcb.113.4.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goldstein BJ, Bittner-Kowalczyk A, White MF, Harbeck M. Tyrosine dephosphorylation and deactivation of insulin receptor substrate-1 by protein-tyrosine phosphatase 1B. Possible facilitation by the formation of a ternary complex with the Grb2 adaptor protein. J Biol Chem. 2000;275:4283–4289. doi: 10.1074/jbc.275.6.4283. [DOI] [PubMed] [Google Scholar]
- 35.Zhang W, Qin C, Zhao J, Wang X. Phospholipase D alpha 1-derived phosphatidic acid interacts with ABI1 phosphatase 2C and regulates abscisic acid signaling. Proc Natl Acad Sci U S A. 2004;101:9508–9513. doi: 10.1073/pnas.0402112101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ahn BH, Kim SY, Kim EH, Choi KS, Kwon TK, Lee YH, Chang JS, Kim MS, Jo YH, Min do S. Transmodulation between phospholipase D and c-Src enhances cell proliferation. Mol Cell Biol. 2003;23:3103–3115. doi: 10.1128/MCB.23.9.3103-3115.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu F, Chernoff J. Protein tyrosine phosphatase 1B interacts with and is tyrosine phosphorylated by the epidermal growth factor receptor. Biochem J. 1997;327(Pt 1):139–145. doi: 10.1042/bj3270139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Myers MP, Andersen JN, Cheng A, Tremblay ML, Horvath CM, Parisien JP, Salmeen A, Barford D, Tonks NK. TYK2 and JAK2 are substrates of protein-tyrosine phosphatase 1B. J Biol Chem. 2001;276:47771–47774. doi: 10.1074/jbc.C100583200. [DOI] [PubMed] [Google Scholar]
- 39.Zheng XM, Resnick RJ, Shalloway D. Mitotic activation of protein-tyrosine phosphatase alpha and regulation of its Src-mediated transforming activity by its sites of protein kinase C phosphorylation. J Biol Chem. 2002;277:21922–21929. doi: 10.1074/jbc.M201394200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peng ZY, Cartwright CA. Regulation of the Src tyrosine kinase and Syp tyrosine phosphatase by their cellular association. Oncogene. 1995;11:1955–1962. [PubMed] [Google Scholar]
- 41.Gervais FG, Veillette A. Reconstitution of interactions between protein-tyrosine phosphatase CD45 and tyrosine-protein kinase p56(lck) in nonlymphoid cells. J Biol Chem. 1997;272:12754–12761. doi: 10.1074/jbc.272.19.12754. [DOI] [PubMed] [Google Scholar]
- 42.Thomas ML. The regulation of antigen-receptor signaling by protein tyrosine phosphatases: a hole in the story. Curr Opin Immunol. 1999;11:270–276. doi: 10.1016/s0952-7915(99)80044-2. [DOI] [PubMed] [Google Scholar]
- 43.Sexl V, Diehl JA, Sherr CJ, Ashmun R, Beach D, Roussel MF. A rate limiting function of cdc25A for S phase entry inversely correlates with tyrosine dephosphorylation of Cdk2. Oncogene. 1999;18:573–582. doi: 10.1038/sj.onc.1202362. [DOI] [PubMed] [Google Scholar]
- 44.Bahassi el M, Hennigan RF, Myer DL, Stambrook PJ. Cdc25C phosphorylation on serine 191 by Plk3 promotes its nuclear translocation. Oncogene. 2004;23:2658–2663. doi: 10.1038/sj.onc.1207425. [DOI] [PubMed] [Google Scholar]
- 45.Greene MW, Garofalo RS. Positive and negative regulatory role of insulin receptor substrate 1 and 2 (IRS-1 and IRS-2) serine/threonine phosphorylation. Biochemistry. 2002;41:7082–7091. doi: 10.1021/bi015992f. [DOI] [PubMed] [Google Scholar]
- 46.Murai H, Okazaki M, Kikuchi A. Tyrosine dephosphorylation of glycogen synthase kinase-3 is involved in its extracellular signal-dependent inactivation. FEBS Lett. 1996;392:153–160. doi: 10.1016/0014-5793(96)00806-x. [DOI] [PubMed] [Google Scholar]
- 47.Liu F, Hill DE, Chernoff J. Direct binding of the proline-rich region of protein tyrosine phosphatase 1B to the Src homology 3 domain of p130(Cas) J Biol Chem. 1996;271:31290–31295. doi: 10.1074/jbc.271.49.31290. [DOI] [PubMed] [Google Scholar]
- 48.Han JM, Kim JH, Lee BD, Lee SD, Kim Y, Jung YW, Lee S, Cho W, Ohba M, Kuroki T, Suh PG, Ryu SH. Phosphorylation-dependent regulation of phospholipase D2 by protein kinase C delta in rat Pheochromocytoma PC12 cells. J Biol Chem. 2002;277:8290–8297. doi: 10.1074/jbc.M108343200. [DOI] [PubMed] [Google Scholar]