Abstract
Background
Self-reported physical health and functioning and direct measures of physical performance are decreased in hemodialysis patients and are associated with mortality and hospitalization.
Study Design
We determined baseline cross-sectional associations of physical performance, health, and functioning with demographics, clinical characteristics, nutritional indexes, laboratory benchmarks, and measures of body composition in participants in the Frequent Hemodialysis Network (FHN) trial.
Setting & Participants
375 persons enrolled in the FHN with data for physical performance, health, and functioning.
Predictors
Explanatory variables were categorized into fixed factors of age, race, comorbid conditions (diabetes mellitus, heart failure, and peripheral arterial disease) and potentially modifiable factors of dialysis dose, phosphorus level, hemoglobin level, equilibrated normalized protein catabolic rate (enPCR), body composition, body mass index, phase angle, and ratio of intracellular water volume to body weight (calculated from bioelectrical impedance).
Outcomes
Scores on tests of physical performance, health, and functioning.
Measurements
Physical performance measured using the Short Physical Performance Battery, self-reported physical health and functioning using the 36-Item Short Form Health Survey (SF-36). Body composition (body mass index and bioimpedance analysis) and laboratory data were obtained from affiliated dialysis providers.
Results
Relative to population norms, scores for all 3 physicality metrics were low. Poorer scores on all 3 metrics were associated with diabetes mellitus and peripheral arterial disease. Poorer scores on the SF-36 Physical Functioning subscale and Short Physical Performance Battery also were associated with age, lower ratio of intracellular water volume to body weight, and lower enPCR. Black race was associated with poorer scores on the Short Physical Performance Battery.
Limitations
This was a cross-sectional study of individuals agreeing to participate in the FHN study and may not be generalizable to the general dialysis population.
Conclusions
Hemodialysis patients show markedly impaired physical performance, health, and functioning relative to population norms. Although some factors associated with these impairments are not modifiable, others may change with improvement in nutritional status or body composition.
Keywords: Cardiovascular disease, congestive heart failure, diabetes, inflammation, intracellular water, muscle mass, phase angle, peripheral arterial disease
The Frequent Hemodialysis Network (FHN) clinical trials were designed to measure the efficacy of increased dialysis frequency on 2 coprimary out-comes: self-reported physical health and left ventricular mass. As part of the comprehensive baseline evaluation, study participants underwent testing of physical performance using the Short Physical Performance Battery (SPPB) score to assess lower-extremity functioning and completed the Medical Outcomes Study (MOS) 36-Item Short Form Health Survey (SF-36). The SF-36 physical health composite score was calculated as an indicator of self-reported physical health, and the Physical Functioning subscale was used as an indicator of self-reported physical functioning.1
Multiple factors may affect physical performance, health, and functioning, including age, years since the start of end-stage renal disease (ESRD; ESRD vintage),2 anemia in some populations,3 comorbid conditions,4 nutritional status, and body composition.4 Many of these factors are unlikely to be modified by a treatment regimen, but nutritional status and body composition potentially are responsive to treatment modality.
Self-reported physical health and functioning5,6 and directly measured physical performance4 are impaired in hemodialysis patients. Impaired physical performance has been associated with increased hospitalizations, as well as morbidity and mortality.7 The Quetélet index (ie, body mass index [BMI]) is associated directly with survival in patients on hemodialysis therapy.8,9 However, obesity generally impairs physical performance.9 Although simple to use and reproducible, BMI fails to distinguish between body cell mass (metabolically active tissue) and adiposity. Bioelectrical impedance analysis (BIA) can provide additional precision in distinguishing fat-free (lean) and fat mass (adiposity) and further distinguishing between intracellular and extracellular fluid,10 providing a measure of body cell mass.
This cross-sectional analysis is presented to describe factors associated with physical performance, health, and functioning in enrolled and randomly assigned FHN participants at baseline. We hypothesized that impaired physical performance, health, and functioning would be associated with advanced age; lower educational attainment; multiple comorbid conditions, including diabetes mellitus and peripheral arterial disease; congestive heart failure; disordered mineral metabolism; malnutrition; anemia; and lower levels of residual kidney function. We also hypothesized that physical performance, self-reported physical health, and physical functioning would be related inversely to obesity, reported at least in part using BMI, and directly to measures of intracellular water volume normalized to body weight as intracellular water volume per kilogram of body weight (ICW/kg), an indicator of body cell mass derived from resistance and reactance measured using single-frequency BIA,10 and wider phase angle (the ratio of measured reactance and resistance using BIA), a parameter associated with both nutrition and body cell mass.11,12
METHODS
Participants and Measurements
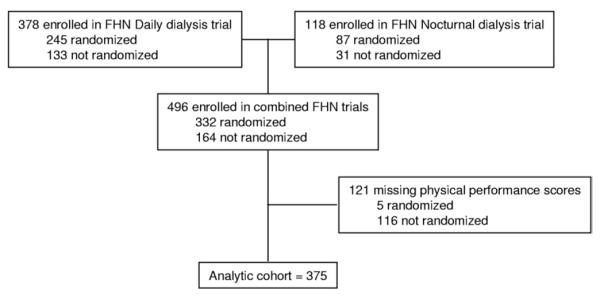
The study design, recruitment strategies, and measurements of the FHN trials have been described previously.1 The present analysis aimed to assess the status of physical performance, self-reported physical health, and physical functioning in FHN participants at baseline (before initiation of the intervention) and determine their clinical correlates. The FHN study consists of 2 separate trials, 1 comparing the effect of in-center 6- to 3-times-weekly in-center hemodialysis (Daily Trial), and the second comparing home nocturnal 6-times-weekly hemodialysis and 3-times-weekly mostly home hemodialysis (Nocturnal Trial). Both trials will examine the effects of frequent hemodialysis on 2 coprimary outcomes: left ventricular mass using cardiac magnetic resonance imaging and the SF-36 physical health composite score. Major exclusion criteria included age younger than 13 (Daily Trial) or younger than 18 years (Nocturnal Trial), inability to achieve a mean estimated Kt/Vurea ≥1.0 on 2 occasions, life expectancy shorter than 6 months, medical need for hemodialysis more than 3 times weekly, history of poor adherence to hemodialysis therapy, medical conditions preventing cardiac magnetic resonance imaging, inability to communicate in English or Spanish, and anticipated kidney transplant or relocation within 14 months. All protocols were reviewed and approved by the institutional review boards at each participating center. Informed consent was obtained from each participant. We included all enrolled participants with physical performance data at baseline, resulting in an analytic cohort of 375 participants (Fig 1).
Figure 1.
Flow chart of derivation of analytic cohort. Abbreviation: FHN, Frequent Hemodialysis Network.
Explanatory variables included demographics (age, sex, and race/ethnicity) and clinical characteristics (ESRD vintage, comorbid conditions [including diabetes mellitus, heart failure, peripheral arterial disease, and stroke]), nutritional indexes (serum albumin level and equilibrated normalized protein catabolic rate [enPCR]), laboratory benchmarks (serum creatinine, hemoglobin, phosphorus, calcium, and parathyroid hormone levels), and measures of body composition: (BMI, phase angle, and derived intracellular water volume per kilogram of body weight).
BMI was calculated using weight measured immediately after a hemodialysis session by means of the Quetélet index (kg/m2). Single-frequency BIA was performed before a midweek dialysis session at 50 kHz for participants with at least one intact leg and arm and used to calculate phase angle, the arc tangent of the reactance (Xc) to resistance (R) ratio. We multiplied the arc tangent of Xc/R by 180/π (57.297) to convert from radians to degrees.13 We also used reactance to estimate total-body potassium using the method of Kotler et al.10 We estimated body cell mass using the following equation14: body cell mass (kg) = 0.00833 × total-body potassium (mmol). Based on St-Onge et al,15 intracellular water volume was calculated as 0.73 × body cell mass. Total intracellular water volume was divided by body weight to derive the fraction of body weight as ICW/kg.
Laboratory variables were measured predialysis at laboratories affiliated with each clinical center, and if more than 1 baseline value existed, the first value was used.
Lower-extremity function was measured empirically using the SPPB, which assessed: (1) gait speed for a 4-m walk, (2) chair stand that measured the ability to stand up and sit down unassisted without the use of arms 5 times, and (3) balance test that measured the ability to stand with the feet in several positions for 10 seconds. Each exercise was scored from 0-4, with higher scores representing fewer limitations. SPPB score was the sum of these component scores and ranged from 0-12.16 The SPBB17 was developed to evaluate lower-extremity function for the Established Populations for Epidemiology Research in the Elderly (EPESE) cohort study for use in the gerontology population. In older persons, the SPPB is highly predictive of death, hospitalization, and need for institutional care.17
Self-reported physical health and functioning were determined using the SF-36.18 The SF-36 has been validated across diverse populations and health care settings and includes 8 scales of self-reported health status: Physical Functioning, Role-Physical, Bodily Pain, General Health, Vitality, Social Functioning, Role-Emotional, and Mental Health.19 These scales are scored from 0-100, with higher scores indicating better functioning.20 The physical health composite score is a weighted average, using the RAND Corp method,21 of all domains except Mental Health. In addition to using the physical health composite score as an assessment of self-reported physical health, we chose the Physical Functioning subscale as a measure of physical functioning, including mild functional losses relevant to independent living.22 The Physical Functioning subscale is composed of 10 questions about limitations in performing various activities.
Data Analysis
Baseline characteristics of the analytic cohort were stratified according to trial and randomization status and characterized using mean ± standard deviation, median (25th percentile, 75th percentile), or frequency (percentage), as appropriate.
We evaluated nonlinear associations between continuous explanatory variables and each outcome graphically and using restricted cubic splines. Significant nonlinear associations of age with physical health composite and Physical Functioning scores were represented using linear splines. We used linear regression to determine the association of explanatory variable with physical health composite and physical functioning. SPPB scores were categorized into 3 ordinal groups for analysis (<6, 7-9, and 10-12), then examined using the proportional odds model for ordinal logistic regression. The proportional odds assumption was tested for all variables in all model settings. For each outcome, explanatory variables were tested individually and after adjusting for case-mix (age, sex, and race/ethnicity [black vs nonblack]). Residuals were examined for normality and heteroscedascity. To meet model assumptions, residual kidney function and parathyroid hormone level were log-transformed after first adding a value of 1. We tested covariate-by-study and covariate-by-randomization status interactions for all explanatory variables.
Covariates significant at the 0.10 level from the case-mix–adjusted analysis became candidates for multivariable models. However, qualifying factors with uncertain cause (primary language not English) or low power (interactions and education level) were not tested further. Multicollinearity among remaining factors was tested using variance inflation factor statistics. Missing values were multiply imputed after incorporating auxiliary variables to reflect factors possibly associated with baseline dropout to validate the assumption that missing observations were associated with the variables used for imputation. Five imputation data sets were created, then sampled with replacement to form 100 bootstrap samples that were each tested for independent associations using the LASSO stepwise selection method23 in tandem with the Schwarz Bayesian information criterion. Of 100 model selection results, variables selected at least 5 times were included in the final multivariable models, which were run using the original set of imputed data as though the variable had been selected a priori. Results were adjusted for uncertainty caused by the imputation of missing values, but we did not factor in uncertainty arising from the model selection process. Body composition measures that did not qualify for inclusion in the final multivariable models were tested separately, adjusting for the qualifying non–body composition factors.
Results from linear regression models were represented with regression coefficients. Ninety-five percent confidence intervals quantified the range of possible coefficients for the univariate and case-mix–adjusted models, with 99% confidence intervals for the multivariable models. Odds ratios with 95% and 99% confidence intervals were used in the same fashion for the ordinal logistic regression results. Univariate and case-mix–adjusted inference tests used α = 0.05. Multivariable models used α = 0.01.
To approximate more generalizable results, ridge regression was used to generate more conservative linear regression estimates, deriving the penalty factor from the average of the heuristic shrinkage estimates24 generated by the bootstrap samples. A corresponding method was used to generate uniformly shrunken odds ratio estimates for SPPB score.
All analyses were conducted using SAS, version 9.2 (SAS Institute, www.sas.com) and S-Plus v6.0 (TIBCO Software Inc, spotfire.tibco.com).
RESULTS
Study Participants and Demographic Characteristics
Data for physical performance, health, and functioning were available for a total of 375 participants enrolled in the combined FHN Daily and Nocturnal Trials. Of these, 243 were randomly assigned in the Daily Trial and 84 were randomly assigned in the Nocturnal Trial (Table 1). Participants in the analytic cohort had a mean age of 50.6 ± 13.7 years, 62% were men, 38% were black, and 41% had diabetes mellitus. Baseline demographic factors were similar in the analytic cohort and excluded persons, except that excluded persons were slightly older (54.4 ± 13.5 years; P = 0.008). Figure 1 graphically summarizes the study cohort.
Table 1.
Participant Characteristics at Enrollment
| Daily Trial |
Nocturnal Trial |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| All (N = 375) |
Randomly Assigned (n = 243) |
Enrolled Only (n = 37) |
Randomly Assigned (n = 84) |
Enrolled Only (n = 11) |
||||||
| Variables | No. | Value | No. | Value | No. | Value | No. | Value | No. | Value |
| Age (y) | 375 | 50.6 ± 13.7 | 243 | 50.3 ± 13.9 | 37 | 48.6 ± 13.1 | 84 | 52.3 ± 13.5 | 11 | 49.2 ± 13.4 |
| Men | 375 | 232 (62) | 243 | 150 (62) | 37 | 20 (54) | 84 | 55 (65) | 11 | 7 (64) |
| Black race | 375 | 143 (38) | 243 | 102 (42) | 37 | 16 (43) | 84 | 22 (26) | 11 | 3 (27) |
| ESRD vintage (y) | 375 | 2.67 (0.96, 6.15) | 243 | 3.64 (1.62, 7.55) | 37 | 1.85 (1.26, 4.64) | 84 | 0.86 (0.27, 3.53) | 11 | 1.71 (0.15, 10.6) |
| Primary language not English |
375 | 63 (17) | 243 | 48 (20) | 37 | 3 (8) | 84 | 10 (12) | 11 | 2 (18) |
| Education level | 367 | 240 | 34 | 83 | 10 | |||||
| >High school graduate | 211 (57) | 132 (55) | 24 (71) | 49 (59) | 6 (60) | |||||
| High school graduate | 86 (23) | 57 (24) | 5 (15) | 21 (25) | 3 (30) | |||||
| <High school graduate | 70 (19) | 51 (21) | 5 (15) | 13 (16) | 1 (10) | |||||
| Lives with family | 371 | 273 (74) | 243 | 166 (68) | 34 | 21 (62) | 84 | 78 (93) | 10 | 8 (80) |
| Insurance status | 372 | 243 | 35 | 84 | 10 | |||||
| Government insurance not awarded by income levela |
91 (24) | 52 (21) | 6 (17) | 29 (35) | 4 (40) | |||||
| Employer group health insurance or privately purchased |
123 (33) | 62 (26) | 12 (34) | 46 (55) | 3 (30) | |||||
| Government insurance for the poorb |
158 (42) | 129 (53) | 17 (49) | 9 (11) | 3 (30) | |||||
| Diabetes | 370 | 153 (41) | 243 | 98 (40) | 32 | 15 (47) | 84 | 35 (42) | 11 | 5 (45) |
| Congestive heart failure | 370 | 69 (19) | 243 | 48 (20) | 32 | 8 (25) | 84 | 11 (13) | 11 | 2 (18) |
| Peripheral arterial disease |
370 | 45 (12) | 243 | 25 (10) | 32 | 5 (16) | 84 | 13 (15) | 11 | 2 (18) |
| Stroke | 370 | 26 (7) | 243 | 19 (8) | 32 | 5 (16) | 84 | 2 (2) | 11 | 0 |
| enPCR (g/kg/d) | 371 | 1.04 ± 0.27 | 243 | 1.03 ± 0.25 | 33 | 1.07 ± 0.35 | 84 | 1.02 ± 0.28 | 11 | 1.20 ± 0.40 |
| Residual renal clearance (mL/min) |
372 | 0 (0, 1.22) | 243 | 0 (0, 0.72) | 34 | 0.49 (0, 5.30) | 84 | 1.13 (0, 2.71) | 11 | 0.83 (0, 3.00) |
| Creatinine (mg/dL) | 372 | 9.96 ± 2.96 | 243 | 10.6 ± 2.70 | 34 | 9.12 ± 3.36 | 84 | 8.83 ± 2.96 | 11 | 8.29 ± 3.29 |
| Phosphate (mg/dL) | 372 | 5.80 ± 1.68 | 243 | 5.78 ± 1.64 | 34 | 5.86 ± 2.08 | 84 | 5.80 ± 1.63 | 11 | 6.04 ± 1.85 |
| Hemoglobin (g/dL) | 346 | 11.9 ± 1.30 | 232 | 11.9 ± 1.30 | 27 | 12.1 ± 1.80 | 79 | 11.8 ± 1.10 | 8 | 12.5 ± 1.00 |
| PTH (pg/mL) | 364 | 317 (183, 548) | 243 | 313 (183, 616) | 27 | 398 (171, 584) | 84 | 326 (195, 439) | 10 | 204 (55.9, 293) |
| Albumin (g/dL) | 372 | 3.92 ± 0.43 | 243 | 3.94 ± 0.42 | 34 | 3.89 ± 0.36 | 84 | 3.91 ± 0.49 | 11 | 3.62 ± 0.48 |
| Calcium (mg/dL) | 364 | 8.96 ± 0.88 | 243 | 9.01 ± 0.92 | 27 | 9.01 ± 0.73 | 84 | 8.82 ± 0.79 | 10 | 8.67 ± 0.85 |
| Bicarbonate (mEq/L) | 363 | 23.3 ± 3.80 | 243 | 23.7 ± 3.70 | 26 | 22.3 ± 4.70 | 84 | 22.7 ± 3.70 | 10 | 22.0 ± 4.10 |
| LV mass (g) | 349 | 141 ± 53.0 | 243 | 141 ± 55.0 | 13 | 165 ± 65.0 | 84 | 137 ± 45.0 | 9 | 152 ± 56.0 |
| BMI (kg/m2) | 353 | 29.0 ± 7.70 | 233 | 28.7 ± 6.80 | 32 | 30.3 ± 11.6 | 79 | 29.5 ± 7.80 | 9 | 27.5 ± 11.3 |
| Phase angle (degrees) | 351 | 5.42 ± 1.50 | 233 | 5.43 ± 1.52 | 32 | 5.39 ± 1.46 | 77 | 5.52 ± 1.49 | 9 | 4.35 ± 1.23 |
| ICW volume/weight (dL/kg) |
351 | 0.260 ± 0.063 | 233 | 0.267 ± 0.062 | 32 | 0.257 ± 0.067 | 77 | 0.268 ± 0.066 | 9 | 0.260 ± 0.074 |
| PHC scorec | 375 | 37.4 ± 10.1 | 37 | 34.6 ± 9.8 | 37 | 34.6 ± 9.8 | 84 | 37.6 ± 9.0 | 11 | 29.7 ± 7.5 |
| PF scale scorec | 375 | 57.9 ± 26.0 | 243 | 58.1 ± 26.9 | 37 | 51.6 ± 27.1 | 84 | 61.9 ± 22.2 | 11 | 42.7 ± 24.0 |
| SPPB score | 375 | 243 | 37 | 84 | 11 | |||||
| 0-6 | 83 (22) | 60 (25) | 9 (24) | 10 (12) | 4 (36) | |||||
| 7-9 | 134 (36) | 80 (33) | 17 (46) | 36 (43) | 1 (9) | |||||
| 10-12 | 158 (42) | 103 (42) | 11 (30) | 38 (45) | 6 (55) | |||||
Note: Results are shown as mean ± standard deviation, median (25th, 75th percentiles), or frequency (percentage), as appropriate. Conversion factors for units: creatinine in mg/dL to μmol/L, ×88.4; hemoglobin and albumin in g/dL to g/L, ×10; calcium in mg/dL to mmol/L, ×0.2495. No conversion necessary for PTH in pg/mL and ng/L and bicarbonate in mEq/L and mmol/L.
Abbreviations: BMI, body mass index; enPCR, equilibrated normalized protein catabolic rate; ESRD, end-stage renal disease; ICW, intracellular water; LV, left ventricular; PF, Physical Functioning; PHC, physical health composite; PTH, parathyroid hormone; SPPB, Short Physical Performance Battery.
Medicare plus Canadian Health.
Medicaid and/or other state/county programs.
From the 36-Item Short Form Health Survey.
Physical Performance
Scores on the SPPB were skewed (Fig 2), whereas the SF-36 physical health composite (Fig 3) and Physical Functioning subscale scores (Fig 4) were somewhat more symmetrically distributed, yet truncated.
Figure 2.
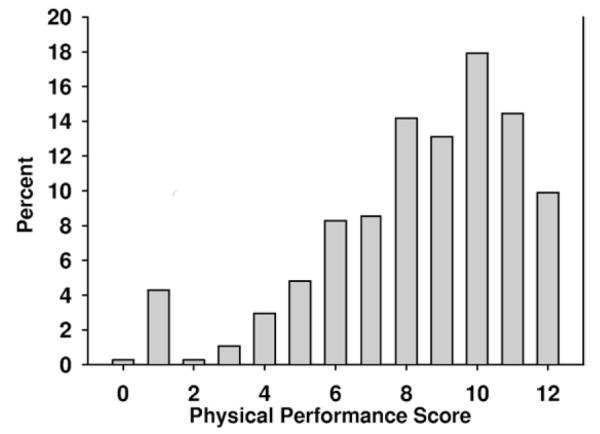
Distribution of Short Physical Performance Battery (SPPB) scores of patients enrolled in the Frequent Hemodialysis Network trial. Lower-extremity function was measured empirically using the SPPB, which measured: (1) gait speed for a 4-m walk, (2) chair stand with a goal of standing unassisted without use of arms 5 times within 60 seconds, and (3) balance score, which measures the capacity to stand with feet together for 10 seconds. Each exercise was scored from 0-4, with higher scores representing fewer limitations. SPPB score was the sum of these component scores. For composing this figure, scores were grouped by 2.
Figure 3.
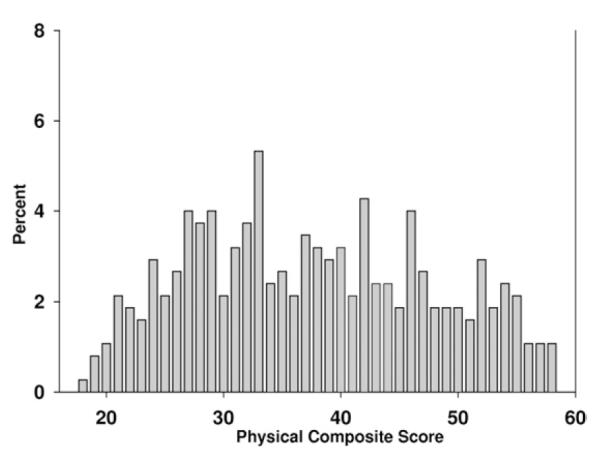
Distribution of physical health composite scores in patients enrolled in the Frequent Hemodialysis Network Trial. The minimum score achieved was 18.
Figure 4.
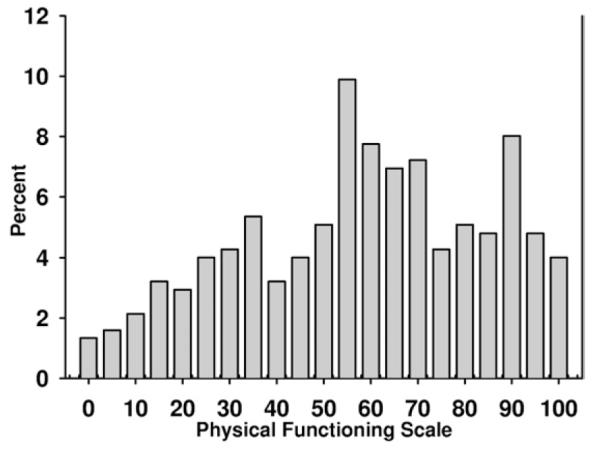
Distribution of Physical Functioning subscale scores measured in patients enrolled in the Frequent Hemodialysis Network Trial. The Physical Functioning subscale is composed of 10 questions about mobility (moving a table, pushing a vacuum, lifting or carrying groceries, climbing several flights of stairs, climbing 1 flight of stairs, bending or stooping, walking 1 mile, walking several blocks, and walking 1 block) and self-care (bathing or dressing oneself). These scales are scored from 0-100, with higher scores indicating better function. For composing this figure, scores were grouped by 5.
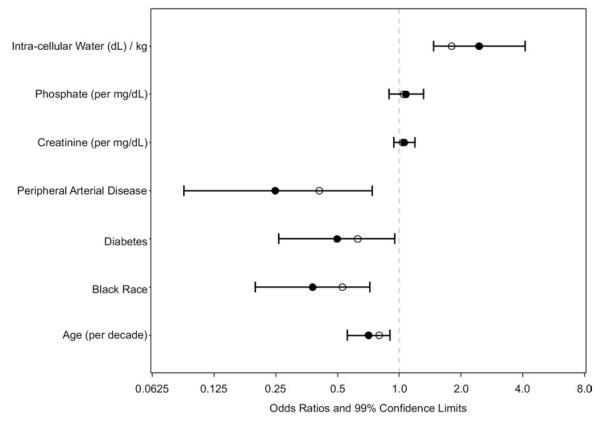
Using univariate analysis, scores on the SPPB (Table 2) were associated inversely with age, female sex, black race, diabetes mellitus, heart failure, history of peripheral arterial disease, history of stroke, BMI, and bicarbonate level and directly with male sex, creatinine level (daily trial only), phosphate level (daily trial only), ICW/kg, and phase angle. After case-mix adjustment, associations with heart failure, history of stroke, serum albumin level, and bicarbonate level became nonsignificant. Using multivariable analysis, SPPB scores were associated inversely with age, black race, diabetes mellitus, and peripheral arterial disease (Fig 5). After controlling for other variables in the multivariable model, ICW/kg and phase angle, tested separately, were directly associated with SPPB scores, but BMI was not.
Table 2.
Univariate and Case-Mix–Adjusted Regression Analysis of Short Physical Performance Battery Score
| Univariate |
Case-Mix–Adjusted |
||||
|---|---|---|---|---|---|
| Variables | No. | OR (95% CI) | P | OR (95% CI) | P |
| Age (/decade) | 375 | 0.61 (0.53-0.71) | <0.001 | 0.60 (0.52-0.70) | <0.001 |
| Male | 375 | 2.03 (1.37-3.00) | <0.001 | 2.01 (1.34-3.01) | <0.001 |
| Black race | 375 | 0.58 (0.40-0.86) | 0.007 | 0.58 (0.39-0.87) | 0.009 |
| ESRD vintage (/1 y) | 375 | 1.01 (0.98-1.05) | 0.4 | 1.00 (0.97-1.03) | 0.9 |
| Primary language not English | 375 | 0.90 (0.55-1.49) | 0.7 | 0.53 (0.30-0.92) | 0.02 |
| Education levela | 372 | ||||
| <High school graduate | 3.80 (0.68-21.3) | 0.1 | 4.81 (0.78-29.7) | 0.09 | |
| High school graduate | 3.80 (0.68-21.0) | 0.1 | 5.98 (0.98-36.5) | 0.05 | |
| Does not live with family | 372 | 0.92 (0.60-1.42) | 0.7 | 0.92 (0.58-1.45) | 0.7 |
| Insurance statusb | 372 | ||||
| Employer group health insurance or privately purchased |
1.16 (0.71-1.89) | 0.5 | 1.49 (0.89-2.48) | 0.1 | |
| Government insurance for the poorc | 0.94 (0.59-1.51) | 0.8 | 0.82 (0.49-1.35) | 0.4 | |
| Diabetes | 370 | 0.30 (0.20-0.45) | <0.001 | 0.36 (0.23-0.54) | <0.001 |
| Congestive heart failure | 370 | 0.47 (0.29-0.77) | 0.003 | 0.77 (0.46-1.28) | 0.3 |
| Peripheral arterial disease | 370 | 0.20 (0.11-0.37) | <0.001 | 0.22 (0.12-0.42) | <0.001 |
| Stroke | 370 | 0.42 (0.20-0.88) | 0.02 | 0.65 (0.30-1.38) | 0.3 |
| enPCR (/0.1 g/kg/d) | 371 | 1.06 (0.99-1.13) | 0.1 | 1.04 (0.96-1.12) | 0.3 |
| Residual renal clearance (/1 mL/min) | 372 | 1.22 (0.74-2.01) | 0.4 | 1.27 (0.75-2.13) | 0.4 |
| Creatinine (/1 mg/dL) | 372 | ||||
| Daily Trial | 1.23 (1.13-1.34) | <0.001 | 1.16 (1.06-1.28) | 0.002 | |
| Nocturnal Trial | 1.02 (0.89-1.16) | 0.8 | 0.99 (0.85-1.16) | 0.9 | |
| Phosphate (/1 mg/dL) | 372 | ||||
| Daily Trial | 1.40 (1.21-1.61) | <0.001 | 1.25 (1.08-1.45) | 0.003 | |
| Nocturnal Trial | 1.02 (0.81-1.28) | 0.9 | 0.98 (0.77-1.24) | 0.8 | |
| Hemoglobin (/1 g/dL) | 346 | 1.05 (0.90-1.23) | 0.5 | 1.05 (0.90-1.24) | 0.5 |
| PTH (/0.1 pg/L) | 364 | 1.76 (1.13-2.93) | 0.9 | 1.68 (1.05-2.86) | 0.9 |
| Albumin (/0.1 g/dL) | 372 | 1.04 (1.00-1.09) | 0.07 | 1.04 (0.99-1.09) | 0.1 |
| Calcium (/1 mg/dL) | 364 | 0.85 (0.69-1.06) | 0.2 | 0.95 (0.76-1.20) | 0.7 |
| Bicarbonate (/1 mmol/L) | 363 | 0.93 (0.88-0.98) | 0.006 | 0.96 (0.91-1.01) | 0.1 |
| LV mass (/10 g) | 349 | 1.01 (0.97-1.05) | 0.7 | 0.97 (0.93-1.01) | 0.2 |
| BMI (/1 kg/m2) | 353 | 0.97 (0.94-0.99) | 0.007 | 0.97 (0.94-1.00) | 0.02 |
| ICW volume/weight (/1 dL/kg) | 351 | 2.97 (2.13-4.15) | <0.001 | 3.88 (2.17-6.93) | <0.001 |
| Phase angle (/0.1°) | 351 | 1.04 (1.03-1.06) | <0.001 | 1.02 (1.01-1.04) | 0.003 |
| Randomly assigned | 375 | 1.37 (0.79-2.41) | 0.3 | 1.53 (0.86-2.72) | 0.1 |
| Nocturnal Trial | 375 | 1.41 (0.91-2.18) | 0.1 | 1.45 (0.92-2.30) | 0.1 |
Note: The Short Physical Performance Battery is a direct measurement of lower-extremity function, measured using: (1) gait speed for a 4-m walk, (2) chair stand with a goal of standing unassisted without use of arms 5 times within 60 seconds, and (3) balance score, which measures the capacity to stand with feet together for 10 seconds. Each exercise was scored from 0-4, with higher scores representing fewer limitations. The Short Physical Performance Battery Score was the sum of these component scores.
Abbreviations: BMI, body mass index; CI, confidence interval; enPCR, equilibrated normalized protein catabolic rate; ESRD, end-stage renal disease; ICW, intracellular water; LV, left ventricular; OR, odds ratio; PTH, parathyroid hormone.
The reference group includes individuals with education beyond high school.
The reference group is government insurance not awarded by income level (Medicare plus Canadian Health).
Medicaid and/or other state/county programs.
Figure 5.
Multivariable associations with physical performance subscale score. Values are shown as odds ratios and 99% confidence limits for independent associations with the Short Physical Performance Battery score. Empty circles represent odds ratio estimates in which maximized fitting to the data from our cohort is offset through penalization methods. These estimates may be more generalizable.
Self-reported Physical Health
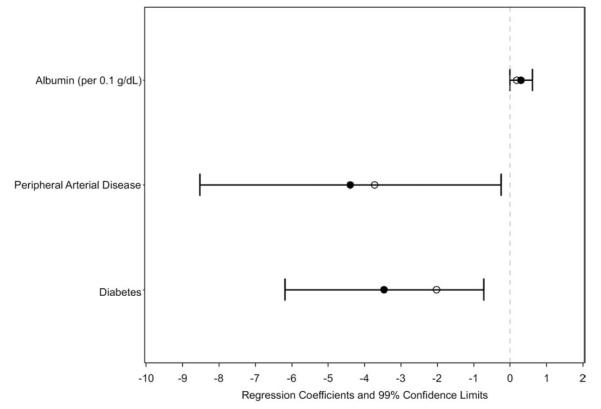
Using univariate analysis, physical health composite score (Table 3) was associated inversely with age, diabetes mellitus, heart failure, peripheral arterial disease, and stroke and directly with age (for participants [>50 years), enPCR, residual kidney function (randomly assigned participants only), serum creatinine level, serum albumin level (daily trial only), and ICW/kg. Randomly assigned participants had higher physical health composite scores than those excluded from randomization after enrollment. After multivariable adjustment, only diabetes mellitus and peripheral arterial disease remained significantly associated with physical health composite score (Fig 6).
Table 3.
Univariate and Case-Mix–Adjusted Regression Analysis of Physical Health Composite Score
| Univariate |
Case-Mix–Adjusted |
||||
|---|---|---|---|---|---|
| Variables | No. | Estimate (95% CI) | P | Estimate (95% CI) | P |
| Age (/decade) | 375 | ||||
| ≤50 y | −2.56 (−4.02 to −1.10) | <0.001 | −2.65 (−4.11 to −1.19) | <0.001 | |
| >50 y | 1.59 (0.19 to 2.99) | 0.03 | 1.69 (0.28 to 3.09) | 0.02 | |
| Male | 375 | 1.90 (−0.21 to 4.01) | 0.08 | 2.12 (0.03 to 4.21) | 0.05 |
| Black race | 375 | −0.08 (−2.20 to 2.04) | 0.9 | 0.48 (−1.62 to 2.59) | 0.7 |
| ESRD vintage (/1 y) | 375 | 0.15 (−0.02 to 0.32) | 0.08 | 0.17 (0.001 to 0.34) | 0.05 |
| Primary language not English | 375 | 2.72 (−0.02 to 5.47) | 0.05 | 2.94 (0.09 to 5.80) | 0.04 |
| Education levela | 372 | ||||
| <High school graduate | 1.06 (−1.69 to 3.80) | 0.5 | 0.61 (−2.14 to 3.36) | 0.7 | |
| High school graduate | 0.26 (−2.29 to 2.80) | 0.8 | 0.20 (−2.32 to 2.72) | 0.9 | |
| Does not live with family | 372 | 1.16 (−1.19 to 3.50) | 0.3 | 1.23 (−1.13 to 3.60) | 0.3 |
| Insurance statusb | 372 | ||||
| Employer group health insurance or privately purchased |
0.39 (−2.24 to 3.02) | 0.8 | 0.53 (−2.07 to 3.14) | 0.7 | |
| Government insurance for the poorc | 0.22 (−2.27 to 2.71) | 0.9 | 0.28 (−2.24 to 2.80) | 0.8 | |
| Diabetes | 370 | −4.55 (−6.60 to −2.49) | <0.001 | −4.11 (−6.27 to −1.95) | <0.001 |
| Congestive heart failure | 370 | −3.46 (−6.10 to −0.82) | 0.01 | −3.16 (−5.86 to −0.46) | 0.02 |
| Peripheral arterial disease | 370 | −5.77 (−8.89 to −2.65) | <0.001 | −6.04 (−9.19 to −2.89) | <0.001 |
| Stroke | 370 | −5.17 (−9.20 to −1.14) | 0.01 | −4.48 (−8.51 to −0.44) | 0.03 |
| enPCR (/0.1 g/kg/d) | 371 | 0.36 (−0.01 to 0.74) | 0.06 | 0.48 (0.09 to 0.86) | 0.01 |
| Residual renal clearance (/1 mL/min)d | 372 | ||||
| Randomized patients | 8.74 (0.04 to 5.72) | 0.04 | 8.29 (0.02 to 5.61) | 0.04 | |
| Not randomized patients | 0.05 (−1.80 to 1.79) | 0.8 | 0.06 (−1.80 to 1.81) | 0.8 | |
| Creatinine (/1 mg/dL) | 372 | 0.61 (0.26 to 0.95) | <0.001 | 0.63 (0.24 to 1.01) | 0.002 |
| Phosphate (/1 mg/dL) | 372 | 0.20 (−0.41 to 0.82) | 0.5 | 0.24 (−0.39 to 0.87) | 0.5 |
| Hemoglobin (/1 g/dL) | 346 | 0.71 (−0.13 to 1.55) | 0.1 | 0.63 (−0.19 to 1.46) | 0.1 |
| PTH (/0.1 pg/L) | 364 | 1.85 (−0.44 to 13.4) | 0.2 | 0.26 (0.001 to 0.53) | 0.05 |
| Albumin (/1 g/dL)d | 372 | ||||
| Daily Trial | 0.56 (0.27 to 0.85) | <0.001 | 0.56 (0.27 to 0.85) | <0.001 | |
| Nocturnal Trial | 0.02 (−0.39 to 0.42) | 0.9 | −0.05 (−0.45 to 0.36) | 0.8 | |
| Calcium (/1 mg/dL) | 364 | 0.78 (−0.40 to 1.96) | 0.2 | 1.09 (−0.09 to 2.28) | 0.07 |
| Bicarbonate (/1 mmol/L) | 363 | −0.06 (−0.34 to 0.21) | 0.6 | −0.07 (−0.35 to 0.20) | 0.6 |
| LV mass (/10 g) | 349 | −0.04 (−0.25 to 0.16) | 0.7 | −0.13 (−0.35 to 0.10) | 0.3 |
| BMI (/1 kg/m2) | 353 | −0.13 (−0.27 to 0.01) | 0.07 | −0.10 (−0.24 to 0.04) | 0.2 |
| ICW volume/weight (/1 dL/kg) | 351 | 2.53 (0.87 to 4.20) | 0.003 | 3.53 (0.72 to 6.33) | 0.01 |
| Phase angle (/0.1°) | 351 | 0.07 (−0.01 to 0.14) | 0.07 | 0.05 (−0.03 to 0.13) | 0.2 |
| Randomly assigned | 375 | 4.43 (1.38 to 7.48) | 0.005 | 4.30 (1.29 to 7.31) | 0.005 |
| Nocturnal trial | 375 | −0.89 (−3.26 to 1.47) | 0.5 | −0.90 (−3.26 to 1.46) | 0.5 |
Note: The physical health composite score is a weighted average of scores deriving from 8 scales of self-reported health status: Physical Functioning, Role-Physical, Bodily Pain, General Health, Vitality, Social Functioning, and Role-Emotional, scored 0-100.
Abbreviations: BMI, body mass index; CI, confidence interval; enPCR, equilibrated normalized protein catabolic rate; ESRD, end-stage renal disease; ICW, intracellular water; LV, left ventricular; PTH, parathyroid hormone.
The reference group is individuals with education beyond high school.
The reference group is government insurance not awarded by income level (Medicare plus Canadian Health).
Medicaid and/or other state/county programs.
Variables showed a statistically significant interaction with trial or randomization status.
Figure 6.
Multivariable associations with self-reported physical health. Values are shown as regression coefficients and 99% confidence limits for independent associations with the 36-Item Short Form Health Survey physical health composite scores. Empty circles represent regression coefficient estimates in which maximized fitting to the data from our cohort is offset through penalization methods. These estimates may be more generalizable.
Self-reported Physical Functioning
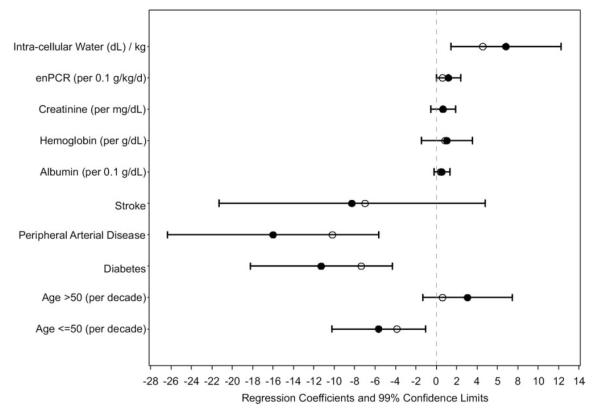
Using univariate analysis, physical functioning (Table 4) was associated inversely with age (for participants ≤50 years), female sex, diabetes mellitus, heart failure, peripheral arterial disease, stroke, bicarbonate level, and BMI and directly with enPCR, serum creatinine level, hemoglobin level, serum albumin level, both intracellular water volume per kilogram of body weight and phase angle, and randomization status. Using multivariable analysis (Fig 7), physical functioning was associated inversely with age of 50 years or younger, diabetes mellitus, and peripheral arterial disease and directly with enPCR and intracellular water volume per kilogram of body weight.
Table 4.
Univariate and Case-Mix–Adjusted Regression Analysis of Physical Function Score
| Univariate Results |
Case-Mix–Adjusted Results |
||||
|---|---|---|---|---|---|
| Variables | No. | Estimate (95% CI) | P | Estimate (95% CI) | P |
| Age (/decade) | 375 | ||||
| ≤50 y | −8.90 (−12.5 to −5.24) | <0.001 | −9.13 (−12.7 to −5.49) | <0.001 | |
| >50 y | −0.34 (−3.86 to 3.18) | 0.9 | −0.09 (−3.59 to 3.41) | 0.9 | |
| Male | 375 | 8.37 (2.98 to 13.7) | 0.002 | 8.66 (3.44 to 13.8) | 0.001 |
| Black race | 375 | −1.83 (−7.28 to 3.62) | 0.5 | −0.44 (−5.68 to 4.80) | 0.9 |
| ESRD vintage (y) | 375 | 0.32 (−0.12 to 0.76) | 0.1 | 0.25 (−0.18 to 0.67) | 0.3 |
| Primary language not English | 375 | 6.69 (−0.36 to 13.7) | 0.06 | 4.77 (−2.36 to 11.9) | 0.2 |
| Education levela | 372 | ||||
| <High school graduate | 1.61 (−5.45 to 8.68) | 0.7 | −0.67 (−7.52 to 6.17) | 0.8 | |
| High school graduate | 2.50 (−4.04 to 9.05) | 0.5 | 1.81 (−4.45 to 8.08) | 0.5 | |
| Does not live with family vs lives with family | 372 | 4.23 (−1.80 to 10.2) | 0.2 | 4.37 (−1.49 to 10.2) | 0.1 |
| Insurance statusb | 372 | ||||
| Employer group health insurance or privately purchased |
−0.75 (−7.51 to 6.02) | 0.8 | 0.71 (−5.78 to 7.20) | 0.8 | |
| Government insurance for the poorc | 1.56 (−4.48 to 7.95) | 0.6 | −0.11 (−6.37 to 6.16) | 0.9 | |
| Diabetes | 370 | −19.2 (−24.2 to −14.1) | <0.001 | −16.3 (−21.5 to −11.0) | <0.001 |
| Congestive heart failure | 370 | −9.81 (−16.6 to −3.01) | 0.005 | −5.45 (−12.2 to 1.31) | 0.1 |
| Peripheral arterial disease | 370 | −24.2 (−32.0 to −16.4) | <0.001 | −22.9 (−30.6 to −15.3) | <0.001 |
| Stroke | 370 | −18.5 (−28.8 to −8.20) | <0.001 | −13.7 (−23.7 to −3.70) | 0.008 |
| enPCR (0.1 g/kg/d) | 371 | 1.17 (0.20 to 2.14) | 0.02 | 1.35 (0.40 to 2.31) | 0.005 |
| Residual renal clearance (mL/min) | 372 | 4.39 (−1.89 to 310.0) | 0.2 | 0.76 (−0.78 to 2.29) | 0.3 |
| Creatinine (mg/dL) | 372 | 2.26 (1.39 to 3.14) | <0.001 | 1.80 (0.84 to 2.75) | <0.001 |
| Phosphate (mg/dL) | 372 | 1.46 (−0.11 to 3.04) | 0.07 | 0.73 (−0.85 to 2.31) | 0.4 |
| Hemoglobin (g/dL) | 346 | 2.69 (0.54 to 4.84) | 0.01 | 2.42 (0.35 to 4.48) | 0.02 |
| PTH (0.1 pg/L) | 364 | 1.93 (−0.96 to 192.0) | 0.6 | 0.19 (−0.47 to 0.86) | 0.6 |
| Albumin (g/dL) | 372 | 1.16 (0.55 to 1.76) | <0.001 | 0.96 (0.37 to 1.55) | 0.002 |
| Calcium (mg/dL) | 364 | 1.06 (−2.00 to 4.11) | 0.5 | 2.25 (−0.71 to 5.20) | 0.1 |
| Bicarbonate (mmol/L) | 363 | −0.79 (−1.49 to −0.09) | 0.03 | −0.59 (−1.27 to 0.09) | 0.09 |
| LV mass (10 g) | 349 | −0.05 (−0.57 to 0.47) | 0.8 | −0.46 (−1.02 to 0.09) | 0.1 |
| BMI (kg/m2) | 353 | −0.56 (−0.91 to −0.21) | 0.002 | −0.47 (−0.81 to −0.13) | 0.007 |
| ICW volume/weight (dL/kg) | 351 | 11.7 (7.60 to 15.8) | <0.001 | 15.0 (8.18 to 21.9) | <0.001 |
| Phase angle (0.1°) | 351 | 0.40 (0.20 to 0.60) | <0.001 | 0.23 (0.04 to 0.43) | 0.02 |
| Randomly assigned vs not randomly assigned | 375 | 9.46 (1.60 to 17.3) | 0.02 | 9.66 (2.15 to 17.1) | 0.01 |
| Nocturnal vs daily trial | 375 | 2.41 (−3.67 to 8.50) | 0.4 | 2.76 (−3.11 to 8.63) | 0.4 |
Note: Physical Functioning is composed of 10 questions about mobility (moving a table, pushing a vacuum, lifting or carrying groceries, climbing several flights of stairs, climbing 1 flight of stairs, bending or stooping, walking 1 mile, walking several blocks, and walking 1 block) and self-care (bathing or dressing oneself).
Abbreviations: BMI, body mass index; CI, confidence interval; enPCR, equilibrated normalized protein catabolic rate; ESRD, end-stage renal disease; ICW, intracellular water; LV, left ventricular; PTH, parathyroid hormone.
The reference group is individuals with education beyond high school.
The reference group is government insurance not awarded by income level (Medicare plus Canadian Health).
Medicaid and/or other state/county programs.
Figure 7.
Multivariable associations with self-reported physical functioning. Values are shown as regression coefficients and 99% confidence limits for independent associations with the 36-Item Short Form Health Survey Physical Functioning subscale score. Empty circles represent regression coefficient estimates in which maximized fitting to the data from our cohort is offset through penalization methods. These estimates may be more generalizable. Abbreviation: enPCR, equilibrated normalized protein catabolic rate.
DISCUSSION
Physical performance was relatively poor in participants in the FHN clinical trials compared with population norms for the elderly population in the EPESE. Scores for self-reported measures of physical health (physical health composite score) and functioning (Physical Functioning subscale) from the SF-36 questionnaire were lower than expected for the age of the studied population.19,25 Physical performance was significantly better in the EPESE population despite an average age 20 years older than the FHN population (Fig S1, available as online supplementary material). Physical health composite and Physical Functioning subscale scores were similar to those reported elsewhere for hemodialysis patients.5,6,26
Diabetes mellitus and peripheral arterial disease were associated with poor physical performance and self-reported physical health and functioning. These were the only variables associated with all 3 measures using multivariable analysis and are unlikely to be modified by changes in dialysis frequency or dose. Kidney disease and dialysis-related variables, specifically ESRD vintage and residual kidney function, were not associated with any of the tests performed, and neither were bicarbonate, calcium, or parathyroid hormone level. enPCR, which at steady state is a reflection of dietary nitrogen intake, was associated directly with physical health composite and Physical Functioning subscale scores using univariate analysis and with Physical Functioning score using multivariable analysis, and serum phosphorus level was associated directly with SPPB score, but not with the physical health composite or Physical Functioning scores. None of these variables remained significantly associated with physical performance, health, or functioning after multivariable adjustment with the exception of enPCR and Physical Functioning score. Because dietary phosphorus closely parallels that of protein,27 these variables may be reflecting dietary intake and nutritional status downstream, including support of lean body mass reflected by ICW/kg or phase angle, as well as improving physical performance through mechanisms not directly reflected in altered body composition.
ICW/kg was associated directly with SPPB and Physical Functioning score using multivariable analysis and with all 3 metrics using univariate analysis. Intracellular water volume is a measure of body cell mass, the body’s pool of metabolically active tissue.15 The principal body cell mass compartment that would vary among individuals would be expected to be muscle.28 Muscle mass tends to decrease over time in patients on dialysis therapy, most likely in response to inflammatory, nutritional, and other factors, including acidemia29 and physical inactivity. Increasing the frequency of dialysis potentially could preserve muscle mass by improving nutritional status, more fully correcting acidemia (by avoidance of prolonged interdialytic periods in which acid accumulates), and perhaps by facilitating physical activity. In contrast to ICW/kg (or if this was omitted from the analysis, phase angle), BMI was associated inversely with both SPPB and Physical Functioning scores, suggesting a separate and potentially deleterious effect of adiposity when adjusted for body cell mass.
The SPPB was developed in an elderly population with a variety of comorbid conditions. Elderly and nonelderly patients on dialysis therapy share multiple clinical characteristics, including high rates of skeletal muscle wasting and weakness, vitamin D deficiency, osteoporosis, and multiple comorbid conditions. Chen et al30 used the SPPB as a primary outcome measure in a strength training study in hemodialysis patients. They reported that SPPB score was associated directly with leg muscle strength at baseline, and changes in scores after training paralleled changes in leg muscle strength.
One other study has reported SPPB scores in older patients with ESRD awaiting transplant. The mean score (8.49) in our population was similar to that reported by Hartmann et al31 (8.35), although our participants were younger (50.7 vs 67.5 years). The distribution of SPPB scores in our population was lower than for the 70-year-old population in EPESE,25 with a lower percentage of patients scoring in the highest quartile (10-12) and a much higher percentage scoring in the lower quartile (4-6; Fig S1).
Older age, black race, diabetes mellitus, peripheral arterial disease, and lower intracellular water volume per kilogram of body weight were all associated with poorer SPPB scores. The study by Hartmann et al31 featured too few patients to stratify by race; however, older age and black race have been associated consistently with lower physical functioning in studies of older adults without chronic kidney disease or ESRD. The associations of diabetes mellitus and peripheral arterial disease with impaired physical performance are not surprising. Comorbidity has been associated with disability32 and functional decline,33-35 and the specific comorbid conditions of diabetes34 and peripheral arterial disease36 have been related specifically to poor physical functioning and disability in several studies.
Interestingly, age was associated with SPPB and Physical Functioning scores (ie, older age was associated with lower levels of physical performance and self-reported physical functioning), but not physical health composite score, suggesting that expectations of overall physical health may be accommodated with advancing age despite objective evidence of impaired physical performance and the inability to perform certain activities of daily living and independent function. Perhaps the most interesting observations in this study relate to the bidirectional effects suggested by BMI and BIA-derived estimates of body composition. Physical performance and functioning were related directly to intracellular water volume per kilogram of body weight and phase angle and inversely to BMI, suggesting that body cell mass may augment physical performance and functioning, whereas adiposity might further impair performance and functioning in the hemodialysis population, similar to the increased physical disability observed in older women who show sarcopenic obesity (ie, low muscle mass to total body mass ratio).37
Inflammation has been noted to be an independent determinant of physical performance,38 and serum albumin level, the only surrogate marker of inflammation obtained in this analysis, was associated strongly with both Physical Functioning and physical health composite scores. It was not associated with SPPB score, an objective measurement of physical performance. Creatinine level, most likely a surrogate for muscle mass, was associated positively with all 3 measures of physical performance using case-mix–adjusted analysis. In contrast, anemia was not associated with any of the performance measures.
The main limitations of the study relate to its cross-sectional design. For example, we cannot determine whether decreased intracellular water volume per kilogram of body weight led to impaired physical performance or vice versa. When the FHN trials conclude, it will be informative to determine rates of change in physical performance, health, and function in groups randomly assigned to conventional 3- or 6-times-weekly hemodialysis. In addition, although the demographic distribution of participants in the FHN trials was similar to the North American hemodialysis population, persons willing to be enrolled and/or randomly assigned into a clinical trial that could potentially result in a major change in lifestyle for 1 year are likely to differ from “all comers” in ways that cannot be measured. In summary, we observed relatively poor levels of physical performance, health, and functioning in a cohort of 375 participants in the FHN trials. Recognizing that the results reported here probably represent the ESRD program’s “best-case example” with regard to physical performance, self-reported physical health, and physical functioning, there is much room for improvement. Impaired physical performance, health, and functioning are common complications of hemodialysis therapy, with broad-ranging effects on lifestyle, quality of life, and longevity. Although the fixed effects of age, sex, diabetes mellitus, and peripheral arterial disease explain much of the variation in physical performance, health, and functioning, differences in body composition also appear to be important determinants and may be modifiable with exercise training and/or other interventions. We are eager to learn whether more frequent dialysis will result in material improvements in physical performance, health, and functioning. Regardless, given the degree of functional impairment in patients with ESRD, prospective testing of multiple interventions is clearly warranted.
Supplementary Material
ACKNOWLEDGEMENTS
Members of the FHN Trial Group are Achinger S, Anderson S, Appel L, Apruzzes R, Atwal J, Augustine B, Ayus J, Bardsley J, Bay W, Beach S, Beck G, Bharti B, Briggs J, Bullas R, Burkart J, Burrowes J, Cabezon E, Callegari J, Carter M, Champagne J, Chan C, Chan W, Chang J, Chertow G, Cheung A, Copland M, Coplon N, Coppley A, Daugirdas J, Dellagrottaglie S, Depner T, Derse A, Dominguez A, Doss S, Eggers P, Eknoyan G, Escalada R, Fensterer A, Finkelstein F, Fofie Y, Franzwa B, Frome R, Fu Z, Garg A, Gassman J, Gayda P, Geller N, Geronemus R, Goodman W, Gorodetskaya I, Gotch F, Greene T, Greenwood R, Grimm R, Gutierrez M, Hall Y, Handelman G, Henderson L, Hernandez A, Higgins H, Hilkin A, Hostetter T, Hoy C, Humphreys M, Hunsicker L, James S, Kariisa M, Kaufman A, Kaufman T, Kaysen G, Ke S, Keene R, Kimmel P, Kliger A, Kotanko P, Kramer C, Kuhlmann M, Kwan S, Kwok S, Lacson E, Larive B, Leavell E, Lemus D, Levin A, Levin N, Li M, Lilli K, Lindsay R, Lockridge R, Luan J, MacKrell J, Manaster R, Mandaci O, Mathew R, Mauck V, Mazzorato A, McCulloch C, McGrath-Chong M, McLeroy S, Mehta R, Meisels I, Miller B, Mohr P, Moossavi S, Nabali A, Narva A, Nissenson A, Ornt D, Painter P, Pepas J, Peterson C, Pierratos A, Pipkin M, Prichard S, Rajagopalan S, Ramos R, Rashid M, Rastogi A, Regozo K, Riley J, Rivas M, Rocco M, Rodriquez R, Roecker E, Roger D, Rogers J, Salusky I, Sanz G, Sanz J, Schiller-Moran B, Schlarb J, Schuessler R, Schulman G, Schweitzer S, Sergeyeva O, Shah S, Sherer S, Sika M, Sioson L, Skelton R, Smith M, Snell C, Somers D, Sonico J, Spanner E, Star R, Steigerwald D, Stokes J, Suri R, Suter M, Tamura M, Tarallo M, Tichy M, Ting G, Tran T, Ulloa D, Unruh M, Vassalotti J, Wallace W, Waterman E, Wei J, Weiss B, West J, Wiggins K, Winchester J.
Support: The funds for the FHN trials were received from the National Institute of Diabetes and Digestive and Kidney Diseases, Centers for Medicare & Medicaid Services, National Institutes of Health Research Foundation, Fresenius Medical Care Canada, the Renal Research Institute, and Satellite Health Care.
Footnotes
Financial Disclosure: The authors declare that they have no relevant financial interests.
SUPPLEMENTARY MATERIAL Figure S1: Distribution of the Short Physical Performance Battery scores of patients enrolled in the FHN trial and EPESE.
Note: The supplementary material accompanying this article (doi:10.1053/j.ajkd.2010.08.021) is available at www.ajkd.org.
REFERENCES
- 1.Suri RS, Garg AX, Chertow GM, et al. Frequent Hemodialysis Network Trial Group Frequent Hemodialysis Network (FHN) randomized trials: study design. Kidney Int. 2007;71:349–359. doi: 10.1038/sj.ki.5002032. [DOI] [PubMed] [Google Scholar]
- 2.Johansen KL, Kaysen GA, Young BS, Hung AM, da Silva M, Chertow GM. Longitudinal study of nutritional status, body composition, and physical function in hemodialysis patients. Am J Clin Nutr. 2003;77:842–846. doi: 10.1093/ajcn/77.4.842. [DOI] [PubMed] [Google Scholar]
- 3.Thein M, Ershler WB, Artz AS, et al. Diminished quality of life and physical function in community-dwelling elderly with anemia. Medicine. 2009;88:107–114. doi: 10.1097/MD.0b013e31819d89d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johansen K, Chertow G, da Silva MD, Carey S, Painter P. Determinants of physical performance in ambulatory patients on hemodialysis. Kidney Int. 2001;60:1586–1591. doi: 10.1046/j.1523-1755.2001.00972.x. [DOI] [PubMed] [Google Scholar]
- 5.DeOreo P. Hemodialysis patient-assessed functional health status predicts continued survival, hospitalization, and dialysis-attendance compliance. Am J Kidney Dis. 1997;30:204–212. doi: 10.1016/s0272-6386(97)90053-6. [DOI] [PubMed] [Google Scholar]
- 6.Lowrie E, Curtin R, LePain N, Schatell D. Medical Outcomes Study Short Form-36: a consistent and powerful predictor of morbidity and mortality in dialysis patients. Am J Kidney Dis. 2003;41:1286–1292. doi: 10.1016/s0272-6386(03)00361-5. [DOI] [PubMed] [Google Scholar]
- 7.Painter P. Physical functioning in end-stage renal disease patients: update 2005. Hemodial Int. 2005;9:218–235. doi: 10.1111/j.1492-7535.2005.01136.x. [DOI] [PubMed] [Google Scholar]
- 8.Johansen KL, Young B, Kaysen GA, Chertow GM. Association of body size with outcomes among patients beginning dialysis. Am J Clin Nutr. 2004;80:324–332. doi: 10.1093/ajcn/80.2.324. [DOI] [PubMed] [Google Scholar]
- 9.Johansen KL, Kutner NG, Young B, Chertow GM. Association of body size with health status in patients beginning dialysis. Am J Clin Nutr. 2006;83:543–549. doi: 10.1093/ajcn.83.3.543. [DOI] [PubMed] [Google Scholar]
- 10.Kotler DP, Burastero S, Wang J, Pierson RN., Jr Prediction of body cell mass, fat-free mass, and total body water with bioelectrical impedance analysis: effects of race, sex, and disease. Am J Clin Nutr. 1996;64(3 suppl):489S–497S. doi: 10.1093/ajcn/64.3.489S. [DOI] [PubMed] [Google Scholar]
- 11.Marra M, Caldara A, Montagnese C, et al. Bioelectrical impedance phase angle in constitutionally lean females, ballet dancers and patients with anorexia nervosa. Eur J Clin Nutr. 2009;63:905–908. doi: 10.1038/ejcn.2008.54. [DOI] [PubMed] [Google Scholar]
- 12.Bellizzi V, Scalfi L, Terracciano V, et al. Early changes in bioelectrical estimates of body composition in chronic kidney disease. J Am Soc Nephrol. 2006;17:1481–1487. doi: 10.1681/ASN.2005070756. [DOI] [PubMed] [Google Scholar]
- 13.Baumgartner RN, Chumlea WC, Roche AF. Bioelectric impedance phase angle and body composition. Am J Clin Nutr. 1988;48:16–23. doi: 10.1093/ajcn/48.1.16. [DOI] [PubMed] [Google Scholar]
- 14.Moore FD, Olesen KH, McMurray JD, Parker HV, Ball MR, Boyden CM. The Body Cell Mass and Its Supporting Environment. Body Composition in Health and Disease. Saunders; Philadelphia, PA: 1963. [Google Scholar]
- 15.St-Onge MP, Wang Z, Horlick M, Wang J, Heymsfield SB. Dual-energy x-ray absorptiometry lean soft tissue hydration: independent contributions of intra- and extracellular water. Am J Physiol Endocrinol Metab. 2004;287(5):E842–E847. doi: 10.1152/ajpendo.00361.2003. [DOI] [PubMed] [Google Scholar]
- 16.Guralnik JM, Simonsick EM, Ferrucci L, et al. A Short Physical Performance Battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home placement. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 17.Guralnik J, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 as a predictor of subsequent disability. N Engl J Med. 1995;43:845–854. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hays RD, Morales LS. The RAND-36 measure of health-related quality of life. Ann Med. 2001;33:350–357. doi: 10.3109/07853890109002089. [DOI] [PubMed] [Google Scholar]
- 19.Ware JE. SF-36 Health Survey Manual and Interpretation Guide. Nimrod Press; Boston, MA: 1993. [Google Scholar]
- 20.Johansen KL, Chertow GM. Chronic kidney disease mineral bone disorder and health-related quality of life among incident end-stage renal-disease patients. J Ren Nutr. 2007;17:305–313. doi: 10.1053/j.jrn.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hays RD, Kallich JD, Mapes DL, et al. Kidney Disease Quality of Life Short Form (KDQOL-SF), version 1.3: A Manual for Use and Scoring. RAND; Santa Monica, CA: 1997. [Google Scholar]
- 22.Anderson C, Laubscher S, Burns R. Validation of the Short Form 36 (SF-36) health survey questionnaire among stroke patients. Stroke. 1996;27:1812–1816. doi: 10.1161/01.str.27.10.1812. [DOI] [PubMed] [Google Scholar]
- 23.Tibshirani R. Regression shrinkage and selection via the lasso. J R Stat Soc B. 1996;58:267–288. [Google Scholar]
- 24.Copas JB. Regression, prediction and shrinkage (with discussion) J R Stat Soc B. 1983;45:311–354. [Google Scholar]
- 25.Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55:M221–M231. doi: 10.1093/gerona/55.4.m221. [DOI] [PubMed] [Google Scholar]
- 26.Painter P, Carlson L, Carey S, et al. Low-functioning hemodialysis patients improve with exercise training. Am J Kidney Dis. 2000;36:600–608. doi: 10.1053/ajkd.2000.16200. [DOI] [PubMed] [Google Scholar]
- 27.Boaz M, Smetana S. Regression equation predicts dietary phosphorus intake from estimate of dietary protein intake. JAm Diet Assoc. 1996;96:1268–1270. doi: 10.1016/S0002-8223(96)00331-8. [DOI] [PubMed] [Google Scholar]
- 28.Kaysen GA, Zhu F, Sarkar S, et al. Estimation of total-body and limb muscle mass in hemodialysis patients by using multifrequency bioimpedance spectroscopy. Am J Clin Nutr. 2005;82(5):988–995. doi: 10.1093/ajcn/82.5.988. [DOI] [PubMed] [Google Scholar]
- 29.Workeneh BT, Mitch WE. Review of muscle wasting associated with chronic kidney disease. Am J Clin Nutr. 2010;91(4):1128S–1132S. doi: 10.3945/ajcn.2010.28608B. [DOI] [PubMed] [Google Scholar]
- 30.Chen JLT, Godfrey S, Ng TT, et al. Effect of intra-dialytic, low-intensity strength training on functional capacity in adult haemodialysis patients: a randomized pilot trial. 2010. pp. 1936–1943. [DOI] [PMC free article] [PubMed]
- 31.Hartmann EL, Kitzman D, Rocco M, et al. Physical function in older candidates for renal transplantation: an impaired population. Clin J Am Soc Nephrol. 2009;4(3):588–594. doi: 10.2215/CJN.03860808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fried LP, Bandeen-Roche K, Kasper JD, Guralnik JM. Association of comorbidity with disability in older women: the Women’s Health and Aging Study. J Clin Epidemiol. 1999;52:27–37. doi: 10.1016/s0895-4356(98)00124-3. [DOI] [PubMed] [Google Scholar]
- 33.Stuck AE, Walthert JM, Nikolaus T, Bula CJ, Hohmann C, Beck JC. Risk factors for functional decline in community-living elderly people: a systematic literature review. Soc Sci Med. 1999;48:445–469. doi: 10.1016/s0277-9536(98)00370-0. [DOI] [PubMed] [Google Scholar]
- 34.Ferrucci L, Penninx BW, Leveille SG, et al. Characteristics of non-disabled older persons who perform poorly in objective tests of lower extremity function. J Am Geriatr Soc. 2000;48:1102–1110. doi: 10.1111/j.1532-5415.2000.tb04787.x. [DOI] [PubMed] [Google Scholar]
- 35.Maggi S, Noale M, Gallina P, et al. ILSA Group: physical disability among older Italians with diabetes. The ILSA study. Diabetetologia. 2004;47:1957–1962. doi: 10.1007/s00125-004-1555-8. [DOI] [PubMed] [Google Scholar]
- 36.Herman SD, Liu K, Tian L, et al. Baseline lower extremity strength and subsequent decline in functional performance at 6-year follow-up in persons with lower extremity peripheral arterial disease. J Am Geriatr Soc. 2009;57:2246–2252. doi: 10.1111/j.1532-5415.2009.02562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zoico E, DiFrancsco V, Guralnik JM, et al. Physical disability and muscular strength in relation to obesity and different body composition indexes in a sample of healthy elderly women. Int J Obes Relat Metab Disord. 2004;28:234–241. doi: 10.1038/sj.ijo.0802552. [DOI] [PubMed] [Google Scholar]
- 38.Brinkley TE, Leng X, Miller ME, et al. Chronic inflammation is associated with low physical function in older adults across multiple comorbidities. J Gerontol A Biol Sci Med Sci. 2009;64:455–461. doi: 10.1093/gerona/gln038. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.