Abstract
Background
There is a daily rhythm in the voluntary intake of ethanol in mice, with greatest consumption in the early night and lowest intake during the day. The role of daily timing of ethanol exposure on the development and control of long-term ethanol self-administration has been neglected. The present study examines these issues using C57BL/6J mice.
Methods
Mice were repeatedly exposed to 10% ethanol for 2 h early in the night or day for several weeks. Subsequently, ethanol was available at the opposite time (Expt 1) or 24 h daily (Expts 1 and 2). Lick sensors recorded the patterns of drinking activity in Experiment 2.
Results
Mice exposed to ethanol during the night drink more than mice exposed during the day. Prior history did not affect ethanol intake when the schedule was reversed. Under 24 hour exposure conditions, mice with a history of drinking during the night consumed significantly more than mice drinking during the day. The circadian patterns of drinking were not altered.
Conclusions
These results demonstrate that the daily timing of ethanol exposure exerts enduring effects of self-administration of ethanol in mice. Understanding how circadian rhythms regulate ethanol consumption may be valuable for modifying subsequent intake.
Keywords: Ethanol, Circadian Rhythms, Self-Administration, Mice
Introduction
In many mammals, including humans, myriad aspects of physiology and behavior are characterized by robust and predictable 24 hour fluctuations. Among these are daily rhythms of ethanol consumption that have been observed in a number of different species (Danel and Touitou, 2004; el Guebaly, 1987, Smith et al., 1980). Mice and rats, for example, voluntarily consume more ethanol in the dark, during the active phase of their cycles than they do in the light or inactive phase (for review see (Hiller-Sturmhofel and Kulkosky, 2001)). In mice given temporally restricted daily access to ethanol, the peak for voluntary consumption has been further localized to a few hours into the dark phase (Rhodes et al., 2005). Humans with alcoholism likewise display a daily rhythm in their craving for their first drink of the day early into the active phase (Danel et al., 2003). In neither species has the temporal context of drinking been fully explored (but see Spanagel et al., 2005b), particularly with regard to its influence on ethanol self-administration and dependence. Thus, we examine the significance of scheduled ethanol intake on subsequent ad libitum consumption in a well established rodent model, the C57 mouse.
The daily rhythm in ethanol intake likely derives from the joint influence of an endogenous circadian clock in the suprachiasmatic nuclei of the anterior hypothalamus (SCN) and environmental (for example, lighting) factors. The influence of endogenously driven circadian rhythmicity on ethanol intake is suggested by altered ethanol intake following manipulations of the circadian clock. For example, repeated shifts in the light/dark cycle alter intake, albeit in different directions, in male versus female rats (Clark et al., 2007). Similarly, some (Spanagel et al., 2005a), but not other (Zghoul et al., 2007) genetic manipulations of the molecular circadian clock mechanism also affect ethanol drinking in mice. Correlations between circadian measures (e.g., period) and ethanol intake have also been reported (Hofstetter et al., 2003; Spanagel et al., 2005b). Conversely, alcohol consumption may feed back on the circadian pacemaker (Rosenwasser et al., 2005a-b). In addition to endogenous factors, some environmental factors, such as light can acutely affect ethanol intake and thereby contribute to the daily rhythm (Geller and Purdy, 1979).
While prior work has addressed the relationship between circadian clocks and concurrent ethanol intake (particularly in animals), and other research has found a role of (non-circadian) timing in increasing subsequent alcohol intake (e.g., intermittent is more effective than continuous ethanol exposure at increasing self-administration and producing dependence with liquids or vapor; Becker and Lopez, 2004; Finn et al., 2005; O'Dell et al., 2004; Wise, 1973), the role of circadian timing of ethanol exposure in subsequent or long term ethanol use has been neglected. Given the highly structured temporal context in which humans tend to consume alcohol, this issue warrants consideration.
Within a circadian context, timing of ethanol exposure may influence self-administration via two routes: first, self-administered drinking at preferred times of day may promote subsequent intake simply by virtue that these animals acquire a history of drinking more ethanol compared with animals offered ethanol at a non-preferred time of day. In this case, drinking at the preferred time of day may be analogous to alcohol sweetening or dilution – two methods useful in establishing self-administration habits in animals that would not drink otherwise (Samson et al., 1999). Thus, drinking at a preferred time might be expected to lead to higher levels of subsequent self-administration, while drinking at a non-preferred time of day might not. Alternatively, repeated daily timed exposure may engage circadian oscillators involved in behavioral reinforcement. Daily timed feedings, for instance, have revealed the existence of a food entrainable oscillator (Pitt et al., 2003; Stephan, 1984) that generates increased activity in advance of the expected food/reward time. In this case, animals will re-entrain their food related activity such that they anticipate and eat according to a circadian rhythm that was determined by their prior circadian-timed schedule of feeding. Analogously, timed ethanol exposure might also entrain anticipatory activity and drinking despite drinking at a preferred or non-preferred time of day leading to equal self-administration. Comparable oscillatory mechanisms have been proposed to play a role in fear conditioning (Cain et al., 2008) as well as in the response to and reinforcement of drugs of abuse (Abarca et al., 2002; McClung et al., 2005), also see (Kosobud et al., 2007) for review.
Here, we ask what influence a history of drinking during a preferred (or non-preferred) time of day has on subsequent voluntary intake. To address this question and the hypotheses above, we present two experiments with C57BL/6J mice, a strain known for its high levels of voluntary ethanol intake. In Experiment 1 we assess whether a history of drinking during highly preferred times of day subsequently elevates drinking at less preferred times of day and vice versa. We then evaluate how these scheduled histories affect ethanol intake when it is available 24 hours/day. Experiment 2 replicates and extends Experiment 1 by employing “skeleton” photoperiods to remove the potentially confounding factor of light exposure (Geller and Purdy, 1979), and further tests whether the timing, per se, of ethanol exposure (versus amount previously consumed) is critical in setting subsequent levels of self-administration. Lastly, we also monitor patterns of ethanol drinking during 24 hour ad lib access to determine how the circadian rhythm is altered by prior drinking history.
Materials and Methods
Subjects
Male C57BL/6J mice (Jackson Laboratories, Sacramento CA) were 10 weeks old at the start of each experiment. Mice were single housed with food (Purina chow) and water available ad libitum. All procedures and animal care was approved by and conducted under the guidelines of the Institutional Animal Care Use Committee at University of California, San Diego and at The Scripps Research Institute.
Lighting Conditions
In Experiment 1, mice were housed under a full 12 hour light/12 hour dark cycle. In Experiment 2, mice were housed under a 13 hour light/11 hour dark cycle prior switching to a skeleton light/dark cycle. During the skeleton light/dark cycle, mice received only two 1-hour pulses of light each followed by 11 hours of darkness. The two light pulses simulate dawn and dusk of a 13 hour day/11 hour night (Rosenwasser et al., 1983).
Drinking Procedures
Two hour two bottle choice ethanol exposure
A 10% (w/v) ethanol solution was prepared using 95% ethyl alcohol and water; a separate water bottle was also prepared for the procedure. Mice were given 50 ml conical tubes fitted with sipper tubes for two hours at a time for the two bottle choice portions of the study. At the end of each two hour exposure, the mouse's normal 16 ounce water bottle was returned to the cage and the 50 ml ethanol and water bottles were weighed to determine g/kg intake as well as ethanol preference (preference calculated= ethanol intake/(ethanol + water intake)). This exposure lasted 5 days each week (Monday-Friday). Mice were weighed at two week intervals.
Twenty-four hour two bottle choice ethanol exposure
Mice were each given one 10% (w/v) 50 ml ethanol bottle together with one 50 ml bottle of water for twenty-four hours at a time. Bottles were weighed every 2-4 days (and divided by number of days to obtain a 24 hour average) to determine g/kg intake and ethanol preference. The twenty-four hour ethanol choice procedure lasted 4 days during each week (starting Monday afternoon and ending Friday afternoon). Mice were weighed at two week intervals.
Experiment 1
Mice (N = 39) were randomly divided into 2 groups at the beginning of the experiment.
2 hour 2 BC: Initial Phase
Mice in the “Night” group (n = 20) received the two hour two bottle choice (2 hour 2 BC) procedure starting at 2 hours into the 12 hour dark phase, which corresponds to their active phase. Mice in the “Day” group (n = 19) were housed in a separate room on a reverse light/dark cycle. Mice in the Day group received ethanol (2 hour 2 BC) at the exact same time as the Night group, but because they were housed on a reverse lighting cycle this exposure started at 2 hours into the 12 hour light phase, when animals are generally inactive. The 2 hour 2 BC procedure lasted for 5 weeks.
2 hour 2 BC: Crossover Phase
After 5 weeks, mice were further subdivided so that half the mice from the Night and Day groups would continue to drink in their original lighting condition (Night/Night group and Day/Day group). The other half were transferred to the opposite lighting/drinking condition (Day/Night and Night/Day groups). Mice placed in opposite lighting conditions were transferred to a different room that was on a reverse light/dark cycle from their original room; they were allowed 2 weeks for re-entrainment to new lighting conditions. No animals received ethanol during this interval. Following this break, the Crossover Phase began and mice received 4 weeks of 2 hour 2 BC starting 2 hours into the dark or light.
24 hour 2 BC: Ethanol Phase
Three days following the last day of 2 hour 2 BC, all mice were given twenty-four hour two bottle choice ethanol exposure for three weeks. This began in the middle of the dark or light phase (10 am) on Monday and bottles were changed daily at non-consistent times between 8 am and 3 pm.
Experiment 2
Mice (N = 30) were randomly divided into 3 groups at the beginning of the experiment. Animals were housed under a skeleton light/dark cycle; prior to skeleton cycles mice in the Subjective Day group were entrained to reverse light/dark cycles so that the Subjective Night and Subjective Day groups were 12 hours out of phase with each other. Locomotor activity was recorded throughout the experiment.
Initial Phase 2 hour 2 BC
Mice in the “Subjective Night” group (n = 10) received the two hour choice procedure during the subjective night starting 2 hours following the “dusk” light pulse (that signals the end of day). Mice in the “Subjective Day” group (n = 10) received the two hour choice procedure during subjective day starting 2 hours after the “dawn” light pulse (that signals the start of day). Subjective night and subjective day are terms used to describe an animal's internal circadian phase as distinguished from environmental phase, which may not match. Under a skeleton light/dark cycle the internal sense of night and day was established by the mouse's prior entrainment to a full light/dark cycle (thus, the subjective sense of night and day is reversed for the two groups of animals, even while they are housed in the same room). Mice in both groups received ethanol at exactly the same time, but because mice were earlier entrained to opposite light/dark cycles before being placed on the common skeleton light/dark cycle, ethanol exposure occurred either during the active (Subjective Night group) or inactive phase (Subjective Day group). Again, as mice were housed under a skeleton light/dark cycle, ethanol exposure took place in darkness in all groups (during both night and day). Mice in the “Restricted Subjective Night” group (n = 10) also received ethanol during subjective night starting 2 hours after the dusk light pulse; however the ethanol choice procedure for this group lasted only 10-15 minutes in order to restrict intake to the same low levels seen in the Subjective Day group during 2 hour 2 BC. This procedure lasted for 5 weeks.
Ad Lib 24 hour 2 BC
Three days following the last day of 2 hour 2 BC, mice were given 24 hour 2 BC ethanol exposure for eight weeks. During Ad Lib 24 hour 2 BC, lickometers were also used to record 24-hour ethanol drinking behavior.
Blood Alcohol Concentrations
One blood alcohol sample was taken from each mouse during the Initial and Crossover Phase of Experiment 1. Tail blood (0.05 ml) was collected into heparinized tubes immediately following the 2 hour exposure and assayed to determine BAL.
Activity Monitoring
In Experiment 2, locomotor activity was recorded continuously by a motion detector mounted to the top of each cage lid (Coral Plus; Visonic, Bloomfield, CT). Drinking activity for ethanol was measured by a contact sensing lickometer circuit. Lickometers were used on each cage every other week, Monday afternoon through Friday afternoon during 24 hour 2 BC. Both locomotor and lickometer data were recorded and compiled into 6 minute bins by Vital View software (Mini Mitter, Bend, OR). Activity histograms were made using Microsoft Excel by taking the average at each 6 minute interval across the 24 hour period for the duration of the experiment. For locomotor data this includes all days throughout the experiment. As mentioned above, for drinking data this includes 4 days per week for weeks 1, 3, 5 and 7 of Ad Lib 24 hour 2 BC.
Data Analysis
Ethanol drinking and preference levels were analyzed by univariate repeated measures ANOVAs using SPSS 15.0 (Chicago, IL) to statistically compare between groups for each experimental phase (e.g. Initial, Crossover and Twenty-four hour phases). This was followed by post-hoc comparisons (corrected using the Bonferroni method). For activity rhythm analysis, data points were averaged over hourly intervals and reduced to 24 data points. Group values were compared at each time point across groups using ANOVAs corrected for multiple comparisons (Bonferroni method).
Results
Experiment 1
2 hour 2 BC
Ethanol intake (g/kg) for each group throughout Experiment 1 is represented in Figure 1. In the Initial 2 hour 2 BC, the Night group consumed approximately 3 times more ethanol than mice in the Day group (F(1, 37) = 415.5; p < 0.001).
Figure 1.
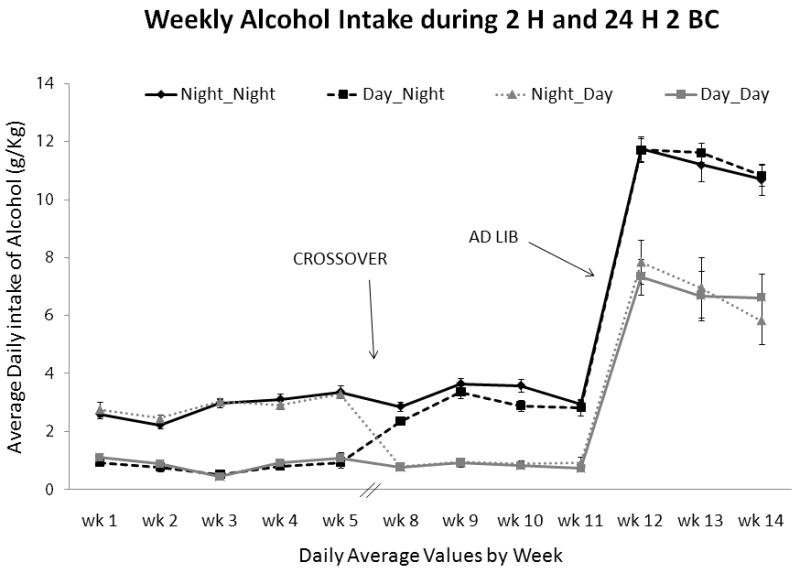
Mean ± SEM daily ethanol intake (g/kg) in each mouse group by week for all parts of Experiment 1. This includes 2 hour 2 BC for both the Initial (5 weeks) and Crossover (4 weeks) phases as well as for 24 hour 2 BC in the Ad Lib phase (3 weeks); the start of Crossover and Ad Lib phases are labeled and marked with arrows. The Dark/Dark group drank at night during both the Initial and Crossover phase; the Dark/Light group drank at night for the Initial phase and during the day (morning) for the Crossover phase; the Light/Light group drank during the day (morning) for both phases; the Light/Dark group drank during the day in the Initial phase and at night during the Crossover phase.
During Crossover, the Night/Night and Day/Night mice (that drank at night) consumed significantly more than the Day/Day and Night/Day mice (F(3, 35) = 84.5; p < 0.001). Drinking condition during the Initial phase had no effect on drinking in the Crossover phase; post-hoc comparisons showed no statistical differences between Day/Day and Night/Day groups (p = 1.0) or between the Night/Night and Day/Night groups (p = 0.27) during the Crossover phase.
Mice in all groups preferred ethanol over water; however, mice who drank at night exhibited a stronger ethanol preference (for the Initial phase F(1, 37) = 32.96; p < 0.001; for the Crossover phase, F(3, 35) = 13.23; p < 0.001). In Crossover there were no differences between groups that drank during the same time of day (post-hoc comparisons between the Night/Night and Day/Night group were n.s. (p = 1.0) as were comparisons between the Day/Day and Night/Day groups (p = 1.0); see Fig. 2.
Figure 2.
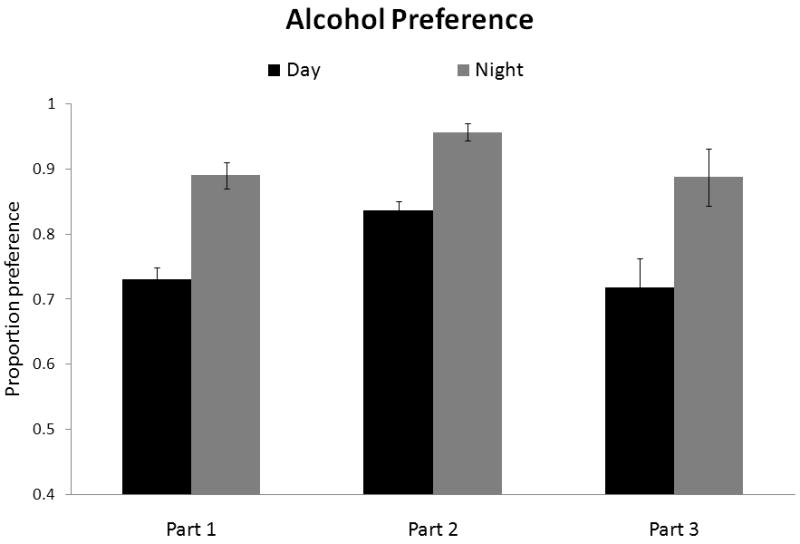
Proportion ethanol preference (versus water) for mice drinking during the dark or light in the Initial and Crossover phases, or with a history of drinking during the dark or light (Crossover phase) prior to Ad Lib 24 hour exposure. Mice show preference for ethanol when drinking in the dark (Initial and Crossover) and continue to show a preference during Ad Lib if they drank in the dark prior to 24 hour exposure (during the Crossover phase).
Blood alcohol samples taken during Initial and Crossover phases following 2 hour 2 BC confirmed higher blood alcohol concentration (BAC) in the Night group compared to the Day group (mean± SEM for Night = 84.0 ± 9.2 mg/dl, Day = 4.5 ± 0.7 mg/dl).
24 hour 2 BC
Under 24 hour, Ad Lib conditions Night/Night and Day/Night mice (that previously drank in night during Crossover) drank more (F(3, 35) = 17.87; p < 0.001) and had a higher preference (F(3, 35) = 4.65; p < 0.01) for ethanol during subsequent weeks of 24 hour exposure than Day/Day and Night/Day mice. Again, these results were influenced by the most recent timed history (Crossover) rather than by Initial conditions as post-hoc comparisons confirmed no statistical differences between the Night/Night and Day/Night groups or between the Day/Day and Night/Day group.
Experiment 2
2 hour 2 BC
Figures 3 and 4 show ethanol intake and preference during Experiment 2. Mice drinking for 2 hours in the Subjective Night group consumed more (F(2, 27) = 68.53; p < 0.001) than mice drinking in the Subjective Day or Restricted Subjective Night groups. Post-hoc comparisons showed significant differences between the Subjective Night and Subjective Day groups (p < 0.001), and between the Subjective Night and Restricted Subjective Night groups (p < 0.001). By design, mice in the Restricted Subjective Night group drank comparable levels with those in the Subjective Day group (p = 0.56). Mice drinking in both the Subjective Night and Restricted Subjective Night groups showed higher preference for ethanol (F(2, 27) = 12.78; p < 0.001) than mice in the Subjective Day group. Specifically, post-hoc comparisons show differences between the Subjective Night and Subjective Day groups (p < 0.001) and between the Restricted Subjective Night and Subjective Day groups (p < 0.04).
Figure 3.
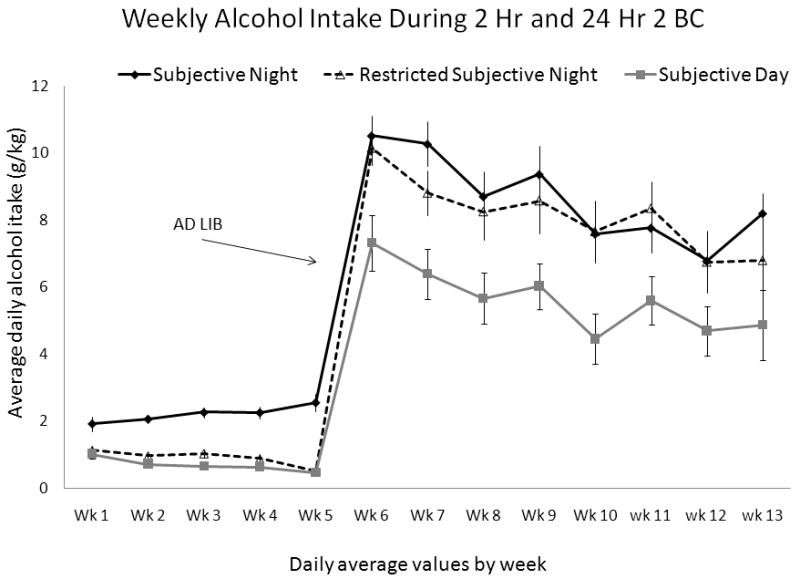
Mean ± SEM daily ethanol intake (g/kg) in each mouse group by week for 2 hour 2 BC (5 weeks) and 24 hour 2 BC (8 weeks) in Experiment 2; the start of Ad Lib intake is labeled on the figure. The Subjective Night group drank during the night (active phase) during 2 hour 2 BC; the Subjective Day group drank during the day (inactive phase) during 2 hour 2 BC; the Restricted Subjective Night group was allowed minimal ethanol access during the night (active phase) approximately 15 minutes starting at the same time as mice in the night group.
Figure 4.
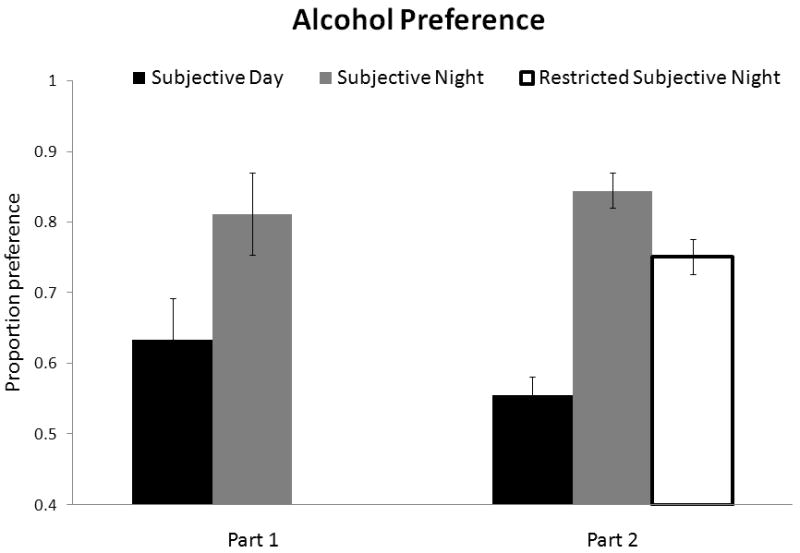
Proportion ethanol preference (versus water) exhibited by mice in each group during 2 hour 2 BC and 24 hour 2 BC. Mice show a higher preference for ethanol when drinking during the night versus the day for 2 hour 2 BC; groups also show a higher preference during Ad Lib with a history of drinking in the dark (Subjective Night and Restricted Subjective Night groups). Note that during 24 hour 2 BC ethanol is available at all times of day but animals still show a preference based on their history of drinking during the night or day during 2 hour 2 BC.
24 hour 2 BC
When ethanol was subsequently freely available 24 hours/day, mice in the Subjective Night and Restricted Subjective Night groups (who previously drank at night) consumed more than those in the Subjective Day group (F(2, 27) = 5.4; p < 0.02). Pairwise comparisons showed significant differences between the Subjective Night group vs. the Subjective Day group (p < 0.02) and between the Restricted Subjective Night vs. the Subjective Day group (p < 0.05). As with volume ethanol intake, preference was also higher in the Subjective Night and Restricted Subjective Night groups than the Subjective Day group (F(2, 27) = 6.35; p < 0.01), see Figure 4.
Drinking and locomotor activity rhythms
Figure 5a shows each group's average daily ethanol drinking profile plotted across the 24 hour day. All groups showed bimodal licking patterns with peaks at the beginning and the end of the active phase. Consistent with volumetric measures, the number of licks was significantly reduced in the Subjective Day group compared to the others (F(2, 27) = 5.45; p < 0.02). Specifically, univariate ANOVAs of hourly average drinking levels across the day showed significantly higher intake during the early and middle hours of the night by mice in the Subjective Night and Restricted Subjective Night groups compared with the Subjective Day group (p < 0.002 at 2, 6 and 7 hours into the night phase). Average daily ingested ethanol volume was highly correlated with average daily number of licks (Pearson's r = 0.86; p < 0.01).
Figure 5A-B.
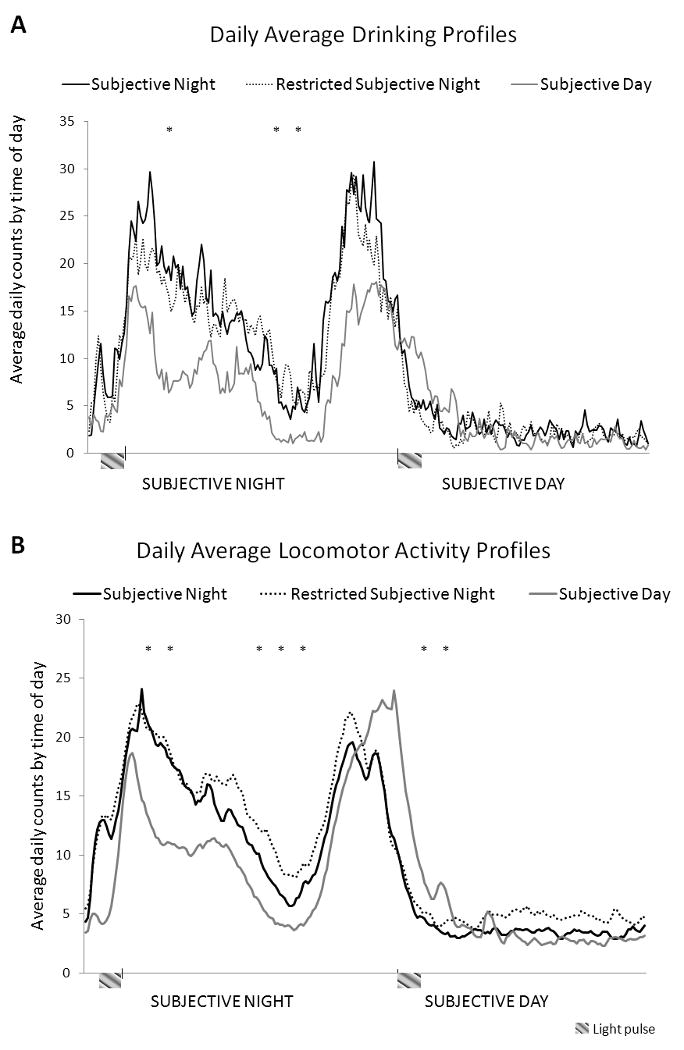
Average daily activity profiles by group for ethanol drinking (A) and locomotor (B) activity (sampled at 6 minute intervals). Axes are 24 hours across and show the average daily pattern of ethanol drinking (A) or locomotor activity (B) for animals in each group. Higher points on the line indicate higher levels of activity for that time of day. Drinking activity (A) was measured continuously every other week of 24 hour Ad Lib exposure (weeks 1, 3, 5 and 7 of Ad Lib). Locomotor activity (B) was measured all weeks throughout the entire experiment (during 2 hour and 24 hour 2 BC). Asterisks (*) on figure indicate the hours at which univariate ANOVAs showed significantly different activity or drinking levels in the Subjective Day group compared with the Subjective and Restricted Subjective Night groups (p < 0.002). Because locomotor activity profiles were largely similar during scheduled and ad lib drinking, data over both intervals are plotted together.
Examination of individual records confirmed that all mice were clearly entrained to their respective dark period. Locomotor activity during Experiment 2 is shown in Figure 5b with each group's average daily locomotor activity plotted across 24 hours. Locomotor activity is also bimodal in all groups with peaks near the beginning and end of the active phase. All groups show similar amounts of daily activity (average daily counts for the Subjective Night group = 2250, Restricted Subjective Night = 2563, Subjective Day group = 1952). However, daily patterns of locomotor activity differed in the Subjective Day group compared with the Subjective Night and Restricted Subjective Night groups. Specifically, mice in the Subjective Night and Restricted Subjective Night groups showed more activity counts early and in the middle of the night phase when compared to the Subjective Day group (univariate ANOVAs showed that p < 0.002 at 1, 2, 5, 6 and 7 hours into the night phase). However, during the early part of the day, mice in the Subjective Day group showed greater amounts of activity when compared to the Subjective Night and Restricted Subjective Night groups (p < 0.002 at 1 and 2 hours into the day phase).
Discussion
These results establish the importance of daily timing of ethanol exposure in the control of voluntary ethanol consumption in mice. As has been previously documented (Rhodes et al., 2005), mice demonstrated varying consumption and preference for ethanol at different times of day. This study breaks ground in exhibiting that the circadian timing of prior ethanol exposure has enduring effects on voluntary drinking during ad lib availability. In humans, we know alcohol consumption also follows predictable temporal patterns as people tend to drink near the end of the day (although as alcohol use disorders develop, drinking often begins earlier in the day). The mouse may be a convenient model to understand the relevance of temporal context for addictive behaviors.
In developing a mouse model of excessive ethanol intake, a number of laboratories have devised protocols to increase consumption by adding exteroceptive cues, for example sucrose, to enhance ethanol consumption, a cue that is faded out and leaves the animal with a self-administration habit that would not have otherwise occurred naturally. Alternatively, animals may be made physically dependent so that self-administration attenuates withdrawal symptoms. In the current experiments, mice showed a significant preference for ethanol over water in both their active and inactive phases, but this preference was significantly stronger at night. Thus, we tested whether the heightened preference of nighttime drinking would serve a role analogous to sweetening, such that high voluntary drinking at night would render ethanol more attractive during the day. However, our Crossover phase in Experiment 1 showed no hint of a history effect on 2 hour daytime versus nighttime drinking. One methodological issue should be noted before fully rejecting this hypothesis. For logistical reasons, animals in Experiment 1 needed to be re-entrained to a reversed light/dark cycle in order to switch their times of ethanol exposure, thus introducing a 2 week break between phases of the experiment. This gap or the process of circadian re-entrainment itself could plausibly diminish the physiological memory or reinforcement associated with drinking during prior conditions. Consistent with such an interpretation is the finding that the alcohol deprivation effect in this same mouse strain is evident after one week of abstinence but not after two (Melendez et al., 2006). On the other hand, in both Experiments 1 and 2, effects of prior timed ethanol exposure persisted for much longer than 2 weeks following final scheduled ethanol access.
Whereas the prior timing of 2 hour scheduled ethanol access did not affect drinking when timing of that exposure was merely shifted, it markedly affected self-administration for up to 8 weeks when ethanol was subsequently available ad lib 24 hours per day. In Experiment 1, the critical aspect of timed exposure could have been the amount of ethanol consumed. But as Experiment 2 equalized the amount of ethanol ingested during the active versus the inactive phase, this explanation is ruled out. (Again, we note that animals scheduled to drink during the inactive phase nevertheless discriminated ethanol from water as indicated from their significant ethanol preference.) Thus, the daily timing of ethanol drinking produced an enduring effect on ongoing self-administration. This finding complements existing literature that documents an importance of ethanol exposure procedures in influencing voluntary alcohol intake (e.g., continuous versus intermittent). The fact that the history effect persists for so long in Experiment 2 suggests either that there is an enduring physiological memory for the reinforcement of ethanol based on the timing of past exposure, or that the elevated 24 hour patterns of ad-lib are self-sustaining. The present data cannot distinguish these possibilities, although the waning of the effect in Experiment 2 suggests the former hypothesis. If the prior reinforcement effects are indeed remembered for 8 weeks, then it must also be the case that the temporal reinforcement effects were abolished or overridden in Experiment 1 by switching the time of daily exposure. That is, animals switched from drinking in the night to drinking in the day disregarded the earlier history in favor of the later despite the fact that fewer than 8 weeks elapsed between nighttime drinking and ad-lib exposure. This raises the intriguing, but yet to be rigorously tested idea that manipulations of circadian rhythms or timing of ethanol exposure could potentially contribute to treatments of alcohol use disorders. It should also be noted that we tested how a history of drinking at a preferred or non-preferred time of day produced differences in subsequent drinking but did not compare drinking levels to mice without pre-exposure who are allowed ethanol 24 hours/day. Thus, our data do not distinguish between a suppression of ad lib drinking by a history of alcohol at non-preferred times versus a facilitation by similar exposure at preferred times.
A central concern among circadian biologists is the degree to which daily rhythms in behavior are internally generated programs versus acute responses to a changing external environment. The use of skeleton photoperiods in Experiment 2 is an attempt to distinguish these possibilities since a literature exists that suggests an acute role of light on ethanol consumption in rats (Burke and Kramer, 1974; Geller, 1971). In contrast to that literature, the main findings of the present study suggest that light during the inactive phase is of no or little significance. The skeleton photoperiods are also useful for examining whether timed ethanol exposure produces marked changes in the circadian activity rhythm. Time limited access to food, for example, induces a marked reorganization of circadian activity rhythms in a number of rodent species (Stephan, 1984). Scheduled ethanol, on the other hand, had no large effects on circadian activity rhythms either during scheduled drinking or after although the intensity of activity early in the active phase was somewhat reduced in mice drinking during their inactive period.
In several species, rewards available at certain times of day are time-stamped so that motivated behaviors are reinforced to be expressed around the time that the reward was scheduled (Kosobud et al., 2007; McClung et al., 2005). The scheduled 2 hour ethanol access in the present study might thus produce changes in the 24 hour ad-lib drinking by reinforcing ethanol consumption at a particular time of day. We found no evidence that scheduled drinking during the inactive phase led animals to drink preferentially at that time of day when ethanol was available around the clock. Instead, all mice avoided daytime drinking in favor of their active phase when ethanol consumption closely tracked the locomotor activity rhythm.
The mechanisms by which circadian timing affects ethanol preference and intake remain to be determined. While Experiment 2 controlled for the amount of ethanol consumed at different circadian phases, at this early stage of our investigation we cannot account for potential differences in pharmacokinetics or metabolism which are known to vary on a circadian basis (Kosobud et al., 2007). Thus, while we document that it is not the amount but the timing of ethanol consumed that influences subsequent 24 hour intake we cannot exclude the possibility that the small amount of ethanol these mice consumed produced BALs transiently equivalent to those of unrestricted mice. The close association of locomotor and drinking activity suggests that circadian rhythms of arousal or alertness levels may mediate these effects. At a more reductionist level, at least one gene (Per2) that plays a central role in the generation of the circadian clock mechanism is associated with elevated ethanol intake in mutant mice (Spanagel et al., 2005a). There is a preliminary suggestion that a genetic variant of the Per2 gene in humans is related to increased alcohol consumption (Spanagel et al., 2005a). More broadly, an estimated 10% of the mouse transcriptome is under circadian control (Panda et al., 2002). Thus, there are myriad mechanisms by which the reinforcing potential of ethanol might be modulated on a temporal basis.
Once they are better understood, the effects reported here may have practical relevance for understanding development of and manipulating human alcohol intake. Behavioral cue exposure (CE) methods for reducing alcohol intake in humans expose subjects to alcohol without allowing them to drink to test how physiological responses to alcohol change over time (Glautier and Drummond, 1994). Though allowing alcohol in treatment is counter-intuitive, it is recognized that a long term change in behavior must utilize both proximate and ultimate goals that do not necessarily agree (DiClemente, 2007); others have made use of alcohol in treatment with positive findings (Sitharthan et al., 1997). For lasting improvements, CE methods incorporate contextual cues during alcohol exposures (Collins and Brandon, 2002; Stasiewicz et al., 2007). While this typically refers to environmental characteristics, we might extend this concept to temporal features. Timing characteristics of human alcohol drinking are currently being studied (Danel and Touitou, 2004), and time of day is a simple context for voluntary drinking that can be manipulated in behavioral treatments of alcohol use disorders. The treatment would not focus on trying to forget or extinguish the reinforcement of alcohol but rather on learning new, non-preferred schedules of intake that change the reinforcing properties of alcohol and ultimately reduce voluntary drinking. In fact, there is substantial evidence that associations are not extinguished as a classical behavioral theory would suggest, but that new alternative ones are learned (Bouton, 2002). Our Experiment 2 results show that the most recent schedule of alcohol exposure controls subsequent intake in an unrestricted environment. Behavioral treatments for alcohol first tried in animals have been extended to humans in the study of context cues (Collins and Brandon, 2002). Here we suggest another based on our results; human behavioral studies could use preferred circadian timing for alcohol ingestion as a context for alcohol use.
Acknowledgments
The authors are grateful to members of the Gorman and Roberts Labs for their support (and in particular to Teri Nguyen, Jen Evans, Coree Levee, and Kelly Rios) as well as to the animal care staff at UCSD (Bob Sundberg and Antonio Mora) and TSRI for their excellent animal care. This work was supported by NIH NICHD 36460 and INIA AA013523.
References
- Abarca C, Albrecht U, Spanagel R. Cocaine sensitization and reward are under the influence of circadian genes and rhythm. Proceedings of the National Academy of Sciences USA. 2002;99(13):9026–9030. doi: 10.1073/pnas.142039099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker HC, Lopez MF. Increased ethanol drinking after repeated chronic ethanol exposure and withdrawal experience in C57BL/6 mice. Alcoholism: Clinical and Experimental Research. 2004;28(12):1829–1838. doi: 10.1097/01.alc.0000149977.95306.3a. [DOI] [PubMed] [Google Scholar]
- Bouton MA. Context, ambiguity, and unlearning: sources of relapse after behavioral extinction. Biological Psychiatry. 2002;52:976–986. doi: 10.1016/s0006-3223(02)01546-9. [DOI] [PubMed] [Google Scholar]
- Burke LP, Kramer SZ. Effects of photoperiod, melatonin and pinealectomy on ethanol consumption in rats. Pharmacology Biochemistry and Behavior. 1974;2:459–463. doi: 10.1016/0091-3057(74)90004-5. [DOI] [PubMed] [Google Scholar]
- Cain SW, McDonald RJ, Ralph MR. Time stamp in conditioned place avoidance can be set to different circadian phases. Neurobiology of Learning and Memory. 2008;89(4):591–594. doi: 10.1016/j.nlm.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Clark JW, Fixaris MC, Belanger GV, Rosenwasser AM. Repeated light-dark phase shifts modulate voluntary ethanol intake in male and female high alcohol-drinking (HAD1) rats. Alcoholism: Clinical and Experimental Research. 2007;31(10):1699–1706. doi: 10.1111/j.1530-0277.2007.00476.x. [DOI] [PubMed] [Google Scholar]
- Collins BN, Brandon TH. Effects of extinction context and retrieval cues on alcohol cue reactivity among nonalcoholic drinkers. Journal of Consulting and Clinical Psychology. 2002;70(2):390–397. [PubMed] [Google Scholar]
- Danel T, Jeanson R, Touitou Y. Temporal pattern in consumption of the first drink of the day in alcohol-dependent persons. Chronobiology International. 2003;20(6):1093–1102. doi: 10.1081/cbi-120025533. [DOI] [PubMed] [Google Scholar]
- Danel T, Touitou Y. Chronobiology of alcohol: From chronokinetics to alcohol-related alterations of the circadian system. Chronobiology International. 2004;21(6):923–935. doi: 10.1081/cbi-200036886. [DOI] [PubMed] [Google Scholar]
- DiClemente CC. Mechanisms, determinants and processes of change in the modification of drinking behavior. Alcoholism: Clinical and Experimental Research. 2007;31(S3):13s–20s. doi: 10.1111/j.1530-0277.2007.00489.x. [DOI] [PubMed] [Google Scholar]
- el Guebaly N. Alcohol, alcoholism, and biological rhythms. Alcoholism: Clinical and experimental research. 1987;2(11):139–143. doi: 10.1111/j.1530-0277.1987.tb01277.x. [DOI] [PubMed] [Google Scholar]
- Finn DA, Belknap JK, Cronise K, Yoneyama N, Murillo A, Crabbe JC. A procedure to produce high alcohol intake in mice. Psychopharamcology. 2005;178:471–480. doi: 10.1007/s00213-004-2039-8. [DOI] [PubMed] [Google Scholar]
- Geller I. Ethanol preference in rats as a function of photoperiod. Science. 1971;173(3995):456–459. doi: 10.1126/science.173.3995.456. [DOI] [PubMed] [Google Scholar]
- Geller I, Purdy RH. Interrelationship between ethanol consumption and circadian rhythms. In: Majchrowicz E, Noble EP, editors. Biochemistry and Pharmacology of Ethanol. Vol. 2. Plenum Press; NY: 1979. pp. 453–465. chapter 23. [Google Scholar]
- Glautier S, Drummond DC. A conditioning approach to the analysis and treatment of drinking problems. British Medical Bulletin. 1994;50(1):186–199. doi: 10.1093/oxfordjournals.bmb.a072877. [DOI] [PubMed] [Google Scholar]
- Hiller-Sturmhofel S, Kulkosky P. Chronobiological regulation of alcohol intake. Alcohol Research and Health. 2001;25(2):141–148. [PMC free article] [PubMed] [Google Scholar]
- Hofstetter JR, Grahame NJ, Mayeda AR. Circadian activity rhythms in high-alcohol-preferring and low-alcohol-preferring mice. Alcohol. 2003;30:81–85. doi: 10.1016/s0741-8329(03)00095-8. [DOI] [PubMed] [Google Scholar]
- Kosobud AEK, Gillman AG, L JK, II, Pecoraro NC, Rebec GV, Timberlake W. Drugs of abuse can entrain circadian rhythms. The Scientific World Journal. 2007;7(S2):203–212. doi: 10.1100/tsw.2007.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung CA, Sidiropoulou K, Vitaterna M, Takahashi JS, White F, Cooper DC, Nestler EJ. Regulation of dopaminergic transmission and cocaine reward by the Clock gene. Proceedings of the National Academy of Sciences USA. 2005;102(26):9377–9381. doi: 10.1073/pnas.0503584102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melendez RI, Middaugh LD, Kalivas PW. Development of an alcohol deprivation and escalation effect in C57BL/6J mice. Alcoholism: Clinical and Experimental Research. 2006;30(12):2017–2025. doi: 10.1111/j.1530-0277.2006.00248.x. [DOI] [PubMed] [Google Scholar]
- O'Dell LE, Roberts AJ, Smith RT, Koob GF. Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcoholism: Clinical and Experimental Research. 2004;28(11):1676–1682. doi: 10.1097/01.alc.0000145781.11923.4e. [DOI] [PubMed] [Google Scholar]
- Panda S, Antoch MP, Miller BH, Su AI, Schook AB, Straume M, Schultz PG, Kay SA, Takahashi JS, Hogenesch JB. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell. 2002;109(3):307–320. doi: 10.1016/s0092-8674(02)00722-5. [DOI] [PubMed] [Google Scholar]
- Pitt S, Perone E, Silver R. Food entrained circadian rhythms are sustained in arrhythmic Clk/Clk mutant mice. American Journal of Physiology, Regulatory, Integrative, and Comparative Physiology. 2003;285(1):R57–67. doi: 10.1152/ajpregu.00023.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiology and Behavior. 2005;84:53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Rosenwasser AM, Boulos Z, Terman M. Circadian feeding and drinking rhythms in the rat under complete and skeleton photoperiods. Physiology and Behavior. 1983;30(3):353–359. doi: 10.1016/0031-9384(83)90138-5. [DOI] [PubMed] [Google Scholar]
- Rosenwasser AM, Fecteau ME, Logan RW. Effects of ethanol intake and ethanol withdrawal on free-running circadian activity rhythms in rats. Physiology and Behavior. 2005a;84:537–542. doi: 10.1016/j.physbeh.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Rosenwasser AM, Logan RW, Fecteau ME. Chronic ethanol intake alters circadian period-responses to brief light pulses in rats. Chronobiology International. 2005b;22(2):227–236. doi: 10.1081/cbi-200053496. [DOI] [PubMed] [Google Scholar]
- Samson HH, Files FJ, Denning C. Chronic ethanol self-administration in a continuous-access operant situation: the use of a sucrose/ethanol solution to increase daily ethanol intake. Alcohol. 1999;19(2):151–155. doi: 10.1016/s0741-8329(99)00032-4. [DOI] [PubMed] [Google Scholar]
- Sitharthan T, Sitharthan G, Hough MJ, Kavanagh DJ. Cue exposure in moderation drinking: a comparison with cognitive-behavior therapy. Journal of Consulting and Clinical Psychology. 1997;65(5):878–882. doi: 10.1037//0022-006x.65.5.878. [DOI] [PubMed] [Google Scholar]
- Smith D, Oei TPS, Ng KT, Armstrong S. Rat self administration of ethanol: enhancement by darkness and exogenous melatonin. Physiology and Behavior. 1980;25:449–455. doi: 10.1016/0031-9384(80)90287-5. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Pendyala G, Abarca C, Zghoul T, Sanchis-Segura C, Magnone MC, Lascorz J, Depner M, Holzberg D, Soyka M, Schreiber S, Matsuda F, Lathrop M, Schumann G, Albrecht U. The clock gene Per2 influences the glutamatergic system and modulates alcohol consumption. Nature Medicine. 2005a;11(1):35–42. doi: 10.1038/nm1163. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Rosenwasser AM, Schumann G, Sarkar DK. Alcohol consumption and the body's biological clock. Alcoholism: Clinical and Experimental Research. 2005b;29(8):1550–1557. doi: 10.1097/01.alc.0000175074.70807.fd. [DOI] [PubMed] [Google Scholar]
- Stasiewicz PR, Brandon TH, Bradizza CM. Effects of extinction context and retrieval cues on renewal of alcohol cue reactivity among alcohol dependent outpatients. Psychology of Addictive Behaviors. 2007;21(2):244–248. doi: 10.1037/0893-164X.21.2.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan FK. Phase shifts of circadian rhythms in activity entrained to food access. Physiology and Behavior. 1984;32(4):663–671. doi: 10.1016/0031-9384(84)90323-8. [DOI] [PubMed] [Google Scholar]
- Wise RA. Voluntary ethanol intake in rats following exposure to ethanol on various schedules. Psychopharmocologia. 1973;29:203–210. doi: 10.1007/BF00414034. [DOI] [PubMed] [Google Scholar]
- Zghoul T, Abarca C, Sanchis-Segura C, Albrecht U, Schumann G, Spanagel R. Ethanol self-administration and reinstatement of ethanol-seeking behavior in Per1Brdm1. Psychopharamcology. 2007;190:13–19. doi: 10.1007/s00213-006-0592-z. [DOI] [PubMed] [Google Scholar]


