Abstract
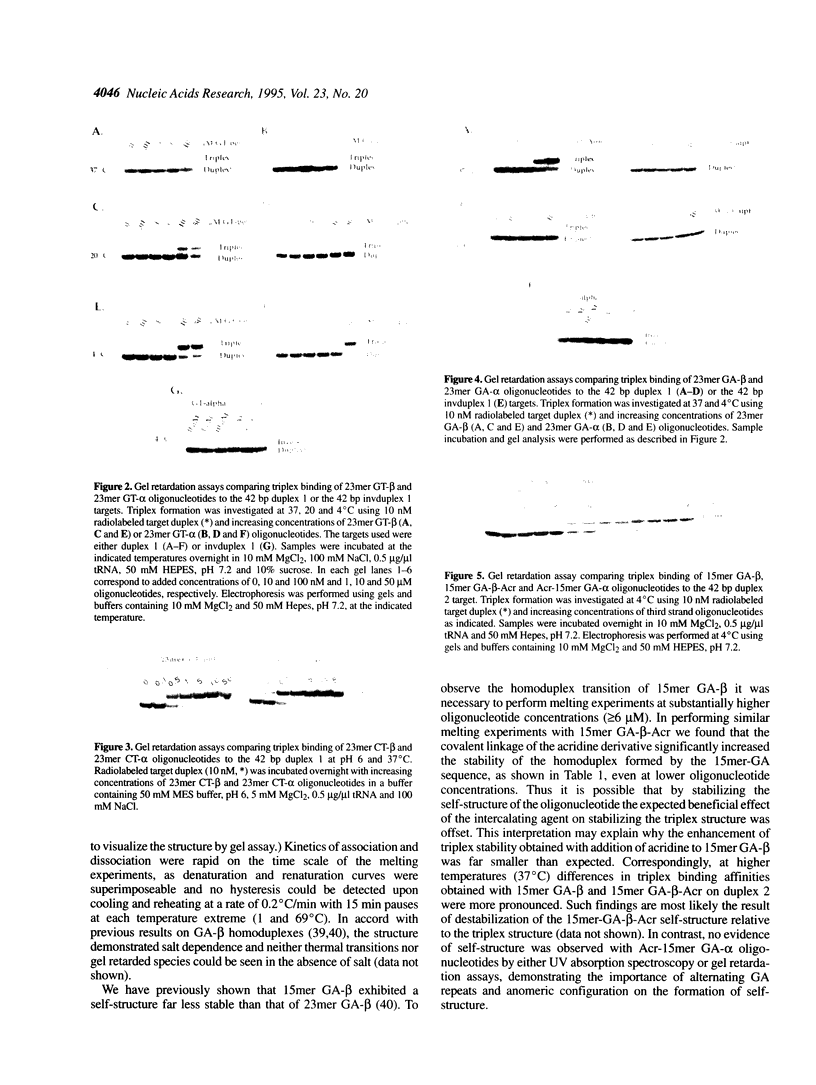
Nuclease-resistant alpha anomers of pyrimidine-rich CT- and purine-rich GA- and GT-containing oligonucleotides were investigated for their triplex-forming potential and compared with their corresponding nuclease-sensitive beta anomers. Both 23mer CT-alpha and 23mer CT-beta had quite similar triplex binding affinities. Synthetic 23mer GT-alpha oligonucleotides were capable of triplex formation with binding affinities slightly lower than corresponding 23mer GT-beta oligonucleotides. The orientation of third strand GT-alpha binding was parallel to the purine strand of the duplex DNA target, whereas the orientation of third strand GT-beta binding was found to be antiparallel. Triplex formation with both GT oligonucleotides showed the typical dependence on magnesium and temperature. In contrast, 23mer GA-alpha oligonucleotides did not support triplex formation in either orientation under a variety of experimental conditions, whereas the corresponding 23mer GA-beta oligonucleotides demonstrated strong triplex formation in the antiparallel orientation. GA-alpha oligonucleotides covalently conjugated to acridine were similarly unable to demonstrate triplex formation. GA-alpha oligonucleotides, in contrast to GT-alpha oligonucleotides, were capable of self-association, detectable by gel retardation and UV spectroscopy, but competing self-association could not fully account for the lack of triplex formation. Thus for in vivo triplex gene regulation strategies using GT oligonucleotides the non-natural alpha anomer may be a feasible alternative to the natural beta anomer, allowing for a comparable degree of triplex formation without rapid cellular degradation. However, alpha anomeric inversion does not appear to be a feasible alternative in applications involving GA oligonucleotides.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asseline U., Toulme F., Thuong N. T., Delarue M., Montenay-Garestier T., Hélène C. Oligodeoxynucleotides covalently linked to intercalating dyes as base sequence-specific ligands. Influence of dye attachment site. EMBO J. 1984 Apr;3(4):795–800. doi: 10.1002/j.1460-2075.1984.tb01887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beal P. A., Dervan P. B. Second structural motif for recognition of DNA by oligonucleotide-directed triple-helix formation. Science. 1991 Mar 15;251(4999):1360–1363. doi: 10.1126/science.2003222. [DOI] [PubMed] [Google Scholar]
- Cantor C. R., Warshaw M. M., Shapiro H. Oligonucleotide interactions. 3. Circular dichroism studies of the conformation of deoxyoligonucleotides. Biopolymers. 1970;9(9):1059–1077. doi: 10.1002/bip.1970.360090909. [DOI] [PubMed] [Google Scholar]
- Cazenave C., Chevrier M., Nguyen T. T., Hélène C. Rate of degradation of [alpha]- and [beta]-oligodeoxynucleotides in Xenopus oocytes. Implications for anti-messenger strategies. Nucleic Acids Res. 1987 Dec 23;15(24):10507–10521. doi: 10.1093/nar/15.24.10507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durland R. H., Kessler D. J., Gunnell S., Duvic M., Pettitt B. M., Hogan M. E. Binding of triple helix forming oligonucleotides to sites in gene promoters. Biochemistry. 1991 Sep 24;30(38):9246–9255. doi: 10.1021/bi00102a017. [DOI] [PubMed] [Google Scholar]
- Escudé C., François J. C., Sun J. S., Ott G., Sprinzl M., Garestier T., Hélène C. Stability of triple helices containing RNA and DNA strands: experimental and molecular modeling studies. Nucleic Acids Res. 1993 Dec 11;21(24):5547–5553. doi: 10.1093/nar/21.24.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escudé C., Sun J. S., Rougée M., Garestier T., Hélène C. Stable triple helices are formed upon binding of RNA oligonucleotides and their 2'-O-methyl derivatives to double-helical DNA. C R Acad Sci III. 1992;315(13):521–525. [PubMed] [Google Scholar]
- Hacia J. G., Wold B. J., Dervan P. B. Phosphorothioate oligonucleotide-directed triple helix formation. Biochemistry. 1994 May 10;33(18):5367–5369. doi: 10.1021/bi00184a002. [DOI] [PubMed] [Google Scholar]
- Hélène C. The anti-gene strategy: control of gene expression by triplex-forming-oligonucleotides. Anticancer Drug Des. 1991 Dec;6(6):569–584. [PubMed] [Google Scholar]
- Ing N. H., Beekman J. M., Kessler D. J., Murphy M., Jayaraman K., Zendegui J. G., Hogan M. E., O'Malley B. W., Tsai M. J. In vivo transcription of a progesterone-responsive gene is specifically inhibited by a triplex-forming oligonucleotide. Nucleic Acids Res. 1993 Jun 25;21(12):2789–2796. doi: 10.1093/nar/21.12.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibler-Herzog L., Kell B., Zon G., Shinozuka K., Mizan S., Wilson W. D. Sequence dependent effects in methylphosphonate deoxyribonucleotide double and triple helical complexes. Nucleic Acids Res. 1990 Jun 25;18(12):3545–3555. doi: 10.1093/nar/18.12.3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibler-Herzog L., Zon G., Whittier G., Mizan S., Wilson W. D. Stabilities of duplexes and triplexes of dA19 + dT19 with alternating methylphosphonate and phosphodiester linkages. Anticancer Drug Des. 1993 Feb;8(1):65–79. [PubMed] [Google Scholar]
- Kim S. G., Tsukahara S., Yokoyama S., Takaku H. The influence of oligodeoxyribonucleotide phosphorothioate pyrimidine strands on triplex formation. FEBS Lett. 1992 Dec 7;314(1):29–32. doi: 10.1016/0014-5793(92)81454-t. [DOI] [PubMed] [Google Scholar]
- Kim S. W., Lajara R., Rotwein P. Structure and function of a human insulin-like growth factor-I gene promoter. Mol Endocrinol. 1991 Dec;5(12):1964–1972. doi: 10.1210/mend-5-12-1964. [DOI] [PubMed] [Google Scholar]
- Latimer L. J., Hampel K., Lee J. S. Synthetic repeating sequence DNAs containing phosphorothioates: nuclease sensitivity and triplex formation. Nucleic Acids Res. 1989 Feb 25;17(4):1549–1561. doi: 10.1093/nar/17.4.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Doan T., Perrouault L., Praseuth D., Habhoub N., Decout J. L., Thuong N. T., Lhomme J., Hélène C. Sequence-specific recognition, photocrosslinking and cleavage of the DNA double helix by an oligo-[alpha]-thymidylate covalently linked to an azidoproflavine derivative. Nucleic Acids Res. 1987 Oct 12;15(19):7749–7760. doi: 10.1093/nar/15.19.7749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy J. L., Guéron M., Mergny J. L., Hélène C. Intramolecular folding of a fragment of the cytosine-rich strand of telomeric DNA into an i-motif. Nucleic Acids Res. 1994 May 11;22(9):1600–1606. doi: 10.1093/nar/22.9.1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liquier J., Letellier R., Dagneaux C., Ouali M., Morvan F., Raynier B., Imbach J. L., Taillandier E. Triple helix formation by alpha-oligodeoxynucleotides: a vibrational spectroscopy and molecular modeling study. Biochemistry. 1993 Oct 12;32(40):10591–10598. doi: 10.1021/bi00091a008. [DOI] [PubMed] [Google Scholar]
- Maher L. J., 3rd Inhibition of T7 RNA polymerase initiation by triple-helical DNA complexes: a model for artificial gene repression. Biochemistry. 1992 Aug 25;31(33):7587–7594. doi: 10.1021/bi00148a021. [DOI] [PubMed] [Google Scholar]
- Manor H., Rao B. S., Martin R. G. Abundance and degree of dispersion of genomic d(GA)n.d(TC)n sequences. J Mol Evol. 1988;27(2):96–101. doi: 10.1007/BF02138367. [DOI] [PubMed] [Google Scholar]
- Mayfield C., Squibb M., Miller D. Inhibition of nuclear protein binding to the human Ki-ras promoter by triplex-forming oligonucleotides. Biochemistry. 1994 Mar 22;33(11):3358–3363. doi: 10.1021/bi00177a029. [DOI] [PubMed] [Google Scholar]
- Morvan F., Rayner B., Imbach J. L., Chang D. K., Lown J. W. alpha-DNA. I. Synthesis, characterization by high field 1H-NMR, and base-pairing properties of the unnatural hexadeoxyribonucleotide alpha-[d(CpCpTpTpCpC)] with its complement beta-[d(GpGpApApGpG)]. Nucleic Acids Res. 1986 Jun 25;14(12):5019–5035. doi: 10.1093/nar/14.12.5019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morvan F., Rayner B., Imbach J. L., Thenet S., Bertrand J. R., Paoletti J., Malvy C., Paoletti C. alpha-DNA II. Synthesis of unnatural alpha-anomeric oligodeoxyribonucleotides containing the four usual bases and study of their substrate activities for nucleases. Nucleic Acids Res. 1987 Apr 24;15(8):3421–3437. doi: 10.1093/nar/15.8.3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen P. E., Egholm M., Berg R. H., Buchardt O. Peptide nucleic acids (PNAs): potential antisense and anti-gene agents. Anticancer Drug Des. 1993 Feb;8(1):53–63. [PubMed] [Google Scholar]
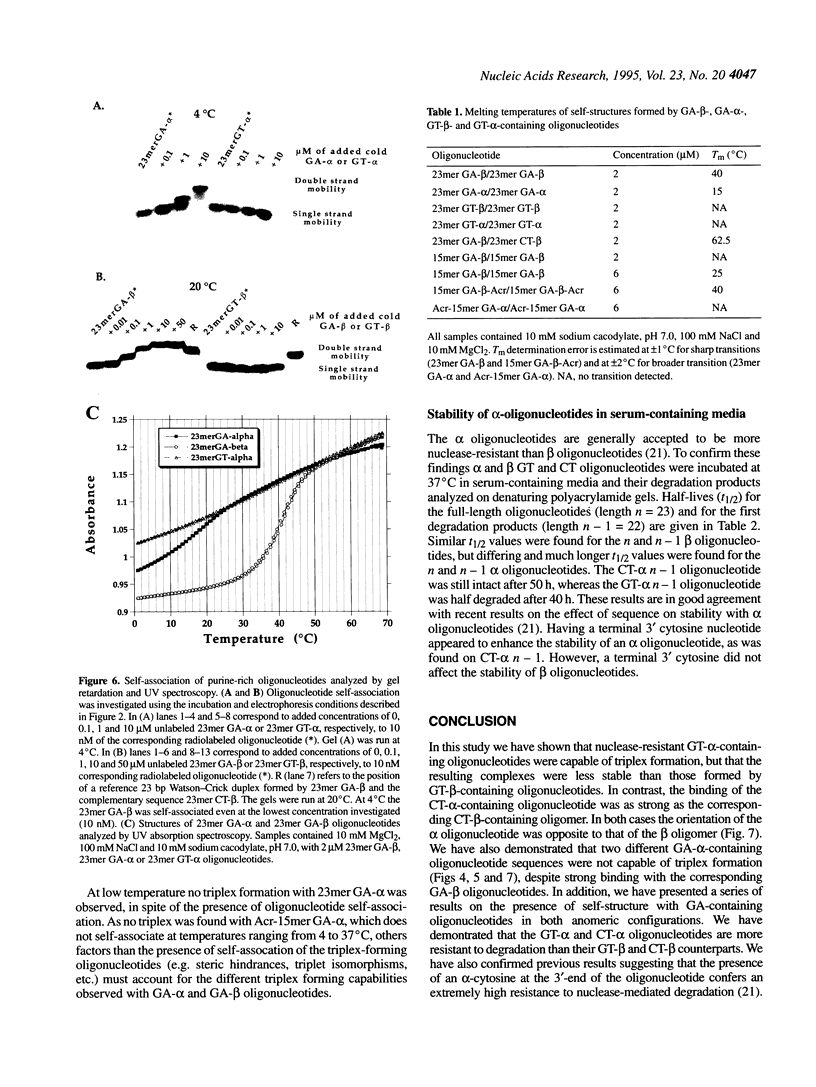
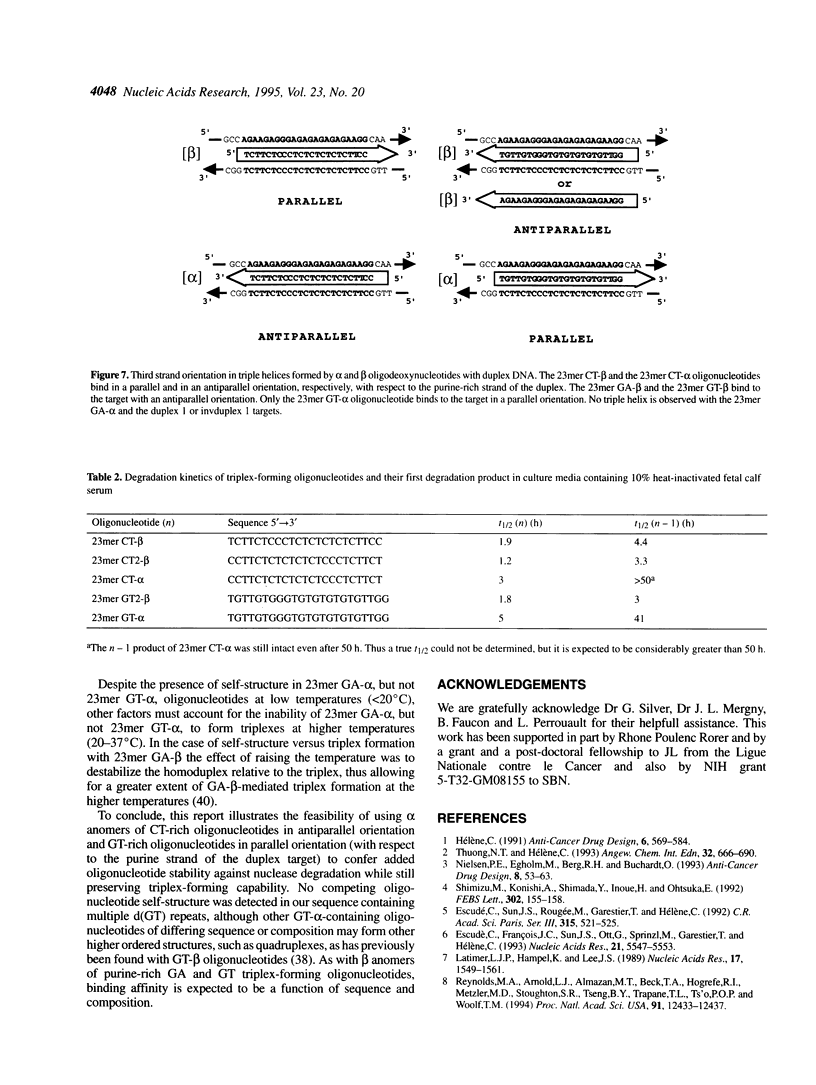
- Noonberg S. B., François J. C., Garestier T., Hélène C. Effect of competing self-structure on triplex formation with purine-rich oligodeoxynucleotides containing GA repeats. Nucleic Acids Res. 1995 Jun 11;23(11):1956–1963. doi: 10.1093/nar/23.11.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivas W. M., Maher L. J., 3rd Competitive triplex/quadruplex equilibria involving guanine-rich oligonucleotides. Biochemistry. 1995 Jan 10;34(1):278–284. doi: 10.1021/bi00001a034. [DOI] [PubMed] [Google Scholar]
- Postel E. H., Flint S. J., Kessler D. J., Hogan M. E. Evidence that a triplex-forming oligodeoxyribonucleotide binds to the c-myc promoter in HeLa cells, thereby reducing c-myc mRNA levels. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):8227–8231. doi: 10.1073/pnas.88.18.8227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praseuth D., Chassignol M., Takasugi M., Le Doan T., Thuong N. T., Hélène C. Double helices with parallel strands are formed by nuclease-resistant oligo-[alpha]-deoxynucleotides and oligo-[alpha]-deoxynucleotides covalently linked to an intercalating agent with complementary oligo-[beta]-deoxynucleotides. J Mol Biol. 1987 Aug 20;196(4):939–942. doi: 10.1016/0022-2836(87)90416-5. [DOI] [PubMed] [Google Scholar]
- Praseuth D., Le Doan T., Chassignol M., Decout J. L., Habhoub N., Lhomme J., Thuong N. T., Hélène C. Sequence-targeted photosensitized reactions in nucleic acids by oligo-alpha-deoxynucleotides and oligo-beta-deoxynucleotides covalently linked to proflavin. Biochemistry. 1988 Apr 19;27(8):3031–3038. doi: 10.1021/bi00408a055. [DOI] [PubMed] [Google Scholar]
- Praseuth D., Perrouault L., Le Doan T., Chassignol M., Thuong N., Hélène C. Sequence-specific binding and photocrosslinking of alpha and beta oligodeoxynucleotides to the major groove of DNA via triple-helix formation. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1349–1353. doi: 10.1073/pnas.85.5.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds M. A., Arnold L. J., Jr, Almazan M. T., Beck T. A., Hogrefe R. I., Metzler M. D., Stoughton S. R., Tseng B. Y., Trapane T. L., Ts'o P. O. Triple-strand-forming methylphosphonate oligodeoxynucleotides targeted to mRNA efficiently block protein synthesis. Proc Natl Acad Sci U S A. 1994 Dec 20;91(26):12433–12437. doi: 10.1073/pnas.91.26.12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rippe K., Fritsch V., Westhof E., Jovin T. M. Alternating d(G-A) sequences form a parallel-stranded DNA homoduplex. EMBO J. 1992 Oct;11(10):3777–3786. doi: 10.1002/j.1460-2075.1992.tb05463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy C. Inhibition of gene transcription by purine rich triplex forming oligodeoxyribonucleotides. Nucleic Acids Res. 1993 Jun 25;21(12):2845–2852. doi: 10.1093/nar/21.12.2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu M., Konishi A., Shimada Y., Inoue H., Ohtsuka E. Oligo(2'-O-methyl)ribonucleotides. Effective probes for duplex DNA. FEBS Lett. 1992 May 11;302(2):155–158. doi: 10.1016/0014-5793(92)80428-j. [DOI] [PubMed] [Google Scholar]
- Sun J. S., Asseline U., Rouzaud D., Montenay-Garestier T., Nguyen T. T., Hélène C. Oligo-[alpha]-deoxynucleotides covalently linked to an intercalating agent. Double helices with parallel strands are formed with complementary oligo-[beta]-deoxynucleotides. Nucleic Acids Res. 1987 Aug 11;15(15):6149–6158. doi: 10.1093/nar/15.15.6149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J. S., De Bizemont T., Duval-Valentin G., Montenay-Garestier T., Hélène C. Extension of the range of recognition sequences for triple helix formation by oligonucleotides containing guanines and thymines. C R Acad Sci III. 1991;313(13):585–590. [PubMed] [Google Scholar]
- Sun J. S., Giovannangeli C., François J. C., Kurfurst R., Montenay-Garestier T., Asseline U., Saison-Behmoaras T., Thuong N. T., Hélène C. Triple-helix formation by alpha oligodeoxynucleotides and alpha oligodeoxynucleotide-intercalator conjugates. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6023–6027. doi: 10.1073/pnas.88.14.6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J. S., Lavery R. Strand orientation of [alpha]-oligodeoxynucleotides in triple helix structures: dependence on nucleotide sequence. J Mol Recognit. 1992 Sep;5(3):93–98. doi: 10.1002/jmr.300050304. [DOI] [PubMed] [Google Scholar]
- Séquin U. Nucleosides and nucleotides. Part 7. Four dithymidine monophosphates with different anomeric configurations, their synthesis and behaviour towards phosphodiesterases. Helv Chim Acta. 1974;57(1):68–81. doi: 10.1002/hlca.19740570108. [DOI] [PubMed] [Google Scholar]
- Thuong N. T., Asseline U., Roig V., Takasugi M., Hélène C. Oligo(alpha-deoxynucleotide)s covalently linked to intercalating agents: differential binding to ribo- and deoxyribopolynucleotides and stability towards nuclease digestion. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5129–5133. doi: 10.1073/pnas.84.15.5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vichier-Guerre S., Pompon A., Lefebvre I., Imbach J. L. New insights into the resistance of alpha-oligonucleotides to nucleases. Antisense Res Dev. 1994 Spring;4(1):9–18. doi: 10.1089/ard.1994.4.9. [DOI] [PubMed] [Google Scholar]
- Xodo L., Alunni-Fabbroni M., Manzini G., Quadrifoglio F. Pyrimidine phosphorothioate oligonucleotides form triple-stranded helices and promote transcription inhibition. Nucleic Acids Res. 1994 Aug 25;22(16):3322–3330. doi: 10.1093/nar/22.16.3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zendegui J. G., Vasquez K. M., Tinsley J. H., Kessler D. J., Hogan M. E. In vivo stability and kinetics of absorption and disposition of 3' phosphopropyl amine oligonucleotides. Nucleic Acids Res. 1992 Jan 25;20(2):307–314. doi: 10.1093/nar/20.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]