Abstract
Genetically engineered Sindbis viruses (SIN) are excellent oncolytic agents in preclinical models. Several human cancers have aberrant Akt signaling, and kinase inhibitors including rapamycin are currently tested in combination therapies with oncolytic viruses. Therefore, it was of interest to delineate possible cross-regulation between SIN replication and PI3K/Akt/mTOR signaling. Here, using HEK293T cells as host, we report the following key findings: a) robust SIN replication occurs in the presence of mTOR specific inhibitors, rapamycin and torin1 or Ly294002- a PI3K inhibitor, suggesting a lack of requirement for PI3K/Akt/mTOR signaling; b) suppression of phosphorylation of Akt, mTOR and its effectors S6, and 4E-BP1 occurs late during SIN infection: a viral function that may be beneficial in counteracting cellular drug resistance to kinase inhibitors; c) Ly294002 and SIN act additively to suppress PI3K/Akt/mTOR pathway with little effect on virus release; and d) SIN replication induces host translational shut off, phosphorylation of eIF2α and apoptosis. This first report on the potent inhibition of Akt/mTOR signaling by SIN replication, bolsters further studies on the development and evaluation of engineered SIN genotypes in vitro and in vivo for unique cytolytic functions.
Keywords: Sindbis, Akt/mTOR, Rapamycin, torin1, translation
Introduction
Addiction to Akt/mTOR signaling is common among human cancers [1,2]. Oncolytic virotherapy in combination with mTOR specific inhibitor, rapamycin is an emerging paradigm in several preclinical settings [3–6]. Sindbis virus (SIN) is one of the best studied oncolytic viruses that selectively targets cancer cells in vivo [7–9]. The effective use of SIN in combination therapy against cancer requires mechanistic studies on the cross-regulation between SIN replication and mTOR signaling both in normal and cancer cells. Virus infection of host cells and the accompanying stress signaling can alter virus replication, host response, and disease outcomes [10–13]. An actively studied pathway during virus infection is the PI3K/Akt/mTOR axis of signaling [14–17]. In this cascade, the lipid kinase, PI3K activates its major downstream effector Akt which in turn activates mTOR [18]. mTOR is a pivotal kinase that integrates a variety of signals from growth factors, nutrients, and microbes to control growth, metabolism, immunity and cancer [19]. Activation of Akt occurs during infection with many DNA and RNA viruses to minimize cell death and promote virus survival [14, 16]. However, a number of other viruses including Vesicular Stomatitis Virus (VSV) do not require Akt signaling for productive infection [20 and references therein].
mTOR forms two multiprotein complexes, namely mTORC1 and mTORC2 [21]. The two important downstream effectors of mTORC1 are S6K1and 4E-BP1 which control translation of mRNAs that contain 5′ cap group [22]. Although the roles of mTORC2 are sketchy, it phosphorylates Akt [21]. Rapamycin, an approved immunosuppressant and allosteric inhibitor of mTOR, blocks mainly mTORC1and its substrates leading to inhibition of translation [21]. However, the presence of a pool of rapamycin-resistant mTORC1override this effect [23]. In addition, mTORC1 exerts feedback inhibition on Akt1 by preventing its phosphorylation through PI3K [24]. While rapamycin does not inhibit mTORC2, prolonged treatment reduces the pool of mTORC2 [21]. Torin1, an ATP-competitive mTOR inhibitor effectively blocks rapamycin-sensitive, and -insensitive mTORC1, and mTORC2 [23]. For example, while rapamycin is a poor inhibitor of herpes virus growth, torin1 effectively blocks rapamycin-resistant mTORC1 and virus replication [25].
SIN and other alphaviruses are positive-sense RNA viruses that replicate in a number of vertebrate cells [26]. Genetically engineered alphaviruses are extensively used in molecular dissection of gene functions [27] vaccine delivery [28], and oncolytic [7] applications. Systemically delivered SIN targets both primary and metastatic tumors, derived from colon and ovarian cancers in mouse xenograft models [7–9]. Although host cell protein synthesis is shut-off in alphavirus infected cells, viral mRNA translation is efficient [29]. SIN infected cells also undergo autophagy, and apoptosis during the course of infection which are regulated by the mTOR [13]. Therefore, we undertook this study to examine the cross-regulation between SIN replication and mTOR signaling using two specific mTOR inhibitors. Here we report that SIN replication does not require PI3K/Akt/mTOR signaling, and later during infection suppresses Akt/mTOR activation in HEK cells. The implications of these findings for the use of alphaviruses as oncolytic agents are discussed.
Materials and methods
Cells, viruses and reagents
Human embryonic kidney cells (HEK 293T) obtained from ATCC were grown in Dulbecco’s modified eagle medium (DMEM, Invitrogen) supplemented with 10% fetal bovine serum. Cell viability checked by trypan blue dye exclusion was more than 90%. Baby Hamster Kidney (BHK) and African green monkey kidney (Vero) cells were maintained in minimal essential medium (Invitrogen) containing 10% fetal bovine serum.
The engineered SIN used in these studies was prepared by in vitro transcription of plasmids SIN Toto1101 and SIN/GFP/TE followed by transfection into BHK cells, propagation and titering in vero cells as previously described [27,30]. Virus preparations were purified by ultracentrifugation at 30K rpm, using 20% sucrose cushion. UV-inactivation of purified virus particles was carried out in a Bio-RAD UV-chamber by two cycles of program C4. No infectious virus was detectable in UV-inactivated virus (UV-SIN) preparations, as confirmed by plaque assay.
Rapamycin and Ly294002 were purchased from Sigma (St. Louis, MO, USA). Torin1 was a kind gift from Nathanael Gray, Boston. Primary antibodies were from cell signaling Technology (Danvers, MA, USA) and the anti-mouse and anti-rabbit secondary antibodies were from Invitrogen. 35S labeled L-methionine was from Perkin Elmer.
Infection and plaque assays
HEK cells were pretreated with 100nM rapamycin, 250nM torin1or 40μm Ly 294002 in 1ml DMEM containing 5% FBS for 1hr and infected with SIN at a MOI of 5 or its equivalent of UV-SIN for 4 and 24h. Culture supernatants were recovered after centrifugation, and stored frozen. The cells were harvested using ice cold PBS. HEK cells infected with SIN/GFP/TE at MOI of 5, for 24hr at 37°C were observed for GFP fluorescence under fluorescent microscope (Nikon Eclipse E2000-S). Virus titer in culture supernatants from HEK cells was determined by plaque assay as described previously [27, 30]
Metabolic labeling and determination of protein synthesis
Cells were labeled for two hours before harvest with 20μCi 35S labeled L-methionine, and lysed with a buffer containing 10mM Tris (pH 7.4), 0.15M NaCl, 1mM EDTA and 0.8% NP40. Protein was precipitated with 20% trichloroacetic acid (TCA) and filtered onto glass microfibre filters (GF/C, Whatman) and washed with 10% TCA using Millipore filtration module. The air dried filters were immersed in scintillation fluid (Amersham) and radioactivity was measured in a liquid scintillation analyzer (Packard Tri-Carb 2300TR).
Apoptosis assay
Annexin/PI staining was done according to manufacturer’s protocol (BD biosciences) to determine the percentage of cells undergoing apoptosis by flow cytometry (Becton Dickinson FACS-Calibur) and the data was analysed by Cell quest pro software.
RNA isolation and quantitative real time PCR
Total RNA from infected and uninfected HEK cells was isolated using Trizol reagent (Invitrogen), treated with DNase (RQ1 RNAse free DNAse, Promega) and cDNA was made using random hexamers by M-MuLV reverse transcriptase[30]. SYBR green based quantitative real time PCR [qPCR] was done using primers specific to the 5′ NTR region of SIN genome (forward, 5′ AAC AGC CGA CCA ATT GCA CTA CCA; reverse, 5′ TTT GCA GTT GCA CGA CAA ACG GAC) with beta actin (forward, 5′ GGA CTT CGA GCA AGA GAT GG; reverse, 5′ AGC ACT GTG TTG GCG TAC AG) as internal control.
Western blotting
At the indicated times, HEK cells were recovered with ice cold PBS and lysed with Laemmli loading dye. An aliquot of the cells was lysed with mammalian protein extraction reagent (Thermo Scientific) for quantification of total protein levels by Bradford method (Sigma). The Laemmli lysates were boiled, sonicated and equal amounts of protein were resolved in 4–15% or 10% SDS–PAGE gels and transferred to PVDF membranes. The membranes were blocked with fat free milk (Cell Signaling) and incubated overnight with the primary antibodies, followed by incubation with horseradish peroxidase (HRP) conjugated anti-rabbit/anti-mouse secondary antibodies. After treating with the substrate (Super signal west Pico, Thermo Scientific) for 1min, the membranes were exposed to X-ray films (Kodak).
Results and discussion
SIN replicates efficiently in HEK 293T cells
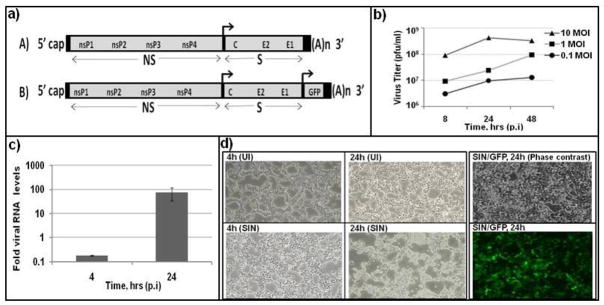
The effective use of SIN as an oncolytic agent in combination with mTOR inhibitors warrants an in depth knowledge on how these inhibitors affect virus replication, and how the kinase inhibitory activity of the drugs are modified by virus infection. In the present work, we addressed this objective using a cell culture based infection model. In the infected host, and in vitro cultures, SIN replicates in a number of cell types including fibroblasts, myocytes, endothelium and neuronal cells [7, 8, 26]. Much of the previous studies in SIN made use of fibroblasts of animal origin including BHK-21, vero and chicken embryo [26, 27, 30]. We chose to study SIN infection of HEK293T cells as a human fibroblast model, since fibroblasts are important targets for SIN, and there is a large research literature for signaling pathways in HEK293T cells. We used Toto1101, a prototype genetically engineered SIN (Fig. 1a), since defined mutations can be easily introduced in the SIN genome for further studies. HEK cells were infected at different multiplicities, and the amount of virus recovered from culture supernatant was determined. Peak levels (4 × 108 pfu/ml, Fig. 1b) of SIN release was observed at 24 hrs (MOI, 10), which is comparable to the amount of virus release observed in other cell lines [26,27]. At the MOI of 0.1 and 1, the virus titer was 10–100 fold lower even at 48 hr. Determination of viral RNA by qPCR indicated a 700 fold lower RNA at 4 hr, implying very low level of genome replication at early times (Fig. 1c). More than 95% of the cells showed fluorescence, when HEK cells were infected with a recombinant SIN expressing GFP reporter, SIN/GFP/TE (Fig. 1d). At 24 hr p.i, SIN infected cells showed distinct morphological changes including cell rounding and brightness. These results indicated that HEK cells are excellent host cells for productive SIN replication.
Fig. 1.
Robust replication of SIN in HEK cells: a) map of engineered SIN, Toto1101 (A), and recombinant SIN expressing GFP (B). NS, non-structural proteins; S, structural proteins. b) Virus release from infected HEK cells. C) Viral RNA accumulates late during infection: The level of virus specific genomic RNA was determined by qPCR using 5′ genome specific primers and beta-actin for normalization. d) Virus induced cytopathology, and GFP expression: Uninfected (UI) and infected cells (SIN, SIN/GFP/TE) were photographed under phase contrast and fluorescent microscopy. Data represent results from at least three different experiments.
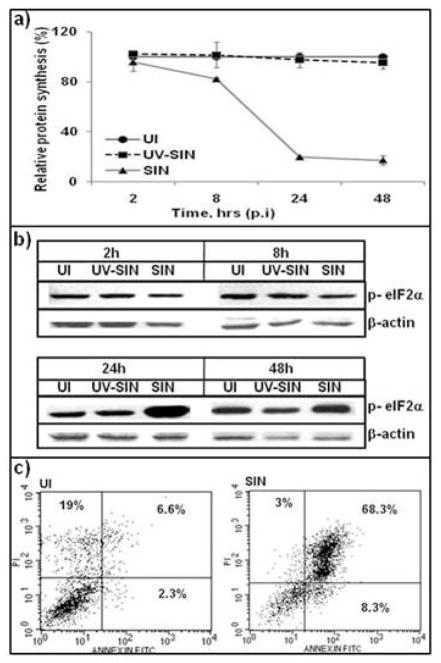
Altered protein synthesis, and apoptosis in SIN infected cells
A salient feature of SIN infected vertebrate cells is the inhibition of host protein synthesis that is attributed to early events in virus replication as well as PKR-dependent and independent pathways [29]. When HEK cells were infected with SIN at a MOI of 10, a 20% inhibition of protein synthesis was observed at 8 hr, and 80% inhibition at 24 hr p.i (Fig. 2a). Cells exposed to UV-SIN showed no inhibition of protein synthesis even at 48 hr compared to uninfected cells, indicating that the inhibition of protein synthesis requires active virus replication. Although a number of pathways lead to inhibition of host protein synthesis [10,11], phosphorylation of eIF2α is an important event that inactivates this protein and makes it unavailable for initiation of translation [31]. Cellular stress due to nutrients, toxins, and virus infections induce different cellular kinases that phosphorylate eIF2α [31]. SIN infected cells showed normal levels of eIF2α at 2 and 8hr p.i (Fig. 2b). However a drastic increase in phosphorylated eIF2α was observed at 24 hr, which also corresponded to the peak inhibition of protein synthesis. Cells exposed to UV-SIN showed no increase in p- eIF2α. Flow cytometric analysis of HEK cells infected with SIN showed that 68% of cells were apoptotic, compared to 8% in uninfected cells (Fig. 2c). These results indicated that SIN infection of HEK cells manifests changes that are characteristic of SIN infection of other vertebrate cells [26] and this infection model is suitable for further studies.
Fig. 2.
SIN induced cellular alterations: a) SIN replication shuts off host protein synthesis. Cells were labeled with 35S-Methionine and TCA precipitable counts were determined. b) Phosphorylation of eIF2α by SIN replication. Cell extracts were analysed by western blotting using p-eIF2α (Ser 51) antibody. c) Induction of apoptosis by SIN replication. HEK cells recovered at 48h, stained with Annexin/PI and analysed by flow cytometry. Data represent results from at least three different experiments.
SIN replication is unaltered by rapamycin and torin1
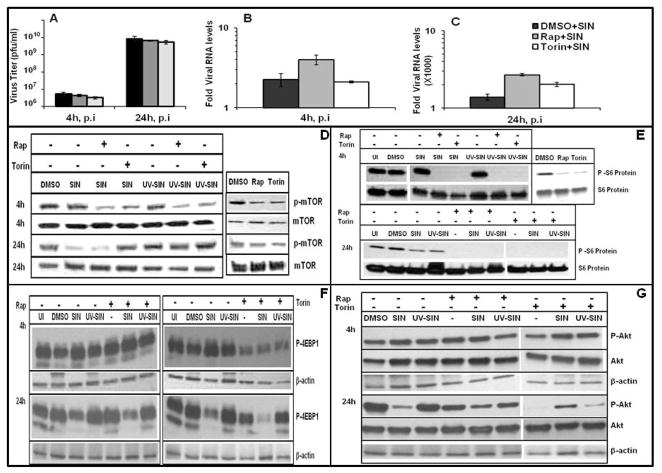
Many viruses activate mTOR pathway to counteract host antiviral strategies [14, 16]. For example, rapamycin has been used to control virus replication in nephropathy or in preclinical testing of oncolytic virotherapy [3–6]. As reported for VSV infection, rapamycin may not affect replication of all viruses [32]. To determine the requirement of mTOR for SIN specific RNA synthesis and virus release, HEK cells were infected with a MOI of 10, in the presence and absence of mTOR inhibitors, rapamycin or torin1. Rapamycin increased viral RNA levels by 2- fold both at 4 and 24h, whereas torin1 increased it by 1.5 fold at 24h (Fig. 3B, C). However, the virus titer in the culture supernatants was largely unaltered by both rapamycin and torin 1 (Fig. 3A). These results indicate that rapamycin and torin1 enhance SIN RNA synthesis moderately, but do not alter virus release from infected cells. Robust replication of SIN in the presence of mTOR inhibitors should bode well for combination virotherapy.
Fig. 3.
Cross regulation between SIN replication and mTOR signaling: A) Rapamycin and torin1 do not block virus replication. B, C) Rapamycin and torin1 mildly upregulate SIN genome levels. Virus specific RNA at 4h (B) and 24h (C) was determined by qPCR. D-G) Alterations in Akt/mTOR signaling by SIN replication and the effect of rapamycin and torin1. Cell extracts were analysed by western blotting using antibodies specific for phosphorylated and total forms of mTOR (D), S6 (E), 4E-BP1 (F) and Akt (G) with actin as loading control. Data represent results from at least three different experiments.
Regulation of mTOR signaling by SIN replication
Although SIN replication is unaffected by mTOR inhibitors, it is possible that SIN replication could positively or negatively impact mTOR function. The details as to how mTOR pathway is altered by virus infections are beginning to emerge [4, 14, 16]. Contrary to herpes viruses, RNA viruses such as poliovirus and VSV, blocked phosphorylation of 4E-BP1, a key substrate in mTOR pathway, and rapamycin treatment of virus infected cells further suppressed phosphorylation of 4E-BP1 [16, 32]. We chose to determine if virus infection alters mTOR signaling by analyzing the phosphorylation status of mTOR (Ser 2448) and its downstream effector molecules S6 (Ser 235/236) and 4E-BP1 (Thr 36/47). At 4 hr, both rapamycin and torin1 reduced the levels of p-mTOR (Fig. 3D). However, SIN as well as UV-SIN showed comparable levels of p-mTOR to that of uninfected cells. Notably at 24 hr, both rapamycin and torin1 showed only a moderate decrease in mTOR phosphorylation, but SIN infected cells showed drastic reduction in p-mTOR levels. Interestingly, while rapamycin treatment did not alter SIN induced suppression of p-mTOR levels, torin1 restores the p-mTOR levels to some extent. These results are suggestive of a mechanistic difference between rapamycin and torin1 in regulating mTOR after prolonged treatment late during SIN replication as previously reported [21].
Next, we examined the downstream effectors of mTOR. Since we had difficulty in detecting p-S6K, we resorted to the determination of its substrate, S6. At 4 hr, both rapamycin and torin1 blocked phosphorylation of S6, while SIN and UV-SIN had no effect on S6, as observed for p-mTOR (Fig. 3E). However, at 24 hr, rapamycin and torin1 eliminated all p-S6, although p-mTOR levels were unaffected. SIN infected cells showed lowered p-S6, although the suppression of p-mTOR was much stronger than that of p-S6. Torin1 inhibited the phosphorylation of 4E-BP1 and the effect was more potent at 4h compared to 24h (Fig. 3F). However, rapamycin did not have any effect on 4E-BP1 phosphorylation. SIN did not alter p4E-BP1levels at 4hr, but drastically reduced the phosphorylation of 4E-BP1 at 24 hr (Fig. 3F), although the total 4E-BP1 levels were unaltered (data not shown). The inhibitory effect of SIN on 4E-BP1 phosphorylation was unaltered by rapamycin, but the effect of torin1 appeared additive along with SIN. Therefore, at 24 hr p.i the drastic inhibition of host protein synthesis in SIN infected cells coincides with suppression of 4E-BP1 phosphorylation, as observed for VSV [32]. In the present study, SIN induced phosphorylation of eIF2a, and dephosphorylation of 4E-BP1 are associated with strong translational shut-off in HEK cells.
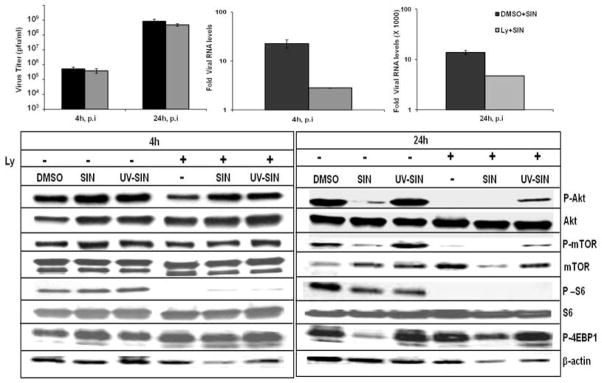
Efficient SIN replication in Ly294002 treated cells
Since mTOR is regulated by Akt and PI3K sequentially, we asked if PI3K activity is required to support SIN replication using PI3K specific inhibitor Ly 294002 (Ly). Unlike rapamycin and torin1, Ly decreased SIN RNA levels by 8-fold at 4hr and 3 fold at 24hr (Fig. 4b, c), However Ly treatment had little effect on virus release from SIN infected cells (Fig. 4a) suggesting that particle assembly and virus release are not dependent on PI3K. At 4hr, Ly decreased Akt phosphorylation (Ser 473) in uninfected cells, but the inhibitory effect was moderately relieved in cells infected with SIN, and UV-SIN (Fig. 4d). Notably, the level of p-mTOR in Ly treated cells was unaltered at 4hr, both in the presence and absence of virus under our culture conditions with 5% serum. As observed for rapamycin, Ly also did not alter the level of p-4E-BP1, but inhibited S6 phosphorylation in the presence and absence of virus, despite minimal changes in p-Akt or p-mTOR (Fig. 4d). This observation suggests that S6 is more sensitive to Ly treatment than mTOR and Akt at 4 hr. At 24 hr, both Ly, and SIN infection strongly lowered the levels of p-Akt, p-mTOR, and S6 suggesting that SIN downregulates PI3/Akt/mTOR signaling. Torin1 drastically downregulated p-Akt levels at 24 hr, whereas rapamycin had only a moderate effect. These results suggest that torin1 is a potent inhibitor of Akt, perhaps due to its effect on mTORC2[23]. SIN replication suppressed Akt phosphorylation both in the presence and absence of rapamycin and torin1. Although both rapamycin and torin1 inhibited Akt phosphorylation variably, the inhibition was slightly reversed in SIN infected cells. Ly completely inhibited phosphorylation of Akt, mTOR and S6 in the presence or absence of SIN at 24h and also did not alter the inhibitory effect of SIN on 4E-BP1 phosphorylation (Fig. 4d).
Fig. 4.
Effect of Ly 294002 and SIN replication and Akt/mTOR signaling: a-c) Ly decreases viral RNA synthesis, but not particle release from HEK cells. d) Alterations in Akt/mTOR signaling by SIN replication in the presence of Ly. Data represent results from at least three different experiments.
Interplay between PI3K/Akt/mTOR signaling and SIN replication machinery
The drastic suppression of phosphorylation of Akt, mTOR, S6, and 4E-BP1, late during SIN infection, along with efficient viral RNA synthesis and particle production is intriguing. This result suggests that SIN replication blocks mTOR pathway, although PI3K and Akt -dependent and -independent mechanisms [2] may also be involved. Dunn et al., recently reported that VSV blocks phosphorylation of Akt and mTOR in a PI3K independent manner [17]. Kinase inhibitors, including rapamycin are meant to slow down growth and proliferation, although the host compensatory pathways might antagonize the effect of these inhibitors [3, 22]. Therefore, the suppression of Akt/mTOR growth signaling by SIN replication could circumvent drug resistance. Viruses including SIN have evolved mechanisms to replicate well by antagonizing host defense pathways such as interferon (IFN) induction and translational shut off [12–14]. Therefore, studies as to how SIN alters Akt/mTOR signaling in IFN-sensitive and –insensitive cancer cells in the presence of kinase inhibitors are needed to fully dissect the outcomes of preclinical animal models of cancer. Screening and defining cognate SIN genotypes that alter cellular balance between growth promotion and apoptosis are prerequisites for effective combination therapy.
Acknowledgments
This work was supported by USPHS grant SC1AI081655. We thank Supriya Pokkali for the initial experiments of this work. We also thank N. Gray and D. Sabatini for torin1, Ilya Frolov and G. Li for SIN/GFP vectors. The use of CHDR/HIV core for flow cytometry, MMC Morphology Core and Molecular Biology Core (U54NS041071, G12RR03032 and U54CA91408) RCMI supported Molecular Biology Core, VICTR and MeTRC resources are gratefully acknowledged.
Abbreviations
- PI3K
phosphatidylinositol 3-kinase
- mTOR
mammalian target of rapamycin
- mTORC1
mTOR complex1
- mTORC2
mTOR complex2
- S6K1
p70 S6 kinase1
- S6
ribosomal protein S6
- 4E-BP1
eIF4E1 binding protein1
- eIF2α
eukaryotic initiation factor 2α
- PKR
ds RNA activated protein kinase
- SIN
Sindbis virus
- UV-SIN
UV inactivated Sindbis virus
- HEK293T
human embryonic kidney cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chen YL, Law PY, Loh HH. Inhibition of PI3K/Akt signaling: an emerging paradigm for targeted cancer therapy. Curr Med Chem Anticancer Agents. 2005;5:575–589. doi: 10.2174/156801105774574649. [DOI] [PubMed] [Google Scholar]
- 2.Memmott RM, Dennis PA. Akt-dependent and -independent mechanisms of mTOR regulation in cancer. Cell Signal. 2009;21:656–664. doi: 10.1016/j.cellsig.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alvarez-Breckenridge C, Kaur B, Chiocca EA. Pharmacologic and chemical adjuvants in tumor virotherapy. Chem Rev. 2009;109:3125–3140. doi: 10.1021/cr900048k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alain T, Lun X, Martineau Y, Sean P, Pulendran B, Petroulakis E, Zemp FJ, Lemay CG, Roy D, Bell JC, Thomas G, Kozma SC, Forsyth PA, Costa-Mattioli M, Sonenberg N. Vesicular stomatitis virus oncolysis is potentiated by impairing mTORC1-dependent type I IFN production. Proc Natl Acad Sci U S A. 2010;107:1576–1581. doi: 10.1073/pnas.0912344107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liacini A, Seamone ME, Muruve DA, Tibbles LA. Anti-BK Virus Mechanisms of Sirolimus and Leflunomide Alone and in Combination: Toward a New Therapy for BK Virus Infection. Transplantation. 2010 doi: 10.1097/TP.0b013e3182007be2. [DOI] [PubMed] [Google Scholar]
- 6.Ottolino-Perry K, Diallo JS, Lichty BD, Bell JC, McCart JA. Intelligent design: combination therapy with oncolytic viruses. Mol Ther. 2010;18:251–263. doi: 10.1038/mt.2009.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tseng JC, Levin B, Hurtado A, Yee H, Perez de Castro I, Jimenez M, Shamamian P, Jin R, Novick RP, Pellicer A, Meruelo D. Systemic tumor targeting and killing by Sindbis viral vectors. Nat Biotechnol. 2004;22:70–77. doi: 10.1038/nbt917. [DOI] [PubMed] [Google Scholar]
- 8.Li C, Gu Y, Andrade D, Liu Y. Susceptibility of colorectal cancer cells to Sindbis virus infection. J Exp Ther Oncol. 2009;8:167–175. [PubMed] [Google Scholar]
- 9.Quetglas JI, Ruiz-Guillen M, Aranda A, Casales E, Bezunartea J, Smerdou C. Alphavirus vectors for cancer therapy. Virus Res. 2010;153:179–196. doi: 10.1016/j.virusres.2010.07.027. [DOI] [PubMed] [Google Scholar]
- 10.Kaariainen L, Ranki M. Inhibition of cell functions by RNA-virus infections. Annu Rev Microbiol. 1984;38:91–109. doi: 10.1146/annurev.mi.38.100184.000515. [DOI] [PubMed] [Google Scholar]
- 11.Holcik M, Sonenberg N. Translational control in stress and apoptosis. Nat Rev Mol Cell Biol. 2005;6:318–327. doi: 10.1038/nrm1618. [DOI] [PubMed] [Google Scholar]
- 12.Randall RE, Goodbourn S. Interferons and viruses: an interplay between induction, signalling, antiviral responses and virus countermeasures. J Gen Virol. 2008;89:1–47. doi: 10.1099/vir.0.83391-0. [DOI] [PubMed] [Google Scholar]
- 13.Orvedahl, Levine B. Autophagy in Mammalian antiviral immunity. Curr Top Microbiol Immunol. 2009;335:267–285. doi: 10.1007/978-3-642-00302-8_13. [DOI] [PubMed] [Google Scholar]
- 14.Cooray S. The pivotal role of phosphatidylinositol 3-kinase-Akt signal transduction in virus survival. J Gen Virol. 2004;85:1065–1076. doi: 10.1099/vir.0.19771-0. [DOI] [PubMed] [Google Scholar]
- 15.Mannova P, Beretta L. Activation of the N-Ras-PI3K-Akt-mTOR pathway by hepatitis C virus: control of cell survival and viral replication. J Virol. 2005;79:8742–8749. doi: 10.1128/JVI.79.14.8742-8749.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buchkovich NJ, Yu Y, Zampieri CA, Alwine JC. The TORrid affairs of viruses: effects of mammalian DNA viruses on the PI3K-Akt-mTOR signalling pathway. Nat Rev Microbiol. 2008;6:266–275. doi: 10.1038/nrmicro1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dunn EF, Connor JH. Dominant Inhibition of Akt/PKB signaling by the Matrix protein of a negative-strand RNA virus. J Virol. 2011 doi: 10.1128/JVI.01671–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol. 2009;10:307–318. doi: 10.1038/nrm2672. [DOI] [PubMed] [Google Scholar]
- 20.Dunn EF, Fearns R, Connor JH. Akt inhibitor Akt-IV blocks virus replication through an Akt-independent mechanism. J Virol. 2009;83:11665–11672. doi: 10.1128/JVI.01092-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, Markhard AL, Sabatini DM. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22:159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 22.Dowling RJ, Topisirovic I, Fonseca BD, Sonenberg N. Dissecting the role of mTOR: lessons from mTOR inhibitors. Biochim Biophys Acta. 2010;1804:433–439. doi: 10.1016/j.bbapap.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 23.Thoreen CC, Kang SA, Chang JW, Liu Q, Zhang J, Gao Y, Reichling LJ, Sim T, Sabatini DM, Gray NS. An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. J Biol Chem. 2009;284:8023–8032. doi: 10.1074/jbc.M900301200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhaskar PT, Hay N. The two TORCs and Akt. Dev Cell. 2007;12:487–502. doi: 10.1016/j.devcel.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 25.Moorman NJ, Shenk T. Rapamycin-resistant mTORC1 kinase activity is required for herpesvirus replication. J Virol. 2010;84:5260–5269. doi: 10.1128/JVI.02733-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strauss JH, Strauss EG. The alphaviruses: gene expression, replication, and evolution. Microbiol Rev. 1994;58:491–562. doi: 10.1128/mr.58.3.491-562.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rice CM, Levis R, Strauss JH, Huang HV. Production of infectious RNA transcripts from Sindbis virus cDNA clones: mapping of lethal mutations, rescue of a temperature-sensitive marker. and in vitro mutagenesis to generate defined mutants. J Virol. 1987;61:3809–3819. doi: 10.1128/jvi.61.12.3809-3819.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pushko P, Parker M, Ludwig GV, Davis NL, Johnston RE, Smith JF. Replicon-helper systems from attenuated Venezuelan equine encephalitis virus: expression of heterologous genes in vitro and immunization against heterologous pathogens in vivo. Virology. 1997;239:389–401. doi: 10.1006/viro.1997.8878. [DOI] [PubMed] [Google Scholar]
- 29.Gorchakov R, Frolova E, Williams BR, Rice CM, Frolov I. PKR-dependent and -independent mechanisms are involved in translational shutoff during Sindbis virus infection. J Virol. 2004;78:8455–8467. doi: 10.1128/JVI.78.16.8455-8467.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.George J, Raju R. Alphavirus RNA genome repair and evolution: molecular characterization of infectious sindbis virus isolates lacking a known conserved motif at the 3′ end of the genome. J Virol. 2000;74:9776–9785. doi: 10.1128/jvi.74.20.9776-9785.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wek RC, Jiang HY, Anthony TG. Coping with stress: eIF2 kinases and translational control. Biochem Soc Trans. 2006;34:7–11. doi: 10.1042/BST20060007. [DOI] [PubMed] [Google Scholar]
- 32.Connor JH, Lyles DS. Vesicular stomatitis virus infection alters the eIF4F translation initiation complex and causes dephosphorylation of the eIF4E binding protein 4E-BP1. J Virol. 2002;76:10177–10187. doi: 10.1128/JVI.76.20.10177-10187.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Proud CG. Regulation of mammalian translation factors by nutrients. Eur J Biochem. 2002;269:5338–5349. doi: 10.1046/j.1432-1033.2002.03292.x. [DOI] [PubMed] [Google Scholar]