Abstract
Human bone marrow mesenchymal stem cells (MSCs) are pleiotrophic cells that differentiate to either adipocytes or osteoblasts as a result of crosstalk by specific signaling pathways including heme oxygenase (HO)-1/-2 expression. We examined the effect of inducers of HO-1 expression and inhibitors of HO activity on MSC differentiation to the osteoblast and following high glucose exposure. MSC cultured in osteogenic medium increased expression of osteonectin, Runt-related transcription factor 2 (RUNX-2), osteocalcin, and alkaline phosphatase. HO-1 expression during differentiation was initially decreased and then followed by a rebound increase after 15 days of culture. Additionally, the effect of HO-1 on osteoblasts appears different to that seen in adipocyte stem cells. On addition of a cobalt compound, the resultant induction of HO-1 decreases adipogenesis. Moreover, glucose (30 mM) inhibited osteoblast differentiation, as evidenced by decreased bone morphogenetic protein (BMP)-2, osteonectin, osteocalcin, and osteoprotegerin (OPG). In contrast, MSC-derived adipocytes were increased by glucose. Increased HO-1 expression increased the levels of osteonectin, OPG, and BMP-2. Inhibition of HO activity prevented the increase in osteonectin and potentiated the decrease of osteocalcin and OPG in cells exposed to high glucose levels. Furthermore, targeting HO-1 expression increased pAMPK and endothelial nitric oxide synthase (eNOS) and restored osteoblastic markers. Our findings suggest that targeting HO-1 gene expression attenuates the hyperglycemia-mediated decrease in MSC-derived osteoblast differentiation. Finally, the mechanism underlying the HO-1-specific cell effect on osteoblasts and adipocytes is yet to be explored. Thus, the targeting of HO-1 gene expression presents a portal to increase osteoblast function and differentiation and attenuate osteoporosis by promoting bone formation.
Keywords: Osteopenia, Osteoporosis, MSC, Diabetes, HO-1
Introduction
Human bone marrow-derived mesenchymal stem cells (MSCs) are multipotent cells that have the potential to proliferate and differentiate into a variety of cell types characteristic of bone, skeletal and cardiac muscle, adipose tissue, and neural cells [1-4]. Diabetes affects dynamic bone formation in both humans and animals, leading to osteopenia and osteoporosis [5, 6]. Bone mineral density and biochemical markers of bone turnover are adversely affected in individuals with diabetes [7]. Reduction of bone mass, occurring with increased frequency in individuals with diabetes mellitus, has been attributed to poor glycemic control, but the pathogenic mechanisms remain unknown. High concentrations of glucose (hyperglycemia) in diabetics exacerbate this complication [7-9]. Osteoblasts secrete growth factors including platelet-derived growth factor, insulin-like growth factors, and bone morphogenetic proteins (BMPs) [10] that are stored in the bone matrix. Whether these factors are affected by diabetes remains to be seen. The molecular mechanism underlying osteoblastic differentiation has not been fully elucidated. Recently, Abraham et al. [12] have shown the essential role of HO-1 in restoration of mice bone marrow-derived stem cells [11] and prevention of type 2 diabetes. HO-1 increases stem cell differentiation to various lineages [13-15].
Heme oxygenase-1 (HO-1) plays a major role during bone marrow stem cell differentiation [16-18]. Heme oxygenase, which exists in two forms, HO-1 (inducible) and HO-2 (constitutive), catalyzes the rate-limiting step in heme degradation, resulting in the formation of carbon monoxide (CO), iron, and biliverdin; the latter is subsequently reduced to bilirubin by biliverdin reductase. Both CO and nitric oxide (NO) protect against tumor necrosis factor-induced apoptosis in osteoblasts [19]. In addition, during fracture repair, activation of hypoxia-inducible factor (HIF)-1 and its target genes, vascular endothelial growth factor (VEGF) and HO-1, regulate osteoclastogenesis and bone reabsorption [20], suggesting a role of HO-1 in bone metabolism. HO-1 expression decreases adipogenesis in obese animals [11, 19-21], suggesting that HO-1 may have a differential effect other than that described for vascular smooth muscle cells and endothelial cells [19, 22].
HO-1 expression is increased as an adaptive response to several injurious stimuli including heme, hyperoxia, hypoxia, endotoxin, and heavy metals [23]. Induction of HO-1 is implicated in numerous clinically relevant disease states including transplant rejection, hypertension, atherosclerosis, lung injury, and endotoxic shock [16, 19, 23]. The proposed role of the HO-1/HO-2 system in osteoblast cell proliferation stems from the observation that HO-1 is a potent regulator of cell growth and angiogenesis. The effect of HO-1-derived CO signaling in promoting angiogenesis in human microvessel endothelial cells is well established [24]. Previously, we have demonstrated that overexpression of the HO-1 gene in endothelial cells caused a significant increase in angiogenesis [25], somatic cell growth [26], and cell proliferation [27]. Changes in HO-1-derived CO modulate vascular calcification [28].
Osteocalcin, an osteoblast-specific protein, is of considerable significance in metabolic disease and is secreted in the circulation from osteoblastic cells [29, 30]. Osteocalcin regulates glucose metabolism and fat mass in genetically modified mice [31]. Osteocalcin-knockout mice display decreased β-cell proliferation, glucose intolerance, and insulin resistance. Osteocalcin administration also regulates gene expression in β cells and adipocytes (including adiponectin expression) and affects the development of obesity and type 2 diabetes in wild-type mice [32].
However, the role of HO-1 expression in MSC development and differentiation to osteoblasts is poorly understood. HO-1 expression and its role in diabetes and other pathologies is a burgeoning area of research [19, 23]. Heme oxygenase is a target gene for the prevention of diabetes and obesity [19]. As seen in obese mice, the apolipoprotein mimetic L-4F or cobalt compounds targeted HO-1 expression, which reduced visceral and subcutaneous adiposity, increased adiponectin levels, and improved insulin sensitivity [11].
In the present study, we hypothesized that increased HO-1 expression serves to counteract the negative effects of high glucose on osteoblastic differentiation but increases adipocyte differentiation by targeting HO-1 expression or inhibition of HO activity by CoPP and SnMP, respectively. We demonstrate that osteoblast differentiation was increased by induction of HO-1, which was associated with a reduction of reactive oxygen species (ROS) formation, thereby permitting the restoration of osteoblastic markers, specifically induction of osteoprotegerin (OPG) and osteocalcin, while increasing the levels of endothelial nitric oxide synthase (eNOS) and pAMPK.
Materials and methods
Chemicals and reagents
Ficoll-Paque PLUS, Dulbecco’s modified essential medium (DMEM), fetal bovine serum (FBS), and antibiotic–antimycotic were purchased from Gibco (Carlsbad, CA, USA). Ascorbic acid, dexamethasone, d-glucose, alizarin red S, and oil red O were purchased from Sigma (St. Louis, MO, USA); β-Glycerophosphate was from Calbiochem (San Diego, CA, USA). Antirabbit polyclonal antibody against HO-1 was from Stressgen (Victoria, BC), antirabbit polyclonal antibodies against pAMPK, AMPK, eNOS, and peroxisome proliferator-activated receptor (PPAR)-γ were from Cell Signalling Technology (Beverly, MA, USA); human receptor activator of nuclear factor kappaB ligand (sRANKL) and OPG ELISA kits were from Bio-Vendor (Modrice, Czech Republic), and the OCN ELISA kit was from BioSource International (Camarillo, CA, USA).
Culture of human bone marrow-derived mesenchymal stem cells (MSCs)
Bone marrow samples were obtained from patients who underwent bone marrow aspirates from donor patients. The fraction of bone marrow mononuclear cells was isolated with a density gradient using Ficoll-Paque PLUS. Mononuclear cells were cultured in flasks coated with polystyrene at a concentration of 2 × 105 cm−2 in the following basic media: DMEM + 2 mM glutamax (Gibco) with 20% fetal bovine serum (FBS) and 1× antibiotic–antimycotic (Gibco), incubated at 37°C in a humidified atmosphere containing 5% CO2. The nonadherent cells were discarded after 72 h, and the adherent cells were incubated in fresh medium for an additional 4 days. The medium was replaced every 3 or 4 days. When the flask was 90% confluent, cells were trypsinized by 0.05% trypsin and 0.53 mM ethylenediaminetetraacetic acid (EDTA) at 37°C for 5 min, washed, and resuspended with basic media. Cells were seeded again at 1:4 density ratios and tested by flow cytometry, with positive results for CD13, CD29, CD44, CD90, CD73, and CD105, but negative results for hematopoietic markers such as CD34 and CD45. The study protocol was approved by the IRB, University of Catania, Italy.
Experimental protocols
Undifferentiated MSCs (control group) and cells that underwent osteoblastic differentiation for 7, 14, and 21 days were analyzed in this study. Osteoblastic differentiation of hMSCs was induced by incubation in an osteogenic induction medium (OM): DMEM + 10% fetal calf serum (FCS) + 100 U/ml penicillin + 100 μg/ml streptomycin, 0.2 mM ascorbic acid (Sigma), 0.1 μm dexamethasone (Sigma), and 10 mM β-glycerophosphate (Calbiochem). Medium was changed every 2 days. In addition, treatment with 5 μM tin (Sn4+)-mesoporphyrin 1X-2Cl (SnMP), 30 mM glucose, 0.5 μM cobalt (Co3+)-protoporphyrin 1X-Cl (CoPP), or media changes were applied every 2 days. The conditioned media was harvested after 7, 14, and 21 days of culture, and the levels of markers such as BMP-2, Runx-2, osteocalcin (OCN), osteonectin, alkaline phosphatase activity, OPG (osteoprotegerin), and receptor activator of nuclear factor kappaB ligand (RANKL) were determined by quantitative reverse transcription-polymerase chain reaction (qRT-PCR) and enzyme-linked immunosorbent assay (ELISA). The change in morphology of MSCs during osteoblastic differentiation was evaluated by light microscopy and by alizarin red staining. HO-1, pAMPK, AMPK, eNOS, and PPAR-γ protein expression was also evaluated during osteoblastic differentiation of MSCs at 7, 14, and 21 days, in the presence or absence of CoPP, SnMP, and glucose (30 mM). Cells were tested for their susceptibility to high glucose concentration; ROS release was measured at the start of the experiment and during differentiation. mRNA adiponectin expression was measured by real-time PCR quantification.
Alkaline phosphatase activity
Cells were plated in six-well plates. Cell layers were washed twice with ice-cold phosphate-buffered saline (PBS), then harvested in 1 ml 50 mM Tris–HCl (pH 7.6), sonicated twice on ice, and then centrifuged at 4°C for 15 min at 1000g. The supernatants were stored at −20°C until analysis for alkaline phosphatase activity, using p-nitrophenylphosphate as substrate. Absorbance was read at 405 nm using a microplate reader (Benchmark; Bio-Rad, Hercules, CA, USA). Alkaline phosphatase activity was expressed as nmol p-nitrophenol released/min per μg DNA. All analyses were done in six replicates. Each experiment was repeated two or three times.
Analysis of cultured cell mineralization
Mineralization was determined using alizarin red S (Sigma) staining and phase-contrast microscopy at 21 days after treatment. Cells were instead incubated with 2% alizarin red with pH 4.2 for 10 min and subsequently washed with distilled water. Subcultured cells were observed by phase-contrast microscopy at 21 days to examine cell morphology and to verify the presence of mineralized nodules.
Immunoblot analysis
The cultures were washed with PBS and trypsinized (0.05% trypsin w/v with 0.02% EDTA). The pellets were lysed in buffer [Tris–Cl 50 mM, EDTA 10 mM, Triton X-100 1% v/v, phenylmethylsulfonyl fluoride (PMSF) 1%, pepstatin A 0.05 mM, and leupeptin 0.2 mM] and, after mixing with sample loading buffer [Tris–Cl 50 mM, sodium dodecyl sulfate (SDS) 10% w/v, glycerol 10% v/v, 2-mercaptoethanol 10% v/v, and bromophenol blue 0.04%] in a ratio of 4:1, were boiled for 5 min. Samples (10 μg protein) were loaded onto 12% gels and subjected to electrophoresis (150 V, 80 min). The separated proteins were transferred to nitrocellulose membranes (Bio-Rad; 1 h, 200 mA per gel). After transfer, the blots were incubated overnight with 5% fatfree milk in Tris-buffered saline (TTBS) followed by incubation with 1:1000 dilution of the primary antibody for 3 h. The polyclonal rabbit antibody directed against the human HO-1 was obtained from Stressgen Biotechnologies (Victoria, BC). After washing with TTBS, the blots were incubated for 2 h with secondary antibody (1:5000) and conjugated with alkaline phosphatase. Finally, the blots were developed using a premixed solution containing 0.56 mM 5-bromo-4-chloro-3-indolyl phosphate (BCIP) and 0.48 mM nitro blue tetrazolium (NBT) in buffer (Tris–HCl 10 mM, NaCl 100 mM, MgCl2 59.3 μM, pH 9.5). The blots were scanned, and the optical density of the bands was measured using Scion (New York, NY) Image software.
mRNA isolation
Total RNA was isolated using tryzol (Invitrogen, Carlsbad, CA, USA). First-strand cDNA was synthesized with Roche (Branford, CT, USA) reverse transcription reagents. Total RNA (1 μg) was analyzed by real-time PCR.
Real-time PCR quantification
The quantitative real-time polymerase chain reaction (qRT-PCR) was performed with the TaqMan gene expression assay on an ABI Prism 7900 sequence analyzer according to the manufacturer’s recommended protocol (Applied Biosystems, Foster City, CA, USA). Each reaction was run in triplicate. The comparative threshold cycle (CT) method was used to calculate the amplification fold as specified by the manufacturer. A value of 10 ng of reverse-transcribed RNA samples was amplified by using the TaqMan Universal PCR Master Mix and TaqMan gene expression assays (ID Hs01055564_m1 for human BMP-2, ID Hs00231692_m1 for RUNX2, ID Hs 00605917_m1 for adiponectin, ID Hs00157965_m1 for HO-1, and ID HS99999901_s1 for 18S as an endogenous control; Applied Biosystems).
Detection of ROS
Generation of ROS was assessed using fluorescent probedihydroethidium (DHE) staining (Sigma). In the presence of superoxide (O2−), DHE is oxidized to fluorescent products, which were monitored by flow cytometry (FC500 Beckman Coulter). Briefly, cells were incubated with 20 μM DHE in culture medium for 30 min at 37°C, and then washed, resuspended in PBS, and subsequently monitored by flow cytometry.
Human bone marrow-derived adipocyte mesenchymal stem cells
Frozen bone marrow mononuclear cells were purchased from Allcells (Emeryville, CA, USA). After thawing the cells, mononuclear cells were resuspended in an α-minimal essential medium (α-MEM; Invitrogen) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Invitrogen) and 1% antibiotic/antimycotic solution (Invitrogen). The cells were plated at a density of 1–5 × 106 cells per 100 cm2 dish. The cultures were maintained at 37°C in a 5% CO2 incubator, and the medium was changed after 48 h and every 3–4 days thereafter. When the MSCs were confluent, the cells were recovered by the addition of 0.25% trypsin/EDTA (Life Technologies, Frederick, MD, USA). MSCs (passage 2–3) were plated in a 60 cm2 dish at a density of 1–2 × 104 and cultured in α-MEM with 10% FBS for 7 days. The medium was replaced with adipogenic medium, and the cells were cultured for an additional 21 days. The adipogenic media consisted of complete culture medium supplemented with OM EM-high glucose, 10% (v/v) FBS, 10 μg/ml insulin, 0.5 mM dexamethasone (Sigma-Aldrich), 0.5 mM isobutylmethylxanthine (Sigma-Aldrich), and 0.1 mM indomethacin (Sigma-Aldrich).
Oil red O staining
For oil red O staining, 0.21% oil red O in 100% isopropanol (Sigma-Aldrich) was used. Briefly, adipocytes were fixed in 10% formaldehyde, washed in oil red O for 10 min, rinsed with 60% isopropanol (Sigma-Aldrich), and eluted with oil red O by adding 100% isopropanol for 10 min. Optical density (OD) was measured at 490 nm for a 0.5-s reading.
ELISA assay
By using a specific ELISA test, according the recommendations of the manufacturer, we evaluated the osteocalcin (OCN; BioSource International), OPG, and RANKL levels in the culture supernatant (BioVendor Laboratory Medicine, Modrice, Czech Republic).
Statistical analysis
Differences among the groups were analyzed by the t test and ANOVA. Values were expressed as mean ± SEM, and differences between groups were considered to be significant at P < 0.05.
Results
Differentiation of mesenchymal stem cells in osteoblastic cells
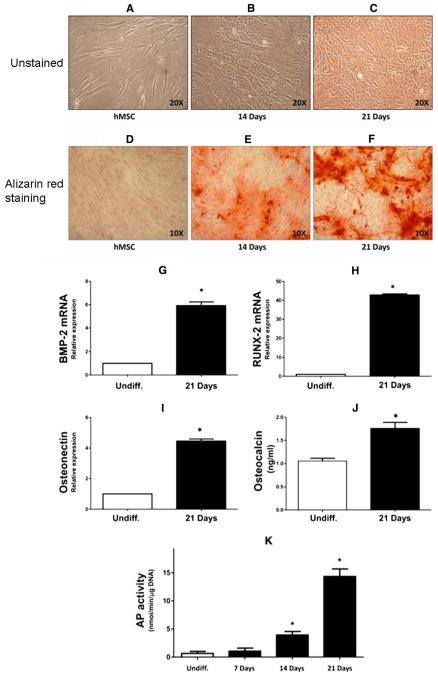
MSCs were cultured in osteogenic differentiation media and collected on days 7, 14, and 21. At 14 days after culture in osteogenic differentiation media, 30% of cells showed osteoblastic cell morphology (Fig. 1b). On day 21, 90% of cells presented typical osteoblastic cell morphology as assessed by light microscopy examination (Fig. 1c). Real-time PCR quantification and ELISA of undifferentiated MSCs and cells at 21 days after culture in differentiation media confirmed that cells were positive for early mature osteoblastic markers such as BMP-2, RUNX-2, osteonectin, and osteocalcin (Fig. 1g–j). Alkaline phosphatase (AP) activity increased in a time-dependent manner during differentiation (Fig. 1k). Furthermore, at 21 days of differentiation, the cultures showed the presence of mineralized nodules following alizarin red staining analysis (Fig. 1d–f). These data support the hypothesis that MSCs can be successfully differentiated into osteoblastic cells when appropriately stimulated in vitro.
Fig. 1.
Osteoblast differentiation and relative markers. a–f Morphological features of undifferentiated and osteoblastic differentiated mesenchymal stem cells (MSCs). Light microscopy analysis of cells after 14 days (b) and 21 days (c) of culture in osteogenic differentiation media shows a gradual increase in the number of osteoblastic cells compared with undifferentiated MSCs (a). d–f Presence of mineralized nodules following alizarin red staining analysis of undifferentiated cells (d) and at 14 (e) and 21 (f) days after culture in osteogenic differentiation media. g–k Expression of osteoblastic markers in differentiated MSCs by quantitative real-time polymerase chain reaction (qRT-PCR) and enzyme-linked immunosorbent assay (ELISA) testing. qRT-PCR reveals a marked increase in bone morphogenetic protein (BMP)-2 (g), Runt-related transcription factor 2 (RUNX-2) (h), and osteonectin (i) at 21 days of osteoblastic differentiation. ELISA shows an increase of osteocalcin secreted in the medium at 21 days with respect to undifferentiated cells (j). k Alkaline phosphatase (AP) activity during osteoblastic differentiation from BM MSCs. Bars represent the mean ± SEM of three independent experiments. *P < 0.05 versus undifferentiated cells; n = 4
HO-1 mRNA and protein expression profile during osteoblastic differentiation from MSCs
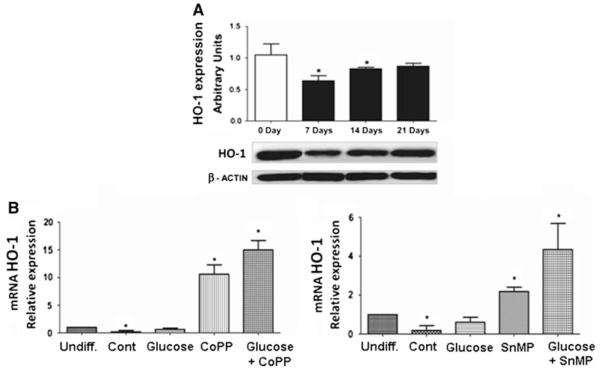
HO-1 mRNA and protein expression during osteoblastic differentiation indicated that HO-1 is significantly downregulated (P < 0.05) at 7 days of culture (Fig. 2a, b), but it was restored and remained constant at days 14 and 21 (Fig. 2a). The presence of high glucose levels had no effect on mRNA, but its level was increased by CoPP, an inducer of HO-1, even in the presence of glucose. These findings suggest that differentiation of MSCs into osteoblastic cells results in a decrease of HO-1 expression. Interestingly, CoPP and SnMP, an inducer and an inhibitor of HO activity, respectively, were able to induce the enzyme expression during differentiation (Fig. 2b). In particular, at 21 days of differentiation CoPP (0.5 μM) was able to increase HO-1 expression by 10.6 fold (P < 0.05) with respect to undifferentiated cells, whereas cells treated with CoPP (0.5 μM) and glucose 30 mM showed an increase of HO-1 expression by 15 fold (P < 0.05) with respect to undifferentiated cells (Fig. 2b, left panel). Treatment with SnMP (5 μM), in the presence or absence of glucose 30 mM, increased HO-1 expression 2.18- and 4.54-fold, respectively, with respect to undifferentiated cells (Fig. 2b, right panel). Finally, in all treated groups (CoPP, SnMP, and high glucose), cell morphology was unchanged.
Fig. 2.
a Development pattern of heme oxygenase (HO)-1 mRNA and protein expression in undifferentiated cells, and at 7, 14, and 21 days. Shown are changes in HO-1 mRNA (upper panel), analyzed by qRT-PCR, and protein expression (lower panel), analyzed by Western blot during osteoblastic differentiation of MSCs. The profile of HO-1 mRNA and protein expression during osteoblastic differentiation shows that HO-1 is significantly downregulated during osteoblastic differentiation. b Effect of CoPP, HO-1 inducers (left panel), and HO inhibitor (SnMP) (right panel) on HO-1 mRNA levels after 21 days of osteoblastic differentiation. Bars represent the mean ± SEM of three independent experiments. *P < 0.05 versus undifferentiated cells (hMSCs); n = 3
The role of the HO system in BMP-2, RUNX-2, and osteonectin expression
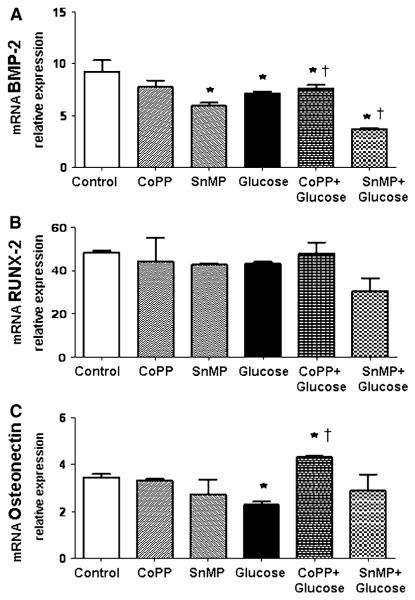
No significant effect of high glucose concentration on RUNX-2 expression during osteoblastic differentiation was detected (Fig. 3a, b). In addition, induction of HO-1 expression following CoPP treatment showed no significant changes in the levels of these markers of differentiation. Osteonectin mRNA during osteoblastic differentiation is significantly downregulated (P < 0.05) following high glucose treatment, whereas CoPP (0.5 μM) and glucose (30 mM) exposure increased osteonectin expression by about threefold (P < 0.05) compared to high glucose treatment and about onefold compared to control (Fig. 3c).
Fig. 3.
Effect of CoPP 0.5 μM, SnMP 5 μM, and glucose 30 mM on BMP-2 (a), RUNX-2 (b), and osteonectin (c) after 21 days of osteoblastic differentiation. Bars represent the mean ± SEM of four independent experiments. *P < 0.05 versus osteogenic medium (OM); †P < 0.05 versus glucose 30 mM (OM + glucose); n = 5
Effect of the HO system and high glucose on ROS formation, osteoprotegerin (OPG), and osteocalcin during differentiation
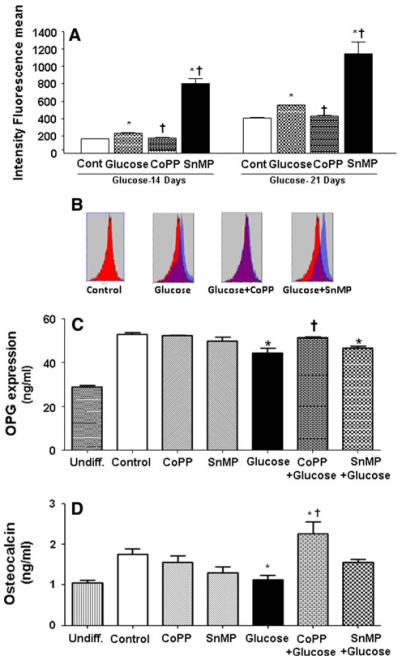
To verify the toxicity of high glucose on osteoblastic differentiation, we quantified levels of reactive oxygen species (ROS) by cytofluorimetric analysis. Osteoblastic differentiation resulted in a slight increase in ROS production commencing at day 14. Furthermore, high glucose increased ROS formation by about 25% with respect to control at both 14 and 21 days of differentiation (Fig. 4a, b). CoPP treatment resulted in a significant (P < 0.05) reduction in ROS release following high glucose at both days 14 and 21, thus restoring values to control levels. Furthermore, ROS formation was increased by SnMP and high glucose treatment with respect to control (Fig. 4a, b). OPG secretion during differentiation showed an increase of ~24 ng/ml with respect to undifferentiated cells, whereas glucose (30 mM) exposure in the presence or absence of SnMP (5 μM) showed a reduction in OPG levels of ~8.5 ng/ml with respect to control. CoPP (0.5 μM) treatment, in the presence of high glucose levels, restored the OPG level to values similar as control (Fig. 4c). Osteocalcin secretion during differentiation showed an increase with respect to undifferentiated cells, whereas glucose (30 mM) exposure showed a significant (P < 0.050) reduction in osteocalcin secretion with respect to differentiated cells (control). CoPP (0.5 μM) treatment in high glucose showed that differentiation was able to induce osteocalcin secretion with respect to exposure to high glucose only (Fig. 4d). These data indicate that increased levels of HO-1 produced an increase in osteocalcin levels when secretion is reduced by high glucose concentration.
Fig. 4.
a Quantification of reactive oxygen species (ROS) by cytofluorimetric analysis during osteoblastic differentiation. Osteoblastic differentiation resulted in a slight ROS production starting from 14 days of differentiation. b ROS emission spectrum obtained after 21 days of osteoblastic differentiation. c Osteoprotegerin (OPG) secretion during differentiation measured in the medium by ELISA. CoPP 0.5 μM treatment in presence of high glucose concentration (OM + glucose + CoPP) is able to restore the OPG level to similar values of OM and osteocalcin secretion. d During differentiation, measured in the medium by ELISA, CoPP 0.5 μM (OM + glucose + CoPP) treatment at high glucose levels, osteoblastic differentiation was able to induce osteocalcin secretion with respect to exposure to high glucose only. CoPP treatment in the presence of glucose 30 mM (OM + glucose + CoPP) shows a decrease of mRNA expression with respect to OM + glucose. Bars represent the mean ± SEM of four independent experiments. *P < 0.05 versus osteogenic medium (OM); †P < 0.05 versus glucose 30 mM (OM + glucose)
Differential effect of HO-1 protein expression and HO activity on adipocyte stem cell differentiation
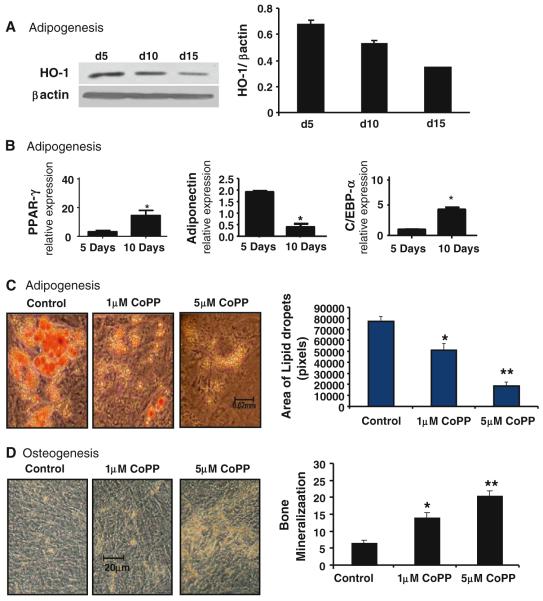
The effects of HO-1 expression on adipocyte stem cell differentiation are shown in Fig. 5a. We show that adipocytes express basal levels of HO-1 (Fig. 5a). HO-1 expression was decreased at days 10 and 15. Adiponectin mRNA levels were also decreased at day 10 when compared to day 5. However, the adipocyte markers PPAR-γ and C/EBP-α were increased during adipogenesis at day 10 (Fig. 5b). We then examined the significance of modulation of HO-1 levels on adipocyte stem cell differentiation by measuring oil red O-stained lipid droplets. As seen, the addition of CoPP to adipocytes resulted in a decrease in the shape and number of lipid droplets compared to control (Fig. 5c). Opposite effects were obtained with osteoblasts (Fig. 5d). These results indicate that upregulation of HO-1 in adipocytes decreases adipogenesis. The opposite was seen in osteoblasts. CoPP treatment decreases adipogenesis in a dose-dependent manner (Fig. 5c). In contrast, CoPP increases osteoblast development and differentiation (Fig. 5d).
Fig. 5.
Effect of increased HO-1 on adipogenesis and osteogenesis in hMSC. a Basal levels of HO-1 in adipogenesis of hMSC on days 5, 10, and 15 were measured by Western blot. b mRNA expression levels of PPAR-γ, adiponectin, and C/EBPα in adipogenesis-treated hMSCs for days 5 and 10, respectively. c hMSCs were also treated with 1 and 5 μM CoPP in adipogenesis media for 14 days. Area and size of lipid droplets were determined by measuring individual lipid droplets (pixel area) from three different fields using ImagePro software. d hMSCs were also treated with 1 and 5 μM CoPP in osteogenesis media for 14 days, and osteocytes were stained with alizarin red. Levels of significance: *P < 0.05, **P < 0.01 control versus CoPP; n = 4
The role of the HO system in eNOS and pAMPK expression
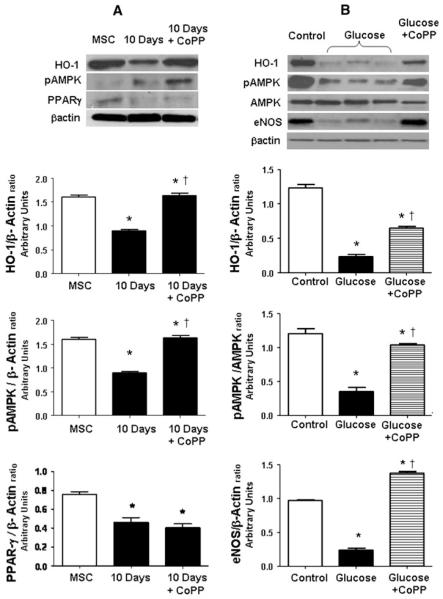
HO-1 protein expression was decreased after 10 days of osteoblastic differentiation, and CoPP treatment was able to increase HO-1 and pAMPK expression (Fig. 6a). Further, osteoblast differentiation under high glucose concentration decreased HO-1 protein (Fig. 6b), and treatment by CoPP was able to increase HO-1 expression with respect to high glucose exposure. Increased HO-1 expression reversed the hyperglycemia-induced suppression and increased pAMPK and eNOS expression (Fig. 6b). These results suggest overproduction of superoxide and subsequent impairment in osteoblast differentiation by a decrease of BMP-2, osteonectin, osteocalcin, and OPG (Fig. 7). Therefore, targeting HO-1 by CoPP increased the levels of osteonectin, OPG, and BMP-2, which may involve increased pAMPK, eNOS, and restored osteoblast markers.
Fig. 6.
a Western blots and densitometer analysis of HO-1, pAMPK, PPAR-γ, and β-actin in undifferentiated cells (MSC) and at 10 days of osteoblast differentiation in presence or absence of CoPP treatment. Bars represent the mean ± SEM of four independent experiments. *P < 0.05 versus undifferentiated cells (MSC); †P < 0.05 versus 10 days. b Western blots and densitometer analysis of HO-1, pAMPK, AMPK, eNOS, and β-actin in differentiated cells (control), in high glucose differentiated cells (glucose), and after CoPP treatment in high glucose osteoblast differentiation. Bars represent the mean ± SEM of four independent experiments. *P < 0.05 versus differentiated cells (control); †P < 0.05 versus glucose; n = 3
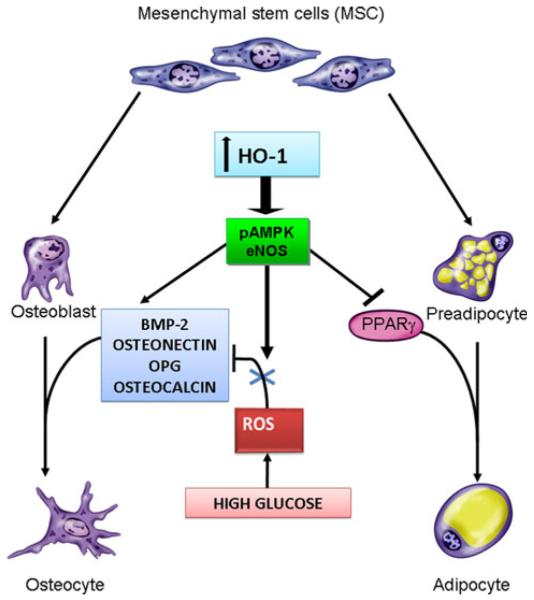
Fig. 7.
Scheme of the mechanisms of HO-1 regulation of osteoblast and adipocyte differentiation. Hyperglycemia causes an increase in ROS, resulting in the overproduction of superoxide and subsequent impairment in osteoblast differentiation by a decrease of BMP-2, osteonectin, osteocalcin, and OPG. Targeting HO-1 by a cobalt compound increased the levels of osteonectin, OPG, and BMP-2. Furthermore, targeting HO-1 expression increased pAMPK and eNOS, reduced ROS formation, and restored osteoblastic markers, suggesting an antiinflammatory or antioxidant role
Discussion
In the present study we show, for the first time, that the upregulation of HO-1 increases MSC-mediated osteoblast lineages but decreases adipocytes. The CoPP-mediated increase in HO-1 levels increases osteoblast proliferation and differentiation and is associated with an increase in osteoblast function via an increase in AKT. A significant increase in CoPP-mediated cell proliferation was observed while, in contrast, SnMP, a competitive inhibitor of HO activity, reversed the CoPP-mediated effect, suggesting that the effect of CoPP was dependent on an increase in both HO-1 expression and HO activity. In addition, osteoblasts cultured in the presence of an inhibitor of HO-1, as in cells exposed to high glucose, exhibited a decrease in the levels of BMP-2, osteonectin, pAMPK, and eNOS. However, upregulation of HO-1 by CoPP in cultured osteoblasts rescued the hyperglycemia-mediated decrease in BMP-2, HO-1, eNOS, and pAMPK. Previous studies have shown that eNOS was expressed in osteoblasts and that a deficiency of this enzyme resulted in a significant reduction in bone formation in mice [33]. Thus, the CoPP-mediated increase in HO-1 and eNOS can be regarded as a pivotal step in bone metabolism through an ability to modulate osteoblast function. eNOS and NO are stimulators of the levels of BMP-2 and increase differentiation of osteoblasts [34, 35].
More recently, we reported that HO-1 overexpression in animal models of both type 1 and type II diabetes attenuates vascular dysfunction via an increase in pAMPK and AKT and a decrease in oxidative stress [19, 36, 37]. Diabetes affects the integrity and functionality of bone tissue [38-40], possibly through increased adiposity [41]. Patients with diabetes frequently show either low bone mass (osteopenia) or increased bone mineral density with an increased risk of fracture and an impairment in bone healing [34], presumably the result of stimulation of osteoblast apoptosis [42], as recently reviewed [43].
Finally, the present data provide a differential effect of HO-1 on MSC-mediated adipocyte stem cells. We investigated the effect of HO-1 expression on differentiation. A clear induction of adipogenic transformation was observed upon exposure of MSC to glucose. The capacity of high glucose to activate adipogenic differentiation has been described in isolated adipocytes [44] and was shown to be dependent on suppression of HO-1. In agreement with these results, glucose increased adipogenesis, and this was associated with the suppression of HO-1 protein levels. We observed, in the present study, the susceptibility of MSCs to high glucose levels, which results in a significant increase in ROS formation commencing on day 14 of differentiation (see Fig. 4a, b). The glucose concentrations used in this study correspond to those reported in healthy individuals (5.5 mM) [45] and to those found in patients with hyperglycemia (glucose = 30 mM) [46]. Glucose has been shown to suppress HO-1 promoter and HO-1 levels [47, 48]. High glucose suppressed HO-1 expression in cell lines [48-50] as well as in animal models [11, 51, 52]. SnMP is known as an inhibitor of HO activity, but it can also increase HO-1 gene expression [19].Thus, fluctuations in osteonectin levels may be regarded as a marker for the onset of oxidative damage in osteoblasts. The understanding that inhibition of HO-1 expression increased the MSC shift toward adipocytes has at least two important conceptual implications. First, high glucose has an adipogenic potential, and a direct link exists between the suppression of HO-1 and the increase in adipogenesis and metabolic syndrome. Second, inhibition of HO-1 increases the ROS need for adipocyte expansion. We recently showed that HO-1 recruits EC-SOD to act as an antioxidant to dissipate H2O2 [51] and in triggering an increase in adiponectin and the signaling pathway pAMPK–pAKT–PPAR-γ. Additionally, Fig. 5 shows that HO-1 expression decreased during differentiation, whereas PPAR-γ levels increased. PPAR-γ is commonly referred to as the master regulator of adipogenesis [53, 54]. Ectopic expression and activation of PPAR-γ are sufficient to induce adipocyte differentiation. Given the role of HO-1 expression in preventing obesity [19], it is possible that the differential role of HO-1 in adipocytes and osteoblast lineage might represent a strategy to curb adiposity and increase osteogenesis.
The results of the present study show that increased HO-1 expression and HO activity are essential for MSC growth to the osteoblast lineage and are consistent with the role of HO-1 in hematopoietic stem cell differentiation [13, 15, 55] in which HO-1 regulates stem cell differentiation to a number of lineages [13-15]. The HO-1/HO-2 system participates in the regulation of cell differentiation in osteoblasts and adipocytes in a cell-specific but very different manner. Although the basal levels of HO-1 protein and HO activity are needed for osteoblast cell growth, an increase in the basal level of HO-1 resulted in the enhancement of osteoblast differentiation. Induction of HO-1 is essential for the resultant increase in pAKT, pAMPK, peNOS level, and NO bioavailability [19, 36]. An increase in NO may be necessary for CoPP-mediated osteoblastic activity [10]; upregulation of NO was shown to play a positive role in bone formation [33-35]. The differential effect of HO-1 in suppression of adipocyte differentiation but increased osteoblast differentiation contributed to the evidence that HO activity and its products, bilirubin, CO, and iron, play a different role in cell proliferation. More recently, it was shown that the elevation of HO-1-derived CO in endothelial cells enhanced endothelial cell proliferation [19, 24, 56]. In contrast, increased HO-1 levels caused a decrease in vascular smooth cells [57]. The effect of HO-1 expression on osteoblasts and adipocytes is mirrored by the effect of HO-1 on endothelial cells and vascular smooth muscle cells [57]. In fact, adipocyte stem cells from both obese rats and mice have low levels of HO-1 protein and HO activity, which may reflect an increase in adiposity [11, 20]. Thus, the site-specific delivery of HO-1 to adipocytes may play a regulatory role in the prevention of adipocyte differentiation in a variety of vascular diseases, including metabolic syndrome [19]. Our results provide direct evidence that HO-1 gene expression has a differential effect on osteoblast and adipocyte cell proliferation and differentiation. Thus, by manipulating the expression of HO-1, it will be possible to attenuate the hyperglycemia-mediated inhibition of osteoblast differentiation while simultaneously inhibiting adipocyte differentiation, thereby offering potential in the management of the metabolic syndrome.
Acknowledgments
This work was supported by NIH grants DK068134, HL55601, and HL34300 (to N.G.A.).
Contributor Information
Ignazio Barbagallo, Department of Biological Chemistry, Medical Chemistry and Molecular Biology, University of Catania, Catania, Italy.
Angelo Vanella, Department of Biological Chemistry, Medical Chemistry and Molecular Biology, University of Catania, Catania, Italy.
Stephen J. Peterson, Department of Medicine, New York Medical College, Valhalla, NY, USA
Dong Hyun Kim, Department of Physiology and Pharmacology, University of Toledo College of Medicine, Health Education Bldg., 3000 Arlington Avenue, Toledo, OH 43614-2598, USA.
Daniele Tibullo, Department of Biomedical Sciences, Section of Hematology, University of Catania, Catania, Italy.
Cesarina Giallongo, Department of Biomedical Sciences, Section of Hematology, University of Catania, Catania, Italy.
Luca Vanella, Department of Physiology and Pharmacology, University of Toledo College of Medicine, Health Education Bldg., 3000 Arlington Avenue, Toledo, OH 43614-2598, USA.
Nunziatina Parrinello, Department of Biomedical Sciences, Section of Hematology, University of Catania, Catania, Italy.
Giuseppe A. Palumbo, Department of Biomedical Sciences, Section of Hematology, University of Catania, Catania, Italy
Francesco Di Raimondo, Department of Biomedical Sciences, Section of Hematology, University of Catania, Catania, Italy.
Nader G. Abraham, Department of Physiology and Pharmacology, University of Toledo College of Medicine, Health Education Bldg., 3000 Arlington Avenue, Toledo, OH 43614-2598, USA; Department of Medicine, New York Medical College, Valhalla, NY, USA
David Asprinio, Department of Orthopedics, New York Medical College, Valhalla, NY, USA.
References
- 1.Ferrari G, Cusella-De AG, Coletta M, Paolucci E, Stornaiuolo A, Cossu G, Mavilio F. Muscle regeneration by bone marrow-derived myogenic progenitors. Science. 1998;279:1528–1530. doi: 10.1126/science.279.5356.1528. [DOI] [PubMed] [Google Scholar]
- 2.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 3.Marie PJ, Fromigue O. Osteogenic differentiation of human marrow-derived mesenchymal stem cells. Regen Med. 2006;1:539–548. doi: 10.2217/17460751.1.4.539. [DOI] [PubMed] [Google Scholar]
- 4.Barbagallo I, Tibullo D, Di RM, Giallongo C, Palumbo GA, Raciti G, Campisi A, Vanella A, Green CJ, Motterlini R. A cytoprotective role for the heme oxygenase-1/CO pathway during neural differentiation of human mesenchymal stem cells. J Neurosci Res. 2008;86:1927–1935. doi: 10.1002/jnr.21660. [DOI] [PubMed] [Google Scholar]
- 5.Hamilton EJ, Rakic V, Davis WA, Chubb SA, Kamber N, Prince RL, Davis TM. Prevalence and predictors of osteopenia and osteoporosis in adults with type 1 diabetes. Diabet Med. 2009;26:45–52. doi: 10.1111/j.1464-5491.2008.02608.x. [DOI] [PubMed] [Google Scholar]
- 6.Fowlkes JL, Bunn RC, Liu L, Wahl EC, Coleman HN, Cockrell GE, Perrien DS, Lumpkin CK, Jr, Thrailkill KM. Runt-related transcription factor 2 (RUNX2) and RUNX2-related osteogenic genes are down-regulated throughout osteogenesis in type 1 diabetes mellitus. Endocrinology. 2008;149:1697–1704. doi: 10.1210/en.2007-1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yaturu S, Humphrey S, Landry C, Jain SK. Decreased bone mineral density in men with metabolic syndrome alone and with type 2 diabetes. Med Sci Monit. 2009;15:CR5–CR9. [PubMed] [Google Scholar]
- 8.Inaba M, Terada M, Koyama H, Yoshida O, Ishimura E, Kawagishi T, Okuno Y, Nishizawa Y, Otani S, Morii H. Influence of high glucose on 1, 25-dihydroxyvitamin D3-induced effect on human osteoblast-like MG-63 cells. J Bone Miner Res. 1995;10:1050–1056. doi: 10.1002/jbmr.5650100709. [DOI] [PubMed] [Google Scholar]
- 9.Terada M, Inaba M, Yano Y, Hasuma T, Nishizawa Y, Morii H, Otani S. Growth-inhibitory effect of a high glucose concentration on osteoblast-like cells. Bone (NY) 1998;22:17–23. doi: 10.1016/s8756-3282(97)00220-2. [DOI] [PubMed] [Google Scholar]
- 10.Bab I, Gazit D, Chorev M, Muhlrad A, Shteyer A, Greenberg Z, Namdar M, Kahn A. Histone H4-related osteogenic growth peptide (OGP): a novel circulating stimulator of osteoblastic activity. EMBO J. 1992;11:1867–1873. doi: 10.1002/j.1460-2075.1992.tb05238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li M, Kim DH, Tsenovoy PL, Peterson SJ, Rezzani R, Rodella LF, Aronow WS, Ikehara S, Abraham NG. Treatment of obese diabetic mice with a heme oxygenase inducer reduces visceral and subcutaneous adiposity, increases adiponectin levels, and improves insulin sensitivity and glucose tolerance. Diabetes. 2008;57:1526–1535. doi: 10.2337/db07-1764. [DOI] [PubMed] [Google Scholar]
- 12.Abraham NG, Li M, Vanella L, Peterson SJ, Ikehara S, Asprinio D. Bone marrow stem cell transplant into intra-bone cavity prevents type 2 diabetes: role of heme oxygenase-adiponectin. J Autoimmun. 2008;30:128–135. doi: 10.1016/j.jaut.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 13.Abraham NG, Nelson JC, Ahmed T, Konwalinka G, Levere RD. Erythropoietin controls heme metabolic enzymes in normal human bone marrow culture. Exp Hematol. 1989;17:908–913. [PubMed] [Google Scholar]
- 14.Abraham NG, Lutton JD, Levere RD. Heme metabolism and erythropoiesis in abnormal iron states: role of delta-aminolevulinic acid synthase and heme oxygenase. Exp Hematol. 1985;13:838–843. [PubMed] [Google Scholar]
- 15.Abraham NG. Molecular regulation: biological role of heme in hematopoiesis. Blood Rev. 1991;5:19–28. doi: 10.1016/0268-960x(91)90004-v. [DOI] [PubMed] [Google Scholar]
- 16.Otterbein LE, Soares MP, Yamashita K, Bach FH. Heme oxygenase-1: unleashing the protective properties of heme. Trends Immunol. 2003;24:449–455. doi: 10.1016/s1471-4906(03)00181-9. [DOI] [PubMed] [Google Scholar]
- 17.Chae HJ, Chin HY, Lee GY, Park HR, Yang SK, Chung HT, Pae HO, Kim HM, Chae SW, Kim HR. Carbon monoxide and nitric oxide protect against tumor necrosis factor-alpha-induced apoptosis in osteoblasts: HO-1 is necessary to mediate the protection. Clin Chim Acta. 2006;365:270–278. doi: 10.1016/j.cca.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 18.Zwerina J, Tzima S, Hayer S, Redlich K, Hoffmann O, Hanslik-Schnabel B, Smolen JS, Kollias G, Schett G. Heme oxygenase 1 (HO-1) regulates osteoclastogenesis and bone resorption. FASEB J. 2005;19:2011–2013. doi: 10.1096/fj.05-4278fje. [DOI] [PubMed] [Google Scholar]
- 19.Abraham NG, Kappas A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol Rev. 2008;60:79–127. doi: 10.1124/pr.107.07104. [DOI] [PubMed] [Google Scholar]
- 20.Peterson SJ, Drummond G, Hyun KD, Li M, Kruger AL, Ikehara S, Abraham NG. L-4F treatment reduces adiposity, increases adiponectin levels and improves insulin sensitivity in obese mice. J Lipid Res. 2008;49:1658–1669. doi: 10.1194/jlr.M800046-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim DH, Burgess AP, Li M, Tsenovoy PL, Addabbo F, McClung JA, Puri N, Abraham NG. Heme oxygenase-mediated increases in adiponectin decrease fat content and inflammatory cytokines, tumor necrosis factor-alpha and interleukin-6 in Zucker rats and reduce adipogenesis in human mesenchymal stem cells. J Pharmacol Exp Ther. 2008;325:833–840. doi: 10.1124/jpet.107.135285. [DOI] [PubMed] [Google Scholar]
- 22.Li Volti G, Wang J, Traganos F, Kappas A, Abraham NG. Differential effect of heme oxygenase-1 in endothelial and smooth muscle cell cycle progression. Biochem Biophys Res Commun. 2002;296:1077–1082. doi: 10.1016/s0006-291x(02)02054-5. [DOI] [PubMed] [Google Scholar]
- 23.Wagener FA, Volk HD, Willis D, Abraham NG, Soares MP, Adema GJ, Figdor CG. Different faces of the heme-heme oxygenase system in inflammation. Pharmacol Rev. 2003;55:551–571. doi: 10.1124/pr.55.3.5. [DOI] [PubMed] [Google Scholar]
- 24.Li Volti G, Sacerdoti D, Sangras B, Vanella A, Mezentsev A, Scapagnini G, Falck JR, Abraham NG. Carbon monoxide signaling in promoting angiogenesis in human microvessel endothelial cells. Antioxid Redox Signal. 2005;7:704–710. doi: 10.1089/ars.2005.7.704. [DOI] [PubMed] [Google Scholar]
- 25.Deramaudt BM, Braunstein S, Remy P, Abraham NG. Gene transfer of human heme oxygenase into coronary endothelial cells potentially promotes angiogenesis. J Cell Biochem. 1998;68:121–127. doi: 10.1002/(sici)1097-4644(19980101)68:1<121::aid-jcb12>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 26.Sabaawy HE, Zhang F, Nguyen X, Elhosseiny A, Nasjletti A, Schwartzman M, Dennery P, Kappas A, Abraham NG. Human heme oxygenase-1 gene transfer lowers blood pressure and promotes growth in spontaneously hypertensive rats. Hypertension. 2001;38:210–215. doi: 10.1161/01.hyp.38.2.210. [DOI] [PubMed] [Google Scholar]
- 27.Kushida T, Quan S, Yang L, Ikehara S, Kappas A, Abraham NG. A significant role for the heme oxygenase-1 gene in endothelial cell cycle progression. Biochem Biophys Res Commun. 2002;291:68–75. doi: 10.1006/bbrc.2002.6403. [DOI] [PubMed] [Google Scholar]
- 28.Zhang B, Tang C, Du J. Changes of heme oxygenase-carbon monoxide system in vascular calcification in rats. Life Sci. 2003;72:1027–1037. doi: 10.1016/s0024-3205(02)02352-4. [DOI] [PubMed] [Google Scholar]
- 29.Hauschka PV, Lian JB, Cole DE, Gundberg CM. Osteocalcin and matrix Gla protein: vitamin K-dependent proteins in bone. Physiol Rev. 1989;69:990–1047. doi: 10.1152/physrev.1989.69.3.990. [DOI] [PubMed] [Google Scholar]
- 30.Price PA. Gla-containing proteins of bone. Connect Tissue Res. 1989;21:51–57. doi: 10.3109/03008208909049995. [DOI] [PubMed] [Google Scholar]
- 31.Achemlal L, Tellal S, Rkiouak F, Nouijai A, Bezza A, Derouiche EM, Ghafir D, El Maghraoui A. Bone metabolism in male patients with type 2 diabetes. Clin Rheumatol. 2005;24:493–496. doi: 10.1007/s10067-004-1070-9. [DOI] [PubMed] [Google Scholar]
- 32.Ferron M, Hinoi E, Karsenty G, Ducy P. Osteocalcin differentially regulates beta cell and adipocyte gene expression and affects the development of metabolic diseases in wild-type mice. Proc Natl Acad Sci USA. 2008;105:5266–5270. doi: 10.1073/pnas.0711119105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Armour KE, Armour KJ, Gallagher ME, Godecke A, Helfrich MH, Reid DM, Ralston SH. Defective bone formation and anabolic response to exogenous estrogen in mice with targeted disruption of endothelial nitric oxide synthase. Endocrinology. 2001;142:760–766. doi: 10.1210/endo.142.2.7977. [DOI] [PubMed] [Google Scholar]
- 34.Strotmeyer ES, Cauley JA, Schwartz AV, Nevitt MC, Resnick HE, Bauer DC, Tylavsky FA, de Rekeneire N, Harris TB, Newman AB. Nontraumatic fracture risk with diabetes mellitus and impaired fasting glucose in older white and black adults: the health, aging, and body composition study. Arch Intern Med. 2005;165:1612–1617. doi: 10.1001/archinte.165.14.1612. [DOI] [PubMed] [Google Scholar]
- 35.Garrett IR, Gutierrez G, Mundy GR. Statins and bone formation. Curr Pharm Des. 2001;7:715–736. doi: 10.2174/1381612013397762. [DOI] [PubMed] [Google Scholar]
- 36.Kruger AL, Peterson SJ, Schwartzman ML, Fusco H, McClung JA, Weiss M, Shenouda S, Goodman AI, Goligorsky MS, Kappas A, Abraham NG. Up-regulation of heme oxygenase provides vascular protection in an animal model of diabetes through its antioxidant and antiapoptotic effects. J Pharmacol Exp Ther. 2006;319:1144–1152. doi: 10.1124/jpet.106.107482. [DOI] [PubMed] [Google Scholar]
- 37.Kruger AL, Peterson S, Turkseven S, Kaminski PM, Zhang FF, Quan S, Wolin MS, Abraham NG. D-4F induces heme oxygenase-1 and extracellular superoxide dismutase, decreases endothelial cell sloughing, and improves vascular reactivity in rat model of diabetes. Circulation. 2005;111:3126–3134. doi: 10.1161/CIRCULATIONAHA.104.517102. [DOI] [PubMed] [Google Scholar]
- 38.Hamada Y, Kitazawa S, Kitazawa R, Fujii H, Kasuga M, Fukagawa M. Histomorphometric analysis of diabetic osteopenia in streptozotocin-induced diabetic mice: a possible role of oxidative stress. Bone (NY) 2007;40:1408–1414. doi: 10.1016/j.bone.2006.12.057. [DOI] [PubMed] [Google Scholar]
- 39.Schwartz AV. Diabetes mellitus: does it affect bone? Calcif Tissue Int. 2003;73:515–519. doi: 10.1007/s00223-003-0023-7. [DOI] [PubMed] [Google Scholar]
- 40.Schwartz AV, Sellmeyer DE, Ensrud KE, Cauley JA, Tabor HK, Schreiner PJ, Jamal SA, Black DM, Cummings SR. Older women with diabetes have an increased risk of fracture: a prospective study. J Clin Endocrinol Metab. 2001;86:32–38. doi: 10.1210/jcem.86.1.7139. [DOI] [PubMed] [Google Scholar]
- 41.Botolin S, Faugere MC, Malluche H, Orth M, Meyer R, McCabe LR. Increased bone adiposity and peroxisomal proliferator-activated receptor-gamma2 expression in type I diabetic mice. Endocrinology. 2005;146:3622–3631. doi: 10.1210/en.2004-1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Al-Mashat HA, Kandru S, Liu R, Behl Y, Desta T, Graves DT. Diabetes enhances mRNA levels of proapoptotic genes and caspase activity, which contribute to impaired healing. Diabetes. 2006;55:487–495. doi: 10.2337/diabetes.55.02.06.db05-1201. [DOI] [PubMed] [Google Scholar]
- 43.Hofbauer LC, Brueck CC, Singh SK, Dobnig H. Osteoporosis in patients with diabetes mellitus. J Bone Miner Res. 2007;22:1317–1328. doi: 10.1359/jbmr.070510. [DOI] [PubMed] [Google Scholar]
- 44.Kinobe RT, Ji Y, Vlahakis JZ, Motterlini R, Brien JF, Szarek WA, Nakatsu K. Effectiveness of novel imidazole-dioxolane heme oxygenase inhibitors in renal proximal tubule epithelial cells. J Pharmacol Exp Ther. 2007;323(3):763–770. doi: 10.1124/jpet.107.119800. [DOI] [PubMed] [Google Scholar]
- 45.Berger AJ, Itzkan I, Feld MS. Feasibility of measuring blood glucose concentration by near-infrared Raman spectroscopy. Spectrochim Acta A Mol Biomol Spectrosc. 1997;53A:287–292. doi: 10.1016/s1386-1425(96)01779-9. [DOI] [PubMed] [Google Scholar]
- 46.Morigi M, Angioletti S, Imberti B, Donadelli R, Micheletti G, Figliuzzi M, Remuzzi A, Zoja C, Remuzzi G. Leukocyte-endothelial interaction is augmented by high glucose concentrations and hyperglycemia in a NF-kB-dependent fashion. J Clin Invest. 1998;101:1905–1915. doi: 10.1172/JCI656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chang SH, Barbosa-Tessmann I, Chen C, Kilberg MS, Agarwal A. Glucose deprivation induces heme oxygenase-1 gene expression by a pathway independent of the unfolded protein response. J Biol Chem. 2002;277:1933–1940. doi: 10.1074/jbc.M108921200. [DOI] [PubMed] [Google Scholar]
- 48.Quan S, Kaminski PM, Yang L, Morita T, Inaba M, Ikehara S, Goodman AI, Wolin MS, Abraham NG. Heme oxygenase-1 prevents superoxide anion-associated endothelial cell sloughing in diabetic rats. Biochem Biophys Res Commun. 2004;315:509–516. doi: 10.1016/j.bbrc.2004.01.086. [DOI] [PubMed] [Google Scholar]
- 49.Chang SH, Garcia J, Melendez JA, Kilberg MS, Agarwal A. Haem oxygenase 1 gene induction by glucose deprivation is mediated by reactive oxygen species via the mitochondrial electron-transport chain. Biochem J. 2003;371:877–885. doi: 10.1042/BJ20021731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abraham NG, Kushida T, McClung J, Weiss M, Quan S, Lafaro R, Darzynkiewicz Z, Wolin M. Heme oxygenase-1 attenuates glucose-mediated cell growth arrest and apoptosis in human microvessel endothelial cells. Circ Res. 2003;93:507–514. doi: 10.1161/01.RES.0000091828.36599.34. [DOI] [PubMed] [Google Scholar]
- 51.Turkseven S, Kruger A, Mingone CJ, Kaminski P, Inaba M, Rodella LF, Ikehara S, Wolin MS, Abraham NG. Antioxidant mechanism of heme oxygenase-1 involves an increase in superoxide dismutase and catalase in experimental diabetes. Am J Physiol Heart Circ Physiol. 2005;289:H701–H707. doi: 10.1152/ajpheart.00024.2005. [DOI] [PubMed] [Google Scholar]
- 52.Di Noia MA, Van DS, Palmieri F, Yang LM, Quan S, Goodman AI, Abraham NG. Heme oxygenase-1 enhances renal mitochondrial transport carriers and cytochrome C oxidase activity in experimental diabetes. J Biol Chem. 2006;281:15687–15693. doi: 10.1074/jbc.M510595200. [DOI] [PubMed] [Google Scholar]
- 53.Schadinger SE, Bucher NL, Schreiber BM, Farmer SR. PPARgamma2 regulates lipogenesis and lipid accumulation in steatotic hepatocytes. Am J Physiol Endocrinol Metab. 2005;288:E1195–E1205. doi: 10.1152/ajpendo.00513.2004. [DOI] [PubMed] [Google Scholar]
- 54.Liu J, Farmer SR. Regulating the balance between peroxisome proliferator-activated receptor gamma and beta-catenin signaling during adipogenesis. A glycogen synthase kinase 3beta phosphorylation-defective mutant of beta-catenin inhibits expression of a subset of adipogenic genes. J Biol Chem. 2004;279:45020–45027. doi: 10.1074/jbc.M407050200. [DOI] [PubMed] [Google Scholar]
- 55.Chertkov JL, Jiang S, Lutton JD, Harrison J, Levere RD, Tiefenthaler M, Abraham NG. The hematopoietic stromal microenvironment promotes retrovirus-mediated gene transfer into hematopoietic stem cells. Stem Cells. 1993;11:218–227. doi: 10.1002/stem.5530110309. [DOI] [PubMed] [Google Scholar]
- 56.Wagener FADTG, Feldman E, de Witte T, Abraham NG. Heme induces the expression of adhesion molecules ICAM-1, VCAM-1, and E selectin in vascular endothelial cells. Proc Soc Exp Biol Med. 1997;216:456–463. doi: 10.3181/00379727-216-44197. [DOI] [PubMed] [Google Scholar]
- 57.Durante W. Heme oxygenase-1 in growth control and its clinical application to vascular disease. J Cell Physiol. 2003;195:373–382. doi: 10.1002/jcp.10274. [DOI] [PubMed] [Google Scholar]