Abstract
Introduction
There are limited data regarding the impact of marijuana (MJ) on cortical development during adolescence. Adolescence is a period of substantial brain maturation and cortical thickness abnormalities may be indicative of disruptions of normal cortical development. This investigation applied cortical-surface based techniques to compare cortical thickness measures in MJ using adolescents compared to non-using controls.
Methods
Eighteen adolescents with heavy MJ use and 18 non-using controls similar in age received MRI scans using a 3T Siemens scanner. Cortical reconstruction and volumetric segmentation was performed with FreeSurfer. Group differences in cortical thickness were assessed using statistical difference maps covarying for age and gender.
Results
Compared to non-users, MJ users had decreased cortical thickness in right caudal middle frontal, bilateral insula and bilateral superior frontal corticies. Marijuana users had increased cortical thickness in the bilateral lingual, right superior temporal, right inferior parietal and left paracentral regions. In the MJ users, negative correlations were found between frontal and lingual regions for urinary cannabinoid levels and between age of onset of use and the right superior frontal gyrus.
Conclusion
This is one of the first studies to evaluate cortical thickness in a group of adolescents with heavy MJ use compared to non-users. Our findings are consistent with prior studies that documented abnormalities in prefrontal and insular regions. Our results suggest that age of regular use may be associated with altered prefrontal cortical gray matter development in adolescents. Furthermore, reduced insular cortical thickness may be a biological marker for increased risk of substance dependence.
Keywords: Marijuana, Adolescents, Imaging, Cortical Thickness
1. INTRODUCTION
There has been a sharp resurgence in adolescent substance abuse since the early 1990’s [1, 2]. Although the effects of illicit drugs on brain structure and function have not been fully characterized, recent findings suggest negative neurobiological consequences of adolescent and young adult substance abuse, including changes in white matter, irregularities in cerebral electrophysiological functioning, and disruptions of homeostatic levels of neurotransmitters and brain metabolites [3–11]. Adolescent brains may be particularly vulnerable to the neurophysiologic effects of illicit substances, as adolescence is a critical period for brain maturation, including synaptic pruning of gray matter [12–15]. Adolescence is also a critical developmental period during which higher cortical functions, such as decision-making, are still developing; thereby rendering adolescence a period of increased vulnerability to substance abuse and rapid drug use escalation [16–20].
At this time, little is known about the impact of marijuana (MJ) use on cortical gray matter development in adolescents. Further, structural imaging studies of adults assessing the impact of MJ use on brain morphometry have produced conflicting results. Some studies have identified no significant anatomical changes associated with MJ use [21–24], while others report findings of cerebral atrophy [25], and decreased concavity of the sulci and thinner sulci in the right frontal lobe [26] in MJ users. One study of adult subjects reported that individuals who started using MJ before age 17 had smaller whole brain volumes, a smaller percentage of cortical gray matter volume and a larger percentage of white matter volume than non-users [27], suggesting earlier age of onset of MJ use may be associated with greater structural brain changes.
Functional magnetic resonance imaging (fMRI) studies in adult MJ users have reported altered activation in prefrontal and insular regions in MJ abusers performing cognitive tasks such as attention [28–31], working memory [32–34], inhibitory control [17, 19, 35, 36] and decision making [16] during acute MJ use, chronic MJ use and abstinence [37]. For example, Kanayama utilized a spatial working memory task to examine brain activity in long-term heavy MJ users and found increased activation in brain regions typically used for spatial working memory tasks such as prefrontal cortex and anterior cingulate compared to non-users [32]. Bolla and colleagues utilized the Iowa Gambling Task, a decision-making task, to evaluate differences in activation patterns in 25-day abstinent MJ users compared to non-users and found dose-related alterations in performance and differences in brain activity between groups in prefrontal regions such as the orbitofrontal cortex (OFC) and the dorsolateral prefrontal cortex (DLPFC) compared to nonusers [16]. Furthermore, Hester and colleagues reported that active chronic MJ users showed a diminished capacity for monitoring their behavior that was associated with hypoactivity in the anterior cingulate (ACC) and right insula compared to non-users on a Go/No-go response inhibition task [19]. Increased levels of hypoactivity in both the ACC and right insula regions were significantly correlated with error-awareness rates in the MJ group [19]. Moreover, numerous functional imaging studies have found both prefrontal and insular activation during conscious drug urges [38–42]. In a study of heavy cigarette smokers a positive correlation was found between intensity of craving and glucose metabolism in the anterior insula bilaterally, DLPFC, and OFC [38]. In an investigation of cocaine related cravings in cocaine dependent participants, increased activation was found in the left insula and ACC when comparing drug use imagery to neutral imagery [39]. In a study of college students at high-risk for alcohol abuse, both alcohol and drug cues produced greater brain activation in the right insula compared to neutral cues [41]. Finally, in an investigation of thirty-eight regular MJ users who were abstinent from use for 72 hours, structures in the reward pathway, including the insula and ACC, demonstrated greater activation in response to a MJ cue as compared with a neutral cue [40]. These findings indicate changes in functionality are present in prefrontal and insular regions in substance abusers. Taken together, prefrontal and insular dysfunction may underlie the abnormal inhibitory control, decision-making and increased cravings that leads to both the initiation and maintenance of aberrant drug use patterns [42–45].
Altered cortical thickness may be indicative of atypical cortical development or disruptions of normative cortical maturation. Previous investigations that have utilized methods to analyze cortical thickness have reported abnormalities in neurodevelopmental disorders such as in Attention-Deficit/Hyperactivity Disorder (ADHD) [46–48] autism spectrum disorder [49], first-episode schizophrenia and in individuals at genetic risk for schizophrenia [50, 51]. Furthermore, recent investigators have applied cortical thickness-based techniques to the study of changes in cortical gray matter from exposure to substances of abuse such as methamphetamine, alcohol, nicotine and MJ [51–54]. For example, in one study, exposure to MJ was associated with cerebral cortical thinning in frontal and parahippocampal region in individuals at moderate to high genetic risk for schizophrenia compared to those at low genetic risk [51]. Therefore, the current investigation used cortical-surface based techniques to compare cortical thickness measures in MJ using adolescents and non-users. Based on previously reported structural and functional imaging studies in adults, we hypothesized that there would be reduced cortical thickness in regions of the prefrontal cortex and insula. Furthermore, because there is growing evidence that age of onset of MJ use is a critical variable in understanding the effects of MJ on structural and neurocognitive impairments [27], we predicted that age of onset would be associated with reduced cortical thickness in both prefrontal and insular regions.
2. MATERIAL and METHODS
2.1 Subjects
The Institutional Review Board at the University of Utah approved this study. All subjects were recruited from the community via local advertisements. Inclusion criteria for all subjects in this analysis were: age 16–19 years old. Inclusion criteria for MJ users included a self-report of heavy MJ use with at least 100 minimum smokes in the previous year. Healthy controls had no DSM-IV Axis I diagnosis based on structured and clinical interviews. Healthy controls had no first-degree family history of bipolar disorder, ADHD, psychosis or any other psychiatric family history. Family history was obtained by clinical interview with participants and/or parents. Exclusion criteria for all subjects included: major sensorimotor handicaps (e.g., deafness, blindness, paralysis); estimated IQ <70 (based on measures of verbal fluency [55] and academic histories) or history of learning disabilities; history of claustrophobia, autism, schizophrenia, anorexia nervosa or bulimia, other drug or alcohol dependence/abuse (during 2 months prior to scan or total past history ≥12 months), active medical or neurological disease, history of ECT; metal fragments or implants; and current pregnancy or lactation. All subjects provided written assent, and their parents (or legal guardians) provided written informed consent for their adolescent’s participation. All adolescents, including non-users, underwent a clinical and diagnostic semi-structured interview by either a board-certified child psychiatrist (MLL) or a trained clinical psychologist (EM). Adolescents under the age of 18 were administered the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Episode (K-SADS-PL) [56] with additional mood onset and offset items derived from the WASH-U K-SADS (K-SADS-PL-W) [57]. The K-SADS-PL is a semi-structured interview used to assess psychiatric disorders in children and adolescents. Since this instrument consists of the K-SADS-PL with supplemental items based on the WASH-U-KADS, we will refer to this instrument as the K-SADS-PL-W. The K-SADS-PL-W was administered to participants under the age of 18. For participants 18 and older, the Structured Clinical Interview for DSM-IV Patient Version (SCID-I/P) [58] was used with the ADHD module from the K-SADS-PL-W. All diagnoses were confirmed via consensus (DYT, MLL, EM). Measures of current psychopathology were obtained using the Profile of Mood States (POMS), [59], the State-Trait Anxiety Inventory (STAI) [60], Hamilton Depression Rating Scale (HAM-D) [61] and the Hamilton Anxiety Scale (HAMA) [62]. The DSM-IV-TR Global Assessment of Functioning (GAF) [63] was used to assess global functioning using a scale from 1 (worst) to 100 (best). All participants underwent a drug screen immediately prior to MRI scanning. Urine samples were subsequently analyzed to obtain urinary cannabinoid levels. In addition, information regarding age of first MJ use, age of regular use, and frequency of MJ use was obtained from all participants via interview and self-report. Total lifetime smoking events was calculated by averaging the number of smoking events per week multiplied by duration of use.
2.2 Magnetic Resonance Imaging
Structural imaging was performed at the Utah Center for Advanced Imaging Research (UCAIR) using a 3T Siemens Trio scanner. Structural acquisitions include a T1-weighted 3D MPRAGE grappa sequence acquired sagittally, with TE/TR/TI=3.38ms/2.0s/1.1s, 8° flip, 256×256 acquisition matrix, 256 mm2 FOV, 160 slices, 1.0 mm slice thickness. The original imaging data were transferred from the scanner in the DICOM format and anonimized.
On first subject specific level analysis, each of the subjects’ cortical thickness was estimated within the FreeSurfer image analysis environment (http://surfer.nmr.mgh.harvard.edu/) [64–66]. First the high resolution T1 MPRAGE volumes were converted to FreeSurfer’s specific format, normalized for intensity and resampled to isotropic voxels of 1 × 1 × 1 mm. Next, the skull was removed from the images using a skull-stripping algorithm [67] and segmented into tissue types. The segmented white matter (WM) volume was used to derive a tessellated surface representing the gray–white boundary. The surface was automatically corrected for topology defects, and expanded to model the pial–gray boundary to produce a second, linked mesh surface. The distance between the gray–white matter boundary and the pial mesh was used to estimate cortical thickness. Individual subject’s cortical thickness were normalized to the spherical-space standard curvature template with a number of deformable procedures including surface inflation and spherical registration that utilized individual cortical folding patterns to match cortical geometry across subjects. The cortex was partitioned using an automated Bayesian segmentation procedure designed to replicate the neuroanatomical parcellation defined by Desikan and colleagues to produce gyral and sulcal cortical thickness measures [68].
The second group specific analysis was done with general linear model (GLM) fit for each vertex, regressing out both age and gender as covariates, in order to test group-wise differences in cortical thickness between MJ adolescent users and non-users. Before GLM fitting, normalized cortical thickness measures were smoothed using a full width half maximum (FWHM) Gaussian kernel of 10 mm, and compared with results for 5 mm, 15 mm, 20 mm, and 25 mm. Group difference z-stat maps were corrected for multiple comparisons across vertices using Gaussian-simulation non-parametric inference testing [69]. Results were considered significant at CWP (cluster wise probability) ≤ 0.001 (10000 simulations, initial cluster-forming threshold at p-uncorrected = 0.05), fully corrected for multiple comparisons.
Each processing step was verified through: a) visual verification of segmentation and label outputs; (b) visual verification of alignment by (i) back-projection of average template sulcal ROIs to individual subject T images and (ii) back-projection of a smaller region of interest (ROI) of significant group difference in the post-central region from the average template to individual subject T1 images; (c) searching for patient outliers in regional cortical thickness measures; and (d) spherical visualization of curvature after alignment.
In order to confirm our findings obtained by the whole-brain clusterwise analysis, we performed a ROI analysis on the cortical thickness measures extracted from the parcellation files constructed from the ‘Destrieux’ cortical atlas [68]. This parcellation scheme (divided the cortex into gyral and sulcal regions) resulted in 156 average cortical thickness measures for both hemispheres. In the present study, it was possible that our 2 samples had unequal variances so we utilized the Welch’s two-sample t-test approach with the number of degrees of freedom (df) estimated with the Satterthwaite’s approximation (shown in 2nd column of table 3). We then identified the 15 most ‘different’ ROIs as measured by p-value (0.05).
Table 3.
The 15 Regions of Interest (ROI) with the Most Significant Cortical Thickness Differences Between Adolescent Marijuana Users and Control Non-Users.
| Average Cortical Thickness in ROIs | p-value | df** | t-statistic |
|---|---|---|---|
| Right Hemisphere | |||
| Sulcal calcarine* | 0.01 | 28.71 | −2.89 |
| Sulcal rectus | 0.05 | 32.07 | 2.02 |
| Sulcal precentral superior-part | 0.09 | 33.68 | −1.75 |
| Gyral insular long | 0.15 | 30.95 | 1.47 |
| Sulcal occipitotemporal lateral | 0.17 | 33.97 | 1.41 |
| Sulcal collateral transverse anterior | 0.19 | 33.89 | 1.33 |
| Gyral cingulate main part | 0.20 | 33.90 | 1.32 |
| Sulcal central insula | 0.22 | 30.44 | 1.27 |
| Sulcal subcentral post | 0.23 | 33.99 | 1.22 |
| Left Hemisphere | |||
| Sulcal central insula* | 0.02 | 33.91 | 2.43 |
| Gyral temporal superior lateral* | 0.04 | 32.92 | 2.09 |
| Sulcal circular insula inferior | 0.10 | 31.55 | 1.72 |
| Sulcal precentral superior part | 0.10 | 32.93 | −1.68 |
| Sulcal paracentral | 0.14 | 33.95 | −1.52 |
| Gyral parietal inferior angular | 0.17 | 33.99 | 1.41 |
ROIs indicative of a rejection of the null hypothesis at the 0.05 significance level.
Degrees of Freedom (df) calculated by Satterthwaite’s approximation
Univariate analyses were performed for total segmented brain volume (TBV), which included ventricles and the cerebellum, controlling for age and gender. Mean cortical thickness values for significant clusters were obtained by creating a ROI around each significant cluster on the statistical map of group differences. Next, each ROI was then mapped onto the analyzed image from each participant in the study and mean cortical thickness measures were extracted. Spearman’s correlations were performed between mean cortical thickness and clinical variables (age, age of regular use, life-time smoking events, and cannabinoid urinary level at time of scanning) for each cluster that differed significantly between diagnostic groups for the clusterwise whole brain analysis.
3. RESULTS
We acquired data from eighteen adolescents (aged 17.8 ± 1.0 years; females: n=2), with heavy MJ use and eighteen non-users similar in age (17.3 ± 0.8 years; females n=6). Verbal fluency was found to be significantly larger (F=5.5, p=0.03) in the MJ group (45.8 ± 9.6) compared to nonusers (37.5 ± 8.7), after covarying for age. All participants were currently enrolled in either high school or college, or had recently graduated high school with plans to attend college in the next 3 months. All participants acknowledged average to above average success in academic achievement. Demographic and clinical characteristics of the groups are shown in Table 1. One MJ subject had a past history of a major depressive episode and was on citalopram, and a second MJ subject had a history of heavy alcohol use lasting less than one year. Three MJ users (16%) also endorsed using alcohol more than once a week, but did not meet criteria for either abuse or dependence. No other MJ subjects had any psychiatric history, used psychotropic medications or had a history of other substance use disorders. Mean age of regular MJ use was 15.7, the average frequency of MJ use was 10.4 ± 8 times/week and the total lifetime smokes was 1346.4 ± 1371.6 (median = 1106, standard error = 323.3, range = 158–5250). The average cannabinoid urine level obtained on the day of MRI scanning was 454.6 ± 351.7 ng/mL.
Table 1.
Demographic and Clinical Characteristics of Adolescent Marijuana Users and Control Non-Users
| MJ (n=18) | HC (n=18) | Between Group Sig. | |
|---|---|---|---|
| Male (%) | 17 (94.4) | 12 (66.6) | 0.04 |
| White, Non-Hispanic (%) | 18 (100) | 12 (66.6) | ≤0.01 |
| Mean (SD*) | Mean (SD) | Between Group Sig. | |
| Age | 17.8 (1.0) | 17.3 (0.8) | ns |
| HAM-D1 | 2.1 (3.0)** | 0.8 (1.4) | ns |
| HAM-A2 | 2.0 (2.7)** | 1.3 (1.9) | ns |
| STAI3 | 63.7 (21.1) | 59.9 (14.8) | ns |
| POMS4 | 18.2 (29.8) | 15.4 (23.7) | ns |
| Verbal Fluency | 45.8 (9.6) | 37.5 (8.7) | 0.03 |
| Age of Regular MJ Use (years) | 15.7 (0.9) | - | - |
| Duration of Heavy MJ use (months) | 18.6 (14.4) | - | - |
| MJ Level (ng/mL) | 454.6 (351.7) | - | - |
| Number of Smoking Events per Week | 10.4 (8.0) | - | - |
| Total Lifetime Smoking Events5 | 1346.4 (1371.6) | - | - |
Hamilton Rating Scale for Depression
Hamilton Rating Scale for Anxiety
State-Trait Anxiety Inventory
Profile of Mood States
Total lifetime smoking events was calculated by averaging the number of smoking events per week multiplied by duration of use.
SD = Standard Deviation
n = 17
3.1 Comparisons of Brain Morphometry
There was no significant difference in total whole brain segmented volume between MJ users (1385.72 ± 103.6 cm3) and non-users (1309.07 ± 103.4 cm3). On clusterwise whole brain analysis, MJ users had decreased cortical thickness in the right caudal middle frontal region, bilateral superior frontal cortex and bilateral insula compared to non-users. Marijuana users had increased cortical thickness in the bilateral lingual, right superior temporal, right inferior and superior parietal and left paracentral regions compared to non-users (See Table 2 for data regarding the clusterwise analysis and Figure 1 showing clusters that were significantly different between groups). In order to verify our findings, thickness measures were extracted from the 156 parcellated brain regions and statistical analyses were performed to determine which 15 brain regions most differentiated between the two groups. We confirmed our results from the whole brain findings for the left central insula (p=0.02). The right calcarine thickness (p=0.01) and left superior-lateral temporal gyral thickness (p=0.04) were also found to significantly distinguish between the two groups (See Table 3).
TABLE 2.
Clusterwise Cortical Thickness Results in Adolescent Marijuana Users and Control Non-Using Participants
| Region | Max | Size [mm] | Talaiarach Coordinates | CWP | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Right Hemisphere | ||||||
| HC > MJ | ||||||
| Insula | 3.7 | 68.9 | 31 | −23 | 14 | ≤0.001 |
| Superior Frontal | 2.6 | 65.5 | 21 | 18 | 45 | ≤0.001 |
| Caudal Middle | ||||||
| Frontal | 2.8 | 37.4 | 29 | 13 | 42 | ≤0.001 |
| MJ > HC | ||||||
| Lingual | −3.3 | 111.0 | 21 | −58 | 3 | ≤0.001 |
| Superior Temporal | −3.3 | 87.7 | 45 | −33 | 13 | ≤0.001 |
| Superior Parietal | −2.3 | 45.1 | 26 | −43 | 59 | ≤0.001 |
| Inferior Parietal | −2.8 | 44.5 | 45 | −49 | 27 | ≤0.001 |
| Left Hemisphere | ||||||
| HC > MJ | ||||||
| Superior Frontal | 2.6 | 172.8 | −9 | 34 | 34 | ≤0.001 |
| Insula | 2.4 | 49.6 | −34 | −13 | 0 | ≤0.001 |
| MJ > HC | ||||||
| Paracentral | −2.8 | 77.1 | −6 | −16 | 67 | ≤0.001 |
| Lingual | −2.4 | 30.5 | −14 | −64 | 4 | ≤0.001 |
Figure 1.
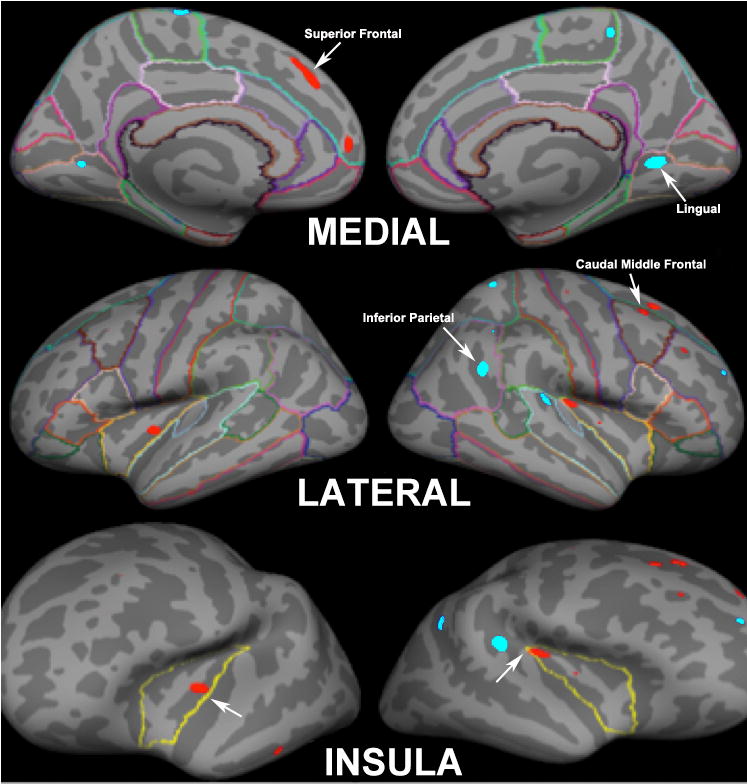
Clusterwise Whole Brain Analysis of Cortical Thickness Between Adolescent Marijuana (MJ) Users and Healthy Controls. Red color indicates cortical thickness is greater in controls compared to MJ users and light blue indicates cortical thickness is greater in MJ users compared to controls.
3.2 Correlations
Mean cortical thickness measures for each individual were extracted from each cluster that was significantly different between groups on whole brain analysis. Spearman’s correlations were performed between the clinical correlates and mean cortical thickness measures for the MJ group. Negative correlations were found between age of regular use and the right superior frontal cortical thickness (r= −0.72, p=0.001). (See Figure 2 for an illustration of the negative correlation of the right superior frontal region and age of regular use). For urinary cannabinoid levels, negative correlations were also found in the right caudal middle frontal (r= −0.63, p=0.01), right lingual (r= −0.63, p=0.01) and left superior frontal gyrus (r= −0.50, p=0.04). Age was not significantly correlated with cortical thickness measures obtained from the significant clusters. Spearman correlations were performed for verbal fluency and significant cortical thickness measures. No significant correlation was found for verbal fluency and any cortical thickness measure in the cannabis group.
Figure 2.

Diagram Showing the Negative Correlation of the Right Superior Frontal Region Cortical Thickness (mm) and Age of Onset of Regular Use (years).
4. DISCUSSION
This is one of the first studies to evaluate cortical thickness in a group of adolescents with heavy MJ use compared to non-users. Compared to non-users, MJ users had reduced cortical thickness in the right caudal middle frontal, bilateral superior frontal cortex and bilateral insula. In addition, MJ users had increased cortical thickness in the bilateral lingual, right superior temporal, right parietal and left paracentral regions compared to non-users. A subsequent ROI analysis provided further evidence that insular thickness was significantly different between groups. Negative correlations were found between age of onset of regular use and the right superior frontal gyrus. Negative correlations were also found in the right caudal middle frontal, right lingual gyrus and left superior frontal regions for urinary cannabinoid levels.
Although we did not detect significant differences in total segmented brain volumes, our findings are consistent with prior morphometric studies in adult MJ users that have documented cortical alterations [25–27]. For instance, in a study by Wilson and colleagues, subjects whose onset of MJ use was before age 17 were found to have smaller whole brain volumes, smaller percent cortical gray matter and larger percent white matter volumes compared to participants who began using MJ after age 17 [27]. In addition, Campbell and colleagues noted cerebral atrophy in adult MJ users measured with air encephalography [25]. Although Mata and colleagues did not find differences in cortical thickness in MJ users compared to non-users, they did find bilaterally decreased concavity of the sulci and thinner sulci in the right frontal lobe in MJ users [26]. In this previous study, healthy controls were also noted to have decreasing gyrification and decreasing cortical thickness with age, which was not noted in MJ users [26]. The authors suggested these findings point to a premature alteration in cortical gyrification in MJ users [26]. The lack of cortical thickness differences between groups in the study by Mata and colleagues as compared to the current study may be related to image resolution associated with scanner strength (3T versus 1.5T), slice thickness (1.0 mm versus 1.5) and methodological differences (whole brain analysis versus lobar level analysis), and the average participant’s age and age range (mean age = 25.7 ± 5.0 versus 17.8 ± 1.0). Additional differences between the two study samples such as frequency of use (26.6×/wk versus 10.4×/week), age of onset of regular use (17.3 versus 15.7), and rates of alcohol (76.7% versus 16%) use may have also contributed to different outcomes between the study performed by Mata and colleagues and this study, respectively [26].
Together, the findings of premature alteration in cortical gyrification from the study by Mata and colleagues [26] and the current study of reduced cortical thickness in frontal and insular brain regions and increased cortical thickness in lingual and temporo-parietal regions suggest there may be differences in the developmental trajectory of adolescents who use MJ. Brain maturation is thought to occur in a back-to-front direction with sensorimotor regions developing first followed by higher-order association areas and lastly, heteromodal association areas found in the prefrontal and lateral temporal cortices [52, 70, 71]. Generally, gray matter maturation occurs via thinning of the cortical mantel and is presumed to be related to synaptic pruning [52, 70, 71]. Our findings of reduced frontal and insular regions suggest that either cortical maturation occurred normally and then there was gray matter tissue loss associated with MJ neurotoxicity or that the MJ adolescent users had an atypical gray matter developmental trajectory and never reached peak cortical thickness as compared to non-users. This atypical cortical development may represent a risk factor for substance use. Furthermore, the increase in cortical thickness measures for lingual and temporo-parietal regions may be related to a premature alteration or delay in cortical thinning in MJ users which is a similar finding to Mata and colleagues [26]. Overall our findings suggest that MJ use does impact cortical brain development in adolescents and that endophenotypic risk makers for substance abuse may be detectable. Cortical thickness-based analytic techniques have been successfully utilized in other neurodevelopmental disorders such as in Attention-Deficit/Hyperactivity Disorder (ADHD) [46–48, 72], autism spectrum disorder [49], and schizophrenia [50, 51] and may be particularly sensitive to the detection of aberrant maturation or atypical patterns of brain development.
Our finding of reduced cortical gray matter in the insula is in line with previous functional imaging studies that have found both abnormal insula activity in abstinent and current adolescent MJ abusers [73, 74] and abnormal insular activation during alcohol and drug urges [42, 75, 76]. Specific to MJ related cravings, one study reported greater activation in the insula in 38 regular adult MJ users, who had abstained for 72 hours, when presented with MJ related cues [76]. Furthermore, in a recent investigation, cigarette smokers with insular lesions were more likely to quite smoking and have reduced cravings to smoke than individuals without insular lesions [77]. In addition, greater right anterior insular gray matter volumes have been correlated with increased accuracy in the subjective sense of the inner body, and with negative emotional experience [78]. Furthermore, Hester and colleagues reported that abnormal insula activity in active chronic MJ users was associated with deficits in behavioral monitoring and error-awareness [19]. Thus it is possible that reduced cortical gray matter in the insula may be associated with the decreased ability to accurately perceive inner subjective or negative emotional states leading to anxiety and increased urges to use [78].
Drug addiction has also been conceptualized as an inability to recognize internal and external drug-related cues [79]. The attenuated awareness of internal and external cues has been linked to insular dysfunction and may lead to the false belief that one does not have a problem with drugs/alcohol and is in control over their substance use behavior despite evidence of impairment [79]. Furthermore, previous studies have linked insular dysfunction with impaired decision making capacity and with the diminished capacity for behavioral monitoring [19, 42–44]. In the current study, cortical insular gray matter was not found to be associated with age of onset measures or total lifetime MJ use, suggesting that reduced insular gray matter may be a trait abnormality. This is consistent with a study by Ray and colleagues who also noted abnormal insula activity may be a biological marker for risk [41]. In this study, alcohol and drug cues produced greater brain activation in the right insula compared to neutral cues in college students at high-risk for alcohol abuse, which is similar to insular activation abnormalities in individuals with substance dependence [41]. Given the role of the insula in both urges and in decision-making, it is unclear whether the observed structural abnormalities in insula structure in the current study of adolescents could reflect a propensity for the development of drug cravings or abnormalities in decision-making capacity, or both.
The finding of a negative correlation with age of onset of regular use in the superior frontal gyrus adds to the growing evidence that age of onset of MJ use is a critical variable in understanding the effects of MJ on structural and neurocognitive measures. Our findings suggest that earlier onset of MJ use may result in a different gray matter developmental trajectory than later onset use as exemplified by having larger cortical thickness measures. In support of our findings, Mata and colleagues also reported that early onset of MJ use may lead to a premature delay in brain maturation [26]. Previous studies have found that MJ users with an age of onset prior to 16 or 17 years of age, have more deficits in cognitive and emotional processes than users with a later onset of use. For example, early-onset MJ use appears to be related to a permanent deficit in visual scanning ability [80, 81]. One study found that individuals who began using MJ before age 16 demonstrated significantly slower reaction times on a visual scanning task than those who used MJ after age 16, suggesting that MJ may interfere with the development of visual processing ability [80]. Becker and colleagues found that earlier age of onset was associated with increased cortical activity in the inferior and superior frontal gyrus, superior temporal gyrus, and the insula while performing verbal working memory task [82]. Pope and colleagues reported that individuals who initiated MJ use prior to age 17 showed poorer cognitive performance, most notably in verbal IQ [83]. Other research has shown that individuals who use MJ before age 18 are more likely to develop substance abuse or dependence than are those who use MJ when they are 18 or older [84]. As discussed earlier, Wilson and colleagues [27] reported smaller whole brain volumes and abnormal gray and white matter content in adolescents with an onset of use prior to age 17 compared to adolescents who began using later. Early onset users also were physically smaller and shorter, and males, displayed significantly elevated cerebral blood flow [27]. Taken together, these previous reports demonstrate that an early age of onset of MJ use may have enduring effects on brain development.
For urinary cannabinoid levels, negative correlations in the right caudal middle frontal and left superior frontal suggests that current severity of cannabis use may preferentially affect cortical thickness in these brain regions leading to tissue loss. Furthermore, a negative correlation was also found for urinary cannabinoid levels for the right lingual gyrus. This finding was surprising as our adolescent MJ users were found to have greater cortical thickness values in this region compared to HC. It is possible that the increased cortical thickness in the lingual gyri in MJ users may be risk markers for current use or severity of use. The lingual gyrus has been linked to visual stimulus processing [85, 86], including early components of facial processing [87, 88], as well as spatial orientation [89]. One recent study also suggests the lingual gyrus as being central for the processing of novel events that occur outside the focus of spatial attention [90]. Interestingly, early-onset MJ use appears to be related to a permanent deficit in visual scanning ability [80, 81]. Furthermore, chronic MJ use has been reported to change response to masked facial affect [91]. Together these findings suggests that MJ may interfere with the development of visual processing ability [80] and potentially to changes in affective interactions. Finally, a study comparing 15 abstinent MJ adolescents with 18 non-using adolescents during a spatial working memory task [34] reported decreased spatial working memory response and increased vigilance response in the MJ group within the lingual gyrus/inferior cuneus and superior portions of the cuneus providing further evidence that lingual gyrus function maybe particularly impaired in MJ users.
Areas of abnormal cortical thickness in key prefrontal brain regions, such as the DLPFC (right caudal middle frontal) and superior frontal corticies may be related to the frontal/executive deficits frequently seen in MJ abusers. For example, investigations of the cognitive effects of MJ following a brief abstinence period have reported that heavy MJ use is associated with deficits in the attentional/executive system [92, 93]. In another investigation of the residual effects of MJ, chronic heavy smokers were enrolled in a 28 day supervised abstinence study using fMRI BOLD techniques and a modified version of the Stroop test [94]. Authors reported that compared to control subjects, heavy MJ smokers demonstrated decreased anterior cingulate activation and increased DLPFC activation during the interference task on Day 1. At Day 28, smokers continued to demonstrate reduced activation within the ACC although the activation within the DLPFC approached levels similar to that of the control subjects. Another investigation utilized a spatial working memory task while comparing 13 adolescents with recent MJ use, 13 adolescents who had been abstinent of MJ use for 27 days, and 18 non-using controls [74]. Relative to abstinent adolescents, those with recent MJ use exhibited increased activation in bilateral insula, medial and left superior prefrontal cortex, bilateral superior frontal gyrus, as well as precentral and inferior frontal gyri. These data suggest increased inhibitory control and working memory updating may be necessary for MJ using adolescents [74].
A recent study of attention with the use of fMRI has also suggested neuroadaptation in the attention network due to chronic MJ exposure [30]. Chang and colleagues administered a visual attention task in 12 active MJ users, 12 abstinent MJ users and 19 comparison subjects. Despite similar task performance between groups, active and abstinent MJ users showed decreased activation in the right prefrontal, medial and dorsal parietal, and medial cerebellar regions, but greater activation in frontal, parietal and occipital brain regions during the visual-attention tasks. Our finding of increased cortical thickness in the bilateral lingual, right superior temporal, right parietal and left paracentral regions in adolescent MJ users compared to non-users supports the idea of neuroadaptation or possible compensatory changes in cortical gray matter in association with MJ use [17, 32, 95]. For instance, Kanayama utilized a spatial working memory task to examine brain activity in long-term heavy MJ users and found increased activation of brain regions typically used for spatial working memory tasks and additional regions not typically used for spatial working memory (such as regions in the basal ganglia) [32]. In another study, Jager and colleagues investigated the effects of adolescent MJ use on working memory and found that MJ users showed excessive activity in prefrontal regions when a task was novel, again suggesting functional compensation [95]. Finally, Gruber and colleagues found that compared to non-users, MJ smokers exhibited different patterns of BOLD response and error response during the Stroop interference condition despite similar task performance [17]. Thus, a number of studies have demonstrated both neuropsychological and neurophysiological changes after MJ use, with some effects lasting after extensive washout of the drug.
Our current findings should be interpreted with care given the modest sample size, group differences in gender and ethnic distributions, cross-sectional nature of the study and the inclusion of youths who were currently using MJ, with presumably different usage patterns. Furthermore, we utilized verbal fluency as an estimate of IQ rather than reporting full-scale IQ scores. Prior studies have found modest to moderate correlations between verbal fluency and estimates of intelligence [55]. Interestingly, our MJ subjects had greater verbal fluency scores, which may have impacted our findings. However, if verbal fluency measures were influencing our results, greater cortical thickness measures would have been expected, particularly in prefrontal and temporal regions in our MJ users, and this was not case [96, 97]. Furthermore, no significant correlation was found for any brain region and verbal fluency in the cannabis group suggesting that differences in IQ were not driving the results of this study. Strengths of this study include the narrow age range of the adolescents and the rigorous clinical assessment of study subjects resulting in the inclusion of adolescents without current comorbid substance abuse disorders and, with the exception of one participant, free of psychiatric comorbidity or history of psychotropic medication use.
5. CONCLUSION
In summary, this is one of the first studies to evaluate cortical thickness in a group of adolescents with heavy MJ use compared to non-users. Our findings of abnormal cortical thickness in prefrontal and insular regions in MJ users compared to non-users is congruent with previous neuroimaging findings that have documented abnormalities in these key brain regions in substance abuse. Although abnormal structure does not imply abnormal function, our findings suggest that abnormal prefrontal and insular cortical gray matter may affect the decision-making capacity and urges to use MJ despite negative consequences. Age of onset of MJ use continues to be an important factor when assessing the impact of MJ on brain substrates and further study is need to determine the long-term and short-term effects of MJ use on brain development. Such studies should include longitudinal and at-risk studies during the critical period of adolescents.
Acknowledgments
This work was supported by research grants from an NIH:1R01 DA020269-01 to DYT and training awards through the American Psychiatric Association’s Program for Minority Research Training in Psychiatry (5T32 MH19126)
None.
Footnotes
AUTHOR DISCLOSURE STATEMENT
Dr. Yurgelun-Todd is a consultant to Kyowa Hakko, Eli Lilly, and Janssen.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bachman JG, Johnson LD, O’Malley PM. Explaining recent increases in students’ marijuana use: impacts of perceived risks and disapproval, 1976 through 1996. Am J Public Health. 1998;88:887–92. doi: 10.2105/ajph.88.6.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weinberg NZ, Rahdert E, Colliver JD, Glantz MD. Adolescent substance abuse: a review of the past 10 years. J Am Acad Child Adolesc Psychiatry. 1998;37:252–61. doi: 10.1097/00004583-199803000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Ennett ST, Flewelling RL, Lindrooth RC, Norton EC. School and neighborhood characteristics associated with school rates of alcohol, cigarette, and marijuana use. J Health Soc Behav. 1997;38:55–71. [PubMed] [Google Scholar]
- 4.Ozaita A, Escriba PV, Ventayol P, Murga C, Mayor F, Jr, Garcia-Sevilla JA. Regulation of G protein-coupled receptor kinase 2 in brains of opiate-treated rats and human opiate addicts. J Neurochem. 1998;70:1249–57. doi: 10.1046/j.1471-4159.1998.70031249.x. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Sevilla JA, Ventayol P, Busquets X, La Harpe R, Walzer C, Guimon J. Marked decrease of immunolabelled 68 kDa neurofilament (NF-L) proteins in brains of opiate addicts. Neuroreport. 1997;8:1561–5. doi: 10.1097/00001756-199705060-00003. [DOI] [PubMed] [Google Scholar]
- 6.Yamanouchi N, Okada S, Kodama K, Hirai S, Sekine H, Murakami A, et al. White matter changes caused by chronic solvent abuse. AJNR Am J Neuroradiol. 1995;16:1643–9. [PMC free article] [PubMed] [Google Scholar]
- 7.Solowij N, Grenyer BF, Chesher G, Lewis J. Biopsychosocial changes associated with cessation of cannabis use: a single case study of acute and chronic cognitive effects, withdrawal and treatment. Life Sci. 1995;56:2127–34. doi: 10.1016/0024-3205(95)00198-f. [DOI] [PubMed] [Google Scholar]
- 8.Dolmatova LS, Ivanets TA. Change in the level of prostaglandins E and glutathione-S-transferase in leukocytes and plasma of hashish addicts. Vopr Med Khim. 1995;41:57–60. [PubMed] [Google Scholar]
- 9.Squeglia LM, Jacobus J, Tapert SF. The influence of substance use on adolescent brain development. Clinical EEG And Neuroscience: Official Journal Of The EEG And Clinical Neuroscience Society (ENCS) 2009;40:31–8. doi: 10.1177/155005940904000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.SA G, Yurgelun-Todd D. Neuropsychological Correlates of Substance Abuse. In: MJ K, editor. Brain Imaging in Substance Abuse: Research, Clinical and Forensic Applications. New York: Elsevier Science; 1999. pp. 199–230. [Google Scholar]
- 11.Bava S, Frank LR, McQueeny T, Schweinsburg BC, Schweinsburg AD, Tapert SF. Altered white matter microstructure in adolescent substance users. Psychiatry Res. 2009;173:228–37. doi: 10.1016/j.pscychresns.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chugani HT. A critical period of brain development: studies of cerebral glucose utilization with PET. Prev Med. 1998;27:184–8. doi: 10.1006/pmed.1998.0274. [DOI] [PubMed] [Google Scholar]
- 13.Giancola PR, Reagin CM, van Weenen RV, Zeichner A. Alcohol-induced stimulation and sedation: relation to physical aggression. J Gen Psychol. 1998;125:297–304. doi: 10.1080/00221309809595339. [DOI] [PubMed] [Google Scholar]
- 14.Moss HB, Talagala SL, Kirisci L. Phosphorus-31 magnetic resonance brain spectroscopy of children at risk for a substance use disorder: preliminary results. Psychiatry Res. 1997;76:101–12. doi: 10.1016/s0925-4927(97)00067-x. [DOI] [PubMed] [Google Scholar]
- 15.Yurgelun-Todd DA, Killgore WD, Young AD. Sex differences in cerebral tissue volume and cognitive performance during adolescence. Psychol Rep. 2002;91:743–57. doi: 10.2466/pr0.2002.91.3.743. [DOI] [PubMed] [Google Scholar]
- 16.Bolla KI, Eldreth DA, Matochik JA, Cadet JL. Neural substrates of faulty decision-making in abstinent marijuana users. Neuroimage. 2005;26:480–92. doi: 10.1016/j.neuroimage.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 17.Gruber SA, Yurgelun-Todd DA. Neuroimaging of marijuana smokers during inhibitory processing: a pilot investigation. Brain Res Cogn Brain Res. 2005;23:107–18. doi: 10.1016/j.cogbrainres.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 18.Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–52. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hester R, Nestor L, Garavan H. Impaired error awareness and anterior cingulate cortex hypoactivity in chronic cannabis users. Neuropsychopharmacology. 2009;34:2450–8. doi: 10.1038/npp.2009.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schweinsburg AD, Brown AB, Tapert SF. The Influence of Marijuana Use on Neurocognitive Functioning in Adolescents. Current Drug Abuse Reviews. 2008;1:99–11. doi: 10.2174/1874473710801010099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Block RI, O’Leary DS, Ehrhardt JC, Augustinack JC, Ghoneim MM, Arndt S, et al. Effects of frequent marijuana use on brain tissue volume and composition. Neuroreport. 2000;11:491–6. doi: 10.1097/00001756-200002280-00013. [DOI] [PubMed] [Google Scholar]
- 22.Delisi LE, Bertisch HC, Szulc KU, Majcher M, Brown K, Bappal A, et al. A preliminary DTI study showing no brain structural change associated with adolescent cannabis use. Harm Reduct J. 2006;3:17. doi: 10.1186/1477-7517-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jager G, Van Hell HH, De Win MM, Kahn RS, Van Den Brink W, Van Ree JM, et al. Effects of frequent cannabis use on hippocampal activity during an associative memory task. Eur Neuropsychopharmacol. 2007;17:289–97. doi: 10.1016/j.euroneuro.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 24.Tzilos GK, Cintron CB, Wood JB, Simpson NS, Young AD, Pope HG, Jr, et al. Lack of hippocampal volume change in long-term heavy cannabis users. Am J Addict. 2005;14:64–72. doi: 10.1080/10550490590899862. [DOI] [PubMed] [Google Scholar]
- 25.Campbell AM, Evans M, Thomson JL, Williams MJ. Cerebral atrophy in young cannabis smokers. Lancet. 1971;2:1219–24. doi: 10.1016/s0140-6736(71)90542-3. [DOI] [PubMed] [Google Scholar]
- 26.Mata I, Perez-Iglesias R, Roiz-Santianez R, Tordesillas-Gutierrez D, Pazos A, Gutierrez A, et al. Gyrification brain abnormalities associated with adolescence and early-adulthood cannabis use. Brain Res. 2010;1317:297–304. doi: 10.1016/j.brainres.2009.12.069. [DOI] [PubMed] [Google Scholar]
- 27.Wilson W, Mathew R, Turkington T, Hawk T, Coleman RE, Provenzale J. Brain morphological changes and early marijuana use: a magnetic resonance and positron emission tomography study. J Addict Dis. 2000;19:1–22. doi: 10.1300/J069v19n01_01. [DOI] [PubMed] [Google Scholar]
- 28.O’Leary DS, Block RI, Koeppel JA, Flaum M, Schultz SK, Andreasen NC, et al. Effects of smoking marijuana on brain perfusion and cognition. Neuropsychopharmacology. 2002;26:802–16. doi: 10.1016/S0893-133X(01)00425-0. [DOI] [PubMed] [Google Scholar]
- 29.O’Leary DS, Block RI, Koeppel JA, Schultz SK, Magnotta VA, Ponto LB, et al. Effects of smoking marijuana on focal attention and brain blood flow. Hum Psychopharmacol. 2007;22:135–48. doi: 10.1002/hup.832. [DOI] [PubMed] [Google Scholar]
- 30.Chang L, Yakupov R, Cloak C, Ernst T. Marijuana use is associated with a reorganized visual-attention network and cerebellar hypoactivation. Brain. 2006;129:1096–112. doi: 10.1093/brain/awl064. [DOI] [PubMed] [Google Scholar]
- 31.Jacobsen LK, Mencl WE, Westerveld M, Pugh KR. Impact of cannabis use on brain function in adolescents. Ann N Y Acad Sci. 2004;1021:384–90. doi: 10.1196/annals.1308.053. [DOI] [PubMed] [Google Scholar]
- 32.Kanayama G, Rogowska J, Pope HG, Gruber SA, Yurgelun-Todd DA. Spatial working memory in heavy cannabis users: a functional magnetic resonance imaging study. Psychopharmacology (Berl) 2004;176:239–47. doi: 10.1007/s00213-004-1885-8. [DOI] [PubMed] [Google Scholar]
- 33.Jacobsen LK, Pugh KR, Constable RT, Westerveld M, Mencl WE. Functional correlates of verbal memory deficits emerging during nicotine withdrawal in abstinent adolescent cannabis users. Biol Psychiatry. 2007;61:31–40. doi: 10.1016/j.biopsych.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 34.Schweinsburg AD, Nagel BJ, Schweinsburg BC, Park A, Theilmann RJ, Tapert SF. Abstinent adolescent marijuana users show altered fMRI response during spatial working memory. Psychiatry Res. 2008;163:40–51. doi: 10.1016/j.pscychresns.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eldreth DA, Matochik JA, Cadet JL, Bolla KI. Abnormal brain activity in prefrontal brain regions in abstinent marijuana users. Neuroimage. 2004;23:914–20. doi: 10.1016/j.neuroimage.2004.07.032. [DOI] [PubMed] [Google Scholar]
- 36.Borgwardt SJ, Allen P, Bhattacharyya S, Fusar-Poli P, Crippa JA, Seal ML, et al. Neural Basis of [Delta]-9-Tetrahydrocannabinol and Cannabidiol: Effects During Response Inhibition. Biological Psychiatry. 2008;64:966–73. doi: 10.1016/j.biopsych.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 37.Martin-Santos R, Fagundo AB, Crippa JA, Atakan Z, Bhattacharyya S, Allen P, et al. Neuroimaging in cannabis use: a systematic review of the literature. Psychol Med. 2010;40:383–98. doi: 10.1017/S0033291709990729. [DOI] [PubMed] [Google Scholar]
- 38.Brody AL, Mandelkern MA, London ED, Childress AR, Lee GS, Bota RG, et al. Brain metabolic changes during cigarette craving. Arch Gen Psychiatry. 2002;59:1162–72. doi: 10.1001/archpsyc.59.12.1162. [DOI] [PubMed] [Google Scholar]
- 39.Kilts CD, Schweitzer JB, Quinn CK, Gross RE, Faber TL, Muhammad F, et al. Neural activity related to drug craving in cocaine addiction. Arch Gen Psychiatry. 2001;58:334–41. doi: 10.1001/archpsyc.58.4.334. [DOI] [PubMed] [Google Scholar]
- 40.Filbey FM, Schacht JP, Myers US, Chavez RS, Hutchison KE. Marijuana craving in the brain. Proc Natl Acad Sci U S A. 2009;106:13016–21. doi: 10.1073/pnas.0903863106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ray S, Hanson C, Hanson SJ, Bates ME. fMRI BOLD response in high-risk college students (part 1): during exposure to alcohol, marijuana, polydrug and emotional picture cues. Alcohol Alcohol. 2010;45:437–43. doi: 10.1093/alcalc/agq042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Naqvi NH, Bechara A. The hidden island of addiction: the insula. Trends Neurosci. 2009;32:56–67. doi: 10.1016/j.tins.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paulus MP. Decision-making dysfunctions in psychiatry--altered homeostatic processing? Science. 2007;318:602–6. doi: 10.1126/science.1142997. [DOI] [PubMed] [Google Scholar]
- 44.Paulus MP, Stein MB. An insular view of anxiety. Biol Psychiatry. 2006;60:383–7. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 45.Churchwell JC, Yurgelun-Todd DA. Neuroimaging, adolescence and risky behavior. In: Bardo M, Fishbein D, Milich R, editors. Inhibitory control and drug abuse prevention: From research to translation. In Press. [Google Scholar]
- 46.Narr KL, Woods RP, Lin J, Kim J, Phillips OR, Del’homme M, et al. Widespread Cortical Thinning Is a Robust Anatomical Marker for Attention-Deficit/Hyperactivity Disorder. J Am Acad Child Adolesc Psychiatry. 2009 doi: 10.1097/CHI.0b013e3181b395c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Almeida LG, Ricardo-Garcell J, Prado H, Barajas L, Fernandez-Bouzas A, Avila D, et al. Reduced right frontal cortical thickness in children, adolescents and adults with ADHD and its correlation to clinical variables: A cross-sectional study. J Psychiatr Res. 2010 doi: 10.1016/j.jpsychires.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 48.Batty MJ, Liddle EB, Pitiot A, Toro R, Groom MJ, Scerif G, et al. Cortical gray matter in attention-deficit/hyperactivity disorder: a structural magnetic resonance imaging study. J Am Acad Child Adolesc Psychiatry. 2010;49:229–38. doi: 10.1016/j.jaac.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wallace GL, Dankner N, Kenworthy L, Giedd JN, Martin A. Age-related temporal and parietal cortical thinning in autism spectrum disorders. Brain. 2010 doi: 10.1093/brain/awq279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schultz CC, Koch K, Wagner G, Roebel M, Schachtzabel C, Gaser C, et al. Reduced cortical thickness in first episode schizophrenia. Schizophr Res. 2010;116:204–9. doi: 10.1016/j.schres.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 51.Habets P, Marcelis M, Gronenschild E, Drukker M, Os JV. Reduced Cortical Thickness as an Outcome of Differential Sensitivity to Environmental Risks in Schizophrenia. Biol Psychiatry. 2010 doi: 10.1016/j.biopsych.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 52.Sowell ER, Mattson SN, Kan E, Thompson PM, Riley EP, Toga AW. Abnormal cortical thickness and brain-behavior correlation patterns in individuals with heavy prenatal alcohol exposure. Cereb Cortex. 2008;18:136–44. doi: 10.1093/cercor/bhm039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lawyer G, Bjerkan PS, Hammarberg A, Jayaram-Lindstrom N, Franck J, Agartz I. Amphetamine dependence and co-morbid alcohol abuse: associations to brain cortical thickness. BMC Pharmacol. 2010;10:5. doi: 10.1186/1471-2210-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kuhn S, Schubert F, Gallinat J. Reduced Thickness of Medial Orbitofrontal Cortex in Smokers. Biol Psychiatry. 2010 doi: 10.1016/j.biopsych.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 55.Strauss E, Sherman EMS, Spreen O. A compendium of neuropsychological tests : administration, norms, and commentary. 3. Oxford ; New York: Oxford University Press; 2006. [Google Scholar]
- 56.Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–8. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 57.Geller B, Zimerman B, Williams M, Bolhofner K, Craney JL, DelBello MP, et al. Reliability of the Washington University in St. Louis Kiddie Schedule for Affective Disorders and Schizophrenia (WASH-U-KSADS) mania and rapid cycling sections. J Am Acad Child Adolesc Psychiatry. 2001;40:450–5. doi: 10.1097/00004583-200104000-00014. [DOI] [PubMed] [Google Scholar]
- 58.First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for the DSM-IV Axis I Disorders. 1996. [Google Scholar]
- 59.McNair DM, Lorr M, Droppleman LF. Revised manual for the Profile of Mood States. San Diego, CA: Educational and Industrial Testing Services; 1992. [Google Scholar]
- 60.Spielberger CD, Gorsuch RL, Lushene RE. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1970. [Google Scholar]
- 61.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hamilton A. British Journal of Psychiatry. 1969. Diagnosis and rating of anxiety; pp. 76–9. Special Publication. [Google Scholar]
- 63.APA. Diagnostic and statistical manual of mental disorders: DSM-IV-TR. 4. Washington, D.C: Author; 2000. Global Assessment of Functioning; p. 32. [Google Scholar]
- 64.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–94. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 65.Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- 66.Fischl B, Sereno MI, Tootell RB, Dale AM. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp. 1999;8:272–84. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Segonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK, et al. A hybrid approach to the skull stripping problem in MRI. Neuroimage. 2004;22:1060–75. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 68.Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–80. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 69.Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101:8174–9. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, et al. Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci. 2008;28:3586–94. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shaw P, Gilliam M, Liverpool M, Weddle C, Malek M, Sharp W, et al. Cortical Development in Typically Developing Children With Symptoms of Hyperactivity and Impulsivity: Support for a Dimensional View of Attention Deficit Hyperactivity Disorder. Am J Psychiatry. 2010 doi: 10.1176/appi.ajp.2010.10030385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tapert SF, Schweinsburg AD, Drummond SP, Paulus MP, Brown SA, Yang TT, et al. Functional MRI of inhibitory processing in abstinent adolescent marijuana users. Psychopharmacology (Berl) 2007;194:173–83. doi: 10.1007/s00213-007-0823-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schweinsburg AD, Schweinsburg BC, Medina KL, McQueeny T, Brown S. The influence of recency of use on fMRI response during spatial working memory in adolescent marijuana users. Journal of Psychoactive Drugs. 2010:42. doi: 10.1080/02791072.2010.10400703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Simpson G, Tate R. Suicidality after traumatic brain injury: demographic, injury and clinical correlates. Psychol Med. 2002;32:687–97. doi: 10.1017/s0033291702005561. [DOI] [PubMed] [Google Scholar]
- 76.Terrio H, Brenner LA, Ivins BJ, Cho JM, Helmick K, Schwab K, et al. Traumatic brain injury screening: preliminary findings in a US Army Brigade Combat Team. J Head Trauma Rehabil. 2009;24:14–23. doi: 10.1097/HTR.0b013e31819581d8. [DOI] [PubMed] [Google Scholar]
- 77.Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315:531–4. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004;7:189–95. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- 79.Goldstein RZ, Craig AD, Bechara A, Garavan H, Childress AR, Paulus MP, et al. The neurocircuitry of impaired insight in drug addiction. Trends Cogn Sci. 2009;13:372–80. doi: 10.1016/j.tics.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ehrenreich H, Rinn T, Kunert HJ, Moeller MR, Poser W, Schilling L, et al. Specific attentional dysfunction in adults following early start of cannabis use. Psychopharmacology (Berl) 1999;142:295–301. doi: 10.1007/s002130050892. [DOI] [PubMed] [Google Scholar]
- 81.Huestegge L, Radach R, Kunert HJ, Heller D. Visual search in long-term cannabis users with early age of onset. Prog Brain Res. 2002;140:377–94. doi: 10.1016/S0079-6123(02)40064-7. [DOI] [PubMed] [Google Scholar]
- 82.Becker B, Wagner D, Gouzoulis-Mayfrank E, Spuentrup E, Daumann J. The impact of early-onset cannabis use on functional brain correlates of working memory. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:837–45. doi: 10.1016/j.pnpbp.2010.03.032. [DOI] [PubMed] [Google Scholar]
- 83.Pope HG, Jr, Gruber AJ, Hudson JI, Cohane G, Huestis MA, Yurgelun-Todd D. Early-onset cannabis use and cognitive deficits: what is the nature of the association? Drug Alcohol Depend. 2003;69:303–10. doi: 10.1016/s0376-8716(02)00334-4. [DOI] [PubMed] [Google Scholar]
- 84.von Sydow K, Lieb R, Pfister H, Höfler M, Sonntag H, Wittchen H-U. The natural course of cannabis use, abuse and dependence over four years: a longitudinal community study of adolescents and young adults. Drug and Alcohol Dependence. 2001;64:347–61. doi: 10.1016/s0376-8716(01)00137-5. [DOI] [PubMed] [Google Scholar]
- 85.Macaluso E, Frith CD, Driver J. Modulation of human visual cortex by crossmodal spatial attention. Science. 2000;289:1206–8. doi: 10.1126/science.289.5482.1206. [DOI] [PubMed] [Google Scholar]
- 86.Bogousslavsky J, Miklossy J, Deruaz JP, Assal G, Regli F. Lingual and fusiform gyri in visual processing: a clinico-pathologic study of superior altitudinal hemianopia. J Neurol Neurosurg Psychiatry. 1987;50:607–14. doi: 10.1136/jnnp.50.5.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mobbs D, Garrett AS, Menon V, Rose FE, Bellugi U, Reiss AL. Anomalous brain activation during face and gaze processing in Williams syndrome. Neurology. 2004;62:2070–6. doi: 10.1212/01.wnl.0000129536.95274.dc. [DOI] [PubMed] [Google Scholar]
- 88.Luks TL, Simpson GV. Preparatory deployment of attention to motion activates higher-order motion-processing brain regions. Neuroimage. 2004;22:1515–22. doi: 10.1016/j.neuroimage.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 89.Committeri G, Galati G, Paradis AL, Pizzamiglio L, Berthoz A, LeBihan D. Reference frames for spatial cognition: different brain areas are involved in viewer-, object-, and landmark-centered judgments about object location. J Cogn Neurosci. 2004;16:1517–35. doi: 10.1162/0898929042568550. [DOI] [PubMed] [Google Scholar]
- 90.Stoppel CM, Boehler CN, Strumpf H, Heinze HJ, Hopf JM, Duzel E, et al. Neural correlates of exemplar novelty processing under different spatial attention conditions. Hum Brain Mapp. 2009;30:3759–71. doi: 10.1002/hbm.20804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gruber SA, Rogowska J, Yurgelun-Todd DA. Altered affective response in marijuana smokers: an FMRI study. Drug Alcohol Depend. 2009;105:139–53. doi: 10.1016/j.drugalcdep.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fletcher JM, Page JB, Francis DJ, Copeland K, Naus MJ, Davis CM, et al. Cognitive correlates of long-term cannabis use in Costa Rican men. Arch Gen Psychiatry. 1996;53:1051–7. doi: 10.1001/archpsyc.1996.01830110089011. [DOI] [PubMed] [Google Scholar]
- 93.Pope HG, Jr, Yurgelun-Todd D. The residual cognitive effects of heavy marijuana use in college students. JAMA. 1996;275:521–7. [PubMed] [Google Scholar]
- 94.Bogorodzki P, Rogowska J, Yurgelun-Todd DA. Structural group classification technique based on regional fMRI BOLD responses. IEEE Trans Med Imaging. 2005;24:389–98. doi: 10.1109/tmi.2004.843183. [DOI] [PubMed] [Google Scholar]
- 95.Jager G, Block RI, Luijten M, Ramsey NF. Cannabis use and memory brain function in adolescent boys: a cross-sectional multicenter functional magnetic resonance imaging study. J Am Acad Child Adolesc Psychiatry. 2010;49:561–72. 72, e1–3. doi: 10.1016/j.jaac.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lange N, Froimowitz MP, Bigler ED, Lainhart JE. Associations between IQ, total and regional brain volumes, and demography in a large normative sample of healthy children and adolescents. Dev Neuropsychol. 2010;35:296–317. doi: 10.1080/87565641003696833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Frangou S, Chitins X, Williams SC. Mapping IQ and gray matter density in healthy young people. Neuroimage. 2004;23:800–5. doi: 10.1016/j.neuroimage.2004.05.027. [DOI] [PubMed] [Google Scholar]


