Abstract
We analyzed HIV viral load (VL) and CD4 count changes, and antibody responses following MMR vaccination of individuals in the U.S. Military HIV Natural History Study cohort. Cases receiving at least one dose of MMR vaccine after HIV diagnosis were matched 1:2 to HIV-positive controls not receiving the vaccine. Baseline was defined as time of vaccination for cases and indexed and matched to the time post-HIV diagnosis for controls. Changes in CD4 count and VL at 6, 12, 18 and 24 months were compared between cases and controls using a general linear model. Available sera from cases were tested for MMR seropositivity at baseline and post-vaccination at 6, 12, 18, and 24 months. Overall mean CD4 count change from baseline through 24 months was 20 (±23) cells/μL greater for cases than controls (p=0.39). Similar non-significant changes in CD4 cell count were seen in the subset of those not on HAART at baseline. VL changes were small and similar between groups (mean differential change −0.04 (±0.18) log10 copies/mL; p=0.84). Of 21 vaccinated participants with baseline serologic testing, 14 (67%) were reactive to measles, 19 (91%) to mumps, and 20 (95%) to rubella. Three (43%) of 7 participants nonreactive to measles developed measles IgG; for mumps, 1 (50%) of 2 developed mumps IgG; for rubella, 1 (100%) developed rubella IgG. MMR vaccination did not result in detrimental immunologic or virologic changes through 24 months post-vaccination.
Keywords: Measles, Mumps, MMR Vaccine, Human Immunodeficiency Virus, Vaccines, Vaccination
Introduction
In the setting of 90–93% coverage with measles, mumps and rubella (MMR) vaccine in the U.S., there continue to be outbreaks of both measles and mumps, highlighting the importance of continued vaccination efforts.[1] In 2007, a Japanese child attending an international youth sporting event in Pennsylvania developed measles. A subsequent investigation of 471 contacts identified 7 additional confirmed cases and 128 contacts, both children and adults, requiring vaccine.[2] The largest U.S. mumps epidemic in almost 20 years occurred in 2006, with 6584 cases reported, largely among college age adults.[3] More recently, an outbreak of mumps occurring at a New York City summer camp in 2009 resulted in 1521 cases.[4] These events serve as a reminder that vaccine-preventable illnesses remain a potential threat, even in a largely immunized population, and raise questions about subpopulations, such as those with HIV infection, which may be more vulnerable despite vaccination.
Despite limited data demonstrating safety and efficacy of MMR vaccination in HIV-infected individuals, the vaccine is currently recommended by both the Advisory Committee on Immunization Practices (ACIP) and the World Health Organization (WHO) for all asymptomatic HIV-infected persons without evidence of severe immunosuppression (defined as CD4 cell count ≤200 cells/μL by ACIP) and for whom measles vaccination would otherwise be indicated, and vaccination should also be considered for mildly symptomatic HIV-infected individuals.[5–7] Several case reports demonstrated severe complications from the use of varicella, vaccinia, Bacille Calmette-Guérin (BCG), and measles vaccines in HIV-infected adults, which lead to concerns about use of live vaccines in HIV-infected individuals with severe immunodeficiency.[8–11] However, more recently, several investigations have reported the safe use of live vaccines in HIV-infected children and adults, specifically with varicella, influenza, BCG, yellow fever, and measles.[12–18] These studies have largely included those with preserved immune function, as reflected by relatively high absolute CD4+ T-cell (CD4) counts, and many individuals who were also treated with HAART.
Investigations of measles, mumps, or rubella vaccines in HIV-infected adults have demonstrated overall safety, but have been limited by the small numbers of subjects (a combined total of 71 individuals, 69% of which were not on HAART), use of different vaccine preparations than those used currently, short durations of follow-up and limited data on HIV viral load (VL) changes following immunization.[12, 16, 19] Other risks may be of concern in those with higher CD4 counts, such as immune activation associated with transient increases in VL and detrimental secondary effects on HIV progression, although studies with various non-live virus vaccines, primarily influenza, have shown that even when demonstrated, changes in CD4 cell count or VL are transient.[20]
While studies of lymphocyte responses in healthy children have shown that measles vaccination results in a significant increase in the absolute CD8+ T-cell (CD8) count, a decrease in the CD4:CD8 ratio, a decrease in percentage of CD4+ T cells (CD4%), and an overall suppression of lymphoproliferative responses that can persist for up to 2–3 years post vaccination,[21–23] similar investigations in HIV-infected adults are limited. In one recent study, there was no detectable change in CD4 counts through 12 months after MMR vaccination of 26 HIV-infected adults, but only 4 subjects were not on HAART.[19] In a case-control study in 1993, 39 HIV-infected prison inmates and 17 healthy controls were vaccinated with measles-rubella vaccine, and no difference in clinical adverse events or alterations in CD4 or CD8 lymphocytes between groups were seen, but follow up was limited to 3 weeks.[16] In 1994, Wallace et al. vaccinated 6 members of the U.S. Navy and Marines against measles and reported their CD4 counts at baseline and at 12 months.[12] Data on VL changes following MMR vaccination are even more limited, with only one study reporting whether subjects on HAART were suppressed or not suppressed.[19] Data regarding those not on HAART to our knowledge are not published. Of note, while vaccine seroresponse rates in HIV-negative adults are approximately 95% for measles,[24] and 79–95% for mumps,[4] the studies highlighted above for HIV-infected adults report suboptimal seroresponse to measles vaccination varying substantially between 0 and 80%. Studies regarding mumps and rubella vaccination seroresponse rates in those with HIV are yet more scarce and primarily limited to children.[15, 16, 25–29]
We conducted an analysis of data and serum samples from HIV-infected U.S. Department of Defense beneficiaries in order to further characterize the effect of MMR vaccination on CD4 counts and on serum VL, and also to describe the prevalence of measles, mumps, and rubella seropositivity and response to MMR vaccination in HIV-infected adults.
Methods
The U.S. Military HIV Natural History Study (NHS) is an ongoing, continuous-enrollment, observational cohort of HIV-infected Department of Defense beneficiaries followed at six military medical centers in the United States and has been previously described.[30] Enrolling since 1986, the NHS has over 5000 participants with signed, written consent. Following enrollment patients are seen every six months. Data collected at each visit includes demographic information, past and interim medical histories and illnesses, medications, vaccinations, and standard clinical laboratory studies. This study was approved by a central institutional review board as designated by the individual centers.
To assess the effect of MMR vaccination on CD4 count and VL, we conducted a retrospective case-control study. Cases were defined as NHS participants who had a documented MMR vaccination following the date of HIV seropositivity with a CD4 count available within 3 months prior to the date of vaccination. HIV-positive controls from the NHS who had not received MMR vaccination after HIV seropositivity were matched to cases in a 2:1 ratio. Baseline was defined as the date of vaccination for cases and matched as an index date to the time post HIV diagnosis for controls. In addition to duration of HIV infection prior to baseline, controls were matched to cases for gender, age at HIV diagnosis within 5 years, baseline CD4 count within 20%, baseline VL within 1 log10, and HAART use (yes/no) at baseline. For 3 cases in which all matching criteria could not be satisfied, gender and HAART use was omitted from the criteria. HAART was defined as any three drug combination expected to be active against HIV-1 similar to previous investigations.[31]
A two-way ANOVA was used to assess the effect of MMR vaccination on changes in CD4 count and VL. At each time point, the case and at least one of two matched controls in each triad required a value for the outcome to be included in the analyses. Thus, cases were compared to controls within each matched triad and pooled. Separate analyses were done for changes in CD4 count, CD4%, CD4:CD8 ratio, and VL at 6, 12, 18, and 24 months. In addition, we performed a longitudinal analysis for each variable using all follow-up data. Since cases tended to be diagnosed with HIV infection later than their matched controls, analyses were also adjusted for year of HIV diagnosis (prior to 1996 versus 1996 or later) using general linear models. We also performed a subset analysis of cases and controls not on HAART at baseline.
To assess baseline seroprevalence of measles, mumps, and rubella and seroresponse to MMR vaccination, available NHS repository specimens from cases were utilized from the following time points: pre-vaccination and ≤ 3, 6, 12, and 24 months post-vaccination (± 3 months). Serum samples from all available time points were thawed at room temperature and tested concurrently using commercially available enzyme immunoassays (EIA) for measles, mumps, and rubella IgG using appropriate controls (Diamedix Corporation, Miami, Florida).[32–34] Seropositivity at baseline was defined as an IgG index value (IV) of ≥ 1.00 for measles and rubella, and ≥ 1.10 for mumps. Response to vaccination at any point through 24 months after vaccination was assessed using two different definitions, evaluated independently: 1) development of a reactive EIA in a participant who was nonreactive prior to baseline and 2) using an index ratio (post-vaccination IV/baseline IV) cut-off which was known to correspond to a four-fold increase in antigen-specific IgG in both seropositive and seronegative participants. Definition 2 of a vaccine response was evaluated because a significant number of participants were expected to have positive baseline EIA results due to receipt of MMR vaccine prior to HIV diagnosis. The index ratios indicating a positive response to vaccine according to the package inserts were ≥2.1 for measles, ≥1.6 for mumps, and ≥2.6 for rubella. The maximum index value in the linear range for each assay was 5.0 for measles and rubella, and 4.1 for mumps. Therefore, the index ratio could not be calculated (i.e. the antigen seroresponse under definition 2 could not be assessed) when either the baseline or post-vaccination IV was greater than the linear range limit. Vaccine response rates were reported with 95% confidence intervals (CI). Statistical analyses were performed using SAS software (version 9.2).
Results
63 patients were identified as having received the MMR vaccine after documented HIV seropositivity. Of those, 49 had CD4 count determination within 3 months prior to vaccination and were included in the analysis. Using pre-specified criteria, 98 controls were identified and matched to immunized participants (Table 1). Year of immunization ranged from 1990–2008 (median 2001). Baseline VL was unavailable for 7 cases, most of whom were immunized prior to 1996 when VL assays were not clinically available. The results of the matching produced cases and controls with similar levels of CD4 and VL at baseline. Other factors were also similar.
Table 1.
Characteristics of MMR vaccinated cases and controls.
| Variable | Vaccinated (n=49)a | Controls (n=98)a | pb |
|---|---|---|---|
| Matching criteria: | |||
| Age at HIV diagnosis (yrs)c | 28.7±5.3 | 28.6±5.6 | 0.92 |
| Duration of HIV at baseline (yrs)c | 5.4±4.6 | 5.4±4.6 | 1.00 |
| Male genderd | 46 (94) | 95 (97) | 0.63e |
| CD4+ T-cells/μL at baselinec | 540.4±279.4 | 530.3±260.4 | 0.83 |
| Less than 200d | 5 (10) | 7 (7) | 0.73e |
| 200–500d | 20 (41) | 39 (40) | 0.91 |
| Greater than 500d | 24 (49) | 52 (53) | 0.64 |
| HIV viral load at baseline (log10 copies/mL)c | 2.8±1.2 (n=42) | 2.6±1.1 (n=65) | 0.42 |
| Undetectabled,f | 20 (48) | 37 (57) | 0.35 |
| HAART use at baselined | 29 (59) | 54 (55) | 0.63 |
| Other variables: | |||
| HIV diagnosis prior to 1996d | 24 (49) | 63 (64) | 0.08 |
| CD4+ T- cells/μL at diagnosisc | 583.8±262.5 | 565.1±237.1 | 0.69 |
| Time on HAART (yrs)c | 3.8±3.2 (n=29) | 3.7±3.0 (n=54) | 0.89 |
| Born prior to 1957d | 3 (6) | 12 (12) | 0.25 |
| Ethnicity:d | 0.64 | ||
| African-American | 23 (47) | 38 (39) | |
| Caucasian | 20 (41) | 46 (47) | |
| Other | 6 (12) | 14 (14) | |
Values represent those from total cases or controls unless otherwise indicated
p-values are 2-tailed and by Mantel-Haenszel chi square unless otherwise indicated
Time, CD4, and viral load data presented as mean±SD
Values expressed as n(%)
by Fisher’s exact test
Lower limits of detectable were <50 or <400 copies/mL depending on date/generation of assay
CD4+ T-cell count and HIV viral load changes
CD4 count trends over time are shown in Figure 1. Unadjusted differential changes (cases minus controls ± standard error) in CD4 count from baseline were +2 ±25 cells/μL (p=0.93) at 6 months, +59 ±36 cells/μL (p=0.11) at 12 months, +108 ±33 cells/μL (p<0.01) at 18 months, and −32 ±46 cells/μL (p=0.49) at 24 months (Table 2). Over the entire follow-up period, the mean change in CD4 count from baseline was 20 ±23 cells/μL greater for cases than controls (p=0.39). After adjustment for HIV diagnosis prior to and after the advent of HAART, differential CD4 count changes were similar; overall, the differential change was 12 ±24 cells/μL (p=0.62). Similar patterns of relative changes were seen in CD4 percent and CD4/CD8 ratio. Analyses for the subgroup of participants not on HAART at baseline (n=20) yielded similar results, an average relative change of −2 ±36 cells/μL (p=0.34).
Figure 1.
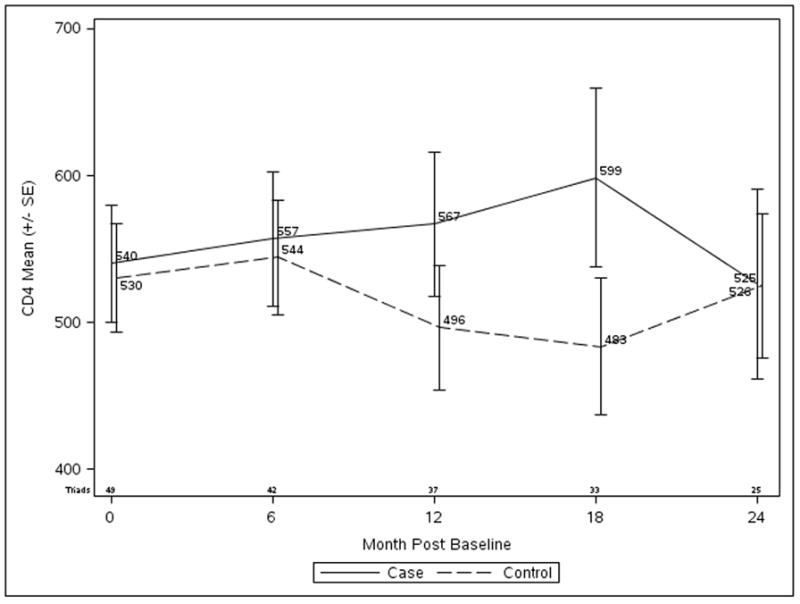
Changes in absolute CD4+ T-cell count in MMR vaccinated cases (solid line) compared to unvaccinated controls (dashed line).
Table 2.
Changes in Immunologic Parameters and HIV-1 Viral Load for MMR vaccinated cases and controls.a
| Unadjusted Model | Adjusted for HAART era | |||||||
|---|---|---|---|---|---|---|---|---|
| Visit | Nb | Vaccinatedc | Controlsc | Differenced | p | Differenced | p | |
| Absolute CD4 count (cells/μL) | Baselinee | 49 | 540 ± 279 | 530 ± 259 | 10 ± 9 | 0.26 | 6 ± 9 | 0.49 |
| 6 months | 42 | +8 ± 121 | +6 ± 122 | 2 ± 25 | 0.93 | −3 ± 26 | 0.92 | |
| 12 months | 37 | +43 ± 138 | −16 ± 163 | 59 ± 36 | 0.11 | 56 ± 38 | 0.15 | |
| 18 months | 32 | +74 ± 165 | −32 ± 133 | 108 ± 33 | <0.01 | 96 ± 32 | <0.01 | |
| 24 months | 25 | −25 ± 191 | +4 ± 178 | −32 ± 46 | 0.49 | −61 ± 46 | 0.20 | |
| Averagef | 48 | +16 ± 134 | −4 ± 133 | 20 ± 23 | 0.39 | 12 ± 24 | 0.62 | |
| CD4%g | Baseline | 49 | 25.9 ± 9.7 | 26.7 ± 9.3 | −0.9 ± 1.1 | 0.44 | −1.5 ± 1.1 | 0.20 |
| 6 months | 42 | +0.7 ± 4.6 | +0.2 ± 3.0 | 0.6 ± 0.8 | 0.47 | 0.5 ± 0.9 | 0.56 | |
| 12 months | 37 | +0.5 ± 5.8 | +0.0 ± 3.4 | 0.5 ± 1.1 | 0.65 | 0.7 ± 1.2 | 0.58 | |
| 18 months | 32 | +1.5 ± 5.5 | −0.4 ± 5.4 | 2.1 ± 1.4 | 0.16 | 1.9 ± 1.5 | 0.19 | |
| 24 months | 25 | −0.7 ± 7.0 | −1.0 ± 5.5 | 0.4 ± 1.5 | 0.82 | −0.3 ± 1.6 | 0.85 | |
| Average | 48 | +0.3 ± 5.1 | −0.1 ± 3.5 | 0.5 ± 0.9 | 0.61 | 0.3 ± 0.9 | 0.73 | |
| CD4/CD8 ratio | Baseline | 49 | 0.63±0.40 | 0.61±0.31 | 0.01±0.05 | 0.87 | −0.03 ± 0.05 | 0.56 |
| 6 months | 42 | +0.02±0.20 | +0.00±0.09 | 0.02±0.03 | 0.49 | 0.03 ± 0.03 | 0.4 | |
| 12 months | 37 | +0.05±0.28 | +0.04±0.14 | 0.01±0.06 | 0.88 | 0.02 ± 0.06 | 0.71 | |
| 18 months | 32 | +0.09±0.20 | −0.01±0.18 | 0.10±0.06 | 0.07 | 0.11 ± 0.06 | 0.07 | |
| 24 months | 25 | +0.05±0.30 | −0.04±0.19 | 0.09±0.07 | 0.21 | 0.08 ± 0.07 | 0.29 | |
| Average | 48 | +0.03±0.23 | +0.01±0.11 | 0.02±0.04 | 0.66 | 0.02 ± 0.04 | 0.54 | |
| Absolute CD4 count (cells/μL) limited to those not on HAART | Baseline | 20 | 509±265 | 487±239 | 22 ± 13 | 0.10 | 16 ± 15 | 0.32 |
| 6 months | 17 | +11±130 | −17±105 | 28 ± 38 | 0.47 | −15 ± 44 | 0.74 | |
| 12 months | 15 | −1±122 | −42±171 | 40 ± 45 | 0.38 | 37 ± 56 | 0.51 | |
| 18 months | 14 | +19±149 | −68±141 | 97 ± 52 | 0.08 | 70 ± 60 | 0.26 | |
| 24 months | 11 | −40±167 | −43±211 | −6 ± 54 | 0.91 | −64 ± 65 | 0.34 | |
| Average | 20 | −4±121 | −35±126 | 31 ± 31 | 0.32 | −2 ± 36 | 0.96 | |
| HIV viral load (log10 copies/mL) | Baseline | 32 | 2.57±1.19 | 2.55±1.10 | 0.03±0.11 | 0.80 | 0.03 ± 0.11 | 0.81 |
| 6 months | 28 | −0.09±1.14 | −0.02±0.29 | −0.07±0.17 | 0.67 | −0.07 ± 0.17 | 0.66 | |
| 12 months | 24 | −0.20±1.49 | −0.19±0.58 | −0.03±0.27 | 0.91 | −0.08 ± 0.28 | 0.78 | |
| 18 months | 20 | −0.07±1.15 | +0.10±0.75 | −0.17±0.31 | 0.59 | −0.18 ± 0.32 | 0.57 | |
| 24 months | 16 | +0.08±1.58 | +0.00±1.07 | 0.16±0.40 | 0.70 | 0.16 ± 0.41 | 0.70 | |
| Average | 31 | −0.08±1.23 | −0.08±0.39 | −0.04±0.18 | 0.84 | −0.04 ± 0.18 | 0.82 | |
Interval changes from baseline
Number of case/control combinations
Values presented as mean ± SD
Case minus control, estimated by general linear model, presented as mean ± SE
Baseline defined as time of vaccination for cases and index visit for matched controls
Average of interval changes
Percentage of total T-lymphocytes which are CD4+
Overall VL measurements were similar for cases and controls with an average differential change of −0.04 ±0.18 log10 copies/mL over the follow up period (p=0.82; Table 2). Analysis of the subset of participants not receiving HAART again showed no significant differences (data not shown).
Of the 5 cases with CD4 count <200 cells/μL at baseline, all were alive at 24 months and one had been hospitalized for Pneumocystis jiroveci pneumonia at 6 months. Three of the 5 were on HAART at the time of MMR immunization. Pre-immunization CD4 counts ranged from 125 to 188 cells/μL with 6 month post-immunization CD4 cell counts ranging from 26 to 243 cells/μL (p=0.66 by paired t-test).
Seropositivity to measles, mumps, and rubella and responses to vaccination
Twenty-one (43%) cases had frozen repository samples available for serologic testing at baseline (Table 3). The mean CD4 count in this group was 620 ± 231 cells/μL; none were less than 200 cells/μL and 9 (43%) were less than 500 cells/μL. Fourteen (67%) were on HAART and 11 (52%) had undetectable VL. Fourteen (67%; 95% CI, 47–87), 19 (91%; 95% CI, 78–100), and 20 (95%; 95% CI, 86–100) were seropositive at baseline for measles, mumps, and rubella, respectively. Three (43%) of the 7 cases seronegative for measles and 1 case each for mumps and rubella developed a positive IgG following vaccination (vaccine response Definition 1). No subject was seronegative to more than one vaccine component at baseline and no patient who was initially seropositive lost immunity during the study period (data not shown). Not all 21 cases with available sera could be assessed for vaccine response using Definition 2, assessing fold-change in IgG, due to EIA reactivity above the linear range at baseline (5 (24%) for measles, 9 (43%) for mumps, and 4 (19%) for rubella). For those in whom vaccine response could be assessed using Definition 2, response rates were low: 13% for measles, 17% for mumps, and 12% for rubella (Table 3).
Table 3.
Baseline Seroprevalence to measles, mumps, and rubella and vaccine responses among MMR vaccinated cases with available sera for testing (N=21).
| Definition 1 | Definition 2 | ||||
|---|---|---|---|---|---|
| Antigen | Baseline Seropositivea | Nb | Categorical Responsea | Nc | Paired Sera Responsea |
| Measles | 14(67) | 7 | 3(43) | 16 | 2(13) |
| Mumps | 19(91) | 2 | 1(50) | 12 | 2(17) |
| Rubella | 20(95) | 1 | 1(100) | 17 | 2(12) |
Expressed as N(%).
Baseline seronegative samples included in analysis for definition 1.
Results above linear range for EIA, thus uninterpretable, in 5 (24%) measles, 9 (43%) mumps, and 4 (19%) rubella.
Discussion
Our retrospective cohort study is the first reported to compare effects of MMR vaccination on CD4 count in HIV-infected adults with HIV-infected controls not receiving the vaccine. Belaunzarán-Zamudio et al. showed a trend toward an increased mean CD4 count following MMR vaccination, but without a control group for comparison it was unclear whether the observed CD4 count trend was related to vaccination. In our study, where vaccinated cases and unvaccinated controls were matched for several variables including baseline CD4 count, VL, and HAART use we found a similar trend in our vaccinated participants. These findings were associated with parallel changes in the CD4:CD8 ratio and CD4%, although the latter was not statistically significant. This may indicate an overall beneficial effect of vaccination, but given previous studies showing the opposite effect in healthy children and considering the difference was not observed until a full 18 months post-vaccination, it would seem more likely that this was due to confounding, such as possible differences in the potency of HAART regimens or other factors. We attempted to correct for this difference by adjusting our analyses for era of HIV diagnosis and found little change in the results. Despite our somewhat small sample size, our results suggest that any impact of MMR vaccination on CD4 cell count is likely to be small and unlikely to be detrimental.
While co-infections and vaccinations are often associated with transient increases in HIV VL,[35] HIV replication has ironically been shown to be transiently suppressed in the acute phase of measles infection in children through measles virus-induced inhibition of lymphocyte proliferation.[36–39] From our study, it appears that MMR vaccination does not significantly impact VL, although we were limited by low numbers of subjects not receiving HAART, by unavailable VL data during the follow-up period, and by long follow-up intervals that may have missed short-term effects of vaccination. This is, however, in agreement with other studies reporting a similar lack of VL change after measles-rubella vaccination in 11 Ugandan children[40] and continued viral suppression in HAART-treated adults after MMR vaccination.[19]
We found a relatively high seroprevalence to mumps and rubella, likely reflective of immunization practices in the United States, both for the general population and the military. From 1989 until recently, the majority of U.S. military service members have received MMR immunization at the time of enlistment regardless of prior vaccination.[41] As many of our study participants entered military service after 1989, many likely received at least 1 MMR dose prior to HIV diagnosis. The high seroprevalence to mumps and rubella (both greater than 90%) suggests that adults vaccinated (or exposed, as 3 of our cases were born before 1957) prior to acquisition of HIV infection may retain immunity.
Unlike the high rates for mumps and rubella, however, we found a seroprevalence rate of 67% for measles. Previous studies have estimated that up to 12% of HIV-infected adults in the U.S. lack antibody to measles virus, and would therefore be susceptible to infection.[42–44] Our low observed rate of measles seroprevalence was unexpected, but our sample size was also relatively limited, yielding a wide 95% confidence interval with an upper bound which approached reported estimates. However, when considering that we captured only those subjects who were vaccinated, one might also consider it somewhat surprising that the majority were seropositive for measles prior to immunization. Considering the rates of baseline seroprevalence for all three antigens, screening HIV-infected adults in the U.S. military for seropositivity to vaccine antigens prior to immunization may be justifiable. Such a practice may also be reasonable for other HIV-infected adults in the US with relatively preserved CD4 counts who likely received MMR vaccine prior to HIV diagnosis.
While data on mumps seroprevalence among HIV-infected adults in the U.S. has not been previously reported, the seroprevalence rate of 91% which we found was similar to that of the general U.S. adult population.[3, 45] This is marginally sufficient for the estimated 88–92% threshold required for mumps herd immunity. In light of recent mumps outbreaks, some have proposed providing additional doses of vaccine as a possible control measure despite high seroprevalence.[3] Unfortunately, the low response rate to mumps vaccine that we observed in HIV-infected adults, many of whom were already seropositive, may preclude use of this as a strategy for preventing mumps infection in those with HIV or controlling an outbreak in this patient population, although our results should be confirmed.
We found low response rates to all antigens regardless of the definition used. Previous reports addressing antibody response to measles vaccination in HIV-infected adults have been limited by small numbers and difficult to compare due to differing definitions of seroresponse. Wallace et al. reported only two of six previously-vaccinated, yet measles-seronegative HIV-infected men responded to revaccination,[12] whereas Sprauer et al. reported serologic response in none of 39 HIV-infected inmates vaccinated with measles-rubella.[16] Both studies were prior to the HAART era. In a more recent study where 85% of subjects were treated with HAART, greater than 80% of HIV-infected subjects had developed a seroresponse at 3 months, although the majority lost protective antibody titers by 12 months.[19] Wallace et al. and Belaunzarán-Zamudio et al. defined seroresponse as change from negative to positive EIA result (similar to our Definition 1), which may overestimate the true immune response, while Sprauer et al. used paired sera to define response (similar to our Definition 2), which may underestimate the true immune response. It is therefore likely, that differences in the definition of a response, as well as differences in study populations, particularly the use of HAART and previous exposure to MMR vaccine, account for discrepant rates of MMR vaccine responses seen in these studies. Not surprisingly, seroresponse rates in our small population, where approximately 55% of participants were receiving HAART and many were seropositive for vaccine antigens at baseline, were intermediate to those previously reported.
There were limitations to our retrospective cohort study. First, 6 month follow-up intervals may not detect short-term transient effects of vaccination on CD4 count or VL, although our goal was to assess long-term changes. Second, we used EIA for assessing MMR vaccine responses. EIA for measles and mumps have been shown to underestimate seropositivity when compared with the gold-standard, plaque reduction neutralization assay (PRN).[46–50] Therefore, seropositivity as measured by PRN may be higher than what we observed using EIA. PRN assays, however, are expensive and labor-intensive and EIAs remain the recommended screening tests for measles, mumps, and rubella immunity although no definitive value has been established as a correlate of clinical protection.[51] The limited linearity of the EIA also made it impossible to assess paired samples with high baseline results, which may have led to an underestimation of response under Definition 2. Our sample size was somewhat limited, and not all subjects had samples available for EIA testing, impacting our ability to assess seroprevalence and vaccine responses. Due to these limited numbers, longitudinal analysis of seroresponse was unable to be performed as planned. We also did not assess cellular immune responses to MMR vaccination, which have been shown to be more persistent than and poorly correlated with humoral immune responses,[19] although the contribution of cellular immunity to vaccine-induced protection is unknown and no correlate of immunity has been defined.[52] Lastly, unique characteristics of our study population, including presumed high rates of MMR vaccination prior to HIV diagnosis, and high CD4 counts at baseline, may limit the ability to generalize our findings to other groups of HIV-infected individuals.
In summary, for HIV-infected adults receiving MMR vaccine with relatively preserved CD4 counts, or receiving HAART, there appears to be low risk of detrimental alterations in CD4 count or VL for 24 months following immunization. However, whether seronegative or seropositive prior to vaccination, response rates to each antigen also appear to be low, raising the question of whether or not MMR vaccination in HIV-infected adults would be clinically effective. Fortunately, the majority of individuals in our study were seropositive for these vaccine antigens, suggesting that they would be protected from developing disease if exposed.
Acknowledgments
Funding source: Support for this work (IDCRP-000-10) was provided by the Infectious Disease Clinical Research Program (IDCRP), a Department of Defense (DoD) program executed through the Uniformed Services University of the Health Sciences. This project has been funded in whole, or in part, with federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH), under Inter-Agency Agreement Y1-AI-5072.
The funding source had no involvement in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication. The content of this publication is the sole responsibility of the authors and does not necessarily reflect the views or policies of the NIH or the Department of Health and Human Services, the DoD or the Departments of the Army, Navy or Air Force. Mention of trade names, commercial products, or organizations does not imply endorsement by the U.S. Government.
The authors would like to thank Ms. Marisa Fernandez for her expertise and assistance in performing the needed laboratory assays for this study. We would also like to express our gratitude to the current members of IDCRP HIV Working Group and the long line of military HIV researchers who have supported the HIV NHS, and to the research coordinators and support staff for their countless hours of work. Most importantly, we would like to thank the patients for their participation, without which this research would not have been possible.
Footnotes
Conflicts of interest: All authors, no conflicts.
Contributors
B. Stermole participated in data analysis and interpretation and manuscript preparation. G. Grandits and M. Roediger participated in data acquisition, analysis, and interpretation. B. Clark participated in study conception and design. A. Ganesan, A. Weintrob, N. Crum-Cianflone, T. Ferguson, G. Macalino, and B. Clark participated in subject enrollment at participating institutions and data interpretation. M. Landrum participated in study conception and design, participant enrollment, data acquisition, data analysis and interpretation, and manuscript preparation. All contributing authors were involved in review and revision of the manuscript and approval prior to submission.
These data were presented in part at the 48th Annual Meeting of the Infectious Diseases Society of America held in Vancouver, Canada, October 21–24, 2010.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Marin M, Broder KR, Temte JL, Snider DE, Seward JF. Use of combination measles, mumps, rubella, and varicella vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2010;59(RR-3):1–12. [PubMed] [Google Scholar]
- 2.Chen TH, Kutty P, Lowe LE, Hunt EA, Blostein J, Espinoza R, et al. Measles outbreak associated with an international youth sporting event in the United States, 2007. Pediatr Infect Dis J. 2010;29(9):794–800. doi: 10.1097/INF.0b013e3181dbaacf. [DOI] [PubMed] [Google Scholar]
- 3.Dayan GH, Quinlisk MP, Parker AA, Barskey AE, Harris ML, Schwartz JM, et al. Recent resurgence of mumps in the United States. N Engl J Med. 2008;358(15):1580–9. doi: 10.1056/NEJMoa0706589. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Brief Update: Mumps Outbreak--New York and New Jersey, June 2009-January 2010. MMWR. 2010;59(5):125–29. [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Recommended adult immunization scheduled--United States, 2009. MMWR. 2009;57:Q1–Q4. [PubMed] [Google Scholar]
- 6.Kroger AT, Atkinson WL, Marcuse EK, Pickering LK. General recommendations on immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2006;55(RR-15):1–48. [PubMed] [Google Scholar]
- 7.WHO position on measles vaccines. Vaccine. 2009;27(52):7219–21. doi: 10.1016/j.vaccine.2009.09.116. [DOI] [PubMed] [Google Scholar]
- 8.Redfield RR, Wright DC, James WD, Jones TS, Brown C, Burke DS. Disseminated vaccinia in a military recruit with human immunodeficiency virus (HIV) disease. N Engl J Med. 1987;316(11):673–6. doi: 10.1056/NEJM198703123161106. [DOI] [PubMed] [Google Scholar]
- 9.Angel JB, Walpita P, Lerch RA, Sidhu MS, Masurekar M, DeLellis RA, et al. Vaccine-associated measles pneumonitis in an adult with AIDS. Ann Intern Med. 1998;129(2):104–6. doi: 10.7326/0003-4819-129-2-199807150-00007. [DOI] [PubMed] [Google Scholar]
- 10.Talbot EA, Perkins MD, Silva SF, Frothingham R. Disseminated bacille Calmette-Guerin disease after vaccination: case report and review. Clin Infect Dis. 1997;24(6):1139–46. doi: 10.1086/513642. [DOI] [PubMed] [Google Scholar]
- 11.Kramer JM, LaRussa P, Tsai WC, Carney P, Leber SM, Gahagan S, et al. Disseminated vaccine strain varicella as the acquired immunodeficiency syndrome-defining illness in a previously undiagnosed child. Pediatrics. 2001;108(2):E39. doi: 10.1542/peds.108.2.e39. [DOI] [PubMed] [Google Scholar]
- 12.Wallace MR, Hooper DG, Graves SJ, Malone JL. Measles seroprevalence and vaccine response in HIV-infected adults. Vaccine. 1994;12(13):1222–4. doi: 10.1016/0264-410x(94)90247-x. [DOI] [PubMed] [Google Scholar]
- 13.King JC, Jr, Treanor J, Fast PE, Wolff M, Yan L, Iacuzio D, et al. Comparison of the safety, vaccine virus shedding, and immunogenicity of influenza virus vaccine, trivalent, types A and B, live cold-adapted, administered to human immunodeficiency virus (HIV)-infected and non-HIV-infected adults. J Infect Dis. 2000;181(2):725–8. doi: 10.1086/315246. [DOI] [PubMed] [Google Scholar]
- 14.Aurpibul L, Puthanakit T, Sirisanthana T, Sirisanthana V. Response to measles, mumps, and rubella revaccination in HIV-infected children with immune recovery after highly active antiretroviral therapy. Clin Infect Dis. 2007;45(5):637–42. doi: 10.1086/520651. [DOI] [PubMed] [Google Scholar]
- 15.Brena AE, Cooper ER, Cabral HJ, Pelton SI. Antibody response to measles and rubella vaccine by children with HIV infection. J Acquir Immune Defic Syndr. 1993;6(10):1125–9. [PubMed] [Google Scholar]
- 16.Sprauer MA, Markowitz LE, Nicholson JK, Holman RC, Deforest A, Dales LG, et al. Response of human immunodeficiency virus-infected adults to measles-rubella vaccination. J Acquir Immune Defic Syndr. 1993;6(9):1013–6. [PubMed] [Google Scholar]
- 17.Levin MJ, Gershon AA, Weinberg A, Song LY, Fentin T, Nowak B. Administration of live varicella vaccine to HIV-infected children with current or past significant depression of CD4(+) T cells. J Infect Dis. 2006;194(2):247–55. doi: 10.1086/505149. [DOI] [PubMed] [Google Scholar]
- 18.Levin MJ, Gershon AA, Weinberg A, Blanchard S, Nowak B, Palumbo P, et al. Immunization of HIV-infected children with varicella vaccine. J Pediatr. 2001;139(2):305–10. doi: 10.1067/mpd.2001.115972. [DOI] [PubMed] [Google Scholar]
- 19.Belaunzaran-Zamudio PF, Garcia-Leon ML, Wong-Chew RM, Villasis-Keever A, Cuellar-Rodriguez J, Mosqueda-Gomez JL, et al. Early loss of measles antibodies after MMR vaccine among HIV-infected adults receiving HAART. Vaccine. 2009;27(50):7059–64. doi: 10.1016/j.vaccine.2009.09.063. [DOI] [PubMed] [Google Scholar]
- 20.Landrum ML, Dolan MJ. Routine Vaccination in HIV-Infected Adults. Infectious Diseases in Clinical Practice. 2008;16:85–93. [Google Scholar]
- 21.Hussey GD, Goddard EA, Hughes J, Ryon JJ, Kerran M, Carelse E, et al. The effect of Edmonston-Zagreb and Schwarz measles vaccines on immune response in infants. J Infect Dis. 1996;173(6):1320–6. doi: 10.1093/infdis/173.6.1320. [DOI] [PubMed] [Google Scholar]
- 22.Leon ME, Ward B, Kanashiro R, Hernandez H, Berry S, Vaisberg A, et al. Immunologic parameters 2 years after high-titer measles immunization in Peruvian children. J Infect Dis. 1993;168(5):1097–104. doi: 10.1093/infdis/168.5.1097. [DOI] [PubMed] [Google Scholar]
- 23.Lisse IM, Aaby P, Knudsen K, Whittle H, Andersen H. Long term impact of high titer Edmonston-Zagreb measles vaccine on T lymphocyte subsets. Pediatr Infect Dis J. 1994;13(2):109–12. doi: 10.1097/00006454-199402000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Preblud SR, Gross F, Halsey NA, Hinman AR, Herrmann KL, Koplan JP. Assessment of susceptibility to measles and rubella. Jama. 1982;247(8):1134–7. [PubMed] [Google Scholar]
- 25.Bekker V, Scherpbier H, Pajkrt D, Jurriaans S, Zaaijer H, Kuijpers TW. Persistent humoral immune defect in highly active antiretroviral therapy-treated children with HIV-1 infection: loss of specific antibodies against attenuated vaccine strains and natural viral infection. Pediatrics. 2006;118(2):e315–22. doi: 10.1542/peds.2005-2616. [DOI] [PubMed] [Google Scholar]
- 26.Frenkel LM, Nielsen K, Garakian A, Cherry JD. A search for persistent measles, mumps, and rubella vaccine virus in children with human immunodeficiency virus type 1 infection. Arch Pediatr Adolesc Med. 1994;148(1):57–60. doi: 10.1001/archpedi.1994.02170010059012. [DOI] [PubMed] [Google Scholar]
- 27.Hilgartner MW, Maeder MA, Mahoney EM, Donfield SM, Evatt BL, Hoots WK. Response to measles, mumps, and rubella revaccination among HIV-positive and HIV-negative children and adolescents with hemophilia. Hemophilia Growth and Development Study. Am J Hematol. 2001;66(2):92–8. doi: 10.1002/1096-8652(200102)66:2<92::AID-AJH1023>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 28.Melvin AJ, Mohan KM. Response to immunization with measles, tetanus, and Haemophilus influenzae type b vaccines in children who have human immunodeficiency virus type 1 infection and are treated with highly active antiretroviral therapy. Pediatrics. 2003;111(6 Pt 1):e641–4. doi: 10.1542/peds.111.6.e641. [DOI] [PubMed] [Google Scholar]
- 29.Oldakowska A, Marczynska M, Szczepanska-Putz M. Effects of measles vaccination in HIV infected children. Przegl Epidemiol. 2001;55(4):523–7. [PubMed] [Google Scholar]
- 30.Weintrob AC, Fieberg AM, Agan BK, Ganesan A, Crum-Cianflone NF, Marconi VC, et al. Increasing age at HIV seroconversion from 18 to 40 years is associated with favorable virologic and immunologic responses to HAART. J Acquir Immune Defic Syndr. 2008;49(1):40–7. doi: 10.1097/QAI.0b013e31817bec05. [DOI] [PubMed] [Google Scholar]
- 31.Crum-Cianflone N, Hullsiek KH, Marconi V, Weintrob A, Ganesan A, Barthel RV, et al. Trends in the incidence of cancers among HIV-infected persons and the impact of antiretroviral therapy: a 20-year cohort study. Aids. 2009;23(1):41–50. doi: 10.1097/QAD.0b013e328317cc2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diamedix Corporation, a subsidiary of IVAX Diagnostics, Inc. Measles IgG Enzyme Immunoassay Test Kit [package insert] Miami, FL: Diamedix Corporation; 2006. pp. 1–5. [Google Scholar]
- 33.Diamedix Corporation, a subsidiary of IVAX Diagnostics, Inc. Mumps IgG Enzyme Immunoassay Test Kit [package insert] Miami, FL: Diamedix Corporation; 2006. pp. 1–5. [Google Scholar]
- 34.Diamedix Corporation, a subsidiary of IVAX Diagnostics, Inc. Rubella IgG Enzyme Immunoassay Test Kit [package insert] Miami, FL: Diamedix Corporation; 2006. pp. 1–8. [Google Scholar]
- 35.Sulkowski MS, Chaisson RE, Karp CL, Moore RD, Margolick JB, Quinn TC. The effect of acute infectious illnesses on plasma human immunodeficiency virus (HIV) type 1 load and the expression of serologic markers of immune activation among HIV-infected adults. J Infect Dis. 1998;178(6):1642–8. doi: 10.1086/314491. [DOI] [PubMed] [Google Scholar]
- 36.Garcia M, Yu XF, Griffin DE, Moss WJ. Measles virus inhibits human immunodeficiency virus type 1 reverse transcription and replication by blocking cell-cycle progression of CD4+ T lymphocytes. J Gen Virol. 2008;89(Pt 4):984–93. doi: 10.1099/vir.0.83601-0. [DOI] [PubMed] [Google Scholar]
- 37.Grivel JC, Garcia M, Moss WJ, Margolis LB. Inhibition of HIV-1 replication in human lymphoid tissues ex vivo by measles virus. J Infect Dis. 2005;192(1):71–8. doi: 10.1086/430743. [DOI] [PubMed] [Google Scholar]
- 38.Moss WJ, Ryon JJ, Monze M, Cutts F, Quinn TC, Griffin DE. Suppression of human immunodeficiency virus replication during acute measles. J Infect Dis. 2002;185(8):1035–42. doi: 10.1086/340027. [DOI] [PubMed] [Google Scholar]
- 39.Moss WJ, Scott S, Ndhlovu Z, Monze M, Cutts FT, Quinn TC, et al. Suppression of human immunodeficiency virus type 1 viral load during acute measles. Pediatr Infect Dis J. 2009;28(1):63–5. doi: 10.1097/INF.0b013e318184eed2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ruel TD, Achan J, Gasasira AF, Charlebois ED, Mehbratu T, Rosenthal PJ, et al. HIV RNA suppression among HIV-infected Ugandan children with measles. J Acquir Immune Defic Syndr. 2008;48(2):225–7. doi: 10.1097/QAI.0b013e31816d9c86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Petruccelli BP. Concordance of Measles and Rubella Immunity with Immunity to Mumps, Enlisted Accessions, U.S. Armed Forces, 2000–2004. Medical Surveillance Monthly Report. 2007;13(2):10–12. [Google Scholar]
- 42.Glaser JB, DeCorato DR, Greifinger R. Measles antibody status of HIV-infected prison inmates. J Acquir Immune Defic Syndr. 1991;4(5):540–1. [PubMed] [Google Scholar]
- 43.Kemper CA, Gangar M, Arias G, Kane C, Deresinski SC. The prevalence of measles antibody in human immunodeficiency virus-infected patients in northern California. J Infect Dis. 1998;178(4):1177–80. doi: 10.1086/515679. [DOI] [PubMed] [Google Scholar]
- 44.Sha BE, Harris AA, Benson CA, Atkinson WL, Urbanski PA, Stewart JA, et al. Prevalence of measles antibodies in asymptomatic human immunodeficiency virus-infected adults. J Infect Dis. 1991;164(5):973–5. doi: 10.1093/infdis/164.5.973. [DOI] [PubMed] [Google Scholar]
- 45.Kutty PK, Kruszon-Moran DM, Dayan GH, Alexander JP, Williams NJ, Garcia PE, et al. Seroprevalence of antibody to mumps virus in the US population, 1999–2004. J Infect Dis. 2010;202(5):667–74. doi: 10.1086/655394. [DOI] [PubMed] [Google Scholar]
- 46.Cohen BJ, Doblas D, Andrews N. Comparison of plaque reduction neutralisation test (PRNT) and measles virus-specific IgG ELISA for assessing immunogenicity of measles vaccination. Vaccine. 2008;26(50):6392–7. doi: 10.1016/j.vaccine.2008.08.074. [DOI] [PubMed] [Google Scholar]
- 47.Cohen BJ, Parry RP, Doblas D, Samuel D, Warrener L, Andrews N, et al. Measles immunity testing: comparison of two measles IgG ELISAs with plaque reduction neutralisation assay. J Virol Methods. 2006;131(2):209–12. doi: 10.1016/j.jviromet.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 48.Chen RT, Markowitz LE, Albrecht P, Stewart JA, Mofenson LM, Preblud SR, et al. Measles antibody: reevaluation of protective titers. J Infect Dis. 1990;162(5):1036–42. doi: 10.1093/infdis/162.5.1036. [DOI] [PubMed] [Google Scholar]
- 49.Ratnam S, Gadag V, West R, Burris J, Oates E, Stead F, et al. Comparison of commercial enzyme immunoassay kits with plaque reduction neutralization test for detection of measles virus antibody. J Clin Microbiol. 1995;33(4):811–5. doi: 10.1128/jcm.33.4.811-815.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mauldin J, Carbone K, Hsu H, Yolken R, Rubin S. Mumps virus-specific antibody titers from pre-vaccine era sera: comparison of the plaque reduction neutralization assay and enzyme immunoassays. J Clin Microbiol. 2005;43(9):4847–51. doi: 10.1128/JCM.43.9.4847-4851.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Atkinson WWS, Hamborsky J, McIntyre L, editors. Epidemiology and Prevention of Vaccine-Preventable Diseases. 11. Washington DC: Public Health Foundation; 2009. Centers for Disease Control and Prevention. [Google Scholar]
- 52.Naniche D, Garenne M, Rae C, Manchester M, Buchta R, Brodine SK, et al. Decrease in measles virus-specific CD4 T cell memory in vaccinated subjects. J Infect Dis. 2004;190(8):1387–95. doi: 10.1086/424571. [DOI] [PubMed] [Google Scholar]


