Recent years have seen a renewed interest in understanding the placebo effect. From the beginning of controlled trials, when putatively active treatments were for the first time compared against “sham” controls, it was recognized that “the passions of the mind [had a wonderful and powerful influence] upon the state and disorder of the body” (Haygarth, 1801). These initial observations of placebo effects have now been corroborated across multitudes of studies in modern times (de Craen et al., 1999). Clinically significant placebo-associated improvements can occur in as few as 5% or as many as 65% of individuals in randomized, controlled trials (RCTs), depending on the central nervous system (CNS) disease process under consideration and the particular study sample under investigation. Certainly, placebo effects add to the variability in responses associated with the pathologies and treatments themselves. As a result, promising results in open, uncontrolled trials without placebo control are oftentimes no longer observable once the placebo effects are taken into account. Similarly, nocebo effects, the development of adverse events or worsening of a condition after the administration of a placebo are reported in a sizable proportion of individuals participating in clinical trials (Drici et al., 1995; Long et al., 1989).
Historically, placebo effects have been reported consistently since the emergence of placebo-controlled trials in the 18th century. In the widely quoted Beecher report of clinical trials of analgesic drugs (Beecher, 1955), it was noted that placebos exerted significant clinical responses in approximately 30% of patients enrolled in inactive treatment groups. However, elements unaccounted for in this and a number of subsequent reports have included the effects of natural history of the disease (no-treatment), which can spontaneously remit or change in severity in the course of the disease without intervention. The presence of other cognitive-emotional biases, such the “halo” effect, related to the individual response to the characteristics of the experimenting or treatment team or individual, or those induced by fact that subjects know that they are being studied, termed the Hawthorne effect, have to be additionally considered in the interpretation of placebo-related responses, particularly so when subjective or simple behavioral measures (e.g., improvement in performance) are the primary outcomes.
More recent work using meta-analytical tools has emphasized the variability present in both active and inactive treatment conditions. These were found to be considerable for subjective outcome measures, with their utilization accounting for a substantial proportion of the variance in the so-called “placebo effects”. However, even from that perspective, analgesic placebo effects were observed to be more substantial than those in other conditions with similarly subjective outcome measures (Hrobjartsson and Gotzsche, 2001; Hrobjartsson and Gotzsche, 2004; Vase et al., 2002). It is then not surprising that a considerable effort has been committed to the study of mechanisms that may account for placebo-induced analgesic effects over the last 3 decades (Benedetti et al., 2005; Price et al., 2008). As will be reviewed in the following pages, there is substantial evidence that cognitive processes associated with the expectation of treatment and recovery are in fact associated with the mobilization of internal mechanisms that can elicit an objectively observable physiological response (a placebo effect).
There is an emerging literature examining the neurobiology of placebo effects across a variety of domains, such as mood and affective regulation (Mayberg, 1997; Petrovic et al., 2005) as well as motor control in Parkinson’s disease (Benedetti et al., 2004; de la Fuente-Fernandez et al., 2001). However, the neurobiology of the placebo effect was born in 1978, when it was shown that placebo analgesia could be blocked by the opioid receptor antagonist naloxone. This indicated an involvement of the endogenous opioid system in the production of placebo-induced analgesic effects (Levine et al., 1978). In patients who had undergone oral surgery 2 hours prior, naloxone, placebo or morphine were administered with the expectation of either pain relief or pain worsening. Naloxone was associated with hyperalgesia, showing that the stress and/or pain associated with the surgical procedure had by itself induced the release of endogenous opioids. The administration of placebo induced a significant reduction in pain ratings in 39% of the subjects, which was fully antagonized by naloxone. In subsequent studies by the same group, in which hidden and machine-driven infusions of placebo and naloxone were introduced (Levine and Gordon, 1984), the effect of naloxone on placebo analgesia was confirmed, and estimated to approximate that of 8 mg of morphine in that particular experimental setting. Subsequent studies by Gracely et al. (Gracely et al., 1983) and Grevert et al.(Grevert et al., 1983) using similar opioid receptor-blocking pharmacological challenges confirmed the existence of opioid-mediated placebo analgesia but also described a time-dependent, non-opioid component that is not reversible by naloxone.
In what has become a classic study of components related to the development of placebo analgesic effects, Amanzio and Benedetti (Amanzio and Benedetti, 1999) explored the contribution of expectations and conditioning to the development of placebo analgesic effects. Utilizing ischemic arm pain as an experimental model, it was demonstrated that contextual cues promoting a credible expectation of analgesia during placebo administration induced analgesic effects that were completely blocked by naloxone (i.e., expectation effects were entirely mediated by the activation of opioid mechanisms). Expectation cues that followed a course of morphine (morphine pre-conditioning group) also produced analgesic responses that were also fully antagonized by naloxone. Naloxone reversibility was also achieved in the absence of cues promoting expectation as long as morphine had been pre-administered (i.e., the volunteers were receiving an inactive agent when morphine would have been normally administered). However, conditioning with the non-steroidal anti-inflammatory drug, ketorolac, paired with additional expectation cues induced a placebo antinociceptive response that was only partially blocked by naloxone, while ketorolac conditioning alone produced analgesia that proved to be naloxone insensitive. Overall, these results show that while purely cognitive factors (expectation of analgesia induced by the placebo administration) are associated with the activation of endogenous opioid systems, conditioning is capable of recruiting other mechanisms in support of analgesia depending on the conditioning agent.
These and other observations have led to the proposition of a number of theoretical constructs to explain the formation of placebo effects, again most typically studied in the context of analgesic responses to pain. All these constructs hinge upon elements of higher order processing involving cognitive and emotional circuits, known to modulate the experience of pain. (A) Expectations and beliefs, whereby cognitive assessments and beliefs of analgesia trigger the placebo effects (Bootzin, 1985; Montgomery and Kirsch, 1997; Price et al., 1999). (B) Anxiety relief, where placebo administration elicits analgesia through reductions in the anxiety experienced by the subjects (Evans, 1985; McGlashan et al., 1969). (C) The conditioning hypothesis emphasizes the engagement of learned responses through the previous exposures to active treatments (Ader, 1997; Gleidman et al., 1957; Herrnstein, 1962; Siegel, 1985; Voudouris et al., 1989; Voudouris et al., 1990; Wickramasekera, 1980). (D) The so-called response appropriate sensations hypothesis further states that pain and analgesia are experienced after a complex, preconscious assessment of sensory and internal stimuli. Pain experience or pain suppression are then engaged as a process of adaptation to environmental circumstances (Wall, 1993).
A number of studies have now shown the involvement of distinct brain structures in responses to cognitive manipulation. Hypnotic suggestions have been used to selectively reduce or increase sensory (intensity) and affective (unpleasantness) qualities of pain, with the effects being associated with changes in the metabolic activity of the somatosensory and anterior cingulate cortex, respectively. (Hofbauer et al., 2001; Rainville et al., 1997; Willoch et al., 2000). Hypnotic suggestions, however, seem to differ from and could not account for typical placebo analgesic responses (Price and Barrell, 2000), albeit some similarities as to the networks involved have emerged (Raz et al., 2005). In fact, data show that certain CNS circuits, known to be involved in the perception and integration of the pain experience, are susceptible to various manipulations. The perception of pain can be either diminished or enhanced, depending on the additional presence of cognitive distractors, or the suggestion of pain enhancement or reduction (Petrovic and Ingvar, 2002). Theories regarding the placebo analgesic effect uniformly acknowledge the interplay between environmental information and their perception and integration by the individual's organism to induce a positive (placebo) or negative (nocebo) response. The presence of these interactions implies the involvement of higher order, CNS associative processes in the production of analgesic placebo effects. This assertion has been elegantly demonstrated by work in which analgesic agents were administered covertly (subjects were not aware of the actual timing of the administration). Substantially lower and even insignificant effects were obtained from even well-recognized analgesic treatments when the context of drug administration was removed from the treatment (Amanzio et al., 2001; Benedetti et al., 2003; Levine et al., 1981). These findings call for the elucidation of mechanisms underlying “mind-body” interactions.
In an initial report, the effects of the short-acting μ-opioid receptor agonist remifentanil on regional cerebral blood flow (rCBF, as measured with positron emission tomography [PET], thought to reflect metabolic demands), were found to overlap with the effects of a placebo under conditions of expectation of analgesia in the rostral anterior cingulate cortex (rACC). Placebo administration increased the correlation between the activity of this region and that of the midbrain periaqueductal gray (PAG), a region known to exert modulatory effects on the experience of pain. Individuals with high placebo analgesic responses further demonstrated greater rCBF responses to remifentanil, suggesting that individual differences in placebo analgesia may involve differences in the concentration or function of μ-opioid receptors (Petrovic et al., 2002). Subsequent work has utilized functional magnetic resonance imaging (fMRI and the blood oxygenation level dependent signal [BOLD]) as well as a covert manipulation to increase individual expectations. This consisted of a reduction in the heat intensity of the probe used to induce pain during the administration of a placebo. In response to the manipulation, placebo-associated reductions in the activity of the rACC, insular cortex and thalamus were observed, correlating with the subjectively rated pain relief afforded by the placebo administration (Wager et al., 2004). Using a similar experimental approach, the opposite effect, activation of the rACC and increased connectivity between this region, the amygdala and PAG during placebo administration were described (Bingel et al., 2006). Other work found increases in the activity of the rACC, prefrontal, insular cortex, supramarginal gyrus and inferior parietal cortex, employing sham acupuncture as a form of placebo intervention (Kong et al., 2006). While these differences in the directionality of findings may seem difficult to reconcile, particularly when using similar placebo enhancement procedures, several methodological differences between the studies have been noted (Kong et al., 2006). Among them is the selection criteria for the subjects entered in the neuroimaging protocols. In one of them (showing placebo-associated reductions in BOLD responses during placebo administration), only subjects demonstrating substantial placebo analgesia in preceding “training” trials were studied (Wager et al., 2004). In contrast, the reminder of the studies (showing placebo-associated increases in regional BOLD activity), did not eliminate non-responder subjects for imaging (Bingel et al., 2006), (Kong et al., 2006). Raz et al. (Raz et al., 2005), reported that only high hypnotizable subjects responded with reductions in rACC BOLD responses during post-hypnotic suggestions in a cognitive conflict resolution task (as opposed to low hypnotizable or volunteers in whom no suggestions were used). This may suggest that differences in subject preselection procedures (e.g., the elimination of non placebo responders) would have contributed to the apparent differences in response directionality between studies. However, they also highlight the non-specificity of the regional BOLD response, thought to reflect the sum of local field potentials across a variety of inputs, neuronal types and neurotransmitter systems (Logothetis et al., 2001).
In our laboratory, we have primarily focused on the examination of in-vivo molecular mechanisms and related circuits involved in the formation of placebo effects. For that purpose, we employed positron emission tomography (PET) and validated models to quantify μ-opioid and DA D2/3 receptors while administering a model of sustained experimental pain. Using these types of functional assays, reductions in the in vivo availability (binding potential, BP) of the respective receptor population reflect placebo-induced activation of either the opioid or DA neurotransmission, respectively. Subjects were studied under baseline conditions (no stimulus), pain expectation (pain intensity is rated, expected but not actually endured) and actual pain. The latter two were performed with and without the administration of a placebo, consisting of isotonic saline infused intravenously, 1 mL every 4 min and with the subject receiving verbal and visual cues at the time of application. The study sample consisted of young healthy males and females, ages 20–30 years. Women were studied in the follicular phase of the menstrual cycle, ascertained by menstrual diaries, timing of menses and plasma levels of estradiol and progesterone prior to scanning. The sustained pain model employed elicits psychophysical responses similar to those of clinical pain states in terms of pain intensity and pain affect (Stohler and Kowalski, 1999). The resulting steady-state of deep muscle pain was maintained for 20 min by a computer-controlled closed-loop system through individually titrated infusion of medication-grade hypertonic saline (5%) into the masseter muscle, aiming for a target pain intensity of 40 visual analog scale (VAS) units (Stohler and Kowalski, 1999; Zhang et al., 1993). Volunteers rated pain intensity every 15 s using an electronic version of a 10-cm VAS, placed in front of the scanner gantry. For trials where subjects expected to receive pain but a non-painful stimulus was applied, the same procedures was followed, except that isotonic instead of hypertonic saline was administered.
In order to study the molecular mechanisms underlying the placebo effect, our model of sustained experimental pain was used in either one of two modes of operation, producing very different experimental conditions: (a) The placebo effect was assessed by measuring the subject-specific infusion volume required to maintain pain at the preset target level for 20 minutes, with or without the administration of the placebo, and (b) by using the subject-specific, pre-established infusion profile with and without the administration of the placebo. In the first condition, the placebo effect is perceptually not transparent to the subject as pain intensity is kept at the preset target level for both the “no placebo” and “placebo” conditions and with the effect of the placebo being expressed by the difference of the rate of infusion required between the two conditions. For the second scenario, the subject is able to recognize the effect of the administered placebo by experiencing either a lessening or worsening of the pain intensity over the course of the trial as a consequence of the placebo administration.
In addition to the momentary assessments of pain intensity acquired every 15 sec, subjects completed the McGill Pain Questionnaire with its sensory and affective subscales (Melzack and Katz, 2000), 0–100 VAS scores of pain intensity and unpleasantness, the Positive and Negative Affectivity Scale (PANAS) measuring internal affective state (Watson et al., 1988), and the Profile of Mood States inventory (POMS), which provides a total mood disturbance score (TMD) (McNair et al., 1992). These rating scales were completed at the end of the challenges for both conditions, with and without placebo administration.
We were interested in the understanding of individual variations in placebo responses, and all eligible subjects were included in the studies without any consideration given to their placebo responsivity. Furthermore, we utilized instructions that were similar to those of typical clinical trials: “We are testing an agent that has been shown to reduce pain in some subjects. It is thought that it does this through the activation of anti-pain mechanisms in our bodies. You will receive both active and inactive agents during the trial”. In the first series of experiments described below, an additional statement was added to deal with the fact that the placebo effect was not transparent to subjects due to the choice of pain model used: “You may not be able to tell whether the agent is working, but the investigators will be able to tell with their equipment”
Placebo-induced activation of regional endogenous opioid neurotransmission
In an initial investigation involving 14 healthy males, we determined the regional activation of endogenous opioid neurotransmission on μ-opioid receptors with PET and the selective μ-opioid radiotracer [11C]carfentanil (Zubieta et al., 2005). In this experiment, the pain model was operated so that the infusion was individually titrated to the preset level of pain intensity, irrespective of whether placebo was administered or not, preventing subjects from experiencing a difference between conditions. It was observed that the administration of the placebo, with expectation that it represented an analgesic agent, was associated with significant activation of μ-opioid receptor mediated neurotransmission in both higher order and subcortical brain regions. These included the pre- and subgenual rACC, the dorsolateral prefrontal cortex (DLPFC), anterior insular cortex (aINS) and the nucleus accumbens (NAC). These regional activations were correlated with lower ratings of pain intensity (rACC, aINS, NAC), pain unpleasantness (rACC), reductions in MPQ sensory (rACC, aINS), affective (NAC) and total (rACC, aINS) scores, as well as in the negative emotional state of the volunteers as measured with the POMS (NAC). The magnitude of μ-opioid system activity in the rACC also correlated positively with the increases in pain tolerance (the increase in algesic volume requirements to maintain pain at the target intensity, r= 0.96) This dataset was the first direct evidence that the administration of a placebo with implied analgesic properties was associated with the activation of a pain and stress inhibitory neurotransmitter system, the endogenous opioid system and μ-opioid receptors, involving a number of brain regions. Furthermore, this activation was associated with quantifiable reductions in the physical and emotional attributes of the stressor, a sustained pain challenge. The regions implicated in this phenomenon included some involved in cognitive and emotional integration, including responses to placebo (rACC); the representation and modulation of internal states, both physical and emotional (INS), and reward and saliency assessments (NAC). The DLPFC was not found to be related to changes in the psychophysical properties of the pain challenge, but instead to the expected analgesic effect of the placebo, as rated by the volunteers prior to its administration. This is consistent with the hypothesized function of this brain region in the cognitive adjustments to environmental information for the control of behavior (Fuster, 2000).
A follow-up analysis, conducted in a larger sample (n=20) (Zubieta et al., 2006), examined the variance in endogenous opioid activity as a function of placebo-associated expectations, and psychophysical characteristics of pain. Perhaps counter-intuitively, the largest proportion of the variance in regional endogenous opioid activity (40–68%, depending on the region) was accounted for by a multiple regression model that included the affective (but not sensory) quality of the pain, the PANAS positive and negative affect ratings, and a measure of individual pain sensitivity (the volume of algesic substance that had to be infused to maintain pain at target intensity level). This indicated that the individual affective experience during pain, whether pain-specific (MPQ pain affect subscale) or not (PANAS ratings of positive and negative internal affective state) were important predictors of the subsequent development of a placebo response, as was the measure of individual pain sensitivity. This concept seems to be consistent with that advanced by observations that placebo analgesia is achieved proportionally to the relief of anxiety afforded by the placebo (Evans, 1985; McGlashan et al., 1969). It is also in line with the assertion that placebo effects result from the organism’s assessment of its internal needs (Wall, 1993), as pain sensitivity was also found to be a predictor of the formation of placebo responses as reflected by endogenous opioid activation.
A second experimental series was conducted with the same radiotracer, labeling μ-opioid receptors, but this time the infusion profile to achieve target pain levels was determined in advance and repeated in the studies with and without placebo (Scott et al., 2008). Pain intensity ratings, acquired every 15 sec, would be expected to be lower with placebo administration than without, this being the primary evidence of a formation of the placebo effect at a psychophysical level. This series also included PET studies with the dopaminergic (DA) tracer [11C]raclopride, labeling DA D2 receptors in the basal ganglia and D2 and D3 receptors in the NAC (Seeman et al., 2006). The data acquired with this radiotracer will be described in the following section.
In these studies, the expected analgesic effects were rated at 48±23 (range 0–95). After the experiments were conducted, the perceived effectiveness of the placebo was rated at 42±29. Significant endogenous opioid activation was observed in the pre- and subgenual rACC, orbitofrontal cortex (OFC), anterior insula (aINS) and pINS, medial thalamus (mTHA), NAC, amygdala (AMY) and periacqueductal gray (PAG). There was a notable lack of involvement of the DLPFC in these results, while activation in the OFC was observed instead. Regional magnitudes of activation correlated with the subjects expected analgesia (NAC, PAG), the update of these expectations by the subjectively perceived efficacy of the placebo (the ratio between observed and expected efficacy) (NAC, AMY), as well as with placebo-induced changes in pain intensity (rACC, NAC, OFC). In view of the previous results, where affective state explained a substantial proportion of the variance in placebo responses, we also examined whether increases in positive affect during the placebo condition were related to the opioid response. Positive correlations were obtained between the increases in PANAS positive affect and the magnitude of placebo-induced endogenous opioid system activity in the NAC.
When individuals were classified as high and low placebo responders using the median reduction in pain intensity during placebo as the split point, it was opioid activity in the NAC that was significantly different between the two groups. A small group of subjects (n=5) showed higher ratings of pain (hyperalgesia) during placebo (consistent with a nocebo effect). When compared to high placebo responders, the placebo and nocebo groups demonstrated changes in the opposite direction: regional opioid system activation was observed in high responders, while deactivations were present in the nocebo group.
Besides demonstrating a dynamic modulation of placebo and nocebo responses by the endogenous opioid system the involvement of NAC opioid neurotransmission in differentiating high and low placebo responders was documented for the first time. This brain region presents high levels of DA innervation arising from the ventral tegmental area (mesolimbic DA circuit) and is known to be involved in responding to rewards and salient stimuli (both rewarding and aversive) (Horvitz, 2000). It is also thought to respond to updates in reward expectations that depend on the emotional response to changing environmental information (so-called counterfactual comparisons) through its connections with the OFC and the AMY (Schultz, 2006; Tobler et al., 2005; Tom et al., 2007). It is also notable that in the 2nd experimental design, when pain intensity was allowed to decline with increasing placebo effects, involvement of the OFC but not the DLPFC was observed. It is possible that OFC-NAC-AMY integration would be more prominent when there is a need for the continuous update of effectiveness information over time (subjective value), while in the absence of this information, previously created expectations (and possibly DLPFC involvement) would have a more prominent role.
Dopaminergic Mechanisms in the Formation of Placebo Analgesic Effects
The previous results point to a distributed network of regions participating in placebo effects, mediated by the endogenous opioid system. The NAC emerged as a prominent part of it, believed to be responding to the saliency or the reward value of the placebo stimulus. Here, endogenous opioid activation was associated with expectations of analgesia, the update of those expectations over time, and placebo-induced analgesic effects.
The NAC lies at the interface of sensorimotor and limbic systems, and through its connections with the OFC, ventral pallidum and the amygdala, forms part of a circuit involved in the integration of cognitive, affective and motor responses in animal models (Kalivas et al., 1999; Mogenson and Yang, 1991). This circuit and additional interconnected regions (e.g., insular and medial prefrontal cortex, medial thalamus) are heavily modulated by the endogenous opioid system and μ-opioid receptors. It has also been proposed as a primary site of interaction between the effects of DA-releasing drugs, novelty and stressors (Badiani et al., 1998; Badiani et al., 1999; Day et al., 2001; Napier and Mitrovic, 1999; Uslaner et al., 2001), typically studied in the context of the administration of reinforcing drugs. A possible role of NAC DA in placebo responding was initially postulated following observations that basal ganglia DA release took place in the placebo arm of a RCT in patients with Parkinson disease. Dorsal basal ganglia DA activity was related to improvements in motor control, while NAC DA was associated with the expectations of improvement reported by the subjects (de la Fuente-Fernandez et al., 2002; de la Fuente-Fernandez et al., 2001). Subsequent work has also shown that placebo administration in a RCT with patients diagnosed with Major Depression increased metabolism in the NAC region, among other brain areas (Mayberg et al., 2002). The expectation of receiving methylphenidate, a psychostimulant, but instead being administered a placebo, activated NAC and rACC metabolism in healthy subjects (Volkow et al., 2006), but not in a drug abusing sample (Volkow et al., 2003). BOLD responses in the NAC have also been shown to be proportional to expectations of anxiety relief in a study in which subjects were preconditioned with a benzodiazepine anxiolytic and presented with negative affective stimuli (Petrovic et al., 2005). This work then suggests an involvement of the ventral basal ganglia in either responding to individual expectations or the novelty of a placebo administration.
To further study these processes in the context of placebo analgesia, the same subjects (n=20) that underwent μ-opioid receptor scanning (2nd experiment above), underwent studies with the DA D2/D3 receptor radiotracer [11C]raclopride (Scott et al., 2008). Opioid and DA scans were randomized in order. As in previous studies, scans included a pain anticipation period (pain was expected but not received) where subjects were administered intramuscular non-painful isotonic saline and rated pain intensity in the same manner as the actual pain scans. During the actual pain scans, the same infusion profile was used for studies with and without placebo (Scott et al., 2008).
Placebo administration was associated with the activation of DA D2/D3 neurotransmission that was exclusively localized in mesolimbic dopaminergic terminal fields, ventral caudate, ventral putamen and NAC. The magnitude of DA activation in the NAC was positively correlated with the individual expectations of analgesia, the update of those expectations during the study period (the ratio of subjectively rated analgesic efficacy over the initial expectations), and the magnitude of analgesia (the change in pain intensity ratings over the 20 min study period). As was the case with the opioid system, DA activation in the NAC was also positively correlated with the increase in PANAS positive affect ratings during placebo. When both regional opioid and DA responses to placebo were examined as to their contribution to placebo analgesia, DA release in the NAC emerged as the most predictive region and neurotransmitter, accounting for 25% of the variance in the formation of placebo analgesic effects. Consistent with the hypothesis that NAC DA responses to placebo constitute a “trigger” that, responding to the saliency and reward value of the placebo would allow for the activation of down-stream adaptive (e.g., opioid) responses, placebo-induced NAC DA release was positively correlated with the magnitude of endogenous opioid release in the NAC, ventral putamen, AMY, aINS, pINS and rACC. Similarly to the opioid system, NAC DA release also differentiated volunteers that were above and below the mean in their analgesic responses (high and low placebo responders) in these trials. For the comparison between high placebo and nocebo responders, nocebo responders demonstrated a deactivation of DA neurotransmission during placebo in the NAC and ventral putamen, an effect opposite in direction to that of high placebo responders.
Partly overlapping with the above sample, we then examined the hypothesis that individual variations in placebo responses may be related to differences in the processing of reward expectation (Scott et al., 2007). For this purpose, healthy males and females (n=30 total) were studied with a combination of molecular PET with [11C]raclopride and functional magnetic resonance imaging (fMRI). In this case, and to avoid motivational mechanisms that maybe related to individual differences in pain sensitivity, placebo-induced DA release was examined during the pain expectation state. Subjects also underwent an fMRI-BOLD study using a variation of the Monetary Incentive Delay (MID) task. This task is known to activate NAC synaptic activity during anticipation of a monetary reward (Knutson et al., 2004). Individual variations in placebo-induced NAC DA release were then compared to the synaptic activity of the same region during anticipation of a monetary reward. Both these measures were also examined as a function of the anticipated analgesic effects of the placebo, deviations from those expectations and the magnitude of placebo analgesic effects in pain challenges. It was hypothesized that in healthy subjects, in the absence of underlying pathology or previous conditioning, individual variations in placebo-induced NAC DA activity and in the synaptic activity of this region during reward anticipation would be related to each other and to the variability in placebo effects obtained in the studies.
In a manner similar to what was observed in actual pain studies, the introduction of the placebo during a pain anticipation state was associated with the activation of DA neurotransmission and D2/D3 receptors in the NAC, bilaterally, in a manner proportional to the anticipated analgesic effects as rated by the volunteers, as well as with the difference between anticipated and subjectively perceived effectiveness of the placebo (i.e., the update of expectations over time).
We then examined whether individual variations in the synaptic activity of the NAC during the MID task would be predictive of the magnitude of placebo effects. It was observed that individuals that activated NAC synaptic function to a greater extent during monetary reward anticipation also showed more profound placebo responses. These included greater positive affect scores during pain expectation periods and greater levels of analgesia in pain trials. The NAC BOLD signal during monetary reward anticipation was further correlated with placebo-induced DA activity as measured with PET. In a regression model, NAC synaptic activation during anticipation of the (high, $5) monetary reward accounted for approximately one third of the variance in the development of placebo-induced analgesia in the pain trials. In a manner similar to the results obtained with NAC DA responses to placebo, the activation of NAC synaptic activity during reward expectation was further correlated to the difference between the anticipated and subjectively perceived analgesic effects of the placebo. It should be noted that the fMRI studies were conducted separately from the pain expectation and pain studies and that the subjects were not aware of any link between the two sets of experiments. Given this situation, these results are believed to reflect intrinsic differences in the response of the NAC during reward anticipation, further defining individual variations in placebo responding.
Conclusions
An emerging literature is demonstrating that cognitive and emotional processes that are engaged during the administration of an otherwise inactive agent, a placebo, are capable of activating internal mechanisms that modify physiology. A network of regions, including the rostral anterior cingulate, dorsolateral prefrontal and orbitofrontal cortices, insula, nucleus accumbens, amygdala, medial thalamus and periaqueductal gray appear involved. Opioid and dopamine neurotransmission in these areas modulate various elements of the placebo effect, which appear to include the representation of its subjective value, updates of expectations over time, changes in affective state and in pain ratings. In some cases, such as that of the nucleus accumbens, a substantial proportion of the variance in placebo analgesic effects seems linked to the capacity to activate this brain region to rewards, a possibility that will need to be replicated and tested in larger trials. In this context, it is of interest to note that both opioid (Harris et al., 2007; Jones et al., 1994; Jones et al., 2004; Willoch et al., 2004) and dopamine (Wood et al., 2007) neurotransmission has been found diminished in chronic pain syndromes. The relationship between these alterations and the capacity to develop placebo effects in these and other clinical conditions remains to be explored.
The circuitry involved in placebo analgesic effects also have the potential to modulate a number of functions beyond pain, as the brain regions involved have been implicated in the regulation of stress responses, neuroendocrine and autonomic functions, mood, reward and integrative cognitive processes, such as decision-making. Both unconditioned, presumably associated with conscious expectations (Lanotte et al., 2005; Pollo et al., 2003) and conditioned effects on some of these mechanisms are starting to be shown in humans (Benedetti et al., 2003; Goebel et al., 2005; Longo et al., 1999; Stockhorst et al., 2000). Besides the perspective that placebo effects confound RCTs, the information so far acquired points to neurobiological systems that when activated by positive expectations, or even pre-conditioning, are capable of inducing physiological change. They should therefore be considered as resiliency mechanisms with the potential to aid in the recovery from challenges to the organism.
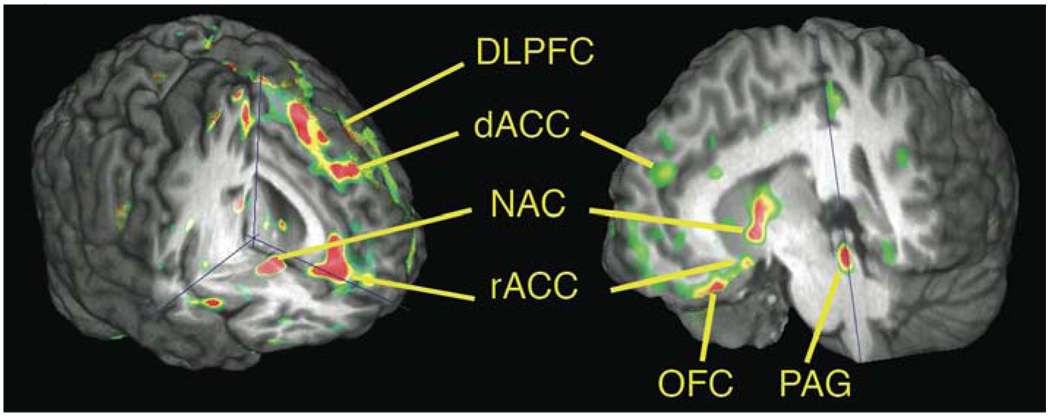
Figure 1.
Placebo-induced activation of regional μ-opioid receptor mediated neurotransmission
Some of the areas in which significant activation of μ-opioid neurotransmission during sustained pain were observed after the introduction of a placebo with expectation of analgesia in two different experimental designs. On the left (Zubieta et al., 2005) there was evidence of dorsolateral prefrontal cortex activation, related to individual expectations of analgesia. On the right (Scott et al., 2008), prefrontal activation was localized in the orbitofrontal cortex, and correlated with nucleus accumbens endogenous opioid and dopamine release. DLPFC = dorsolateral prefrontal cortex, OFC = orbitofrontal cortex, rACC = rostral anterior cingulate (BA 25), dACC = dorsal area of the rostral anterior cingulate (BA 24), NAC = nucleus accumbens, PAG = periaqueductal gray.
Acknowledgments
Support was provided by National Center for Complementary and Alternative Medicine grant R01 AT 001415 and R01 DA 016423 to J.K.Z.
References
- Ader R. The role of conditioining in pharmacotherapy. In: Harrington A, editor. The placebo effect: an interdisciplinary exploration. Cambridge, MA: Harvard UP; 1997. pp. 138–165. [Google Scholar]
- Amanzio M, Benedetti F. Neuropharmacological dissection of placebo analgesia: expectation-activated opioid systems versus conditioning-activated specific subsystems. J Neurosci. 1999;19:484–494. doi: 10.1523/JNEUROSCI.19-01-00484.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amanzio M, Pollo A, Maggi G, Benedetti F. Response variability to analgesics: a role for non-specific activation of endogenous opioids. Pain. 2001;90:205–215. doi: 10.1016/S0304-3959(00)00486-3. [DOI] [PubMed] [Google Scholar]
- Badiani A, Oates MM, Day HE, Watson SJ, Akil H, Robinson TE. Amphetamine-induced behavior, dopamine release, and c-fos mRNA expression: modulation by environmental novelty. J Neurosci. 1998;18:10579–10593. doi: 10.1523/JNEUROSCI.18-24-10579.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badiani A, Oates MM, Day HE, Watson SJ, Akil H, Robinson TE. Environmental modulation of amphetamine-induced c-fos expression in D1 versus D2 striatal neurons. Behav Brain Res. 1999;103:203–209. doi: 10.1016/s0166-4328(99)00041-8. [DOI] [PubMed] [Google Scholar]
- Beecher H. The powerful placebo. JAMA. 1955;159:1602–1606. doi: 10.1001/jama.1955.02960340022006. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Colloca L, Torre E, Lanotte M, Melcarne A, Pesare M, Bergamasco B, Lopiano L. Placebo-responsive Parkinson patients show decreased activity in single neurons of subthalamic nucleus. Nat Neurosci. 2004;7:587–588. doi: 10.1038/nn1250. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Mayberg HS, Wager TD, Stohler CS, Zubieta JK. Neurobiological mechanisms of the placebo effect. J Neurosci. 2005;25:10390–10402. doi: 10.1523/JNEUROSCI.3458-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti F, Pollo A, Lopiano L, Lanotte M, Vighetti S, Rainero I. Conscious expectation and unconscious conditioning in analgesic, motor, and hormonal placebo/nocebo responses. J Neurosci. 2003;23:4315–4323. doi: 10.1523/JNEUROSCI.23-10-04315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingel U, Lorenz J, Schoell E, Weiller C, Buchel C. Mechanisms of placebo analgesia: rACC recruitment of a subcortical antinociceptive network. Pain. 2006;120:8–15. doi: 10.1016/j.pain.2005.08.027. [DOI] [PubMed] [Google Scholar]
- Bootzin R. The role of expectancy in behavior change. In: White L, Tursky B, Schwartz G, editors. Placebo: theory, research and mechanisms. New York: Guilford; 1985. [Google Scholar]
- Day HE, Badiani A, Uslaner JM, Oates MM, Vittoz NM, Robinson TE, Watson SJ, Jr, Akil H. Environmental novelty differentially affects c-fos mRNA expression induced by amphetamine or cocaine in subregions of the bed nucleus of the stria terminalis and amygdala. J Neurosci. 2001;21:732–740. doi: 10.1523/JNEUROSCI.21-02-00732.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Craen AJ, Kaptchuk TJ, Tijssen JG, Kleijnen J. Placebos and placebo effects in medicine: historical overview. J R Soc Med. 1999;92:511–515. doi: 10.1177/014107689909201005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fuente-Fernandez R, Phillips AG, Zamburlini M, Sossi V, Calne DB, Ruth TJ, Stoessl AJ. Dopamine release in human ventral striatum and expectation of reward. Behav Brain Res. 2002;136:359–363. doi: 10.1016/s0166-4328(02)00130-4. [DOI] [PubMed] [Google Scholar]
- de la Fuente-Fernandez R, Ruth TJ, Sossi V, Schulzer M, Calne DB, Stoessl AJ. Expectation and dopamine release: mechanism of the placebo effect in Parkinson's disease. Science. 2001;293:1164–1166. doi: 10.1126/science.1060937. [DOI] [PubMed] [Google Scholar]
- Drici MD, Raybaud F, De Lunardo C, Iacono P, Gustovic P. Influence of the behaviour pattern on the nocebo response of healthy volunteers. Br J Clin Pharmacol. 1995;39:204–206. doi: 10.1111/j.1365-2125.1995.tb04434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans FJ. Expectancy, therapeutic instructions, and the placebo response. In: White L, Tursky B, Schwartz G, editors. Placebo: theory, research and mechanisms. New York: Guilford; 1985. pp. 215–228. [Google Scholar]
- Fuster JM. Executive frontal functions. Exp Brain Res. 2000;133:66–70. doi: 10.1007/s002210000401. [DOI] [PubMed] [Google Scholar]
- Gleidman L, Gantt W, Teitelbaum H. Some implications of conditioned reflex studies for for placebo research. Am J Psychiatry. 1957;113:1103–1107. doi: 10.1176/ajp.113.12.1103. [DOI] [PubMed] [Google Scholar]
- Goebel MU, Hubell D, Kou W, Janssen OE, Katsarava Z, Limmroth V, Schedlowski M. Behavioral conditioning with interferon beta-1a in humans. Physiol Behav. 2005;84:807–814. doi: 10.1016/j.physbeh.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Gracely RH, Dubner R, Wolskee PJ, Deeter WR. Placebo and naloxone can alter post-surgical pain by separate mechanisms. Nature. 1983;306:264–265. doi: 10.1038/306264a0. [DOI] [PubMed] [Google Scholar]
- Grevert P, Albert L, Goldstein A. Partial antagonism of placebo analgesia by naloxone. Pain. 1983;16:129–143. doi: 10.1016/0304-3959(83)90203-8. [DOI] [PubMed] [Google Scholar]
- Harris RE, Clauw DJ, Scott DJ, McLean SA, Gracely RH, Zubieta JK. Decreased central mu-opioid receptor availability in fibromyalgia. J Neurosci. 2007;27:10000–10006. doi: 10.1523/JNEUROSCI.2849-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haygarth J. Of the imagination, as a cause and as a cure of disorders of the body, exemplified by ficticious tractors, and epidemical convulsions. Bath: Crutwell; 1801. [Google Scholar]
- Herrnstein R. Placebo effect in the rat. Science. 1962;138:677–678. doi: 10.1126/science.138.3541.677. [DOI] [PubMed] [Google Scholar]
- Hofbauer RK, Rainville P, Duncan GH, Bushnell MC. Cortical representation of the sensory dimension of pain. J Neurophysiol. 2001;86:402–411. doi: 10.1152/jn.2001.86.1.402. [DOI] [PubMed] [Google Scholar]
- Horvitz J. Mesolimbic and nigrostriatal dopamine responses to salient non-rewarding stimuli. Neuroscience. 2000;96:651–656. doi: 10.1016/s0306-4522(00)00019-1. [DOI] [PubMed] [Google Scholar]
- Hrobjartsson A, Gotzsche P. Is the placebo powerless? An analysis of clinical trials comparing placebo with no treatment. N Engl J Med. 2001;344:1594–1602. doi: 10.1056/NEJM200105243442106. [DOI] [PubMed] [Google Scholar]
- Hrobjartsson A, Gotzsche PC. Is the placebo powerless? Update of a systematic review with 52 new randomized trials comparing placebo with no treatment. J Intern Med. 2004;256:91–100. doi: 10.1111/j.1365-2796.2004.01355.x. [DOI] [PubMed] [Google Scholar]
- Jones AK, Cunningham VJ, Ha-Kawa S, Fujiwara T, Luthra SK, Silva S, Derbyshire S, Jones T. Changes in central opioid receptor binding in relation to inflammation and pain in patients with rheumatoid arthritis. Br J Rheumatol. 1994;33:909–916. doi: 10.1093/rheumatology/33.10.909. [DOI] [PubMed] [Google Scholar]
- Jones AK, Watabe H, Cunningham VJ, Jones T. Cerebral decreases in opioid receptor binding in patients with central neuropathic pain measured by [11C]diprenorphine binding and PET. Eur J Pain. 2004;8:479–485. doi: 10.1016/j.ejpain.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Churchill L, Romanides A. Involvement of the pallidal-thalamocortical circuit in adaptive behavior. Ann N Y Acad Sci. 1999;877:64–70. doi: 10.1111/j.1749-6632.1999.tb09261.x. [DOI] [PubMed] [Google Scholar]
- Knutson B, Bjork JM, Fong GW, Hommer D, Mattay VS, Weinberger DR. Amphetamine modulates human incentive processing. Neuron. 2004;43:261–269. doi: 10.1016/j.neuron.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Kong J, Gollub RL, Rosman IS, Webb JM, Vangel MG, Kirsch I, Kaptchuk TJ. Brain activity associated with expectancy-enhanced placebo analgesia as measured by functional magnetic resonance imaging. J Neurosci. 2006;26:381–388. doi: 10.1523/JNEUROSCI.3556-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanotte M, Lopiano L, Torre E, Bergamasco B, Colloca L, Benedetti F. Expectation enhances autonomic responses to stimulation of the human subthalamic limbic region. Brain Behav Immun. 2005;19:500–509. doi: 10.1016/j.bbi.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Levine J, Gordon N, Fields H. The mechanism of placebo analgesia. Lancet. 1978;2:654–657. doi: 10.1016/s0140-6736(78)92762-9. [DOI] [PubMed] [Google Scholar]
- Levine JD, Gordon NC. Influence of the method of drug administration on analgesic response. Nature. 1984;312:755–756. doi: 10.1038/312755a0. [DOI] [PubMed] [Google Scholar]
- Levine JD, Gordon NC, Smith R, Fields HL. Analgesic responses to morphine and placebo in individuals with postoperative pain. Pain. 1981;10:379–389. doi: 10.1016/0304-3959(81)90099-3. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- Long DM, Uematsu S, Kouba RB. Placebo responses to medical device therapy for pain. Stereotact Funct Neurosurg. 1989;53:149–156. doi: 10.1159/000099531. [DOI] [PubMed] [Google Scholar]
- Longo DL, Duffey PL, Kopp WC, Heyes MP, Alvord WG, Sharfman WH, Schmidt PJ, Rubinow DR, Rosenstein DL. Conditioned immune response to interferon-gamma in humans. Clin Immunol. 1999;90:173–181. doi: 10.1006/clim.1998.4637. [DOI] [PubMed] [Google Scholar]
- Mayberg HS. Limbic-cortical dysregulation: a proposed model of depression. J Neuropsychiatry Clin Neurosci. 1997;9:471–481. doi: 10.1176/jnp.9.3.471. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Silva JA, Brannan SK, Tekell JL, Mahurin RK, McGinnis S, Jerabek PA. The functional neuroanatomy of the placebo effect. Am J Psychiatry. 2002;159:728–737. doi: 10.1176/appi.ajp.159.5.728. [DOI] [PubMed] [Google Scholar]
- McGlashan TH, Evans FJ, Orne MT. The nature of hypnotic analgesia and placebo response to experimental pain. Psychosom Med. 1969;31:227–246. doi: 10.1097/00006842-196905000-00003. [DOI] [PubMed] [Google Scholar]
- McNair D, M L, Droppleman L. EdITS manual for the Profile of Mood States. San Diego, CA: Educational and Industrial Testing Service (EdITS); 1992. [Google Scholar]
- Melzack R, Katz J. The McGill Pain Questionnaire: Appraisal and Current Status. In: Turk D, Melzack R, editors. Handbook of Pain Assessment. New York: Guilford Press; 2000. pp. 152–168. [Google Scholar]
- Mogenson GJ, Yang CR. The contribution of basal forebrain to limbic-motor integration and the mediation of motivation to action. Adv Exp Med Biol. 1991;295:267–290. doi: 10.1007/978-1-4757-0145-6_14. [DOI] [PubMed] [Google Scholar]
- Montgomery G, Kirsch I. Classical conditioning and the placebo effect. Pain. 1997;72:107–113. doi: 10.1016/s0304-3959(97)00016-x. [DOI] [PubMed] [Google Scholar]
- Napier T, Mitrovic I. Opioid modulation of ventral pallidal inputs. Ann N Y Acad Sci. 1999;877:176–201. doi: 10.1111/j.1749-6632.1999.tb09268.x. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Dietrich T, Fransson P, Andersson J, Carlsson K, Ingvar M. Placebo in emotional processing--induced expectations of anxiety relief activate a generalized modulatory network. Neuron. 2005;46:957–969. doi: 10.1016/j.neuron.2005.05.023. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Ingvar M. Imaging cognitive modulation of pain processing. Pain. 2002;95:1–5. doi: 10.1016/s0304-3959(01)00467-5. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Kalso E, Petersson KM, Ingvar M. Placebo and opioid analgesia--imaging a shared neuronal network. Science. 2002;295:1737–1740. doi: 10.1126/science.1067176. [DOI] [PubMed] [Google Scholar]
- Pollo A, Vighetti S, Rainero I, Benedetti F. Placebo analgesia and the heart. Pain. 2003;102:125–133. doi: 10.1016/s0304-3959(02)00345-7. [DOI] [PubMed] [Google Scholar]
- Price D, Milling L, Kirsch I, Duff A, Montgomery G, Nicholls S. An analysis of factors that contribute to the magnitude of placebo analgesia in an experimental paradigm. Pain. 1999;83:147–156. doi: 10.1016/s0304-3959(99)00081-0. [DOI] [PubMed] [Google Scholar]
- Price DD, Barrell JJ. Mechanisms of analgesia produced by hypnosis and placebo suggestions. Prog Brain Res. 2000;122:255–271. doi: 10.1016/s0079-6123(08)62144-5. [DOI] [PubMed] [Google Scholar]
- Price DD, Finniss DG, Benedetti F. A comprehensive review of the placebo effect: recent advances and current thought. Annu Rev Psychol. 2008;59:565–590. doi: 10.1146/annurev.psych.59.113006.095941. [DOI] [PubMed] [Google Scholar]
- Rainville P, Duncan G, Price D, Carrier B, Bushnell M. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science. 1997;277:968–971. doi: 10.1126/science.277.5328.968. [DOI] [PubMed] [Google Scholar]
- Raz A, Fan J, Posner MI. Hypnotic suggestion reduces conflict in the human brain. Proc Natl Acad Sci U S A. 2005;102:9978–9983. doi: 10.1073/pnas.0503064102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Behavioral theories and the neurophysiology of reward. Annu Rev Psychol. 2006;57:87–115. doi: 10.1146/annurev.psych.56.091103.070229. [DOI] [PubMed] [Google Scholar]
- Scott DJ, Stohler CS, Egnatuk CM, Wang H, Koeppe RA, Zubieta JK. Individual differences in reward responding explain placebo-induced expectations and effects. Neuron. 2007;55:325–336. doi: 10.1016/j.neuron.2007.06.028. [DOI] [PubMed] [Google Scholar]
- Scott DJ, Stohler CS, Egnatuk CM, Wang H, Koeppe RA, Zubieta JK. Placebo and nocebo effects are defined by opposite opioid and dopaminergic responses. Arch Gen Psychiatry. 2008;65:220–231. doi: 10.1001/archgenpsychiatry.2007.34. [DOI] [PubMed] [Google Scholar]
- Seeman P, Wilson A, Gmeiner P, Kapur S. Dopamine D2 and D3 receptors in human putamen, caudate nucleus, and globus pallidus. Synapse. 2006;60:205–211. doi: 10.1002/syn.20298. [DOI] [PubMed] [Google Scholar]
- Siegel S. Drug anticipatory responses in animals. In: White L, Tursky B, Schwartz G, editors. Placebo: theory, research and mechanisms. New York: Guilford; 1985. pp. 288–305. [Google Scholar]
- Stockhorst U, Steingruber HJ, Scherbaum WA. Classically conditioned responses following repeated insulin and glucose administration in humans. Behav Brain Res. 2000;110:143–159. doi: 10.1016/s0166-4328(99)00192-8. [DOI] [PubMed] [Google Scholar]
- Stohler C, Kowalski C. Spatial and temporal summation of sensory and affective dimensions of deep somatic pain. Pain. 1999;79:165–173. doi: 10.1016/s0304-3959(98)00171-7. [DOI] [PubMed] [Google Scholar]
- Tobler PN, Fiorillo CD, Schultz W. Adaptive coding of reward value by dopamine neurons. Science. 2005;307:1642–1645. doi: 10.1126/science.1105370. [DOI] [PubMed] [Google Scholar]
- Tom SM, Fox CR, Trepel C, Poldrack RA. The neural basis of loss aversion in decision-making under risk. Science. 2007;315:515–518. doi: 10.1126/science.1134239. [DOI] [PubMed] [Google Scholar]
- Uslaner J, Badiani A, Day HE, Watson SJ, Akil H, Robinson TE. Environmental context modulates the ability of cocaine and amphetamine to induce c-fos mRNA expression in the neocortex, caudate nucleus, and nucleus accumbens. Brain Res. 2001;920:106–116. doi: 10.1016/s0006-8993(01)03040-2. [DOI] [PubMed] [Google Scholar]
- Vase L, Riley JL, 3rd, Price DD. A comparison of placebo effects in clinical analgesic trials versus studies of placebo analgesia. Pain. 2002;99:443–452. doi: 10.1016/S0304-3959(02)00205-1. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Ma Y, Fowler JS, Wong C, Jayne M, Telang F, Swanson JM. Effects of expectation on the brain metabolic responses to methylphenidate and to its placebo in non-drug abusing subjects. Neuroimage. 2006;32:1782–1792. doi: 10.1016/j.neuroimage.2006.04.192. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Ma Y, Fowler JS, Zhu W, Maynard L, Telang F, Vaska P, Ding YS, Wong C, Swanson JM. Expectation enhances the regional brain metabolic and the reinforcing effects of stimulants in cocaine abusers. J Neurosci. 2003;23:11461–11468. doi: 10.1523/JNEUROSCI.23-36-11461.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voudouris NJ, Peck CL, Coleman G. Conditioned response models of placebo phenomena: further support. Pain. 1989;38:109–116. doi: 10.1016/0304-3959(89)90080-8. [DOI] [PubMed] [Google Scholar]
- Voudouris NJ, Peck CL, Coleman G. The role of conditioning and verbal expectancy in the placebo response. Pain. 1990;43:121–128. doi: 10.1016/0304-3959(90)90057-K. [DOI] [PubMed] [Google Scholar]
- Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, Kosslyn SM, Rose RM, Cohen JD. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science. 2004;303:1162–1167. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- Wall P. Pain and the placebo response. New York: Wiley; 1993. [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Personal Soc Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Wickramasekera I. A conditioned response model of the placebo effect predictions from the model. Biofeedback Self Regul. 1980;5:5–18. doi: 10.1007/BF00999060. [DOI] [PubMed] [Google Scholar]
- Willoch F, Rosen G, Tolle T, Oye I, Wester H, Berner N, Schwaiger M, Bartenstein P. Phantom limb pain in the human brain: unraveling neural circuitries of phantom limb sensations using positron emission tomography. Ann Neurol. 2000;48:842–849. [PubMed] [Google Scholar]
- Willoch F, Schindler F, Wester HJ, Empl M, Straube A, Schwaiger M, Conrad B, Tolle TR. Central poststroke pain and reduced opioid receptor binding within pain processing circuitries: a [11C]diprenorphine PET study. Pain. 2004;108:213–220. doi: 10.1016/j.pain.2003.08.014. [DOI] [PubMed] [Google Scholar]
- Wood PB, Schweinhardt P, Jaeger E, Dagher A, Hakyemez H, Rabiner EA, Bushnell MC, Chizh BA. Fibromyalgia patients show an abnormal dopamine response to pain. Eur J Neurosci. 2007;25:3576–3582. doi: 10.1111/j.1460-9568.2007.05623.x. [DOI] [PubMed] [Google Scholar]
- Zhang X, Ashton-Miller JA, Stohler CS. A closed-loop system for maintaining constant experimental muscle pain in man. IEEE Trans Biomed Eng. 1993;40:344–352. doi: 10.1109/10.222327. [DOI] [PubMed] [Google Scholar]
- Zubieta JK, Bueller JA, Jackson LR, Scott DJ, Xu Y, Koeppe RA, Nichols TE, Stohler CS. Placebo effects mediated by endogenous opioid activity on mu-opioid receptors. J Neurosci. 2005;25:7754–7762. doi: 10.1523/JNEUROSCI.0439-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubieta JK, Yau WY, Scott DJ, Stohler CS. Belief or Need? Accounting for individual variations in the neurochemistry of the placebo effect. Brain Behav Immun. 2006;20:15–26. doi: 10.1016/j.bbi.2005.08.006. [DOI] [PubMed] [Google Scholar]