Abstract
Context
Cochlear implantation (CI) is a surgical alternative to traditional amplification (hearing aids) that can facilitate spoken language development in young children with severe-to-profound sensorineural hearing loss (SNHL).
Objective
To prospectively assess spoken language acquisition following CI in young children with adjustment of co-variates.
Design, Setting, and Participants
Prospective, longitudinal, and multidimensional assessment of spoken language growth over a 3-year period following CI. Prospective cohort study of children who underwent CI before 5 years of age (n=188) from 6 US centers and hearing children of similar ages (n=97) from 2 preschools recruited between November, 2002 and December, 2004. Follow-up completed between November, 2005 and May, 2008.
Main Outcome Measures
Performance on measures of spoken language comprehension and expression.
Results
Children undergoing CI showed greater growth in spoken language performance (10.4;[95% confidence interval: 9.6–11.2] points/year in comprehension; 8.4;[7.8–9.0] in expression) than would be predicted by their pre-CI baseline scores (5.4;[4.1–6.7] comprehension; 5.8;[4.6–7.0] expression). Although mean scores were not restored to age-appropriate levels after 3 years, significantly greater annual rates of language acquisition were observed in children who were younger at CI (1.1;[0.5–1.7] points in comprehension per year younger; 1.0;[0.6–1.5] in expression), and in children with shorter histories of hearing deficit (0.8;[0.2,1.2] points in comprehension per year shorter; 0.6;[0.2–1.0] for expression). In multivariable analyses, greater residual hearing prior to CI, higher ratings of parent-child interactions, and higher SES associated with greater rates of growth in comprehension and expression.
Conclusions
The use of cochlear implants in young children was associated with better spoken language learning than would be predicted from their pre-implantation scores. However, discrepancies between participants’ chronologic and language age persisted after CI, underscoring the importance of early CI in appropriately selected candidates.
INTRODUCTION
Young children who experience severe-to-profound sensorineural hearing loss (SNHL) face challenges in developing spoken language because of an inability to detect acoustic-phonetic cues that are essential for speech recognition, even when fitted with traditional amplification devices (hearing aids). Over half the children identified with early, severe-to-profound SNHL are treated with cochlear implantation (CI).1 CI systems are comprised of an externally worn microphone and a microprocessor programmed to extract intensity, frequency and timing cues from acoustic signals. The system transforms these acoustic cues into a physiologically useful electrical code. Internally, a surgically-placed receiver relays the transmitted code to an implanted array of contacts in the cochlea to stimulate surviving auditory neurons.2 With experience, children understand speech, environmental sounds, and music with varying degrees of success after CI.3–6
Intervening at early ages with CI is predicated on behavioral data suggesting language performance is more accurate the earlier children can be implanted. 3–8 Early implantation may take advantage of neuronal flexibility inherent in critical periods of auditory-based learning. 9 Safety and technical concerns regarding early CI have been addressed with continued refinements of medical and surgical approaches. 10 Support for early implantation, however, must be tempered until sufficient longitudinal data are available. Behavioral studies supporting CI primarily use retrospective and case-series designs11 and variance in observed results is notoriously high.3–7 As a consequence, criteria remain unspecified regarding the timing of CI, especially those concerning the level of hearing loss and the associated delays in verbal language that should prompt CI.
To better understand the child, family, and clinical factors that promote verbal language growth after early CI, the Childhood Development after Cochlear Implantation (CDaCI) investigative group initiated a prospective study of spoken language outcomes in a cohort of children who underwent CI prior to the age of 5 years at 6 major US implant centers.12–15
METHODS
Study Design and Setting
Details of the study design have been published previously.14 Participants were enrolled between 11/02 and 12/04; 3-year follow-up was completed between 11/05 and 5/08. Children with SNHL were enrolled prior to CI through 6 large implant centers situated in different regions of the US. Children with normal hearing were enrolled from 2 private preschools affiliated with each of 2 implant centers. The study was approved by the centers’ Institutional Review Boards and written informed consent was obtained from the parent(s) of each enrolled child.
Study Population
For the experimental group, children under the age of 5 years with severe-to-profound SNHL were consecutively screened for CI based on the absence of medical contraindications and an inability to amplify the acoustic-phonetic cues of speech to audible levels. Children deemed candidates for CI were enrolled based on developmental criteria described below and a willingness of their parents to participate in a longitudinal study. Inclusion of a normal-hearing group served as a reference for longitudinal assessment of language development. Normal hearing children within the age range of children undergoing CI who met the same developmental criteria were enrolled based on family willingness to participate.
Inclusion criteria for both groups required that 1) parents speak English and either plan to, or had already enrolled their child in English-speaking schools, and 2) the child attained scores within 2 SDs of the norms on the Bayley Scales of Infant Development Motor Scale (BSID II)16 or Leiter International Performance Scale Revised (Leiter-R)17. Performance within 2 SDs served as an indicator that the child demonstrated cognitive and motor skills appropriate for their chronological age16, 17 Children were also excluded if they had any condition that prevented testing with the Reynell Developmental Language Scales (RDLS).18,19
Data Collection and Testing
At baseline, parents completed questionnaires on family demographics, communication, educational services, and their child’s hearing and medical history. Birth and medical records were used to determine periods of normal hearing, hearing loss without intervention, and amplification prior to implantation. Residual hearing was assessed in each ear as thresholds for pure-tones at 500, 1000, 2000 and 4000 Hz. Hearing was determined for each ear, and the average threshold for the better hearing ear served as a proxy for residual hearing.
A battery of tests (Table 1) was administered at each data point. In addition to the RDLS scores, measures of speech recognition and videotaped parent-child interactions were collected and coded to assess their co-variation with RDLS performance. All measures were administered pre-CI (baseline) and at follow-up visits scheduled for 6, 12, 24 and 36 months post-CI activation. For hearing children, follow-up visits were scheduled for the same time intervals as CI, but anchored at 6 weeks after baseline to correspond to the delay imposed by post-surgical healing and activation of the implant in children who underwent CI.
Table 1.
Measures: Domain Probed, Age Administered, Respondent, Construct(s) Measured and Interpretation
| Domain/Use | Test | Age | Respondent | Construct(s) Measured | Interpretation |
|---|---|---|---|---|---|
| Cognition/Screening | Bayley Scales of Infant Development16 | <2 years | Child | Physical and mental development | Exclusion from study if >2 SDs from age-normed scores established in typical childhood populations |
| Leiter Brief Form17 | >2 years | Child | Nonverbal cognitive abilities | ||
| Language/1° Outcome | Reynell Developmental Language Scales18,19 | 1 year – 6 years 11 months | Child | Comprehension and expressive skills of spoken language | Score relative to age-normed scores established in typical childhood populations |
| Speech Recognition Index13,20/2° Outcome | Infant-toddler meaningful auditory Integration scale (IT-MAIS)21 | 1–3 years | Parent | Parent perception of everyday communication behaviors | Hierarchical battery of tests of speech recognition The SRI generates scores between 0–600 based on: IT-MAIS/MAIS: 0–100 ESP: 101–200 PSI: 201–300 MLNT/LNT: 301–400 PBK: 401–500 HINT-C: 501–600 |
| Meaningful auditory integration scale (MAIS)22 | >4 years | Parent | Parent perception of everyday communication behaviors | ||
| Early speech perception (low verbal)23 | >2 years | Child | Word perception using toys | ||
| Early speech perception (standard)23 | 3–7 years | Child | Word perception requiring a picture-pointing response | ||
| Pediatric Speech Intelligibility (PSI-Format II) (standard)24 | 3–7 years | Child | Word/sentence stimuli requiring a picture-pointing response | ||
| Multisyllabic/lexical Neighborhood Test (MLNT)25 | >3 years | Child | Word recognition ability requiring verbal response | ||
| Lexical Neighborhood Test (LNT)25 | >4 years | Child | Word recognition ability requiring verbal response | ||
| Phonetically balanced word list (PBK)26 | >5 years | Child | Word recognition ability requiring verbal response | ||
| Hearing in Noise Test (HINT-C)27 | >5 years | Child | Word and sentence recognition requiring verbal response | ||
| Video analysis of child/provider interaction/2° Outcome | Parent-child interactions videotaped and coded28:
|
Toy sets selected for age of child | Parent-Child interaction | Respect for child autonomy, positive regard, cognitive stimulation, shared visual attention, bi-directional interaction | Mean scaled scores of 2 observers rating parental sensitivity, time in joint attention, and communicative competence |
Main Outcome Measures
The RDLS18,19 comprehension and expressive language scales were administered as interdependent measures of spoken language performance at baseline (pre-CI for children undergoing CI. Age-equivalent language level (language age) was determined based on RDLS scores. If language level is “on par” with the mean established by normative data from hearing children of the same age, the gap between a child’s chronologic and language ages would be 0; if scores match those expected of a child 1 year younger, the gap is 1 year.
Secondary measures addressed emergent speech recognition and characteristics of parent-child interactions. Speech recognition was represented by the Speech Recognition Index (SRI) (Table 1) summarizing the scores collected from a hierarchical battery of speech recognition tests and developed specifically for this study to track speech recognition performance via growth curve analysis.13,20 Videos of parent-child interactions were coded for respect for child autonomy, positive regard, cognitive stimulation, shared visual attention, and bi-directional interaction (Table 1).12,28
Sample Size Determination
Sample size was based upon 80% statistical power to detect a 1.3 points/year growth in RDLS raw score between 3 equal-sized (2 CI and 1 normal hearing) subgroups in a 3 year, longitudinal analysis. The needed parameters for the calculation were adopted from reported RDLS scores of children undergoing CI.18, 29
Statistical Analysis
Baseline demographic, socioeconomic and medical history factors were characterized as means and standard deviations for continuous variables and as frequency distributions for categorical variables. Baseline comparisons between the children undergoing CI and hearing controls were tested using analysis of variance (ANOVA) for continuous variables and chi-square tests for categorical variables. Stratified analyses based on age at implantation were conducted for the CI group to identify factors associated with age at implantation and to explore post-CI spoken language growth and language age gaps.
Developmental trajectories of verbal language in different sub-groups were explored using nonparametric regression with locally-weighted smoothing scatterplot (lowess)30 to identify the mean trajectories without assuming a priori its parametric forms. The identified mean trajectories were then modeled using mixed-effects linear regression models, approximating any identified non-linear mean trajectories using segment-linear models for ease of interpretation. Child-specific intercepts and slopes over the period of follow-up were included as random effects, while other co-variates were modeled as fixed effects. Follow-up time, based on actual visit dates, provided the time variable.
Co-variates included child characteristics related to hearing thresholds and speech recognition performance; family characteristics related to caregiver sensitivity to communication and income; and intervention characteristics related to time spent in normal hearing, SNHL, length of traditional amplification, age at implantation, mode of communication and bilateral CI. Emergent speech recognition and bilateral CI were treated as time-dependent co-variates. Associations were adjusted for demographic characteristics and other baseline variables related to center, gender, race, ethnicity, maternal education (high school graduate or not), hearing onset, cognition, baseline RDLS scores, and time to CI activation. We performed sensitivity analyses evaluating the effect of censoring those children who underwent bilateral CI after their 2nd implant.
The rate of language growth, as modified by a given covariate, was modeled by including the cross-product of the follow-up time by co-variate as an interaction term. All longitudinal analyses were adjusted for centers. SAS version 9.1 (SAS Institute, Cary, NC) was used for all analyses, and all tests were two-sided (α= 0.05).
RESULTS
Study Population
For the experimental group, 425 children with severe-to-profound SNHL were consecutively screened; 268 were deemed appropriate candidates for CI and met inclusion criteria.. Families of 188 children (70%) were willing to participate and were enrolled. Families of 80 (30%) of these children did not wish to participate. Children with SNHL enrolled in the study did not differ from non-participants in average age or socioeconomic status, although there was a difference in racial background, with African American families constituting 19% of non-participants and 9% of participants.14 A total of 97 children with normal hearing who were within the age range of children undergoing CI were enrolled. The mean age at enrollment was 2.2 years for the CI group and 2.3 years for the normal hearing children. Thirty-one (17.4%) of the children undergoing CI received a second, contralateral ear implant after enrollment.
Table 2 shows baseline measures of hearing status, child and family demographics, and language status. Children undergoing CI and hearing controls differed in family income, scores of RDLS comprehension and expression, parents’ perception of communication behaviors (IT-MAIS/MAIS) 21,22, ratings of parent-child interactions, and cognition, as well as hearing thresholds.
Table 2.
Baseline Characteristics of CDaCI Participants
| Characteristic | Children Undergoing Cochlear Implantation Age at Implantation | Normal Hearing Children | |||
|---|---|---|---|---|---|
| <18 months (n=72) | 18–36 months (n=64) | >36–60 months (n=52) | All 9 months– 5 years (n=188) | 9 months–5 years (n=97) | |
| Means (SD) | |||||
| Age at baseline, months+ | 12.6 (3.1) | 27.1 (5.5) | 46.1 (7.3) | 26.7 (14.5) | 27.5 (13.1) |
| Age at SNHL dx, months+ | 3.8 (4.0) | 12.2 (8.5) | 18.0 (13.1) | 10.6 (10.5) | n/a |
| Time in hearing pre-op, months+ | 0.7 (2.4) | 1.6 (4.1) | 7.4 (12.2) | 2.8 (7.4) | n/a |
| Median {25th, 75th percentile} | 0.0 {0.0, 0.0} | 0.0 {0.0, 0.0} | 0.0 {0.0, 12.0} | 0.0 {0.0, 0.0} | |
| Time in hearing loss, months+ | 5.0 (3.4) | 13.4 (7.8) | 13.3 (10.3) | 10.1 (8.4) | n/a |
| Median {25th, 75th percentile} | 3.0 {4.0, 6.0} | 13.0 {6.0, 20.0} | 12.0 {4.0, 21.5} | 6.0 {3.0, 15.5} | |
| Time in amplification, months+ | 6.8 (3.4) | 11.9 (8.9) | 24.9 (15.3) | 13.5 (12.1) | n/a |
| Median {25th, 75th percentile} | 7.0 {4.4, 9.3} | 5.3 {2.2, 10.0} | 23.3 {12.9, 38.9} | 9.5 {5.3, 17.8} | |
| Age at activation, months+ | 15.5 (3.2) | 29.4 (5.6) | 48.5 (7.4) | 29.4 (14.4) | n/a |
| % time in hearing pre-op+ | 4.9 (15.8) | 6.1 (15.6) | 16.0 (26.8) | 8.2 (19.8) | n/a |
| % time in hearing loss+ | 39.1 (21.6) | 50.4 (27.9) | 29.8 (24.0) | 40.4 (25.7) | n/a |
| % time in amplification+ | 55.7 (25.3) | 43.0 (29.5) | 52.3 (28.9) | 50.4 (28.2) | n/a |
| 4 tone hearing threshold average, dB, better ear+ | 108.9 (15.6) | 105.1 (15.6) | 100.0 (17.4) | 105.1 (16.4) | 14.2 (7.1)* |
| RDLS Verbal Comprehension+ | 0.8 (1.7) | 3.9 (6.4) | 13.5 (13.4) | 5.8 (9.8) | 30.1 (18.3)* |
| RDLS Expressive Language+ | 3.4 (1.8) | 8.0 (6.2) | 17.9 (12.9) | 9.5 (10.0) | 27.6 (15.2)* |
| MAIS+ | 12.4 (15.0) | 22.7 (23.0) | 38.0 (31.4) | 23.0 (25.3) | 97.1 (7.7)* |
| Average Parent-Child Interaction score | 5.4 (0.7) | 5.2 (0.8) | 5.1 (0.6) | 5.2 (0.7) | 5.7 (0.6)* |
| Cognitive Status | |||||
| Bayley PDI (< 2 years)+ | 94.7 (18.3) [n=61] | 95.7 (19.4) [n=51] | 79.5 (18.3) [n=18] | 93.0 (19.4) | 108.2 (14.7)* [n=41] |
| Leiter Brief IQ (> 2 years) | 102.5 (21.7) [n=10] | 100.3 (13.4) [n=12] | 106.3 (21.0) [n=33] | 104.3 (19.5) | 109.4 (11.5) [n=53] |
| Combined1 | 97.6 (19.9) | 97.7 (19.5) | 93.4 (24.9) | 96.5 (21.2) | 106.9 (15.1)* |
| N (%) | |||||
| Race, white | 58 (81) | 42 (66) | 40 (77) | 140 (74) | 77 (79) |
| Gender, female+ | 32 (44) | 31 (48) | 35 (67) | 98 (52) | 60 (62) |
| Maternal Education, HS graduate+ | 70 (97) | 60 (94) | 43 (83) | 173 (93) | 91 (95) |
| Hispanic | 9 (13) | 17 (27) | 11 (22) | 37 (20) | 9 (10) |
| Household income, $50,000+ | 43 (60) | 24 (38) | 21 (40) | 88 (46) | 76 (78)* |
| Congenital+ | 56 (78) | 31 (51) | 18 (38) | 105 (56) | n/a |
| Communication Mode+ | |||||
| Speech Only | 19 (26) | 8 (13) | 10 (19) | 37 (20) | n/a |
| Speech/Sign: Speech emphasis+ | 14 (19) | 13 (20) | 8 (15) | 35 (19) | n/a |
| Speech/Sign: Sign emphasis+ | 1 (1) | 4 (6) | 7 (13) | 12 (6) | n/a |
| Sign Only | 12 (17) | 19 (30) | 4 (8) | 35 (19) | n/a |
| Other/NR | 26 (36) | 20 (31) | 23 (44) | 69 (37) | n/a |
Cognitive Status measured Bayley Psychomotor Developmental Index score for children < 24 months of age and by Leiter Brief IQ score for children > 24 months of age.
Differences among children undergoing CI at <18 months; 18–36 months; and >36 months (P<0.05).
Differences between children undergoing CI and normal hearing children (P<0.05).
Children undergoing CI were stratified into 3 groups by age of implantation: <18 months (N=72; 38%); 18–36 months (N=64; 34%), and >36 months (N=52; 28%). These 3 groups demonstrated significantly different lengths of time spent in hearing, SNHL and amplification (Table 1). There were significant differences in their baseline RDLS comprehension and expression and MAIS scores, gender, maternal education, family income, congenital onset of SNHL, and communication mode. They did not differ in mean baseline scores of parent-child interactions or cognition.
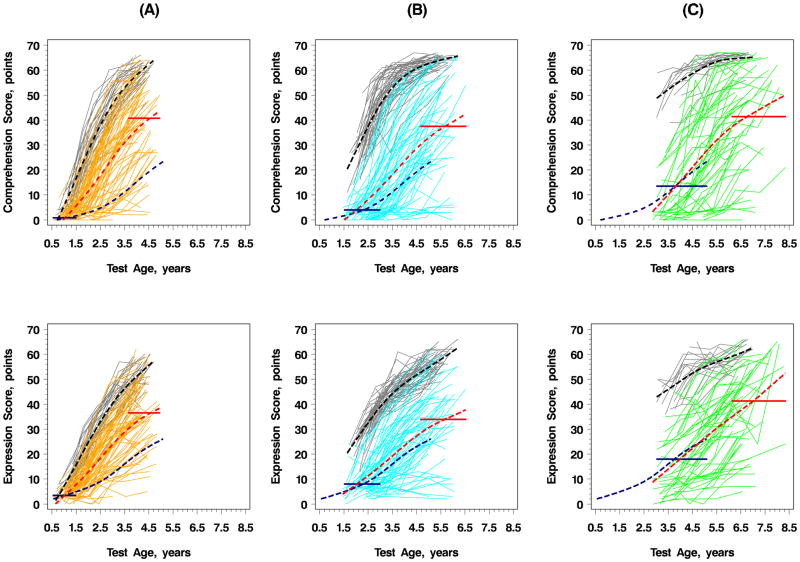
Figure 1 demonstrates the trajectories of raw score changes on the RDLS comprehension and expressive language scales over the 3 year follow-up. Children who underwent CI demonstrated slower and more variable language trajectories compared with hearing children. However, children undergoing CI produced steeper growth trajectories than those predicted by their baseline comprehension and expression scores. Significantly higher rates of comprehension and expression were noted in children undergoing CI at <18 months of age, compared with children undergoing CI between 18–36 months, and >36 months. The majority of children implanted prior to 18 months revealed trajectories of growth that paralleled those of hearing controls. CI after 18 months of age was associated with less favorable trajectories of growth in performance and greater variability in measures of both comprehension and expression.
Figure 1.
Developmental Trajectories of RDLS Raw Scores of Comprehension and Expression Grouped by Age At Baseline
Hearing children in gray (A) <18 months, (B) 18–36 months, and (C) >36 months; mean trajectories of sub-groups by age of enrollment in black. Children undergoing CI: (A) Implant age <18 months in orange, (B) 18–36 months in blue, (C) >36 months in green. Mean trajectories for respective age group in red dash. Mean score after 3 years designated by horizontal line, the width of which spans the age range at time of testing. Mean scores established by baseline (pre-CI) scores of children undergoing CI are shown in each plot in navy blue dash.
Unadjusted, mixed-effects modeling analyses revealed that after 3 years, children who underwent CI had a mean deficit of 22.3;(95% confidence interval:19.4–25.2) points in comprehension and 19.8;(17.3–22.3) points in expression compared with hearing peers after 3 years. When stratified by age, the average deficit in comprehension scores for children undergoing CI was 8.1;(6.2–9.9) for those implanted at <18 months, 27.0;(23.8–30.1) for those implanted between 18–36 months, and 38.7;(34.2–43.2) for those implanted >36 months. The average deficit in expression scores for children undergoing CI was 8.2;(6.4–9.9) for those implanted <18 months, 21.7;(19.3–24.1) for those implanted between 18–36 months, and 29.4;(24.1–34.7) for those implanted after 36 months.
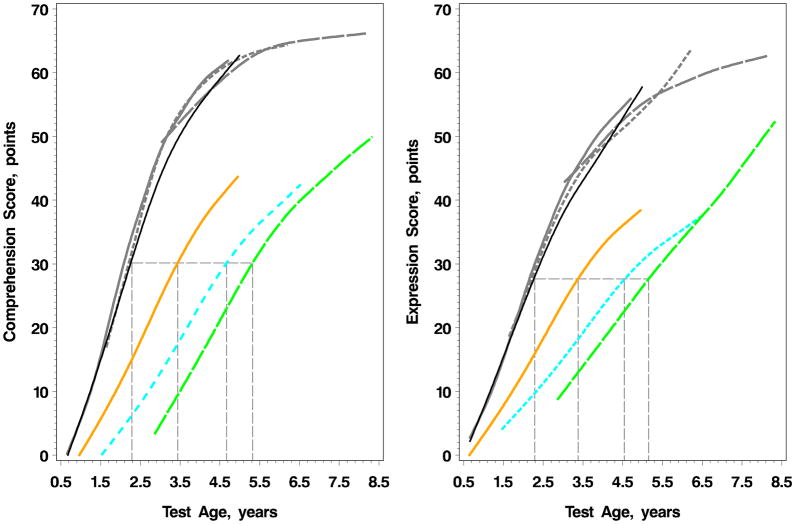
Trajectories of change in speech recognition capacity are shown in eFigure 1. Children undergoing CI showed mean rates of progress through the SRI hierarchy of speech recognition measures that were parallel to that of normal hearing children. Figure 2 demonstrates the chronologic age at which RDLS raw scores of 30.1 and 27.6 were obtained for comprehension and expression, respectively, representing the mean scores for the normal hearing children at baseline (at their enrollment age of 2.3 years). For children undergoing CI, this reference comprehension score was attained at 3.4, 4.7, and 5.3 years for children undergoing CI at <18 months, 18–36 months, and >36 months, respectively. For verbal expression, the reference score was attained at 3.4, 4.5, and 5.2 years for children undergoing CI at <18 months, 18–36 months, and >36 months, respectively.
Figure 2.
Nonparametric Fit of RDLS Raw Scores of Comprehension and Expression Stratified by Age at Baseline and Test Age
Children with normal hearing in gray lines (categorized by age at baseline: solid gray line < 18 months; short dashed gray line 18–36 months; and long dashed gray line between >36 months). Children undergoing CI: <18 months at implant age in orange, 18–36 months in cyan dash, and >36 months in green dash. Mean trajectories established by baseline scores of all normal hearing children in black. For comparative assessment, the horizontal, gray line projects the chronological age at which the mean scores of normal hearing children at baseline (30.1 for comprehension and 27.6 for expression) were obtained by sub-group of children undergoing CI at different age.
The vertical drop-lines indicate ages at which this score was obtained for each group of children. On the comprehension scale, the ages were 2.3 years for hearing children; 3.4 years for CI <18 months at implant age, 4.7 years for CI 18–36 months at implant age, 5.3 years for CI >36 months at implant age. On the expression scale, the ages were 2.3 years for hearing children; 3.4 years for CI <18 months at implant age, 4.5 years for CI 18–36 months at implant age, and 5.2 years for CI >36 months at implant age.
eFigure 2 demonstrates the mean trajectories of growth in comprehension and expression scores. Faster average rates of growth in verbal comprehension and language expression trajectories were associated with earlier age of CI (age at implant <18 months).
Table 3 compares the child’s chronologic age with their language age equivalent. Whereas the gap in language growth between children undergoing CI at <18 months and normal hearing children did not widen during follow-up, larger gaps accrued in children implanted at older ages.
Table 3.
Mean [95% confidence Interval] Estimates of Language Age for Children Undergoing CI and Normal Hearing Children at Baseline and 3Year Follow-up Based on Comprehension and Expression
| Children undergoing Cochlear Implantation | Normal Hearing | ||||||
|---|---|---|---|---|---|---|---|
| CI < 12† months | CI 12–18 months | <18 months | 18–36 months | 36–60 months | All | All | |
| BASELINE | N=33 | N=39 | N=72 | N=64 | N=52 | N=188 | N=97 |
| Chronologic Age, months | 9.8 [6.8–12.8] | 14.9 [12.2–17.7] | 12.6 [10.5–14.6] | 27.1 [24.9–29.3] | 46.1 [43.7–48.5] | 26.2 [24.2–28.1] | 27.5 [24.7–30.3] |
| Comprehension | |||||||
| N | --- | 34 | --- | 59 | 50 | 163 | 97 |
| Language Age, months | --- | < 13.0* | --- | 13.7 [11.5–16.0] | 18.3 [15.8–20.7] | 14.8 [13.5–16.2] | 28.6 [26.8–30.4] |
| ‡CDaCI Comprehension Age, months | --- | 9.6 [6.2–12.9] | --- | 11.5 [9.0–13.9] | 17.6 [14.9–20.3] | 12.7 [11.1–14.2] | 29.2 [27.2–31.2] |
| Gap Chronologic Age less Language Age | --- | --- | --- | 12.8 [11.4–14.3] | 27.2 [25.6–28.7] | 12.9 [11.3–14.5] | −0.3 [−2.4–1.7] |
| Gap Chronologic Age less CDaCI Age | --- | 5.6 [3.7–7.5] | --- | 15.1 [13.7–16.6] | 27.8 [26.2–29.3] | 15.1 [13.7–16.6] | −1.0 [−2.9–0.9] |
| Expression | |||||||
| Language Age, months | --- | < 16.0* | --- | 16.5 [14.5–18.6] | 21.2 [19.0–23.4] | 17.7 [16.5–19.0] | 27.6 [25.9–29.2] |
| ‡CDaCI Expressive Age, months | --- | 8.2 [4.5–11.9] | --- | 11.9 [9.1–14.7] | 20.4 [17.4–23.4] | 13.2 [11.5–15.0] | 29.8 [27.5–32.1] |
| Gap Chronologic Age less Language Age | --- | --- | --- | 10.0 [8.6–11.5] | 24.2 [22.6–25.8] | 10.0 [8.4–11.6] | 0.7 [−1.4–2.8] |
| Gap Chronologic Age less CDaCI Age | --- | 7.0 [4.7–9.3] | --- | 14.7 [13.0–16.4] | 25.0 [23.2–26.9] | 14.6 [13.2–16.0] | −1.6 [−3.4–0.3] |
| 36 MONTH FOLLOW-UP | N=32 | N=37 | N=69 | N=61 | N=50 | N=180 | N=84 |
| Chronologic Age, months | 49.5 [46.5–52.5] | 53.5 [50.7–56.4] | 51.6 [49.5–53.7] | 65.7 [63.5–67.9] | 85.0 [82.6–87.4] | 65.8 [63.7–67.9] | 64.1 [61.0–67.2] |
| Comprehension | |||||||
| N | 31 | 37 | 68 | 59 | 41 | 167 | 84 |
| Language Age, months | 35.5 [31.2–39.9] | 35.2 [31.3–39.1] | 35.4 [32.5–38.3] | 33.9 [30.7–37.0] | 37.2 [33.6–40.9] | 35.3 [33.5–37.2] | 58.0 [55.4–60.5] |
| ‡CDaCI Comprehension Age, months | 35.7 [31.8–39.6] | 35.0 [31.4–38.6] | 35.3 [32.7–38.0] | 33.5 [30.7–36.4] | 35.7 [32.3–39.1] | 34.8 [33.1–36.5] | 59.3 [56.9–61.7] |
| Gap Chronologic Age less Language Age | 13.6 [8.4–18.8] | 17.2 [12.4–22.0] | 15.5 [12.0–19.1] | 32.0 [28.2–35.8] | 46.3 [41.9–50.7] | 29.2 [26.4–31.9] | 5.9 [2.1–9.8] |
| Gap Chronologic Age less CDaCI Age | 13.7 [9.8–17.6] | 17.6 [13.9–21.3] | 15.8 [13.1–18.5] | 32.2 [29.4–35.1] | 47.8 [44.4–51.3] | 29.5 [27.2–31.9] | 4.5 [1.2–7.9] |
| Expression | |||||||
| Language Age, months | 34.4 [30.1–38.8] | 33.5 [29.5–37.5] | 33.9 [31.0–36.9] | 33.4 [30.2–36.6] | 40.4 [36.7–44.0] | 35.4 [33.5–37.3] | 56.2 [53.6–58.9] |
| ‡CDaCI Expressive Age, months | 35.8 [31.6–40.0] | 34.2 [32.0–39.8] | 35.9 [33.0–38.7] | 34.2 [31.2–37.3] | 40.2 [36.6–43.8] | 36.3 [34.5–38.2] | 59.4 [56.8–62.0] |
| Gap Chronologic Age less Language Age | 14.7 [9.7–19.7] | 19.2 [14.5–23.9] | 17.1 [13.7–20.5] | 32.5 [28.8–36.1] | 43.4 [39.2–47.6] | 29.3 [26.7–31.8] | 7.5 [3.9–11.1] |
| Gap Chronologic Age less CDaCI Age | 13.6 [9.5–17.8] | 16.9 [12.9–20.9] | 15.3 [12.5–18.2] | 31.5 [28.5–34.5] | 43.6 [40.0–47.2] | 28.1 [25.8–30.4] | 4.5 [1.2–7.8] |
The RDLS is not validated for age < 12 months, hence the oral language development is numerically not ascertainable through RDLS for the group “CI < 12” at Baseline.
The floor of RDLS language age measure is coded as “< 13 months” for comprehension, and “< 16 months” for expression. Language age measures below the RDLS floor are numerically not ascertainable through RDLS.
The CDaCI Comprehension Age and CDaCI Expression Age are scored according to the estimated mean ages when normal hearing children in the CDaCI Study attained these respective RDLS comprehension and expression scores
Independent associations of child-, family- and treatment variables with growth in comprehension and expressive skills are shown in Table 4. RDLS comprehension growth was positively associated with the amount of pre-CI residual hearing. Better baseline hearing thresholds (e.g. 85dB HL versus 105dB HL) were associated with greater growth in comprehension and expression. Although higher RDLS comprehension scores at baseline were associated with higher comprehension scores over the course of follow-up (P<0.0001), baseline comprehension was not significantly related to the rate of comprehension development.
Table 4.
Multivariable Adjusted† Mixed-Effects Modeling Analyses for Children Undergoing CI: Associations with RDLS Raw Scores of Comprehension and Expression at Baseline and Rate of Gain over 3 Years
| Comprehension | Expression | |||||
|---|---|---|---|---|---|---|
| Factors associated with RDLS raw scores at Baseline | Estimate | 95% Confidence Interval | P-value | Estimate | 95% Confidence Interval | P-value |
| Child Characteristics | ||||||
| Hearing threshold average, per 20 dB ↑ | −0.31 | −1.11–0.49 | 0.4491 | −0.17 | −0.75–−0.41 | 0.5668 |
| Family Characteristics | ||||||
| Mean Rating of Parent-Child Interactions, per point ↑ | 0.51 | −0.15–1.16 | 0.1306 | 0.59 | 0.06–1.12 | 0.0299 |
| Self-reported Income < $50k @ BL | 0.28 | −0.41–0.96 | 0.4251 | −0.24 | −0.72–0.24 | 0.3202 |
| Intervention Characteristics | ||||||
| Length Normal Hearing, per 6 months ↑ | 0.71 | 0.05–1.38 | 0.0360 | 0.62 | 0.24–0.99 | 0.0014 |
| Length Hearing Loss (prior to amplification), per 6 months ↑ | 0.01 | −0.42–0.44 | 0.9713 | 0.07 | −0.25–0.39 | 0.6800 |
| Length Amplification, per 6 months ↑ | −0.14 | −0.49–0.22 | 0.4432 | −0.02 | −0.32–0.28 | 0.8928 |
| Oral Communication Mode @ BL | 0.08 | −0.52–0.69 | 0.7839 | −0.14 | −0.53–0.26 | 0.5031 |
| Factors associated with differential rate of gain in RDLS raw scores over 3 Years | Estimate | 95% Confidence Interval | P-value | Estimate | 95% Confidence Interval | P-value |
| Child Characteristics | ||||||
| Hearing threshold average, per 20 dB ↑ | −2.28 | −4.31–−0.25 | 0.0280 | −2.24 | −4.20–−0.27 | 0.0257 |
| Speech Recognition Index, Time Dependent*, per 100 points ↑ | 3.76 | 3.07–4.45 | <.0001 | 1.98 | 1.41–2.54 | <.0001 |
| Family Characteristics | ||||||
| Mean Rating of Parent-Child Interactions, per point ↑ | 3.75 | 1.77–5.73 | 0.0002 | 3.45 | 1.60–5.29 | 0.0003 |
| Self-reported Income < $50k @ BL | −2.20 | −3.93–−0.47 | 0.0126 | −0.82 | −2.45–0.82 | 0.3290 |
| Intervention Characteristics | ||||||
| Length Normal Hearing, per 6 months ↑ | −1.45 | −2.70–−0.19 | 0.0236 | −2.20 | −3.60–−0.81 | 0.0021 |
| Length Hearing Loss (prior to amplification), per 6 months ↑ | −0.74 | −1.91–0.43 | 0.2120 | −0.51 | −1.63–0.60 | 0.3663 |
| Length Amplification, per 6 months ↑ | −1.31 | −2.11–−0.52 | 0.0013 | −1.12 | −1.79–−0.44 | 0.0012 |
| Oral Communication Mode @ BL | 1.57 | −0.22–3.35 | 0.0848 | 1.69 | −0.02–3.41 | 0.0523 |
| Bilateral CI Status, time-dependent | 2.19 | −0.86–2.55 | 0.1049 | 1.67 | −0.94–4.27 | 0.2087 |
Each variable is adjusted for study centers, gender, race, ethnicity, maternal education (high school graduate or not), hearing onset, cognition, baseline RDLS raw scores, time to CI activation, time dependent bilateral implant status, and all other variables listed in the Table.
SRI scores were included as a time-dependent variable. The average gain in SRI scores over the 3-year follow-up period was 349 points moving from an average of 45 point at Baseline to 395 points after 3 years.
Comprehension growth was not associated gender, congenital onset of SNHL, or baseline cognition level (Table 4). Growth in speech recognition was significantly associated with improvements in verbal language (P<0.0001).
Table 4 summarizes associations of language growth with characteristics of the child’s family. Higher parent-child interaction scores were significantly associated with higher growth rates of comprehension and expressive language. Although lower family income was strongly associated with reduced rates of growth in comprehension and expression using bi-variable analyses (both P <0.0001), these negative associations were either attenuated (comprehension, P<.05) or diminished (expression, P=.329) after adjustment (Table 4).
Longer periods of normal hearing (prior to onset of hearing loss) were associated with higher language scores at baseline after accounting for duration of hearing loss and pre-implant amplification. A reduced rate of language development after CI was associated with longer periods of hearing loss, without or with pre-CI amplification. Prolonged periods of hearing loss without and with amplification contributed to an older age at implantation and were associated with slower growth in language comprehension. eFigure 3 highlights the slow growth in comprehension and expression associated with extended hearing aid use in children who underwent CI at a later age.
Exclusive use of spoken communication at baseline did not significantly associate with RDLS growth in children undergoing CI (Table 4). Centers were found to be significantly associated with different rates of growth in comprehension scores. Bilateral implantation was associated with a statistically insignificant tendency for positive growth in RDLS comprehension and expression scores compared to unilateral implantation after adjusting for other variables. Results from the multivariable model analyses present in Table 4 (exc. Bilateral Status) were unchanged for 3-year comprehension and expression.
COMMENT
Parents commonly seek CI because they want their children with SNHL to hear and speak like children with normal hearing.14,31 Language learning through listening and speaking serves as an effective marker of later school performance in children with normal hearing.32,33
CI associated with significant improvement in comprehension and expression of spoken language over the first 3 years. Growth of spoken language was positively associated with earlier, as opposed to later, ages of CI and greater residual hearing prior to CI. Unfortunately, results also revealed that gaps in spoken language growth between normal hearing children and those who underwent CI were not eliminated in the first 3 years of implant use.
Consistent with the critical period concept for language learning, early implantation in infants and toddlers associates with significantly accelerated spoken language learning. Two indices of spoken language growth revealed the association between age of CI and spoken language outcome: Performance scores in children implanted younger are closer to scores of normal hearing controls (Figures 1 & 2) and older age at CI associated with greater gaps between chronological and language ages (Table 3).
The rate of growth in performance on spoken language measures was less steep in children undergoing CI at later ages. At birth, normal hearing infants discriminate speech sounds used in all languages.34 Hearing infants lose the ability to discriminate the sounds of other languages as they develop more precision with their native language, typically between 7 and 10 months of age.35, 36 Accurate native language discrimination at 7.5 months of age is associated with accelerated language abilities; conversely, continued non-native language discrimination is related to reduced language abilities later.37 Infants between 8 and 18 months of age use the statistical distributional properties of speech to identify the patterns contributing to the words of their language which, in turn, makes learning word meanings possible.38 Models of verbal language development stress that sound pattern learning requires a neural commitment to the acoustic properties of a native language.39 Children with early severe-to-profound SNHL do not experience similar neural commitments and, as such, spoken language growth is altered.
The decision to pursue CI must weigh the potential benefit of CI versus continued amplification of residual hearing. Here we observed that higher language scores at baseline were associated with greater residual hearing prior to CI. However, significantly reduced language learning was associated with the prolonged use of hearing aids prior to CI. These findings suggest that delaying implantation in order to extend hearing aid use for children with severe-to-profound hearing loss may be detrimental to language growth following CI. Spoken language learning relies on effective hearing, characterized as a young infant’s ability to perceive the acoustic-phonetic cues of speech. Close monitoring of performance with hearing aids can determine whether speech is effectively amplified to allow spoken language acquisition to progress without imposing cumulative delays.
Although spoken language outcomes were significantly associated with the age of implantation and residual hearing, associations with environmental factors were also evident. Maternal engagement in early communication reflected in greater scores of parent-child interactions associated with growth in spoken language skills. Language comprehension and expression are influenced by parent-child interactions in bidirectionalspoken communication.28, 40, 41 Language exposure and caregivers’ mentoring provide the context for language learning. Neuro-developmental mechanisms that support early language learning rely on interactional cues available almost exclusively in social settings.42
Family income above $50,000 was associated with better language performance at baseline (pre-CI). This factor also favorably associated with growth rates in language comprehension. These findings are consistent with studies of the persistent problem of lower language growth experienced by children of poor families.32 The notion that children reared in disadvantaged environments may have fewer early language experiences that are associated with optimal language development may extend to children undergoing CI. However, multivariable adjustment attenuated associations with income. Higher family income was associated with higher maternal education and greater maternal engagement in communication.
Some limitations of our study deserve comment. The observational design of this study and the absence of a control group of SNHL children without CI preclude causal conclusions. For ethical reasons, we could not randomize or match children with similar levels of SNHL who continue to use hearing aids. Such trials could formally test the efficacy of CI in children who are implanted at different ages or stages of linguistic development. Instead, baseline, pre-CI performance across candidates, provided an estimate of language learning trajectory of children with severe-to-profound SNHL and without CI. Although we employed rigorous adjustment procedures to mitigate the impact of potential confounds, residual confounding cannot be ruled out in this observational study.
Although bilateral CI associated with greater spoken language growth, this observation must be viewed cautiously given the brief period between the 2nd CI and the end of follow-up. Continued assessment will allow us to more carefully determine how such factors associate with longterm trends in the acquisition of spoken language skills.
The generalizability of our results beyond major implant centers is uncertain. The representativeness of the children with SNHL was likely influenced by access to participating centers and our inclusion criteria. Outcomes may have been influenced by the expertise of participating clinicians and other caregivers. Regional and other characteristics specific to participating implant centers were not directly evaluated.
CONCLUSIONS
Results from this study carry implications for the clinical management of children with severe-to-profound SNHL. Though not determinative, age at implantation and residual hearing are variables associated with growth rates for the acquisition of spoken language in children with cochlear implants. These findings underscore the need to develop objective tools that can monitor the benefit of amplification in supporting the emergence of early skills that support spoken language acquisition and guide timely intervention with CI. Environmental factors significantly associated with performance on measures of spoken language after CI and may further account for variation in observed outcomes.
Supplementary Material
Acknowledgments
The CDaCI was supported by Grant R01 DC004797 from the National Institute On Deafness And Other Communication Disorders, The CityBridge Foundation and the Sidgmore Familyl Foundation. The National Institute on Deafness And Other Communication Disorders of the National Institutes of Health, The CityBridge Foundation, and the Sidgmore Family Foundation played no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Warranties on the implant devices used by children with implants in this study were discounted by 50% by the Advanced Bionics Corporation, Cochlear Corporation, and the MedEl Corporation.
All authors are without financial interests and relationships and affiliations relevant to the subject of this manuscript. Dr. Niparko serves on advisory boards without remuneration for two cochlear implant manufacturers: Advanced Bionics Corporations and the Cochlear Corporation. The terms of this arrangement are being managed by the Johns Hopkins University in accordance with its conflict of interest policies.
External Advisors received honoraria for their review of the study protocol and progress reports. Dr. Niparko had full access to the entirety of the data in this study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
CDaCI Investigative Team
House Ear Institute, Los Angeles: Laurie S. Eisenberg, PhD, CCC-A (PI); Karen Johnson, PhD, CCC-A (coordinator); Traci Critton, PhD (data collection); Jean DesJardin, PhD (data collection); Melinda Gillinger (data collection); William Luxford, MD (surgeon); Amy Martinez, MA, CCC-A (data collection); Louise Mebane, PhD (data collection); Jennifer Regnery, M.S. (data collection); Leslie Visser-Dumont, MA, CCC-A (data collection).
Johns Hopkins University, Listening Center, Baltimore: John K. Niparko, MD (PI); Steve Bowditch, MS, CCC-A (data collection); Jill Chinnici, MA, CCC-A (data collection); James Clark, MB (data analyst); Howard Francis, MD (surgeon); Jennifer Mertes, AuD, CCC-A (coordinator); Rick Ostrander, EDD (data collection); Jennifer Yeagle, MEd, CCC-A (data collection), Jiovani Visaya, MD (data analyst).
Johns Hopkins University, The River School, Washington, DC: Nancy Mellon (administration); Mary O’Leary Kane, MA, CCC-SLP (coordinator); Sarah Wainscott (data collection); Jennifer Wallace, MS, CCC-SLP (data collection).
University of Miami, Miami: Annelle Hodges, PhD (PI); Thomas Balkany, MD (surgeon); Alina Lopez, MA, CCC-SLP/A (coordinator); Leslie Goodwin, MSN, CCRC (data collection); Stacy Payne, MA, CCC-A (data collection).
University of Michigan, Ann Arbor: Teresa Zwolan, PhD (PI); Amy Donaldson, MA, CCC-A (coordinator); H. Alexander Arts, MD (surgeon); Brandi Butler, MA, CCC-A (data collection); Hussam El-Kashlam, MD (surgeon); Krista Heavner, MS, CCC-SLP (data collection); Mary Beth O’Sullivan, MS, CCC-A (data collection); Steve Telian, MD (surgeon); Ellen Thomas, MA, CCC-SLP (data collection); Anita Vereb, MS, CCC-A (former coordinator).
University of North Carolina, Carolina Children’s Communicative Disorders Program, Chapel Hill: Carolyn J. Brown, MS (PI); Holly F.B. Teagle, AuD, (coordinator); Craig A. Buchman, MD (surgeon); Carlton Zdanski, MD (surgeon); Hannah Eskridge, MSP (data collection); Harold C. Pillsbury, MD (surgeon).
University of Texas at Dallas, Callier Advanced Hearing Research Center, Dallas: Emily A. Tobey, PhD, CCC-SLP (PI); Betty Loy, AuD, CCC-A (coordinator); Paul Bauer, MD (surgeon); Angela Boyd, BA (data collection); Laura Cantu, BS (data collection); Carol Cokely, PhD, CCC-A (data collection); Sarah Florence, MS, CCC-A (data collection); Janee Gisclair, MS, CCC-A (data collection); Laura Levitan, BA (data collection); Joy Penrad (data collection); Shannon Raby, MA, CCC-SLP (data collection); Jamie Rasmus, BS (data collection); Peter Roland, MD (surgeon); Heather MacFadyen, MS, CCC-SLP (data collection); Donise Pearson, MS, CCC-SLP (data collection); Deborah M. Rekart, PhD (former coordinator); Lauren Sacar, BA (data collection); Melissa Sweeney, MS, CCC-SLP (data collection); Linsey Wagner, BA (data collection); Nicole Weissner, BA (data collection); Berkley Williams, MA, CCC-SLP (data collection).
Resource Centers
Data Coordinating Center, Johns Hopkins University, Welch Center for Prevention, Epidemiology & Clinical Research, Baltimore: Nancy E. Fink, MPH (PI); Patricia Bayton (data assembly); Daniel Habtemarian (data assembly); Neil R. Powe, MD, MPH, MBA (Senior Epidemiologist); Thelma Vilche (data assembly); Nae-Yuh Wang, PhD (Co-PI, Biostatistician, data assembly and analysis).
Psychometrics Center, University of Miami, Department of Psychology, Coral Gables: Alexandra Quittner, PhD (PI); David Barker (data analysis); Pam Leibach (data analysis); Ivette Cruz (data analysis).
Study Oversight Committees
Executive Committee: John K. Niparko, MD (chair); Laurie S. Eisenberg, PhD; Nancy E. Fink, MPH; Alexandra L. Quittner; Donna Thal, PhD; Emily A. Tobey, PhD, Nae-Yuh Wang, PhD.
External Advisors: Noel Cohen, MD; Julia Evans, PhD; Ann Geers, PhD; Karen Iler Kirk, PhD, Anil Lalwani, MD.
Footnotes
Web address for published article: http://jama.ama-assn.org/cgi/reprint/303/15/1498
Reference List
- 1.Bradham T, Jones J. Cochlear implant candidacy in the United States: prevalence in children 12 months to 6 years of age. Int J Pediatr Otorhinolaryngol. 2008;72(7):1023–1028. doi: 10.1016/j.ijporl.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 2.Niparko JK. Speech, language, and reading skills after early cochlear implantation. JAMA. 2004;291(19):2378–2380. doi: 10.1001/jama.291.19.2378. [DOI] [PubMed] [Google Scholar]
- 3.Dettman SJ, Pinder D, Briggs RJ, Dowell RC, Leigh JR. Communication development in children who receive the cochlear implant younger than 12 months: risks versus benefits. Ear Hear. 2007;28(2 Suppl):11S–18S. doi: 10.1097/AUD.0b013e31803153f8. [DOI] [PubMed] [Google Scholar]
- 4.Geers A, Tobey E, Moog J, Brenner C. Long-term outcomes of cochlear implantation in the preschool years: from elementary grades to high school. Int J Audiol. 2008;47 (Suppl 2):S21–S30. doi: 10.1080/14992020802339167. [DOI] [PubMed] [Google Scholar]
- 5.Holt RF, Svirsky MA. An exploratory look at pediatric cochlear implantation: is earliest always best? Ear Hear. 2008;29(4):492–511. doi: 10.1097/AUD.0b013e31816c409f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nicholas JG, Geers AE. Will they catch up? The role of age at cochlear implantation in the spoken language development of children with severe to profound hearing loss. J Speech Lang Hear Res. 2007;50(4):1048–1062. doi: 10.1044/1092-4388(2007/073). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Svirsky MA, Sloan RB, Caldwell M, Miyamoto RT. Speech intelligibility of prelingually deaf children with multichannel cochlear implants. Ann Otol Rhinol Laryngol Suppl. 2000;185:123–125. doi: 10.1177/0003489400109s1254. [DOI] [PubMed] [Google Scholar]
- 8.Tobey EA, Geers AE, Brenner C, Altuna D, Gabbert G. Factors associated with development of speech production skills in children implanted by age five. Ear Hear. 2003;24(1 Suppl):36S–45S. doi: 10.1097/01.AUD.0000051688.48224.A6. [DOI] [PubMed] [Google Scholar]
- 9.Sharma A, Tobey E, Dorman M, et al. Central auditory maturation and babbling development in infants with cochlear implants. Arch Otolaryngol Head Neck Surg. 2004;130(5):511–516. doi: 10.1001/archotol.130.5.511. [DOI] [PubMed] [Google Scholar]
- 10.Francis HW, Buchman CA, Visaya JM, et al. Surgical factors in pediatric cochlear implantation and their early effects on electrode activation and functional outcomes. Otol Neurotol. 2008;29(4):502–508. doi: 10.1097/MAO.0b013e318170b60b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marschark M, Rhoten C, Fabich M. Effects of cochlear implants on children’s reading and academic achievement. J Deaf Stud Deaf Educ. 2007;12(3):269–282. doi: 10.1093/deafed/enm013. [DOI] [PubMed] [Google Scholar]
- 12.Barker DH, Quittner AL, Fink NE, Eisenberg LS, Tobey EA, Niparko JK. Predicting behavior problems in deaf and hearing children: the influences of language, attention, and parent-child communication. Dev Psychopathol. 2009;21(2):373–392. doi: 10.1017/S0954579409000212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eisenberg LS, Johnson KC, Martinez AS, et al. Speech recognition at 1-year follow-up in the childhood development after cochlear implantation study: Methods and preliminary findings. Audiol Neurootol. 2006;11(4):259–268. doi: 10.1159/000093302. [DOI] [PubMed] [Google Scholar]
- 14.Fink NE, Wang NY, Visaya J, et al. Childhood Development after Cochlear Implantation (CDaCI) study: design and baseline characteristics. Cochlear Implants Int. 2007;8(2):92–116. doi: 10.1179/cim.2007.8.2.92. [DOI] [PubMed] [Google Scholar]
- 15.Lin FR, Wang NY, Fink NE, et al. Assessing the use of speech and language measures in relation to parental perceptions of development after early cochlear implantation. Otol Neurotol. 2008;29(2):208–213. doi: 10.1097/mao.0b013e31812f6fa6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bayley N. Bayley Scales of Infant Development. 2. San Antonio, TX: The Psychological Corporation; 1993. [Google Scholar]
- 17.Roid G, Miller L. Leiter International Performance Scale--Revised. Wood Dale, IL: Stoelting Co; 2002. [Google Scholar]
- 18.Reynell J, Huntley M. Reynell development language scales ± second revision. Windsor, England: NFER-Nelson; 1985. [Google Scholar]
- 19.Reynell J, Gruber C. Reynell Developmental Language Scales: U.S. Edition. Los Angeles, CA: Western Psychological Services; 1990. [Google Scholar]
- 20.Wang NY, Eisenberg LS, Johnson KC, et al. Tracking development of speech recognition: longitudinal data from hierarchical assessments in the Childhood Development after Cochlear Implantation Study. Otol Neurotol. 2008 Feb;29(2):240–5. doi: 10.1097/MAO.0b013e3181627a37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robbins AM, Renshaw J, Berry S. Evaluating meaningful auditory integration in profoundly hearing-impaired children. American Journal of Otology. 1991;12(Suppl):144–150. [PubMed] [Google Scholar]
- 22.Zimmerman-Phillips S, Robbins AM, Osberger MJ. Assessing cochlear implant benefit in very young children. Annals of Otology, Rhinology, & Laryngology. 2000;109(suppl 185):42–42. doi: 10.1177/0003489400109s1217. [DOI] [PubMed] [Google Scholar]
- 23.Moog JS, Geers AE. Early Speech Perception Test for profoundly hearing-impaired children. St. Louis: Central Institute for the Deaf; 1990. [Google Scholar]
- 24.Jerger S, Jerger J. Pediatric Speech Intelligibility test. Auditec of St. Louis; 1984. [Google Scholar]
- 25.Kirk KI, Pisoni DB, Osberger MJ. Lexical effects on spoken word recognition by pediatric cochlear implant users. Ear and Hearing. 1995;16(5):470–481. doi: 10.1097/00003446-199510000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haskins H. Unpublished master’s thesis. Evanston, IL: Northwestern University; 1949. A phonologically balanced test of speech discrimination for children. [Google Scholar]
- 27.Nilsson M, Soli SD, Gelnett DJ. Development of the Hearing in Noise Test for children (HINT-C) Los Angeles: House Ear Institute; 1996. [Google Scholar]
- 28.Quittner AL, Leibach P, Marciel K. The impact of cochlear implants on young deaf children: new methods to assess cognitive and behavioral development. Arch Otolaryngol Head Neck Surg. 2004;130(5):547–554. doi: 10.1001/archotol.130.5.547. [DOI] [PubMed] [Google Scholar]
- 29.Svirsky MA, Robbins AM, Kirk KI, et al. Language development in profoundly deaf children with cochlear implants. Psychol Sci. 2000 Mar;11(2):153–8. doi: 10.1111/1467-9280.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cleveland WS, Devlin SJ. Locally weighted regression: an approach to regression analysis by local fitting. J Am Stat Assoc. 1988;83:596–610. [Google Scholar]
- 31.Nikolopoulos TP, Dyar D, Archbold S, O’Donoghue GM. Development of spoken language grammar following cochlear implantation in prelingually deaf children. Arch Otolaryngol Head Neck Surg. 2004;130(5):629–633. doi: 10.1001/archotol.130.5.629. [DOI] [PubMed] [Google Scholar]
- 32.Walker D, Greenwood C, Hart B, Carta J. Prediction of school outcomes based on early language production and socioeconomic factors. Child Dev. 1994;65(2 Spec):606–621. [PubMed] [Google Scholar]
- 33.Geers A, Tobey E, Moog J, Brenner C. Long-term outcomes of cochlear implantation in the preschool years: from elementary grades to high school. Int J Audiol. 2008;47 (Suppl 2):S21–S30. doi: 10.1080/14992020802339167. [DOI] [PubMed] [Google Scholar]
- 34.Streeter LA. Language perception of 2-month-old infants shows effects of both innate mechanisms and experience. Nature. 1976;259(5538):39–41. doi: 10.1038/259039a0. [DOI] [PubMed] [Google Scholar]
- 35.Werker JF. Baby steps to learning language. J Pediatr. 2003;143(4 Suppl):S62–S69. doi: 10.1067/s0022-3476(03)00403-7. [DOI] [PubMed] [Google Scholar]
- 36.Kuhl PK. Learning and representation in speech and language. Curr Opin Neurobiol. 1994;4(6):812–822. doi: 10.1016/0959-4388(94)90128-7. [DOI] [PubMed] [Google Scholar]
- 37.Kuhl PK, Coffey-Corina S, Padden D, Dawson G. Links between social and linguistic processing of speech in preschool children with autism: behavioral and electrophysiological measures. Dev Sci. 2005;8(1):F1–F12. doi: 10.1111/j.1467-7687.2004.00384.x. [DOI] [PubMed] [Google Scholar]
- 38.Saffran JR, Aslin RN, Newport EL. Statistical learning by 8-month-old infants. Science. 1996;274(5294):1926–1928. doi: 10.1126/science.274.5294.1926. [DOI] [PubMed] [Google Scholar]
- 39.Graf EK, Evans JL, Alibali MW, Saffran JR. Can infants map meaning to newly segmented words? Statistical segmentation and word learning. Psychol Sci. 2007;18(3):254–260. doi: 10.1111/j.1467-9280.2007.01885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spencer PE, Meadow-Orlans KP. Play, language, and maternal responsiveness: a longitudinal study of deaf and hearing infants. Child Dev. 1996;67(6):3176–3191. [PubMed] [Google Scholar]
- 41.Moeller MP, Schick B. Relations between maternal input and theory of mind understanding in deaf children. Child Dev. 2006;77(3):751–766. doi: 10.1111/j.1467-8624.2006.00901.x. [DOI] [PubMed] [Google Scholar]
- 42.Kuhl PK. Is speech learning ‘gated’ by the social brain? Dev Sci. 2007;10(1):110–120. doi: 10.1111/j.1467-7687.2007.00572.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.