Abstract
Alzheimer’s disease is thought to be caused by an imbalance between amyloid-β (Aβ) production and clearance leading to Aβ accumulation in the Central Nervous System (CNS). Aβ production and clearance are key targets in the development of disease modifying therapeutic agents for Alzheimer’s disease. However, there has not been direct evidence of altered Aβ production or clearance in Alzheimer’s disease. Using metabolic labeling, we measured Aβ42 and Aβ40 production and clearance rates in the CNS of patients with Alzheimer’s disease and cognitively normal controls. Clearance rates for both Aβ42 and Aβ40 were impaired in Alzheimer’s disease compared to controls. On average, there were no differences in Aβ42 or Aβ40 production rates. Thus, the common late-onset form of Alzheimer’s disease may involve an overall impairment of Aβ clearance.
Alzheimer’s disease (AD) is characterized by increased amounts of soluble and insoluble amyloid-β (Aβ), predominantly in the form of Aβ42 in amyloid plaques and Aβ40 in amyloid angiopathy. The amyloid hypothesis proposes that AD is caused by an imbalance between Aβ production and clearance (1), resulting in increased amounts of Aβ in various forms such as monomer, oligomer, insoluble fibrils, and plaques in the Central Nervous System (CNS). High levels of Aβ then initiate a cascade of events culminating in neuronal damage and death manifesting as progressive clinical dementia of the Alzheimer type (2).
In rare cases of AD, genetic alterations increase the production of Aβ (3). However, Aβ dysregulation in the far more common late onset “sporadic” AD is less well understood. Possible mechanisms of increased Aβ production for late onset AD include an increase in the amount of beta secretase or in beta-secretase activity. Alternatively, impaired clearance of Aβ may also cause late onset AD through interactions with ApoE4, decreased catabolism of Aβ via reduced proteolytic enzymes, impaired transport across the blood-brain barrier or impaired cerebrospinal fluid (CSF) transport.
To measure the production and clearance of Aβ in AD, we developed a method to measure human CNS Aβ production and clearance (fig. S1) (4), and compared Aβ42 and Aβ40 production and clearance rates in individuals with symptomatic AD and cognitively normal persons to determine if either or both is altered in AD.
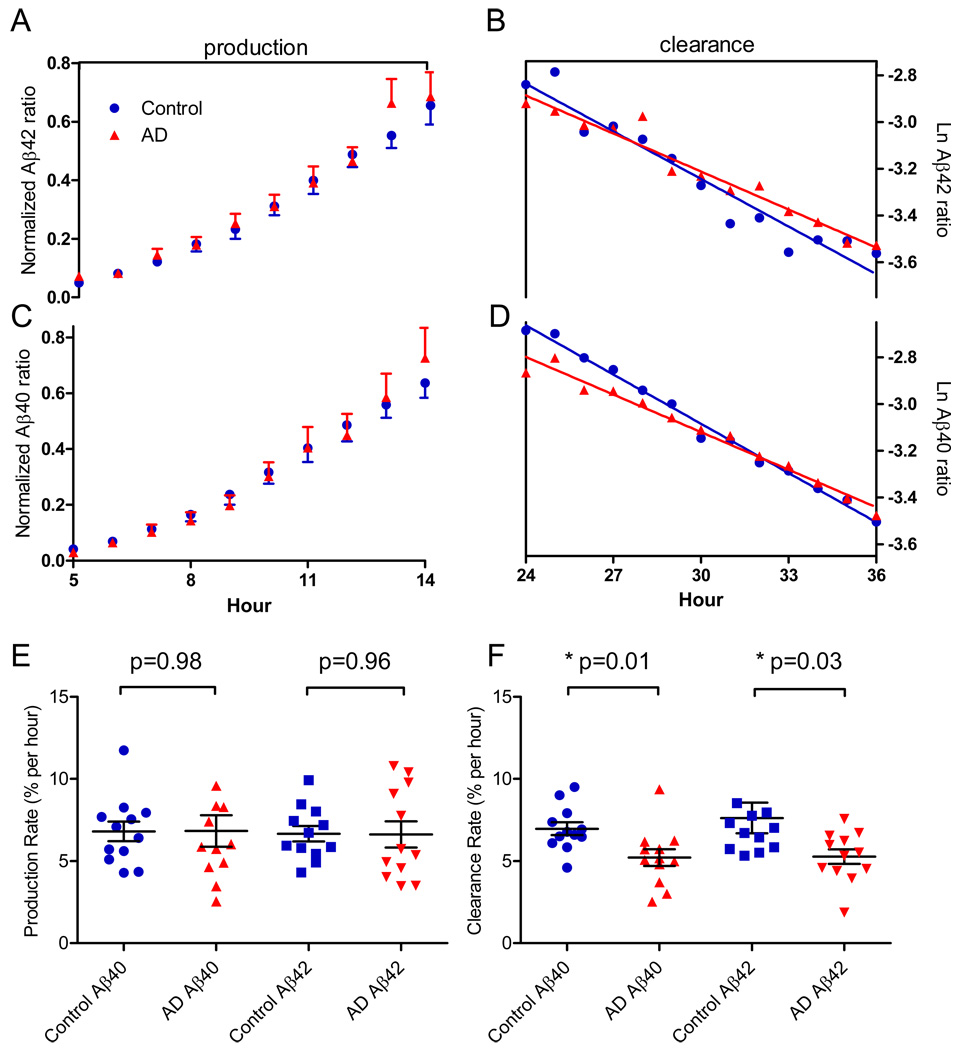
We plotted the average time course results of labeled Aβ42 and Aβ40 for the production phase (hours 5 to 14) and clearance phase (hours 24 to 36) (fig. 1). The production and clearance rates were calculated for each participant and compared by group status (AD versus control). The average Aβ42 production rate did not differ between the control (6.7%/hr) and AD (6.6%/hr) groups (p=0.96), nor did Aβ40 production rate differ between groups (6.8%/hr for controls and 6.8%/hr for the AD group; p=0.98). The average clearance rate of Aβ42 was slower for AD individuals compared with cognitively normal controls (5.3%/hr vs. 7.6%/hr, p=0.03), as was the average clearance rate of Aβ40 (5.2%/hr for AD individuals vs. 7.0%/hr for controls; p=0.01).
Figure 1.
Aβ kinetics in the CNS of twelve AD participants (red triangles) and twelve controls (blue circles). The amount of labeled Aβ42 and Aβ40 was measured and compared between groups to measure production and clearance rates of both Aβ species. (A) The average normalized labeled Aβ42 time course. (B) Aβ42 clearance rate during the clearance phase (hours 24–36). (C) Normalized labeled Aβ40 time course. (D) Aβ40 clearance in AD compared to controls. (E) The average fractional synthesis rates of Aβ42 and Aβ40 in AD participants and cognitively normal controls. (F) The average fractional clearance rates of Aβ42 and Aβ40.
To determine the balance of Aβ production to clearance rates in AD versus controls, we measured the ratios of production to clearance (fig. S2). The ratio of Aβ42 production to clearance rates were balanced for cognitively normal participants (0.95), however, due to decreased clearance in the AD participants; there was an imbalance in the Aβ42 production to clearance ratio (1.35). Similarly, there we observed an imbalance in AD Aβ40 production to clearance ratio (1.37), compared with cognitively normal participants (0.99).
The pathophysiologic changes that lead to AD are important for developing improved treatments. However, the pathogenic events that ultimately lead to AD are not well understood in sporadic, late onset AD. The amyloid hypothesis predicts that Aβ over-production or impaired clearance leads to downstream events that cause neuronal dysfunction and death, manifesting as clinical dementia. The technique of measuring Aβ production and clearance has been used to measure effects of drugs which target Aβ generation, demonstrating decreases in production (5). We found that late onset AD is associated with a 30% impairment in the clearance of both Aβ42 and Aβ40, indicating that Aβ clearance mechanisms may be critically important in the development of AD (6). Estimates based on the 30% decrease in Aβ clearance rates measured here suggest that brain Aβ accumulates over approximately 10 years in AD. Thus, decreased clearance rates may be an early predictor of risk for AD. The impaired clearance of both Aβ40 and Aβ42 is consistent with prior findings of significant biochemical deposition of Aβ40 in cerebral amyloid angiopathy in approximately 80% of cases of AD (7) and also in parenchymal plaques, in addition to Aβ42 predominant deposition in amyloid plaques.
Limitations of this study are the relatively small numbers (twelve in each group) and the association of impaired Aβ clearance with AD does not prove causality. In addition to decreased CNS Aβ clearance, CSF Aβ42 concentrations are decreased in AD compared to controls (fig. S3). Taken together, these may be consistent with decreased Aβ42 clearance (efflux) from the brain to the CSF. However, the relationship between decreased concentrations of CSF Aβ42 with decreased CNS clearance of labeled Aβ (fig. S4) is not fully understood. For example, both Aβ42 clearance rates and Aβ42 concentrations demonstrated significant correlations with dementia diagnosis, however, did not correlate with each other. Additional possibilities include more than one pool of Aβ in CSF, undetected pools of Aβ in CSF by ELISA (e.g. oligomers), or a combined increase in Aβ production with impaired efflux from parenchyma to CSF. Overall, these results indicate a significant impairment in the metabolism of Aβ in AD compared to controls.
Supplementary Material
Footnotes
Supporting Online Material
Materials and Methods
Figs. S1 to S4
References
References and Notes
- 1.Hardy J, Selkoe DJ. Science. 2002 Jul 19;297:353. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 2.Cummings JL. N Engl J Med. 2004 Jul 1;351:56. doi: 10.1056/NEJMra040223. [DOI] [PubMed] [Google Scholar]
- 3.Scheuner D, et al. Nat Med. 1996 Aug;2:864. doi: 10.1038/nm0896-864. [DOI] [PubMed] [Google Scholar]
- 4.Bateman RJ, et al. Nat Med. 2006 Jul;12:856. doi: 10.1038/nm1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bateman RJ, et al. Annals of Neurology. 2009;66:48. doi: 10.1002/ana.21623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeMattos RB, et al. Neuron. 2004 Jan 22;41:193. doi: 10.1016/s0896-6273(03)00850-x. [DOI] [PubMed] [Google Scholar]
- 7.Ellis RJ, et al. Neurology. 1996 Jun;46:1592. doi: 10.1212/wnl.46.6.1592. [DOI] [PubMed] [Google Scholar]
- 8.Acknowledgements: We are grateful to the participants for their time and effort. The authors thank J. X. Wang for GC/MS analysis, and R. Connors and R. Potter for IP/MS analysis. Special thanks to D. M. Holtzman, for his mentoring, support, and review of the manuscript. This work was supported by grants from the US National Institutes of Health (NIH) grants K08 AG027091, K23 AG030946, R01 NS065667, P50 AG05681, P01 AG03991, UL1 RR024992, P41 RR000954, P60 DK020579, P30 DK056341, and also grants from an Anonymous Foundation, an Eli Lilly research grant, The Knight Initiative for Alzheimer Research, and The James and Elizabeth McDonnell Fund for Alzheimer Research. Potential conflicts of interest: R.J.B. and D.M.H. are cofounders of a company (C2N Diagnostics) that has licensed a pending Washington University patent on the technology described in this article.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.