Abstract
Stem cells from human exfoliated deciduous teeth (SHED) have been identified as a novel population of postnatal stem cells capable of differentiating into neural cells, odontogenic cells, and adipocytes. SHED were reported to differentiate into neural cells based on cellular morphology and the expression of early neuronal markers when cultured under neural inductive conditions. This study therefore investigated the therapeutic efficacy of SHED in alleviating Parkinson's disease (PD) in a rat model. We found that SHED could be induced to form neural-like spheres in a medium optimized for neural stem cells in vitro. After incubation with a cocktail of cytokines including sonic hedgehog, fibroblast growth factor 8, glial cell line-derived neurotrophic factor, and forskolin, these SHED-derived spheres further differentiated into a cell population that contained specific dopaminergic neurons. Moreover, transplantation of SHED spheres into the striatum of parkinsonian rats partially improved the apomorphine-evoked rotation of behavorial disorders compared to transplantation of control SHED. Our data indicate that SHED, potentially derived from neural crest cells, may be an optimal source of postnatal stem cells for PD treatment.
Introduction
Parkinson's disease (PD), one of the most common neurodegenerative diseases, is caused by the loss of dopaminergic (DAergic) neurons in the substantia nigra pars compacta, leading to symptoms of tremor, rigidity, and bradykinesia [1]. Intracerebral transplantation of DAergic neurons or progenitors derived from fetal neural tissues has been proved a promising approach for PD treatment [2–6]. Adult stem or progenitor cells, such as mesenchymal stem cells (MSCs), may possess greater potential as therapeutic cells due to their greater availability and because they can be expanded ex vivo to enrich cell numbers without serious ethical or technical problems. Numerous studies have shown that rat and human MSCs can differentiate into cells that display neuronal or even DAergic characteristics both in vitro and in vivo [7–11]. Transplantation of MSCs in conjunction with MSC-mediated expression of tyrosine hydroxylase (TH) (required for dopamine [DA] synthesis) improves parkinsonian behaviors in animal models [12,13].
Recently, stem cells from human exfoliated deciduous teeth (SHED) have been demonstrated as a novel population of adult stem cells capable of multi-differentiation potential. They could generate different cell types including neural cells, odontogenic cells, and adipocytes in vitro, and were reported to have a higher proliferation rate compared to adult bone marrow MSCs and dental pulp stem cells (DPSCs) [14]. This suggests that SHED could facilitate the expansion of these cells in vitro before transplantation. Because SHED are derived from a naturally occurring exfoliated organ, they may be a unique stem cell resource for potential clinical applications. Dental pulp tissue is thought to be derived from migrating neural crest cells during development [15], and has been shown to possess various populations of multipotent stem or progenitor cells [14,16,17]. Previous investigations into the neural potential of human adult DPSCs and SHED have shown that under non-neuronal inductive conditions, these cells expressed the neural progenitor marker, nestin, and the glial marker, glial fibrillary acidic protein (GFAP), at both the mRNA and protein levels [14,17]. In vitro neural differentiation studies of rat and human adult dental pulp cells (DPCs) and SHED demonstrated that these stem or precursor cell populations were able to differentiate into neural cells. In addition, it has been documented that rat DPCs and SHED survived and expressed neuronal markers when transplanted into the adult rodent brain [14,18]. Bone marrow-derived MSCs have been used to treat neurodegenerative diseases; however, these cells originate from the mesoderm but not from neural crest cells. We consider that neural crest cell-derived SHED may possess a greater propensity for neuronal differentiation. Furthermore, SHED can be easily obtained from naturally exfoliated deciduous teeth, implying their potential application merit. Until now, interest in dental pulp stem or stromal cell (DPSC and SHED) research has been focused on mineralization and tooth repair [19,20]. In this study, we developed an approach to induce SHED to differentiate into DAergic neurons, which could alleviate behavioral impairment in parkinsonian rats. Thus, exfoliated teeth may offer a unique stem cell resource for PD treatment.
Materials and Methods
Cell culture
Normal exfoliated human deciduous upper and lower incisors (n = 3) were collected from 7- to 8-year-old children after informed parental consent was obtained. Permission to use exfoliated human deciduous teeth was granted by the Ethical Committee of Capital Medical University. SHED were isolated and cultured as previously described [14]. SHED used in this study were at 1–3 passages.
SHED-derived spheres culture and neural induction assay
In order to induce SHED to undergo DAergic differentiation, a 2-step method was used. First, SHED was induced into neural stem cells (NSCs)-like spheres, named SHED-derived spheres. SHED-derived spheres were cultured in accordance with a modified version of a recently described method [21]. The cells were cultured on super-hydrophilic plates (Costar, Cambridge, MA) in Neurobasal A media (GIBCO/Invitrogen, Carlsbad, CA) supplemented with B27 (Invitrogen, Carlsbad, CA), 20 ng/mL basic fibroblast growth factor (bFGF; Invitrogen) and 20 ng/mL epidermal growth factor (EGF; Invitrogen). Cell suspensions were plated on super-hydrophilic 6-well culture plates at a density of 1.0 × 106 cells/well. Fresh culture medium including EGF and bFGF was added to the medium after 4–5 days of culture. SHED-derived spheres cultured for 7–9 days were used for experiments.
For further neural differentiation, SHED-derived spheres were plated onto poly-l-lysine-coated glass coverslips for immunostaining or seeded onto culture plates for western blot analysis. The cells were induced with a cocktail of 200 ng/mL sonic hedgehog (SHH), 100 ng/mL fibroblast growth factor 8 (FGF8), 10 ng/mL glial cell line-derived neurotrophic factor (GDNF; R&D Systems, Minneapolis, MN), and 10 μM forskolin (PeproTech EC, London, England). Cells were then cultured at 37°C with 5% CO2 for 7 days. Half of the medium was replaced every 2–3 days. After 7 days of cultivation, the cells differentiated into neuronal cells and were tested for neural cell marker expression. The whole induction time was about 2–3 weeks. Intact SHED referred to those without any induction, and were used as a control to the SHED-derived spheres in the experiment.
Immunofluorescence staining of cultured cell preparations
Cells on coverslips were fixed with 4% paraformaldehyde. SHED-derived spheres were fixed with 4% paraformaldehyde and immersed in 30% sucrose overnight. After embedding in OCT and quick refrigeration, SHED-derived spheres were sliced by cryoultramicrotome at 10 μm and mounted on poly-l-lysine-coated slides.
Cultures were subsequently processed for immunocytochemical staining using various primary antibodies. Mouse antibodies used: STRO-1 (1:50; R&D Systems, Minneapolis, MN), CD29 (1:500; Chemicon, Temecula, CA), CD90 (1:100; Chemicon), CD146 (1:1,000; Abcam, Cambridge, MA), CD34 (1:500; Chemicon), vimentin (1:100; Chemicon), nestin (1:200; Chemicon), and TH (1:10,000; Sigma-Aldrich, St. Louis, MO). Rabbit antibodies used: MAP2 (1:500; Chemicon), dentin sialoprotein (DSP, 1:100; Santa Cruz Biotechnology, Santa Cruz, CA), and βIII-tubulin (1:500; Sigma).
In brief, fixed cultures were incubated in 0.025% (w/v) Triton X-100 and blocked with 5% goat serum. Cells were then incubated with primary antibody followed by secondary antibody (1:200 goat anti-mouse Alexa 488, and 1:200 goat anti-rabbit Alexa 488; Invitrogen, Carlsbad, CA). After nuclear staining using 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI, 1:10,000; Invitrogen), slices were washed and mounted in VECTASHIELD mounting medium (Vector Laboratories, Burlingame, CA) and then examined by fluorescence microscopy. Isotype-matched control antibodies (Invitrogen) were used under the same conditions.
Fluorescence-activated cell sorting
SHED were fixed with 4% paraformaldehyde. Primary STRO-1 (1:50; R&D Systems, Minneapolis, MN) and nestin (1:100; R&D Systems) antibodies were added to the tubes, followed by fluorescein-conjugated secondary antibody (1:200 goat anti-mouse Alexa 488; Invitrogen, Carlsbad, CA). The percentage of SHED staining positive for STRO-1 and nestin were assessed using a FACSCalibur flow cytometer (Becton Dickinson Immunocytometry Systems, San Jose, CA). Isotype-matched control antibodies were used under the same conditions.
Western blot analysis
Protein lysates were prepared in the presence of a protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO). Equal amounts of protein were loaded under reducing conditions onto a 10% SDS gel. After electrophoresis, the proteins were transferred to nitrocellulose membranes. The membranes were blocked with 5% nonfat dry milk in PBS containing 0.2% Tween 20 for 1 h, and then incubated with mouse anti-TH and mouse anti-actin antibodies (1:10,000; Sigma) overnight at 4°C. Membranes were then treated with IRDyeTM800 (green)- or IRDyeTM700 (red) (1:10,000; Rockland Immunochemicals, Gilbertsville, PA)-conjugated affinity-purified anti-mouse secondary IgG. Fluorescent bands were visualized using an LI-COR Odyssey infrared double-fluorescence imaging system (LI-COR Biosciences, Lincoln, NE). Normal rat striatum was used as the positive control.
Real-time PCR
To detect the expression of neural cell markers in DAergic neuron development including nestin, βIII-tubulin, MAP2, and TH, we performed real-time PCR using the Stratagene/SYBR green system. Total mRNA was extracted using Trizol reagent (Invitrogen). Total RNA (2 μg) was converted to cDNA in accordance with the instructions for the SSIII cDNA synthesis kit (Invitrogen). A housekeeping gene β-actin was used as an internal control to quantify and normalize the results. Primer pairs used for PCR amplifications are listed below. Primers nestin (accession NM 006617) forward (5′-AGCCCTGACCACTCCAGTTTAG-3′), reverse (5′-CCCTCTATGGCTGTTTCTTTCTCT-3′); βIII-tubulin (accession NM 006086) forward (5′-CCTGGAACCCGGAACCAT-3′), reverse (5′-AGGCCTGAAGAGATGTCCAAA-3′); MAP2 (accession NM 001039538) forward (5′-GCCCAGAAAAGCGCTCTTC-3′), reverse (5′-GCCGAGGAGGGAGAATGG-3′); TH (accession NM 000360) forward (5′-CCGAGCTGTGAAGGTGTTTGA-3′), reverse (5′-CGGGCCGGGTCTCTAGAT-3′); and β-actin (accession NM 001101) forward (5′-ACCGAGCGCGGCTACAG-3′), reverse (5′-CTTAATGTCACGCACGATTTCC-3′). Real-time PCR was performed using the SYBR green system in a total volume of 20 μL in 96-well microwell plates. The 0.4 μL purified cDNA, 10 μL Brilliant SYBR green QPCR Master Mix (Stratagene), 100 nM of primers, and 0.3 μL reference dye with 500-fold diluted were added to each microwell. RNase-free distilled water was added to reach a total volume of 20 μL per well. The PCR program was as follows: 95°C for 5 min, then 40 cycles of 95°C for 30 s, 59°C for 1 min, and 72°C for 30 s. The specificity of the reaction is given by the detection of the Tms of the amplification products immediately after the last reaction cycle. Data analysis was performed according to quick protocol of MxproTM QPCR software for Mx3000P system (Stratagene). The value 2−ΔΔCT was used to comparative quatitation. All PCRs were performed in triplicates.
Parkinsonian rat model generation
Fifty adult female Sprague-Dawley rats (8 weeks of age, 250–350 g) were used. All surgical interventions and animal care were in accordance with the Guide for the Care and Use of Laboratory Animal Models of Capital Medical University. Hemi-parkinsonian rats were generated by unilateral injection of 4 μL of 6-hydroxydopamine (6-OHDA; Sigma, St. Louis, MO; 2 μg/μL dissolved in 0.9% saline containing 0.2 mg/mL ascorbic acid) into the right medial forebrain bundle (MFB) at the following coordinates: AP = −4.3 mm, ML = −1.5 mm, and DV = −7.5 mm [22]. Apomorphine-induced rotational behavior was assessed at 4 weeks after 6-OHDA injection. For that, the rats were placed in individual hemispherical bowls and allowed to habituate for 5 min before being injected with an intraperitoneally dose of apomorphine (0.5 mg/kg). Left and right full-body turns were counted. Their rotational scores were collected for 40 min in a computer-assisted rotometer system (Rota-count 8; Columbus instruments, Columbus, OH). Animals showing 6 or more net rotations per minute ipsilaterally toward the intact side after a single dose of apomorphine were considered successful parkinsonian models and were selected for grafting. All behavioral tests were performed in a closed and dark room to avoid any environmental disturbance and assessed by an independent observer blind to the treatments.
Cell transplantation and behavioral test
Parkinsonian rats (successful models, n = 36) were randomly placed into 3 groups that were transplanted, respectively, with basic medium (negative control), intact SHED, and SHED-derived spheres created by a combination of EGF and bFGF for 7 days in vitro. Cells for transplantation were dispersed and suspended in Neurobasal A media with no cytokines, and were counted by Trypan blue exclusion with viability above 95%. Live cells for transplantation were set at a density of 200,000/μL [23]. The cell suspension was injected into 2 DA-depleted striatum sites in rats (2.5 μL per site). The coordinates were set as AP = +1.0 mm, ML = −3.0 mm, and DV = −4.1/−5.0 mm. All of the animals were immunosuppressed with cyclosporin A (10 mg/kg/day injected intraperitoneally; Novartis Pharma AG, Switzerland) 24 h before transplantation and then throughout the entire survival period. Behavioral improvements were tested by apomorphine-induced rotation examination as described above at 2, 4, 6, and 8 weeks after transplantation.
Assessment of cell transplantation
Five rats from each group were killed at 6 weeks post-transplantation and 3 rats from each group were kept for up to 8 weeks. Rats were deeply anesthetized and intracardiac perfusion was performed with 0.1 M PBS followed by 4% paraformaldehyde. Brain tissues were dissected, post-fixed, and cryoprotected as described above. Fixed brains were sliced on a freezing microtome (40 μm) and serial sections at 240 μm intervals throughout the graft were collected for further processing. Grafted human cell survival was assessed by immunostaining of human nuclei-positive cells, and DAergic neurons in the striatum were estimated by counting the number of TH-positive cells in the graft sites of each animal at 240 μm intervals through the graft. Sections were pretreated with 0.3% H2O2 to quench endogenous peroxidase, blocked with 3% normal horse serum, and then incubated with mouse anti-human nuclei (1:200; Chemicon, Temecula, CA) and mouse anti-TH (1:10,000; Sigma, St. Louis, MO) primary antibodies overnight at 4°C. Next, they were incubated with rat-adsorbed biotinylated horse anti-mouse secondary antibody (1:200; Vector Laboratories, Burlingame, CA). After incubation with peroxidase-conjugated avidin–biotin complex (1:100; Vector Laboratories), signals were developed by exposure to 0.02% 3,3-diaminobenzidine and 0.03% H2O2.
High-performance liquid chromatography analysis of dopamine concentration
The culture medium was acidified with 0.1 M perchloric acid and centrifuged at 10,000g for 10 min. The supernatant was immediately frozen in liquid nitrogen and stored at −70°C until analysis. Rats were killed 6 weeks after transplantation by decapitation (n = 4). The striatum was immediately collected, weighed, homogenized in 0.1 M perchloric acid, and centrifuged. DA was determined by high-performance liquid chromatography (HPLC) with electrochemical detection using a reverse-phase column. Results were presented as the ratio of the content of lesioned (right) side versus that of contralateral (left) side.
Statistical analysis
All data were expressed as the mean ± the standard error of the mean (s.e.m.). Behavioral recovery in animals and DA content was analyzed using a one-way analysis of variance (ANOVA, SPSS software) where appropriate, followed by a Bonferroni (Dunn) comparison of groups using least squares-adjusted means. In all of the statistical analyses, P < 0.05 was considered to be statistically significant.
Results
Characterization of isolated SHED
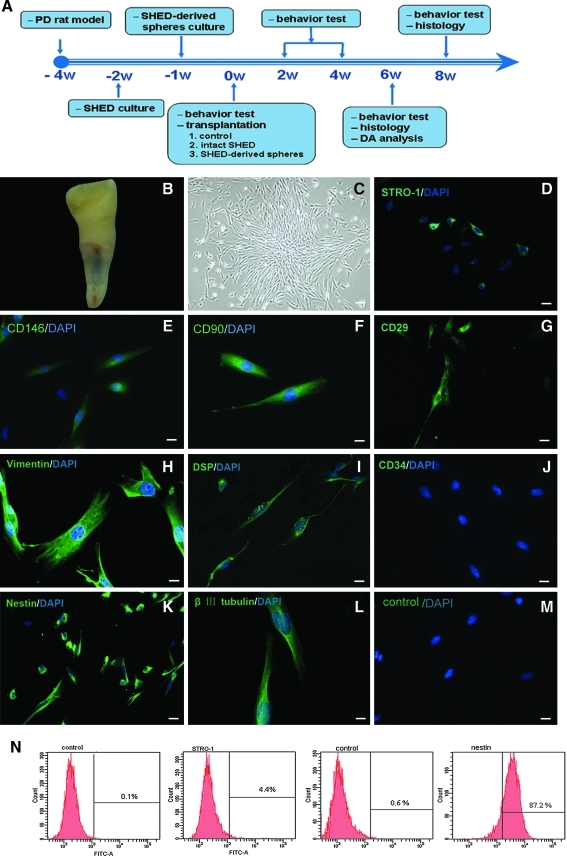
Figure 1A shows a schematic timeline of the procedures conducted for this study. The crowns of exfoliated deciduous teeth contained a living pulp remnant (Fig. 1B). Isolated SHED proliferated to cover about 80% of the dish surface after 2 weeks of cultivation (Fig. 1C). These cells expressed the cell surface molecules STRO-1 (Fig. 1D) and CD 146 (Fig. 1E), 2 early MSCs markers previously found to be present in bone marrow MSCs and DPSCs [14]. Cultured SHED also expressed the mesenchymal and dental-related markers CD90, CD29, vimentin, and DSP (Fig. 1F–1I, but were negative for CD34 (Fig. 1J), a marker of primitive hematopoietic progenitors and endothelial cells. To investigate the potential of SHED to differentiate into neurons, we examined the expression of neural markers. Cultured SHED expressed a variety of neural cell markers, including neural precursor marker, nestin, and early neuronal marker, βIII-tubulin (Fig. 1K and 1L). A low amount (4.4%) of in vitro-expanded SHED stained positive for STRO-1, while a large proportion (87.2%) of SHED stained positive for nestin, consistent with previous reports [14,24] (Fig. 1N).
FIG. 1.
Characterization of isolated human-exfoliated deciduous teeth (SHED). (A) Schematic timeline of the procedures conducted for this study. (B) Exfoliated deciduous incisor containing dental pulp. Cultured SHED had a fibroblast-like morphology (C) and expressed STRO-1 (D), CD146 (E), CD90 (F), CD29 (G), vimentin (H), DSP (I), nestin (K), and βIII-tubulin (L), but were negative for CD34 (J), control (M). (N) Flow cytometry analysis of SHED. Numbers in panels represent mean fluorescent intensity of the cells expressing each marker. Scale bars: 20 μm (C–E, G, I–K, and M) and 10 μm (F, H, and L).
In vitro differentiation of SHED
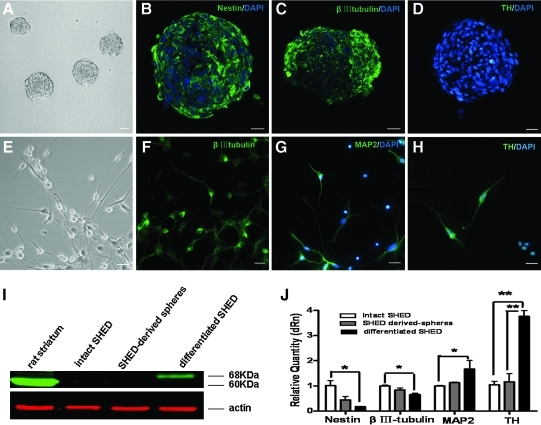
SHED formed sphere-like clusters when cultured in serum-free medium supplemented with EGF and bFGF (Fig. 2A). These spheres contained nestin-positive progenitors (Fig. 2B) and βIII-tubulin-positive neuronal cells (Fig. 2C), but were negative for TH expression (Fig. 2D). These data indicate that SHED-derived spheres are similar to neurospheres with regard to neural marker expression. In order to further induce the DAergic differentiation, SHED-derived spheres were incubated with the growth factor cocktail for another 7 days. After that, the cultures generated some neuron-like cells with multiple neurites (Fig. 2E) expressing 2 neuronal markers βIII-tubulin (Fig. 2F) and MAP2 (Fig. 2G). Furthermore, TH-positive neurons were detected (10.25% ± 2.45%, Fig. 2H), which was absent in SHED-derived spheres. The up-regulated TH expression induced by the cytokine mixture was further confirmed by western blot analysis (Fig. 2I). These results showed that TH was expressed only in SHED spheres induced by the cytokines cocktail. Real-time PCR analysis indicated that when SHED derived from 3 different donors were cultured in neuronal inductive media, nestin transcript levels appeared to be down-regulated compared to uninduced SHED (Fig. 2J). Similarly, there was a minor decrease in the expression of βIII-tubulin. Conversely, there was a significant increase in MAP2 and TH transcript levels after induction compared to cells maintained in control media (P < 0.05), which indicated that SHED-derived spheres could generate some mature neurons and a number of committed differentiated DAergic neurons. Collectively, these data suggest that in response to the neuronal inductive stimuli, a large proportion of SHED acquired a phenotype resembling mature neurons. This indicated that SHED could potentially differentiate into DAergic neuronal-like cells in an appropriate environment. To test the neuronal function of differentiated cells, we examined the synthesis and secretion of neurotransmitter, DA. As assayed by HPLC, the amount of DA in the culture supernatant of the differentiated SHED induced from SHED-derived spheres after induction rose to a concentration of 154 ± 13 ng/mL. DA was not detected in the supernatant of SHED and SHED-derived spheres. This suggested that DAergic neurons were effectively generated in our induction system.
FIG. 2.
Differentiation of SHED in vitro. (A) SHED formed sphere-like clusters when cultured in serum-free medium supplemented with epidermal growth factor (EGF) and basic fibroblast growth factor (bFGF). Most cells in the SHED-derived spheres expressed nestin (B) and βIII-tubulin (C), but not TH (D). After incubation with a cytokine cocktail for 7 days, SHED-derived spheres generated neuron-like cells with multiple neurites (E) that expressed βIII-tubulin (F), MAP2 (G), and TH (H). Nuclei were counterstained with DAPI (blue). (I) Expression of TH (68 kDa) was confirmed by western blot analysis (positive control: rat TH [60 kDa]). (J) Real-time PCR revealed that gene transcription levels of the neuronal precursor, nestin, and the early neuronal marker, βIII-tubulin, in differentiated SHED induced from SHED-derived spheres were down-regulated, while expression of the mature neuronal marker MAP2 and TH, the rate-limiting enzyme during DA synthesis, was up-regulated (n = 3). Samples were normalized to the control gene actin. Scale bars: 50 μm (A) and 20 μm (B–H). *P < 0.05; **P < 0.01.
Transplantation and behavioral recovery in parkinsonian rats
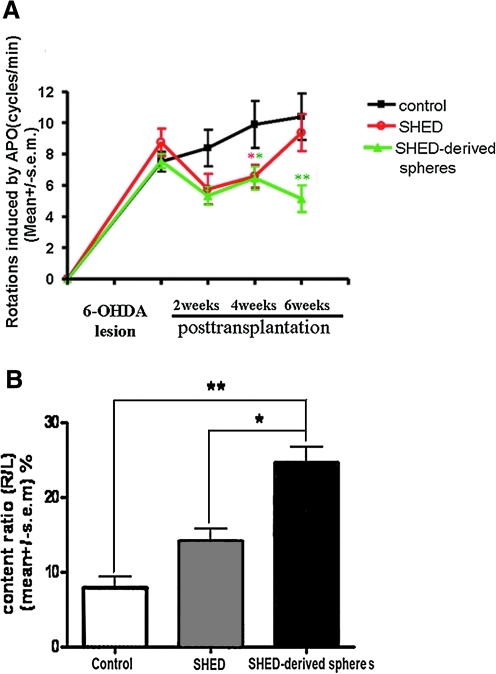
Injection of 6-OHDA into one-side MFB (the axonal pathway from substantia nigra to striatum) induced an almost complete lesion on dopamine system in this side. The effects of stem cells transplantation were examined in 6-OHDA-lesioned animals by quantification of rotations in response to apomorphine. In the group transplanted with SHED-derived spheres, the rats (n = 12) displayed significant behavioral recovery compared with negative controls; the recovery began 2 weeks after transplantation and behavioral improvement was sustained for up to 6 weeks. The average rotation score decreased from 7.56 ± 0.46 to 5.73 ± 0.58 at 2 weeks (P > 0.05), 6.47 ± 0.74 at 4 weeks (P < 0.05), and 5.15 ± 0.85 at 6 weeks (P < 0.01) after transplantation. This recovery was similar to the intact SHED graft group (n = 12) for 2–4 weeks after transplantation, but was significantly different after 4 weeks. The control group rats that received injections of basic medium without any cytokine in the DA-denervated striatum showed no significant improvement. There were no significant differences in the 3 groups' rotational scores 8 weeks after transplantation (Fig. 3A).
FIG. 3.
Behavioral and biochemical analysis of parkinsonian rats. (A) Apomorphine-induced rotation in the group of rats transplanted with SHED-derived spheres (n = 12) was ameliorated to a greater extent compared to the negative control group (n = 12), as observed starting 2 weeks after transplantation and then throughout the observation period. This recovery was similar to the intact SHED graft group (n = 12) 2–4 weeks after transplantation, but a better recovery effect was evident in the SHED-derived spheres group. The rats in the control group showed no significant improvement. (B) DA content in the striatum, which reflects the level of active neurotransmitter and its usage efficiency, was measured by high-performance liquid chromatography (HPLC). To avoid variation among individual rats, the result was presented as the ratio of the DA level of the lesion side versus the contralateral (normal) side, which may reflect the actual degree to which synthesis of the neurotransmitter recovered. DA content in the group transplanted with SHED-derived spheres (n = 4) was significantly elevated 6 weeks after transplantation compared to the control group (n = 4) and intact SHED group. *P < 0.05; **P < 0.01.
HPLC results revealed that in the group transplanted with pre-differentiated neurospheres, the DA content was significantly elevated to 24.61% ± 2.09% (P < 0.05) compared to the negative control group (7.95% ± 1.45%), while the intact SHED grafts had a non-significant elevation of DA (14.26% ± 1.55%) (Fig. 3B).
Survival and differentiation of transplanted cells
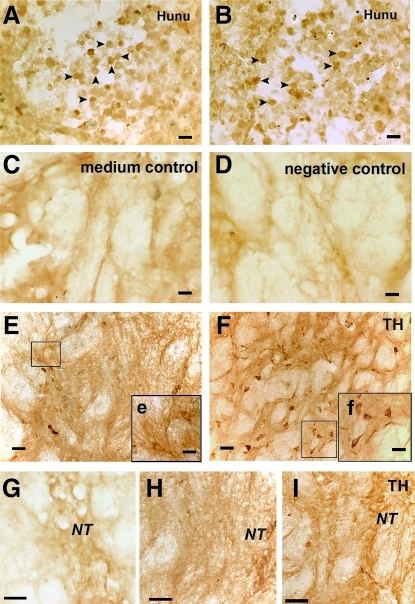
To investigate cell survival 6 weeks after transplantation, we counted the total number of human nuclei-positive cells in a series of sections spaced at 240 μm intervals throughout each rat brain graft. The average number for the intact SHED group was 2,782 ± 225 human nuclei-positive cells/section (Fig. 4A), and the number for the SHED-derived sphere group was 3,052 ± 334 human nuclei-positive cells/section (Fig. 4B, P > 0.05 compared with intact SHED group). We detected, but did not find human nuclei-positive cells in the brains of any of the rats at 8 weeks post-transplantation.
FIG. 4.
Survival and DAergic differentiation of engrafted human cells in parkinsonian rats. Engrafted intact SHED (A) and SHED-derived spheres (B) survived in the striatum of parkinsonian rats 6 weeks post-transplantation and had similar survival rates, as tested by human nuclei staining (Hunu, arrowheads). The group transplanted with basic medium serviced as the medium control in C, and the negative control for anti-human nuclei antibody was shown (D). TH-positive cells with different morphologies were detected around the needle track in the striatum transplanted with intact SHED and SHED-derived spheres. Most of intact SHED possessed fewer and shorter neurites (E, e) and some TH-positive cells of SHED-derived spheres indeed extended more and longer neuritis from the neurosome (F, f), exhibiting typical matured DAergic neurons. More TH-positive fibers (I) were detected around the needle track in the group transplanted with SHED-derived spheres compared to those transplanted with intact SHED grafts (H). (G) Fibers in negative control striatum. Inserts were the higher magnifi cent images. Scale bars: 20 μm (A–D), 40 μm (E–I), 20 μm (e and f). Abbreviation: Hunu, human nuclei; TH, tyrosine hydroxylase; NT, needle track.
Normally, there are very few or no DAergic neurons in the striatum. Thus, TH-positive cells in the striatum were likely associated with SHED implantation. Under higher magnification, TH-positive cells with different morphologies were detected around the needle track in the striatum at 6 weeks post-transplantation with intact SHED and SHED-derived spheres. Most of intact SHED possessed fewer and shorter neurites (Fig. 4E, e) and some TH-positive cells of SHED-derived spheres indeed extended more and longer neuritis from the neurosome (Fig. 4F, f), exhibiting typical matured DAergic neurons. Grafts of SHED-derived spheres had 70.67 ± 14.25 TH-positive cells/section, while the intact SHED grafts had 20.34 ± 15.34 TH-positive cells/section (P < 0.05), which indicated that appropriate pre-induction might be benefit for the differentiation of grafted cells, thereby for the efficacy of PD transplantation therapy. No TH-positive cells were found in the negative control group (Fig. 4G). Interestingly, rats transplanted with SHED (Fig. 4H) or SHED-derived spheres (Fig. 4I) exhibited TH-positive cells in their grafted striatum; re-innervation of host striatum also occurred after these transplants, which could contribute to therapeutic efficacy.
Discussion
Our study demonstrated that SHED derived from exfoliated deciduous teeth possess the potential to differentiate into DAergic neuron-like cells. During this procedure, we simulated the proliferation and differentiation of NSCs, and used a 2-step induction method. The first step was to induce SHED to form neurosphere-like aggregates. NSCs proliferate by the way of neurospheres, which is also an important property of NSCs. If SHED form floating spheres under the conditioned media, it suggests that SHED might possess the conversion potential to NSCs. The results from present study showed that SHED could form neurospheres in serum-free culture medium supplemented with EGF and bFGF, which was in accordance with what we previously found [14]. Furthermore, our previously published results demonstrated that a combination of forskolin, GDNF, and FGF8 promoted differentiation to a DAergic phenotype in human-derived neural progenitors [25]. In the present study, we modified the above protocol for DAergic induction. That is, SHED-derived spheres were induced into DAergic neurons with a cocktail of SHH, FGF8, GDNF, and forskolin. Under this condition, SHED-derived spheres generated a number of βIII-tubulin- and MAP2-positive neurons, and some of them were TH-positive cells. Moreover, the increasing amounts of DA detected by HPLC in the culture medium suggested that controlled differentiation of SHED by the cytokine cocktail led to the activation of catalytically active TH and the induction of functional neurons.
Previous studies indicated that transplanting DAergic neurons induced from human MSCs spheres was difficult, and that these cells were likely too mature to survive engraftment [26]. Thus, in our in vivo studies, instead of induced DAergic neurons, we used an earlier stage of development, SHED-derived spheres, for transplantation into the striatum of 6-OHDA-treated rats. Implantation of SHED-derived spheres, which were isolated from dental pulp and expanded in vitro, could potentially provide neurons to injured nervous tissue in vivo. Notably, SHED expressed neuronal and glial cell markers probably due to the neural crest cell origins of dental pulp [15]. Neural crest cells play a pivotal role in embryonic development, giving rise to a variety of cell types such as neural cells, pigment cells, smooth muscle, craniofacial cartilage, and bone [27]. Therefore, SHED could be a resource for neurodegenerative disease treatment. From a practical perspective, SHED would be an accessible tissue resource for autologous transplantation. Moreover, SHED maintain a higher proliferation rate than bone marrow MSCs and have the capacity to provide sufficient numbers of cells for clinical therapies.
In the present study, we found that pre-differentiation could be a key step for PD transplantation therapy [28]. We found that intrastriatal transplantation of SHED or SHED-derived spheres ameliorated behavioral deficits in parkinsonian rats; however, the efficacy of the SHED-derived spheres was far higher. Consistent with the behavioral recovery that we observed, the DA content in the striatum was higher in the group transplanted with SHED-derived spheres. The differential DAergic differentiation between SHED and SHED-derived sphere grafts indicated that pre-differentiating SHED-derived spheres prior to transplantation could lead to the generation of a more desired phenotype following grafting; more TH-positive cells were detected in this group, which likely contributed to the differences in behavioral recovery. It also suggested that in present induction system in vitro, some transcriptional factors for the regulation of TH expression might be activated in SHED-derived spheres. Once these cells were transplanted to an appropriate niches (striatum), they probably responded to the microenvironmental signals to undergo the committed differentiation. Therefore, pre-differentiated SHED-derived spheres should be transplanted to increase behavioral recovery in parkinsonian rats.
Our results indicated that TH-positive neurons could be found in rat striatum, but the number was small. Therefore, we considered that except for the DA increase, paracrine effect of grafted SHED-derived spheres may also contribute to the therapeutic function. It has been known that DPCs could produce neurotrophic factors and even rescue motoneurons after spinal cord injury [16]. Implantation of DPSCs into the hippocampus of mice stimulated proliferation of endogenous neural progenitor cells. Previous study demonstrated that DPSCs could enhance the expression of ciliary neurotrophic factor (CNTF), vascular endothelial growth factor (VEGF), and FGF for up to 30 days post-implantation [29]. DPSCs also could secrete multiple pro-angiogenic and anti-apoptotic factors [30]. However, further experiments should be performed to determine the possible factors responsible for this recovery.
In order to increase the cell survival capacity, we transplanted the SHED-derived spheres instead of the quite matured differentiation neurons, because the latter has been reported poor survival rate [26]. Nevertheless, this improvement did not achieve the satisfactory result. We considered that there might be many factors involved in this procedure including the immunological rejection, severe inflammation reaction, and the correct integration of grafted cells with host brains. These facets should be further investigated.
In summary, the present study demonstrated that a cytokine cocktail including SHH, FGF8, GDNF, and forskolin induced SHED to differentiate in vitro into a population that contained DAergic neurons. SHED and SHED-derived spheres survived and differentiated into DAergic neurons in parkinsonian rats; SHED-derived spheres gave rise to more DAergic neurons than SHED. Intrastriatal transplantation of SHED and SHED-derived spheres ameliorated behavioral impairment in parkinsonian rats. Contrary to intact SHED, pre-differentiation in vitro is a key step to improve the disordered behaviors of parkinsonian animal models after transplantation. SHED may prove to be a promising stem cell resource for the treatment of neurodegenerative diseases.
Acknowledgments
This work was supported by grants from the National Basic Research Program of China (2007CB947304 and 2010CB944801 to S.W.; 2006CB500700 to X.W.); the National Natural Science Foundation of China (30700941 and 30428009 to Y.L., S.W., and S.S.; 30500255 to X.W.), the Funding Project for Academic Human Resources Development in Institutions of Higher Learning Under the Jurisdiction of Beijing Municipality (PHR20090510 to S.W.), the Beijing Major Scientific Program (D0906007000091 to S.W.), and the United States National Institutes of Health (NIDCR R01DE17449 to S.S.).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Dauer W. Przedborski S. Parkinson's disease: mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 2.Lindvall O. Brundin P. Widner H. Rehncrona S. Gustavii B. Frackowiak R. Leenders KL. Sawle G. Rothwell JC. Marsden CD. Grafts of fetal dopamine neurons survive and improve motor function in Parkinson's disease. Science. 1990;247:574–577. doi: 10.1126/science.2105529. [DOI] [PubMed] [Google Scholar]
- 3.Freed CR. Breeze RE. Rosenberg NL. Schneck SA. Kriek E. Qi JX. Lone T. Zhang YB. Snyder JA. Wells TH. Survival of implanted fetal dopamine cells and neurologic improvement 12 to 46 months after transplantation for Parkinson's disease. N Engl J Med. 1992;327:1549–1555. doi: 10.1056/NEJM199211263272202. [DOI] [PubMed] [Google Scholar]
- 4.Olanow CW. Kordower JH. Freeman TB. Fetal nigral transplantation as a therapy for Parkinson's disease. Trends Neurosci. 1996;19:102–109. doi: 10.1016/s0166-2236(96)80038-5. [DOI] [PubMed] [Google Scholar]
- 5.Greely HT. Hamm T. Johnson R. Price CR. Weingarten R. Raffin T. The ethical use of human fetal tissue in medicine. Stanford University Medical Center Committee on Ethics. N Engl J Med. 1989;320:1093–1096. doi: 10.1056/NEJM198904203201624. [DOI] [PubMed] [Google Scholar]
- 6.Lie DC. Dziewczapolski G. Willhoite AR. Kaspar BK. Shults CW. Gage FH. The adult substantia nigra contains progenitor cells with neurogenic potential. J Neurosci. 2002;22:6639–6649. doi: 10.1523/JNEUROSCI.22-15-06639.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woodbury D. Schwarz EJ. Prockop DJ. Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000;61:364–370. doi: 10.1002/1097-4547(20000815)61:4<364::AID-JNR2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 8.Wislet-Gendebien S. Hans G. Leprince P. Rigo JM. Moonen G. Rogister B. Plasticity of cultured mesenchymal stem cells: switch from nestin-positive to excitable neuron-like phenotype. Stem Cells. 2005;23:392–402. doi: 10.1634/stemcells.2004-0149. [DOI] [PubMed] [Google Scholar]
- 9.Tropel P. Platet N. Platel JC. Noel D. Albrieux M. Benabid AL. Berger F. Functional neuronal differentiation of bone marrow-derived mesenchymal stem cells. Stem Cells. 2006;24:2868–2876. doi: 10.1634/stemcells.2005-0636. [DOI] [PubMed] [Google Scholar]
- 10.Sanchez-Pernaute R. Studer L. Ferrari D. Perrier A. Lee H. Vinuela A. Isacson O. Long-term survival of dopamine neurons derived from parthenogenetic primate embryonic stem cells (cyno-1) after transplantation. Stem Cells. 2005;23:914–922. doi: 10.1634/stemcells.2004-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hellmann MA. Panet H. Barhum Y. Melamed E. Offen D. Increased survival and migration of engrafted mesenchymal bone marrow stem cells in 6-hydroxydopamine-lesioned rodents. Neurosci Lett. 2006;395:124–128. doi: 10.1016/j.neulet.2005.10.097. [DOI] [PubMed] [Google Scholar]
- 12.Li Y. Chen J. Wang L. Zhang L. Lu M. Chopp M. Intracerebral transplantation of bone marrow stromal cells in a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson's disease. Neurosci Lett. 2001;316:67–70. doi: 10.1016/s0304-3940(01)02384-9. [DOI] [PubMed] [Google Scholar]
- 13.Fu YS. Cheng YC. Lin MY. Cheng H. Chu PM. Chou SC. Shih YH. Ko MH. Sung MS. Conversion of human umbilical cord mesenchymal stem cells in Wharton's jelly to dopaminergic neurons in vitro: potential therapeutic application for parkinsonism. Stem Cells. 2006;24:115–124. doi: 10.1634/stemcells.2005-0053. [DOI] [PubMed] [Google Scholar]
- 14.Miura M. Gronthos S. Zhao M. Lu B. Fisher LW. Robey PG. Shi S. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci USA. 2003;100:5807–5812. doi: 10.1073/pnas.0937635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chai Y. Jiang X. Ito Y. Bringas P. Han J., Jr Rowitch DH. Soriano P. McMahon AP. Sucov HM. Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development. 2000;127:1671–1679. doi: 10.1242/dev.127.8.1671. [DOI] [PubMed] [Google Scholar]
- 16.Nosrat IV. Widenfalk J. Olson L. Nosrat CA. Dental pulp cells produce neurotrophic factors, interact with trigeminal neurons in vitro, and rescue motoneurons after spinal cord injury. Dev Biol. 2001;238:120–132. doi: 10.1006/dbio.2001.0400. [DOI] [PubMed] [Google Scholar]
- 17.Gronthos S. Brahim J. Li W. Fisher LW. Cherman N. Boyde A. DenBesten P. Robey PG. Shi S. Stem cell properties of human dental pulp stem cells. J Dent Res. 2002;81:531–535. doi: 10.1177/154405910208100806. [DOI] [PubMed] [Google Scholar]
- 18.Nosrat IV. Smith CA. Mullally P. Olson L. Nosrat CA. Dental pulp cells provide neurotrophic support for dopaminergic neurons and differentiate into neurons in vitro; implications for tissue engineering and repair in the nervous system. Eur J Neurosci. 2004;19:2388–2398. doi: 10.1111/j.0953-816X.2004.03314.x. [DOI] [PubMed] [Google Scholar]
- 19.Seo BM. Sonoyama W. Yamaza T. Coppe C. Kikuiri T. Akiyama K. Lee JS. Shi S. SHED repair critical-size calvarial defects in mice. Oral Dis. 2008;14:428–434. doi: 10.1111/j.1601-0825.2007.01396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cordeiro MM. Dong Z. Kaneko T. Zhang Z. Miyazawa M. Shi S. Smith AJ. Nor JE. Dental pulp tissue engineering with stem cells from exfoliated deciduous teeth. J Endod. 2008;34:962–969. doi: 10.1016/j.joen.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 21.Sasaki R. Aoki S. Yamato M. Uchiyama H. Wada K. Okano T. Ogiuchi H. Neurosphere generation from dental pulp of adult rat incisor. Eur J Neurosci. 2008;27:538–548. doi: 10.1111/j.1460-9568.2008.06026.x. [DOI] [PubMed] [Google Scholar]
- 22.Paxinos G. Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; San Diego: 1986. [Google Scholar]
- 23.Fitoussi N. Sotnik-Barkai I. Tornatore C. Herzberg U. Yadid G. Dopamine turnover and metabolism in the striatum of parkinsonian rats grafted with genetically-modified human astrocytes. Neuroscience. 1998;85:405–413. doi: 10.1016/s0306-4522(97)00635-0. [DOI] [PubMed] [Google Scholar]
- 24.Zheng Y. Liu Y. Zhang C. Zhang H. Li W. Shi S. Le A. Wang S. Stem cells from deciduous teeth repair mandibular defect in swine. J Dent Res. 2009;88:249–254. doi: 10.1177/0022034509333804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang X. Li X. Wang K. Zhou H. Xue B. Li L. Wang X. Forskolin cooperating with growth factor on generation of dopaminergic neurons from human fetal mesencephalic neural progenitor cells. Neurosci Lett. 2004;362:117–121. doi: 10.1016/j.neulet.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 26.Suon S. Yang M. Iacovitti L. Adult human bone marrow stromal spheres express neuronal traits in vitro and in a rat model of Parkinson's disease. Brain Res. 2006;1106:46–51. doi: 10.1016/j.brainres.2006.05.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.LaBonne C. Bronner-Fraser M. Molecular mechanisms of neural crest formation. Annu Rev Cell Dev Biol. 1999;15:81–112. doi: 10.1146/annurev.cellbio.15.1.81. [DOI] [PubMed] [Google Scholar]
- 28.Wang X. Lu Y. Zhang H. Wang K. He Q. Wang Y. Liu X. Li L. Wang X. Distinct efficacy of pre-differentiated versus intact fetal mesencephalon-derived human neural progenitor cells in alleviating rat model of Parkinson's disease. Int J Dev Neurosci. 2004;22:175–183. doi: 10.1016/j.ijdevneu.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 29.Huang AH. Snyder BR. Cheng PH. Chan AW. Putative dental pulp-derived stem/stromal cells promote proliferation and differentiation of endogenous neural cells in the hippocampus of mice. Stem Cells. 2008;26:2654–2663. doi: 10.1634/stemcells.2008-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gandia C. Arminan A. Garcia-Verdugo JM. Lledo E. Ruiz A. Minana MD. Sanchez-Torrijos J. Paya R. Mirabet V. Carbonell-Uberos F. Llop M. Montero JA. Sepulveda P. Human dental pulp stem cells improve left ventricular function, induce angiogenesis, and reduce infarct size in rats with acute myocardial infarction. Stem Cells. 2008;26:638–645. doi: 10.1634/stemcells.2007-0484. [DOI] [PubMed] [Google Scholar]