Abstract
There is a significant flux of the neurotoxic oxysterol 27-hydroxycholesterol (27OHC) from the circulation across the blood-brain barrier. Because there is a correlation between 27OHC and cholesterol in the circulation and lipoprotein-bound cholesterol does not pass the blood-brain barrier, we have suggested that 27OHC may mediate the effects of hypercholesterolemia on the brain. We previously demonstrated a modest accumulation of 27OHC in brains of patients with sporadic Alzheimer's disease (AD), consistent with a role of 27OHC as a primary pathogenetic factor. We show here that there is a 4-fold accumulation of 27OHC in different regions of the cortexes of patients carrying the Swedish amyloid precursor protein (APPswe) 670/671 mutation. The brain levels of sitosterol and campesterol were not significantly different in the AD patients compared with the controls, suggesting that the blood-brain barrier was intact in the AD patients. We conclude that accumulation of 27OHC is likely to be secondary to neurodegeneration, possibly a result of reduced activity of CYP7B1, the neuronal enzyme responsible for metabolism of 27OHC. We discuss the possibility of a vicious circle in the brains of the patients with familial AD whereby neurodegenerative changes cause an accumulation of 27OHC that further accelerates neurodegeneration.
Keywords: cholesterol/metabolism, Alzheimer's disease, cytochrome P450, oxysterols, phytosterols
The large pool of cholesterol in the brain is efficiently isolated from other pools of cholesterol in the body by the efficient blood-brain barrier (for reviews, see 1, 2). In contrast to the lipoprotein-bound cholesterol in the circulation, side-chain oxidized cholesterol metabolites are able to cross the blood-brain barrier and fluxes of oxysterols have been demonstrated in both directions. We (3, 4) and others (5) have shown that about two-thirds of the synthesis of cholesterol in the brain is compensated for by a flow of 24S-hydroxycholesterol (24OHC) from the brain into the circulation. We also demonstrated a flux of the extracerebral metabolite 27-hydroxycholesterol (27OHC) from the circulation into the brain with a net uptake of this oxysterol (6). Most of the 27OHC present in the cerebrospinal fluid originates from the circulation (7).
Despite the substantial uptake of 27OHC by the brain, the levels of this oxysterol in the brain are very low, reaching only 5–10% of the levels of 24OHC (3, 8). The reason for this is a highly efficient metabolism. We have shown that the end metabolite of 27OHC in the brain is a steroid acid, 7α-hydroxy-3-oxo-4-cholestenoic acid, and that there is a continuous flux of this acid from the brain into the circulation (9). The nature of this acid as an end metabolite is illustrated by a very substantial accumulation of it in encapsulated brain hematomas (10). The rate-limiting step in the conversion of 27OHC into the steroid acid appears to be the introduction of a 7α-hydroxyl group catalyzed by the enzyme oxysterol 7α-hydroxylase, CYP7B1 (9). This enzyme is exclusively located in neuronal cells in the brain (11).
Despite the fact that lipoprotein-bound cholesterol does not pass the blood-brain barrier (1, 2), hypercholesterolemia is regarded to be a risk factor for Alzheimer's disease (AD) (12, 13), in particular in midlife (14). Because there is a close correlation between cholesterol and 27OHC in the circulation (15), hypercholesterolemia is likely to result in an increased uptake of this oxysterol. We have discussed the possibility that 27OHC is mediating the effect of hypercholesterolemia and that this oxysterol may be a primary pathogenetic factor in the development of AD (16, 17). The findings from a number of in vitro studies are thus consistent with the possibility that 27OHC may accelerate neurodegeneration. In cultured neuroblastoma cells, 27OHC counteracts the inhibiting effect of 24OHC on generation of amyloid (18) and in retinal pigment epithelial cells, it increases β-amyloid and oxidative stress (19). It has also been shown to increase β-amyloid formation in hippocampal slices and in neuronal preparations from rabbits (20). In addition to this, 27OHC reduces the production of the “memory protein” activity-regulated cytoskeleton-associated protein (arc) in mouse brain (21).
We have demonstrated modestly increased levels of 27OHC both in the brain (8) and cerebrospinal fluid (CSF) (22) of patients with the sporadic form of AD. There are three different possibilities for the increased levels: 1) increased uptake of 27OHC from the circulation due to hypercholesterolemia; 2) reduced efficiency of the blood-brain barrier; and 3) reduced metabolism of 27OHC due to loss of CYP7B1 activity as a consequence of neuronal loss. The first two possibilities are consistent with the influx of 27OHC as a primary pathogenetic factor whereas the last possibility means that the accumulation of 27OHC is secondary to the neurodegeneration.
In order to discriminate between the different possibilities, we measured 27OHC in brain autopsy samples from patients with a familial form of AD in which the primary pathogenetic factor is a double mutation in the gene encoding the amyloid precursor protein (APP) (23). In the present study, we found a marked accumulation of 27OHC in the brains of these patients, and the levels of 27OHC were considerably higher than the levels we previously observed in the brains of patients with the sporadic form of the disease (8). We conclude that accumulation of 27OHC is likely to be secondary to the neurodegeneration. Evidence is presented that the accumulation is more likely to be due to reduced CYP7B1 activity than to increased permeability of the blood-brain barrier or increased synthesis in the brain.
MATERIALS AND METHODS
Human brain postmortem tissues from AD patients carrying the APPswe mutation and age-matched control subjects
Autopsy brain tissue from the temporal, parietal, and occipital cortexes was obtained from three AD patients carrying the Swedish APP 670/671 mutation (age 63 ± 4 years, mean ± SEM) at specific request from the Huddinge University Hospital Brain Bank (courtesy of Dr. Nenad Bogdanovic). These AD patients had received the clinical diagnosis of dementia according to the National Institute of Neurological and Communicative Disorders and Stroke - Alzheimer's Disease and Related Disorders Association criteria (24) and the diagnosis of definitive AD was confirmed by neuropathological examination using Consortium to Establish a Registery for Alzheimer's Disease criteria (25). Cerebral cortical tissue from four age-matched control subjects (age 65 ± 2 years, mean ± SEM) with no clinical history of psychiatric or neurological disorders and no neuropathological changes indicative for dementia were included in this study. The main cause of death for controls was myocardial infarction and for AD patients pneumonia or cachexia and dehydration. In compliance with the Swedish law, the deceased were kept first at room temperature for a minimum of 3 h and then transferred to a cold room until the clinical autopsy was performed. At autopsy, samples from the different brain regions were collected from the left hemisphere with a postmortem interval of 5–22 h for AD patients and 11–44 h for controls. The tissue samples were frozen immediately and stored at −80°C until use.
Ethical aspects
Permission to use autopsy brain material in the experimental procedures was granted by the Regional Human Ethics committee in Stockholm and the Swedish Ministry of Health. For all subjects included in the study, consent from next of kin was given for research studies prior to autopsy.
Labeled steroids
2H3-labeled 24OHC and 2H5-labeled 27OHC were prepared as described previously (26). 2H6-labeled 5-cholestene-3β,7α,27-triol was prepared from commercially available 2H6-labeled 7α-hydroxycholesterol (Avanti Polar Lipids) by enzymatic 27-hydroxylation using recombinant human CYP27A1, adrenodoxin, and adrenodoxin reductase essentially under the conditions described earlier for 27-hydroxylation of other C27-steroids (27). The product, 2H6-labeled 5-cholesten-3β,7α,27-triol, had the expected mass spectrum with prominent peaks at m/z 129, m/z 550 (M-90), and m/z 460 (M-2 × 90).
Oxysterol and sterol analyses
Brain tissue was homogenized according to Folch's method (28) with minor modifications. Approximately 0.1 g of each cortical region was homogenized in 1ml of buffer (5 mM EDTA, 50µg/ml BHT in PBS, pH 7.4) using a polytron homogenizer. Three ml of choloroform:methanol (2:1, v/v) were added to the disrupted brain tissue homogenate. The homogenate was mixed by shaking (about 100 rpm) for 24 h in 4°C. The samples were centrifuged at 5000 × g for 5 min (room temperature) and the organic phase was collected in a new glass tube. The extraction procedure was repeated and both organic phases were combined. The extract was evaporated under argon gas, redissolved in 5 ml Folch, and stored at −20°C until analysis of the oxysterol content.
A volume corresponding to 10 mg of the extract was analyzed with respect to 24OHC, 27OHC, and 5-cholestene-3β,7α,27-triol using GC/MS and deuterium labeled internal standards as previously described (26). Prior to GC/MS analysis the residue was silyated with 200 µl pyridine:hexamethylsilyldisilazane:trimethylchlorosilane (3:2:1, v/v/v) for 30 min at 60°C. The solvent was evaporated under argon gas and redissolved in 80 µl hexane and subjected to GC/MS. The ions at m/z 413, 456, and 544 were used for tracing of unlabeled 24OHC, 27OHC, and 5-cholestene-3β,7α,27-triol, respectively, and the ions at m/z 416, 461, and 550 for tracing of 2H3-24OHC, 2H5-27OHC, and 2H6-5-cholestene-3β,7α,27-triol, respectively. Analysis of 5-cholesten-3β,7α,27-triol by isotope dilution has not been described previously. Replicate analyses of the same biological sample gave a coefficient of variation of 7%.
Cholesterol, cholesterol precursors, and plant sterols were analyzed by isotope dilution mass spectrometry as described (29) using the same extract as above.
Western blotting
Microsomes prepared from the above brain material were subjected to electrophoresis on a 10% SDS or Bis/Tris polyacrylamide gel and transferred to nitrocellulose membranes. The membranes were incubated for 2 h at room temperature in blocking buffer (5% milk in PBS, 0,05% Tween) followed by incubation overnight in a cold room with an anti-CYP46 antibody (a generous kind gift from Prof. D. Russell, University of Texas Southwestern Medical Center, Dallas,TX) and an anti-β-actin antibody. As a secondary antibody, Goat-anti-rabbit coupled with HRP (Pierce) was used and incubated at room temperature for 2 h. The membranes were incubated in Super Signal West Dura Extended Duration Substrate (Prod#34075) from Thermo Scientific (Pierce) according to the manufacturer's instructions. The signal (around 50 kDa) was detected by equipment from Bio Rad (Universal hood II).
Mitochondria were also prepared from the same brain material and subjected to electrophoresis and the same procedures as above with the exception that the anti-CYP46 antibody was replaced by an anti-CYP27 antibody (8).
RESULTS
Accumulation of 27OHC in the brains of AD patients carrying the APPswe mutation
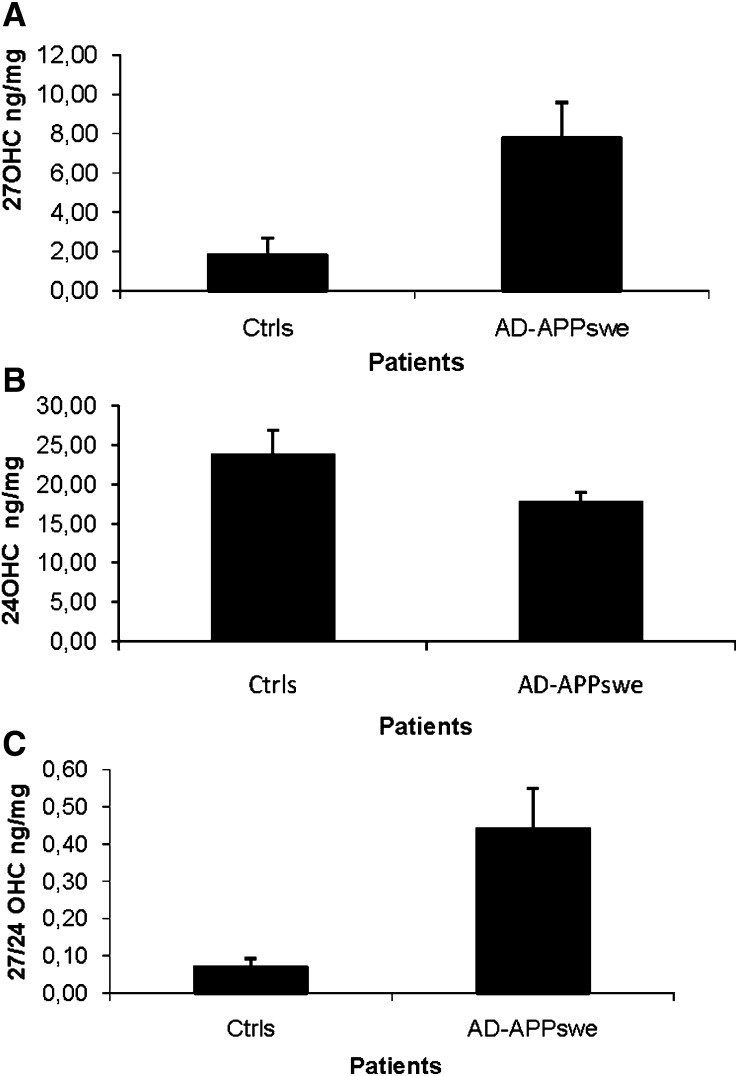
As shown in Fig. 1A, the levels of 27OHC in brain homogenates from the temporal cortexes of the AD patients were 7.8 ± 1.8 ng /mg tissue as compared with 1.8 ± 0.8 ng/mg tissue in the corresponding material from the controls (P = 0.02, Student's t-test). The level of 24OHC was 18 ± 1 ng/mg tissue in the AD patients as compared with 24 ± 3 ng/mg tissue in the controls (P > 0.05). The ratio between 27OHC and 24OHC was 0.44 ± 0.11 in the temporal cortexes of the AD patients and 0.07 ± 0.02 in the corresponding material from the controls (P = 0.02, Student's t-test).
Fig. 1.
Levels of 27OHC (A), 24OHC (B), and ratio 27OHC/24OHC (C) in brain homogenates from the temporal cortex of control subjects (n = 4) and AD patients carrying the APPswe mutation (n = 3). The levels correspond to ng/mg wet tissue.
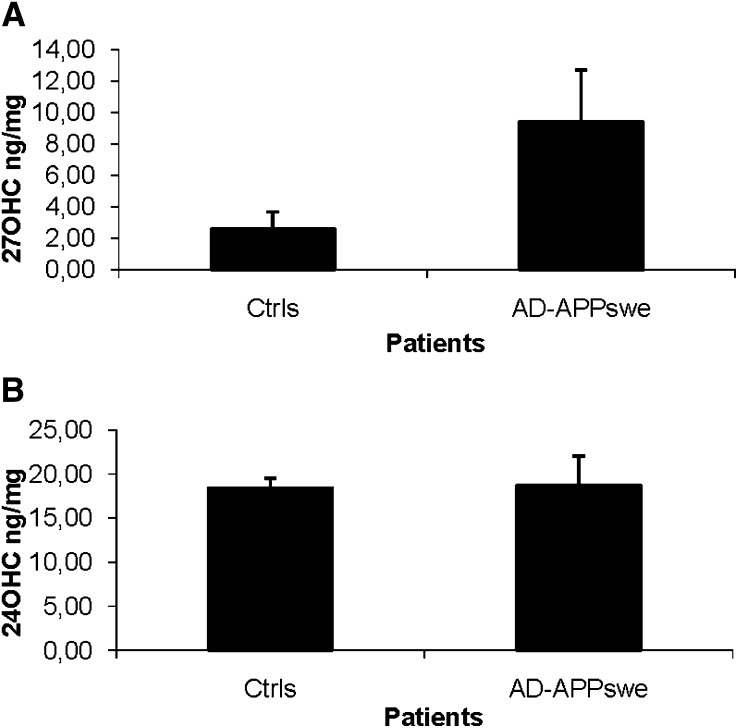
As shown in Fig. 2, the levels of 27OHC in brain homogenates from the parietal cortexes of the AD patients were 9.4 ± 3.3 ng/mg tissue as compared with 2.6 ± 1.1 ng/mg tissue in the corresponding material from the controls (P = 0.03, Student's t-test). The corresponding figures for 24OHC were 18 ± 1 ng/mg tissue in the patients and 19 ± 1 ng/mg tissue in the controls (P > 0.05).
Fig. 2.
Levels of 27OHC (A), 24OHC (B) in brain homogenates from the parietal cortex of control subjects (n = 4) and AD patients carrying the APPswe mutation (n = 3). The levels correspond to ng/mg wet tissue.
Similar results as obtained above were obtained in the analyses of brain homogenates from the occipital cortexes of AD patients and controls (results not shown).
Levels of 5-cholestene-3β,7α,27-triol in the brains of AD patients carrying the APPswe mutation
If the activity of the metabolizing enzyme CYP7B1 is the limiting factor for elimination of 27OHC from the brain and if this activity is reduced in the brains of patients with AD, reduced levels of the product 5-cholestene-3β,7α,27-triol would be expected, or at least a reduced ratio between this product and 27OHC. The levels of 5-cholestene-3β,7α,27-triol were found to be 31 ± 5 pg/mg tissue in the brain homogenates from the parietal cortexes of the AD patients as compared with 25 ± 4 pg/mg tissue in the corresponding materials from the controls (P > 0.05). The corresponding figures obtained in the analysis of 5-cholestene-3β,7α,27-triol in brain homogenates from the temporal cortexes of the AD patients was 63 ± 8 pg/mg tissue and 26 ± 2 pg/mg tissue, respectively (P < 0.05). The absolute levels of the product are thus similar or higher in the brains of patients with AD than in the controls, whereas the ratio between product and substrate is lower in AD. Data are consistent with but do not prove a reduced capacity to convert 27OHC into 5-cholestene-3β,7α,27-triol in the brains of the AD patients.
Levels of plant sterols and lathosterol in the brains of AD patients carrying the APPswe mutation
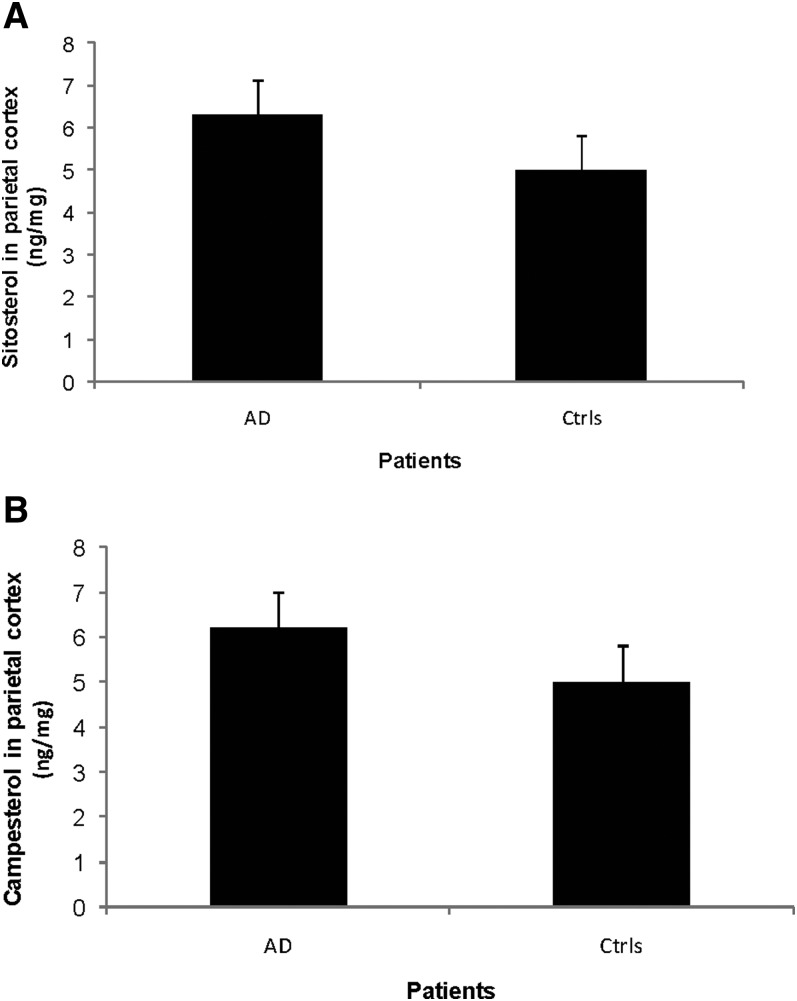
As shown in Fig. 3A, the levels of sitosterol in the brain homogenates from the parietal cortexes of the AD patients were 6.3 ± 0.8 ng/mg tissue as compared with 5.0 ± 0.8 ng/mg tissue in the controls (P > 0.05). The levels of campesterol in the same area of the brains of AD patients were 6.2 ± 0.8 ng/mg tissue as compared with 5.0 ± 0.8 ng/mg tissue in the controls (Fig. 3B, P > 0.05).
Fig. 3.
Levels of sitosterol (A) and campesterol (B) in the parietal cortex of control subjects (n = 3) and AD patients carrying the APPswe mutation (n = 3). The levels correspond to ng/mg wet tissue.
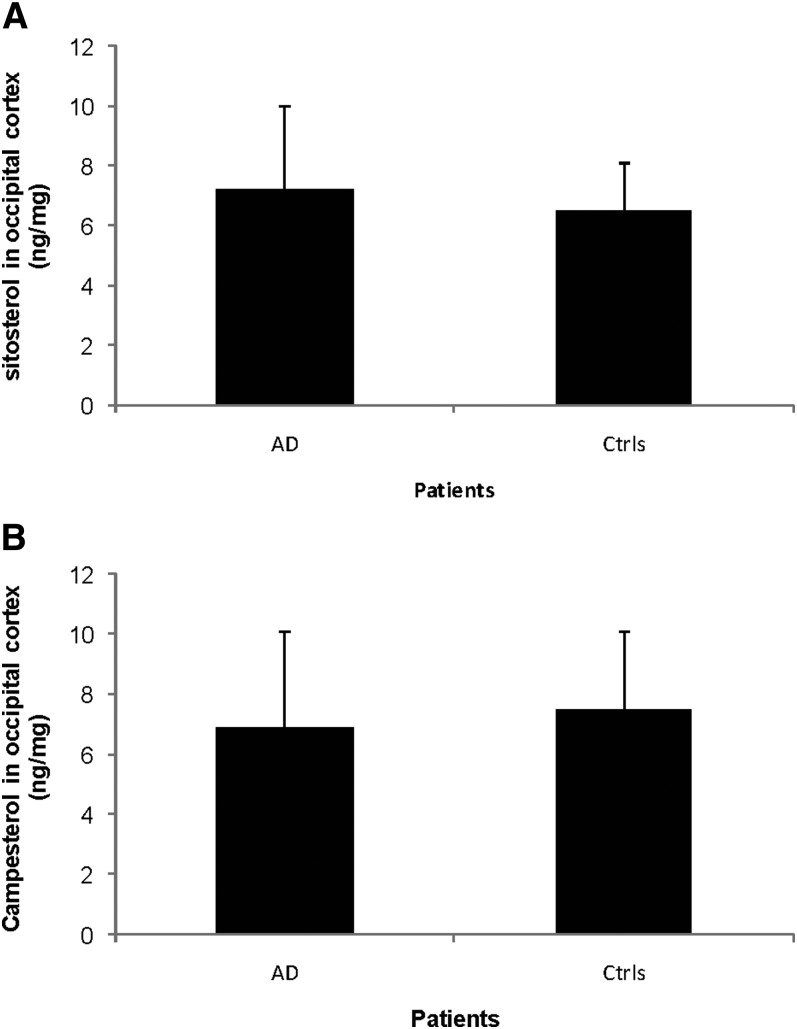
The levels of sitosterol in brain homogenates from the occipital cortexes of the AD patients were 7.2 ± 2.8 ng/mg tissue as compared with 6.5 ± 1.6 ng/mg tissue in the controls (Fig. 4A, P > 0.05). The levels of campesterol in brain homogenates from the same area were 6.9 ± 2.7 ng/mg tissue in the AD patients as compared with 7.5 ± 2.6 ng/mg tissue in the controls (Fig. 4B, P > 0.05).
Fig. 4.
Levels of sitosterol (A) and campesterol (B) in the occipital cortex of control subjects (n = 3) and AD patients carrying the APPswe mutation (n = 3). The levels correspond to ng/mg wet tissue.
Thus, there were no significant differences between AD and controls with respect to levels of plant sterols in the brain. Because the plant sterols are of exogenous origin the results are consistent with an intact blood-brain barier in the AD patients.
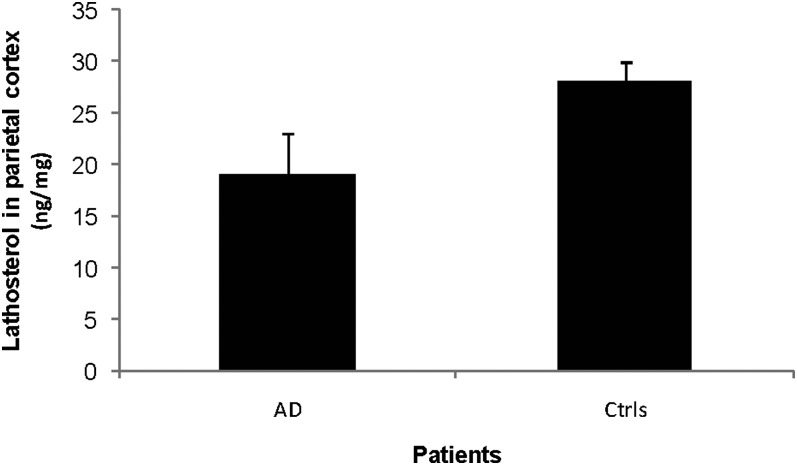
Lathosterol is a marker for cholesterol synthesis. As shown in Fig. 5, the levels of this steroid in the parietal cortex were 19 ± 4 ng/mg tissue as compared with 28 ± 2 ng/mg tissue in the controls (P < 0.05). In the brain homogenates from the occipital cortex, the corresponding levels were 19 ± 2 ng/mg tissue and 22 ± 3 ng/mg tissue (P > 0.05) (not shown). The results indicate a reduced synthesis of cholesterol in the parietal cortexes but not in the temperal cortexes of the AD patients.
Fig. 5.
Levels of lathosterol in the parietal cortex of control subjects (n = 3) and AD patients carrying the APPswe mutation (n = 3). The levels correspond to ng/mg wet tissue.
Levels of CYP46A1 and CYP27A1 in the brains of AD patients carrying the APPswe mutation
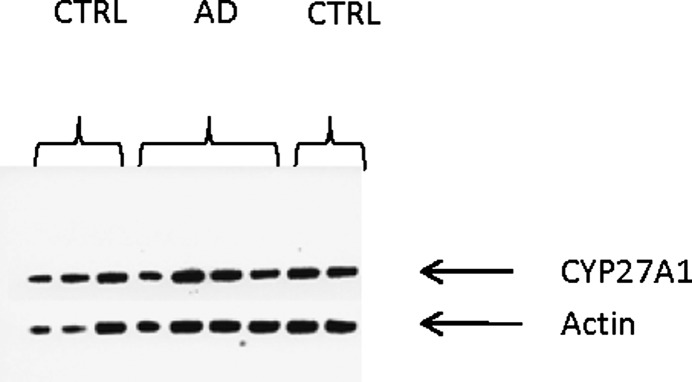
Theoretically, the high levels of 27OHC could be due to increased synthesis. As shown in Fig. 6, Western blotting with antibodies toward human CYP27A1 gave similar signals in the analysis of the mitochondrial fraction of brain tissue from the AD patients as in the analysis of the corresponding fraction from the brains of the controls. Western blotting was also performed with antibodies toward human CYP46A1 and β-actin on the microsomal fraction of brain homogenates from the patients and the controls. No significant difference was observed between the patients and the controls (not shown). The results are consistent with unchanged levels of CYP27A1 and CYP46A1 in the brains of the AD patients.
Fig. 6.
Western blotting of the sterol 27-hydroxylase (CYP27) in mitochondrial preparations from the parietal and occipital cortex of control subjects and AD patients carrying the APPswe mutation. The blots show the results obtained in the analysis of mitochondria from the occipital region of three controls and two AD patients and parietal region of two controls and two AD patients.
DISCUSSION
The brain levels of 27OHC and 24OHC in the present control subjects were found to be almost identical to those of the control subjects studied previously using the same methodology (8). In that study, we reported that the levels of 27OHC were increased by 40–80% in the temporal, parietal, and occipital cortexes of sporadic AD patients. In the present work, we demonstrate that brain levels of 27OHC are increased by a factor of about four in AD patients carrying the Swedish APP 670/671 mutation. The APP 670/671 mutation on chromosome 21 in this Swedish family is considered to be the cause of the disease because it alters APP metabolism leading to an accumulation of Aβ peptides and Aβ plaque formation (30–33). A history of AD has been traced through eight generations of this family and the onset of the disease occurs between 44 and 61 years of age (34).
In our previous investigation, there was a slight (15–20%) decrease in the levels of 24OHC in the brains of the patients with the sporadic form of AD (8). In the present work, a similar decrease of these levels was observed in the temporal cortexes but not in the parietal cortexes of the APPswe patients. The level of CYP46A1 as measured by Western blotting was not significantly different between patients and controls. Due to the location of CYP46 in the neuronal cells, the loss of such cells in the brains of AD patients would be expected to be associated with a reduction in the level of 24OHC. On the other hand, we have shown that the loss of CYP46 in the neuronal cells may at least in part be compensated for by an abnormal expression of CYP46 in glial cells in the brains of patients with AD (35).
It is of interest to compare the present findings in patients with the familial form of AD with a transgenic mouse model for AD expressing the same Swedish APP mutation. Up to the age of 9 months, we found that APP23 transgenic mice have levels of 27OHC identical to those found in the control wild-type mice (8). At the ages of 12 and 18 months, there was a modest (20–30%) increase in the levels of 27OHC. The latter increase was associated with a similar increase in the levels of plant sterols in the brain. Because the latter increase is likely to be due to an increased permeability of the blood-brain barrier, it seems likely that the slight accumulation of 27OHC in the brains of the animal model is due to a less efficient blood-brain barrier rather than due to a direct effect of the neurodegeneration. It is obvious that there are differences between patients with an APP mutation and the corresponding mouse model. Part of the explanation for the lower accumulation of 27OHC in the brains of the APP23 mice may be the 4-fold lower levels of this oxysterol in the circulation as compared with the corresponding levels in humans. It may be speculated that this difference in circulating levels is related to a higher rate of metabolism in mice compared with that of man.
It is evident that the high accumulation of 27OHC in the brains of the patients with familial AD and a known pathogenetic mechanism causing the disease is a consequence rather than a cause of the neurodegeneration. However, given the properties of 27OHC, it cannot be excluded that the accumulation of this oxysterol may promote the disease process and that a vicious circle is created in which the neurodegeneration causes increased accumulation, which may further exacerbate neurodegeneration. The considerably higher accumulation of 27OHC in the brains of the patients with an autosomal dominant disease-causing mutation may reflect the early and more clinically aggressive disease progression in these patients compared with that of sporadic AD (36). The age of the patient at the time of death may be of importance for the accumulation of 27OHC and the present patients were relatively young (mean age 63 years). It may be mentioned, however, that in our previous studies on brain autopsy samples from patients between 66 and 90 years of age (mean age 82 years) there was no correlation between the age at the time of death and the accumulation of 27OHC (8).
The reason for the increased accumulation of 27OHC in the AD patients bearing the APP Swedish mutation is not likely to be increased permeability of the blood-brain barrier. Thus, there was no significant accumulation of plant sterols in the brains of the patients. Because the protein level of the sterol 27-hydroxylase enzyme (CYP27) was similar in the brains of the AD patients and the controls, a local production of 27OHC in the brains of the AD patients is also excluded. The most likely explanation seems to be a reduced level of CYP7B1, the neuronal enzyme responsible for metabolism of 27OHC. It has been reported previously that the levels of CYP7B1 are reduced in the brains of patients with AD (11). A number of attempts in our laboratory to measure CYP7B1 by Western blotting in human brain samples using different preparations of antibodies have failed thus far. We found a reduced ratio between 5-cholestene-3β,7α,27-triol and 27OHC in the brains of APP 670/671 AD patients. The absolute levels of the product were, however, similar or higher in the brains of the AD patients. The data are consistent with but do not prove that the activity of CYP7B1 is reduced in the AD patients.
Recently it was shown that a small subgroup of patients with hereditary spastic paresis (HSP SPG5) have a mutation in the gene coding for CYP7B1 (37). As a consequence, these patients have high levels of 27OHC in plasma and CSF (38) and most probably also in the brain. Whether or not the accumulation of 27OHC in these patients is part of the pathogenesis is not known at this stage. Interestingly, most of these patients do not develop neurodegeneration, but a more detailed characterization is lacking. The possibility has been discussed that motor neurons may be more sensitive to 27OHC than other neuronal cells, but definite evidence for this is lacking (38).
In summary, patients with the present familial form of AD have a 4-fold accumulation of 27OHC in the brain, considerably higher than the corresponding accumulation in the brains of patients with the sporadic form of the disease and in the brains of a transgenic mouse model for AD. Here, we provide evidence that the accumulation is a consequence of the neurodegeneration. We discuss the possibility of a vicious circle in the brains of patients with AD whereby neurodegenerative changes cause an accumulation of 27OHC that further accelerates neurodegeneration.
Footnotes
Abbreviations:
- AD
- Alzheimer's disease
- APPswe
- Swedish amyloid precursor protein
- 24OHC
- 24S-hydroxycholesterol
- 27OHC
- 27-hydroxycholesterol
This work was supported by the Swedish Science Council (projects 05871 and 03141), Swedish Brain Power, the Söderberg Foundation, the Swedish Alzheimer's Foundation, and the National Institutes of Health (GM062882 and AG024336 to I.P.). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or other granting agencies.
REFERENCES
- 1.Dietschy J. M., Turley S. D. 2004. Thematic review series: Brain lipids. Cholesterol metabolism in the central nervous system during early development and in the mature animal. J. Lipid Res. 45: 1375–1397. [DOI] [PubMed] [Google Scholar]
- 2.Bjorkhem I., Meaney S. 2004. Brain cholesterol: long secret life behind a barrier. Arterioscler. Thromb. Vasc. Biol. 24: 806–815. [DOI] [PubMed] [Google Scholar]
- 3.Lutjohann D., Breuer O., Ahlborg G., Nennesmo I., Siden A., Diczfalusy U., Bjorkhem I. 1996. Cholesterol homeostasis in human brain: evidence for an age-dependent flux of 24S-hydroxycholesterol from the brain into the circulation. Proc. Natl. Acad. Sci. USA. 93: 9799–9804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bjorkhem I., Lutjohann D., Breuer O., Sakinis A., Wennmalm A. 1997. Importance of a novel oxidative mechanism for elimination of brain cholesterol. Turnover of cholesterol and 24(S)-hydroxycholesterol in rat brain as measured with 18O2 techniques in vivo and in vitro. J. Biol. Chem. 272: 30178–30184. [DOI] [PubMed] [Google Scholar]
- 5.Russell D. W., Halford R. W., Ramirez D. M., Shah R., Kotti T. 2009. Cholesterol 24-hydroxylase: an enzyme of cholesterol turnover in the brain. Annu. Rev. Biochem. 78: 1017–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heverin M., Meaney S., Lutjohann D., Diczfalusy U., Wahren J., Bjorkhem I. 2005. Crossing the barrier: net flux of 27-hydroxycholesterol into the human brain. J. Lipid Res. 46: 1047–1052. [DOI] [PubMed] [Google Scholar]
- 7.Leoni V., Masterman T., Patel P., Meaney S., Diczfalusy U., Bjorkhem I. 2003. Side chain oxidized oxysterols in cerebrospinal fluid and the integrity of blood-brain and blood-cerebrospinal fluid barriers. J. Lipid Res. 44: 793–799. [DOI] [PubMed] [Google Scholar]
- 8.Heverin M., Bogdanovic N., Lutjohann D., Bayer T., Pikuleva I., Bretillon L., Diczfalusy U., Winblad B., Bjorkhem I. 2004. Changes in the levels of cerebral and extracerebral sterols in the brain of patients with Alzheimer's disease. J. Lipid Res. 45: 186–193. [DOI] [PubMed] [Google Scholar]
- 9.Meaney S., Heverin M., Panzenboeck U., Ekstrom L., Axelsson M., Andersson U., Diczfalusy U., Pikuleva I., Wahren J., Sattler W., et al. 2007. Novel route for elimination of brain oxysterols across the blood-brain barrier: conversion into 7alpha-hydroxy-3-oxo-4-cholestenoic acid. J. Lipid Res. 48: 944–951. [DOI] [PubMed] [Google Scholar]
- 10.Nagata K., Takakura K., Asano T., Seyama Y., Hirota H., Shigematsu N., Shima I., Kasama T., Shimizu T. 1992. Identification of 7 alpha-hydroxy-3-oxo-4-cholestenoic acid in chronic subdural hematoma. Biochim. Biophys. Acta. 1126: 229–236. [DOI] [PubMed] [Google Scholar]
- 11.Yau J. L., Rasmuson S., Andrew R., Graham M., Noble J., Olsson T., Fuchs E., Lathe R., Seckl J. R. 2003. Dehydroepiandrosterone 7-hydroxylase CYP7B: predominant expression in primate hippocampus and reduced expression in Alzheimer's disease. Neuroscience. 121: 307–314. [DOI] [PubMed] [Google Scholar]
- 12.Launer L. J., White L. R., Petrovitch H., Ross G. W., Curb J. D. 2001. Cholesterol and neuropathologic markers of AD: a population-based autopsy study. Neurology. 57: 1447–1452. [DOI] [PubMed] [Google Scholar]
- 13.Pappolla M. A., Bryant-Thomas T. K., Herbert D., Pacheco J., Fabra Garcia M., Manjon M., Girones X., Henry T. L., Matsubara E., Zambon D., et al. 2003. Mild hypercholesterolemia is an early risk factor for the development of Alzheimer amyloid pathology. Neurology. 61: 199–205. [DOI] [PubMed] [Google Scholar]
- 14.Kivipelto M., Helkala E. L., Hanninen T., Laakso M. P., Hallikainen M., Alhainen K., Soininen H., Tuomilehto J., Nissinen A. 2001. Midlife vascular risk factors and late-life mild cognitive impairment: a population-based study. Neurology. 56: 1683–1689. [DOI] [PubMed] [Google Scholar]
- 15.Babiker A., Dzeletovic S., Wiklund B., Pettersson N., Salonen J., Nyyssonen K., Eriksson M., Diczfalusy U., Bjorkhem I. 2005. Patients with atherosclerosis may have increased circulating levels of 27-hydroxycholesterol and cholestenoic acid. Scand. J. Clin. Lab. Invest. 65: 365–375. [DOI] [PubMed] [Google Scholar]
- 16.Bjorkhem I., Heverin M., Leoni V., Meaney S., Diczfalusy U. 2006. Oxysterols and Alzheimer's disease. Acta Neurol. Scand. Suppl. 185: 43–49. [DOI] [PubMed] [Google Scholar]
- 17.Bjorkhem I., Cedazo-Minguez A., Leoni V., Meaney S. 2009. Oxysterols and neurodegenerative diseases. Mol. Aspects Med. 30: 171–179. [DOI] [PubMed] [Google Scholar]
- 18.Famer D., Meaney S., Mousavi M., Nordberg A., Bjorkhem I., Crisby M. 2007. Regulation of alpha- and beta-secretase activity by oxysterols: cerebrosterol stimulates processing of APP via the alpha-secretase pathway. Biochem. Biophys. Res. Commun. 359: 46–50. [DOI] [PubMed] [Google Scholar]
- 19.Dasari B., Prasanthi J. R., Marwarha G., Singh B. B., Ghribi O. 2010. The oxysterol 27-hydroxycholesterol increases beta-amyloid and oxidative stress in retinal pigment epithelial cells. BMC Ophthalmol. 10: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghribi O. 2008. Potential mechanisms linking cholesterol to Alzheimer's disease-like pathology in rabbit brain, hippocampal organotypic slices, and skeletal muscle. J. Alzheimers Dis. 15: 673–684. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Mateos L., Akterin S., Gil-Bea F. J., Spulber S., Rahman A., Bjorkhem I., Schultzberg M., Flores-Morales A., Cedazo-Minguez A. 2009. Activity-regulated cytoskeleton-associated protein in rodent brain is down-regulated by high fat diet in vivo and by 27-hydroxycholesterol in vitro. Brain Pathol. 19: 69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leoni V., Masterman T., Mousavi F. S., Wretlind B., Wahlund L. O., Diczfalusy U., Hillert J., Bjorkhem I. 2004. Diagnostic use of cerebral and extracerebral oxysterols. Clin. Chem. Lab. Med. 42: 186–191. [DOI] [PubMed] [Google Scholar]
- 23.Mullan M., Crawford F., Axelman K., Lillius L., Winblad B., Lannfelt L. 1992. A pathogenetic mutation for probable Alzheimer's disease in the APP gene at the N-terminus of beta-amyloid. Nat. Genet. 1: 345–347. [DOI] [PubMed] [Google Scholar]
- 24.McKhann G., Drachman D., Folstein M., Katzman R., Price D., Stadlan E. M. 1984. Clinical diagnosis of Alzheimer's disease: report of NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services task forces on Alzheimer's disease. Neurology. 34: 939–944. [DOI] [PubMed] [Google Scholar]
- 25.Mirra S.S., Heyman A., McKeel D., Sumi S.M., Crain B.J., Brownlee L.M., Vogel F.S., Hughes J.P., Van Belle G., Berf L., participating CERAD neuropathologists 1991. The Consortium to Establish a Registery for AD (CERAD). Part II: Standardization of the neuropathological assessment of Alzheimer's disease. Neurology. 41: 479–486. [DOI] [PubMed] [Google Scholar]
- 26.Dzeletovic S., Breuer O., Lund E., Diczfalusy U. 1995. Determination of cholesterol oxidation products in human plasma by isotope dilution-mass spectrometry. Anal. Biochem. 225: 73–80. [DOI] [PubMed] [Google Scholar]
- 27.Pettersson H., Norlin M., Andersson U., Pikuleva I., Bjorkhem I., Misharin A. Y., Wikvall K. 2008. Metabolism of a novel side chain modified Delta8(14)-15-ketosterol, a potential cholesterol lowering drug: 28-hydroxylation by CYP27A1. Biochim. Biophys. Acta. 1781: 383–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Folch J., Lees M., Sloane Stanley G. H. 1957. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226: 497–509. [PubMed] [Google Scholar]
- 29.Acimovic J., Lövgen-Sandblom A., Monostery K., Rozman D., Golicnik M., Lutjohann D., Björkhem I. 2009. Combined gas chromatographic-mass spectrometric analysis of cholesterol precursors and plant sterols in cultured cells. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 877: 2081–2086. [DOI] [PubMed] [Google Scholar]
- 30.Citron M., Oltersdorf T., Haass G., McConglogue L., Hung A., Seubert P., Vigo-Pelfrey C., Lieberburg I., Selkoe D. 1992. Mutation of the beta amyloid precursor protein in familal AD increases beta-protein production. Science. 360: 672–674. [DOI] [PubMed] [Google Scholar]
- 31.Lannfelt L., Bogdanovic N., Appelgren H., Axelman K., Lillius L., Hansson G., Schenk D., Hardy J., Winblad B. 1994. Amyloid precursor protein mutation causes Alzheimer's disease in a Swedish family. Neurosci. Lett. 168: 254–256. [DOI] [PubMed] [Google Scholar]
- 32.Hellström-Lindahl E., Viitanen M., Marutle A. 2009. Comparison of Abeta levels in the brain of familial and sporadic Alzheimer's disease. Neurochem. Int. 5: 243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marutle A., Warpman U., Bogdanovic N., Lannfelt L., Nordberg A. 1992. Neuronal nicotinic receptor deficits in Alzheimer's patients with the Swedish amyloid precursor protein 670/671 mutation. J. Neurochem. 1972: 1161–1169. [DOI] [PubMed] [Google Scholar]
- 34.Axelman K., Basun H., Winblad B., Lannfelt L. 1994. A clinical and genealogical investigation of large Swedish Alzheimer family with a codon 670/671 amyloid precursor protein mutation. Arch. Neurol. 57: 1193–1197. [DOI] [PubMed] [Google Scholar]
- 35.Bogdanovic N., Bretillon L., Lund E. G., Diczfalusy U., Lannfelt L., Winblad B., Russell D., Björkhem I. 2001. On the turnover of brain cholesterol in patients with Alzheimer's disease. Abnormal induction of the cholesterol-catabolic enzyme CYP46 in glial cells. Neurosci. Lett. 314: 45–48. [DOI] [PubMed] [Google Scholar]
- 36.Rabinovici G. D., Furst A. J., Alkalay A., Racine C. A., O'Neil J. P., Janabi M., Baker S. L., Agarwal N., Bonasera S. J., Mormino E. C., et al. 2010. Increased metabolic vulnerability in early-onset Alzheimer's disease is not related to amyloid burden. Brain. 133: 512–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsaousidou M. K., Ouahchi K., Warner T. T., Yang Y., Simpson M. A., Laing N. G., Wilkinson P. A., Madrid R. E., Patel H., Hentati F., et al. 2008. Sequence alteration within CYP7B1 implicate defective cholesterol homeostasis in motor-neuron degeneration. Am. J. Hum. Genet. 82: 510–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schule R., Siddique T., Deng H. X., Yang Y., Donkervoort S., Hansson M., Madrid R. E., Siddique N., Schöls L., Björkhem I. 2010. Marked accumulation of 27- hydroxycholesterol in patients with hereditary spastic paresis (SPG5) and a mutation in the gene coding for the oxysterol 7 alpha hydroxylase (CYP7B1). J. Lipid Res. 51: 819–893. [DOI] [PMC free article] [PubMed] [Google Scholar]