Abstract
Experimental studies showed that paraoxonase-3 (PON3) retards lipoprotein oxidation. Our objective was to describe a new assay to measure serum PON3 concentrations and report their reference values in a population-based study. The influence of PON3 promoter polymorphisms and their relationships with PON1 and lipid profile were also studied. We generated an anti-PON3 antibody by inoculating rabbits with a synthetic peptide specific to mature PON3. This antibody was used to develop an ELISA. The average regression line of standard plots (n = 8) was y = 0.9587 (0.3392) log10x + 1.9466 (0.0861) [r2 = 0.924 (0.0131); P < 0.001]. There was no cross reaction with PON1. Detection limit was 0.24 mg/l. Imprecision was ≤13.2%. Reference interval (n = 356) was 1.00-2.47 mg/l. PON3 was observed in HDL particles containing apolipoprotein (apo)A-I and PON1, but not apoA-II or apoE. Serum PON3 concentrations showed a moderate influence (about 10% variation) by PON3 promoter polymorphisms. Our study describes for the first time a method to measure serum PON3 concentrations. This method offers new opportunities in the investigation of the properties and role of PON3 in cardiovascular disease, with possible implications in clinical practice.
Keywords: analytical methods, antioxidants, atherosclerosis, lipoproteins, oxidative stress
The paraoxonase (PON) enzyme family comprises three members, PON1, PON2, and PON3, whose genes are located adjacent to each other on chromosome 7q21-22 (1, 2). In humans, PON1 and PON3 genes are produced in many cell types (3, 4), and their protein products are found in the circulation bound to HDL (5, 6). Conversely, PON2 is an intracellular enzyme that is not found in plasma (7). PON1 has paraoxonase, arylesterase, and lactonase activities and is involved in the protection against xenobiotic toxicity. PON2 and PON3 are not active against organophosphate substrates, but they have lactonase activity (8). PONs are able to retard LDL oxidation and cellular oxidative stress, and PON2 has been shown to prevent apoptosis in vascular endothelial cells (9–12). For these reasons, PONs are described as having anti-atherogenic properties. Furthermore, studies using a variety of mouse models of atherosclerosis have consistently shown that human PON1, PON2, or PON3 expression inhibits or reverses the development of atherosclerosis via mechanisms involving the reduction of oxidative stress, the promotion of cholesterol efflux from macrophages, and the normalization of vascular endothelium function (13–16).
Although knowledge about PON1 and PON2 structure and function is rapidly expanding, not much is known about the PON3 protein. Its gene was identified in 1996 when Primo-Parmo et al. (1) detected a large number of cDNA sequences in the Genome Data Base with significant similarity, but not identical, to human PON1. The percentage identity among human PON1, PON2, and PON3 genes is high (about 70%), and the genes are believed to derive from a common precursor (17). It soon became apparent that, in humans, PON3 is present in HDL particles and prevents LDL oxidation in vitro. In addition, the adenovirus-mediated expression of human PON3 protected against the development of atherosclerosis in apolipoprotein (apo)E-deficient mice. Recent experimental studies showed that PON3 is not only associated with cardiovascular disease but also with obesity (15). Transgenic mice overexpressing human PON3 had lower body weight, decreased adiposity, and decreased plasma leptin concentration compared with their corresponding littermates. Interestingly, PON3 protected against early atherosclerosis onset in these animals. This important report opens new perspectives in the treatment of human obesity and its associated disorders.
Despite the potential relevance of PON3 in the protection against oxidative stress and inflammation, research on this protein has been hampered by the lack of reliable methods to measure its levels in the circulation. In the present study, we describe a new enzyme-linked immunosorbent assay (ELISA) to measure serum PON3 concentrations and report their reference values in a population-based study. The influence of PON3 promoter polymorphisms and their relationship with PON1 and lipid profile were also studied.
MATERIALS AND METHODS
Study participants
We analyzed samples from a population-based study conducted in our area. Details of this study have been published (18). The participants were healthy subjects (n = 356; 156 women, 200 men; mean age: 47 years, range 18-81 years) of Caucasian ethnic origin from the Mediterranean region of Catalonia. All the volunteers had been invited to attend a clinical examination and to provide a fasting blood sample. There was no clinical or analytical evidence of renal insufficiency, liver damage, neoplasia, or neurological disorders. One-hundred and twenty participants declared they were active smokers at the time of data collection. Blood samples were collected after an overnight fast into tubes with no anticoagulants to obtain serum or into tubes with K2-EDTA for genetic analyses. Measurements were either performed immediately or aliquots of material were stored at −80°C for subsequent batched analyses. The study was approved by the Ethics Committee (Institutional Review Board) of the Hospital Universitari de Sant Joan, and written, informed consent was obtained from all the participants.
Reagents for the PON3 ELISA
The anti-PON3 antibody was generated by inoculating rabbits with the peptide CRVNASQEVEPVEPEN, which is specific to mature PON3 (19, 20). This peptide was synthesized at the University of Manchester Biological Sciences Unit using an inhouse protocol. The rabbit immunoglobulin G (IgG) was separated on a protein-A-sepharose column. For the assay, stock antibody solutions (0.6 g/l) were diluted at 1:1600 in PBS with 0.1% BSA (pH 7.4).
As a standard, we used a pool of normal sera that was calibrated against purified PON3 (21). Standards were diluted in 0.05 M carbonate buffer (pH 9.6) to produce a standard curve of 0.09-3.00 mg/l.
There are no commercial control materials to use in performance studies of PON3 concentration measurement. Instead, we used three pools of sera prepared from samples from the healthy population described above. Sera with the lowest, highest, and intermediate levels were pooled, gently mixed for two h at 4°C and divided into aliquots for storage at –40°C. The three pools were termed “Pool A” (2.64 ± 0.26 mg/l), “Pool B” (1.37 ± 0.09 mg/l), and “Pool C” (0.89 ± 0.05 mg/l).
The secondary antibody was an anti-rabbit-IgG peroxidase conjugate (Sigma Aldrich Co., St. Louis, MO), diluted 1/2500 PBS/BSA. The enzyme substrate was tetramethylbenzidine (Sigma) plus hydrogen peroxide. Two mg tetramethylbenzidine was diluted in 200 μl dimethylsulfoxide (Sigma) and added to 20 ml of 0.1 M citrate/acetate buffer (pH 6.0) with 72 μl urea hydrogen peroxide.
PON3 ELISA procedure
Human serum samples were diluted 1:10,000. An amount of 100 μl of sample or standard were added in duplicate to the plate wells and incubated overnight at room temperature. After one wash with PBS, we added 100 μl of PBS/1% BSA to block the remaining absorption sites and incubated for 1 h at room temperature. After three washes, we added 100 μl of diluted antibody and incubated again for 1 h at room temperature. After washing, we added 200 μl of secondary antibody and incubated the plates again for 1 h at room temperature. After washing, we added 200 μl of enzyme/substrate solution and incubated for 15 min. The reaction was stopped with 50 μl of 2 M H2SO4, and the absorbance was read at 450 nm in an automated microplate reader (BioTek Instruments Inc., Winooski, VT). All the incubations were under gentle shaking. The assays were performed on 96-well, low-binding plates (Scientific Laboratory Supplies Ltd., Yorkshire, UK).
Other biochemical analyses
Serum PON1 lactonase activity was analyzed by measuring the hydrolysis of 5-thiobutyl butyrolactone (TBBL) as described (22, 23). Lactonase activity was measured in an assay reagent containing 1 mM CaCl2, 0.25 mM TBBL, and 0.5 mM 5,5′-dithio-bis-2-nitrobenzoic acid (DTNB) in 0.05 mM Tris-HCl buffer (pH = 8.0). The change in absorbance was monitored at 412 nm. Activities were expressed as U/L (1 U = 1 mmol of TBBL hydrolyzed per minute). The shelf-life of TBBL solution is approximately two months, and stocks were replenished during this period. Serum PON1 paraoxonase activity was determined by measuring the rate of hydrolysis of paraoxon at 410 nm and 37°C in a 0.05 mM glycine buffer (pH 10.5) with 1 mM CaCl2 (24). Activities were expressed as U/L (1 U = 1 μmol of paraoxon hydrolyzed per minute). Paraoxon substrate had to be prepared fresh for every batch of measurements. Serum PON1 concentration was determined as previously reported (25). Serum cholesterol, triglycerides, HDL-cholesterol, apoA-I and apoB concentrations were measured with reagents obtained from Beckman-Coulter in a Synchron LXi automated analyzer (Beckman-Coulter, Fullerton, CA).
HPLC of HDL particles
We fractioned HDL by a dual-detection HPLC system (26, 27) to investigate PON3 distribution in these lipoproteins. Briefly, 100 μl of serum from three healthy volunteers were applied onto two columns of TSK gel Lipopropak XL connected in tandem and eluted at a flow rate of 0.7 ml/min with 50 mM Tris acetate buffer (pH 8.0) containing 0.3 M sodium acetate, 0.05% sodium azide, and 0.005% Brij-35. Fractions were collected, and the concentrations of apoA-I, apoA-II, apoE, and PON1 were measured as reported (25).
PON3 genotyping
Genomic DNA was obtained from leukocytes (Puregene DNA Isolation reagent set, Gentra Systems Inc., Minneapolis, MN). Selected single nucleotide polymorphisms (SNP) of the PON3 promoter were analyzed by the Iplex Gold MassArray™ method (Sequenom Inc., San Diego, CA) at the Spanish National Genotyping Center (Barcelona, Spain).
Statistical analyses
The normality of distributions was determined using the Kolmogorov-Smirnov test. Differences between two groups were assessed with the Student's t-test (parametric) or the Mann-Whitney U test (non-parametric). Differences between multiple groups were analyzed by the Kruskal-Wallis test. Pearson or Spearman correlation coefficients were used to evaluate the degree of association between variables. Each SNP was tested for Hardy-Weinberg equilibrium using Haploview 4.0 software (28). Estimates of linkage disequilibrium between SNPs were calculated using Fisher's test. Haplotypic association analyses were determined with the EM algorithm (29) and the Markov Chain Monte Carlo method (30). Results are shown as means and SD in parentheses (parametric) or as medians and 95% CI (non-parametric). The SPSS 18.0 package was employed for all statistical calculations.
RESULTS
Performance evaluation of serum PON3 ELISA
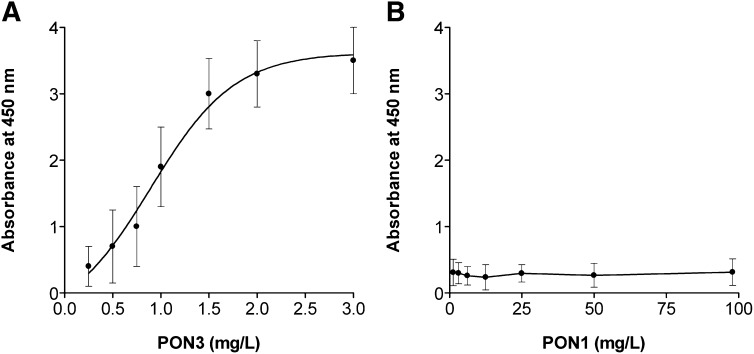
Standard plots of PON3 concentration versus absorbance were curvilinear (Fig. 1A) and could be transformed to linear after logarithmic conversion. The valid range of measurement was 0.25-3.0 mg/l, and the average regression line (n = 8) was y = 0.9587 (0.3392) log10x + 1.9466 (0.0861) [r2 = 0.924 (0.0131); P < 0.001; numbers in parentheses are the SD]. Cross-reactivity studies showed that there was no cross reaction with PON1 (Fig. 1B). To determine the detection limit, the absorbance of the reagent blank (buffer) was measured 20 times, the mean and SD were calculated, and the detection limit was defined as the concentration corresponding to an absorbance equal to the mean of the reagent blank value plus three times SD. The calculated detection limit was 0.24 mg/l. Intra-assay imprecision was determined with 20 replicate analyses of the three pools performed in the same run. To assess inter-assay imprecision, aliquots of these pools stored at –40°C were analyzed over 20 consecutive days. The coefficients of variation (CV) of the measurements ranged between 5.6 and 13.2% (supplementary Table I). The analytical recovery was calculated by adding a known concentration of purified PON3 (0.44 mg/l) to the three pools, and it was expressed as percentage of the analyzed values on the theoretical values. Results were 94.3 ± 3.0% for Pool A, 86.3 ± 2.6% for Pool B, and 95.4 ± 4.9 for Pool C (n = 3 for each pool).
Fig. 1.
A: Representative example of serum PON3 ELISA standard plots (N = 8). B: Cross-reactivity with PON1. The anti-PON3 antibody was incubated with the specified concentrations of PON1. There was no evidence of cross-reaction at the PON1 range studied.
Reference interval
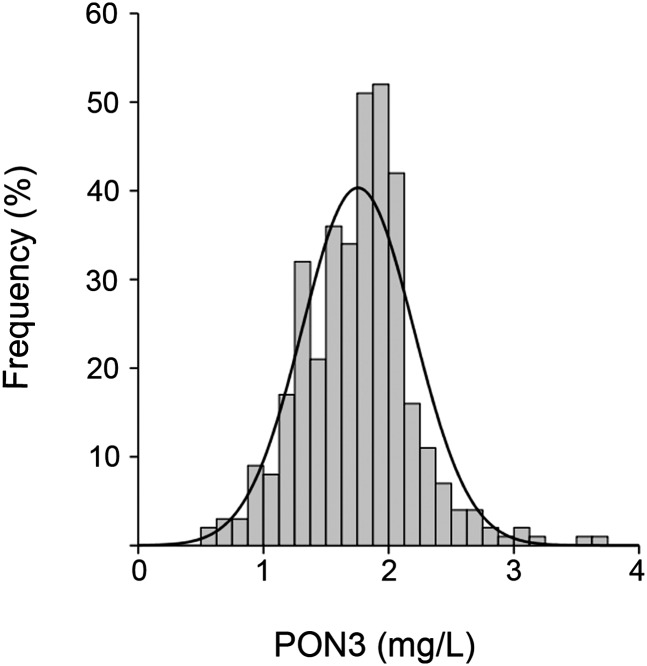
Results of the analytic and demographic variables in the studied population are shown in supplementary Table II. Serum PON3 concentrations were distributed according to a nonGaussian plot (P = 0.001; Fig. 2). Reference interval (n = 356) was the following: median = 1.78 mg/l; 95% CI, range 1.00-2.47 mg/l.
Fig. 2.
Frequency distribution of serum PON3 concentration in the normal population (N = 356).
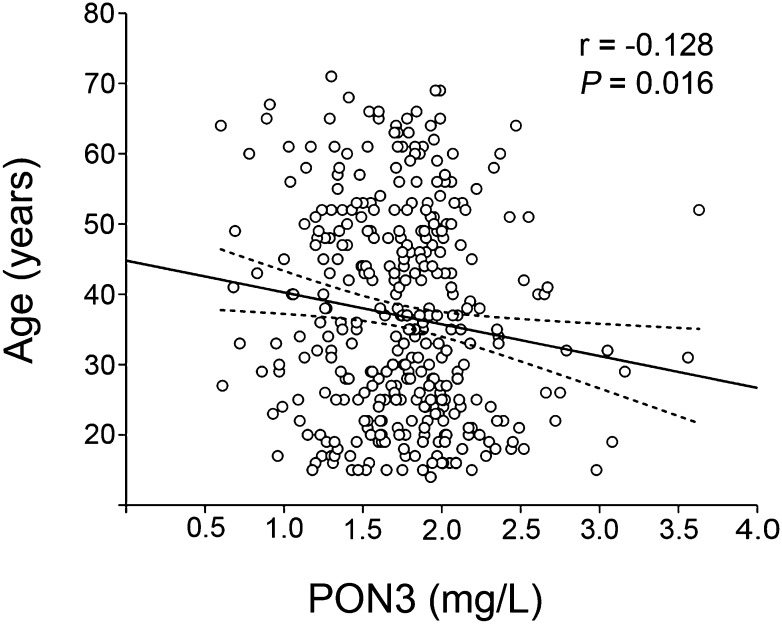
Serum PON3 concentrations significantly decreased with age (Fig. 3). We did not show any significant relationships with gender (men = 1.77 ± 0.47 mg/l; women = 1.74 ± 0.41 mg/l) or smoking status (non-smokers = 1.73 ± 0.43 mg/l; smokers = 1.77 ± 0.49 mg/l). There were no significant relationships between serum PON3 concentration and the body mass index, serum PON1 concentration, lactonase and paraoxonase activities, cholesterol, HDL-cholesterol, or apoA-I (supplementary Table III).
Fig. 3.
Relationship between serum PON3 concentration and age. The dashed lines represent the 95% CI of the regression line.
PON3 distribution in HDL particles
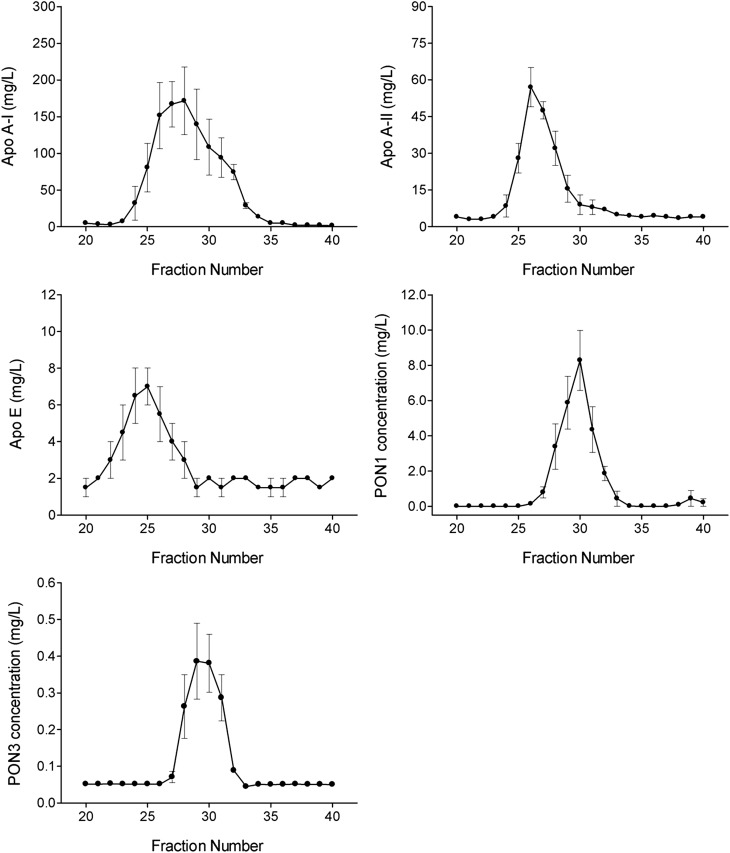
Analysis of the HPLC apolipoprotein profile of HDL particles showed that PON3 elutes basically between fractions 28 and 31, together with PON1, in those particles containing apoA-I, but not apoA-II or apoE (Fig. 4).
Fig. 4.
Distribution of apolipoproteins and paraoxonases in HDL particles.
Influence of PON3 gene polymorphisms on serum PON3 concentrations
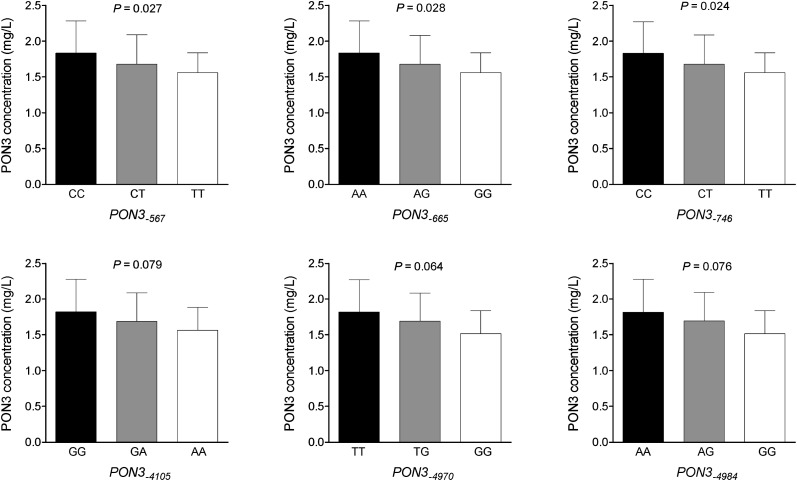
The frequency distributions of the selected PON3 promoter polymorphisms are shown in supplementary Table IV. Bivariate statistical analyses showed that PON3-567, PON3-665, and PON3-746 polymorphisms were associated with moderate but significant changes in serum PON3 concentrations, while the association between the other studied polymorphisms and PON3 did not reach statistical significance (Fig. 5).
Fig. 5.
Relationship between serum PON3 concentrations and PON3 promoter polymorphisms. P values are calculated by the Kruskal-Wallis test.
All six PON3 promoter polymorphisms were strongly linked in a single haplotype (supplementary Fig. I). There were three possible combinations: CACGTA (frequency = 77.6%), TGTAGG (frequency = 19.6%), and TGTGTA (frequency = 1.3%). Haplotypes were significantly associated with serum PON3 concentrations when adjusted for age, sex, and body mass index (Table 1).
TABLE 1.
Association between PON3 promoter haplotypes and serum PON3 concentrations
| B |
95% CI for B |
|||
|---|---|---|---|---|
| Variable | P | (mean effect on PONS) | Lower Limit | Upper Limit |
| Constant | <0.001 | 2.295 | 2.027 | 2.563 |
| Haplotype | 0.002 | −0.098 | −0.161 | −0.035 |
| Age | 0.001 | −0.004 | −0.007 | −0.002 |
| Gender | 0.633 | −0.017 | −0.088 | 0.053 |
| Body mass index | 0.070 | −0.007 | −0.014 | 0.001 |
DISCUSSION
The production of antibodies against synthetic peptides derived from specific sequences of the different PONs has been demonstrated to provide effective tools to analyze the protein expression of these enzymes in lipoproteins and tissues, either by Western blot or by immunohistochemical methods (7, 8, 19, 20, 31). Using this approach, we previously described an ELISA to measure serum PON1 concentration that has been successfully employed to investigate PON1 alterations in a variety of inflammatory diseases (25, 32–34). To the best of our knowledge, the present study reports for the first time an ELISA for measuring serum PON3 concentrations. The assay is practical and has rapid, high throughput. The antibody has been generated against a peptide identical to a specific sequence of PON3, and our study demonstrated that there is no cross-reactivity with PON1. This sequence is highly conserved throughout evolution, which means that the assay can also be used for studies in experimental animals (20). It requires a low volume of sample (1-2 μl), which is an important requisite for epidemiological research purposes in which the sample volume is often a serious limitation. Imprecision was ≤13.2%, which is acceptable for a noncommercial method in which new reagents have to be prepared regularly. Reproducibility of the standard curves was good over the measurement range, and the detection limit was sufficient for human and experimental animal studies. The analytical accuracy, measured by the percentage of recovery, was excellent. Using this method, we have defined a serum PON3 concentration reference range in a wide population sample representative of Reus (Catalonia) on the Spanish Mediterranean coast. Serum PON3 concentration in these subjects was about 50-60 times lower on average to that of PON1, although the ratio between these enzymes varied considerably among individuals. These results are not very different from those obtained by Draganov et al. (35), who found a PON3/PON1 ratio of about 1/200 when they studied rabbit purified PON1 and PON3. They also reported that rabbit PON3 is about 100 times more potent per mg of protein than PON1 in protecting LDL against lipid peroxidation, suggesting that serum PON3 may be as important as PON1 as an antioxidant, atheroprotective enzyme, despite its lower concentrations. We observed moderate but significant trends for PON3 to decrease with age, although no relationships were observed with gender, body mass index, or smoking status. The epidemiological relevance of these observations has to be determined by further investigations, although they agree with the general pro-atherogenic profile of advanced age.
Previous studies already showed that, in humans, PON3 circulates in plasma associated with HDL. Draganov et al. (35) observed that PON3 copurified with apoA-I and PON1 through agarose columns, and Reddy et al. (19) and Marsillach et al. (20) detected PON3 protein expression in HDL by Western blot. However, to-date it was not known whether PON1 and PON3 coexisted on the same subset of HDL particles. The present study showed an identical co-elution pattern of both PON enzymes; i.e., they are present in particles containing apoA-I but not apoA-II and apoE. Perhaps both PONs need the same physicochemical environment, with similar lipid composition and anchoring to apolipoproteins in the HDL particles.
Information about PON3 genotypes is scarce (36, 37), and their influence on enzyme levels is unknown. In the present study, we have investigated six PON3 promoter polymorphisms. Their influence on PON3 circulating levels was moderate. On average, the least frequent variant was associated with about a 10% reduction of serum PON3 concentration, compared with the most frequent genotype. The degree of influence was practically identical, and the frequency distribution of the different isoforms was very similar. This finding suggests strong linkage disequilibrium among the six polymorphisms. Haplotype analysis confirmed the existence of three haplotypes, CACGTA being by far the most frequent. Haplotypes were significantly associated with changes in serum PON3 concentration when adjusted for gender, age, and body mass index.
In summary, the present study reports a simple and robust ELISA assay to measure serum PON3 concentrations. It could be a valuable tool for epidemiological studies and investigation of the relationships between this still mysterious enzyme and oxidative and inflammatory diseases. By using this assay, we have been able to ascertain the distribution of PON3 in HDL subfractions, and we have determined that six promoter polymorphisms are functional, with significant changes in the serum concentration of this enzyme. This study offers new opportunities in the investigation of the properties and role of the PON family in cardiovascular disease, with possible implications in clinical practice.
Supplementary Material
Acknowledgments
The authors thank Dr. John Teiber (University of Texas, Dallas, TX) and Dr. Dan Tawfik (Weizmann Institute of Science, Rehovot, Israel) for the generous gifts of purified PON1 and PON3, and the TBBL reagent, respectively. The authors are indebted to Pilar Hernández for her help with statistical analyses.
Footnotes
Abbreviations:
- apo
- apolipoprotein
- PON
- paraoxonase
- SNP
- single nucleotide polymorphism
- TBBL
- 5-thiobutyl butyrolactone
This work was supported by Grant PI08/1175 from the Instituto de Salud Carlos III (ISCIII), Madrid, Spain.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of one figure and four tables.
REFERENCES
- 1.Primo-Parmo S. L., Sorenson R. C., Teiber J., La Du B. N. 1996. The human serum paraoxonase/arylesterase gene (PON1) is one member of a multigene family. Genomics. 33: 498–507. [DOI] [PubMed] [Google Scholar]
- 2.Sorenson R. C., Primo-Parmo S. L., Camper S. A., La Du B. N. 1995. The genetic mapping and gene structure of mouse paraoxonase/arylesterase. Genomics. 30: 431–438. [DOI] [PubMed] [Google Scholar]
- 3.Rodríguez-Sanabria F., Rull A., Beltrán-Debón R., Aragonès G., Camps J., Mackness B., Mackness M., Joven J. 2010. Tissue distribution and expression of paraoxonases and chemokines in mouse: the ubiquitous and joint localisation suggest a systemic and coordinated role. J. Mol. Histol. 41: 379–386. [DOI] [PubMed] [Google Scholar]
- 4.Leviev I., Negro F., James R. W. 1997. Two alleles of the human paraoxonase gene produce different amounts of mRNA. An explanation for differences in serum concentrations of paraoxonase associated with the (Leu-Met54) polymorphism. Arterioscler. Thromb. Vasc. Biol. 17: 2935–2939. [DOI] [PubMed] [Google Scholar]
- 5.Sierksma A., van der Gaag M. S., van Tol A., James R. W., Hendriks F. J. 2002. Kinetics of HDL cholesterol and paraoxonase activity in moderate alcohol consumers. Alcohol. Clin. Exp. Res. 26: 1430–1435. [DOI] [PubMed] [Google Scholar]
- 6.Jaouad L., de Guise C., Berrougui H., Cloutier M., Isabelle M., Fulop T., Payette H., Khalil A. 2006. Age-related decreased in high-density lipoproteins antioxidant activity is due to an alteration in the PON1’s free sulfhydryl groups. Atherosclerosis. 185: 191–200. [DOI] [PubMed] [Google Scholar]
- 7.Ng C. J., Wadleigh D. J., Gangopadhyay A., Hama S., Grijalva V. R., Navab M., Fogelman A. M., Reddy S. T. 2001. Paraoxonase-2 is a ubiquitously expressed protein with antioxidant properties and is capable of preventing cell-mediated oxidative modification of low density lipoprotein. J. Biol. Chem. 276: 44444–44449. [DOI] [PubMed] [Google Scholar]
- 8.Camps J., Marsillach J., Joven J. 2009. The paraoxonases: role in human diseases and methodological difficulties in measurement. Crit. Rev. Clin. Lab. Sci. 46: 83–106. [DOI] [PubMed] [Google Scholar]
- 9.Aviram M., Rosenblat M. 2004. Paraoxonases 1, 2, and 3, oxidative stress, and macrophage cell formation during atherosclerosis development. Free Radic. Biol. Med. 37: 1304–1316. [DOI] [PubMed] [Google Scholar]
- 10.Mackness B., Hine D., Liu Y., Mastorikou M., Mackness M. 2004. Paraoxonase-1 inhibits oxidised LDL-induced MCP-1 production by endothelial cells. Biochem. Biophys. Res. Commun. 318: 680–683. [DOI] [PubMed] [Google Scholar]
- 11.Mastorikou M., Mackness M., Mackness B. 2006. Defective metabolism of oxidized phospholipid by HDL from people with type 2 diabetes. Diabetes. 55: 3099–3103. [DOI] [PubMed] [Google Scholar]
- 12.Horke S., Witte I., Wilgenbus P., Krüger M., Strand D., Förstermann U. 2007. Paraoxonase-2 reduces oxidative stress in vascular cells and decreases endoplasmic reticulum stress-induced caspase activation. Circulation. 115: 2055–2064. [DOI] [PubMed] [Google Scholar]
- 13.Mackness B., Quarck R., Verreth W., Mackness M., Holvoet P. 2006. Human paraoxonase-1 overexpression inhibits atherosclerosis in a mouse model of metabolic syndrome. Arterioscler. Thromb. Vasc. Biol. 26: 1545–1550. [DOI] [PubMed] [Google Scholar]
- 14.Ng C. J., Hama S. Y., Bourquard N., Navab M., Reddy S. T. 2006. Adenovirus mediated expression of human paraoxonase 2 protects against the development of atherosclerosis in apolipoprotein E-deficient mice. Mol. Genet. Metab. 89: 368–373. [DOI] [PubMed] [Google Scholar]
- 15.Shih D. M., Xia Y. R., Wang X. P., Wang S. S., Bourquard N., Fogelman A. M., Lusis A. J., Reddy S. T. 2007. Decreased obesity and atherosclerosis in human paraoxonase 3 transgenic mice. Circ. Res. 100: 1200–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ng C. J., Bourquard N., Hama S. Y., Shih D., Grijalva V. R., Navab M., Fogelman A. M., Reddy S. T. 2007. Adenovirus-mediated expression of human paraoxonase 3 protects against the progression of atherosclerosis in apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 27: 1368–1374. [DOI] [PubMed] [Google Scholar]
- 17.Bourquard N., Shih D. M., Ng C. J., Villa-García N., Nakamura K., Stoltz D. A., Ozer E., Grijalva V., Rozengurt N., Hama S. Y., et al. 2008. The role of PON2 and PON3 in atherosclerosis and related traits. The Paraoxonases: Their Role in Disease Development and Xenobiotic Metabolism. Mackness B., Mackness M., Aviram M., Paragh G., Springer, Dordrecht: 103–128. [Google Scholar]
- 18.Bertran N., Camps J., Fernández-Ballart J., Arija V., Ferré N., Tous M., Joven J. 2005. Diet and lifestyle are associated with serum C-reactive protein concentrations in a population-based study. J. Lab. Clin. Med. 145: 41–46. [DOI] [PubMed] [Google Scholar]
- 19.Reddy S. T., Wadleigh D. J., Grijalva V., Ng C., Hama S., Gangopadhyay A., Shih D. M., Lusis A. J., Navab M., Fogelman A. M. 2001. Human paraoxonase-3 is an HDL-associated enzyme with biological activity similar to paraoxonase-1 protein but is not regulated by oxidized lipids. Arterioscler. Thromb. Vasc. Biol. 21: 542–547. [DOI] [PubMed] [Google Scholar]
- 20.Marsillach J., Mackness B., Mackness M., Riu F., Beltrán R., Joven J., Camps J. 2008. Immunohistochemical analysis of paraoxonases-1, 2, and 3 expression in normal mouse tissues. Free Radic. Biol. Med. 45: 146–157. [DOI] [PubMed] [Google Scholar]
- 21.Draganov D. I., Teiber J. F., Speelman A., Osawa Y., Sunahara R., La Du B. N. 2005. Human paraoxonases (PON1, PON2, and PON3) are lactonases with overlapping and distinct substrate specificities. J. Lipid Res. 46: 1239–1247. [DOI] [PubMed] [Google Scholar]
- 22.Gaidukov L., Tawfik D. 2007. The development of human sera tests for HDL-bound serum PON1 and its lipolactonase activity. J. Lipid Res. 48: 1637–1646. [DOI] [PubMed] [Google Scholar]
- 23.Marsillach J., Aragonès G., Beltrán R., Caballeria J., Pedro-Botet J., Morcillo-Suárez C., Navarro A., Joven J., Camps J. 2009. The measurement of the lactonase activity of paraoxonase-1 in the clinical evaluation of patients with chronic liver impairment. Clin. Biochem. 42: 91–98. [DOI] [PubMed] [Google Scholar]
- 24.Ferré N., Camps J., Prats E., Vilella E., Paul A., Figuera L., Joven J. 2002. Serum paraoxonase activity: a new additional test for the improved evaluation of chronic liver damage. Clin. Chem. 48: 261–268. [PubMed] [Google Scholar]
- 25.Marsillach J., Aragonès G., Mackness B., Mackness M., Rull A., Beltrán-Debón R., Pedro-Botet J., Alonso-Villaverde C., Joven J., Camps J. 2010. Decreased paraoxonase-1 activity is associated with alterations of high-density lipoprotein particles in chronic liver impairment. Lipids Health Dis. 9: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Usui S., Hara Y., Hosaki S., Okazaki M. 2002. A new on-line dual enzymatic method for simultaneous quantification of cholesterol and triglycerides in lipoproteins by HPLC. J. Lipid Res. 43: 805–814. [PubMed] [Google Scholar]
- 27.Magoori K., Kang M. J., Ito M. R., Kakuuchi H., Ioka R. X., Kamataki A., Kim D. H., Asaba H., Iwasaki S., Takei S. A., et al. 2003. Severe hypercholesterolemia, impaired fat tolerance, and advanced atherosclerosis in mice lacking both low density lipoprotein receptor-related protein 5 and apolipoprotein E. J. Biol. Chem. 278: 11331–11336. [DOI] [PubMed] [Google Scholar]
- 28.Barrett J. C., Fry B., Maller J., Daly M. J. 2005. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 21: 263–265. [DOI] [PubMed] [Google Scholar]
- 29.Niu T. 2004. Algorithms for inferring haplotypes. Genet. Epidemiol. 27: 334–347. [DOI] [PubMed] [Google Scholar]
- 30.Durrant C., Mott R. 2010. Bayesian quantitative trait locus mapping using inferred haplotypes. Genetics. 184: 839–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marsillach J., Camps J., Beltrán-Debón R., Rull A., Aragonès G., Maestre-Martínez C., Sabench F., Hernández M., del Castillo D., Joven J., et al. 2011. Immunohistochemical analysis of paraoxonases-1 and 3 in human atheromatous plaques. Eur. J. Clin. Invest. 41: 308–314. [DOI] [PubMed] [Google Scholar]
- 32.Parra S., Marsillach J., Aragonès G., Beltrán R., Montero M., Coll B., Mackness B., Mackness M., Alonso-Villaverde C., Joven J., et al. 2010. Paraoxonase-1 gene haplotypes are associated with metabolic disturbances, atherosclerosis, and immunologic outcome in HIV-infected patients. J. Infect. Dis. 201: 627–634. [DOI] [PubMed] [Google Scholar]
- 33.Gaita L., Manzi B., Sacco R., Lintas C., Altieri L., Lombarda F., Pawlowski T. L., Redman M., Craig D. W., Huentelman M. J., et al. 2010. Decreased serum arylesterase activity in autism spectrum disorders. Psychiatry Res. 180: 105–113. [DOI] [PubMed] [Google Scholar]
- 34.Marsillach J., Checa M. A., Pedro-Botet J., Carreras R., Joven J., Camps J. 2010. Paraoxonase-1 in female infertility: a possible role against oxidative stress-induced inflammation. Fertil. Steril. 94: 1132–1134. [DOI] [PubMed] [Google Scholar]
- 35.Draganov D. I., Stetson P. I., Watson C. E., Billecke S. S., La Du B. N. 2000. Rabbit serum paraoxonase 3 (PON3) is a high density lipoprotein-associated lactonase and protects low density lipoprotein against oxidation. J. Biol. Chem. 275: 33435–33442. [DOI] [PubMed] [Google Scholar]
- 36.Campo S., Sardo A. M., Campo G. M., Avenoso A., Castaldo M., D'Ascola A., Giunta E., Calatroni A., Saitta A. 2004. Identification of paraoxonase 3 gene (PON3) missense mutations in a population of southern Italy. Mutat. Res. 546: 75–80. [DOI] [PubMed] [Google Scholar]
- 37.Sánchez R., Levy E., Seidman E., Amre D., Costea F., Sinnett D. 2006. Paraoxonase 1, 2 and 3 DNA variants and susceptibility to childhood inflammatory bowel disease. Gut. 55: 1820–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.