Abstract
Progression of human arteriosclerosis is associated with and promoted by induction of the endoplasmic reticulum (ER) stress pathway known as the unfolded protein response (UPR). Most studies that assess UPR markers in atherosclerosis rely on methodologies that suffer from low signal sensitivity, nonspecific immunohistochemistry, or inability to resolve differences between cellular subsets. To accurately monitor the UPR independently of artifacts generated postmortem, we describe here the first in vivo reporter for ER stress during atherosclerosis. Mice transgenic for the fluorescent XBP-1 ER stress indicator Erai were bred onto the Ldlr−/− background and fed an atherogenic diet. Subsequently, ERAI fluorescence at aortic roots was quantified and colocalized with lesional cell type. We found that the ERAI fluorescent signal increased as a function of time on the atherogenic diet and, in advanced lesions, was found close to necrotic cores. The majority of ERAI fluorescence localized to macrophages, and to a lesser extent, to intimal smooth muscle cells and patches of endothelial cells. These mice provide a valuable tool to monitor activation of the UPR in atherosclerosis and will be useful for future studies investigating relationships between pharmacologic and genetic modulators of UPR and atherosclerosis.
Keywords: macrophage, atherosclerosis, ER stress, unfolded protein response
Accumulating evidence in vivo implicates the cellular endoplasmic reticulum (ER) stress response known as the unfolded protein response (UPR) in atherosclerosis progression in both humans and experimental animals. In human coronary arteries, strong correlations have been reported between positive immunohistochemistry of UPR markers and the presence of rupture-prone necrotic plaques (1). In mice, molecular genetic evidence has now surfaced implicating the ER stress effector CHOP as causally promoting advanced atherosclerotic progression and features of plaque instability (2, 3). Such unstable plaques are the precursor to acute atherothrombosis, myocardial infarction, and stroke. Induction of the UPR appears to occur in each of the primary cell types that populate the plaque. In macrophages, initial work by Feng et al. (4) and later, Zhou et al. (5), showed that ER stress is prominently induced during atherosclerosis in chow-fed or Western diet-fed Apoe−/− mice. In endothelial cells, sustained activation of UPR XBP-1 splicing can lead to endothelial apoptosis and atherosclerosis development in response to disturbed flow (6). Finally, in vascular smooth muscle cells (VSMCs), exposure of human coronary artery-derived VSMCs to 7-ketocholesterol, which is found in rupture-prone shoulder regions of thin-capped human coronary atheromata, led to the induction of CHOP and UPR chaperones (1). A growing list of ER stressors and UPR-inducing pathways has been identified in atheromata. These include oxidized lipids, unesterified cholesterol, saturated fatty acids, homocysteine, and oxidative stress. Moreover, insulin resistance in lesional macrophages amplifies the UPR response, and insulin resistance and obesity are a growing epidemic driving atherosclerosis (7–10). Together, these pieces of experimental data support an important role for UPR-induced progression of atherosclerotic disease.
To date, studies of UPR activation in plaques are often limited to postmortem analyses that include harvesting of heterogeneous extracts and immunohistochemistry. These techniques represent an indirect measure that is subject to artificial antigen modification, nucleic acid degradation, and the possibility of nonspecific antibody interactions (11). These potential problems can lead to false-positive signals or postfixation artifacts that include loss of antigenic epitopes and therefore signal underestimation or, conversely, false-positive signals. Gene expression analysis by laser capture micro-dissection is useful for monitoring UPR mRNA expression in situ (12), but this technique may be compromised by RNA degradation effects and single cell examination is technically challenging. Finally, spatial correlations between defined regions of interest, such as plaque necrosis and areas of UPR activation, may provide unique insight into the biology of plaque subregions subject to ER stress. Thus, an endogenous fluorescent reporter induced during ER stress in vivo, could significantly reduce such complications and further increase resolution to the level of individual cells, which is an important goal in the context of a heterogenous plaque population (13). In this context, Iwawaki et al. (14) created a mouse transgenic for a UPR reporter consisting of UPR X-box binding protein 1 (XBP-1) fused to venus fluorescent protein and driven by the universal chicken β-actin promoter. Murine cells that are transgenic for this marker fluoresce after activation of the UPR and therefore can be used to monitor physiological and pathological ER stress in vivo.
To monitor endogenous cell-type specific UPR in vivo during atherosclerosis, we crossed the aforementioned ERAI reporter mouse (Erai) to the atherogenic Ldlr−/− background. Transgenic Erai, Ldlr −/− mice developed atherosclerotic lesions in which lesional cell fluorescence could be localized to differing extents to macrophage, endothelial, and vascular smooth muscle cells during lesion progression. Given the strong association between cardiovascular disease and UPR activation, this model will be a useful tool for future studies that test the temporal and spatial relationship between ER stress induction and atherosclerosis progression under various therapeutic and disease stimuli.
MATERIALS AND METHODS
Mice and diets
Erai mice have been previously described (14). Transgenic mice were screened by PCR using primers 5′ GAA CCA GGA GT AAG ACA GC 3′ and 5′ GAA CAG CTC CTC GCC CTT GC 3′. Erai mice were bred onto C57BL/6; Ldlr−/− background to generate Erai, Ldlr−/− mice. At 8–10 weeks of age, both Ldlr−/− and Erai, Ldlr−/− mice were fed a high-fat (21.2%), high-cholesterol (0.2%), Western-type diet (Catalog # TD88137) from Harlan Teklad (Madison, WI) for durations of 4, 8, 12, 16, and 20 weeks. Mice were subsequently euthanized for aortic root analysis as described below. All animals were housed and cared for according to National Institutes of Health and IACUC guidelines in a barrier facility at Columbia University Medical Center, New York, NY.
Vascular tissue preparation
At the end of the feeding protocols, mice were fasted, weighed, and anesthetized with isoflurane. Hearts were perfused in situ with ice-cold PBS and the upper half removed by cutting the ascending aorta midway between the aortic root and brachiocephalic artery. Roots were rapidly harvested and frozen in OCT. Fifty sections per mouse were cut at a thickness of 6 µm starting at the valves at the base of the aortic root. Total intimal lesion area (between internal elastic lamina to the lumen) per cross-section was quantified by taking the average of six sections spaced 30 µm apart beginning at the base of the aortic root. Six cross-sections per mouse, each representing the average lesion area per mouse, were used for quantification of ERAI and cell-type immunohistochemistry. Five transgenic mice and five control mice were analyzed per time point except for week 16, in which 10 transgenic mice were compared with 10 controls.
Lesion analysis
Slides were fixed in cold-acetone and blocked in 3% BSA prior to epifluorescence microscopy or immunohistochemistry. All section images were normalized to equal exposure and contrast settings between control and transgenics. Under these settings, less than 1% of cells in the nonErai control lesions were autofluorescent. To control for possible differences in fat-induced autofluorescence in Erai mice, we also measured and found no differences in Oil Red O staining versus control. Staining of endothelial cells was performed with rat anti-mouse CD31 (PECAM-1;GPIIa) from AbD Serotec (MCA2388T). Macrophages were stained with anti-F4/80 from Serotec. Anti-actin was from Sigma. Phospho-eIF2α (Ser51) (119A11) was from Cell Signaling. For endothelial cells, ∼1,000 endothelial cells were counted per mouse. Percent ERAI+ macrophages was determined by colocalization of F4/80+ nuclei with ERAI signal. Apoptotic cells in atherosclerotic lesions were detected by Tdt-mediated dUTP nick end labeling (TUNEL) using the in situ cell death detection kit, TMR red from Roche. The treated sections were incubated in TdT reaction mixture containing TMR red dUTP for 45 min at 37°C in a humidified chamber. Nuclei were counterstained with Hoechst for 5 min. The slides were viewed and imaged by fluorescent microscopy on an Olympus IX-70 inverted fluorescent microscope equipped with a Cool Snap charge-coupled device camera and imaging software (Roper Scientific). Fluorescent images were captured and analyzed with image Photoshop analysis software (Adobe Systems, San Jose, CA) and ImageJ. All settings were normalized to equal exposures.
Statistics
Data are displayed as mean ± SEM. Statistically significant differences of normally distributed data sets were determined by ANOVA.
RESULTS
A UPR reporter mouse for in vivo studies of ER stress in atherosclerosis
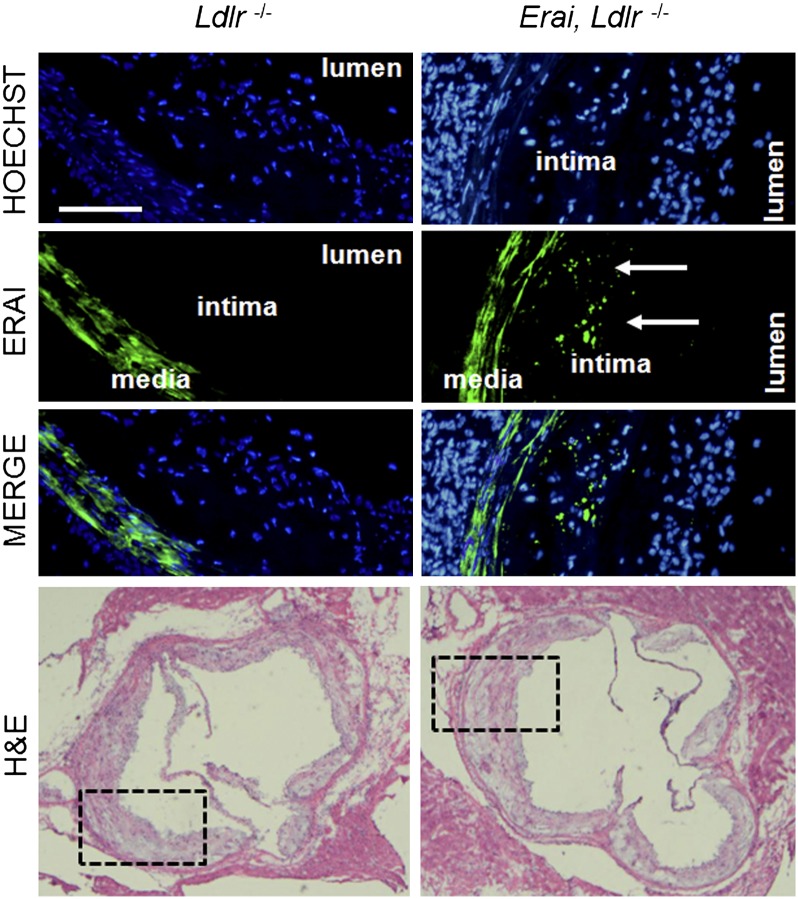
An in vivo ER stress reporter in plaque would be a useful tool for monitoring the effects of experimental and therapeutic manipulations on UPR activation within the complex cellular milieu of atheromata. To achieve this goal, mice transgenic for a fluorescent ER stress-activated indicator (Erai) were crossed to atherosclerosis-prone Ldlr−/− mice to generate Erai, Ldlr−/− mice. Erai mice are healthy, fertile, and without developmental defects as previously described (14). Briefly, these mice are transgenic for a fusion protein consisting of venus fluorescent protein downstream of the XBP1 ER stress-specific intron and driven by the universal chicken β-actin promoter. Under homeostatic conditions, ERAI message is not spliced and translation terminated at a stop codon upstream of the venus open reading frame. During induction of the UPR, the 26 nucleotide intron is spliced out and a frame shift permits translation of fluorescent venus. The reporter does not contain a DNA binding domain and does not modulate, induce, nor inhibit ER stress responses (14). As an initial characterization, Ldlr−/− and Erai,Ldlr−/− mice were fed a Western diet for 16 weeks. Total plasma cholesterol and body weights of control and ERAI mice were not significantly different (data not shown). In addition, no differences in lesion size or Phospho-eIF2α by immunohistochemistry were found between control and transgenic groups. Although the arterial media showed green autofluorescence in both groups, only the Erai,Ldlr−/− mice exhibited significant levels of green fluorescence in the intima when the lesions from the two groups were imaged under identical exposure settings (Fig. 1). These data are consistent with our findings and others that have previously documented expression of UPR markers in advanced plaque (5, 15).
Fig. 1.
UPR reporter mice for studies of atherosclerosis. Mice transgenic for an XBP-1-venus fusion protein [Iwawaki et al.(14)] were bred onto the C57/Ldlr−/− background. Subsequently, control Ldlr−/− (left) and Erai,Ldlr−/− (right) mice were fed a hypercholesterolemic Western type diet and hearts dissected for analysis at the aortic root. Green = VFP signal. Blue is nuclear stain Hoechst. Bar, 100 μm. Medial staining is vascular smooth muscle autofluorescence. Areas of fluorescent photomicrographs are demarcated in corresponding hematoxylin and eosin sections below.
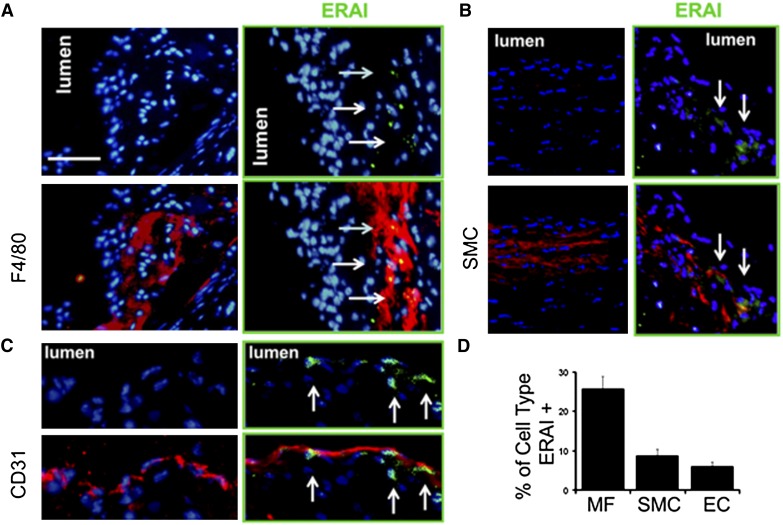
Atheromatous plaque is populated by a heterogeneous population of cells, including smooth muscle cells, endothelial cells, and macrophages, which are the most numerous cells in lesions (16–18). To ascertain the identity of cells in plaque that were positive for the UPR reporter, we performed immunohistochemistry for markers of macrophages, VSMCs, and endothelial cells from 16 week diet-fed lesions. In advanced lesion, the ERAI signal was most often found associated with intimal macrophages (Fig. 2A). The ERAI signal was also found to a lesser extent in endothelial cells (Fig. 2B). Endothelial cells that were positive for ERAI signal were often found in clusters. Previous reports documented that ER stress occurs in endothelial cells near regions of disturbed flow (19). Finally, the fluorescent signal was also found in a small percentage of intimal VSMCs (Fig. 2C). The summary of this quantification by cell type is shown in Fig. 2D.
Fig. 2.
ER stress reporter distribution in lesional cell types. Aortic root sections were subjected to immunohistochemistry with (A) anti-F4/80 for macrophage phagocytes (B) anti-mouse smooth muscle actin in the intima (C) and anti-CD31 for endothelial cells. D: Enumeration of percent macrophages, VSMCs, and endothelial cells that were also ERAI+ (* indicates P < 0.05 relative to other cell types). Bar, 50 μm. Red is antibody staining. Blue is nuclear stain Hoechst. Lesions are from 16 week feedings.
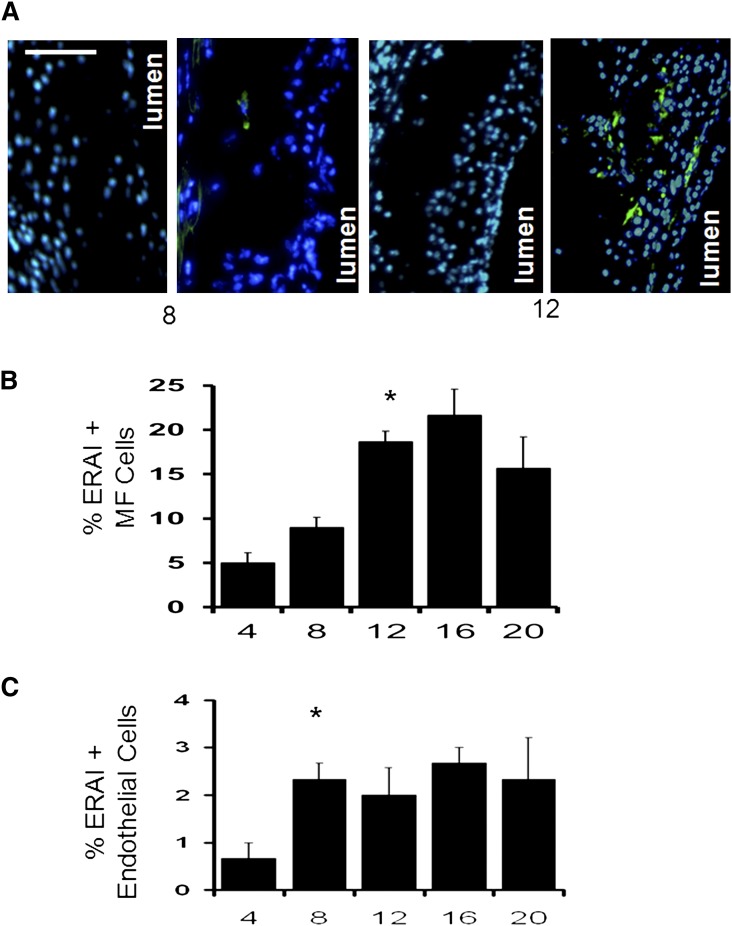
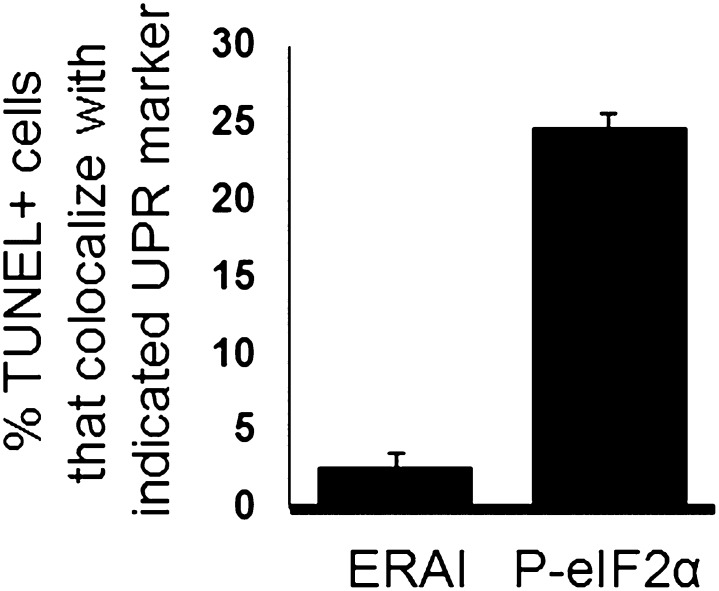
Activation of the UPR in mice and humans has been shown by immunohistochemistry to increase with age and disease burden (2, 5). To determine if fluorescent signal in Erai, Ldlr−/− recapitulated these previous reports, we next assessed the kinetics of ERAI+ cell expression during atherosclerosis progression after feeding mice high-cholesterol diet for increasing fixed durations. As exemplified by the images in Fig. 3A, early lesions contained very low levels of ERAI signal relative to more advanced and complex lesions. ERAI signal in advanced lesions was often found proximal to and within necrotic cores, areas that characteristically stain for macrophage debris (20). A quantified analysis for intimal macrophage staining for mice on the atherogenic diet for 4, 8, 12, 16, and 20 weeks is shown in Fig. 3B. The data show that lesions from mice on diet for 4 and 8 weeks exhibited significantly less reporter gene activation compared with the more advanced lesions starting at 12 weeks of diet (Fig. 3B). Previous reports have implicated endothelial ER stress as a relatively early event during atherogenesis (21–23). Quantification of endothelial ERAI fluorescence showed a prominent increase at 8 weeks (Fig. 3C), which is consistent with earlier activation in this cell type than in macrophages. Finally, previous work by Lin et al. (24) indicates that under chronic ER stress, the IRE-1/XBP-1 pathway is attenuated whereas PKR-like ER kinase (PERK)/CHOP signaling is sustained during programmed cell death. Consistent with their observation, we have found the vast majority of apoptotic/TUNEL positive cells (greater than 95%) to be ERAI negative. In contrast, significantly more TUNEL positive cells costained for activated markers of the UPR PERK pathway, specifically phospo-eIF2α (Fig. 4).
Fig. 3.
Kinetics of ER stress reporter activation in atheromata as a function of atherosclerotic maturation. A: Representative photomicrographs of control (left) and transgenic (right) Ldlr−/− and Erai,Ldlr−/− mice after 8 and 20 weeks of Western-diet feeding. B, C: Quantification of ERAI+ macrophage and endothelial nuclei after indicated weeks of diet. (* indicates P < 0.05 relative to prior time point). Bar, 100 μm.
Fig. 4.
ERAI signal does not colocalize with TUNEL positive intimal cells. TUNEL positive cells were enumerated for percent ERAI positivity. In parallel, TUNEL positive cells were costained for Phospho-eIF2α and Percent TUNEL positive, P-eIF2αcells enumerated.
DISCUSSION
During atherosclerosis, ER stress and ER chaperones are activated at different levels and in multiple cell types of the vascular wall (4, 5, 15). In endothelial cells of Erai, Ldlr−/− mice, ERAI signal was identified. In endothelial cells, the potentially pro-atherogenic molecule homocysteine causes activation of ER stress-induced growth arrest (25). Also in endothelial cells, IRE-1-mediated XBP1 splicing leads to endothelial apoptosis and atherosclerosis development in response to disturbed arterial flow (6). ER transmembrane signal transducer IRE1α has been shown to be activated in endothelial cells at atherosclerosis-prone regions of the aortic arch (19). This is consistent with our findings of ERAI expression, which is downstream of IRE1α signaling. It will be informative to determine in future studies the relative levels of reporter gene expression in endothelial cells at other aortic sites of atherosclerotic susceptibility, including at sites of arterial bifurcation or curvature, where atherogenesis predictably originates (26).
ERAI signal was also found in VSMCs. During advanced stages of atherosclerosis, VSMCs populate the subendothelium to form a protective fibrous cap. Current evidence indicates that athero-relevant ER stressors can promote smooth muscle cell death (27, 28). In advanced plaque, VSMC death compromises plaque integrity and weakens the fibrous cap (29). Exposure of human coronary artery-derived VSMCs to 7-ketocholesterol can lead to the induction of CHOP and UPR chaperones (1). This consequently may affect the ability of plaques to generate cap-protective collagen. Moreover, fibrous caps of thin-cap human atheroma lesions were found to be positive for UPR markers (1). The role of VSMC ER stress is relatively less well understood and further experimental approaches, including in vivo tests of agents that modulate VSMC ER stress, will be beneficial.
Macrophages play a critical role during atherogenesis by ingesting lipoprotein-derived cholesterol and accumulating cholesterol ester as lipid-laden foam cells in early lesions. In advanced lesions, a number of cellular and signaling events contribute to vulnerable plaque formation (30). A process known to be prominent in advanced plaque is activation of ER stress signal transduction pathways (31). In humans, there is a very strong correlation between expression of ER stress markers and plaque necrosis and rupture in human coronary artery lesions (1). In experimental animals, ER stress effectors are causally linked to advanced atherosclerotic progression (2). In advanced lesions, ERAI signal was predominantly localized to macrophages. This is in line with well-documented in vitro and in vivo findings that illustrate activation of macrophage ER stress pathways in response to atherogenic stimuli (4).
To determine the kinetics of ER stress in Erai, Ldlr−/− mice, we monitored ERAI signal as a function of time and high-cholesterol diet. Earlier lesions from Erai, Ldlr−/− mice exhibited a lesser amount of reporter gene activation compared with more advanced lesions. Increases in fluorescent signal occurred between 8 and 12 weeks. Under conditions of prolonged ER stress, a major function of the UPR is to trigger programmed cell death/apoptosis (32). A major death effector of the UPR is CHOP, and we recently reported that CHOP deficiency reduces apoptotic cell accumulation in advanced atherosclerotic plaques (2). In cultured ER-stressed macrophages, CHOP induction precedes markers of programmed cell death (4). CHOP expression typically is elevated after IRE1-XBP-1 signaling (24). We therefore predicted that the lesional ERAI signal would not colocalize with TUNEL positive cells. This was indeed the case as in advanced lesions, though ERAI-TUNEL double positive nuclei could be found, the majority (greater than 95%) of TUNEL positive intimal nuclei were ERAI negative, consistent with reported prosurvival effects of the IRE1-XBP-1 axis. In contrast, significantly more TUNEL positive cells costained for activated markers of the UPR PERK pathway, specifically phospo-eIF2α.
Erai,Ldlr−/− mice will be useful for a number of scenarios, including the effects of chemical chaperones during ER stress and atherosclerosis (33, 34). These mice will also be useful in monitoring the in vivo dynamics of ER stress in the context of hypercholesterolemia at sites peripheral to the plaque. An important future direction will be to examine the regulation of specific branches of the UPR in vivo during athero-progression. Whether or not activation of the three major branches of the UPR and their downstream effectors differentially control athero-progression is an open question. The generation of mice that track the signaling of these distinct branches of the UPR will be informative. Given that Erai, Ldlr−/− mice exhibit patterns of the UPR consistent with reports in human infarct-associated plaque, these mice will be useful for future studies that accurately test the in vivo relationship between pharmacologic and genetic modulators of the UPR and atherosclerotic progression.
Footnotes
Abbreviations:
- CHOP
- C/EBP homologous protein
- ER
- endoplasmic reticulum
- TUNEL
- Tdt-mediated dUTP nick end labeling
- UPR
- unfolded protein response
- VSMC
- vascular smooth muscle cells
- XBP-1
- UPR X-box binding protein-1
This work was supported by National Institutes of Health grants HL54591 and HL75662 (to I.T.). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Myoishi M., Hao H., Minamino T., Watanabe K., Nishihira K., Hatakeyama K., Asada Y., Okada K., Ishibashi-Ueda H., Gabbiani G., et al. 2007. Increased endoplasmic reticulum stress in atherosclerotic plaques associated with acute coronary syndrome. Circulation. 116: 1226–1233. [DOI] [PubMed] [Google Scholar]
- 2.Thorp E., Li G., Seimon T. A., Kuriakose G., Ron D., Tabas I. 2009. Reduced apoptosis and plaque necrosis in advanced atherosclerotic lesions of Apoe−/− and Ldlr−/− mice lacking CHOP. Cell Metab. 9: 474–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsukano H., Gotoh T., Endo M., Miyata K., Tazume H., Kadomatsu T., Yano M., Iwawaki T., Kohno K., Araki K., et al. 2010. The endoplasmic reticulum stress-CHOP pathway-mediated apoptosis in macrophages contributes to the instability of atherosclerotic plaques. Arterioscler. Thromb. Vasc. Biol. 30: 1925–1932. [DOI] [PubMed] [Google Scholar]
- 4.Feng B., Yao P. M., Li Y., Devlin C. M., Zhang D., Harding H. P., Sweeney M., Rong J. X., Kuriakose G., Fisher E. A., et al. 2003. The endoplasmic reticulum is the site of cholesterol-induced cytotoxicity in macrophages. Nat. Cell Biol. 5: 781–792. [DOI] [PubMed] [Google Scholar]
- 5.Zhou J., Lhotak S., Hilditch B. A., Austin R. C. 2005. Activation of the unfolded protein response occurs at all stages of atherosclerotic lesion development in apolipoprotein E-deficient mice. Circulation. 111: 1814–1821. [DOI] [PubMed] [Google Scholar]
- 6.Zeng L., Zampetaki A., Margariti A., Pepe A. E., Alam S., Martin D., Xiao Q., Wang W., Jin Z. G., Cockerill G., et al. 2009. Sustained activation of XBP1 splicing leads to endothelial apoptosis and atherosclerosis development in response to disturbed flow. Proc. Natl. Acad. Sci. USA. 106: 8326–8331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tabas I., Seimon T., Timmins J., Li G., Lim W. 2009. Macrophage apoptosis in advanced atherosclerosis. Ann. N. Y. Acad. Sci. 1173 (Suppl 1): E40–E45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hossain G. S., van Thienen J. V., Werstuck G. H., Zhou J., Sood S. K., Dickhout J. G., de Koning A. B., Tang D., Wu D., Falk E., et al. 2003. TDAG51 is induced by homocysteine, promotes detachment-mediated programmed cell death, and contributes to the development of atherosclerosis in hyperhomocysteinemia. J. Biol. Chem. 278: 30317–30327. [DOI] [PubMed] [Google Scholar]
- 9.Gargalovic P. S. 2009. Atherogenesis on the chopping block. Cell Metab. 9: 399–401. [DOI] [PubMed] [Google Scholar]
- 10.Han S., Liang C. P., DeVries-Seimon T., Ranalletta M., Welch C. L., Collins-Fletcher K., Accili D., Tabas I., Tall A. R. 2006. Macrophage insulin receptor deficiency increases ER stress-induced apoptosis and necrotic core formation in advanced atherosclerotic lesions. Cell Metab. 3: 257–266. [DOI] [PubMed] [Google Scholar]
- 11.Haataja L., Gurlo T., Huang C. J., Butler P. C. 2008. Many commercially available antibodies for detection of CHOP expression as a marker of endoplasmic reticulum stress fail specificity evaluation. Cell Biochem. Biophys. 51: 105–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trogan E., Fisher E.A. 2005. Laser capture microdissection for analysis of macrophage gene expression from atherosclerotic lesions. Methods Mol. Biol. 293: 221–231. [DOI] [PubMed] [Google Scholar]
- 13.Weber C., Zernecke A., Libby P. 2008. The multifaceted contributions of leukocyte subsets to atherosclerosis: lessons from mouse models. Nat. Rev. Immunol. 8: 802–815. [DOI] [PubMed] [Google Scholar]
- 14.Iwawaki T., Akai R., Kohno K., Miura M. 2004. A transgenic mouse model for monitoring endoplasmic reticulum stress. Nat. Med. 10: 98–102. [DOI] [PubMed] [Google Scholar]
- 15.Feng B., Zhang D., Kuriakose G., Devlin C. M., Kockx M., Tabas I. 2003. Niemann-Pick C heterozygosity confers resistance to lesional necrosis and macrophage apoptosis in murine atherosclerosis. Proc. Natl. Acad. Sci. USA. 100: 10423–10428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lusis A. J. 2000. Atherosclerosis 32. Nature. 407: 233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Libby P., Nahrendorf M., Pittet M. J., Swirski F. K. 2008. Diversity of denizens of the atherosclerotic plaque: not all monocytes are created equal. Circulation. 117: 3168–3170. [DOI] [PubMed] [Google Scholar]
- 18.Glass C. K., Witztum J. L. 2001. Atherosclerosis. the road ahead. Cell. 104: 503–516. [DOI] [PubMed] [Google Scholar]
- 19.Civelek M., Manduchi E., Riley R. J., Stoeckert C. J., Jr, Davies P. F. 2009. Chronic endoplasmic reticulum stress activates unfolded protein response in arterial endothelium in regions of susceptibility to atherosclerosis. Circ. Res. 105: 453–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ball R. Y., Stowers E. C., Burton J. H., Cary N. R., Skepper J. N., Mitchinson M. J. 1995. Evidence that the death of macrophage foam cells contributes to the lipid core of atheroma. Atherosclerosis. 114: 45–54. [DOI] [PubMed] [Google Scholar]
- 21.Gargalovic P. S., Imura M., Zhang B., Gharavi N. M., Clark M. J., Pagnon J., Yang W. P., He A., Truong A., Patel S., et al. 2006. Identification of inflammatory gene modules based on variations of human endothelial cell responses to oxidized lipids. Proc. Natl. Acad. Sci. USA. 103: 12741–12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gargalovic P. S., Gharavi N. M., Clark M. J., Pagnon J., Yang W. P., He A., Truong A., Baruch-Oren T., Berliner J. A., Kirchgessner T. G., et al. 2006. The unfolded protein response is an important regulator of inflammatory genes in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 26: 2490–2496. [DOI] [PubMed] [Google Scholar]
- 23.Feaver R. E., Hastings N. E., Pryor A., Blackman B. R. 2008. GRP78 upregulation by atheroprone shear stress via p38-, alpha2beta1-dependent mechanism in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 28: 1534–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin J. H., Li H., Yasumura D., Cohen H. R., Zhang C., Panning B., Shokat K. M., Lavail M. M., Walter P. 2007. IRE1 signaling affects cell fate during the unfolded protein response. Science. 318: 944–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Outinen P. A., Sood S. K., Pfeifer S. I., Pamidi S., Podor T. J., Li J., Weitz J. I., Austin R. C. 1999. Homocysteine-induced endoplasmic reticulum stress and growth arrest leads to specific changes in gene expression in human vascular endothelial cells. Blood. 94: 959–967. [PubMed] [Google Scholar]
- 26.Schwartz C. J., Mitchell J. R. 1962. Observations on localization of arterial plaques. Circ. Res. 11: 63–73. [PubMed] [Google Scholar]
- 27.Kedi X., Ming Y., Yongping W., Yi Y., Xiaoxiang Z. 2009. Free cholesterol overloading induced smooth muscle cells death and activated both ER- and mitochondrial-dependent death pathway. Atherosclerosis. 207: 123–130. [DOI] [PubMed] [Google Scholar]
- 28.Werstuck G. H., Khan M. I., Femia G., Kim A. J., Tedesco V., Trigatti B., Shi Y. 2006. Glucosamine-induced endoplasmic reticulum dysfunction is associated with accelerated atherosclerosis in a hyperglycemic mouse model. Diabetes. 55: 93–101. [PubMed] [Google Scholar]
- 29.Geng Y. J., Libby P. 2002. Progression of atheroma: a struggle between death and procreation. Arterioscler. Thromb. Vasc. Biol. 22: 1370–1380. [DOI] [PubMed] [Google Scholar]
- 30.Tabas I. 2005. Consequences and therapeutic implications of macrophage apoptosis in atherosclerosis: the importance of lesion stage and phagocytic efficiency. Arterioscler. Thromb. Vasc. Biol. 25: 2255–2264. [DOI] [PubMed] [Google Scholar]
- 31.Marciniak S. J., Ron D. 2006. Endoplasmic reticulum stress signaling in disease. Physiol. Rev. 86: 1133–1149. [DOI] [PubMed] [Google Scholar]
- 32.Ron D., Walter P. 2007. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 8: 519–529. [DOI] [PubMed] [Google Scholar]
- 33.Erbay E., Babaev V. R., Mayers J. R., Makowski L., Charles K. N., Snitow M. E., Fazio S., Wiest M. M., Watkins S. M., Linton M. F., et al. 2009. Reducing endoplasmic reticulum stress through a macrophage lipid chaperone alleviates atherosclerosis. Nat. Med. 15: 1383–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dong Y., Zhang M., Liang B., Xie Z., Zhao Z., Asfa S., Choi H. C., Zou M. H. 2010. Reduction of AMP-activated protein kinase alpha2 increases endoplasmic reticulum stress and atherosclerosis in vivo. Circulation. 121: 792–803. [DOI] [PMC free article] [PubMed] [Google Scholar]