Abstract
Mitochondrial acyl-CoA:glycerol-sn-3-phosphate acyltransferase 1 (mtGPAT1) controls the first step of triacylglycerol (TAG) synthesis and is critical to the understanding of chronic metabolic disorders such as primary nonalcoholic fatty liver disease (NAFLD). Anthocyanin, a large group of polyphenols, was negatively correlated with hepatic lipid accumulation, but its impact on mtGPAT1 activity and NAFLD has yet to be determined. Hepatoma cell lines and KKAy mice were used to investigate the impact of anthocyanin on high glucose-induced mtGPAT1 activation and hepatic steatosis. Treatment with anthocyanin cyanidin-3-O-β-glucoside (Cy-3-g) reduced high glucose-induced GPAT1 activity through the prevention of mtGPAT1 translocation from the endoplasmic reticulum to the outer mitochondrial membrane (OMM), thereby suppressing intracellular de novo lipid synthesis. Cy-3-g treatment also increased protein kinase C ζ phosphorylation and membrane translocation in order to phosphorylate the mtF0F1-ATPase β-subunit, reducing its enzymatic activity and thus inhibiting mtGPAT1 activation. In vivo studies further showed that Cy-3-g treatment significantly decreases hepatic mtGPAT1 activity and its presence in OMM isolated from livers, thus ameliorating hepatic steatosis in diabetic KKAy mice. Our findings reveal a novel mechanism by which anthocyanin regulates lipogenesis and thereby inhibits hepatic steatosis, suggesting its potential therapeutic application in diabetes and related steatotic liver diseases.
Keywords: mitochondrial acyl-CoA:glycerol-sn-3-phosphate acyltransferase 1, protein kinase C ζ, hepatocytes
Nonalcoholic fatty liver disease (NAFLD) is considered to be the most common hepatic manifestation of metabolic syndromes such as diabetes and obesity (1). The dysregulation of triacylglycerol (TAG) metabolism may contribute to the derangement of hepatic lipid homeostasis and chronic liver damage (2). Thus, understanding the steps involved in the regulation of hepatic TAG synthesis would likely provide potential new targets for the treatment and prevention of this condition.
Glycerol-sn-3-phosphate acyltransferase (GPAT) controls the rate-limiting step in the glycerol 3-phosphate pathway, converting glycerol-3-phosphate and acyl-CoA into phosphatidic acid, which is the precursor of TAG and glycerophospholipids (3). Currently, two GPAT activities exist in most mammalian cells and tissues, the first of which (msGPAT) is located in the endoplasmic reticulum (ER) and the second of which is located in the mitochondria, which is associated with the outer mitochondrial membrane (OMM) (4, 5). The activities of mtGPAT and microsomal GPAT can be differentiated by their selective sensitivity to sulfhydryl group reactive reagents such as N-ethylmaleimide (NEM) (6). Previous in vitro studies have demonstrated that the overexpression of mtGPAT primarily results in the trafficking of exogenous fatty acids into the TAG pool rather than the incorporation of these acids into phospholipid fractions or into oxidative degradation (7). Thus, it has been proposed that the mtGPAT1 isoform might serve as a valve that channels fatty-acid derivatives preferentially toward glycerolipid synthesis, counteracting their partitioning into the β-oxidation pathway. On the contrary, deletion of mtGPAT in mice substantially limits the incorporation of acyl-CoA species into the sn-glycerol 3-phosphate pathway in liver, resulting in markedly lower levels of hepatic lipid synthesis and the prevention of hepatic steatosis (8, 9). Because the liver is among the organs displaying the highest specific mtGPAT enzyme activity, mtGPAT1 was regarded as a novel and potential target for the treatment of hepatic steatosis.
Anthocyanins are naturally occurring pigments in the plant kingdom and are therefore widely distributed as plant polyphenols. Recently, anthocyanins have received more attention as potential agents to halt, reverse, and improve the symptoms of many degenerative diseases (10–13). Recent studies have demonstrated that anthocyanin-rich foods improve hyperlipidemia and significantly ameliorate hepatic steatosis (14) but the underlying molecular mechanism is incomplete. Because GPAT1 is a major lipogenic enzyme implicated in the development of hepatic steatosis, we currently evaluated the effects of anthocyanin on high glucose-induced mtGPAT1 activation in vitro and in vivo and its impact on hepatic steatosis. Notably, we demonstrated that anthocyanin in hepatocytes abolishes high glucose-induced mtGPAT1 activation through a mechanism involving PKCζ activation and thus ameliorates hepatic steatosis in obese diabetic KKAy mice.
MATERIALS AND METHODS
Cell culture and transfection
HepG2 cells (human hepatoma cell line, ATCC #HB 8065, Manassas, VA) were cultured in DMEM (Gibco, Carlsbad, CA) supplemented with 10% FBS and antibiotics at 37°C in a humidified, 5% CO2/95% air atmosphere.
For PKCζ activation studies, HepG2 cells were seeded at a density of 106 per 60 mm diameter dish for 24 h prior to transfection. Cells were transfected by the Lipofectamine method with 400 to 600 ng of constitutively active PKCζ (myr-PKCζ-FLAG) and an empty vector for a total of 2 mg of plasmid DNA. Cells were starved in serum-free DMEM for 24 h prior to lysis, and lysates were prepared at 48 h posttransfection. Myristoylation of PKCζ targets it to the membrane, resulting in a constitutively active kinase whose basal activity was 6- to 7-fold higher than that of wild-type PKCζ (15).
An F1-ATPase β-subunit clone was generated by PCR using an F1-ATPase β-subunit forward primer (5′-GAGAGGAGCTCCACTATTGCTATGGATGGC-3′, SacI recognition site underlined) and an F1-ATPase β-subunit reverse primer (5′-GAGAGAAGCTTCACGACCCATGCTC-3′, HindIII recognition site underlined) as described (16). The PCR product was cloned into the pCMV5 vector (Clontech, Palo Alto, CA) between the SacI and HindIII sites and then transformed into DH5α cells. Transformants were screened, and positive clones containing plasmid with the correct orientation of the insert were cultured. For transfection, 800 ng of the ATPase β-subunit and 2.4 μl of Fugene 6 (Roche) were incubated in 100 μl of Opti-MEM (Invitrogen) for 30 min at room temperature for 24 h.
Subcellular fraction preparation
Total cell lysates were prepared in lysis buffer (1% Triton X-100, 150 mM NaCl, 10 mM Tris, pH 7.4, 1 mM EDTA, 1 mM EGTA, 0.2 mM phenylmethylsulfonyl fluoride, 0.2 mM sodium orthovanadate, and 0.5% NP-40) and protein contents were measured using the Bradford assay. Cytosolic and membrane fractions were prepared as described previously (17). Mitochondrial fractions from the hepatocytes and livers of mice were prepared as described previously (17) and then solubilized in Tris buffer containing 1% digitonin, or 1% 3-[(3-cholamidopropyl) dimethyl-ammonio]1-propane sulfonate (CHAPS) at 4°C as reported previously (18).
Western blot
Equal amounts of proteins (30 μg) from total cell lysate, cytosolic fractions, membrane fractions, or liver tissue were resolved by SDS-PAGE and transferred to polyvinylidene difluoride membranes. The membranes were then probed with primary antibodies against phospho-PKC-ζ Thr-410 and PKCζ (Cell Signaling Technology, Danvers, MA), GPAT1 (Abcam, Cambridge, MA), ER marker-KDEL (Abcam), mitochondrial marker-VDAC (Abcam), alkaline phosphatase (Abcam), lactate dehydrogenase (Abcam), and β-actin (Cell Signaling Technology). The membranes were then incubated with appropriate secondary antibodies and visualized with the Super Signal West Pico chemiluminescent substrate (Roche).
RNA interference
ATP5B small interfering (si)RNA (sc-40565, Santa Cruz) is a pool of three target-specific 19-25 nt siRNAs designed to knock down the human F1-ATPase β-subunit gene expression.
RNA interference to knock down the human PKCζ gene was composed of four pooled siRNA duplexes with “UU” overhangs (siRNA1: “GGUUGUUCCUGG UCAUUGA”; siRNA2: “GACCAAAUUUACGCCAUGA”; siRNA3:“GAACGAG GACGCCGACCUU”; siRNA4: “CGUCAAAGCCUCCCAUGUU”; Cat no. M-003526-02; Dharmacon, Lafayette, CO). A nonrelated siRNA pool (Cat no. D-001206-13-20, Dharmacon) that does not target any known genes was used as a control. The cells were transfected with these molecules using lipofectamine reagent.
Measurement of GPAT activity
HepG2 cells or approximately one-third of a fresh mouse liver was homogenized using a Dounce homogenizer in an ice-cold homogenization buffer containing 10 mM Tris-HCl, pH 7.4, 250 mM sucrose, 1 mM DTT, 1 mM EDTA, and complete proteinase inhibitor cocktail (Roche Applied Science). Membrane debris and nuclei were removed by centrifugation at 800 g at 4°C for 5 min. Supernatant was transferred to clean tubes and centrifuged at 12,000 g at 4°C for 10 min to pellet the mitochondria. The pellet was then resuspended with the ice-cold homogenization buffer for protein quantification and enzymatic assay. Prior to assay of mtGPAT1 activity, the mitochondrial suspension was incubated on ice for 15 min in the presence of 2 mM NEM (Sigma Aldrich) to inactivate other GPAT activities. The mtGPAT activities were assayed at steady-state equilibrium conditions using 10 μg of protein at 25°C for 1 h in assay buffer (75 mM Tris-HCl, pH 7.4, 4 mM MgCl2, and 8 mM NaF) containing 100 μM palmitoyl-CoA (Avanti Polar Lipids, Alabaster, AL), 1 mM glycerol-3-phosphate, 2 mg/ml BSA and 1 μCi [3H]glycerol-3-phosphate (American Radiolabeled Chemicals, St. Louis, MO). The reaction was stopped by adding five volumes of water-saturated 1-butanol. After thorough mixing to extract the lysophosphatidic acid product, phase separation was achieved by centrifugation at 13,000 g in a microcentrifuge for 10 min. The aqueous phase was transferred to clean tubes, and further separation was achieved using two back extractions with water-saturated 1-butanol. The top layer was then transferred to a scintillation vial and counted for radioactivity (7, 19). The use of NEM sensitivity on a single high-speed spin pellet has been previously demonstrated to effectively differentiate mitochondrial (NEM-resistant) from ER-associated (NEM-sensitive) GPAT activity (20).
F0F1-ATPase activity
Mitochondrial F0F1-ATPase activity was measured spectrophotometrically at 340 nm by coupling the production of ADP to the oxidation of NADPH via the pyruvate kinase and lactate dehydrogenase reaction (coupled assay). Specific F0F1-ATPase activity in all cases was determined in the presence of the enzyme inhibitors oligomycin or efrapeptin. To study the possible effects of anthocyanin on the other enzymes used in the coupled assay with ATPase, i.e., pyruvate kinase and lactate dehydrogenase, ATP was omitted from the buffer and the reaction was started by the addition of 0.2 mM ADP (21).
Measurement of ADP/ATP ratio
The ADP/ATP ratio of the HepG2 cells was determined with an ADP/ATP Ratio Assay Kit (Bioluminescent, ab65313, Abcam). The assay can be fully automated for high-throughput and is highly sensitive.
Fatty acid uptake assay
The HepG2 cells were incubated with Cy-3-g (1 μM, 10 μM, 100 μM) for 2 h in serum-free medium. The cells were then treated with [3H]BSA-palmitate (1 μCi per well) for 4 min. We washed the cells with HBSS and lysed them with 1 N NaOH. Radioactivity was quantified with a liquid scintillation counter (LKB Instruments) (22).
Lipid biosynthesis
Hepatocytes were incubated with [3H] labeled-palmitic acid as described. Cells were rinsed with PBS and scraped from the plates using PBS containing 1% Triton X-100. Lipids were extracted from either cellular homogenate or media in methanol-chloroform (1:2), and the radioactivity incorporated into lipids was determined by scintillation vials and and counting radioactivity (7).
Triglyceride content in liver and cultured hepatocytes
Liver (100 mg) was homogenized in water and lipids were extracted into CHCl3, dried, and resuspended in 1 ml of CHCl3. A fraction of the original lipid extract was dried and resuspended in isopropyl alcohol, 1% Triton X-100 at room temperature for 1 h. The triglyceride level was determined using triglyceride assay reagents (Sigma Aldrich) (23). The quantitative estimation of hepatic triglyceride accumulation in HepG2 cells was performed by the extraction of hepatic lipids from cell homogenates using chloroform-methanol (2:1) and the enzymatic assay of triglyceride mass using the EnzyChrom™ triglyceride assay kit (Bioassay Systems) (24).
Histology of liver
Tissues were fixed in 4% buffered paraformaldehyde. For hematoxylin and eosin (HE) staining, tissues were dehydrated and embedded in paraffin. For Oil Red O staining, livers were embedded in Tissue-Tek OCT, snap-frozen, and stored at −80°C. Images were captured using an Olympus BX60 camera (Tokyo, Japan) (25).
PKCζ activity
PKCζ kinase activity was assayed using an HTScan® PKCζ Kinase Assay Kit (#7607, Cell Signaling Technology).
KKAy mice study
Male KKAy mice at 6 wks of age were purchased from the Jackson Laboratory (Bar Harbor, ME). The mice were maintained on a standard purified mouse diet (AIN-93, control) or on AIN-93 plus Cy-3-g (100 mg/kg) ad libitum for 12 wks. Anthocyanin Cy-3-g was mixed to homogeneity during the preparation of these diets. Chow diet was never permitted to exceed 30°C and was kept away from light whenever possible to ensure the stability of Cy-3-g. Stock Cy-3-g and all chow was stored in the dark at −40°C, and food was provided in cages for no more than 1 wk. To identify the critical role of PKCζ, some mice also received TAT-conjugated peptide PKCζ inhibitor (0.2 mg/kg). The peptide PKCζ inhibitor was given by intraperitoneal injection. All drugs were prepared in sterile saline and controls received equal volumes of the saline vehicle.
Determination of hepatic triglyceride secretion
We used a protocol modified as previously demonstrated (26). Briefly, mice were injected with 100 μl of 10% Tyloxapol (Triton WR1339, which inhibits all lipoprotein lipases and therefore the clearance of TG from the blood) per animal by intravenous injection, and blood was collected to check TG at 1 h. Plasma was separated and assayed for triglycerides. TG secretion rates were expressed as milligram per kilogram per h after normalizing with their liver weight.
Statistical analysis
Results were expressed as mean ± SEM. Comparison between groups was performed by Student's paired two-tailed t-test. One-way ANOVAs were used to examine differences in response to treatments between groups with post hoc analysis performed by the method of Student-Newman-Keuls. P < 0.05 was considered significant.
RESULTS
Anthocyanin reduces high glucose-induced GPAT1 translocation and inhibits lipid accumulation in human HepG2 cells
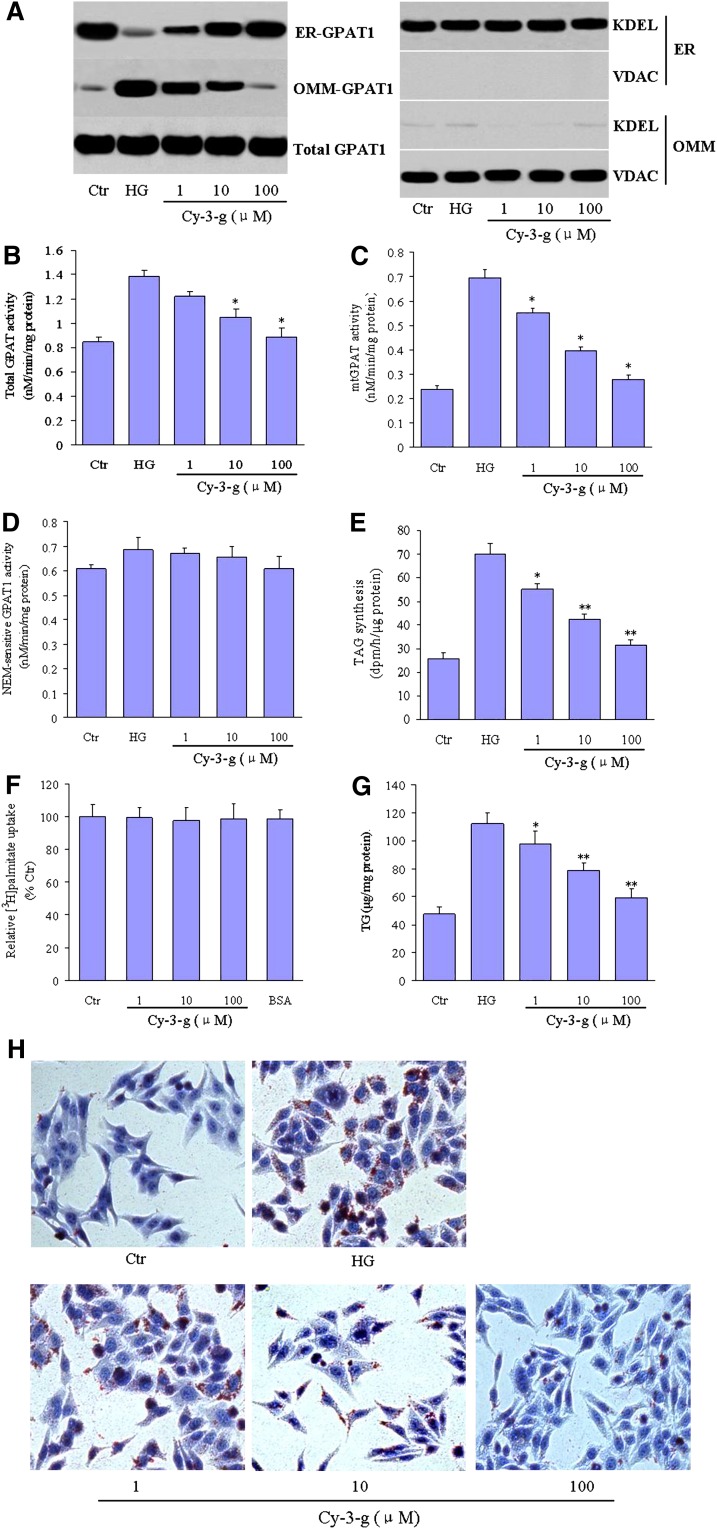
We first examined the effects of anthocyanin upon GPAT1 protein expression. HepG2 cells were treated with normal (5.5 mM) and high (25 mM) concentrations of glucose either alone or in the presence of anthocyanin Cy-3-g for 24 h. GPAT1 protein was mainly present in the ER fractions when the cells were cultured in a normal glucose medium. However, the presence of high glucose levels caused a significant decrease in GPAT1 protein expression in the ER and an increase in the OMM, indicating that a high concentration of glucose induces GPAT1 translocation in HepG2 cells. Significantly, this translocation of GPAT1 to the OMM was inhibited by anthocyanin Cy-3-g in a dose-dependent manner (Fig. 1A). These effects were not attributable to the alteration of GPAT1 expression because the level of total GPAT1 was not changed by anthocyanin. The purity of subcellular fractions was verified with the specific markers: the presence of KDEL and the absence of VDAC in ER preparations, and the presence of VDAC and the absence of KDEL in OMM fractions.
Fig. 1.
Anthocyanin prevents high glucose-induced mtGPAT1 activation. HepG2 cells were stimulated with high glucose levels (25 mM, HG) in the absence or presence of 1, 10, or 100 μM Cy-3-g for 24 h. Normal glucose (5.5 mM) was used as a control (Ctr). A: Cellular subfractions containing the endoplasmic reticulum (ER) and outer mitochondrial membrane (OMM) were isolated and GPAT1 expression was measured by Western blot. Anti-KDEL and anti-VDAC antibodies were used to assess the purity of the ER and mitochondria fractions, respectively. The blot data are representative of three separate experiments. B: Total GPAT1 activity, (C) mtGPAT1, and (D) NEM-sensitive GPAT1 activity was quantified. E: Cellular triacylglycerol (TAG) were extracted and isolated by thin-layer chromatography. F: The uptake of [3H]palmitate by HepG2 cells was measured as described in Materials and Methods and expressed as percent of control. G, H: Hepatic lipid accumulation was determined by (G) TG mass measurements (expressed as μg of lipid/mg of protein) and identified by (H) Oil Red O staining (200×). Data are expressed as the means ± SEM from three independent experiments. *P < 0.05 and **P < 0.01 versus HG-treated cells.
A previous study reported that GPAT1 is more active in OMM (27). We thus evaluated the impact of GPAT1 translocation inhibition by anthocyanin upon its enzymatic activity in HepG2 cells. Compared with normal concentrations of glucose, high glucose treatment caused a significant increase in total GPAT activity (Fig. 1B) and mtGPAT1 activity (Fig. 1C), both of which were inhibited by Cy-3-g in a dose- dependent fashion. However, NEM-sensitive GPAT1 activity was unchanged after treatment by Cy-3-g (Fig. 1D).
To further determine whether the reduction of mtGPAT1 activity by anthocyanin is of functional relevance, we investigated de novo lipid biosynthesis. Cells incubated with high glucose levels showed a significant increase in intracellular TAG synthesis (Fig. 1E), which was markedly inhibited by anthocyanin. However, anthocyanin Cy-3-g treatment did not affect the uptake of palmitate into hepatocytes (Fig. 1F). More importantly, Cy-3-g markedly suppressed intracellular lipid accumulation as revealed by the direct TG mass measurements (Fig. 1G) and Oil Red O staining (Fig. 1H).
F1-ATPase mediates high glucose-induced GPAT1 translocation and activity
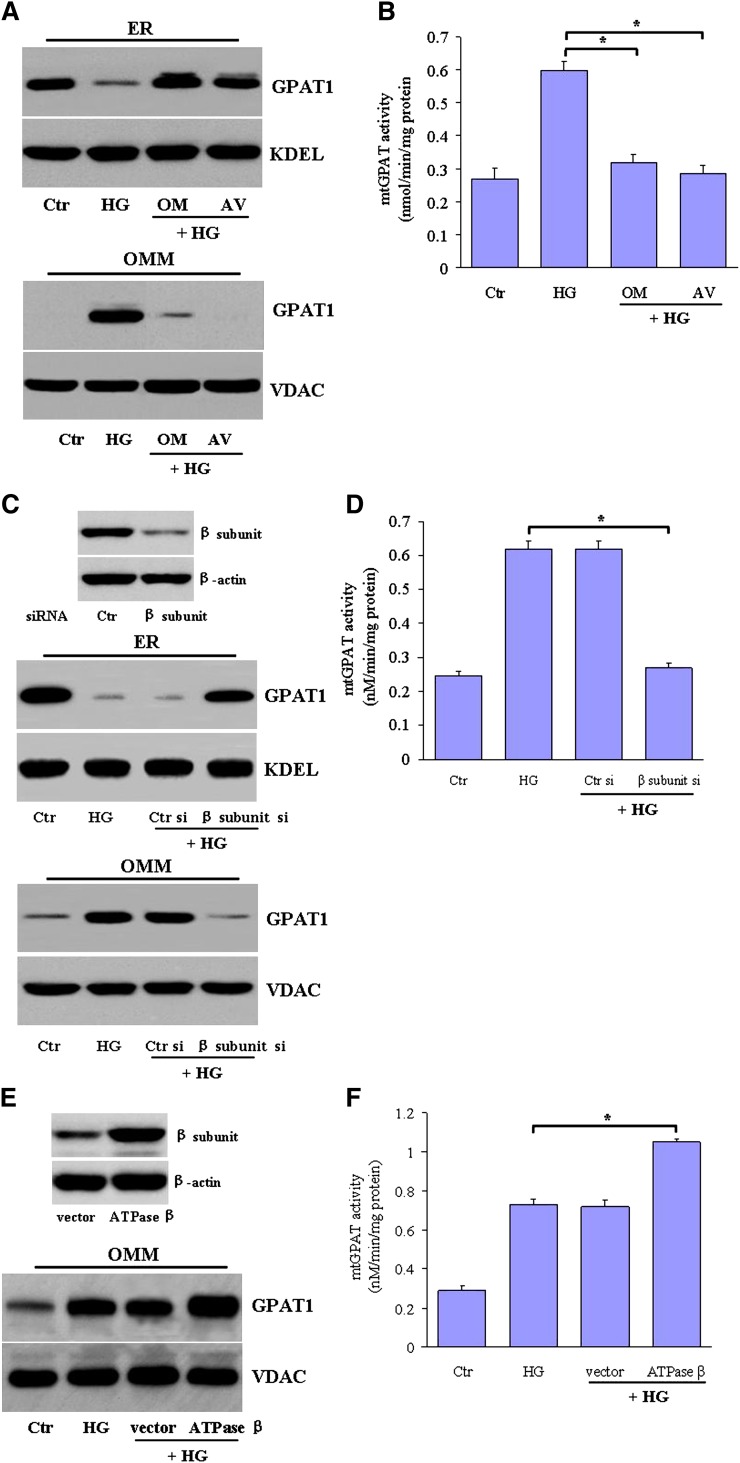
Mitochondrial F0F1-ATP synthase is a mitochondrial inner membrane-bound enzyme composed of two main domains, including F0 and F1 (28). We then assessed the possible role of F1-ATPase in high glucose-induced mtGPAT1 activation. Both oligomycin, a specific inhibitor of the F0F1-ATPase via the blockade of rotary proton translocation in F0, and aurovertin, a specific inhibitor of the F1 sector of the mitochondrial ATPase, completely suppressed the high glucose-induced GPAT1 translocation (Fig. 2A) and activity (Fig. 2B).
Fig. 2.
F0F1-ATPase is both necessary and sufficient for high glucose-induced mtGPAT1 activation. A, B: HepG2 cells were stimulated with high glucose levels (25 mM) in the absence or presence of oligomycin (OM) and aurovertin (AV) for 24 h. Normal glucose (5.5 mM) was used as a control. A: GPAT1 expression in the ER and OMM was determined by Western blot. B: The mtGPAT1 activity was measured in OMM fractions. C, D: HepG2 cells were transfected with vectors encoding the F1-ATPase β-subunit siRNA (β si) and control siRNA (Ctr si) and then incubated with high glucose levels for 24 h. C, inset: Representative blot showing β-subunit protein expression in hepatocytes transfected with control or β-subunit siRNA. E, F: HepG2 cells were transfected with a plasmid encoding F1-ATPase β-subunit (ATPase β) or empty vector. After that, the transfected hepatocytes were stimulated with high glucose levels (25 mM) for 24 h. E: The expression levels of GPAT1 and (F) the enzymatic activity was measured in the OMM fractions. E, inset: Representative blot showing β-subunit protein expression in hepatocytes transfected with empty vector or ATPase β-subunit. Data are shown as representative blots or are expressed as the means ± SEM by three independent assays from three independent experiments.
F1-ATPase is made up of five subunits (α3β3γδϵ), with the catalytic site located within the β-subunit (29).We then transfected HepG2 cells with a specific siRNA against the human β-subunit to further verify the role of F1-ATPase on GPAT1 translocation. Notably, we observed that β-subunit silencing abrogated the high glucose-mediated activation of GPAT1 translocation from the ER to the OMM (Fig. 2C) and increase in mtGPAT1 activity (Fig. 2D). On the contrary, overexpression of the β-subunit potently enhanced the high glucose-induced GPAT1 translocation (Fig. 2E) and mtGPAT1 activity (Fig. 2F), indicating that the β-subunit of F1-ATPase is essential for high glucose-mediated mtGPAT1 activation.
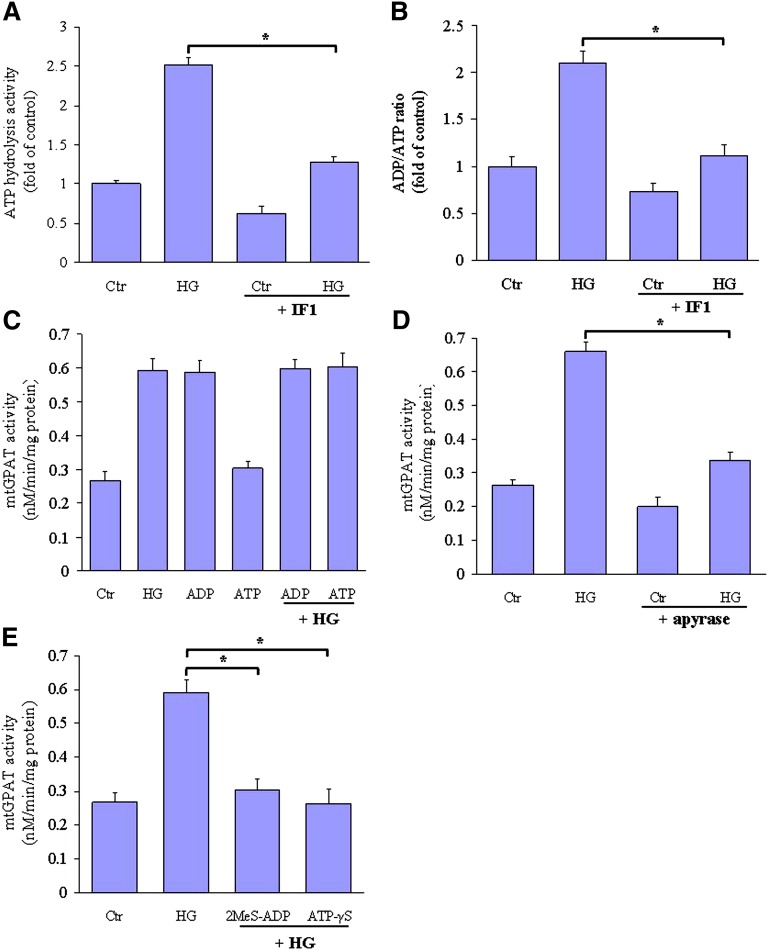
We further measured the functional activity of cell-surface ATPase. Evidently, high glucose level incubation resulted in the significant increase of the ATP hydrolysis activity (Fig. 3A) and the ADP/ATP ratio (Fig. 3B). The purified IF1 protein, a natural inhibitor of mitochondrial F1-ATPase that interacts with the β-subunit to inhibit the ATP hydrolysis activity (30), could reduce both the basal (38.4% reduction) and the high glucose-stimulated hydrolysis activity (49.3% reduction). Because ATP hydrolase activity is a main feature of a whole F1-ATPase complex, our data indicate that, on the plasma membrane of hepatocytes, there is a complete F1-ATPase that functions as an ATP hydrolase and can be stimulated by high glucose levels.
Fig. 3.
F1-ATPase-dependent ATP hydrolysis is critical for high glucose-induced mtGPAT1 activation. A, B: HepG2 cells were incubated with normal glucose (Ctr, 5.5 mM), or high glucose (HG, 25 mM) in the absence or presence the purified IF1 protein (100 nM). A: The ATP hydrolysis activity was measured with an ATP-regenerating system. B: The ADP/ATP ratio was measured by bioluminescent detection. In some experiments, (C) ADP (100 nM) or ATP (100 nM), (D) apyrase (0.2 u/ml) and (E) 2MeS-ADP (100 nM) or ATP-γS (100 nM) was added to the incubation medium, respectively. The mtGPAT1 activity was assayed, and data are expressed as means ± SEM from three independent experiments. *P < 0.05.
Furthermore, 100 nM ADP (the only compound produced by ATP synthase at the hepatocyte cell surface) stimulated the activity of mtGPAT1 by 2.2-fold, similar to the amount stimulated by high glucose levels (Fig. 3C). The effect of both agents was not additive, suggesting that they affect a similar mtGPAT1 activation pathway. By contrast, 100 nM ATP stimulated only a minor increase in mtGPAT1 activation. In addition, apyrase, which hydrolyzes both ATP and ADP, completely abolished both the basal and the high glucose-stimulated mtGPAT1 activation (Fig. 3D). Moreover, 2MeS-ADP, a nonhydrolysable analog of ADP, showed a weak stimulation of mtGPAT1 activation. By contrast, ATP-γS, a nonhydrolysable analog of ATP, had no effect (Fig. 3E). Together, these data indicate that there may be a specific ADP-dependent pathway for mtGPAT1 activation.
Anthocyanin suppresses high glucose-induced mtF0F1-ATPase activity through stimulation of PKCζ activation
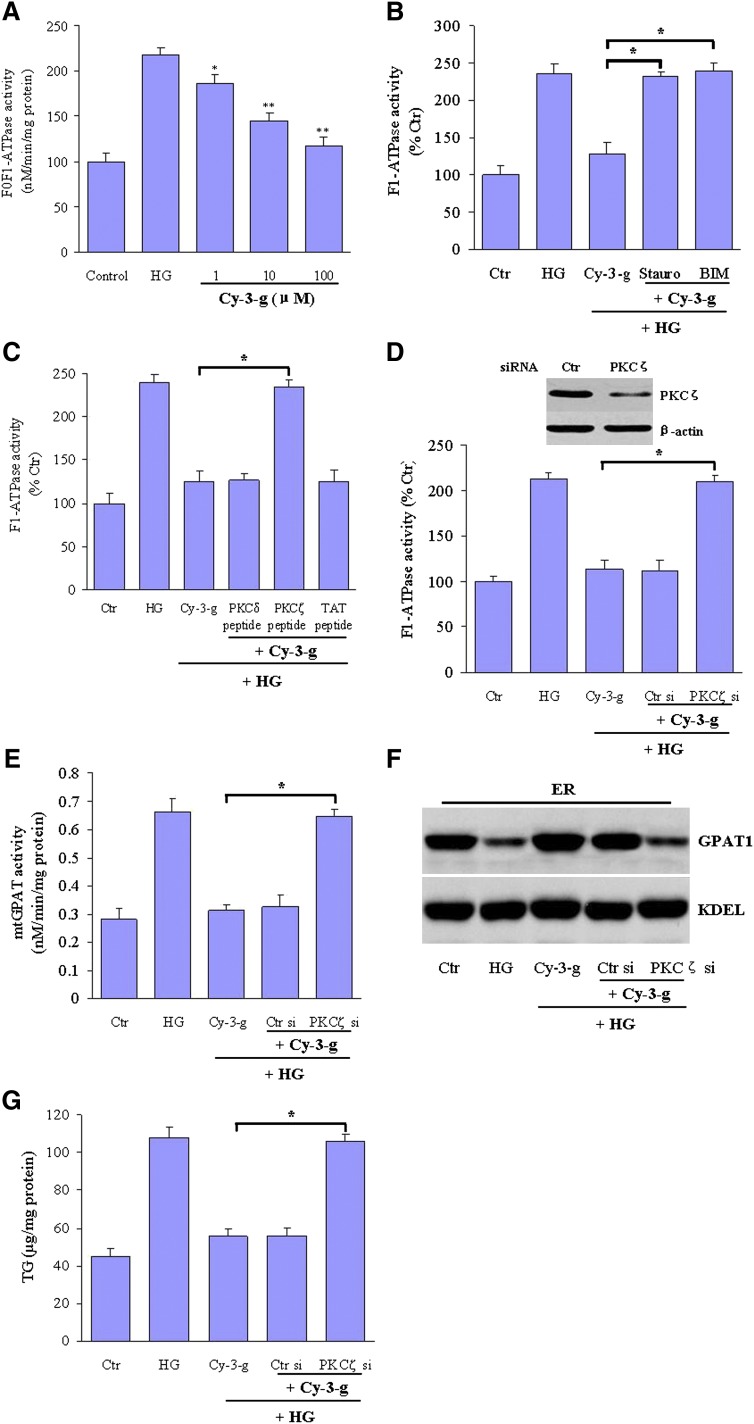
We next examined whether the suppressive effects of anthocyanin upon mtGPAT1 activity were dependent on the inhibition of mtF0F1-ATPase. Anthocyanin Cy-3-g treatment was found to inhibit F0F1-ATPase activity in a concentration-dependent manner (Fig. 4A). Dynamic analysis further showed that the IC50 of Cy-3-g was 11.8 μM but was 10 μM for digitonin-solubilized and CHAPS-solubilized preparations, respectively, indicating that anthocyanin Cy-3-g is a potent antagonist of F0F1-ATPase (supplementary Fig. I). Anthocyanin itself did not have any effect upon other enzymes, i.e., pyruvate kinase and lactate dehydrogenase, which were used in the ATPase activity assay.
Fig. 4.
PKCζ mediates the inhibition of high glucose-induced mtF0F1-ATPase activity by anthocyanin. A: HepG2 cells were incubated with normal glucose (control, 5.5 mM) or stimulated with high glucose (HG, 25 mM) in the absence or presence of 1, 10, or 100 μM Cy-3-g for 24 h. After that, the F0F1-ATPase activity hepatic mitochondrial preparations was assayed as indicated. Data are expressed as means ± SEM from three independent experiments. *P < 0.05, **P < 0.01 versus HG. B–D: Cultured HepG2 cells were incubated with normal glucose, high glucose levels (HG), HG plus Cy-3-g, or HG plus Cy-3-g in the presence of (B) the PKC inhibitor staurosporine (Stauro, 200 nM) or bisindolymaleimide I hydrochloride (BIM, 5 μM) for 24 h; (C) peptide inhibitors of PKCζ, PKCδ, or the TAT carrier peptide (all 1 mM); or (D) PKCζ siRNA (PKCζ si) or control siRNA (Ctr si). F1-ATPase activity was then determined as indicated in Materials and Methods. D, inset: Representative blot showing PKCζ protein expression in HepG2 cells transfected with control or PKCζ siRNA. E: HepG2 cells were incubated with normal glucose (Ctr), high glucose levels (HG), HG plus Cy-3-g, or HG plus Cy-3-g in the presence of PKCζ siRNA (PKCζ si) or control siRNA (Ctr si). The mtGPAT1 activity was then determined. F: GPAT1 expression in ER fractions was measured by Western blot. The blot is representative of three separate experiments. G: Hepatic TG concentrations were analyzed and expressed as μg of lipid/mg of protein. Data are expressed as the means ± SEM from three independent experiments. *P < 0.05.
PKCζ is an atypical isoform of the PKC family and has been implicated as a potential target for treating obesity- and diabetic-associated abnormalities (31, 32). To elucidate the participation of PKCζ activation in the anthocyanin-mediated inhibition of mtF0F1-ATPase, we exposed HepG2 cells to staurosporine or bisindolylmaleimide (BIM), two potent PKC antagonists. Notably, in the presence of staurosporine or BIM, the reduction in mtF0F1-ATPase activity by anthocyanin was markedly reversed, indicating that PKC activation is involved in the effects of this chemical (Fig. 4B). We then sought to identify the responsible PKC isoform using specific peptide inhibitors (33). The inhibition of high glucose-stimulated mtF0F1-ATPase by anthocyanin was significantly blocked by a TAT-conjugated PKCζ peptide inhibitor, but not by a TAT-conjugated PKCδ inhibitor or by the TAT carrier peptide alone (Fig. 4C). To further confirm the critical role of PKCζ, we employed specific PKCζ siRNA and found consistently that transfection of HepG2 cells with this molecule also significantly and specifically reduced anthocyanin-mediated inhibition of high glucose-induced mtF0F1-ATPase activation (Fig. 4D). Furthermore, this reduction was strongly attenuated by the PKCζ-specific siRNA (Fig. 4E). More significantly, PKCζ silencing strongly abolished the beneficial effects of anthocyanin upon GPAT1 translocation (Fig. 4F) and intracellular lipid accumulation in HepG2 cells (Fig. 4G).
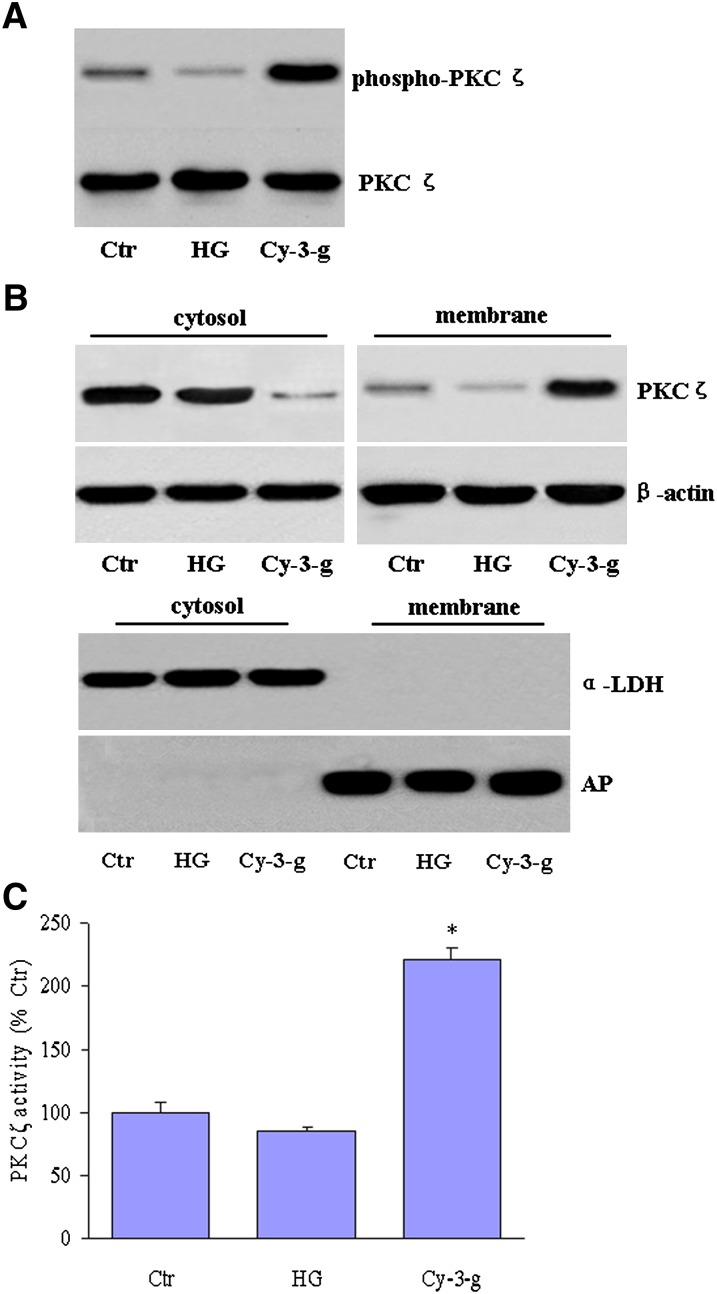
Because the phosphorylation of a critical threonine in the activation loop of PKCs is the essential first step of PKC activation (34), we then determined the impact of anthocyanin on PKCζ activation in HepG2 cells. The levels of phosphorylated PKCζ were found to be very low in HepG2 cells under high glucose-stimulated conditions. In contrast, anthocyanin treatment significantly enhanced the PKCζ Thr410/403 phosphorylation levels (Fig. 5A). The translocation of PKCζ is considered a critical step in PKCζ activation, and the exposure of HepG2 cells to anthocyanin significantly increased the levels of PKCζ in membrane fractions but lowered these levels in the cytosol (Fig. 5B, upper panel). The purity of these subcellular fractions was confirmed by incubation with antibodies against specific protein markers of the cytosol (lactate dehydrogenase) and plasma membrane (alkaline phosphatase) (Fig. 5B, lower panel) (15). In parallel, anthocyanin significantly increased the specific PKCζ kinase activity in HepG2 cells (Fig. 5C).
Fig. 5.
Anthocyanin increases PKCζ phosphorylation and translocation. A: Confluent HepG2 cells were exposed to normal glucose (Ctr), high glucose levels (HG), or HG plus Cy-3-g (10 μM) for 1 h, and PKCζ phosphorylation was assayed by immunoblot. B: Anthocyanin increases the translocation of PKCζ from the cytosol to the membrane (upper panel). The purity of the subcellular fractions was detected by Western blot with the use of specific antibodies against marker enzymes. All the blots are a representative of three blots obtained from three independent experiments. C: PKCζ activity was analyzed as described in Materials and Methods. Data are the means ± SEM from three independent experiments. *P < 0.05 versus HG+Cy-3-g.
PKCζ activation induces the phosphorylation of F1-ATPase β-subunit
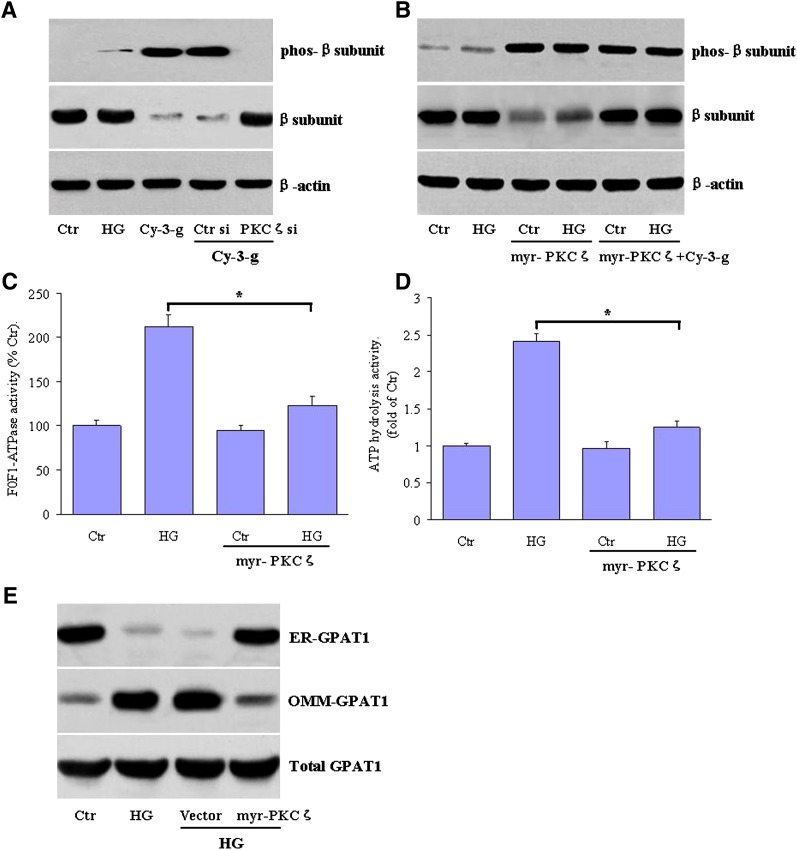
PKC has been demonstrated to be involved in controlling the function of other proteins through the phosphorylation of serine and threonine amino acid residues on these proteins (35). Several F0F1-ATPase subunits have PKC consensus amino acid motifs (36), and we further determined the subunit, which is phosphorylated by PKCζ. We found that anthocyanin treatment resulted in the increased phosphorylation of the F0F1-ATPase β-subunit, which was largely blocked by PKCζ siRNA (Fig. 6A).
Fig. 6.
PKCζ induces the phosphorylation of the β-subunit of F0F1-ATPase. A: HepG2 cells were incubated with normal glucose (Ctr), high glucose levels (HG), HG plus Cy-3-g, or HG plus Cy-3-g in the presence of PKCζ siRNA (PKCζ si) or control siRNA (Ctr si). Serine phosphorylation of the β-subunit of F0F1-ATPase was assessed by immunoprecipitation from hepatocytes and detection with the specific antibody. Data are representative of three independent analyses. B: HepG2 cells, transfected with constitutively active PKCζ (myr-PKCζ-FLAG), were left untreated or incubated with Cy-3-g. A representative autoradiogram from three independent assays of the phosphorylation of the β-subunit of F0F1-ATPase was shown. C, D: HepG2 cells were transfected with myr-PKCζ-FLAG and then incubated with high glucose levels for 24 h. C: F0F1-ATPase activity and (D) the ATP hydrolysis activity were measured as indicated and expressed as fold of control. E: Representative blots showing GPAT1 expression in ER, OMM, and total GPAT1 levels. Data are the means ± SEM from three independent experiments. *P < 0.05 versus HG.
We further wanted to determine whether PKCζ could regulate the phosphorylation of the β-subunit. A constitutively active and membrane-targeted PKCζ (Myr-PKCζ- FLAG) alone increased Thr phosphorylation in the F0F1-ATPase β-subunit (lane 3) almost 3-fold over the basal level of phosphorylation observed in unstimulated and high glucose-stimulated HepG2 cells (lanes 1 and 2), resulting in the reduction of β-subunit expression levels (Fig. 6B). This impact was not further augmented by anthocyanin Cy-3-g (Fig. 6B). These data indicate that PKCζ mediates the anthocyanin-responsive phosphorylation and degradation of the β-subunit of F0F1-ATPase.
To investigate the functional consequences of the phosphorylation of the β-subunit of F0F1-ATPase by PKCζ, we performed an in vitro assay to determine whether this event alters the enzyme activity. The higher activity of F0F1-ATPase induced by high glucose levels was decreased by 42% in the presence of constitutively active PKCζ (Fig. 6C), leading to lowered ATP hydrolysis activity (Fig. 6D). More evidently, transfection of HepG2 cells with constitutively active PKCζ also prevented the translocation of GPAT1 from the ER to OMM (Fig. 6E). These results indicate that β-subunit phosphorylation by PKCζ has an inhibitory effect upon F1F0-ATPase.
Anthocyanin inhibits mtGPAT1 and lipogenesis in diabetic KKAy mice
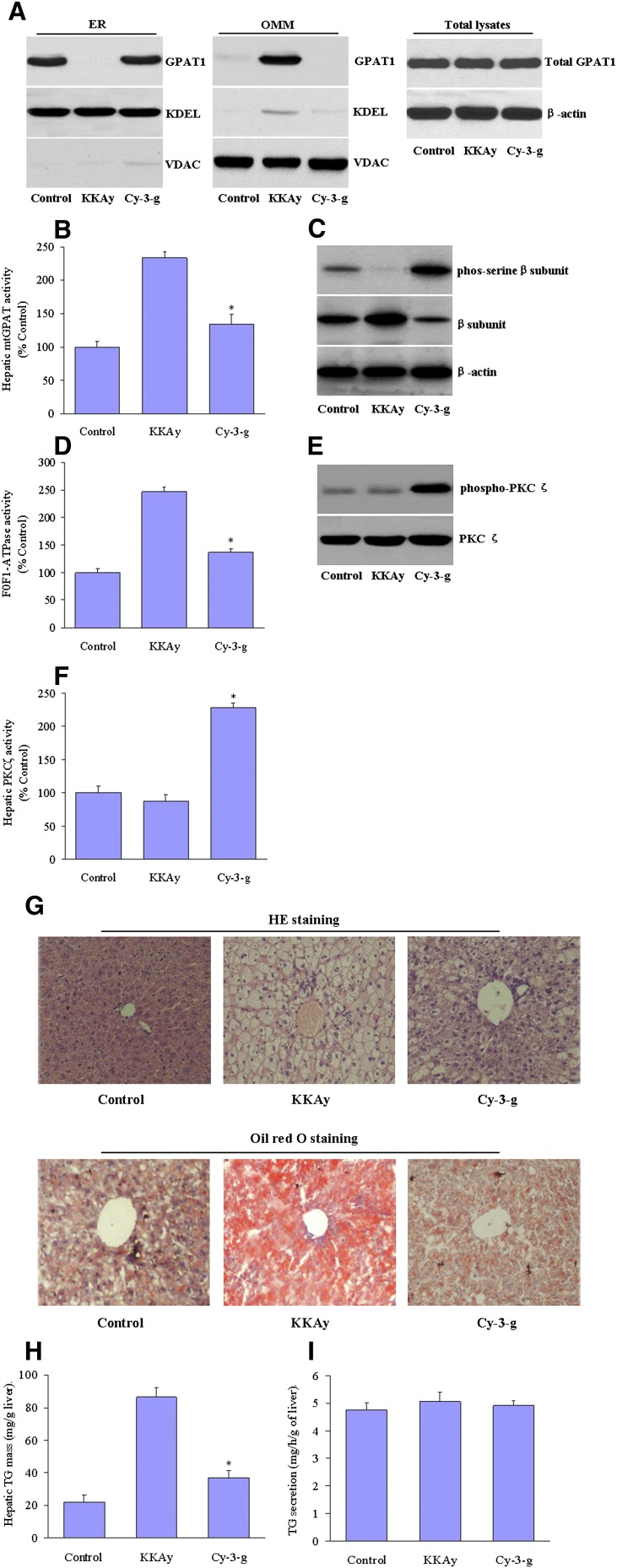
To confirm the effects of anthocyanin on mtGPAT1 activity and hepatic steatosis in vivo, obese diabetic KKAy mice were fed normal chow or chow supplemented with anthocyanin Cy-3-g (100 mg/kg). After 12 wks, the GPAT1 protein was found to mainly accumulate in the ER fractions from the livers isolated from control C57BL/6J mice but was mostly found in the mitochondria in the livers of KKAy mice, indicating that exposure to high glucose levels may promote the translocation of this protein. Remarkably, this translocation was largely blocked in the livers of KKAy mice supplemented with anthocyanin (Fig. 7A). In addition, the mtGPAT1 activity was increased in mitochondrial fractions from the livers of KKAy mice versus C57BL/6J mice. In contrast, anthocyanin supplementation significantly decreased this mtGPAT1 activity (Fig. 7B). These findings indicate that anthocyanin prevents mtGPAT1 translocation from the ER to the mitochondria, where it is activated. Furthermore, anthocyanin treatment also alters F1-ATPase β-subunit phosphorylation levels (Fig. 7C) and enzymatic activity (Fig. 7D) in KKAy mice. However, anthocyanin supplementation did not affect the plasma glucose concentration but significantly reduced TG levels (supplementary Table I).
Fig. 7.
Anthocyanin induces PKCζ activation and inhibits mtGPAT1 activation in KKAy mice. Male KKAy mice were fed on a normal chow diet or chow supplemented with Cy-3-g at a dose of 100 mg/kg for 12 wks. C57BL/6J mice were used as a control (Ctr). A: Representative blots showing GPAT1 expression in the ER and OMM isolated from the mouse livers. B: Hepatic mtGPAT1 activity. C: Representative blots of the phosphorylation levels of F0F1-ATPase β-subunit. D: Hepatic F0F1-ATPase activity. * P < 0.05 versus KKAy mice. E: The PKCζ phosphorylation and (F) activity were assayed in protein samples from the livers of KKAy mice. G: Fixed liver sections were stained with HE (upper panel) and Oil Red O (lower panel) to detect intracellular neutral lipids under microscopy. H: Hepatic total TG mass (mg/g of liver). I: TG secretion rate to plasma (mg/h/g of liver). The results are representative of eight mice in each group.
We next evaluated PKCζ phosphorylation and its kinase activity in KKAy mice. Anthocyanin treatment strongly increased the levels of phospho-PKCζ at Thr410/403 (Fig. 7E), which is required for the full catalytic activity of this kinase. Accordingly, anthocyanin caused a 2.3-fold induction of PKCζ activity compared with untreated KKAy mice (Fig. 7F). In addition, Oil Red O and HE staining showed that the livers from KKAy mice had more lipid droplet accumulation than those of the control C57BL/6J mice. Anthocyanin supplementation of KKAy mice remarkably reduced the number (or size) of lipid droplets (Fig. 7G) and TG mass (Fig. 7H) in the liver, indicating an alleviation of hepatic steatosis. Hepatic steatosis could also result from a relative reduction in VLDL secretion. To test this possibility, lipoprotein lipase activity was pharmacologically blocked with Tyloxapol, and VLDL secretion rates were measured in vivo. Following Tyloxapol injection, the plasma TG accumulation was identical in untreated and anthocyanin-treated mice, suggesting that the total TG secretion from the liver was not affected in the presence of anthocyanin (Fig. 7I). These data suggest that the decreased hepatic TG by anthocyanin may be caused by the reduction of TG synthesis rather than TG secretion.
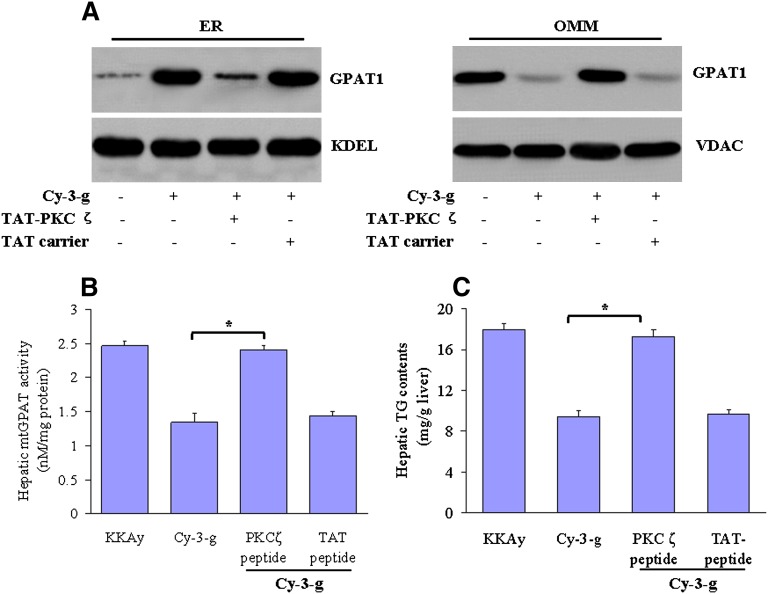
To further substantiate the critical role of PKCζ in the improvement of hepatic steatosis by anthocyanin Cy-3-g in vivo, we performed a knockdown of the PKCζ expression by intraperitoneal injection of TAT-conjugated peptide PKCζ inhibitor or TAT carrier peptide. PKCζ inhibition abrogates the protective effects of anthocyanin on GPAT1 translocation (Fig. 8A), activity (Fig. 8B), and lipid accumulation in livers (Fig. 8C).
Fig. 8.
PKCζ inhibition prevents the anthocyanin-mediated hepatic mtGPAT1 activation and lipid accumulation in vivo. Male KKAy mice at 6 wks of age were maintained on a standard purified mouse diet (AIN-93) or on AIN-93 plus Cy-3-g (100 mg/kg) for 12 wks. Some mice fed Cy-3-g also received TAT-conjugated peptide PKCζ inhibitor (0.2 mg/kg) or TAT-carrier (0.2 mg/kg), respectively. The peptide PKCζ inhibitor was given by intraperitoneal injection. A: Cellular subfractions containing the endoplasmic reticulum (ER) and outer mitochondrial membrane (OMM) were isolated and GPAT1 expression was measured by Western blot. Anti-KDEL and anti-VDAC antibodies were used to assess the purity of the ER and mitochondria fractions, respectively. B: The mtGPAT1 activity was measured in the OMM fractions. The results are expressed as the means ± SEM from eight mice.*P < 0.05. C: Hepatic TG levels were assessed directly. The results are expressed as the means ± SEM from eight mice.*P<0.05.
DISCUSSION
The major findings of our current study are that anthocyanin Cy-3-g stimulates PKCζ activation in HepG2 cells by inducing its phosphorylation at Thr410/403 and its membrane translocation. The activation of PKCζ then phosphorylates the β-subunit of mtF0F1-ATPase, suppresses its activity, and inhibits mtGPAT1 activation, leading to the reduction of de novo lipid biosynthesis induced by high glucose levels. Furthermore, in vivo studies showed that supplementation of Cy-3-g increased PKCζ activity and decreased mtGPAT1 activity in the mouse liver, thereby ameliorating the hepatic steatosis in diabetic KKAy mice. The novel mechanism we have deduced is one in which anthocyanin beneficially regulates hepatic lipid metabolism through the PKCζ-F0F1 ATPase-mtGPAT1 pathway. The ability of anthocyanin to modulate mtGPAT1 activation and improve hepatic lipid metabolism also suggests that it might be a valuable tool in treating NAFLD.
Anthocyanins are widely integrated in the human diet through the consumption of plant-based foods (10, 11). Many reports have discussed the important role of anthocyanins as dietary antioxidants that protect against oxidative damage, and recent studies including ours have further unveiled the important roles of anthocyanin in lipid metabolism (10–12, 37). Although the mtGPAT1 is shown to be an important regulator in lipid homeostasis and obesity-related disorders, no evidence as yet links mtGPAT1 to the antilipogenic effects of anthocyanin. In this study, we demonstrated that anthocyanin treatment strongly inhibits mtGPAT1 activation in response to high glucose levels by interrupting GPAT1 translocation from the ER to the mitochondria in hepatocytes, resulting in a potent suppression of mtGPAT1 activity and the lipogenic process.
F0F1-ATPase/ATP synthase acts as the powerhouse of the cell by synthesizing ATP in the inner membranes of mitochondria (38). It can also operate in the reverse direction, i.e., by hydrolyzing ATP and pumping protons under certain conditions. Our current data show that depletion of F0F1-ATPase largely abolishes GPAT1 incorporation into the OMM. ADP treatment augments mitochondrial GPAT activity, but a nonhydrolysable analog does not induce this response, suggesting that the functional F1-ATPase may function as an ATP hydrolase in regulation of GPAT1 translocation, which can be stimulated by high glucose levels. Thus, we uncovered a novel finding, that mtF0F1-ATPase mediates the high glucose-induced activation of GPAT1 by accelerating the movement of GPAT1 from the ER to the OMM in hepatocytes. In accordance with the previous study showing that ATP synthase is a target for dietary phytochemicals (18), we currently demonstrated that anthocyanin Cy-3-g treatment also significantly suppressed the F0F1-ATPase activity and simultaneously affected the ADP/ATP ratio. Thus, the interaction of anthocyanin with these enzymes could be the principal mechanism underlying the actions of these phytochemicals because apyrase, a calcium-activated plasma membrane-bound enzyme, catalyses the hydrolysis of both ATP and ADP to yield AMP. Furthermore, increased AMP-to-ATP ratio leads to a conformational change in the γ-subunit leading to the activation of AMP-activated protein kinase (AMPK), a heterotrimeric energy-sensing protein, decreases lipid synthesis in liver tissues (39, 40). Thus, our data could not exclude the role of AMP and AMPK in effects of Cy-3-g.
Atypical protein kinase C (aPKC) isoforms mediate insulin effects on glucose transport in muscle and adipose tissues and lipid synthesis in liver and support other metabolic processes (41). Hepatic aPKC-dependent activation of sterol regulatory binding protein-1c and nuclear factor-κB contributes to the development of hepatic lipogenesis, hyperlipidemia, and systemic insulin resistance. Accordingly, hepatic aPKC is considered as a potential target for treating obesity-associated abnormalities (32). PKCζ is a serine/threonine protein kinase that belongs to the atypical subfamily of PKC isoforms (42, 43); however, few reports link it to lipid metabolism. Here, we presented the original data that PKCζ is primarily responsible in the anthocyanin-mediated inhibition of mtGPAT1 activation and lipid synthesis induced by high glucose levels both in vitro and in vivo. Pharmacological or genetic ablation of PKCζ largely reversed the impact of Cy-3-g upon mtGPAT1 activation in hepatocytes, indicating that PKCζ is primarily responsible for the anti-lipogenesis effects of anthocyanin. However, blockade of PKCδ, another isoform of PKC, exerts little influence. More importantly, anthocyanin Cy-3-g treatment was found to induce PKCζ phosphorylation, and this finding is further supported by the results of PKCζ activity. Protein phosphorylation, a posttranslational modification event that produces changes in enzymatic conformation and activity, may represent one of the mechanisms responsible for regulation of F0F1-ATPase activity (44). Indeed, we have observed that exogenous expression of PKCζ in hepatocytes causes the phosphorylation at the serine site on subunit β and promotes the catabolism of F0F1-ATPase protein, leading to the suppression of the enzymatic activity of F0F1-ATPase. Thus, we provide an important insight into mechanisms underlying the biology of our findings that anthocyanin may regulate proteolysis through PKCζ-mediated phosphorylation targeting the β-subunit.
To further authenticate our findings, we applied dietary intervention experiments using KKAy mice, a genetic animal model of type 2 diabetes with hyperglycemia and nonalcoholic fatty liver (45). In line with cellular studies, Cy-3-g significantly alleviated lipid deposition in livers and improved hepatic steatosis. This result is not likely due to the hypoglycemic effects of anthocyanin, as Cy-3-g did not evidently affect blood glucose concentration. Of particular note, the effects of Cy-3-g upon the fat accumulation of livers from KKAy mice recapitulate those observed by stimulating PKCζ activation, increasing F1-ATPase β-subunit phosphorylation, and hence, decreasing hepatic GPAT1 translocation and activity as described in hepatocytes. Simultaneous administration of PKCζ peptide inhibitor notably blocked the protective effects of anthocyanin on hepatic mtGPAT1 activation and lipid accumulation. These results indicate a mechanism by which PKCζ can facilitate long-term potentiation. Further studies are necessary to identify precisely the mechanisms involved in anthocyanin-mediated PKCζ activation. Finally, it remains to be established whether the effects reported herein are a class-family effect of the anthocyanins or are specific to Cy-3-g.
In conclusion, for the first time, we identify a dominant role of PKCζ in inhibiting GPAT1 activation by anthocyanin via the phosphorylation of the β-subunit of F0F1-ATPase. As a result of these changes, anthocyanin suppresses lipogenesis and ameliorates hepatic steatosis in hepatocytes and obese diabetic KKAy mice. Thus, our findings provide the evidence that targeting PKCζ by anthocyanin may contribute to the prevention and treatment of steatotic liver associated with obesity and type 2 diabetes.
Supplementary Material
Footnotes
Abbreviations:
- AMPK
- AMP-activated protein kinase
- aPKC
- atypical protein kinase C
- CHAPS
- 3-[(3-cholamidopropyl) dimethyl-ammonio]1-propane sulfonate
- Cy-3-g
- cyanidin-3-O-beta-glucoside
- ER
- endoplasmic reticulum
- HE
- hematoxylin and eosin
- mtGPAT1
- mitochondrial acyl-CoA:glycerol-sn-3-phosphate acyltransferase 1
- NAFLD
- primary nonalcoholic fatty liver disease
- NEM
- N-ethylmaleimide
- OMM
- outer mitochondrial membrane
- PKCζ
- protein kinase C ζ
- TAG
- triacylglycerol
This research was supported by grants from the National Natural Science Foundation (No.81072301, 30800913, and 30730079), grants from the Guangdong Natural Science Foundation (8451200501000168 and 9151012003000002), Program for New Century Excellent Talents in University (NCET-10-0813), a Foundation for the Author of National Excellent Doctoral Dissertation of P. R. China (No.200978), and the Fundamental Research Funds for the Central Universities of Sun Yat-Sen University (10ykzd04).
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of one figure and one table.
REFERENCES
- 1.Flegal K. M., Carroll M. D., Ogden C. L., Curtin L. R. 2010. Prevalence and trends in obesity among US adults. JAMA. 303: 235–241. [DOI] [PubMed] [Google Scholar]
- 2.Gaemers I. C., Groen A. K. 2006. New insights in the pathogenesis of non-alcoholic fatty liver disease. Curr. Opin. Lipidol. 17: 268–273. [DOI] [PubMed] [Google Scholar]
- 3.Coleman R. A., Lee D. P. 2004. Enzymes of triacylglycerol synthesis and their regulation. Prog. Lipid Res. 43: 134–176. [DOI] [PubMed] [Google Scholar]
- 4.Lewin T. M., Schwerbrock N. M., Lee D. P., Coleman R. A. 2004. Identification of a new glycerol-3-phosphate acyltransferase isoenzyme, mtGPAT2, in mitochondria. J. Biol. Chem. 279: 13488–13495. [DOI] [PubMed] [Google Scholar]
- 5.Dircks L. K. 1997. Mammalian mitochondrial glycerol-3-phosphate acyltransferase. Biochim. Biophys. Acta. 1348: 17–26. [DOI] [PubMed] [Google Scholar]
- 6.Sul H. S., Wang D. 1998. Nutritional and hormonal regulation of enzymes in fat synthesis: studies of fatty acid synthase and mitochondrial glycerol-3-phosphate acyltransferase gene transcription. Annu. Rev. Nutr. 18: 331–351. [DOI] [PubMed] [Google Scholar]
- 7.Lindén D., William-Olsson L., Rhedin M., Asztély A. K., Clapham J. C., Schreyer S. 2004. Overexpression of mitochondrial GPAT in rat hepatocytes leads to decreased fatty acid oxidation and increased glycerolipid biosynthesis. J. Lipid Res. 45: 1279–1288. [DOI] [PubMed] [Google Scholar]
- 8.Neschen S., Morino K., Hammond L. E., Zhang D., Liu Z. X., Romanelli A. J., Cline G. W., Pongratz R. L., Zhang X. M., Choi C. S., et al. 2005. Prevention of hepatic steatosis and hepatic insulin resistance in mitochondrial acyl-CoA:glycerol-sn-3-phosphate acyltransferase 1 knockout mice. Cell Metab. 2: 55–65. [DOI] [PubMed] [Google Scholar]
- 9.Wendel A. A., Li L. O., Li Y., Cline G. W., Shulman G. I., Coleman R. A. 2010. Glycerol-3-phosphate acyltransferase 1 deficiency in ob/ob mice diminishes hepatic steatosis but does not protect against insulin resistance or obesity. Diabetes. 59: 1321–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams C. A., Grayer R. J. 2004. Anthocyanins and other flavonoids. Nat. Prod. Rep. 21: 539–573. [DOI] [PubMed] [Google Scholar]
- 11.Kong J. M., Chia L. S., Goh N. K., Chia T. F., Brouillard R. 2003. Analysis and biological activities of anthocyanins. Phytochemistry. 64: 923–933. [DOI] [PubMed] [Google Scholar]
- 12.Xia M., Ling W. H., Ma J., Kitts D. D., Zawistowski J. 2003. Supplementation of diets with the black rice pigment fraction attenuates atherosclerotic plaque formation in apolipoprotein e deficient mice. J. Nutr. 133: 744–751. [DOI] [PubMed] [Google Scholar]
- 13.Xia X., Ling W., Ma J., Xia M., Hou M., Wang Q., Zhu H., Tang Z. 2006. An anthocyanin-rich extract from black rice enhances atherosclerotic plaque stabilization in apolipoprotein E-deficient mice. J. Nutr. 136: 2220–2225. [DOI] [PubMed] [Google Scholar]
- 14.Seymour E. M., Singer A. A., Kirakosyan A., Urcuyo-Llanes D. E., Kaufman P. B., Bolling S. F. 2008. Altered hyperlipidemia, hepatic steatosis, and hepatic peroxisome proliferator-activated receptors in rats with intake of tart cherry. J. Med. Food. 11: 252–259. [DOI] [PubMed] [Google Scholar]
- 15.Romanelli A., Martin K. A., Toker A., Blenis J. 1999. P70 S6 kinase is regulated by protein kinase Cζ and participates in a phosphoinositide 3-kinase-regulated signalling complex. Mol. Cell. Biol. 19: 2921–2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park M., Farrell J., Lemmon K., York D. A. 2009. Enterostatin alters protein trafficking to inhibit insulin secretion in Beta-TC6 cells. Peptides. 30: 1866–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoffmann K., Blaudszun J., Brunken C., Höpker W. W., Tauber R., Steinhart H. 2005. New application of a subcellular fractionation method to kidney and testis for the determination of conjugated linoleic acid in selected cell organelles of healthy and cancerous human tissues. Anal. Bioanal. Chem. 381: 1138–1144. [DOI] [PubMed] [Google Scholar]
- 18.Zheng J., Ramirez V. D. 2000. Inhibition of mitochondrial proton F0F1-ATPase/ATP synthase by polyphenolic phytochemicals. Br. J. Pharmacol. 130: 1115–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng J., Ramirez V. D. 1999. Purification and identification of an estrogen binding protein from rat brain: oligomycin sensitivity-conferring protein (OSCP), a subunit of mitochondrial F0F1-ATP synthase/ATPase. J. Steroid Biochem. Mol. Biol. 68: 65–75. [DOI] [PubMed] [Google Scholar]
- 20.Igal R. A., Wang S., Gonzalez-Baró M., Coleman R. A. 2001. Mitochondrial glycerol phosphate acyltransferase directs the incorporation of exogenous fatty acids into triacylglycerol. J. Biol. Chem. 276: 42205–42212. [DOI] [PubMed] [Google Scholar]
- 21.Muoio D. M., Seefeld K., Witters L. A. 1999. AMP-activated kinase reciprocally regulates triacylglycerol synthesis and fatty acid oxidation in liver and muscle: evidence that sn-glycerol-3-phosphate acyltransferase is a novel target. Biochem. J. 338: 783–791. [PMC free article] [PubMed] [Google Scholar]
- 22.Park S. G., Kang Y. S., Kim J. Y., Lee C. S., Ko Y. G., Lee W. J., Lee K. U., Yeom Y. I., Kim S. 2006. Hormonal activity of AIMP1/p43 for glucose homeostasis. Proc. Natl. Acad. Sci. USA. 103: 14913–14918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagle C. A., An J., Shiota M. 2007. Hepatic overexpression of glycerol-sn-3-phosphate acyltransferase 1 in rats causes insulin resistance. J. Biol. Chem. 282: 14807–14815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim H. S., Xiao C., Wang R. H., Lahusen T., Xu X., Vassilopoulos A., Vazquez-Ortiz G., Jeong W. I., Park O., Ki S. H., et al. 2010. Hepatic-specific disruption of SIRT6 in mice results in fatty liver formation due to enhanced glycolysis and triglyceride synthesis. Cell Metab. 12: 224–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qin X., Xie X., Fan Y., Tian J., Guan Y., Wang X., Zhu Y., Wang N. 2008. Peroxisome proliferator-activated receptor-delta induces insulin-induced gene-1 and suppresses hepatic lipogenesis in obese diabetic mice. Hepatology. 48: 432–441. [DOI] [PubMed] [Google Scholar]
- 26.Tietge U. J., Bakillah A., Maugeais C., Tsukamoto K., Hussain M., Rader D. J. 1999. Hepatic overexpression of microsomal triglyceride transfer protein (MTP) results in increased in vivo secretion of VLDL triglycerides and apolipoprotein B. J. Lipid Res. 40: 2134–2139. [PubMed] [Google Scholar]
- 27.Pellon-Maison M., Montanaro M. A., Coleman R. A., Gonzalez-Baró M. R. 2007. Mitochondrial glycerol-3-P acyltransferase 1 is most active in outer mitochondrial membrane but not in mitochondrial associated vesicles (MAV). Biochim. Biophys. Acta. 1771: 830–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boyer P. D. 1997. The ATP synthase–a splendid molecular machine. Annu. Rev. Biochem. 66: 717–749. [DOI] [PubMed] [Google Scholar]
- 29.Menz R. I., Walker J. E., Leslie A. G. R. 2001. Structure of bovine mitochondrial F(1)-ATPase with nucleotide bound to all three catalytic sites: implications for the mechanism of rotary catalysis. Cell. 106: 331–341. [DOI] [PubMed] [Google Scholar]
- 30.Cabezón E., Arechaga I., Jonathan P., Butler G., Walker J. E. 2000. Dimerization of bovine F1-ATPase by binding the inhibitor protein, IF1. J. Biol. Chem. 275: 28353–28355. [DOI] [PubMed] [Google Scholar]
- 31.Dempsey E. C., Newton A. C., Mochly-Rosen D., Fields A. P., Reyland M. E., Insel P. A., Messing R. O. 2000. Protein kinase C isozymes and the regulation of diverse cell responses. Am. J. Physiol. Lung Cell. Mol. Physiol. 279: L429–L438. [DOI] [PubMed] [Google Scholar]
- 32.Sajan M. P., Standaert M. L., Nimal S., Varanasi U., Pastoor T., Mastorides S., Braun U., Leitges M., Farese R. V. 2009. The critical role of atypical protein kinase C in activating hepatic SREBP-1c and NFkappaB in obesity. J. Lipid Res. 50: 1133–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brennan A. M., Suh S. W., Won S. J., Narasimhan P., Kauppinen T. M., Lee H., Edling Y., Chan P. H., Swanson R. A. 2009. NADPH oxidase is the primary source of superoxide induced by NMDA receptor activation. Nat. Neurosci. 12: 857–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xie Z., Dong Y., Scholz R., Neumann D., Zou M. H. 2008. Phosphorylation of LKB1 at serine 428 by protein kinase C-ζ is required for metformin-enhanced activation of the AMP-activated protein kinase in endothelial cells. Circulation. 117: 952–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Geraldes P., King G. L. 2010. Activation of protein kinase C isoforms and its impact on diabetic complications. Circ. Res. 106: 1319–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu X., Godwin M. L., Nowak G. 2004. Protein kinase C-alpha inhibits the repair of oxidative phosphorylation after S-(1,2-dichlorovinyl)-L-cysteine injury in renal cells. Am. J. Physiol. Renal Physiol. 287: F64–F73. [DOI] [PubMed] [Google Scholar]
- 37.Xia M., Hou M., Zhu H., Ma J., Tang Z., Wang Q., Li Y., Chi D., Yu X., Zhao T., et al. 2005. Anthocyanins induce cholesterol efflux from mouse peritoneal macrophages: the role of the peroxisome proligerator-activated receptor γ-liver X receptor α-ABCA1 pathway. J. Biol. Chem. 280: 36792–36801. [DOI] [PubMed] [Google Scholar]
- 38.Pedersen P. L., Amzel L. M. 1993. TP synthases. Structure, reaction center, mechanism, and regulation of one of nature's most unique machines. J. Biol. Chem. 268: 9937–9940. [PubMed] [Google Scholar]
- 39.Lin C. L., Huang H. C., Lin J. K. 2007. Theaflavins attenuate hepatic lipid accumulation through activating AMPK in human HepG2 cells. J. Lipid Res. 48: 2334–2343. [DOI] [PubMed] [Google Scholar]
- 40.Brusq J. M., Ancellin N., Grondin P., Guillard R., Martin S., Saintillan Y., Issandou M. 2006. Inhibition of lipid synthesis through activation of AMP kinase: an additional mechanism for the hypolipidemic effects of berberine. J. Lipid Res. 47: 1281–1288. [DOI] [PubMed] [Google Scholar]
- 41.Farese R. V., Sajan M. P. 2010. Metabolic functions of atypical protein kinase C: “good” and “bad” as defined by nutritional status. Am. J. Physiol. Endocrinol. Metab. 298: E385–E394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roffey J., Rosse C., Linch M. 2009. Protein kinase C intervention: the state of play. Curr. Opin. Cell Biol. 21: 268–279. [DOI] [PubMed] [Google Scholar]
- 43.Steinberg S. F. 2008. Structural basis of protein kinase C isoform function. Physiol. Rev. 88: 1341–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang F. X., Pan W., Hutchins J. B. 1995. Phosphorylation of F1F0 ATPase delta-subunit is regulated by platelet-derived growth factor in mouse cortical neurons in vitro. J. Neurochem. 65: 2812–2815. [DOI] [PubMed] [Google Scholar]
- 45.Furukawa S., Fujita T., Shimabukuro M., Iwaki M., Yamada Y., Nakajima Y., Nakayama O., Makishima M., Matsuda M., Shimomura I. 2004. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Invest. 114: 1752–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.