Abstract
11β-hydroxysteroid dehydrogenase 1 (11β-HSD1) converts inactive 11-keto derivatives to active glucocorticoids within tissues and may play a role in the metabolic syndrome (MS). We used an antisense oligonucleotide (ASO) to knock down 11β-HSD1 in livers of C57BL/6J mice consuming a Western-type diet (WTD). 11β-HSD1 ASO-treated mice consumed less food, so we compared them to ad libitum-fed mice and to food-matched mice receiving control ASO. Knockdown of 11β-HSD1 directly protected mice from WTD-induced steatosis and dyslipidemia by reducing synthesis and secretion of triglyceride (TG) and increasing hepatic fatty acid oxidation. These changes in hepatic and plasma lipids were not associated with reductions in genes involved in de novo lipogenesis. However, protein levels of both sterol regulatory element-binding protein (SREBP) 1 and fatty acid synthase were significantly reduced in mice treated with 11β-HSD1 ASO. There was no change in hepatic secretion of apolipoprotein (apo)B, indicating assembly and secretion of smaller apoB-containing lipoproteins by the liver in the 11β-HSD1-treated mice. Our results indicate that inhibition of 11β-HSD1 by ASO treatment of WTD-fed mice resulted in improved plasma and hepatic lipid levels, reduced lipogenesis by posttranslational regulation, and secretion of similar numbers of apoB-containing lipoproteins containing less TG per particle.
Keywords: fatty acid/oxidation, fatty acid/synthesis, lipoproteins/metabolism, apolipoproteins, liver, steroid hormones, triglycerides
The phenotypic similarities between individuals with the metabolic syndrome (MS) and individuals with Cushing's syndrome have stimulated considerable interest in the role of endogenous glucocorticoids in the pathogenesis of MS (1). Insulin resistance appears to play a central role in the pathophysiology of MS, and glucocorticoids have been shown to induce insulin resistance in multiple tissues (2–4). However, whereas plasma concentrations of glucocorticoids are elevated in Cushing's syndrome, circulating glucocorticoid levels are normal in patients with the MS (3, 4). Glucocorticoid action on target tissues depends not only on circulating hormone levels, but also on intracellular glucocorticoid receptors and the activities of both 11β-hydroxysteroid dehydrogenase (11β-HSD) 1 and 2 (5–9). 11β-HSD1 is an NADP+/NADPH-dependent oxidoreductase that reversibly interconverts inactive cortisone and 11-dehydrocorticosterone in humans and rodents, respectively, to active cortisol and corticosterone. In liver and adipose tissue, the direction of the reaction is from inactive to active glucocorticoids (3, 4, 7). 11β-HSD1 is widely expressed, most notably in liver, adipose, vasculature, brain, and macrophages (3, 4, 7, 10), where it increases intracellular cortisol levels. 11β-HSD1 does not participate in adrenal cortisol biosynthesis from cholesterol.
Genetic manipulations that either deleted 11β-HSD1 in the whole animal (6, 11, 12) or resulted in overexpression in either adipose tissue (5) or liver (13) indicated significant roles for this enzyme in the regulation of body weight, energy metabolism, hepatic glucose and lipid metabolism, and blood pressure. Based on those studies, as well as the demonstration of increased 11β-HSD1 activity in adipose tissue of obese rodents and humans (3, 4, 14, 15), inhibition of 11β-HSD1 has become a major therapeutic target for MS, type 2 diabetes mellitus (T2DM), and hypertension (6, 16–20). However, results of many of these studies have been difficult to interpret because systemic or adipose-specific inhibition of 11β-HSD1 activity appears to alter food intake. Specifically, on a C57BL/6J background, knockout of 11β-HSD1 expression increased food intake (12), whereas adipose-specific deactivation of 11β-HSD1 by overexpression of 11β-HSD2 decreased food intake (21).
In view of the expression pattern of 11β-HSD1 (3) and the previously described tissue distribution of other intraperitoneally administered ASO (22, 23), we hypothesized that we would see relatively specific inhibition of both hepatic and adipose tissue 11β-HSD1 by the cognate ASO. Importantly, we conducted the present studies, which focus on the effects of ASO-mediated inhibition of 11β-HSD1 on hepatic metabolism, with both ad libitum-fed and pair-fed mice receiving control ASO. This enabled us to differentiate direct effects of reduced 11β-HSD1 activity on lipid and carbohydrate metabolism from effects secondary to decreased food intake and concomitant differences in body weight. We present here results demonstrating that inhibition of 11β-HSD1 results in protection from a Western-type diet (WTD)-induced hepatic steatosis by directly reducing hepatic de novo lipogenesis and increasing hepatic β-oxidation.
MATERIALS AND METHODS
Animals, diets, and ASO
We studied male C57BL/6J wide-type mice purchased from The Jackson Laboratory (Bar Harbor, ME). All mice were maintained on a 12 h light/12 h dark cycle (light cycle was 7 AM to 7 PM) and fed a WTD TD.88137 (Harlan Laboratories) composed, by calories, of 42% fat (anhydrous milk fat), 43% carbohydrate, 15% protein with 0.2% cholesterol by weight. This diet has been used by many groups studying lipoprotein metabolism in mice (24). In our first set of experiments, we observed reduced food intake in 11β-HSD1 ASO-treated mice compared with control ASO-treated mice. Therefore, we added a food-matched control (FMC) ASO group. Mice in both control and 11B-HSD1 ASO-treated groups were fed ad libitum for 12 weeks, after which the control group received an in-house universal control ASO and the 11B-HSD1 ASO-treated group received an ASO to 11B-HSD1. The FMC group started receiving control ASO treatment one week later, as this group was delayed one week to match food consumption to the 11β-HSD1 ASO-treated group. Each ASO was injected for 12 weeks at a dose of 50 mg/kg of body weight intraperitoneally twice a week. During the last four weeks of treatment, we determined rates of TG and apolipoprotein (apo)B secretion in vivo and rates of lipogenesis, β-oxidation in primary hepatocytes, etc. Animal studies were approved by the Institutional Animal Care and Use Committee of Columbia University College of Physicians and Surgeons.
ASO to 11β-HSD1 (ISIS 146039) has the following sequence: 5′TGTTGCAAGAATTTCTCATG3′. The in-house universal control ASO (ISIS 141923) has the sequence: 5′CCTTCCCTGAAGGTTCCTCC3′.
Body weight and food intake
Body weights were recorded once a week. Mice were housed individually, and daily food intake was calculated as the difference between the food remaining and original food provided, divided by seven days. For the food-matched control ASO treatment group, food was provided every afternoon at 5 PM.
Blood metabolites and hormones
Blood for various plasma metabolites and hormones was obtained around 1 PM after a 4-6 h fast. Because limited food was provided to FMC mice, they likely ate all their food rapidly as soon as it was available; therefore, the fasting period of FMC mice was almost certainly longer than that of ad libitum control and 11β-HSD1 ASO-treated mice. Plasma total cholesterol (TC), triglyceride (TG) and free fatty acid (FFA) concentrations were measured enzymatically (Wako Pure Chemicals). Corticosterone concentrations were determined by radioimmunoassay (MP Biomedicals, Inc.).
Determination of hepatic VLDL TG and apoB secretion rates
TG and apoB were determined as described previously (25). Briefly, 4 h-fasted mice were injected intravenously with a mixture of 200 mCi of [35S]methionine (1175 Ci/mmol, PerkinElmer Life Sciences) and 500 mg/kg Triton WR1339 (Sigma-Aldrich) in 0.9% sodium chloride. They were bled prior to injection and at 30, 60, 90, and 120 min. Because plasma VLDL clearance is completely inhibited in mice under these conditions, the accumulation of plasma TG and apolipoproteins in plasma after injection of Triton can be used to estimate rates of secretion of VLDL into the plasma compartment. Under the experimental conditions, secretion is linear for at least 2 h, and we used the difference between plasma TG at the 120 and 0 min time points as indicative of the rate at which TG was secreted from the liver. ApoB secretion is estimated from the accumulation of [35S]methionine-labeled apoB in a plasma sample from the 120 min time point. ApoB100 and apoB48 were isolated by 4% SDS-PAGE, and the appearance of newly secreted, labeled proteins was estimated by autoradiography and scintillation counting of each band.
Hepatic lipids determination
Mice were fasted 6-7 h, and then sacrificed. Liver tissues were isolated, snap-frozen in liquid nitrogen, and maintained at −80°C. These tissues were analyzed for hepatic lipids determination, quantitative real-time PCR, and immunoblots. Total liver lipids were extracted by a modification of the method of Folch and Lees (26).
Quantitative real-time PCR
Total RNA samples were used for cDNA synthesis with oligo-dT primers with a commercial kit from Invitrogen. Quantitative real-time PCR (QPCR) was done using SYBR Green PCR Master Mix (Agilent Technology) in triplicate using the Mx3005p Multiplex Quantitative PCR system (Stratagene). Expression of each target gene was quantified by transformation against a standard curve and normalized to β-ACTIN expression. The primers used in the QPCR are shown in Table 1.
TABLE 1.
Primer sequences of genes used for QPCR
| Gene | Forward (5′→3′) | Reverse (5′→3′) |
|---|---|---|
| 11β-HSD1 | AGTACACCTCGCTTTTGCGT | CTCTCTGTGTCCTTGGCCTC |
| BETA-ACTIN | GTATCCATGAAATAAGTGGTTACAGG | GCAGTACATAATTTACACAGAAGCA |
| SREBP1C | GGCACTAAGTGCCCTCAACCT | GCCACATAGATCTCTGCCAGTGT |
| FAS | CCTGGATAGCATTCCGAACCT | AGCACATCTGCAAGGCTACACA |
| SREBP2 | CAGGGAACTCTCCCACTTGA | GAGACCATGGAGACCCTCAC |
| ABCA1 | TCCGGATTCTTTGTCAGCTT | AGCCGTAGATGGACAGGATG |
| ABCG5 | GGCTGCTTATTGGATCTGGA | TTGGATCCACCACAAGTGAA |
| ABCG8 | CACCTTCCACATGTCCTCCT | GCAGGCACTATCCACAGGTT |
| HMG-CoA reductase | CCAGCTTGTGGTAGCTTTCAG | GTGGGAACTCTGGTTCTTTCC |
| PCSK9 | ATGGACTCTTGCCACACACA | AGGTCCTTCAGAGCAGGTCA |
| LDLr | GCAAGGACAAGTCAGATGAGG | CATTGACGCAGCCGAGCTCG |
| APOA1 | ACCCACCTGAAGACACTTGG | GGCCTTGTCGATCACACTCT |
| LXRα | GCTCTGCTCATTGCCATCAG | TGTTGCAGCCTCTCTACTTGGA |
| ACC1 | GGAGGACCGCATTTATCGA | TGACCAGATCAGAGTGCCT |
| DGAT1 | GTGCACAAGTGGTGCATCAG | CAGTGGGATCTGAGCCATC |
| DGAT2 | AGTGGCAATGCTATCATCATCGT | AAGGAATAAGTGGGAACCAGATCA |
| SCD1 | TGCGATACACTCTGGTGCTC | AGGATATTCTCCCGGGATTG |
| PPARα | AGGGTTGAGCTCAGTCAGGA | GGTCACCTACGAGTGGCATT |
| CPT1α | CATGTCAAGCCAGACGAAGA | TGGTAGGAGAGCAGCACCTT |
| ACOX1 | CTTGGATGGTAGTCCGGAGA | TGGCTTCGAGTGAGGAAGTT |
| HMC-CoA synthase 2 | TGAATCCTGGGTGTCTCTCC | CTGTGGGGAAAGATCTGCAT |
| MCAD | GAGCCCGGATTAGGGTTTAG | TCCCCGCTTTTGTCATATTC |
| L-PBE | CTACCTGAGGAGGCTGGTTG | CCATACATGGCAAAATGCAG |
| PPARγ2 | AACTCTGGGAGATTCTCCTGTTGA | TGGTAATTTCTTGTGAAGTGCTCATA |
| ADRP | AGCATCGGCTACGACGACACC | CAGCATTGCGGAATACGGAG |
| CIDEa | CTCGGCTGTCTCAATGTCAA | CAGGAACTGTCCCGTCATCT |
| CIDEb | CCAAAGCAACAGGGAGAGAG | GCTGAGTTCCAGACCCTACG |
| FSP27 | CAGAAGCCAACTAAGAAGATCG | TGTAGCAGTGCAGGTCATAG |
| PGC1α | GAGTCTGAAAGGGCCAAACA | ACGGTGCATTCCTCAATTTC |
| PEPCK | ATCTTTGGTGGCCGTAGACCT | GCCAGTGGGCCAGGTATTT |
| G6Pase | TCCTCTTTCCCATCTGGTTC | TATACACCTGCTGCGCCCAT |
| ChREBP | GTCCGATATCTCCGACACACTCTT | CATTGCCAACATAAGCATCTTCTG |
| CREB | ATGTTCCCTGGTTGCTTGTC | GGTTGCTCTGCAGAAAGGAG |
| GR | CCTCTCTGTCGGGGTAGCAC | ACAGACTTTCGGCTTCTGGA |
| HES1 | AGCCACTGGAAGGTGACACT | GCCAATTTGCCTTTCTCATC |
Immunoblots
Protein extracts were prepared by using T-per tissue protein extraction buffer (Thermo Scientific) containing a complete, EDTA-free protein inhibitor cocktail tablet (Roche Diagnostics Corp.). The primary antibodies used were anti-11β-HSD1 (Cayman Chemical), anti-SREBP1 (provided by Jay D. Horton), anti-FAS and anti-MTP (BD Biosciences Pharmingen), anti-apoAI (Calbiochem), and monoclonal anti-β-ACTIN (Sigma-Aldrich). Densitometry was carried out using Image J software from the US National Institutes of Health (27).
Measurement of hepatic de novo lipogenesis
The rate of hepatic de novo lipogenesis was determined by measuring the amount of newly synthesized FA present in the liver 1 h after intraperitoneal injection of 3H2O 1 mCi into 4 h-fasted mice as previously described (28). 3H-Labeled fatty acids were isolated by saponification of liver samples in KOH. After extraction of nonsaponifiable lipids and acidification with H2SO4, the 3H-labeled fatty acids were extracted and separated by thin layer chromatography. The plate was stained with iodine; the FA “spot” was scraped off the plate, and the isolated FA was added to scintillation fluid (National Diagnostics) and counted in a liquid scintillation counter (LS6500 Beckman Coulter). The specific activity of body water was determined and used to calculate de novo lipogenesis as micromoles of 3H2O incorporated into FA/h/g liver protein.
Beta-oxidation in primary hepatocytes
Beta-oxidation in primary hepatocytes was measured as previously described (29) with modifications. Briefly, mice were fasted for 4 h before livers were perfused through the vena cava with perfusion buffer (oxygenated Hanks’ balanced saline solution, buffered with 10 mM Hepes), pH 7.4, at 37°C for 5 min. Then the perfusion buffer was changed to isolation buffer containing 30 mg of collagenase (Type I, 245 units/mg, Worthington) and 5 mm Ca2+, pH 7.6, at 37°C for 18 min. 5.0 × 105 hepatocytes were plated into a 25 ml flask with a center well in 2 ml DMEM containing 0.1 mM oleic acid (OA), 1.5% BSA, and [14C]OA (0.5 µCi/ml) (NEC317, PerkinElmer Life Sciences). The same cell amount was saved in −80°C for protein concentration. The flasks were sealed with a stopper and rocked for 2 h at 37°C with 5% CO2. Prior studies in our laboratory showed that CO2 secretion was linear for the first 2 h (data not shown). After 2 h, 200 µl of 70% perchloric acid was injected into the bottom of the flask, 200µL of 1M KOH was injected onto filter paper, and the flasks were incubated for an additional 1 h at 37°C. Then the saturated filter paper containing trapped [14C]CO2 was put into vials with 5 ml liquid scintillation and assessed for radioactivity in a liquid scintillation counter. The media was spun in a microcentrifuge tube for 2 min at 12,000 g, and 200 µl of supernatant was counted to assess the amount of 14C-labeled acid-soluble metabolites (ASM), which represent labeled ketone bodies.
Fast performance liquid chromatography analysis of lipoproteins
An amount 4 h-fasted plasma samples from 10 mice each group (500 µl total) was pooled and subjected to FPLC analysis using two Superose 6 columns in series (GE Healthcare Bio-Sciences Corp.). A total of 55 0.5-ml fractions were collected. Cholesterol determinations of each fraction were made using commercial kits (Wako Pure Chemicals) adapted to 96-well microtiter plates.
Statistics
Values are expressed as mean ± SEM. The significance of the differences in mean values among three different treatment groups was evaluated by general linear model with treatment as the model factor and day as a covariate because the FMC group received its ASO treatment one week later followed by post hoc analysis with Bonferroni's test. P < 0.05 was considered statistically significant.
RESULTS
11β-HSD1 ASO decreased hepatic 11β-HSD1 expression, food intake, and body weight
All mice received a WTD composed, by calories, of 42% fat (anhydrous milk fat), 43% carbohydrate, and 15% protein with 0.2% cholesterol by weight (24). After 12 weeks on the WTD, mice were treated intraperitoneally with either control ASO or 11β-HSD1 ASO at 50 mg/kg body weight twice weekly for 8 weeks while continuing on the WTD. Compared with control ASO, intraperitoneal treatment with 11β-HSD1 ASO resulted in dramatic reductions of mRNA and protein levels of the enzyme in the liver (supplemental Fig. IA, B). 11β-HSD1 ASO also reduced average food consumption by about 12% (supplemental Fig. IC) and average body weight by about 11% (supplemental Fig. ID). The effects of 11β-HSD1 treatment on fasting plasma parameters are presented in supplemental Table I. 11β-HSD1 ASO treatment did not change circulating corticosterone levels, but it decreased fasting concentrations of TC and TG. There was no change in fasting plasma FFA concentrations.
11β-HSD1 ASO-protected mice from WTD-induced hepatic steatosis by diminishing lipogenesis and increasing fatty acid oxidation
Effects of 11β-HSD1 treatment on hepatic lipid metabolism are depicted in supplemental Fig. IIA-G. Compared with control ASO-treated mice, ASO-mediated knockdown of 11β-HSD1 resulted in lower TG content (supplemental Fig. IIA) without changes in hepatic TC mass (supplemental Fig. IIB) and lower rates of hepatic TG secretion (supplemental Fig. IIC) without changes in secretion of either apolipoprotein (apo)B48 or apoB100 (supplemental Fig. IID). Reductions in 11β-HSD1 were associated with reduced hepatic de novo lipogenesis, as determined indirectly by demonstration of lower sterol regulatory element-binding protein (SREBP) 1 (supplemental Fig. IIE) and FAS protein (supplemental Fig. IIF), despite the absence of change in either SREBP1C or FAS mRNA levels (supplemental Fig. IIG). Microsomal triglyceride transfer protein (MTP) mRNA, APOB mRNA, and MTP protein levels in the liver were not affected by 11β-HSD1 ASO treatment (data not shown).
Decreased food intake and/or weight loss accounted for reductions in fasting plasma TG but not plasma TC in 11β-HSD1 ASO-treated mice
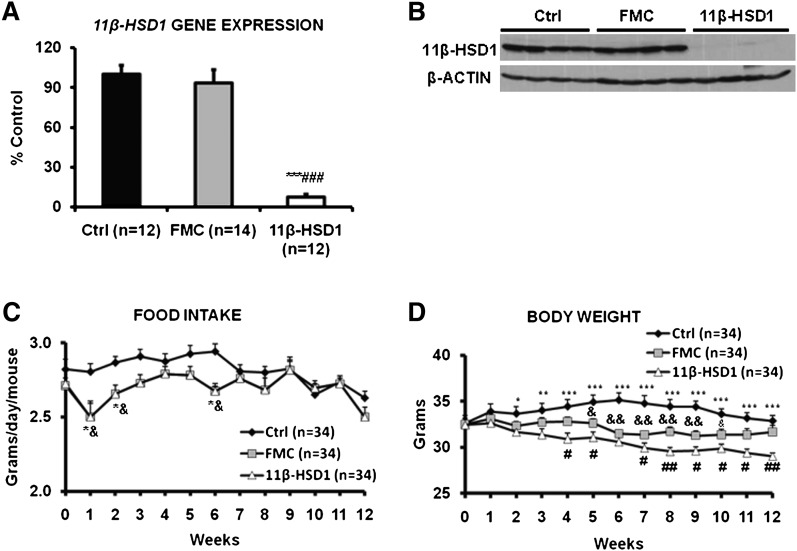
Because we observed significant reductions in food intake and weight over the 8 weeks of 11β-HSD1 ASO treatment in preliminary studies, we carried out all of the experiments with two control ASO groups, one that was fed ad libitum (hereafter referred to as the control ASO group) and one that was pair-fed (hereafter referred to as the food-matched control or FMC ASO group) to match the food intake of the 11β-HSD1 ASO-treated group. The ASO treatment period was from 8-12 weeks, depending on the specific studies performed. As in the first series of experiments, intraperitoneal treatment with 11β-HSD1 ASO decreased hepatic 11β-HSD1 mRNA by approximately 90% (Fig. 1A) and 11β-HSD1 protein by almost 100% (Fig. 1B) compared with either the control ASO or the FMC ASO group. Food restriction alone had no effect on either hepatic 11β-HSD1 mRNA expression or protein levels. 11β-HSD1 ASO treatment also reduced mRNA levels of the enzyme in some, but not all, adipose tissue depots and in lung, heart, and kidney. Brain and muscle expression of 11β-HSD1 were not affected by ASO treatment. Liver had by far the greatest expression of 11β-HSD1 mRNA and the largest percent suppression (supplemental Fig. III). We will present the effects of 11β-HSD1 ASO on adipose tissue in a separate report.
Fig. 1.
11β-HSD1 ASO treatment decreased hepatic 11β-HSD1 transcript and protein, and it reduced food intake and body weight. Food-matched mice receiving control ASO had the exact same food intake but higher body weight than the 11β-HSD1 ASO-treated group. A: The level of 11β-HSD1 mRNA, measured by QPCR with mouse β-ACTIN as an internal control, in liver after 12 weeks of treatment with a control ASO (in both control and FMC mice) or an ASO against 11β-HSD1. B: 11β-HSD1 protein levels determined by immunoblotting with mouse β-ACTIN as an internal control. The average values of densitometry scans from control ASO-treated mice were set as 100%. A representative Western blot is depicted. C, D: Food intake and body weight were determined during 12 weeks of ASO treatment. Food consumption was restricted in the FMC group according to the food consumption of 11β-HSD1 ASO-treated mice during the preceding week. Data are presented as means ± SEM (n = 12-34 per group). *P < 0.05, **P < 0.01, ***P < 0.001, 11β-HSD1 versus control; #P < 0.05, ##P < 0.01, ###P < 0.001, 11β-HSD1 versus FMC; &P < 0.05, &&P < 0.01, FMC versus control.
Compared with control ASO, 11β-HSD1 ASO treatment was associated with about 5% reduction in food consumption and about 10% decrease in body weight. In the FMC ASO group, although food intake (Fig. 1C) was matched to the 11β-HSD1 ASO-treated group, body weight decreased by only 5.5% (Fig. 1D). Weights of mice in the 11β-HSD1 ASO-treated group were significantly lower than weights of mice in the FMC ASO group. Notably limited food supply caused lower body weight of FMC than of control ASO. Levels of alanine transaminase measured after 8-12 weeks of ASO treatment were in the normal range (30-40 IU/l) in all three groups, and there were no differences among the groups (data not shown).
Plasma corticosterone levels were similar in the control ASO and 11β-HSD1 ASO groups, but they were significantly elevated in FMC ASO mice, a sign of stress that has been observed before in FMC mice (19) (Table 2). In accordance with the data on ad libitum-fed animals (supplemental Table I) compared with the control ASO group, 11β-HSD1 ASO treatment significantly decreased fasting TC (47%) and tended to reduce TG levels (22%). There was no effect of 11β-HSD1 treatment on fasting plasma FFA concentration. However, when compared with FMC, 11β-HSD1 ASO treatment had no significant effect on fasting levels of TG, whereas reduced TC concentration was still evident.
TABLE 2.
Fasting plasma lipid and hormone levels after 8-12 weeks ASO treatment
| Control | FMC | 11β-HSD1 | |
|---|---|---|---|
| Corticosterone (ng/ml) | 120.5 ± 7.0 | 156.4 ± 8.5bd | 102.8 ± 5.7 |
| Total cholesterol (mg/dl) | 209.2 ± 6.0 | 194.4 ± 7.9 | 111.5 ± 8.4ac |
| Triglyceride (mg/dl) | 33.4 ± 3.1 | 26.3 ± 1.8 | 26.1 ± 2.5 |
| Free fatty acid (mM) | 0.503 ± 0.033 | 0.485 ± 0.030 | 0.506 ± 0.038 |
Data are expressed as means ± SEM (n = 12-14).
P < 0.001, 11β-HSD1 versus control.
P < 0.05, 11β-HSD1 versus FMC.
P < 0.001, 11β-HSD1 versus FMC.
P < 0.05, control versus FMC.
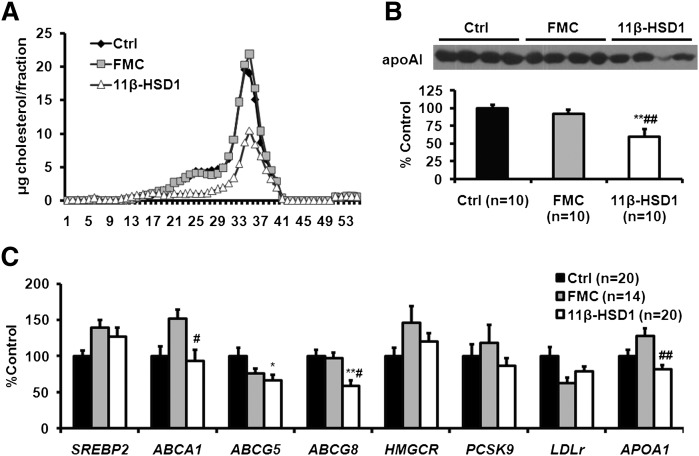
11β-HSD1 ASO treatment had direct effects on plasma lipoproteins
To determine the basis for the reduction in TC in 11β-HSD1 ASO-treated mice, we performed FPLC separation of plasma lipoproteins. FPLC results indicated that LDL and HDL cholesterol levels were decreased in 11β-HSD1 ASO-treated mice (Fig. 2A). Concomitant with lower HDL cholesterol levels, we found that apoAI concentrations in plasma were also reduced in 11β-HSD1 ASO-treated mice (Fig. 2B).
Fig. 2.
11β-HSD1 ASO treatment decreased fasting plasma levels of LDL cholesterol, HDL cholesterol, and plasma apoAI. Expression of apoAI and several ABC transporters was also reduced. A: Plasma samples from 10 mice in each treatment group were pooled for FPLC separation of lipoproteins using two Superose 6 columns in series. B: Plasma apoAI was determined by immunoblotting with mouse β-ACTIN as an internal control. A representative Western blot is shown. C: Genes related to cholesterol metabolism were determined using QPCR and normalized by β-ACTIN mRNA. Data are presented as means ± SEM (n = 10-20 per group). *P < 0.05, **P < 0.01, 11β-HSD1 versus control; #P < 0.05, ##P < 0.01, 11β-HSD1 versus FMC.
A survey of the expression of hepatic genes involved in cholesterol, LDL, and HDL metabolism indicated that 11β-HSD1 ASO treatment reduced expression of ABCA1, ABCG5, ABCG8, and APOAI compared with at least one of the two control groups (Fig. 2C). Of note, SREBP2, HMGCR, PCSK9, and LDLR mRNA levels were not affected by 11β-HSD1 ASO treatment. LDLR protein in the liver was actually reduced in mice treated with 11β-HSD1 ASO compared with FMC and control ASO-treated mice (supplemental Fig. IV). Together, these data indicate that although decreased food intake and/or weight loss accounted for the reductions in fasting plasma concentrations of TG, 11β-HSD1 ASO treatment had direct effects on LDL and HDL cholesterol concentrations. The mechanisms underlying these effects are unclear, although a direct effect of 11β-HSD1 ASO treatment on apoAI gene expression may be the basis for the reduction in the HDL cholesterol level.
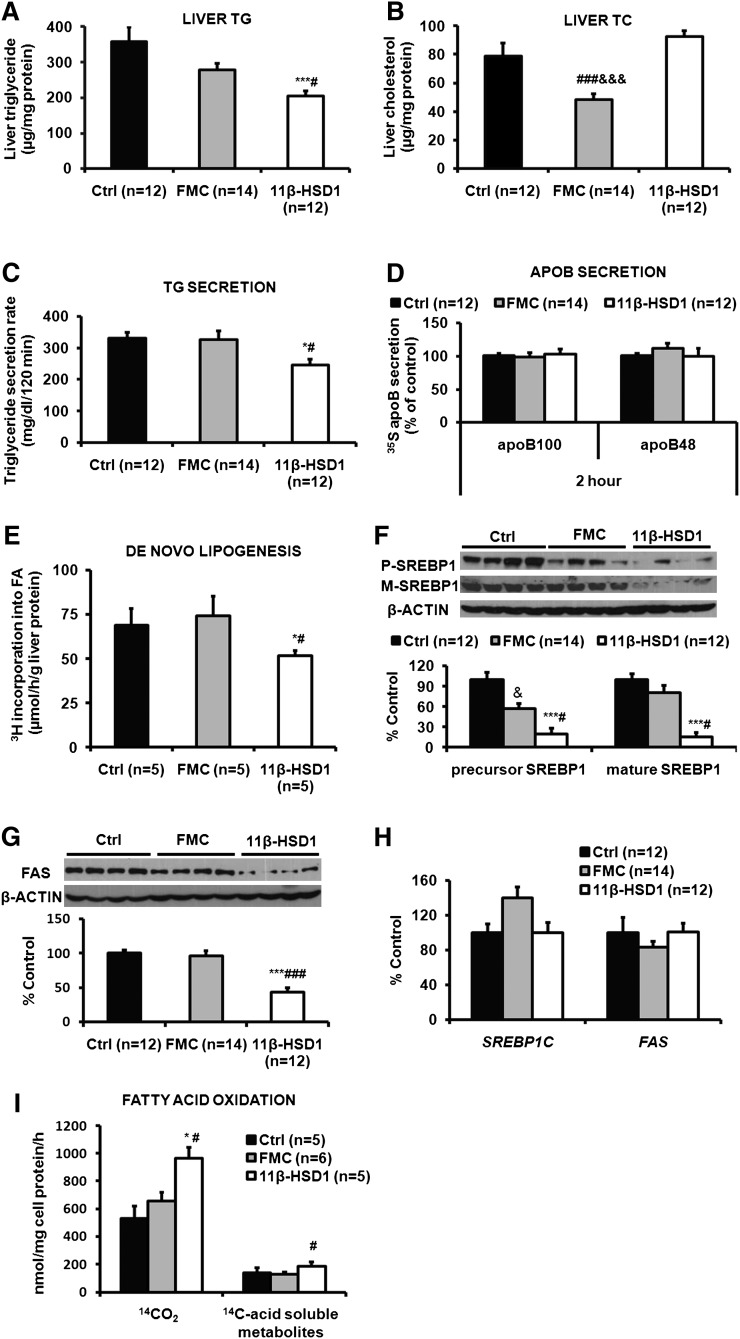
11β-HSD1 ASO treatment directly decreased hepatic TG, TG secretion, and de novo lipogenesis, and it increased hepatic fatty acid oxidation
Compared with control ASO-treated mice and FMC ASO treated mice, ASO-mediated knockdown of 11β-HSD1 resulted in 43% and 30% reductions in hepatic TG content (Fig. 3A). ASO-mediated knockdown of 11β-HSD1 did not affect hepatic cholesterol compared with control ASO-treated mice. The FMC ASO-treated mice did, however, have reduced hepatic cholesterol content (Fig. 3B). There was a 26% decrease in TG secretion in mice with reduced 11β-HSD1 compared with either control group (Fig. 3C), and this occurred without changes in hepatic apoB48 or apoB100 secretion (Fig. 3D). Hepatic de novo lipogenesis, measured in vivo, fell by 25% versus either control group (Fig. 3E). The lower rates of de novo lipogenesis were associated with decreased levels of both the precursor and mature forms of SREBP1 protein (Fig. 3F) and reductions in FAS protein (Fig. 3G), despite no effects of 11β-HSD1 ASO on mRNA levels of SREBP1C and FAS (Fig. 3H). β-oxidation, measured ex vivo in primary hepatocytes isolated from each group of mice, was increased by 81% in the 11β-HSD1 ASO-treated mice compared with control ASO mice and 55% compared with FMC ASO controls (Fig. 3I). Neither MTP nor APOB mRNA levels were altered by 11β-HSD1 ASO treatment compared with controls (data not shown). The same was true for MTP protein levels.
Fig. 3.
11β-HSD1 ASO treatment significantly decreased hepatic TG content, TG secretion, lipogenic proteins, and de novo lipogenesis, and it increased β-oxidation. Treatment with 11β-HSD1 ASO did not alter hepatic TC content, apoB secretion, or expression of lipogenic genes. Total liver TG (A) and TC (B) were measured enzymatically after extraction. TG secretion (C) was determined using Triton WR1339 as described in Materials and Methods. Secretion of newly synthesized apoB (D) was estimated using Triton WR1339 and [35S]methionine. Hepatic de novo lipogenesis (E) was measured in vivo with 3H20. Hepatic levels of the precursor (p-) and mature (m-) forms of SREBP1 protein (F) and FAS protein (G) were determined by immunoblotting and densitometry; the average densitometry values from control ASO-treated mice were set as 100% for each protein. SREBP-1c and FAS gene expression (H) was estimated using QPCR. Beta-oxidation (I) was measured ex vivo in primary hepatocytes using 14C-oleic acid. All data are presented as means ± SEM (n = 5-14 per group). *P < 0.05, ***P < 0.001, 11β-HSD1 versus control; #P < 0.05, ###P < 0.001, 11β-HSD1 versus FMC; &&&P < 0.001, FMC versus control.
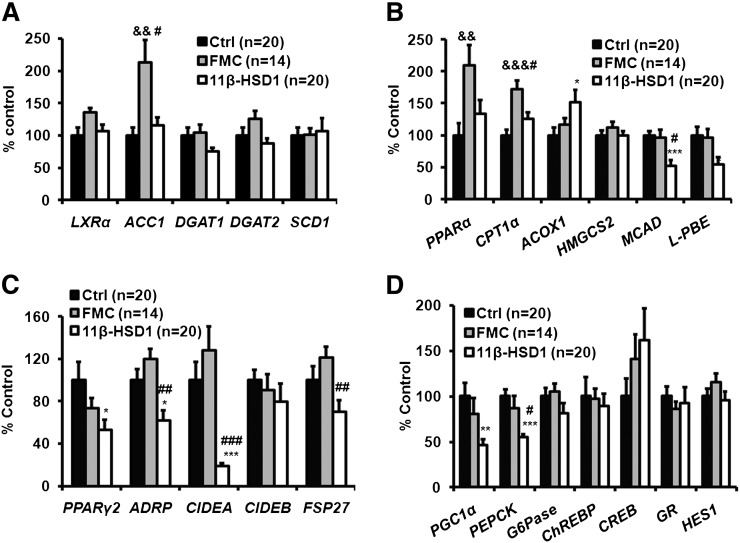
11β-HSD1 ASO treatment had variable effects on hepatic gene expression profile
The expression of most lipogenic genes, including SREBP1C, FAS (Fig. 3H), LXRα, DGAT1, DGAT2, and SCD1 (Fig. 4A), was not altered by 11β-HSD1 ASO treatment. ACC1 expression increased in the FMC ASO group. Genes involved in fatty acid oxidation were not uniformly affected by 11β-HSD1 ASO treatment; ACOX1 expression was increased, but mRNA levels of both MCAD and L-PBE were decreased (Fig. 4B). PPARα and CPT1 levels were increased in the FMC ASO controls. PPARγ2 mRNA levels were reduced in 11β-HSD1 ASO-treated mice, as were expression levels of several of its downstream targets, including ADRP, CIDEA, and FSP27 (Fig. 4C). These changes were not observed in the FMC group. Key gluconeogenic genes PGC1α and PEPCK were significantly decreased, and G6Pase tended to be lower in the 11β-HSD1 ASO-treated mice, but no obvious differences were found in other genes in the gluconeogenic pathway, including ChREBP, CREB, GR, and HES1 (Fig. 4D). The HES1 protein was not increased either (data not shown). Of note, there were no differences in mRNA levels of gluconeogenic genes between the control ASO and the FMC ASO groups.
Fig. 4.
11β-HSD1 ASO treatment had variable effects on hepatic genes involved in TG synthesis and fatty acid oxidation but significantly decreased genes involved in hepatic lipid droplet formation and gluconeogenesis. Expression of genes was determined using QPCR after 12 weeks of ASO treatment and normalized with β-ACTIN mRNA. Lipogenic (A), beta-oxidation (B), lipid droplet formation (C), and gluconeogenesis(D) genes. Expression of each gene in ad libitum-fed control mice was set at 100%. Data are presented as means ± SEM (n = 14-20). *P < 0.05, **P < 0.01, ***P < 0.001, 11β-HSD1 versus control; #P < 0.05, ##P < 0.01, ###P < 0.001, 11β-HSD1 versus FMC; &&P < 0.01, &&&P < 0.001, FMC versus control.
DISCUSSION
Soon after the purification (30) and cloning (31) of 11β-HSD1, a series of papers from Seckl et al. highlighted the therapeutic potential of inhibiting this enzyme in adipose tissue and liver. Beginning with the first, brief report of the total body knockout in 1997 (6), then followed by more detailed examinations of the knockout mouse (11, 12) as well as generation of mice overexpressing 11β-HSD1 in adipose tissue (5) and liver (13), interest has continued to increase. Indeed, oral inhibitors developed by several pharmaceutical companies have demonstrated beneficial effects on glucose (16, 32, 33), lipid metabolism (17), and/or atherosclerosis (18), albeit almost exclusively in rodents.
Although these studies have clearly increased our knowledge in this area, several important issues regarding the role of 11β-HSD1 in energy metabolism remain incompletely characterized. For example, in several rodent species, inhibition of glucocorticoid activation either in the whole body (12, 18, 19) or adipose tissue (21) is associated with weight loss, often with reduced food intake. Of note, increased 11β-HSD1 activity in adipose tissue (5) caused increased body weight and food intake, whereas isolated overexpression of 11β-HSD1 in the liver had no effect on those parameters (13). 11β-HSD1 effects on food intake may occur via hypothalamic signaling (34). The importance of the food intake issue was acknowledged in previous studies (5, 17, 19), but the effects of reduced energy intake and/or decreased body weight has not, however, been consistently addressed in the published studies. Thus, although decreased energy intake and weight loss would be beneficial clinical effects of pharmacologic inhibitors of 11β-HSD1, such effects observed in rodents may not be nearly as dramatic in humans (16), making demonstration of direct effects of 11β-HSD1 inhibition crucial. Additionally, while consistent benefits of 11β-HSD1 inhibition on glucose tolerance and insulin resistance have been reported (albeit usually with concomitant weight loss), effects of enzyme inhibition on hepatic lipid metabolism have been variable and seemingly dependent on the rodent model studied.
In the present study, after identifying an effect of 11β-HSD1 ASO treatment on food intake and weight gain (see supplemental online data), we utilized food-matched ASO controls to allow clear characterization of the effects of 11β-HSD1 inhibition that are independent of food intake. It is important to realize that FMC controls are not, however, perfect controls. In our studies, we found that reduced availability of food in the FMC ASO control mice was associated with increased levels of plasma corticosterone (Table 2), reduced levels of hepatic cholesterol, and increased expression of genes associated with hepatic fatty acid oxidation. Thus, our interpretation of the effects of simultaneous knockdown of hepatic and adipose tissue 11β-HSD1 takes into account the results in both the ASO control and the FMC ASO control mice.
We first examined the role of 11β-HSD1 in the regulation of plasma lipid levels. Dyslipidemia, with increased plasma TG levels, decreased HDL cholesterol levels, and variable concentrations of small, cholesterol-ester-depleted LDL, is a characteristic abnormality of the insulin-resistant state in humans (35, 36). Previous studies of the effects of deletion, knockdown, or inhibition of 11β-HSD1 have reported varying results. In the first studies of lipid metabolism in the 11β-HSD1 knockout mouse, Morton et al. (11) noted that MF1/129 mice had lower ad libitum plasma TG levels and increased HDL cholesterol concentrations on a chow diet; there were no food intake or weight data in that report. In later studies, after the knockout mice had been bred to a C57BL/6J background, these investigators found lower plasma TG levels on a high-fat diet in association with less weight gain (12). HDL cholesterol levels on that same diet were not shown (12). Adipose-specific overexpression of 11β-HSD1 resulted in increased plasma TG levels associated with significantly greater weight gain and food intake (5). On the other hand, hepatic overexpression of 11β-HSD1, which did not affect food intake or weight gain, did not alter plasma TG levels on either low- or high-fat diets (13). In the latter studies, HDL cholesterol was higher in the hepatic 11β-HSD1 transgenic mice on a low-fat diet, but it was lower on the high-fat diet. We found that plasma levels of fasting TG tended to fall in the 11β-HSD1 ASO-treated mice compared with control mice (supplemental Table I), but this appeared to be due completely to reduced food intake (Table 2). Our finding differs from those of Wang et al. (19), who observed lower TG levels in mice treated with a pharmacologic inhibitor of 11β-HSD1, even when compared with TG levels in pair-fed controls. They also differ from the results reported by Berthiaume et al. (17), who demonstrated a robust reduction of plasma TG with a pharmacologic inhibitor that did not affect food intake or weight. We did find that ASO-mediated inhibition of 11β-HSD1 was associated with lower levels of both LDL and HDL cholesterol concentrations independent of caloric intake. Examination of genes relevant to the regulation of LDL cholesterol and HDL metabolism revealed reduced expression of apoA-I and ABCA1; these changes are consistent with known effects of glucocorticoids on expression of these genes (37, 38). Both of these changes could be directly involved in the reduced HDL levels we observed. Of note, short-term administration of glucocorticoids results in increased HDL cholesterol levels in humans (39). In summary, when reduced food intake is accounted for, 11β-HSD1 inhibition does not affect plasma TG levels, but it does reduce plasma cholesterol in both LDL and HDL in C56BL/6J mice on a WTD. HDL cholesterol was reduced mainly due to a decrease in apoA-I synthesis, at least as judged by reduced apoA-I mRNA levels; if a similar decrease occurs in humans, this would limit the benefits of a strategy to lower 11β-HSD1 expression or activity for the treatment of metabolic diseases. We do not, at present, have an explanation for the reduction in LDL cholesterol observed with 11β-HSD1 ASO treatment. Relevant genes, including SREBP2, PCSK9, and the LDL receptor were not altered. LDL receptor protein was unexpectedly decreased. Additional studies of hepatic cholesterol flux to bile or to the circulation via lipoprotein secretion will be required to define mechanisms for this observation. In any event, reduced levels of LDL cholesterol, if demonstrated in humans, would be an additional positive effect of inhibiting 11β-HSD1.
We next characterized the effects of 11β-HSD1 inhibition on hepatic lipid metabolism. High levels of circulating glucocorticoids are associated with fatty liver in humans (40) and mice (41), although the mechanisms linking this association have not been fully characterized. We demonstrated that 11β-HSD1 ASO treatment protected C57BL/6J mice from hepatic steatosis induced by a WTD by decreasing de novo lipogenesis and increasing β-oxidation compared with both control ASO and FMC ASO groups. Of note, we did not observe changes in the expression of key genes involved in either lipogenesis or fatty acid oxidation. We did, however, find that FAS and SREBP1 proteins (both precursor and mature forms) were significantly reduced in 11β-HSD1 ASO-treated mice compared with both control and FMC ASO groups. Expression of SREBP1C and FAS were also not different in 11β-HSD1 knockout mice in ad libitum-fed and 24 h-fasted states studied by Morton et al. (11). However these lipogenic genes (SREBP1C and FAS) were upregulated more in 11β-HSD1 knockout mice than in wild-type mice after a 24 h fast followed by either 4 h or 24 h period of refeeding (11). Morton et al. (11) concluded that this result was due to enhanced insulin sensitivity in the 11β-HSD1 knockout mice. Our results cannot be directly compared with those of Morton et al. because we did not conduct studies using the fasting-refeeding paradigm; we measured liver parameters after a 4-6 h fast. On the other hand, Morton et al. (11) did not measure SREBP-1c or FAS proteins. Although the fasting-refeeding paradigm is not physiologic, we acknowledge that hepatic de novo lipogenesis is very sensitive to ambient nutrient flux and hepatic insulin signaling, and our results might have been different if we had studied the mice while they were eating.
In mice with hepatic overexpression of 11β-HSD1 (13), hepatic TG was increased with a chow diet but tended to be lower than wild-type on a high-fat diet. On chow, overexpression of 11β-HSD1, which did not affect food intake or weight, was associated with increased expression of FAS but not SREBP1C; on the high-fat diet, transgenic expression of 11β-HSD1 was actually associated with lower expression of those genes. Lemke et al. (9) reported reduced steatosis in db/db mice on a chow diet after treatment with an adenovirus carrying shRNA for the glucocorticoid receptor; this did not affect food intake or weight gain. They also could not link reduced hepatic fat to changes in lipogenic gene expression, but they did not measure protein levels of FAS and SREBP1 or lipogenesis itself. Posttranslational regulation of FAS (42) and SREBP1 (43) have been demonstrated under other circumstances; the mechanism for such an effect by glucocorticoids will require further investigation.
We also demonstrated increased fatty acid oxidation measured directly in hepatocytes isolated from mice treated with 11β-HSD1 ASO. We did not, however, find a clear pattern of changes in the expression of genes involved in hepatic fatty acid oxidation; mRNA levels for PPARα and CPT1α were slightly, but not significantly, higher compared with control mice, but they were reduced compared with levels in the FMC ASO controls. Furthermore, although ACOX1 mRNA levels were increased in 11β-HSD1 ASO-treated mice compared with control mice, the expression of the other two fatty acid oxidation-related genes, MCAD and L-PBE, was decreased. Together, our findings support posttranslational regulation of fatty acid oxidation by glucocorticoids. In contrast, when the 11β-HSD1 gene was knocked out (11), hepatic expression of PPARα, CPT-1, and ACO was increased; there were no data on food intake or weight in this study, although it is likely that the knockout mice weighed less than the controls (12). A recent in vivo report demonstrated that pharmacologic inhibition of 11β-HSD1 activity, which did not affect food intake or body weight, significantly increased hepatic fatty acid oxidation; hepatic gene expression was also variable in that study, with no changes in PPARα or CPT-1 mRNA levels but with an increase in some (but not all) of the mitochondrial oxidation genes (44). We cannot fully explain the differences in our findings, but overall, it seems clear that reductions in 11β-HSD1 activity results in increased fatty acid oxidation by the liver.
Lemke et al. (9), utilizing the alternative approach of knockdown of the glucocorticoid receptor in the liver, also observed increases in circulating levels of ketones as well as increased CPT1α and ACAA2 mRNA levels in db/db mice. In addition to reducing the glucocorticoid receptor instead of 11β-HSD1 expression, another major difference between the report by Lemke et al. (9) and our studies is their identification of Hes1 as a key regulator of hepatic steatosis. HES1 mRNA and protein levels were increased in mice with shRNA knockdown of the glucocorticoid receptor, but they were reduced in mice and hepatocytes treated with dexamethasone. Additionally, replenishment of Hes1 by adenoviral overexpression in db/db mice was associated with reductions in steatosis (9). HES1 mRNA and protein levels (data not shown) were not affected by 11β-HSD1 ASO treatment in our study. We cannot explain these different results. In summary, our studies, done with proper controls for food intake, demonstrate significant direct effects of 11β-HSD1 inhibition on hepatic lipogenesis and oxidation that resulted in protection from WTD-induced steatosis in C57BL/6J mice.
We also demonstrated that inhibition of 11β-HSD1 was associated with reductions in TG secretion and unchanged apoB secretion; 11β-HSD1 inhibition resulted, therefore, in the assembly and secretion of smaller VLDL particles. Our findings are concordant with those of Berthiaume et al. (17), who observed significant reductions in liver TG content and TG secretion during treatment of rats with a pharmacologic inhibitor of 11β-HSD1. Importantly, the pharmacologic inhibitor used by Berthiaume et al. did not affect food intake or weight (17). Lemke et al. (9) also demonstrated decreased secretion of TG concomitant with knockdown of the glucocorticoid receptor. Neither of those groups measured apoB secretion. It is well known that livers can secrete VLDL across a range of size and density by varying the quantity of TG secreted with each apoB (45). In particular, increased hepatic TG as a consequence of increased de novo lipogenesis has been linked to secretion of the same number of larger VLDL particles (46); our results suggested that dissociation of TG and apoB secretion also occurred when de novo lipogenesis was reduced.
A limitation of this study is the uptake of ASO not only by the liver but also by adipose tissue (supplemental Fig III). Although 11β-HSD1 expression is much lower in adipose tissue compared with the liver, ASO treatment significantly reduced that expression, compared with FMC mice, in both epididymal and subcutaneous fat depots, and it tended to reduce expression in mesenteric fat. There was no effect of 11β-HSD1 ASO treatment on either mesenteric or brown adipose tissue expression of the enzyme. Furthermore, there was no effect of ASO treatment on 11β-HSD1 in muscle or brain. We did observe changes in adipose tissue metabolism and mass, and this will be the subject of a separate report. On the other hand, plasma levels of free fatty acids did not differ between any of the experimental groups.
In conclusion, this report demonstrates that selective 11β-HSD1 inhibition in the liver improves WTD-induced obesity, dyslipidemia, and steatosis, independent of reduced food intake and weight loss in the WTD model of obesity in C57BL/6J mice. Inhibition of 11β-HSD1 in the liver had direct effects on hepatic de novo lipogenesis, fatty acid oxidation, and VLDL secretion independent of food intake and weight loss. These findings indicate that inhibition of the activation of glucocorticoids by 11β-HSD1 in liver should have beneficial effects in humans, even if food intake and weight are unaffected. The mechanisms underlying the weight loss in 11β-HSD1 ASO-treated mice compared with FMC controls suggests greater energy expenditure in the former group; studies are underway to examine this possibility.
Supplementary Material
Footnotes
Abbreviations:
- 11β-HSD1
- 11β-hydroxysteroid dehydrogenase 1
- ACC
- acetyl CoA carboxylase
- apo
- apolipoprotein
- ASO
- antisense oligonucleotide
- FMC
- food-matched control
- FPLC
- fast performance liquid chromatography
- LDL-C
- LDL cholesterol
- MS
- metabolic syndrome
- QPCR
- quantitative real-time PCR
- SREBP
- sterol regulatory element-binding protein
- T2DM
- type 2 diabetes mellitus
- TC
- total cholesterol
- TG
- triglyceride
- WTD
- Western-type diet
This work was supported by National Institutes of Health Grants R01 HL-73030 (H.N.G.) and R01 HL-55638 (H.N.G.). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health. The antisense used for this work was provided by ISIS Pharmaceuticals, Inc. No other support was provided. R. M. Crooke and M. G. Graham are employees of ISIS Pharmaceuticals, which has other antisense treatments in clinical trials. H. N. Ginsberg has consulted for ISIS during the past five years but not on anything related to 11beta HSD1.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of four figures and one table.
REFERENCES
- 1.Walker B. R., Seckl J. R. 2001. Cortisol metabolism. In International Textbook of Obesity. Bjorntorp P., editor John Wiley & Sons, London: 241–268. [Google Scholar]
- 2.Morgan S. A., Sherlock M., Gathercole L. L., Lavery G. G., Lenaghan C., Bujalska I. J., Laber D., Yu A., Convey G., Mayers R. A. 2009. 11β-hydroxyteroid dehydrogenase type 1 regulates glucocorticoid-induced insulin resistance in skeletal muscle. Diabetes. 58: 2506–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seckl J. R., Morton N. M., Chapman K. E., Walker B. R. 2004. Glucocorticoids and 11β-hydroxysteroid dehydrogenase in adipose tissue. Recent Prog. Horm. Res. 59: 359–393. [DOI] [PubMed] [Google Scholar]
- 4.Morton N. M., Seckl J. R. 2008. 11β-hydroxysteroid dehydrogenase type 1 and obesity. Front. Horm. Res. 36: 146–164. [DOI] [PubMed] [Google Scholar]
- 5.Masuzaki H., Paterson J., Shinyama H., Morton N. M., Mullins J. J., Seckl J. R., Flier J. S. 2001. A transgenic model of visceral obesity and the metabolic syndrome. Science. 294: 2166–2170. [DOI] [PubMed] [Google Scholar]
- 6.Kotelevtsev Y., Holmes M. C., Burchell A., Houston P. M., Schmoll D., Jamieson P. M., Best R., Brown R., Edwards C. R., Seckl J. R., et al. 1997. 11β-hydroxysteroid dehydrogenase type 1 knockout mice show attenuated glucocorticoid inducible responses and resist hyperglycaemia on obesity or stress. Proc. Natl. Acad. Sci. USA. 94: 14924–14929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paterson J. M., Seckl J. R., Mullins J. J. 2005. Genetic manipulation of 11β-hydroxysteroid dehydrogenases in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 289: R642–R652. [DOI] [PubMed] [Google Scholar]
- 8.Kotelevtsev Y., Brown R. W., Fleming S., Kenyon C., Edwards C. R., Seckl J. R., Mullins J. J. 1999. Hypertension in mice lacking 11β-hydroxysteroid dehydrogenase type 2. J. Clin. Invest. 103: 683–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lemke U., Krones-Herzig A., Berriel D. M., Narvekar P., Ziegler A., Vegiopoulos A., Cato A. C., Bohl S., Klingmuller U., Screaton R. A., et al. 2008. The glucocorticoid receptor controls hepatic dyslipidemia through Hes1. Cell Metab. 8: 212–223. [DOI] [PubMed] [Google Scholar]
- 10.Chapman K. E., Coutinho A. E., Gray M., Gilmour J. S., Savill J. S., Seckl J. R. 2009. The role and regulation of 11β-hydroxysteroid dehydrogenase type 1 in the inflammatory response. Mol. Cell. Endocrinol. 301: 123–131. [DOI] [PubMed] [Google Scholar]
- 11.Morton N. M., Holmes M. C., Fievet C., Staels B., Tailleux A., Mullins J. J., Seckl J. R. 2001. Improved lipid and lipoprotein profile, hepatic insulin sensitivity, and glucose tolerance in 11β-hydroxsteroid dehydrogenase type 1 null mice. J. Biol. Chem. 276: 41293–41300. [DOI] [PubMed] [Google Scholar]
- 12.Morton N. M., Paterson J. M., Masuzaki H., Holmes M. C., Staels B., Fievet C., Walker B. R., Flier J. S., Mullins J. J., Seckl J. R. 2004. Novel adipose tissue-mediated resistance to diet-induced visceral obesity in 11β−hydroxysteroid dehydrogenase type 1-deficient mice. Diabetes. 53: 931–938. [DOI] [PubMed] [Google Scholar]
- 13.Paterson J. M., Morton N. M., Fievet C., Kenyon C. J., Holmes M. C., Staels B., Seckl J. R., Mullins J. J. 2004. Metabolic syndrome without obesity: hepatic overexpression of 11β-hydroxysteroid dehydrogenase type 1 in transgenic mice. Proc. Natl. Acad. Sci. USA. 101: 7088–7093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rask E., Olsson T., Soderberg S., Andrew R., Livingstone D. E. W., Johnson O., Walker B. R. 2001. Tissue-specific dysregulation of cortisol metabolism in human obesity. J. Clin. Endocrinol. Metab. 86: 1418–1421. [DOI] [PubMed] [Google Scholar]
- 15.Walker B. R., Andrew R. 2006. Tissue production of cortisol by 11β-hydroxysteroid dehydrogenase type 1 and metabolic disease. Ann. N. Y. Acad. Sci. 1083: 165–184. [DOI] [PubMed] [Google Scholar]
- 16.Rosenstock J., Banarer S., Fonseca V. A., Inzucchi S. E., Sun W., Yao W., Hollis G., Flores R., Levy R., Williams W. V., et al. 2010. The 11 -β-hydroxysteroid dehydrogenase type 1 inhibitor INCB13739 improves hyperglycemia in patients with type 2 diabetes inadequately cotrolled by metformin monotherapy. Diabetes Care. 33: 1516–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berthiaume M., Laplante M., Festuccia W. T., Cianflone K., Turcotte L. P., Joanisse D. R., Olivecrona G., Thieringer R., Deshaies Y. 2007. 11β-HSD1 inhibition improves triglyceridemia through reduced liver VLDL secretion and partitions lipids toward oxidative tissues. Am. J. Physiol. Endocrinol. Metab. 293: E1045–E1052. [DOI] [PubMed] [Google Scholar]
- 18.Hermanowski-Vosatka A., Balkovec J. M., Cheng K., Chen H. Y., Hernandez M., Koo G. C., Grand L. C. B., Li Z., Metzger J. M., Mundt S. S., et al. 2005. 11β-HSD1 inhibition ameliorates metabolic syndrome and prevents progression of atherosclerosis in mice. J. Exp. Med. 202: 517–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang S. J., Birtles S., de Schoolmeester J., Swales J., Moody G., Hislop D., O'Dowd J., Smith D. M., Turnbull A. V., Arch J. R. 2006. Inhibition of 11β-hydroxysteroid dehydrogenasse type 1 reduces food intake and weight gain but maintains energy expenditure in diet-induced obese mice. Diabetologia. 49: 1333–1337. [DOI] [PubMed] [Google Scholar]
- 20.Lloyd D. J., Helmering J., Cordover D., Bowsman M., Chen M., Hale C., Fordstrom P., Zhou M., Wang M., Kaufman S. A. 2009. Antidiabetic effects of 11β-HSD1 inhibition in a mouse model of combined diabetes, dyslipidaemia and atherosclerosis. Diabetes Obes. Metab. 11: 688–699. [DOI] [PubMed] [Google Scholar]
- 21.Kershaw E. E., Morton N. M., Dhillon H., Ramage L., Seckl J. R., Flier J. S. 2005. Adipocyte-specific glucocorticoid inactivation protects against diet-induced obesity. Diabetes. 54: 1023–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koizumi M., Takagi-Sato M., Okuyama R., Araki K., Sun W., Nakai D. 2007. In vivo antisense activity of ENA oligonucleotides targeting PTP1B mRNA in comparison of that of 2’-MOE-modified oligonucleotides. Nucleic Acids Symp. Ser. 51: 111–112. [DOI] [PubMed] [Google Scholar]
- 23.Nagai Y., Yonemitsu S., Erion D. M., Iwasaki T., Stark R., Weismann D., Dong J., Zhang D., Jurczak M. J., Loffler M. G., et al. 2009. The role of peroxisome proliferator-activated receptor gamma coactivator-1 beta in the pathogenesis of fructose-induced insulin resistance. Cell Metab. 9: 252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hayek T., Ito Y., Azrolan N., Verdery R. B., Aalto-Setala K., Walsh A., Breslow J. L. 1993. Dietary fat increases high density lipoprotein (HDL) levels both by increasing the transport rates and decreasing the fractional catabolic rates of HDL cholesterol ester and lipoprotein (Apo) A-I. J. Clin. Invest. 91: 1665–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siri P., Candela N., Ko C., Zhang Y., Eusufzai S., Ginsberg H. N., Huang L. S. 2001. Post-transcriptional stimulation of the assembly and secretion of triglyceride-rich apolipoprotein B-lipoproteins in a mouse with selective deficiency of brown adipose tissue, obesity and insulin resistance. J. Biol. Chem. 276: 46064–46072. [DOI] [PubMed] [Google Scholar]
- 26.Folch J., Lees, and G. H. Stanley Sloane M. 1957. A simple method for the isolation and purifcation of total lipids from animal tissues. J. Biol. Chem. 226: 497–509. [PubMed] [Google Scholar]
- 27.Abramoff M. D., Magelhaes P. J., Ram S. J. 2004. Image Processing with Image. J. Biophotonics International. 11: 36–42. [Google Scholar]
- 28.Zhang Y. L., Hernandez-Ono A., Siri P., Weisberg S., Conlon D., Graham M. J., Crooke R. M., Huang L. S., Ginsberg H. N. 2006. Aberrant hepatic expression of PPARgamma2 stimulates hepatic lipogenesis in a mouse model of obesity, insulin resistance, dyslipidemia, and hepatic steatosis. J. Biol. Chem. 281: 37603–37615. [DOI] [PubMed] [Google Scholar]
- 29.Erol E., Kumar L. S., Cline G. W., Shulman G. I., Kelly D. P., Binas B. 2004. Liver fatty acid binding protein is required for high rates of hepatic fatty acid oxidation but not for the action of PPARalpha in fasting mice. FASEB. 18: 347–349. [DOI] [PubMed] [Google Scholar]
- 30.Lakshmi V., Monder C. 1998. Purification and characterization of the corticosteroid 11 beta-dehydrogenase component of the rat liver 11 beta-hydroxysteroid dehydrogenase complex. Endocrinology. 123: 2390–2398. [DOI] [PubMed] [Google Scholar]
- 31.Agarwal A. K., Monder C., Eckstein B., White P. C. 1989. Cloning and expression of rat cDNA encoding corticosteroid 11 β-dehydrogenase. J. Biol. Chem. 264: 18939–18943. [PubMed] [Google Scholar]
- 32.Alberts P., Nilsson C., Selen G., Engblom L. O., Edling N. H., Norling S., Klingstrom G., Larsson C., Forsgren M., Ashkzari M., et al. 2003. Selective inhibition of 11 β-hydroxysteriod dehydrogenase type 1 improves hepatic insulin sensitivity in hyperglycemic mice strains. Endocrinology. 144: 4755–4762. [DOI] [PubMed] [Google Scholar]
- 33.Veniant M. M., Hale C., Hungate R. W., Gahm K., Emery M. G., Jona J., Joseph S., Adams J., Hague A., Moniz G., et al. 2010. Discovery of a potent, orally active 11beta-hydroxysteroid dehydrogenase type 1 inhibitor for clinical study: identification of (S)-2-((1S,2S,4R)-bicyclo[2.2.1]heptan-2-ylamino)-5-isopropyl-5-methylthiazol-4(5H)-one (AMG 221). J. Med. Chem. 53: 4481–4487. [DOI] [PubMed] [Google Scholar]
- 34.Densmore V. S., Morton N. M., Mullins J. J., Seckl J. R. 2006. 11β-hydroxysteroid dehydrogenase type 1 induction in the arcuate nucleus by high-fat feeding: a novel constraint to hyperphagia? Endocrinology. 147: 4486–4495. [DOI] [PubMed] [Google Scholar]
- 35.Chahil T. J., Ginsberg H. N. 2006. Diabetic dyslipidemia. Endocrinol. Metab. Clin. North Am. 35: 491–510. [DOI] [PubMed] [Google Scholar]
- 36.Ginsberg H. N., Zhang Y. L., Hernandez-Ono A. 2005. Regulation of plasma triglycerides in insulin resistance and diabetes. Arch. Med. Res. 36: 232–240. [DOI] [PubMed] [Google Scholar]
- 37.Hargrove G. M., Junco A., Wong N. C. 1999. Hormonal regulation of apolipoprotein A1. J. Mol. Endocrinol. 22: 103–111. [DOI] [PubMed] [Google Scholar]
- 38.Sporstol M., Mousavi S. A., Eskild W., Roos N., Berg T. 2007. ABCA1, ABCG1 and SR-B1: hormonal regulation in primary rat hepatocytes and human cell lines. BMC Mol. Biol. 8: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brotman D. J., Girod J. P., Garcia M. J., Patel J. V., Gupta M., Posch A., Saunders S., Lip G. Y., Worley S., Reddy S. 2005. Effects of short-term glucocorticoids on cardiovascular biomarkers. J. Clin. Endocrinol. Metab. 90: 3202–3208. [DOI] [PubMed] [Google Scholar]
- 40.Itoh S., Igarashi M., Tsukada Y., Ichinoe A. 1997. Nonalcoholic fatty liver with alcoholic hyalin after long-term glucocorticoid therapy. Acta Hepatogastroenterol. (Stuttg.). 24: 415–418. [PubMed] [Google Scholar]
- 41.Vegiopoulos A., Herzig S. 2007. Glucocorticoids, metabolism and metabolic diseases. Mol. Cell. Endocrinol. 275: 43–61. [DOI] [PubMed] [Google Scholar]
- 42.Kim K. S., Park S. W., Kim Y. S. 1992. Regulation of fatty acid synthase at transcripional and post-transcriptional levels in rat liver. Yonsei Med. J. 33: 199–208. [DOI] [PubMed] [Google Scholar]
- 43.Horton J. D., Goldstein J. L., Brown M. S. 2002. SREBPs: activators of the complete program of cholesterol and fatty acidsynthesis in the liver. J. Clin. Invest. 109: 1125–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Berthiaume M., Laplante M., Festuccia W. T., Berger J. P., Thieringer R., Deshaies Y. 2010. Preliminary report: pharmacologic 11beta-hydroxysteroid dehydrogenase type 1 inhibition increases hepatic fat oxidation in vivo and expression of related genes in rats fed an obesogenic diet. Metabolism. 59: 114–117. [DOI] [PubMed] [Google Scholar]
- 45.Fisher E. A., Ginsberg H. N. 2002. Complexity in the secretory pathway: the assembly and secretion of apolipoprotein B-containing lipoproteins. J. Biol. Chem. 277: 17377–17380. [DOI] [PubMed] [Google Scholar]
- 46.Wiegman C. H., Bandsma R. H. J., Ouwens M., van der Sluijs F. H., Havinga R., Boer T., Reijngoud D-J., Romijn J. A., Kuipers F. 2003. Hepatic VLDL production in ob/ob mice is not stimulated by massive de novo lipogenesis but is less sensitive to the suppressive effects of insulin. Diabetes. 52: 1081–1089. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.