Abstract
Chronic elevations of plasma apolipoprotein B (apoB) are strongly associated with cardiovascular disease. We have previously demonstrated that inhibition of hepatic apoB mRNA using antisense oligonucleotides (ASO) results in reductions of apoB, VLDL, and LDL in several preclinical animal models and humans. In this study, we evaluated the anti-atherogenic effects of a murine-specific apoB ASO (ISIS 147764) in hypercholesterolemic LDLr deficient (LDLr−/−) mice. ISIS 147764 was administered weekly at 25-100 mg/kg for 10-12 weeks and produced dose-dependent reductions of hepatic apoB mRNA and plasma LDL by 60-90%. No effects on these parameters were seen in mice receiving control ASOs. ApoB ASO treatment also produced dose-dependent reductions of aortic en face and sinus atherosclerosis from 50-90%, with high-dose treatment displaying less disease than the saline-treated, chow-fed LDLr−/− mice. No changes in intestinal cholesterol absorption were seen with apoB ASO treatment, suggesting that the cholesterol-lowering pharmacology of 147764 was primarily due to inhibition of hepatic apoB synthesis and secretion. In summary, ASO-mediated suppression of apoB mRNA expression profoundly reduced plasma lipids and atherogenesis in LDLr−/− mice, leading to the hypothesis that apoB inhibition in humans with impaired LDLr activity may produce similar effects.
Keywords: antisense oligonucleotides, apolipoprotein B, hypercholesterolemia, lipoproteins
Atherosclerosis, a key contributor to cardiovascular disease, is a chronic inflammatory process developed in the context of elevated plasma cholesterol (1, 2). Exhaustive epidemiological, clinical, and animal studies have firmly established the central role of LDL in atherogenesis (3). As a result, management of LDL with HMG-CoA reductase inhibitors (statins) and other lipid-lowering therapeutics has become the cornerstone of the treatment guidelines used in cardiovascular disease prevention (4, 5).
Apolipoprotein B (apoB) is an essential structural and receptor-binding component of LDL, and accordingly, increasing plasma levels of apoB are directly correlated with atherosclerotic disease progression (6). ApoB is a large amphipathic protein that exists in two forms: apoB100 and, after posttranscriptional editing, apoB48. In humans, apoB100 is essential for the intracellular assembly of VLDL in the liver, and apoB48 is used for chylomicron assembly in the gut. In contrast to humans, in mice the liver is capable of generating both apoB100 and apoB48 particles. VLDL is the metabolic precursor of IDL and LDL, and strategies aimed at reducing VLDL secretion from the liver offer a powerful means of reducing plasma LDL. Common therapeutics effective in reducing LDL, such as statins and bile acid sequestrants, utilize similar mechanisms of action principally by facilitating plasma LDL clearance via increased expression of hepatic LDL receptors (LDLr).
Inhibition of apoB and VLDL biosynthesis offers a unique therapeutic approach for reducing LDL (7, 8). Directly inhibiting apoB production would be predicted to be especially beneficial when LDLr function is impaired or absent, as is the case in homozygous familial hypercholesterolemia (hoFH). While efforts are currently underway to evaluate an apoB-specific peptide vaccine in animal models, this approach has not been demonstrated to be safe or efficacious in nonhuman primates or man (9, 10).
Antisense oligonucleotides (ASO) are short, single-strand, synthetic analogs of natural nucleic acids that are designed to hybridize to a target RNA in a sequence-specific manner and promote degradation of the RNA by endogenous nucleases (e.g., RNase H) or modulate the processing and function of the target mRNA (11). We have demonstrated, both in animal models and with the human apoB ASO mipomersen in the clinic, that ASO suppression of hepatic apoB mRNA produces substantial reductions in all atherogenic apoB-containing lipoproteins (12–15). Furthermore, the efficacy of mipomersen in reducing plasma apoB is maintained in hoFH patients, as well as when co-administered with HMG-CoA reductase inhibitors (statins) (16, 17).
Herein, we tested the hypothesis that the suppression of hepatic apoB mRNA would reduce the progression of atherosclerosis in an experimental model of hoFH. The effects of ASO-mediated inhibition of apoB were evaluated in hypercholesterolemic LDLr−/− mice using ISIS 147764, a murine-specific apoB ASO.
MATERIALS AND METHODS
ASOs
All ASOs used in this report were 20-mer phosphorothioate oligonucleotides containing 2’-0-(2-methoxyethyl)-modified ribonucleosides (2’-MOE) groups at positions 1 to 5 and 16 to 20 with 2’-deoxynucleosides at positions 6 to 15. ASOs were synthesized and purified as described previously (18). The in vitro and in vivo characterization of the apoB ASO (5′-GTCCCTGAAGATGTCAATGC-3′) used in this study, ISIS 147764, has been previously described (15). Two control ASOs of the same chemical class as ISIS 147764, but not hybridizing to any known murine RNA sequences, were used and had the following nucleotide sequence: control ASO1, 5′-CCTTCCCTGAAGGTTCCTCC-3′; control ASO2, 5′-AGCATAGTTAACGAGCTCCC-3′. All ASOs were administered via intraperitoneal injection twice weekly.
Animal treatment protocols
All animal procedures were reviewed and approved by the Isis Institutional Animal Care and Use Committee (IACUC) and conducted in conformity with the Public Health Service (PHS) Policy on Humane Care and Use of Laboratory Animals. LDLr−/− mice were purchased from the Jackson Laboratory (stock #002207). These mice have been backcrossed to C57BL/6J mice for 10 generations. Mice were housed in a pathogen-fee environment and given food and water ad libitum. Three studies are presented here.
In Study I, the effect of ASO-mediated reduction of apoB was assessed in LDLr−/− mice fed two different hypercholesterolemic diets (TD.94059 and TD.97234, Harlan Teklad). The fat and cholesterol composition of the diets were as follows: the TD.94059 (HFC diet) was 15.8% fat (wt/wt) and 1.25% cholesterol; and the TD.97234 (HC diet) was 4.4% fat (wt/wt) and 0.5% cholesterol. Female mice (8-10 weeks old, n = 4/group) were fed either diet for 2 weeks prior to initiation of ASO treatment. ISIS 147764 was given at 100 mg/kg/wk for 10 weeks.
In Study II, the effects of ISIS 147764 administered at three dose levels (25, 50, and 75 mg/kg/wk, respectively) were evaluated in male LDLr−/− mice (8-10 weeks old, n = 10/group) fed the HC diet described above. This study also included two control ASOs (ASO1 and ASO2) administered at 75 mg/kg/wk to better characterize and understand potential off-target (i.e., target hybridization-independent) ASO effects. These control ASOs do not hybridize to any known murine RNA transcript; however, we cannot exclude the possibility that they may interact with other nucleic acids and/or proteins in a manner distinct from each other or 147764. It has been appreciated that ASOs can have unpredictable effects due to small sequence variations, which was the rationale of using two control ASOs to better characterize class- and sequence-dependent effects. Therefore, the control ASOs provided additional control groups to understand the class effects of ASOs and the apoB-dependent lowering effects of 147764. However, because every ASO can have unpredictable and distinct effects, all comparisons of treatment effects were performed using the saline group as the comparator. Two saline control groups were also included; in the first, mice were fed normal chow, while in the second, animals were fed the HC diet.
Study III was similar to Study II, except ISIS 147764 was administered for five weeks.
Plasma measurements
Plasma concentrations of alanine aminotransferase (ALT), aspartate aminotransferase (AST), triglycerides (TG), total plasma cholesterol (TPC) and LDL were determined with an Olympus AU400e automated clinical chemistry analyzer (Melville, NY).
Lipoprotein analysis
Plasma lipoprotein cholesterol profiling via size-exclusion chromatography was performed as previously described (15) using a Beckman System Gold 126 HPLC system, 507e refrigerated autosampler, 126 photodiode array detector (Beckman Instruments; Fullerton, CA), and a Superose 6 HR 10/30 column (GE Healthcare) coupled inline with an enzymatic colorimetric cholesterol reagent (Roche). Lipoprotein cholesterol chromatograms were generated from 15 μl (for samples with TPC < 500 mg/dl) or 7 μl (for samples with TPC > 500 mg/dl) aliquots of unpooled plasma samples. Cholesterol concentrations were measured continuously inline from absorbance values taken at a wavelength of 505 nm and validated with a cholesterol calibration kit (Sigma). Additionally, plasma samples (unpooled, 120 μl neat) were sent to LipoScience for their LipoProfile® NMR lipoprotein particle size analysis (LipoScience, Raleigh, NC).
Immunoblotting
Intestinal protein (isolated from the proximal 1/3 of the intestine) and liver protein were homogenized in lysis buffer [50 mM Tris, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 0.5% NP40, 1% Triton, 0.25% sodium deoxycholate, 0.1% SDS, protease inhibitor cocktail 1:100 (Calbiochem, #535140), 0.2 mM sodium orthovanadate], and protein concentrations were measured with the BioRad DC kit (BioRad; Hercules, CA). Plasma samples (diluted 1:1,000) or 50-80 μg of intestinal or liver protein were applied per lane and separated on a 4-12% gradient Tris-Glycine or Bis-Tris gel (Invitrogen). Proteins were then transferred to a PVDF membrane and subsequently blocked in 5% nonfat dry milk in TBS-T. Antibodies against AMPKα (used at 1:1,000; Cell Signaling, #2532) and apoB (used at 1:1,000 and kindly provided by Dr. Stephen G. Young (Department of Medicine, University of California at Los Angeles) were used for overnight incubations at 4°C. Blots were washed in TBS-T and incubated with horseradish peroxidase-conjugated IgG secondary antibodies (1:15,000; BD Biosciences; San Diego, CA) for 1 h. Following final washing in TBS-T, specific protein bands were detected using ECL (GE Healthcare). To improve the detection of faint apoB protein bands, the LI-COR Odyssey imaging system was utilized on some blots, which required the use of near-infrared secondary antibodies (1:10,000; LI-COR).
RT-PCR
Quantitative RT-PCR analysis was performed as described previously (15) using the TaqMan primer probe sets listed in supplementary Table VII. Briefly, total liver mRNA was isolated from 100-200 mg liver samples collected in Qiagen RLT buffer containing 1% β-mercaptoethanol, and it was immediately homogenized with a rotor. RNA was isolated using the RNeasy Mini Kit (Qiagen). RNA samples (50 ng) were subjected to qRT-PCR analysis using commercial reagents (Invitrogen) and analyzed using an ABI Prism 7700 Sequence Detector (Invitrogen). After 40 amplification cycles, absolute values were obtained with SDS analysis software (Invitrogen). Gene expression was normalized to cyclophilin A and/or G3PDH expression and reported as a fraction of the amount measured in the saline, HC diet-fed group. PCR arrays (SA Biosciences) were performed according to the manufacturer's protocol, with 1.09 μg of total RNA used for each cDNA reaction. Only genes with adequate expression (Ct < 35) were analyzed.
Evaluation of atherosclerosis
Two measures of atherosclerosis were performed: en face aortic lesion quantitation and aortic sinus lesion volume as previously described (19).
Intestinal cholesterol absorption
Mice were administered a gavage of 0.1 μCi of [14C] cholesterol and 0.2 μCi of [3H] sitostanol dissolved in 200 μl of olive oil. Feces were collected for three days following gavage and [14C] cholesterol and [3H] sitostanol counts were measured in a scintillation spectrometer in hexane-isolated fecal lipids as previously discussed (20). Short-term [14C] cholesterol absorption studies were performed in mice after an overnight fast. Mice were administered the poloxamer P-407 (ip 1 mg/g body wt), and 1 h later they were administered iv 1 μCi of 14C cholesterol in a 100 μl olive oil solution containing 1 mg/ml “cold” cholesterol. A positive control group treated with ezetimibe (0.005% added to the diet two weeks prior to the assay) was included. At 1.5 and 3 h following the [14C] administration, mice were bled.
Postprandial plasma triglyceride response
The postprandial response to a 200 μl of olive oil gavage was evaluated from triglyceride measurements performed on plasma collected via retro-orbital bleeds taken at baseline (mice were fasted overnight) and 1 and 2 h postgavage.
Body composition analysis
Whole body lean and fat mass was measured with MRI body composition analyzer (Echo MRI, Echo Medical System, Houston, TX).
Hepatic lipid measurements
Liver triglyceride and cholesterol ester (CE) concentrations were determined from a Folch lipid extraction as described previously (21).
Statistical analysis
Plots are expressed as the mean ± SEM. Where appropriate, a two-way repeated measures ANOVA test, one-way ANOVA test, or t test was performed. The analysis of factor level effects was done by the Holm-Sidak test of multiple comparisons versus the saline control group. All statistical analyses were performed using SigmaStat 3.00 software (SPSS, Inc.). A value of P < 0.05 was considered significant.
RESULTS
ApoB ASO treatment reduced apoB liver mRNA and plasma apoB protein while preventing diet-induced hypercholesterolemia in LDLrminus/minus mice
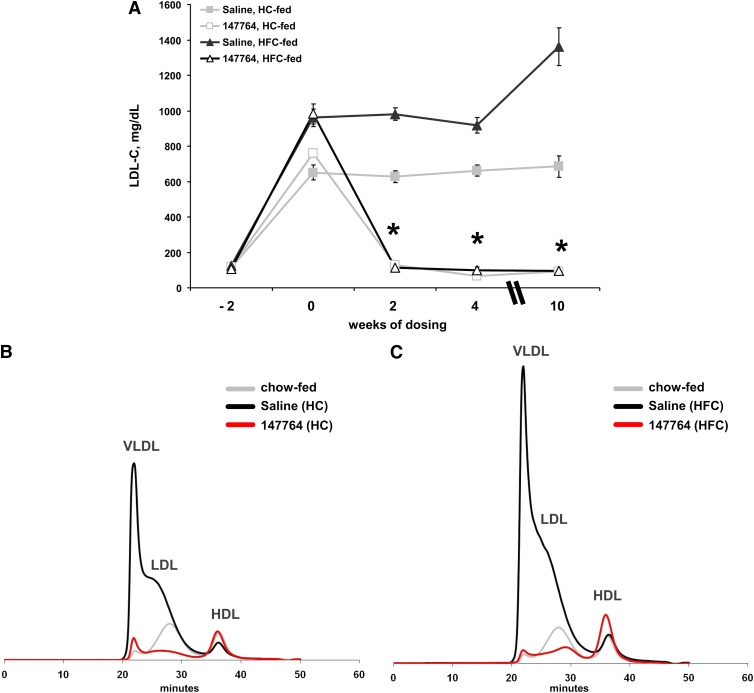
Study I was a preliminary test to determine whether a murine apoB ASO could reduce hepatic apoB mRNA and plasma lipids in LDLr−/− mice fed atherogenic diets that produce different levels of hypercholesterolemia. Relative to mice receiving saline, 10 weeks of 100 mg/kg/wk of ISIS 147764 produced an 85% (HC group) and 87% (HFC group) reduction in hepatic apoB mRNA expression (Table 1). This suppression in apoB mRNA produced a profound and sustained reduction of LDL, which effectively abolished the diet-induced hypercholesterolemia (Fig. 1A). Regardless of diet, ISIS 147764 treatment reduced LDL to the same level (<100 mg/dl) despite the large differences in LDL in the saline HFC (>1,300 mg/dl) and HC (<700 mg/dl) groups (Fig. 1 and Table 1). Size-exclusion chromatography of plasma samples after 4 weeks of treatment demonstrated that the VLDL and LDL fractions in mice fed either the HC or HFC diets and treated with ISIS 147764 were reduced below levels seen in chow-fed LDLr−/− mice (Fig. 1B).
TABLE 1.
Hepatic apoB expression, plasma lipids, and atherosclerotic lesion severity Study I mice
| Hepatic apoB mRNA % saline | % Δ | TPC (T10) | % Δ | LDL (T10) | % Δ | TG (T10) | % Δ | En Face Athero | % Δ | Sinus Athero | % Δ | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Saline, HC | 100 ± 12 | 889 ± 65 | 685 ± 60 | 131 ± 22 | 6.3 ± 0.5 | 0.130 ± 0.019 | ||||||
| ISIS 147764, HC | 15 ± 1 | −85 | 194 ± 13 | −78 | 90 ± 7 | −87 | 89 ± 8 | NS | 1.4 ± 0.3 | −78 | 0.026 ± 0.003 | −80 |
| Saline, HFC | 100 ± 15 | 1854 ± 180 | 1361 ± 106 | 274 ± 42 | 10.4 ± 1.5 | 0.265 ± 0.018 | ||||||
| ISIS 147764, HFC | 13 ± 1 | −87 | 237 ± 23 | −87 | 95 ± 13 | −93 | 99 ± 13 | −64 | 0.8 ± 0.04 | −92 | 0.037 ± 0.009 | −86 |
Values are mean ± SEM. Percentage change reflects statistically significant difference versus the diet-matched saline group at P < 0.05. One-way ANOVA multiple comparisons versus saline (HC) group Holm-Sidak posthoc test. Units: Total plasma cholesterol (TPC) = LDL = TG = mg/dl; En face Athero = % lesions on aorta; Sinus Athero = mm3. NS, not significant.
Fig. 1.
Effect of 100 mg/kg/wk ISIS 147764 on LDL in Study I mice. (A) Time course of LDL before and after ISIS 147764 treatment. HPLC chromatograms of plasma cholesterol after four weeks of treatment in HC-fed (B) and HFC-fed (C) mice. Each trace represents the average of three plasma samples. Error bars = SEM. *Statistically significant difference versus saline HC or saline HFC group at P < 0.05.
Study II was conducted to determine the lipid lowering and anti-atherogenic properties of ISIS 147764 across a range of doses in HC-fed LDLr−/− mice. The HC diet was used as it has been previously shown to produce extensive atherosclerosis without the metabolic syndrome-like complications (e.g., insulin resistance, adiposity, hypertriglyceridemia, hepatic steatosis, elevated systemic inflammation) associated with Western-style diets enriched in both fat and cholesterol (22). Therefore, the HC diet would allow for an evaluation of the impact of specific changes in LDL on atherosclerosis with minimal alterations in other processes that might independently impact atherosclerosis.
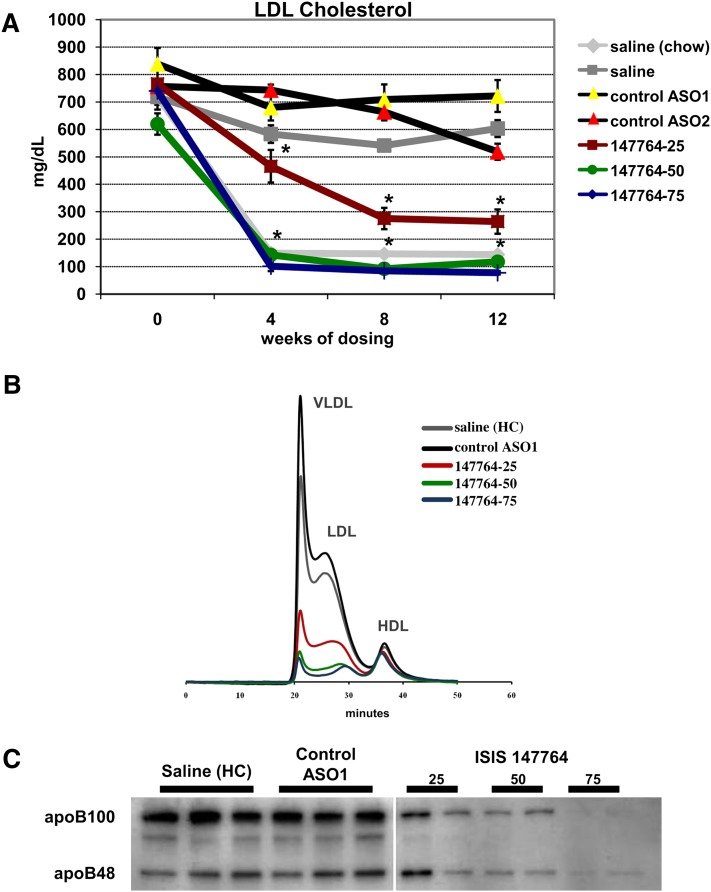
HC-fed LDLr−/− mice administered 25, 50, and 75 mg/kg/wk of ISIS 147764 for 12 weeks displayed dose-dependent reductions in hepatic apoB mRNA expression of 57%, 77%, and 85%, respectively (Table 2), with changes in plasma LDL and TPC roughly mirroring those observed with hepatic apoB suppression. For example, after 12 weeks of dosing, LDL reductions of 56%, 80%, and 87% were observed with 25, 50, and 75 mg/kg/wk ISIS 147764, respectively (Table 2). As expected, the two control ASOs did not affect apoB expression, LDL, or TPC.
TABLE 2.
Hepatic apoB expression, plasma lipids, and atherosclerotic lesion severity in mice treated with 25, 50, or 75 mg/kg/wk ISIS 147764 for 12 weeks
| Hepatic apoB mRNA % saline | % Δ | TPC (T12) | % Δ | LDL (T12) | % Δ | TG (T12) | % Δ | En Face Athero | % Δ | Sinus Athero | % Δ | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Saline, chow | 120 ± 4 | NS | 251 ± 9 | −67 | 144 ± 6 | −76 | 115 ± 4 | NS | 2.3 ± 0.5 | −77 | 0.037 ± 0.002 | −72 |
| Saline, HC | 100 ± 4 | 750 ± 31 | 603 ± 31 | 116 ± 4 | 10.1 ± 1.0 | 0.130 ± 0.010 | ||||||
| Control ASO1 | 120 ± 7 | NS | 899 ± 67 | NS | 722 ± 58 | NS | 139 ± 10 | NS | 10.1 ± 1.8 | NS | 0.127 ± 0.009 | NS |
| Control ASO2 | 127 ± 5 | NS | 659 ± 28 | NS | 519 ± 29 | NS | 163 ± 17 | +41 | 8.8 ± 0.4 | NS | 0.132 ± 0.016 | NS |
| ISIS 147764-25 | 43 ± 5 | −57 | 381 ± 54 | −49 | 264 ± 44 | −56 | 131 ± 12 | NS | 5.5 ± 0.8 | −46 | 0.071 ± 0.010 | −46 |
| ISIS 147764-50 | 23 ± 2 | −77 | 205 ± 19 | −73 | 118 ± 16 | −80 | 86 ± 5 | NS | 1.8 ± 0.4 | −82 | 0.036 ± 0.006 | −72 |
| ISIS 147764-75 | 15 ± 1 | −85 | 167 ± 10 | −78 | 78 ± 6 | −87 | 86 ± 7 | NS | 1.1 ± 0.2 | −89 | 0.017 ± 0.002 | −87 |
Values are mean ± SEM (relative to saline HC group). Percentage change reflects statistically significant difference versus Saline (HC) group at P < 0.05. One-way ANOVA multiple comparisons versus saline (HC) group Holm-Sidak posthoc test. Units: Total plasma cholesterol (TPC) = LDL = TG = mg/dl; En face Athero = % lesions on aorta; Sinus Athero = mm3. NS, not significant.
The LDL lowering effect of ISIS 147764 was both dose- and time-dependent (Fig. 2A). An amount of 25 mg/kg/wk of ISIS 147764 produced maximal LDL lowering by 8 weeks, whereas 50 and 75 mg/kg/wk ISIS 147764 produced maximal LDL lowering by 4 weeks. Both the 50 and 75 mg/kg/wk dose groups had LDL levels at or below those observed in untreated chow-fed LDLr−/− mice, similar to Study I. Size-exclusion chromatography of plasma after 4, 8, and 12 weeks of dosing demonstrated dose-responsive reductions in all apoB-containing lipoproteins (Fig. 2B and supplementary Fig. I). A NMR-based lipoprotein particle analysis of plasma samples after 12 weeks of treatments confirmed the reductions in all apoB-containing lipoproteins (supplementary Fig. II).
Fig. 2.
Effect of ISIS 147764 on plasma LDL and apoB in Study II mice. (A) Time course of LDL during ASO treatments. (B) HPLC chromatograms of plasma cholesterol after eight weeks of treatment. Each trace represents the average of three plasma samples. (C) Representative immunoblot of plasma apoB after eight weeks of treatment. Error bars = SEM. *Statistically significant difference versus saline HC group at P < 0.01.
ApoB plasma immunoblots similarly demonstrated dose-responsive reductions in both apoB-100 and apoB-48 after ISIS 147764 treatments, with a near absence of the apoB100 signal in samples from the 75 mg/kg/wk group (Fig. 2C). The LI-COR Odyssey near infrared fluorescent imaging system was used to improve the detection of the faint apoB bands, and this method demonstrated that the substantial reduction of plasma apoB was both time- and dose-dependent (supplementary Fig. III). Comparing these changes to those observed with LDL suggests that although the near-maximal LDL lowering effect of ISIS 147764 was achieved with 50 mg/kg/wk, the 75 mg/kg/wk dose resulted in further decreases in plasma apoB.
Reduction of atherosclerosis with apoB ASO treatment
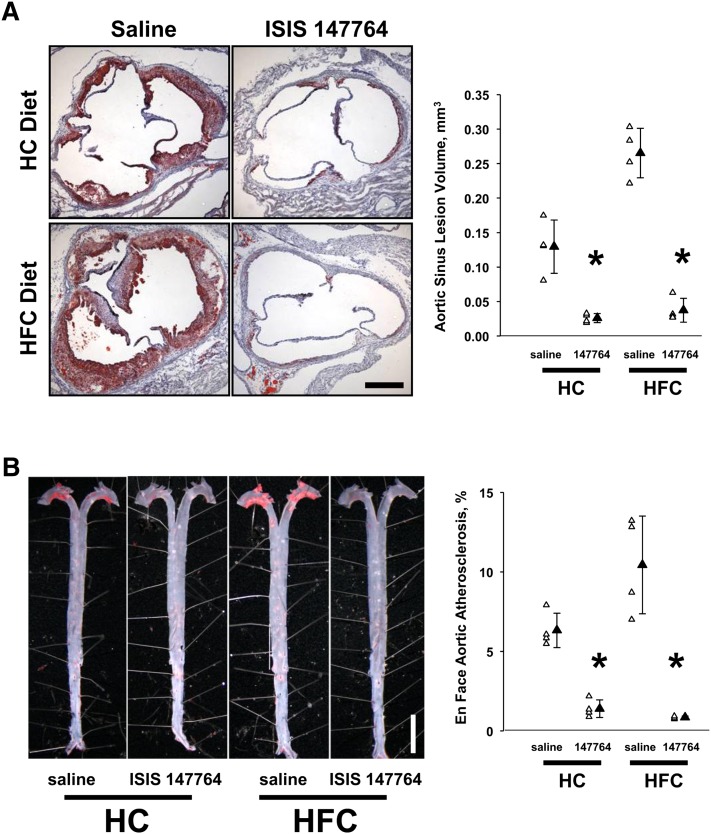
Atherosclerotic disease severity within the aortic sinus (valve) and aorta was quantified in both Study I and II mice. In Study I mice, regardless of dietary regimen, apoB ASO treatment ameliorated lesion development assessed at either the aortic sinus (Fig. 3A) or the aorta (Fig. 3B). Notably, in both HC- and HFC-fed mice, ISIS 147764 reduced atherosclerosis to similar absolute levels, despite the HFC diet resulting in a 2-fold increase aortic sinus atherosclerosis in saline-treated HFC-fed mice versus saline-treated HC-fed mice (0.265 versus 0.130 mm3, P < 0.01; Table 1).
Fig. 3.
Atherosclerosis lesion severity in Study I mice. The anti-atherosclerotic effect of ISIS 147764 was similar at the aortic sinus (A) and aorta (B). At both sites of lesion development, ISIS 147764 reduced lesion severity, and this reduction was diet-independent. In saline-treated mice, there was a diet-dependent effect on lesion severity, as the HFC diet was more atherosclerotic. *Statistically significant difference versus saline HC or saline HFC group at P < 0.05.
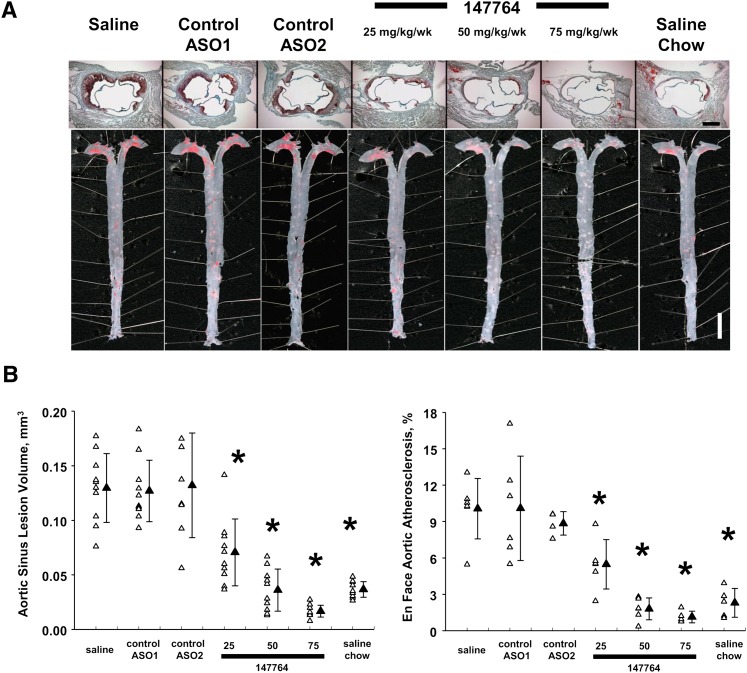
Evaluation of lesion severity in Study II mice demonstrated dose-dependent reductions of 46-89% in atherosclerosis with increasing dose levels of ISIS 147764 (Fig. 4A, B and Table 2). Treatment with the control ASOs did not affect lesion size. Treatment of 50 or 75 mg/kg/wk of ISIS 147764 reduced lesion severity to levels at or below those seen in chow-fed mice. Furthermore, although the differences in plasma cholesterol levels were only slightly reduced or unchanged between the 50 and 75 mg/kg/wk ISIS 147764 treatments, there was a 53% reduction of aortic sinus atherosclerosis in the 75 mg/kg/wk group relative to the 50 mg/kg/wk apoB ASO group (0.017 versus 0.036 mm3, P = 0.05; Table 2). Such atheroprotective effects of the higher dose apoB ASO treatment is consistent with the further reduction of plasma apoB protein observed with the 75 mg/kg/wk dose relative to the 50 mg/kg/wk dose described earlier. Additionally, 75 mg/kg/wk of ISIS 147764 reduced aortic sinus lesion severity by 54% relative to saline, chow-fed LDLr−/− mice (0.017 versus 0.037 mm3, P = 0.02; Table 2).
Fig. 4.
Atherosclerosis lesion severity in Study II mice. (A) Representative aortic sinus and aortic samples. Dimension bars = 0.5 mm (aortic sinus), 0.5 cm (aorta). (B) Quantification of aortic sinus and aortic atherosclerosis. Error bars = SEM. *Statistically significant difference versus saline HC group at P < 0.05.
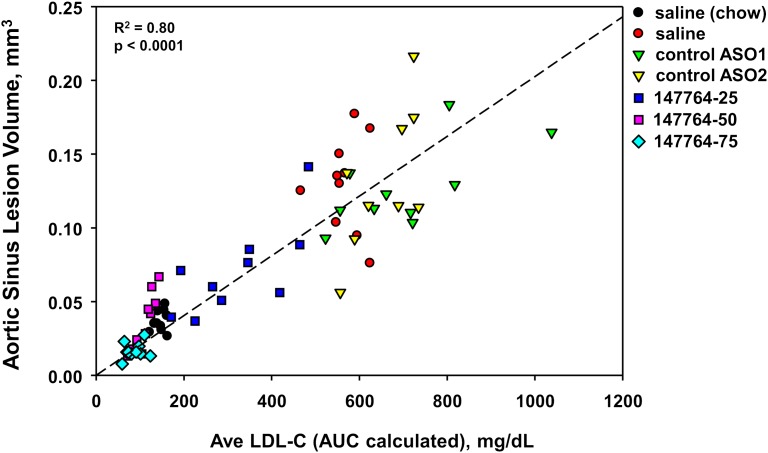
A scatter plot of aortic sinus atherosclerosis and the average LDL during ASO treatment demonstrated a strong linear relationship across all Study II mice (Fig. 5). Hence, regardless of the method of cholesterol lowering (i.e., by diet or by hepatic apoB inhibition), a predictable reduction in disease severity was produced.
Fig. 5.
Scatter plot of LDL and atherosclerosis in Study II mice. The average LDL was calculated from the AUC of the LDL measurements taken at weeks 4, 8, and 12.
Effects of apoB ASO treatment on intestinal apoB expression and lipid absorption
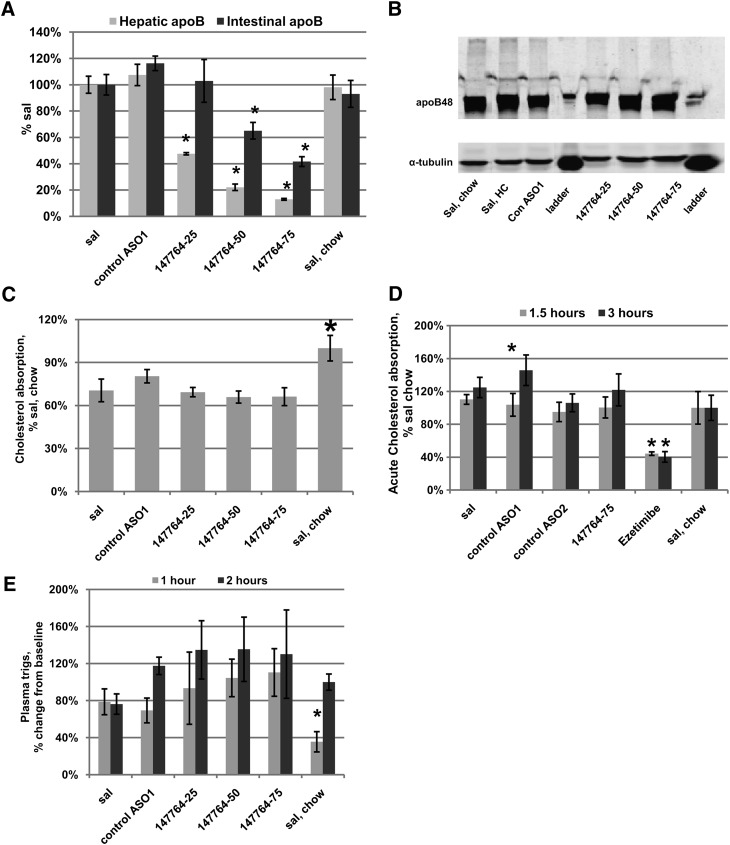
Experiments were conducted to investigate the intestinal activity of the apoB ASO. Study III mice were treated for five weeks with the control ASO1 (75 mg/kg/wk) or the apoB ASO 147764 (25, 50, and 75 mg/kg/wk). Fig. 6A demonstrates the dose-dependency of ISIS 147764 treatment on apoB mRNA in the liver and intestine. As expected, there were dose-dependent reductions of hepatic apoB mRNA with 147764 of 52%, 78%, and 87% for the 25, 50, and 75 mg/kg/wk dose groups, respectively. However, in the intestine, significant reductions in apoB mRNA were seen only with 50 and 75 mg/kg/wk of 147764; these reductions were 35% and 58%, respectively. An immunoblot of intestinal protein (Fig. 6B) demonstrated no discernable changes in apoB across any of the treatment groups. Therefore, although there were reductions in intestinal apoB mRNA with 50 and 75 mg/kg/wk of 147764, this did not translate into measurable changes in apoB intestinal protein. These results are consistent with previous studies reported in high fat-fed C57BL/6 mice treated with 100 mg/kg/wk 147764, which demonstrated changes in intestinal apoB mRNA but not in intestinal apoB protein (15).
Fig. 6.
Effect of apoB ASO treatment on intestinal apoB expression and lipid absorption in Study III mice. (A) ApoB mRNA expression in the liver and intestine. (B) Western blot of intestinal apoB. (C) Measurement of intestinal cholesterol absorption by the fecal dual isotope technique. (D) Measurement of acute intestinal cholesterol absorption following P-407 treatment. (E) Postprandial plasma triglyceride response to an olive oil gavage. Error bars = SEM. *Statistically significant difference versus saline HC group at P < 0.05.
To investigate any differences in functional parameters of intestinal lipid absorption, we measured intestinal cholesterol absorption and postprandial lipemia in the Study III mice. No changes in intestinal cholesterol absorption, measured by the fecal dual isotope ratio as well as an acute cholesterol absorption in poloxamer P-407-treated mice, were observed with apoB ASO treatments (Fig. 6C, D). These results were also consistent to those reported with [14C] oleic acid absorption in apoB ASO-treated high fat-fed C57BL/6 mice (15). No differences were observed in plasma triglycerides in fasted mice that received a 200 μl olive oil gavage (Fig. 6E).
Tolerability and effects of ASO treatments on body composition and hepatic lipids
After 12 weeks of treatment, ISIS 147764 treatments did not affect plasma ALT or AST levels, although a trend of increased plasma ALT was detected with the high dose 147764 treatment (Table 3). No differences were observed in body weight or lean body mass in mice treated with ISIS 147764 (Table 4). As described previously (15), ASO inhibition of apoB in mice did not result in an increase in hepatic triglyceride content in Study I or Study II (Table 5). However, in mice fed the HC diet, hepatic total cholesterol and cholesterol ester content was increased with apoB ASO treatment. (Table 5). A caveat with these data is the effect of the control ASO1 and ASO2 on liver triglycerides; both ASOs resulted in reductions of liver triglycerides. ASO-mediated reductions of liver triglycerides have been observed in rodents for many different ASOs. This appears to be a class effect of ASOs; therefore, we cannot determine how much of this effect is occurring with 147764 treatments.
TABLE 3.
Effect of ASO treatments on plasma transaminase levels in Study II mice
| Saline, chow | Saline, HC | Control ASO1 | Control ASO2 | ISIS 147764-25 | ISIS 147764-50 | ISIS 147764-75 | ||
|---|---|---|---|---|---|---|---|---|
| ALT U/l | T0 | 22.6 ± 6.9 | 48.2 ± 19.1 | 12.9 ± 2.3 | 24.2 ± 4.9 | 42.2 ± 11.0 | 51.6 ± 18.0 | 39.0 ± 12.2 |
| T12 | 22.3 ± 3.0 | 17.8 ± 2.7 | 31.4 ± 3.5 | 106.7 ± 24.9a | 21.2 ± 2.1 | 47.4 ± 9.7 | 68.7 ± 6.4b | |
| AST U/l | T0 | 73.8 ± 11.8 | 74.4 ± 8.4 | 62.3 ± 4.1 | 77.2 ± 9.4 | 75.2 ± 7.3 | 89.2 ± 11.9 | 66.8 ± 5.9 |
| T12 | 67.3 ± 6.2 | 53.7 ± 6.6 | 56.8 ± 3.2 | 111.3 ± 22.7a | 51.0 ± 4.6 | 63.5 ± 8.3 | 69.7 ± 5.8 |
Values in units/liter are mean ± SEM. Two-way repeated measures ANOVA and Holm-Sidak posthoc test.
Statistically significant difference versus the Saline (HC) group at P < 0.05.
Statistically significant difference versus the Saline (HC) group at P < 0.10.
TABLE 4.
Body composition in Study II mice
| Body Weight (g) |
MRI Measurement (T10) |
||||
|---|---|---|---|---|---|
| T0 | T10 | Lean Mass (g) | Fat Mass (g) | Body Fat (%) | |
| Saline, chow | 22.7 ± 0.5 | 25.0 ± 0.4a | 19.1 ± 0.4 | 2.3 ± 0.1 | 9.3 ± 0.5 |
| Saline, HC | 23.1 ± 0.6 | 27.3 ± 0.7 | 20.4 ± 0.5 | 2.6 ± 0.2 | 9.6 ± 0.5 |
| Control ASO1 | 24.1 ± 0.6 | 28.7 ± 0.4 | 22.1 ± 0.2 | 2.1 ± 0.2 | 7.2 ± 0.6 |
| Control ASO2 | 23.7 ± 0.5 | 27.5 ± 0.6 | 21.1 ± 0.6 | 1.9 ± 0.2a | 7.1 ± 0.8 |
| ISIS 147764-25 | 22.9 ± 0.5 | 25.8 ± 0.4 | 19.7 ± 0.5 | 2.2 ± 0.2 | 8.5 ± 0.7 |
| ISIS 147764-50 | 23.2 ± 0.4 | 26.5 ± 0.4 | 20.8 ± 0.4 | 1.9 ± 0.1a | 7.0 ± 0.5 |
| ISIS 147764-75 | 23.8 ± 0.4 | 26.9 ± 0.4 | 21.3 ± 0.4 | 1.6 ± 0.1a | 6.1 ± 0.4a |
Values are mean ± SEM. One-way ANOVA, multiple comparisons versus saline (HC) group Holm-Sidak posthoc test.
Statistically significant difference versus the Saline (HC) group at P < 0.05.
TABLE 5.
Liver lipid composition following apoB ASO treatments
| Liver Triglycerides | Liver Total Cholesterol | Liver Free Cholesterol | Liver Cholesterol Esters | ||
|---|---|---|---|---|---|
| μg/mg | μg/mg | μg/mg | μg/mg | ||
| Study I | Saline, HC | 160 ± 12 | 8.8 ± 2.4 | 2.2 ± 0.4 | 11.1 ± 3.7 |
| ISIS 147764, HC | 101 ± 5a | 17.9 ± 1.1a | 3.1 ± 0.2 | 24.8 ± 1.6a | |
| Saline, HFC | 129 ± 26 | 16.6 ± 0.8 | 3.1 ± 0.3 | 22.5 ± 1.1 | |
| ISIS 147764, HFC | 125 ± 9 | 22.4 ± 2.0a | 6.2 ± 0.5a | 27.1 ± 3.3 | |
| Study II | Saline, chow | 101 ± 14a | 2.0 ± 0.2 | 1.3 ± 0.1 | 1.2 ± 0.2a |
| Saline, HC | 147 ± 10 | 7.1 ± 0.4 | 1.9 ± 0.1 | 8.8 ± 0.7 | |
| Control ASO1 | 63 ± 6a | 6.5 ± 0.6 | 2.2 ± 0.1 | 7.3 ± 1.0 | |
| Control ASO2 | 72 ± 18a | 8.0 ± 0.5 | 3.6 ± 0.1 | 7.2 ± 0.8 | |
| ISIS 147764-25 | 161 ± 24 | 16.3 ± 2.8a | 2.2 ± 0.3 | 23.5 ± 4.2a | |
| ISIS 147764-50 | 114 ± 12 | 19.3 ± 1.3a | 2.3 ± 0.4 | 28.5 ± 1.6a | |
| ISIS 147764-75 | 101 ± 20a | 18.6 ± 1.5a | 2.2 ± 0.2 | 27.3 ± 2.3a |
Values are mean ± SEM. For Study I, t-test comparisons were performed within each diet subgroup. For Study II, One-way ANOVA, multiple comparisons versus saline (HC) group Holm-Sidak posthoc test was performed.
Statistically significant difference versus the respective Saline control group at P < 0.05.
To explore the mechanism(s) of unaltered hepatic triglyceride content in apoB ASO-treated mice, pathways of hepatic lipogenesis and fatty acid oxidation were transcriptionally profiled. Similar to what was observed in high fat-fed C57BL/6 mice (15), expression of several lipogenic genes, which are targets of PPAR-γ, were reduced in the livers from LDLr−/− mice treated with ISIS 147764 (Table 6). These included ACC1, SCD-1, LIPC, Cidec, and adipsin. Contrary to expectations, we could not detect transcriptional increases in pathways of fatty acid oxidation; in fact, there were decreases in AOX, CPT1, and VLCAD with apoB ASO treatment. Some of these changes may not be a result of apoB inhibition, as the control ASOs reduced AOX and also modestly reduced CPT1 and VLCAD.
TABLE 6.
Effect of ASO treatments on hepatic genes involved in fat metabolism in Study II mice
| Saline, Chow | Saline, HC | Control ASO1 | Control ASO2 | ISIS 147764-25 | ISIS 147764-50 | ISIS 147764-75 | |
|---|---|---|---|---|---|---|---|
| ACC1 | 84 ± 5 | 100 ± 5 | 143 ± 12a | 74 ± 5 | 68 ± 11 | 65 ± 5a | 63 ± 3a |
| SCD-1 | 39 ± 2a | 100 ± 7 | 100 ± 8 | 80 ± 5 | 41 ± 5a | 41 ± 4a | 26 ± 2a |
| LIPC | 96 ± 5 | 100 ± 4 | 94 ± 5 | 65 ± 6a | 36 ± 3a | 24 ± 1a | 22 ± 1a |
| PPAR-γ | 83 ± 8 | 100 ± 10 | 111 ± 9 | 162 ± 11a | 54 ± 5a | 93 ± 7 | 65 ± 4a |
| Cidec | 38 ± 4a | 100 ± 16 | 81 ± 14 | 75 ± 18 | 32 ± 3a | 24 ± 2a | 23 ± 2a |
| Adipsin | 7 ± 1a | 100 ± 24 | 73 ± 13 | 21 ± 11a | 13 ± 2a | 10 ± 2a | 9 ± 2a |
| AOX | 101 ± 8 | 100 ± 11 | 55 ± 6a | 71 ± 3a | 51 ± 6a | 56 ± 7a | 68 ± 3a |
| CPT1 | 106 ± 7 | 100 ± 10 | 79 ± 11 | 81 ± 6 | 52 ± 5a | 62 ± 8a | 78 ± 4a |
| VLCAD | 100 ± 8 | 100 ± 9 | 80 ± 12 | 76 ± 9 | 56 ± 7a | 65 ± 9a | 82 ± 6 |
Values are normalized to Saline, HC group and represent mean ± SEM (relative to Saline, HC group). One-way ANOVA or ANOVA on ranks, multiple comparisons versus saline (HC) group Holm-Sidak posthoc test.
Statistically significant difference versus the Saline (HC) group at P < 0.05.
A pathway-focused PCR gene array was used to identify the impact of apoB ASO treatment on cholesterol metabolism and lipoprotein receptors and signaling. Supplementary Fig. IV lists the 84 genes that were profiled in liver samples from Study III mice. Supplementary Fig. V lists all genes significantly changed from the HC-fed saline group that fall into three categories: (a) significantly changed in all apoB ASO treatment groups, but not control ASO1; (b) significantly changed in the mid- and high-dose apoB ASO treatment groups, but not control ASO1; and (c) significantly changed in all ASO treatment groups. A fourth category is included for other genes of interest. Of particular note are the genes that displayed robust dose-dependent changes in expression with the apoB ASO treatments; these included Abca2, Apof, Apol8, Idi2, and Ldlr. Of these, Apol8, Idi2, and Ldlr were the most upregulated genes observed with apoB ASO treatment, and are all targets of SREBP1 or 2 (23, 24). Interestingly, other targets of SREBP2 were also elevated with apoB ASO treatment, such as Idi1, Hmgcr, and SQS (23). Targets of LXR activation, such as apoe, Abca1, Abcg1, CYP7A1, and SREBP-1c (25), which were examined on the PCR array, were not increased with apoB ASO treatments. Finally, apoB ASO treatments had a consistent effect to increase hepatic AMPKα2 expression (supplementary Figs. V and VI).
DISCUSSION
Previous studies in our laboratory demonstrate that administration of ISIS 1467764 in multiple murine models produces significant and prolonged reductions in hepatic apoB mRNA and plasma apoB-100 that result in concomitant decreases in TPC and LDL (15). We hypothesized that antisense inhibition of apoB should also have a significant impact on the development of atherosclerosis in hypercholesterolemic LDLr−/− mice.
Results from the current study confirm and extend our previous findings and demonstrated that ASO-mediated inhibition of hepatic apoB expression dramatically mitigated diet-induced hypercholesterolemia and atherosclerosis in LDLr−/− mice. Study I demonstrated that, regardless of the severity of diet-induced hypercholesterolemia in LDLr−/− mice, the efficacy of ISIS 147764 in reducing LDL and mitigating atherosclerosis was equivalent, with both LDL and atherosclerosis reduced to the same level in HC- and HFC-fed mice. This was noteworthy given the differences of the severity of hypercholesterolemia in HC- versus HFC-fed LDLr−/− mice. In fact, in both HF- and HFC-fed mice, apoB ASO treatment reduced plasma LDL to levels less than that observed in chow-fed LDLr−/− mice, suggesting that when hepatic apoB expression is reduced by >85% in LDLr−/− mice, plasma LDL is determined exclusively by LDL clearance rates, not dietary cholesterol intake or absorption. Additionally, such changes were more profound than that reported with ezetimibe (a potent inhibitor of cholesterol absorption in rodents) in either apoE−/− or LDLr−/− mice (26, 27).
Study II demonstrated the linearity of the pharmacologic effects of increasing doses of ISIS 147764 on inhibition of hepatic apoB expression, LDL, and atherosclerosis in hypercholesterolemic LDLr−/− mice. Specifically, we observed near dose-linear reductions in hepatic apoB mRNA (60-90%), plasma LDL (60-90%), and atherosclerosis (50-90%) with 25-75 mg/kg/wk of ISIS 147764. Importantly, atherosclerotic lesion severity in the aorta and aortic sinus were reduced to levels at or below those present in chow-fed LDLr−/− mice. Furthermore, the linear relationship observed between LDL and atherosclerosis underscored the causal relationship between LDL and atherosclerosis in this model of hoFH and demonstrated that regardless of the means of lowering plasma LDL, whether by diet or apoB ASO treatment, predictable reductions in atherosclerosis were observed.
A comparison of the effects of 50 versus 75 mg/kg/wk of ISIS 147764 on plasma LDL, apoB, and aortic sinus atherosclerosis suggested that plasma apoB reduction is atheroprotective beyond the reductions in all LDL. For example, despite little to no difference in LDL in mice dosed with 50 and 75 mg/kg/wk ISIS 147764, immunoblots of plasma apoB demonstrated large reductions in apoB with the higher dose treatment. Interestingly, there was a 53% reduction of aortic sinus atherosclerosis in mice given 75 mg/kg/wk relative to the 50 mg/kg/wk treatment. Strong evidence exists that apoB is atherogenic due to its ability to bind and retain lipoproteins on the vascular wall and thereby facilitate oxidative modifications of LDL (28–30).
The profound reductions in circulating LDL with apoB ASO treatments were not accompanied by increased plasma ALT or AST, nor did they impact body weight or lean body mass. This was noteworthy given the dramatic phenotype of diet-induced hypercholesterolemia in this animal model and its complete reversal with apoB ASO treatment. The plasma cholesterol lowering of apoB ASO treatment was principally a result of inhibition of hepatic apoB synthesis and secretion, as no changes in intestinal lipid absorption could be detected with apoB ASO treatment.
As reported previously (14) and here, relative to saline-treated mice, liver triglyceride levels were not changed in apoB ASO-treated mice. A caveat is that ASOs generally reduce liver triglycerides in mice, as can be seen with both control ASOs in Study II. Using control ASOs to approximate such an effect can be misleading because every sequence can produce a unique and distinct effect. However, when comparing apoB ASO treatment at the same dose level of control ASO1 and ASO2 (75 mg/kg/wk), we observed reductions of liver triglycerides in all ASO treatments relative to saline. This may have been primarily a consequence of reduced hepatic lipogenesis as indicated by the reduced expression of key hepatic lipogenic genes regulated by PPAR-γ, such as ACC1, SCD-1, LIPC, Cidec, and adipsin. Importantly, reductions in SCD-1, Cidec, and PPAR-γ were most likely due to specific reductions of apoB, as the control ASOs did not reduce hepatic expression of these genes.
Activation and/or overexpression of PPAR-γ have been shown to contribute to hepatic steatosis in several models of dyslipidemia (31–33). Although the expression of key genes of fatty acid oxidation - AOX, CPT1 and VLCAD - were reduced with apoB ASO treatment, it is difficult to determine whether these transcriptional changes were related to apoB inhibition because the control ASO also reduced these genes. Additionally, the largest reductions in AOX, CPT1, and VLCAD were observed in the low-dose apoB ASO group, with dose-dependent increases in activity of AOX, CPT1, and VLCAD observed with increasing doses of the apoB ASO. Consistent with previous studies, apoB ASO treatment upregulated hepatic AMPKα2 expression (15). AMPK is a key sensor of cellular energy levels that affects multiple catabolic and anabolic metabolic pathways (34, 35). Upregulation of AMPK is thought to decrease cholesterol and fatty acid biosynthesis and increase fatty acid oxidation through several mechanisms. Collectively, these data suggest that the lack of hepatic steatosis with apoB inhibition in this mouse model may principally be a result of reductions in hepatic lipogenesis, with changes in pathways of fatty acid oxidation a consequence of secondary adaptations to these lipogenic transcriptional changes and/or a consequence of chemical class effects.
The mechanism of increased hepatic cholesterol ester content with apoB ASO treatment is unknown and may warrant further study. A similar phenotype of increased hepatic cholesterol ester content without a change in hepatic triglycerides is reported in FABP-deficient mice (36). Future studies will be necessary to identify whether similar mechanisms exist between these two models. Preliminary data suggested an increase in fecal neutral sterol excretion with apoB ASO treatment that would mitigate an accumulation of hepatic sterols (data not shown). However, an analysis of LXR target genes in this study did not indicate LXR activation. Additionally, future studies will be needed to delineate the effects of apoB ASO treatment on SREBP activation, as several SREBP targets were upregulated with apoB ASO treatment.
LDLr−/− mice, like hoFH patients, are characterized by high LDL due to the liver's inability to remove circulating LDL. HoFH patients do not achieve their target LDL level through conventional therapeutics and/or dietary regimens and, therefore, undergo routine LDL-apheresis to manage plasma cholesterol (37). Hence, an apoB ASO therapeutic, such as mipomersen, with a mechanism of action distinct from all currently available hypocholesterolemic therapies (e.g., statins, bile acid sequestrants, ezetimibe) may offer an alternative route for effective dyslipidemia management.
Acknowledgments
The authors thank Drs. Stanley T. Crooke, Brett Monia, and Brenda Baker for their helpful discussions and review of the manuscript.
Footnotes
Abbreviations:
- Abcg1
- ATP-binding cassette, sub-family G, member 1
- ACC1
- acetyl-CoA carboxylase alpha 1
- ALT
- alanine aminotransferase
- AMPK
- 5' adenosine monophosphate-activated kinase
- AOX
- acyl-Coenzyme A oxidase
- apo
- apolipoprotein
- AST
- aspartate aminotransferase
- ASO
- antisense oligonucleotide
- CE
- cholesterol ester
- Cidec
- cell death-inducing DFFA-like effector c
- CPT1
- carnitine palmitoyltransferase-1
- CYP7A1
- cholesterol 7 alpha-hydroxylase
- FABP
- fatty acid-binding protein
- G3PDH
- glyceraldehyde-3-phosphate dehydrogenase
- HC
- high cholesterol diet
- HFC
- high fat and cholesterol diet
- Hmgcr
- HMG-CoA reductase
- hoFH
- homozygous familial hypercholesterolemia
- Idi
- isopentenyl-diphosphate delta-isomerase
- LDLr
- LDL receptor
- LIPC
- hepatic lipase
- LXR
- liver X receptor
- PPAR
- peroxisome proliferator-activated receptor
- SCD-1
- stearoyl-CoA desaturase-1
- SQS
- squalene synthase
- SREBP
- sterol regulatory element binding protein
- TG
- triglyceride
- TPC
- total plasma cholesterol
- VLCAD
- very long chain acyl-Coenzyme A dehydrogenase
This work was supported by Isis Pharmaceuticals, Inc., Carlsbad, CA.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of six figures.
REFERENCES
- 1.Steinberg D. 1989. The cholesterol controversy is over. Why did it take so long? Circulation. 80: 1070–1078. [DOI] [PubMed] [Google Scholar]
- 2.Lusis A. J., Fogelman A. M., Fonarow G. C. 2004. Genetic basis of atherosclerosis: part I: new genes and pathways. Circulation. 110: 1868–1873. [DOI] [PubMed] [Google Scholar]
- 3.Tabas I., Williams K. J., Boren J. 2007. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation. 116: 1832–1844. [DOI] [PubMed] [Google Scholar]
- 4.Steinberg D., Witztum J. L. 1990. Lipoproteins and atherogenesis. Current concepts. JAMA. 264: 3047–3052. [PubMed] [Google Scholar]
- 5.Grundy S. M., Cleeman J. I., Merz C. N., Brewer H. B., Jr, Clark L. T., Hunninghake D. B., Pasternak R. C., Smith S. C., Jr, Stone N. J. 2004. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Arterioscler. Thromb. Vasc. Biol. 24: e149–e161. [DOI] [PubMed] [Google Scholar]
- 6.Olofsson S. O., Boren J. 2005. Apolipoprotein B: a clinically important apolipoprotein which assembles atherogenic lipoproteins and promotes the development of atherosclerosis. J. Intern. Med. 258: 395–410. [DOI] [PubMed] [Google Scholar]
- 7.Burnett J. R. 2006. Drug evaluation: ISIS-301012, an antisense oligonucleotide for the treatment of hypercholesterolemia. Curr. Opin. Mol. Ther. 8: 461–467. [PubMed] [Google Scholar]
- 8.Ito M. K. 2007. ISIS 301012 gene therapy for hypercholesterolemia: sense, antisense, or nonsense? Ann. Pharmacother. 41: 1669–1678. [DOI] [PubMed] [Google Scholar]
- 9.Fredrikson G. N., Bjorkbacka H., Soderberg I., Ljungcrantz I., Nilsson J. 2008. Treatment with apo B peptide vaccines inhibits atherosclerosis in human apo B-100 transgenic mice without inducing an increase in peptide-specific antibodies. J. Intern. Med. 264: 563–570. [DOI] [PubMed] [Google Scholar]
- 10.Ginsburg G. S. 2007. Regression of atherosclerosis with therapeutic antibodies pipe cleaner or pipe dream? J. Am. Coll. Cardiol. 50: 2319–2321. [DOI] [PubMed] [Google Scholar]
- 11.Crooke S. T., editor 2008. Antisense Drug Technology: Principles, Strategies, and Applications. 2nd ed. Taylor & Francis Group, Boca Raton, FL. [Google Scholar]
- 12.Merki E., Graham M. J., Mullick A. E., Miller E. R., Crooke R. M., Pitas R. E., Witztum J. L., Tsimikas S. 2008. Antisense oligonucleotide directed to human apolipoprotein B-100 reduces lipoprotein(a) levels and oxidized phospholipids on human apolipoprotein B-100 particles in lipoprotein(a) transgenic mice. Circulation. 118: 743–753. [DOI] [PubMed] [Google Scholar]
- 13.Kastelein J. J., Wedel M. K., Baker B. F., Su J., Bradley J. D., Yu R. Z., Chuang E., Graham M. J., Crooke R. M. 2006. Potent reduction of apolipoprotein B and low-density lipoprotein cholesterol by short-term administration of an antisense inhibitor of apolipoprotein B. Circulation. 114: 1729–1735. [DOI] [PubMed] [Google Scholar]
- 14.Graham M. J., Tae-won K., Lemonidis K. M., Subramaniam A., Crooke R. M. 2005. Suppression of apolipoprotein B by antisense oligonucleotides produces concomitant downstream reductions in lipogenic genes in mice and nonhuman primates. Circulation (Scientific Sessions Abstract). 97: 1199–1206. [Google Scholar]
- 15.Crooke R. M., Graham M. J., Lemonidis K. M., Whipple C. P., Koo S., Perera R. J. 2005. An apolipoprotein B antisense oligonucleotide lowers LDL cholesterol in hyperlipidemic mice without causing hepatic steatosis. J. Lipid Res. 46: 872–884. [DOI] [PubMed] [Google Scholar]
- 16.Stein E. A. 2009. Other therapies for reducing low-density lipoprotein cholesterol: medications in development. Endocrinol. Metab. Clin. North Am. 38: 99–119. [DOI] [PubMed] [Google Scholar]
- 17.Raal F. J., Santos R. D., Blom D. J., Marais A. D., Charng M. J., Cromwell W. C., Lachmann R. H., Gaudet D., Tan J. L., Chasan-Taber S., et al. 2010. Mipomersen, an apolipoprotein B synthesis inhibitor, for lowering of LDL cholesterol concentrations in patients with homozygous familial hypercholesterolaemia: a randomised, double-blind, placebo-controlled trial. Lancet. 375: 998–1006. [DOI] [PubMed] [Google Scholar]
- 18.Baker B. F., Lot S. S., Condon T. P., Cheng-Flournoy S., Lesnik E. A., Sasmor H. M., Bennett C. F. 1997. 2’-O-(2-Methoxy)ethyl-modified anti-intercellular adhesion molecule 1 (ICAM-1) oligonucleotides selectively increase the ICAM-1 mRNA level and inhibit formation of the ICAM-1 translation initiation complex in human umbilical vein endothelial cells. J. Biol. Chem. 272: 11994–12000. [DOI] [PubMed] [Google Scholar]
- 19.Mullick A. E., Tobias P. S., Curtiss L. K. 2005. Modulation of atherosclerosis in mice by Toll-like receptor 2. J. Clin. Invest. 115: 3149–3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Temel R. E., Lee R. G., Kelley K. L., Davis M. A., Shah R., Sawyer J. K., Wilson M. D., Rudel L. L. 2005. Intestinal cholesterol absorption is substantially reduced in mice deficient in both ABCA1 and ACAT2. J. Lipid Res. 46: 2423–2431. [DOI] [PubMed] [Google Scholar]
- 21.Carr T. P., Andresen C. J., Rudel L. L. 1993. Enzymatic determination of triglyceride, free cholesterol, and total cholesterol in tissue lipid extracts. Clin. Biochem. 26: 39–42. [DOI] [PubMed] [Google Scholar]
- 22.Hartvigsen K., Binder C. J., Hansen L. F., Rafia A., Juliano J., Horkko S., Steinberg D., Palinski W., Witztum J. L., Li A. C. 2007. A diet-induced hypercholesterolemic murine model to study atherogenesis without obesity and metabolic syndrome. Arterioscler. Thromb. Vasc. Biol. 27: 878–885. [DOI] [PubMed] [Google Scholar]
- 23.Horton J. D., Goldstein J. L., Brown M. S. 2002. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Invest. 109: 1125–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duchateau P. N., Pullinger C. R., Cho M. H., Eng C., Kane J. P. 2001. Apolipoprotein L gene family: tissue-specific expression, splicing, promoter regions; discovery of a new gene. J. Lipid Res. 42: 620–630. [PubMed] [Google Scholar]
- 25.Edwards P. A., Kast H. R., Anisfeld A. M. 2002. BAREing it all: the adoption of LXR and FXR and their roles in lipid homeostasis. J. Lipid Res. 43: 2–12. [PubMed] [Google Scholar]
- 26.Davis H. R., Jr, Compton D. S., Hoos L., Tetzloff G. 2001. Ezetimibe, a potent cholesterol absorption inhibitor, inhibits the development of atherosclerosis in ApoE knockout mice. Arterioscler. Thromb. Vasc. Biol. 21: 2032–2038. [DOI] [PubMed] [Google Scholar]
- 27.Repa J. J., Turley S. D., Quan G., Dietschy J. M. 2005. Delineation of molecular changes in intrahepatic cholesterol metabolism resulting from diminished cholesterol absorption. J. Lipid Res. 46: 779–789. [DOI] [PubMed] [Google Scholar]
- 28.Gustafsson M., Levin M., Skalen K., Perman J., Friden V., Jirholt P., Olofsson S. O., Fazio S., Linton M. F., Semenkovich C. F., et al. 2007. Retention of low-density lipoprotein in atherosclerotic lesions of the mouse: evidence for a role of lipoprotein lipase. Circ. Res. 101: 777–783. [DOI] [PubMed] [Google Scholar]
- 29.Khalil M. F., Wagner W. D., Goldberg I. J. 2004. Molecular interactions leading to lipoprotein retention and the initiation of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 24: 2211–2218. [DOI] [PubMed] [Google Scholar]
- 30.Nakano T., Nakajima K., Niimi M., Fujita M. Q., Nakajima Y., Takeichi S., Kinoshita M., Matsushima T., Teramoto T., Tanaka A. 2008. Detection of apolipoproteins B-48 and B-100 carrying particles in lipoprotein fractions extracted from human aortic atherosclerotic plaques in sudden cardiac death cases. Clin. Chim. Acta. 390: 38–43. [DOI] [PubMed] [Google Scholar]
- 31.Yu S., Matsusue K., Kashireddy P., Cao W. Q., Yeldandi V., Yeldandi A. V., Rao M. S., Gonzalez F. J., Reddy J. K. 2003. Adipocyte-specific gene expression and adipogenic steatosis in the mouse liver due to peroxisome proliferator-activated receptor gamma1 (PPARgamma1) overexpression. J. Biol. Chem. 278: 498–505. [DOI] [PubMed] [Google Scholar]
- 32.Gavrilova O., Haluzik M., Matsusue K., Cutson J. J., Johnson L., Dietz K. R., Nicol C. J., Vinson C., Gonzalez F. J., Reitman M. L. 2003. Liver peroxisome proliferator-activated receptor gamma contributes to hepatic steatosis, triglyceride clearance, and regulation of body fat mass. J. Biol. Chem. 278: 34268–34276. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y. L., Hernandez-Ono A., Siri P., Weisberg S., Conlon D., Graham M. J., Crooke R. M., Huang L. S., Ginsberg H. N. 2006. Aberrant hepatic expression of PPARgamma2 stimulates hepatic lipogenesis in a mouse model of obesity, insulin resistance, dyslipidemia, and hepatic steatosis. J. Biol. Chem. 281: 37603–37615. [DOI] [PubMed] [Google Scholar]
- 34.Browning J. D., Horton J. D. 2004. Molecular mediators of hepatic steatosis and liver injury. J. Clin. Invest. 114: 147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hardie D. G. 2003. Minireview: the AMP-activated protein kinase cascade: the key sensor of cellular energy status. Endocrinology. 144: 5179–5183. [DOI] [PubMed] [Google Scholar]
- 36.Martin G. G., Danneberg H., Kumar L. S., Atshaves B. P., Erol E., Bader M., Schroeder F., Binas B. 2003. Decreased liver fatty acid binding capacity and altered liver lipid distribution in mice lacking the liver fatty acid-binding protein gene. J. Biol. Chem. 278: 21429–21438. [DOI] [PubMed] [Google Scholar]
- 37.Thompsen J., Thompson P. D. 2006. A systematic review of LDL apheresis in the treatment of cardiovascular disease. Atherosclerosis. 189: 31–38. [DOI] [PubMed] [Google Scholar]