Abstract
Angiogenesis and lymphangiogenesis mediated by vascular endothelial growth factors (VEGFs) are main features of chronic inflammation and tumors. Secreted phospholipases A2 (sPLA2s) are overexpressed in inflammatory lung diseases and cancer and they activate inflammatory cells by enzymatic and receptor-mediated mechanisms. We investigated the effect of sPLA2s on the production of VEGFs from human macrophages purified from the lung tissue of patients undergoing thoracic surgery. Primary macrophages express VEGF-A, VEGF-B, VEGF-C, and VEGF-D at both mRNA and protein level. Two human sPLA2s (group IIA and group X) induced the expression and release of VEGF-A and VEGF-C from macrophages. Enzymatically-inactive sPLA2s were as effective as the active enzymes in inducing VEGF production. Me-Indoxam and RO092906A, two compounds that block receptor-mediated effects of sPLA2s, inhibited group X-induced release of VEGF-A. Inhibition of the MAPK p38 by SB203580 also reduced sPLA2-induced release of VEGF-A. Supernatants of group X-activated macrophages induced an angiogenic response in chorioallantoic membranes that was inhibited by Me-Indoxam. Stimulation of macrophages with group X sPLA2 in the presence of adenosine analogs induced a synergistic increase of VEGF-A release and inhibited TNF-α production through a cooperation between A2A and A3 receptors. These results demonstrate that sPLA2s induce production of VEGF-A and VEGF-C in human macrophages by a receptor-mediated mechanism independent from sPLA2 catalytic activity. Thus, sPLA2s may play an important role in inflammatory and/or neoplastic angiogenesis and lymphangiogenesis.
Angiogenesis, which is the outgrowth of new from preexisting vessels, is a main feature of inflammatory diseases and cancer (1). Lymphangiogenesis, which is the development of new lymphatic vessels, is important in tumor metastases and chronic inflammation (2). Vascular endothelial growth factors (VEGFs) are the most potent mediators involved in angiogenesis and lymphangiogenesis (1, 2). The VEGF family is constituted by VEGF-A, VEGF-B, VEGF-C, VEGF-D, and placental growth factor (PlGF). VEGF-A, VEGF-B, and PlGF are key regulators of blood vessel growth, whereas VEGF-C and VEGF-D regulate primarily lymphangiogenesis (1, 2).
Macrophages play a pivotal role in such pathological processes as chronic inflammation and cancer growth (3, 4). These cells are the predominant immune cells in normal and inflamed tissues (3) as well as in various solid tumors where they are referred to as tumor-associated macrophages (TAMs) (5). Different TAM phenotypes (i.e., M1 versus M2) enable these cells to exert distinct and often opposite effects on tumor-specific immune responses (stimulation versus suppression) and tumor growth (inhibition versus enhancement) (6). In particular, once tumor cells escape immune surveillance, TAMs change their phenotype from proinflammatory M1 macrophages, which contribute to tumor rejection, to proangiogenic M2 macrophages, which stimulate tumor growth (6, 7). Macrophages can promote cancer progression either directly, by stimulating the proliferation of tumor cells, or indirectly, by producing angiogenic and lymphangiogenic factors (7). It is believed that these proangiogenic programs are induced in TAMs by local activation signals resulting from metabolic conditions (e.g., hypoxia, lactate, pyruvate, or hydrogen ions) or from mediators produced at sites of tissue injury (e.g., IL-10, M-CSF, LPS, adenosine) (4, 6, 7).
Phospholipases A2 (PLA2s) are a family of enzymes that mobilize free fatty acids including arachidonic acid and lysophospholipids from membrane phospholipids (8, 9). This family includes 10 isoforms of low m.w. secreted PLA2s (sPLA2s) that have been identified in human cells and tissues (8, 9). Large quantities of sPLA2s are released in biological fluids during local or systemic inflammation that supports their role as proinflammatory mediators (reviewed in Ref. 10). sPLA2 levels are increased in the airways of patients with acute (e.g., pneumonia and adult respiratory distress syndrome) and chronic inflammatory diseases of the lung (e.g., bronchial asthma, chronic obstructive pulmonary disease [COPD], interstitial lung fibrosis, and sarcoidosis) (11). In addition, sPLA2s are overexpressed by certain tumors in humans (12–16). Both human and animal studies indicate that two major sPLA2 isoforms, namely, group IIA (GIIA) and group X (GX), are involved in inflammatory diseases of the lung (17–20) and in several types of cancer (15, 16, 21, 22). In particular, GX contributes to colon tumorigenesis by generating PGE2 and other lipid mediators (23, 24).
sPLA2s exert multiple biological effects related to their enzymatic activity as well as to their capacity to activate inflammatory cells by nonenzymatic mechanisms (10, 25). Macrophages are a major target of sPLA2s because they can be activated by both mechanisms (10, 25). Through their enzymatic activity sPLA2s contribute to the biosynthesis of proinflammatory lipid mediators (PGs, leukotrienes, lipoxins, and platelet-activating factor) (26). In addition, sPLA2s activate exocytosis, cytokine and chemokine production, NO generation, and cell adhesion by nonenzymatic mechanisms involving the interaction with specific (M-type) or promiscuous receptors (mannose receptor and integrins) (27–31). Both human GIIA (hGIIA) and human GX (hGX) are capable to stimulate cytokine production and exocytosis in human lung macrophages in vitro (28, 31, 32). However, the capacity of any sPLA2s to induce the production of angiogenic and lymphangiogenic factors from macrophages has not yet been investigated.
Materials and Methods
Reagents
The following were purchased: LPS (from Escherichia coli serotype 026:B6), 5′-(N-ethylcarboxamido)-adenosine (NECA), 2-p-(2-Carboxyethyl)phenethylamino-5′-N-ethylcarboxamidoadenosine (CGS21680), 2-Chloro-N6-(3-iodobenzyl)-adenosine-5′-N-methyluronamide (Cl-IB-MECA), Pipes, BSA, Percoll, l-glutamine, antibiotic-antimycotic solution (10,000 UI/ml penicillin, 10 mg/ml streptomycin, and 25 μg/ml amphotericin B), Triton X-100, Polymyxin B (Sigma-Aldrich, St. Louis, MO); RPMI 1640, FCS, and guanidine hydrochloride (MP Biomedicals Europe, Illkirch, France); rabbit polyclonal anti–VEGF-B (H-70), anti–VEGF-C (H-190), and anti–VEGF-D (H-144) Abs, goat polyclonal anti-PlGF (C-20) and anti-GAPDH (V-18) Abs, normal rabbit and goat IgG Abs, HRP-conjugated donkey anti-rabbit and anti-goat IgG Abs (Santa Cruz Biotechnology, Santa Cruz, CA); goat polyclonal anti–VEGF-A Ab, human recombinant VEGF-A165, and VEGF-A121 (R&D System, Minneapolis, MN); SB203580 (Calbiochem, La Jolla, CA); PD98059 (Cell Signaling, Beverly, MA). The sPLA2 inhibitors Me-Indoxam and RO092906A (33, 34), recombinant hGIIA and hGX sPLA2s (34), and the H48Q mutants of hGIIA and hGX (hGIIA-H48Q and hGX-H48Q) (35) were prepared as described. All sPLA2 preparations were routinely checked for LPS contamination (Limulus amebocyte test, MP Biomedicals) and discarded if the LPS concentration was above the detection limit of the assay (0.125 EU/ml).
Isolation and purification of human lung macrophages
Macrophages were obtained according to human subject research guidelines established by the University of Naples Federico II, Naples, Italy, and the University of Washington, Seattle, WA. The study protocol was approved by the Ethics Committee of the University of Naples Federico II and informed consent was obtained from patients undergoing thoracic surgery. Macrophages were isolated by mechanical dispersion from the lung parenchyma of patients undergoing thoracic surgery as previously reported (27, 32). Briefly, macroscopically normal tissue was minced finely with scissors, washed extensively with Pipes buffer (27) over Nytex cloth (120-μm pore size, Sefar, Milan, Italy) and the dispersed cells recovered. The macrophage suspension was enriched (75–85%) by flotation over Percoll density gradients. The cells were suspended (106 cells/ml) in RPMI 1640 containing 5% FCS, 2 mM l-glutamine, and 1% antibiotic-antimycotic solution and incubated in 6-24-well plates (Falcon, Becton Dickinson, Franklin Lakes, NJ) at 37°C in a humidified atmosphere of 5% CO2 and 95% air. After 12 h, more than 98% of adherent cells were macrophages as assessed by CD14/CD71/HLA-DR triple staining by flow cytometry (27). In the experiments with freshly isolated macrophages, the enriched macrophage suspension was layered onto additional Percoll gradients to reach a purity ranging between 95% and 99%, as assessed by CD14/CD71/HLA-DR triple staining by flow cytometry (27, 32).
Cell incubations
Adherent human lung macrophages (HLMs) were incubated (37°C, 3–48 h) in RPMI 1640 containing various concentrations (0.1–10 μg/ml) of hGIIA, hGX, hGIIA-H48Q, hGX-H48Q, or LPS. In selected experiments, the cells were preincubated (37°C, 30 min) with or without actinomycin D (Act-D, 1 μg/ml), cycloheximide (CHX, 10 μg/ml) or brefeldin A (BFA, 10 μg/ml) and then stimulated (37°C, 24 h) with hGX (3 μg/ml). In other experiments, hGX (3 μg/ml) was preincubated (37°C, 15 min) with increasing concentrations (0.01–10 μM) of Me-Indoxam or RO092906A. HLMs were then washed and incubated (37°C, 24 h) in RPMI 1640 containing hGX (3 μg/ml) or combinations of hGX with various concentrations of the inhibitors. In the experiments with the inhibitors of MAPKs, HLMs were preincubated (37°C, 1 h) with optimal concentrations of SB203580 (30 μM), PD98059 (50 μM), or a combination of SB203580 plus PD98059, and then stimulated (37°C, 24 h) with hGX (3 μg/ml). In another series of experiments, the cells were incubated with medium alone, increasing concentrations (0.1–10 μM) of NECA, CGS21680, Cl-IB-MECA, hGX (3 μg/ml), or combinations of adenosine analogs plus hGX. At the end of incubation, supernatants were removed, centrifuged twice (1000 × g, 4°C, 5 min) and stored at −280°C for subsequent quantification of VEGF-A, VEGF-C, and TNF-α. Remaining cells were lysed with 0.1% Triton X-100 for total protein quantification by a Bradford-based assay (Biorad Laboratories, Milan, Italy).
RT -PCR of VEGF-A, VEGF-B, VEGF-C, VEGF-D, and PlGF
Total cellular RNA was isolated from HLMs using the SV RNA isolation system (Promega, Madison, WI), treated with RNase-free DNase I and resuspended in DEPC water. RNA concentration and quality were assessed by spectrophotometry. One microgram total RNA was reverse transcribed with oligo(dT) (50 μM) and Superscript III Reverse Transcriptase (200 U, Invitrogen, Carlsbad, CA) as described elsewhere (32).
For semiquantitative PCR, equivalent amounts of cDNA were amplified using target-specific primers for: VEGFA, VEGFB, VEGFC, VEGFD, PlGF, and GAPDH as a housekeeping gene (Table I). The PCR products were separated on 2.0% agarose gel, stained with ethidium bromide and digitalized with ChemidocXRS (Biorad).
Table I.
Primer sequences and conditions for semiquantitative PCR and qPCR
| Target | Product Length (bp) | Annealing Temperature (°C) | Primer (5′-3′) | Genbank Accession No. or Reference |
|---|---|---|---|---|
| PlGF | 440 (Is1) | 51 | Forward: GAGGCTGTTCCCTTGCTTC | NM_002632 |
| (sPCR) | 516 (Is2) | Reverse: GGTTACCTCCGGGGAACAG | ||
| VEGF-A | 100 (Is121) | 54 | Forward: GTGAATGCAGACCAAAGAAAG | NM_003376 |
| (sPCR) | 210 (Is165) | Reverse: AAACCCTGAGGGAGGCTC | ||
| 282 (Is189) | ||||
| VEGF-A165 | 79 | 54.4 | Forward: GCCTTGCCTTGCTGCTCTAC | NM_003376 |
| (qPCR) | Reverse: TGATTCTGCCCTCCTCCTTCTG | |||
| VEGF-B | 230 (Is167) | 59 | Forward: TGTCCCTGGAAGAACACAGCC | NM_003377 |
| (sPCR) | 336 (Is186) | Reverse: GCCATGTGTCACCTTCGCA | ||
| VEGF-C | 197 | 52 | Forward: ATGTTTTCCTCGGATGCTGGA | NM_005429 |
| (s/qPCR) | Reverse: CATTGGCTGGGGAAGAGTTT | |||
| VEGF-D | 226 | 52 | Forward: GTATGGACTCTCGCTCAGCAT | NM_004469 |
| (sPCR) | Reverse: AGGCTCTCTTCATTGCAACAG | |||
| GAPDH | 141 | 55 | Forward: GTCCACTGGCGTCTTCAC | 32 |
| (s/qPCR) | Reverse: CTTGAGGCTGTTGTCATACTTC |
Real-time quantitative PCR (qPCR) was performed as previously described (36) on the iCycler (Biorad) using the Platinum SYBR Green qPCR kit (Invitrogen). Target-specific primers for VEGFA165, VEGFC, and GAPDH suitable for qPCR are reported in Table I. PCR efficiency and specificity were evaluated by analyzing amplification curves with serial dilutions of the template cDNA and their dissociation curves. Each cDNA sample was analyzed in triplicate, and the corresponding no-RT mRNA sample was included as a negative control. The data were analyzed with iCycler iQ analysis software (Biorad), the mRNA signals in each sample were normalized to that of the GAPDH mRNA, and the changes in VEGFA165 and VEGFC mRNAs were expressed as fold increase in treated versus unstimulated cells.
Western blot analysis for VEGF-A, VEGF-B, VEGF-C, VEGF-D, and PlGF
The cells (2–4 × 106 per sample) were lysed as previously described (27). In selected experiments, HLMs (6 × 106/4 ml) were incubated (37°C, 24 h) with RPMI 1640 alone or hGX (3 μg/ml), and at the end of incubation, cell-free supernatants were concentrated by centrifugation (4000 × g, 4°C, 15 min) over Amicon Ultra-4 filter units (Millipore, Billerica, CA) with a cutoff of 10 kDa. Protein content in cell lysates or supernatants was determined by the BCA Protein Assay (Novagen, Merck Biosciences, San Diego, CA) and protein extracts were then diluted in lithium dodecyl-sulfate sample buffer containing (reducing conditions) or not (nonreducing conditions) 2.5% 2β-mercaptoethanol and stored at −280°C. Equal protein extracts (40 μg per sample) were separated on 4–12% Bis-Tris gels (NuPAGE, Novex, Invitrogen) and transferred to a nitrocellulose membrane (Schleicher and Schuell, Dassel, Germany) together with a biotinylated protein ladder (Cell Signaling, Beverly, MA), and human recombinant VEGF-A165, protein extracts of MCF-7 cells, protein extracts of EBNA cells expressing PlGF-1, or protein extracts of RAW 264.7 cells used as positive controls. Membranes were then probed with the indicated primary Abs followed by HRP-conjugated secondary Abs as previously described (36). Membrane-bound Abs were visualized with the ECL detection system (GE Healthcare, Buckinghamshire, U.K.) and digitalized with ChemidocXRS (Biorad).
ELISA for VEGF-A, VEGF-C, and TNF-α
Cytokine release in the supernatant of macrophages was measured in duplicate determinations using commercially available ELISA kits for VEGF-A, VEGF-C, and TNF-α (R&D Systems). The results were normalized for the total protein content in each well. Inhibition of VEGF-A release was expressed as percentage of the maximal response calculated as (R – Rb)/(Rmax – Rb) × 100, where R is the release in samples treated with the combination hGX plus inhibitor, Rb is the release in unstimulated samples, and Rmax is the release in samples stimulated with hGX alone.
Chick embryo chorioallantoic membrane assay
Adherent HLMs were incubated (37°C, 24 h) in RPMI 1640 alone, with hGX (3 μg/ml), Me-Indoxam (10 μM) or a combination of hGX plus Me-Indoxam. At the end of incubation, cell-free supernatants were used for subsequent determination of angiogenic activity in chick embryo chorioallantoic membranes (CAMs) as previously described (36). Briefly, fertilized White Leghorn chicken eggs were incubated under constant humidity at 37°C. CAMs were prepared as previously described (36) and then treated at day 8 with supernatants of HLMs adsorbed on 1-mm3 sterilized gelatin sponges (Gelfoam, Upjohn, Kalamazoo, MI). Sponges containing vehicle alone or 50 ng human recombinant VEGF-A165 were used as negative and positive controls, respectively. Additional controls were performed with supernatants of unstimulated HLMs in which hGX (3 μg/ml) was added after removal of the cells. CAMs were examined daily until day 12 and photographed in ovo with a stereomicroscope SR equipped with the Zeiss Camera System MC63. Blood vessels entering the sponge within the focal plane of the CAM were counted by two observers in a double-blind fashion at ×50 magnification.
Statistical analysis
The data are expressed as mean values ± SE of the indicated number of experiments. Statistical analysis was performed by one-way ANOVA, followed by Dunnett's test (when comparison was made against a control) or Bonferroni's test (when comparison was made between each pair of groups) by means of Analyze-it for Microsoft Excel (Microsoft, Redmond, WA), version 2.16 (Analyze-it Software, Leeds, U.K.) (32). A p value of 0.05 or lower was considered to be significant.
Results
HLMs express VEGFs
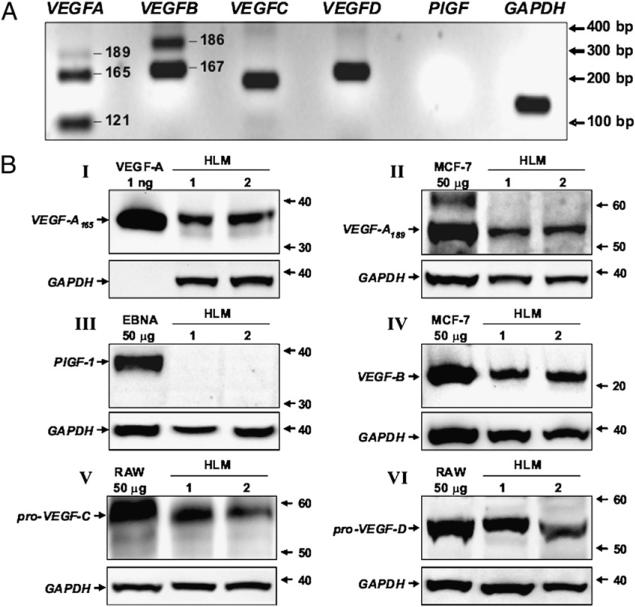
The expression profile of VEGFs produced by primary human macrophages has not yet been reported. Therefore, we initially investigated the constitutive expression of VEGFs in human macrophages purified from lung tissue (see experimental conditions in Table I). Fig. 1A shows the RT-PCR amplification signals for VEGFs and GAPDH obtained in one representative experiment. HLMs constitutively expressed three VEGFA isoforms (VEGFA121, VEGFA165, and VEGFA189) and two VEGFB isoforms (VEGFB167 and VEGFB186). Moreover, mRNA for VEGFC and VEGFD, two mediators of lymphatic development (2), was also detected in these cells. By contrast, neither of the two major isoforms of PlGF (PlGF1 and PlGF2) was expressed in HLMs (Fig. 1A).
FIGURE 1.
HLMs constitutively express different forms of VEGF. A, Expression of VEGF mRNAs. RNA extraction from resting HLMs and RT-PCR was performed as described under Materials and Methods. Specific RT-PCR amplification products for VEGFA (isoforms 189, 165, and 121), VEGFB (isoforms 186 and 167), VEGFC, VEGFD, PlGF, and GAPDH were separated on 2% agarose gel, stained with ethidium bromide, and visualized with an image analysis system. The experiment shown is representative of three separate experiments. B, Detection of VEGF proteins. HLM protein extracts (40 μg per sample) were immunoblotted with anti–VEGF-A (gel I and II), anti-PlGF (gel III), anti–VEGF-B (gel IV), anti–VEGF-C (gel V), and anti–VEGF-D (gel VI) Abs. rhVEGF-A165, MCF-7 cells, EBNA expressing PlGF-1, RAW 264.7 cells were used as positive controls. Stripped membranes were reprobed with anti-GAPDH Ab to confirm equal protein content of each sample. Each Western blot shown is representative of three separate experiments.
To verify the presence of VEGF proteins in resting HLMs, we examined cell lysates by Western blot analysis. Protein extracts of HLMs were initially probed with anti-VEGF-A and anti-PlGF Abs under non reducing conditions because preliminary experiments showed that reducing agents impede VEGF-A and PlGF detection (data not shown). Under these experimental conditions, immunoreactive bands of ~38 kDa (Fig. 1B, gel I) and ~55 kDa (Fig. 1B, gel II) were detected in two different preparations of HLMs. These bands comigrated with the dimers of rhVEGF-A165 (Fig. 1B, gel I), and VEGF-A189 expressed in the human MCF-7 cells (Fig. 1B, gel II) used as positive controls (37). By contrast, VEGF-A121 (data not shown) and PlGF (Fig. 1B, gel III) proteins were not detected in human macrophages. When protein extracts were probed with an anti-VEGF-B Ab under reducing conditions, an immunoreactive band of ~22 kDa was identified in HLMs (Fig. 1B, gel IV). This band comigrated with VEGF-B167 present in MCF-7 cells (37). By contrast, the VEGF-B186 protein was not detected (data not shown). VEGF-C and VEGF-D are synthesized after proteolytic processing of precursor proteins (38, 39). Therefore, we looked for VEGF-C and VEGF-D in HLMs under reducing conditions by using Abs raised against the precursors of VEGF-C and VEGF-D. Two immunoreactive bands of ~58 kDa (Fig. 1B, gel V), and ~53 kDa (Fig. 1B, gel VI) were detected in the two HLM preparations. These bands correspond to the precursor proteins of VEGF-C (38) and VEGF-D (39), respectively, contained in RAW 264.7 macrophages (40). Thus, primary HLMs constitutively express and synthesize the major isoforms of the angiogenic VEGFs (A and B) as well as the precursors of the lymphangiogenic VEGFs (C and D).
hGX and hGIIA sPLA2s induce VEGF production from HLMs
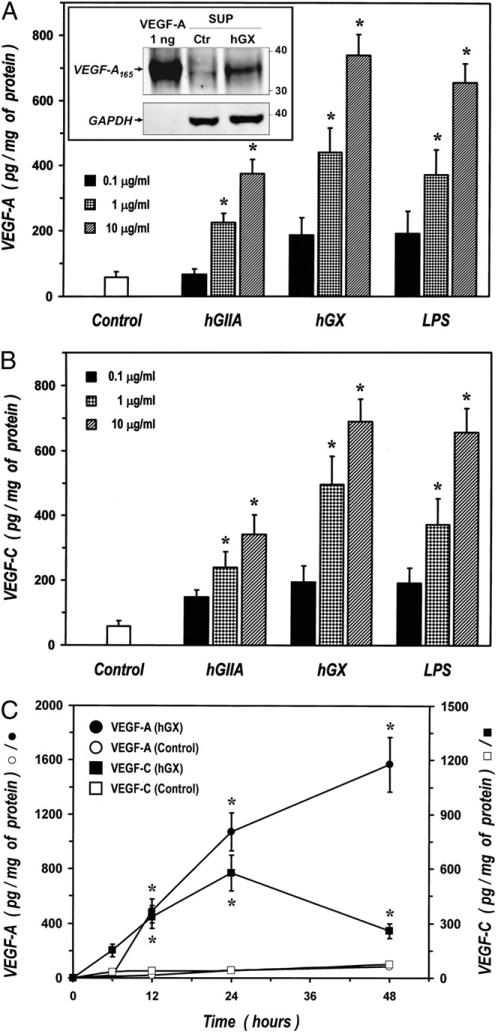
We previously showed that hGIIA and hGX induce cytokine and chemokine production and exocytosis from HLMs (27, 28, 31, 32). We therefore evaluated whether these sPLA2s also induce the production of VEGF-A and VEGF-C. Fig. 2 shows that hGIIA and hGX induced release of both VEGF-A (Fig. 2A) and VEGF-C (Fig. 2B) in a concentration-dependent manner. The effect of both sPLA2s was significant at 1 μg/ml and hGX was more potent than hGIIA in inducing the release of the two angiogenic factors. Interestingly, the release of VEGF-A and VEGF-C induced by hGX was comparable to that induced by LPS, a major stimulus for human macrophages (41, 42). Although sPLA2 preparations were routinely checked by Limulus amebocyte test, to exclude the possibility that VEGF release could be due to contamination of sPLA2 preparations by LPS we repeated these experiments in the presence of polymyxin B (50 μg/ml), a known inhibitor of LPS (43). Polymyxin B did not influence VEGF-A production induced by hGIIA and hGX whereas it completely suppress that induced by LPS (data not shown).
FIGURE 2.
sPLA2s induce the release of VEGF-A and VEGF-C from HLMs. A and B, HLMs were incubated (37°C, 24 h) with RPMI 1640 alone (Control) or with the indicated concentrations of hGIIA, hGX, or LPS. VEGF-A (A) and VEGF-C (B) release was determined by ELISA. Data are mean ± SEM of four experiments. *p < 0.05 versus control. A, inset, HLMs were incubated (37°C, 24 h) with RPMI 1640 alone (Ctr) or hGX (3 μg/ml). At the end of incubation, supernatants were concentrated by ultrafiltration and protein extracts (40 μg per sample) were immunoblotted with anti–VEGF-A Ab. rhVEGF-A165 was used as positive control. Stripped membranes were reprobed with anti-GAPDH Ab to confirm equal protein loading. The Western blot shown is representative of three separate experiments. C, HLMs were incubated for the time indicated with RPMI 1640 alone [control, (○) and (□)] or with hGX [10 μg/ml, (●) and (■)]. VEGF-A [(○) and (●)] and VEGF-C [(□) and (■)] release was determined by ELISA. Data are mean ± SEM of four experiments. *p < 0.05 versus control.
To identify the isoform of VEGF-A released by HLMs on sPLA2 stimulation, cell-free supernatants collected from cells either unstimulated or activated by hGX were concentrated by ultrafiltration and examined by Western blot. The inset in Fig. 2A shows that the supernatans of HLMs kept in culture for 24 h (control [ctr]) contained low levels of VEGF-A165 and that stimulation with hGX greatly increased the amount of this VEGF-A isoform in HLM supernatants. By contrast, the amount of VEGF-A189 released by macrophages was not increased by sPLA2 stimulation, and VEGF-A121 was not detected in the supernatants of either unstimulated or hGX-activated HLMs (data not shown). These results indicate that the major isoform secreted by sPLA2-activated macrophages was VEGF-A165.
In the next group of experiments we evaluated the kinetics of VEGF release in unstimulated and hGX-activated macrophages. Resting HLMs kept in culture for up to 48 h spontaneously released small amounts of VEGF-A (83 ± 16 pg/mg of proteins) and VEGF-C (59 ± 20 pg/mg of proteins). The hGX (10 μg/ml) induced a time-dependent release of both VEGF-A and VEGF-C (Fig. 2C). However, the kinetics of activation were different: VEGF-A release started 6 h after stimulation and then progressively increased up to 48 h, whereas the release of VEGF-C peaked at 24 h and declined thereafter.
sPLA2s induce mRNA expression of VEGF in HLMs
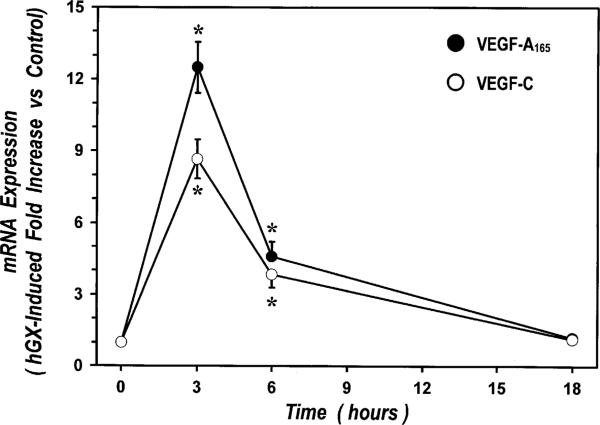
The observation that sPLA2-induced release of VEGF-A and VEGF-C was detected several hours after stimulation suggests that these events depend on protein synthesis. Thus, we examined the effects of Act-D, a transcription inhibitor, CHX, a protein synthesis inhibitor, and BFA, an inhibitor of intracellular protein transport, on VEGF release induced by hGX. Preincubation (30 min) of HLMs with Act-D (1 μg/ml), CHX (10 μg/ml), or BFA (10 μg/ml) completely blocked hGX-induced release of VEGF-A and VEGF-C from HLMs (Table II), indicating that it was an active metabolic process involving mRNA transcription, protein synthesis and transport. In particular, the inhibitory effect of Act-D indicates that sPLA2s induce VEGF production by enhancing mRNA expression. We therefore examined the kinetics of expression of VEGFA165 and VEGFC in HLMs stimulated with hGX by real-time qPCR. Fig. 3 shows that hGX induced a marked increase of mRNA expression for both VEGFA165 and VEGFC with similar kinetics. In particular, the expression of VEGFA165 and VEGFC peaked after 3 h of incubation with hGX, declined after 6 h, and returned to near control levels within 18 h. It is noteworthy that hGX exerted greater effect on VEGFA expression (12.49- ± 1.06-fold increase versus control) than on VEGFC expression (8.67- ± 0.82-fold increase versus control).
Table II.
Effects of metabolic inhibitors on hGX-induced release of VEGF-A and VEGF-C from HLMs
| VEGF-A (pg/mg of Protein) | VEGF-C (pg/mg of Protein) | |
|---|---|---|
| Vehicle + hGX | 650 ± 128 | 516 ± 79 |
| Act-D + hGX | 74 ± 18* | 53 ± 23* |
| CHX+ hGX | 70 ± 13* | 56 ± 18* |
| BFA + hGX | 64 ± 15* | 45 ± 19* |
HLMs were preincubated (37°C, 30 min) with vehicle, Act-D (1 μg/ml), CHX (10 μg/ml), or BFA (10 μg/ml) and then stimulated (37°C, 24 h) with hGX (3 μg/ml). VEGF-A and VEGF-C release was determined in the supernatants by ELISA and normalized for the total protein content. Data are mean ± SEM of three experiments.
p < 0.05 versus vehicle and hGX.
FIGURE 3.
Kinetics of hGX-induced mRNA expression of VEGFA165 and VEGFC in HLMs. HLMs were incubated for the time indicated with RPMI 1640 alone or hGX (10 μg/ml). Real-time quantitative PCR was performed as described under Materials and Methods. The results were normalized for GAPDH. hGX-induced expression of VEGFA165 (●) and VEGFC mRNA (○) was expressed as fold increase versus unstimulated cells. Data are mean ± SEM of three experiments. *p < 0.05 versus control.
sPLA2s induce VEGF-A production by a receptor-mediated mechanism
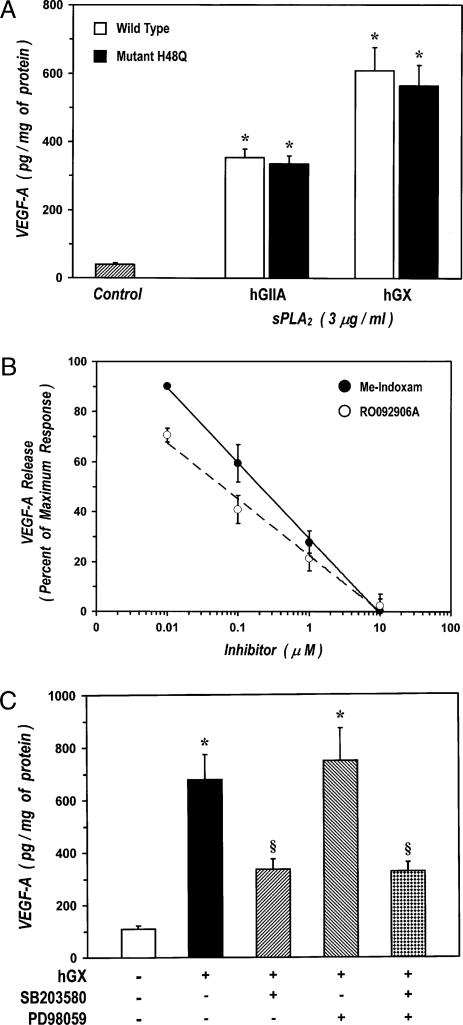
The sPLA2s can exert biological effects either through enzymatic activity or by interacting with surface receptors expressed also on macrophages (25). To clarify the mechanism responsible for sPLA2-induced production of VEGF from HLMs, we used a multiple experimental strategy. Initially, we examined the capacity of two sPLA2s, whose catalytic activity was suppressed by site-directed mutagenesis, to induce the release of VEGF-A from HLMs. We used active site variants of hGIIA and hGX in which the catalytic residue histidine-48 was replaced by glutamine (mutation H48Q). These mutants lack catalytic activity (<1% of the activity of the wt enzyme), whereas their three-dimensional structure, stability and M-type receptor-binding capacity are only slightly changed (35, 44, 45). Fig. 4A shows that hGIIA-H48Q and hGX-H48Q retained their ability to induce the release of VEGF-A from HLMs, which indicates that catalytic activity is not required to elicit VEGF-A production in HLMs.
FIGURE 4.
sPLA2-induced production of VEGF-A is independent from enzymatic activity and involves a receptor-mediated activation of HLMs. A, Effect of H48Q mutants of sPLA2s on VEGF-A release. HLMs were incubated (37°C, 24 h) with RPMI 1640 alone (ctr), with the wt hGIIA and hGX, or the mutants H48Q of hGIIA and hGX. VEGF-A release was determined by ELISA. Data are mean ± SEM of three experiments. *p < 0.05 versus control. B, Effect of Me-Indoxam and RO092906A on hGX-induced VEGF-A release. hGX (3 μg/ml) was preincubated (37°C, 15 min) with RPMI 1640 alone, or with the indicated concentrations of Me-Indoxam (●) or RO092906A (○). HLMs were then incubated (37°C, 24 h) with hGX (3 μg/ml) alone or with the various combinations of hGX with Me-Indoxam (●) or RO092906A (○). VEGF-A release was determined by ELISA. Data are expressed as percent inhibition of the maximum response induced by hGX alone calculated as (R – Rb)/(Rmax – Rb) × 100, where R is the release in samples treated with the combination hGX plus inhibitor, Rb is the release in unstimulated samples, and Rmax is the release in samples stimulated with hGX alone. Data are the mean ± SEM of three experiments. VEGF-A release induced by hGX alone was 661 ± 83 pg/mg protein. The unbroken and broken lines represent the best fit for inhibition of Me-Indoxam and RO092906A, respectively. C, Effect of MAPK inhibitors on sPLA2-induced VEGF-A release. The cells were preincubated (37°C, 1 h) with SB203580 (30 μM), PD98059 (50 μM), or both (30 μM SB203580 and 50 μM PD988059) and then stimulated (37°C, 24 h) with hGX (3 μg/ml). VEGF-A release was determined by ELISA. Data are the mean ± SEM of three experiments. *p < 0.05 versus control; §p < 0.05 versus hGX alone.
In the second group of experiments, we used two active-site sPLA2 inhibitors that block both enzymatic activity and binding to sPLA2 receptors, such as the M-type (46). In fact, Me-Indoxam and other competitive sPLA2 inhibitors, such as RO092906A (33), protrude out of the catalytic groove when bound to sPLA2s thereby leading to steric hindrance and hence interfering with the sPLA2-receptor interaction (46). Thanks to this property, we were able to show, in previous papers, that Me-Indoxam prevented receptor-mediated activation of HLMs stimulated with sPLA2s (27, 28). In three experiments, Me-Indoxam and RO092906A (0.01–10 μM) did not influence the basal secretion of VEGF-A from HLMs (data not shown). Preincubation (15 min, 37°C) of hGX (3 μg/ml) with increasing concentrations of Me-Indoxam or RO092906A before addition to HLMs, resulted in dose-dependent inhibition of VEGF-A release (Fig. 4B). The IC50 values of Me-Indoxam and RO092906A were 221 ± 37 nM and 69 ± 17 nM, respectively. These values are consistent with the affinities of Me-Indoxam (IC50 value of 400–1000 nM) and RO092906A (IC50 value of 40–100 nM) for hGX (33, 34).
Several studies have shown that activation of the M-type receptor induces phosphorylation of the MAPKs p38 and ERK1/2 whose activity mediates several cellular responses evoked by sPLA2s (reviewed in Ref. 25). Thus, to gain further insight into the mechanism involved in sPLA2-induced production of VEGF we examined the effect of specific inhibitors of the MAPKs p38 (SB203580) and ERK1/2 (PD98059) (47). In three experiments, the cells were pre-incubated (37°C, 1 h) with optimal concentrations of SB203580 (30 μM), PD98059 (50 μM), or with a combination of the two inhibitors before stimulation with hGX. SB203580 but not PD98059 significantly inhibited hGX-induced release of VEGF-A up to 60% (Fig. 4C). Incubation of HLMs with both inhibitors caused a reduction of VEGF-A release comparable to that induced by SB203580 alone.
The results of these three groups of experiments indicate that VEGF production induced by sPLA2s does not require the catalytic activity, is prevented by inhibitors of sPLA2-receptor interaction and is reduced by blockade of a signaling pathway associated with activation of the M-type receptor.
Supernatants of HLMs activated by sPLA2s induce angiogenesis in the chick embryo CAM
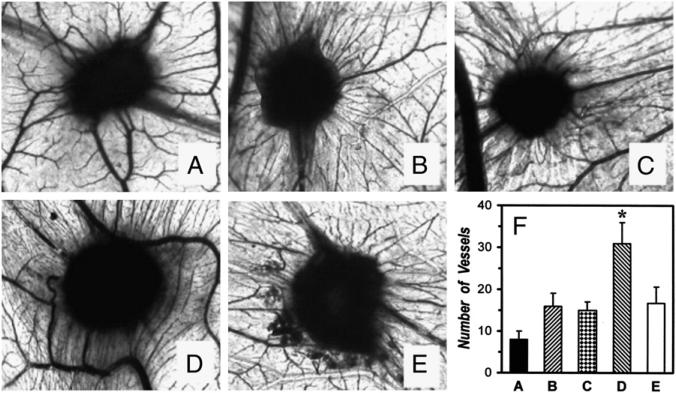
To verify that macrophages stimulated with sPLA2s would produce sufficient VEGFs to elicit angiogenesis in vivo we investigated whether supernatants of sPLA2-activated HLMs induce an angiogenic response in chick embryo CAMs. In three experiments, gelatin sponges adsorbed with supernatants of macrophages, either unstimulated or treated with hGX, were implanted in the CAMs at day 8. At day 12 of incubation, no vascular reaction was detected around the sponges treated with medium alone (Fig. 5A, 5F). Supernatants of unstimulated macrophages kept in culture for 24 h induced the formation of a few vessels at the sponge-CAM boundary (Fig. 5B, 5F). This response was not modified by the addition of Me-Indoxam (10 μM; Fig. 5C, 5F). Supernatants of macrophages treated with hGX (3 μg/ml) greatly enhanced the angiogenic response in the CAMs as witnessed by the presence of allantoic vessels spreading radially toward the sponge in a spoked-wheel pattern (Fig. 5D, 5F). A comparable response was observed in implants treated with 50 ng of VEGF-A165, used as positive control (data not shown). Preincubation (15 min, 37°C) of hGX with Me-Indoxam before the addition to HLMs completely blocked the angiogenic response induced by hGX (Fig. 5E, 5F). Statistical analysis of the three experiments confirmed that supernatants of macrophages treated with hGX significantly increased the number of vessels at the sponge-CAM boundary and that Me-Indoxam suppressed this response (Fig. 5F). To verify whether sPLA2s per se had direct angiogenic activity in the CAM assay, an additional control was performed by adding hGX (3 μg/ml) to the cell-free supernatants of unstimulated HLMs after removal of the cells. The angiogenic response of supernatants supplemented with hGX (15 ± 4 vessels at the sponge-CAM boundary) was comparable to that of untreated supernatants (16 ± 3 vessels at the sponge-CAM boundary), thereby excluding that hGX per se exerts an angiogenic effect in the CAM assay.
FIGURE 5.
Supernatants of HLMs activated by sPLA2 induce an angiogenic response in the chick embryo CAM. Chick embryo CAMs at day 8 of incubation were implanted with gelatin sponges adsorbed with RPMI 1640 alone (A), supernatants of HLMs kept in culture for 24 h at 37°C with RPMI 1640 alone (control, B), with Me-Indoxam alone (10 μM, C), with hGX alone (3 μg/ml, D), or with the combination hGX plus Me-Indoxam (E). At day 12 of incubation, CAMs were examined as described under Materials and Methods. A–E, original magnification ×50. The experiment shown is representative of three separate experiments. In F, data are expressed as number of vessels at the sponge-CAM boundary and are the mean ± SEM of three experiments. *p < 0.05 versus control.
Effect of NECA on sPLA2-induced production of VEGF-A and TNF-α from HLMs
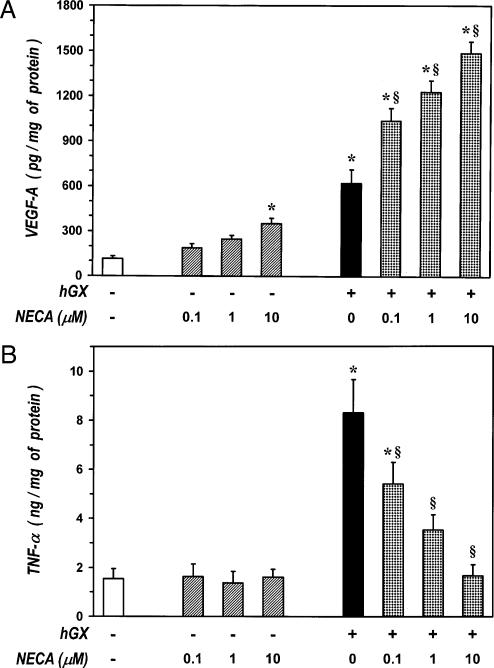
Adenosine is an endogenous purine nucleoside that modulates immune responses by interacting with four subtypes (A1, A2A, A2B, and A3) of membrane receptors expressed on target cells (48). This mediator has been implicated in the pathogenic mechanisms of chronic inflammatory disorders of the airways, such as asthma and COPD, through either increased production and increased density of its receptors (49). In addition, adenosine accumulates in solid tumors where it plays a number of cancer-promoting functions (50). We have shown that sPLA2s are potent stimuli for TNF-α production from HLMs (27, 28, 32). Thus, we evaluated the effects of NECA, a metabolically stable analog of aden-osine, on sPLA2-induced release of VEGF-A and TNF-α from HLMs. Incubation (37°C, 24 h) of HLMs with increasing concentrations (0.1–10 μM) of NECA alone induced a significant release of VEGF-A at the highest concentration examined (Fig. 6A), whereas it did not affect the release of TNF-α (Fig. 6B). Incubation of macrophages with hGX (3 μg/ml) in the presence of increasing concentrations (0.1–10 μM) of NECA caused a synergistic release of VEGF-A compared with that induced by the sPLA2 and NECA alone (Fig. 6A). Considering 100% as the sum of the net release of VEGF-A induced by NECA and hGX alone, the synergistic effect of NECA increased in a concentration-dependent fashion, namely, from ~160% at 0.1 μM to ~190% at 10 μM. By contrast, NECA concentration-dependently suppressed the release of TNF-α induced by hGX (Fig. 6B). Both enhancement of VEGF-A and inhibition of TNF-α were significant at 0.1 μM of NECA and maximal at 10 μM. These results indicate that NECA induces an angiogenic switch in sPLA2-activated HLMs by promoting the production of VEGF-A and by inhibiting that of TNF-α.
FIGURE 6.
Adenosine induces an angiogenic switch in sPLA2-activated HLMs. A, Effect of NECA on hGX-induced release of VEGF-A. HLMs were incubated (37°C, 24 h) with RPMI 1640 alone (ctr), NECA alone (0.1–10 μM), hGX alone (3 μg/ml), or the indicated combinations of hGX plus NECA. VEGF-A releasewas determined by ELISA. Data are the mean ± SEM of three experiments. *p < 0.05 versus control; §p < 0.05 versus hGX alone. B, Effect of NECA on hGX-induced release of TNF-α. HLMs were incubated (37°C, 24 h) as mentioned in A. TNF-α release was determined by ELISA. Data are the mean ± SEM of three experiments. *p < 0.05 versus control; §p < 0.05 versus hGX alone.
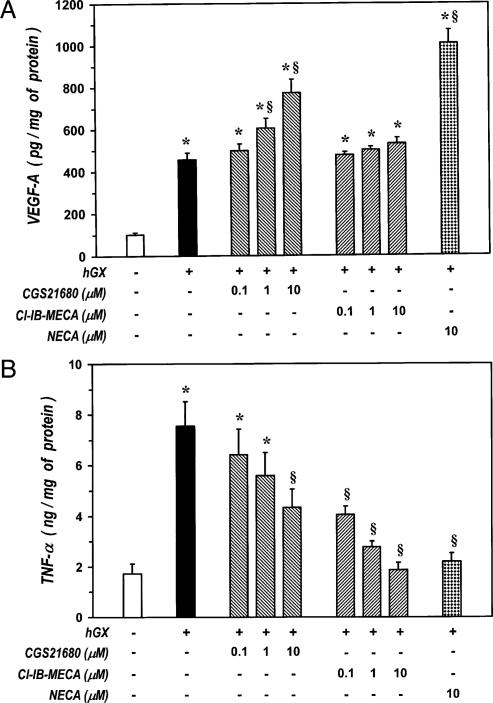
Previous studies have shown that adenosine receptors A2A and A3 modulate the production of VEGF-A and TNF-α in macrophages stimulated by LPS in the presence of adenosine analogs (51–53). To further investigate the adenosine receptor(s) involved in the angiogenic switch of HLMs stimulated with sPLA2s, we evaluated the effect of agonists of A2A (CGS21680) and A3 receptors (Cl-IB-MECA) (54). Both these compounds did not modify the spontaneous release of VEGF-A or TNF-α from HLMs (data not shown). Incubation of macrophages with hGX (3 μg/ml) in the presence of increasing concentrations (0.1–10 μM) of CGS21680 caused a synergistic release of VEGF-A compared with that induced by hGX alone (Fig. 7A). By contrast, Cl-IB-MECA did not enhance sPLA2-induced release of VEGF-A. The synergistic effect of CGS21680 was concentration-dependent ranging from ~135% at 1 μM to ~158% at 10 μM, and it was comparable to that induced by 10 μM of NECA (~175%). In contrast, the A3 receptor agonist Cl-IB-MECA suppressed hGX-induced release of TNF-α (Fig. 7B), whereas CGS21680 only partially inhibited (~50%) this response (Fig. 7B). The inhibitory effect of Cl-IB-MECA was comparable to that of NECA because their IC50 values were both in the nanomolar range (Cl-IB–MECA = 25 ± 11 nM, NECA = 110 ± 23 nM). By contrast, the IC50 value of CGS21680 was >5 μM. These values are consistent with the reported affinity of the various compounds for the adenosine A3 receptor (54). Together these results indicate that adenosine induces a synergistic increase of VEGF-A primarily through the engagement of A2A receptors, whereas it inhibits the production of TNF-α mainly through binding to A3 receptors. Therefore, the angiogenic switch promoted by adenosine in sPLA2-activated macrophages is likely due to a cooperation between A2A and A3 receptors.
FIGURE 7.
The angiogenic switch induced by adenosine in sPLA2-activated HLMs involves a cooperation between A2A and A3 receptors. A, Effect of CGS21680, Cl-IB-MECA, and NECA on hGX-induced release of VEGF-A. HLMs were incubated (37°C, 24 h) with RPMI 1640 alone (ctr), hGX alone (3 μg/ml), or combinations of hGX plus CGS21680 (0.1–10 μM), Cl-IB-MECA (0.1–10 μM), or NECA (10 μM). VEGF-A release was determined by ELISA. Data are the mean ± SEM of three experiments. *p < 0.05 versus control; §p < 0.05 versus hGX alone. B, Effect of CGS21680, Cl-IB-MECA, and NECA on hGX-induced release of TNF-α. HLMs were incubated (37°C, 24 h) as mentioned in A. TNF-α release was determined by ELISA. Data are the mean ± SEM of three experiments. *p < 0.05 versus control; §p < 0.05 versus hGX alone.
Discussion
In this study, we demonstrate that primary human macrophages purified from lung tissue constitutively express and synthesize angiogenic (VEGF-A and VEGF-B) and lymphangiogenic (VEGF-C and VEGF-D) factors. Two isoforms of human sPLA2 (hGIIA and hGX) induced the expression and release of VEGF-A and VEGF-C from HLMs and enhanced the angiogenic activity of these cells by a receptor-mediated mechanism independent of catalytic activity. Activation of HLMs by sPLA2s in the presence of adenosine analogs induced a functional switch of macrophages by increasing VEGF-A and suppressing TNF-α through a cooperation between A2A and A3 adenosine receptors.
Studies performed on tissue biopsies from patients with inflammatory or neoplastic disorders have shown that macrophages express VEGF-A, VEGF-C, and VEGF-D (reviewed in Refs. 4 and 7). However, the pattern of VEGF expression can change depending on the pathophysiological conditions to which macrophages are exposed (55–58). In this study, we show that primary human macrophages isolated from whole lung tissue constitutively express VEGF-A, VEGF-B, VEGF-C, and VEGF-D at both mRNA and protein level. HLMs spontaneously release small amounts of VEGF-A and VEGF-C and their supernatants induce the formation of blood vessels in an in vivo model of angiogenesis. This means that human macrophages possess an intrinsic capacity to produce VEGFs, which induce angiogenesis in normal tissues as well as during inflammatory and neoplastic conditions.
The role of sPLA2s in tissue inflammation and tumor growth is increasingly being appreciated (8–10, 59). The effects of sPLA2s in angiogenesis have been only marginally investigated. Rizzo et al. first showed that sPLA2s of both human and venom (snake and bee) origin induce the migration of endothelial cells by a mechanism involving, at least in part, their catalytic activity (60). In addition, indirect evidence obtained by using an inhibitor of sPLA2 enzymatic activity suggests that sPLA2s are involved in endothelial cell proliferation and migration induced by FGFβ or VEGF-A (61). In this study, we report for the first time that two major human sPLA2s, namely, hGIIA and hGX, induce the expression and release of VEGF-A and VEGF-C from primary human macrophages. VEGF-A is the most potent mediator of angiogenesis in inflamed and neoplastic tissues (1). In contrast, VEGF-C is the main factor responsible for the lymphangiogenesis occurring during tumor progression and metastasis (2). Therefore, our study implies that sPLA2s produced at sites of inflammatory reactions and tumor growth can modulate angiogenesis and lymphangiogenesis by inducing the production of VEGF-A and VEGF-C from macrophages.
The range of concentrations of sPLA2s (1–10 μg/ml) that elicited the production of VEGFs from macrophages includes those detectable in vivo, at least in the case of hGIIA. In fact, concentrations between 1 and 10 μg/ml have been estimated in the airways of patients with inflammatory lung diseases (e.g., bronchial asthma, COPD, and adult respiratory distress syndrome) when the amounts of sPLA2 measured in the bronchoalveolar fluid is corrected for the dilution of the lavage (11). In this context, our observations are particularly relevant because hGIIA and hGX are expressed in the human lung and they are released during lung inflammation and cancer (12, 18–20). Therefore, it is conceivable that production of angiogenic factors by macrophages occurs in the human airways when sPLA2s, such as hGIIA and hGX, are released in vivo.
The concentrations of hGIIA and hGX needed to induce as ignificant release of VEGF-A and VEGF-C are comparable, although hGX is more potent than hGIIA in inducing both VEGFs. These results agree with our previous data showing that hGX is more potent than hGIIA in inducing cytokine production from macrophages (28, 31, 32). Importantly, the release of VEGF-A and VEGF-C induced by hGX is comparable to that induced by the potent activator of macrophages LPS. In particular, at submicromolar concentrations (10 μg/ml = 700 nM), hGX induced quantities of VEGF-A (~1600 pg/mg protein) and VEGF-C (~800 pg) that are among the largest produced by stimulated macrophages (41, 42, 52). Furthermore, the supernatants of hGX-stimulated macrophages induced a strong angiogenic response in the CAM system. This effect is likely due to the production of VEGFs because hGX per se did not stimulate angiogenesis in this experimental model. Thus, hGX appears to be one of the most potent endogenous activators of VEGF production from macrophages. However, further studies are needed to fully evaluate the importance of this proangiogenic mechanism in vivo.
Experiments with metabolic inhibitors showed that Act-D, CHX, and BFA blocked sPLA2-induced release of VEGF-A and VEGF-C indicating that this is an active metabolic process involving mRNA transcription. This was confirmed by our observation that hGX enhanced the mRNA expression of both VEGF-A and VEGF-C. Although the kinetics of mRNA expression of these factors were similar, the kinetics of secretion were different. Indeed, VEGF-A release extended over 12–48 h, whereas VEGF-C release peaked at 24 h and declined thereafter. The latter observation can be explained either by VEGF-C reuptake via a VEGFR-3–mediated internalization (57) or by VEGF-C degradation by lysosomal enzymes secreted in response to sPLA2s (31).
Data obtained with the catalytically inactive sPLA2s (H48Q mutants) indicate that the enzymatic activity is not involved in sPLA2-induced VEGF production. The experiments with the dual sPLA2 inhibitors (Me-Indoxam and RO092906A) and with MAPK inhibitors (SB203580 and PD98059) point to a receptor-mediated mechanism involving either the M-type receptor, which is expressed on HLMs (28), or another, as yet unidentified receptor (35). Although Me-Indoxam and other related compounds were initially characterized as inhibitors of sPLA2 enzymatic activity (34), it was later found that binding with the inhibitor dramatically reduces the affinity of sPLA2s for the M-type receptor (46). The observation that the IC50 values of Me-Indoxam and RO092906A for inhibition of VEGF-A release are similar to those for inhibition of enzymatic activity (33, 34) and of binding to the M-type receptor (46) would indicate that the receptor involved in sPLA2-induced production of VEGFs is the M-type. This hypothesis is supported by the observation that inhibition of MAPK p38, a signaling kinase associated with activation of the M-type receptor (reviewed in Ref. 25), reduces sPLA2-induced production of VEGF-A. However, it should be mentioned that the inhibitory effect of Me-Indoxam and RO092906A does not exclude an interaction of hGIIA and hGX with other membrane targets. In particular, these competitive inhibitors might prevent the interaction of sPLA2s with other unidentified receptors (35) that would bind sPLA2s through a domain close to the catalytic site, as occurs in binding to the M-type receptor (46). It is also noteworthy that hGIIA induces adhesion and proliferation of the macrophage cell line U937 by interacting with certain integrins (i.e., αvβ3 and α4β1) through a domain distant from the catalytic site (30). Although the ability of Me-Indoxam to block such interactions remains to be tested, the possibility exists that hGIIA and hGX activate VEGF production in macrophages by binding to membrane targets other than the M-type receptor.
It is worth noting that blockade of MAPK p38 by SB203580 caused a 60% inhibition of sPLA2-induced release of VEGF-A, whereas blockade of ERK1/2 by PD98059 did not modify this response. These results are in line with previous studies showing that p38 MAPK is involved in VEGF-A production in macrophages activated by different stimuli (62–64). Although ERK1/2 activation has been implicated in VEGF-A production in THP-1 macrophages stimulated by C-reactive protein (65), our data suggest that this pathway is not involved in sPLA2-induced production of VEGF.
Stimulation of macrophages by hGX in the presence of adenosine analogs induced a synergistic increase of VEGF-A mediated by aden-osine receptors A2A and inhibition of TNF-α mediated by A3 receptors. These results depict a functional cooperation between A2A and A3 receptors that causes the proangiogenic switch of human macrophages. This model is reminiscent of the proangiogenic switch previously described in murine macrophages stimulated with adenosine and LPS (or other bacterial products) in which both upregulation of VEGF-A and downregulation of TNF-α were mediated by a synergy between TLRs and the adenosine receptor A2A (51, 52). On the other hand, our results also confirm a role for A3 receptors in adenosine-induced inhibition of TNF-α, as previously reported in human macrophages stimulated with LPS (53).
Like LPS, sPLA2s are multipotent mediators that activate several macrophage functions (reviewed in Ref. 25). However, sPLA2s are also endogenous mediators released during inflammatory lung diseases (11) and tumors (59). These pathological processes are usually associated with an increase of local adenosine production (49, 50). Therefore, it is likely that in vivo, sPLA2s and adenosine are produced simultaneously at sites of lung inflammation and tumor growth and cooperate to promote the vascular remodeling by inducing the production of angiogenic factors from macrophages. In this context, our observation that the combination of sPLA2 and adenosine analogs promotes the angiogenic activity of macrophages provides new information on the tumor-derived factors involved in the development of TAMs. In fact, most TAMs exhibit the proangiogenic M2 pheno-type, which is characterized by increased production of VEGF-A and reduced ability to produce TNF-α (5–7). It is currently believed that this proangiogenic phenotype is induced by local mediators produced at sites of cancer development (4, 5, 7). Thus, it is conceivable that the simultaneous presence of sPLA2s and adenosine at sites of tumor growth (50, 59) may contribute to switching the macrophage phenotype from the tumor-inhibiting M1 to the tumor-supporting M2.
In conclusion, we report a novel function of sPLA2s and expand the spectrum of pathophysiological effects induced by these molecules in human inflammatory cells. Inhibition of sPLA2-induced angiogenic responses may be a strategy with which to reduce angiogenesis and lymphangiogenesis associated with inflammatory diseases of the lung and cancer.
Acknowledgments
This work was supported by grants from the Ministero dell’Istruzione, dell’Università e della Ricerca (to G.M., D.R., and M.T.), the Regione Campania (to G.M.), the Fondazione Cassa di Risparmio di Puglia (Bari, Italy) (to D.R.), Grants HL50040 and HL36235 from the National Institutes of Health (to M.H.G.), the Centre National de la Recherche Scientifique (to G.L.), and the Association pour la Recherche sur le Cancer (to G.L.).
Abbreviations used in this paper
- Act-D
actinomycin D
- BFA
brefeldin A
- CAM
chorioallantoic membrane
- CGS21680
2-p-(2-Carboxyethyl)phenethylamino-5′-N-ethylcarboxamidoadenosine
- CHX
cycloheximide
- Cl-IB-MECA
2-Chloro-N6-(3-iodobenzyl)-adenosine-5′-N-methyluronamide
- COPD
chronic obstructive pulmonary disease
- ctr
control
- GIIA
group IIA
- GX
group X
- hGIIA
human GIIA
- hGX
human GX
- HLM
human lung macrophage
- NECA
5′-(N-ethylcarboxamido) adenosine
- PlGF
placental growth factor
- PLA2
phospholipase A2
- qPCR
quantitative PCR
- sPLA2
secreted phospholipase A2
- TAM
tumor-associated macrophage
- VEGF
vascular endothelial growth factor
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Ferrara N. Vascular endothelial growth factor: pathophysiology and clinical implications. In: Ferrara N, editor. Angiogenesis. From basic science to clinical applications. CRC; New York: 2007. pp. 1–36. [Google Scholar]
- 2.Alitalo K, Tammela T, Petrova TV. Lymphangiogenesis in development and human disease. Nature. 2005;438:946–953. doi: 10.1038/nature04480. [DOI] [PubMed] [Google Scholar]
- 3.Fujiwara N, Kobayashi K. Macrophages in inflammation. Curr. Drug Targets Inflamm. Allergy. 2005;4:281–286. doi: 10.2174/1568010054022024. [DOI] [PubMed] [Google Scholar]
- 4.Ono M. Molecular links between tumor angiogenesis and inflammation: inflammatory stimuli of macrophages and cancer cells as targets for therapeutic strategy. Cancer Sci. 2008;99:1501–1506. doi: 10.1111/j.1349-7006.2008.00853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mantovani A, Schioppa T, Porta C, Allavena P, Sica A. Role of tumor-associated macrophages in tumor progression and invasion. Cancer Metastasis Rev. 2006;25:315–322. doi: 10.1007/s10555-006-9001-7. [DOI] [PubMed] [Google Scholar]
- 6.Allavena P, Sica A, Garlanda C, Mantovani A. The Yin-Yang of tumor-associated macrophages in neoplastic progression and immune surveillance. Immunol. Rev. 2008;222:155–161. doi: 10.1111/j.1600-065X.2008.00607.x. [DOI] [PubMed] [Google Scholar]
- 7.Lamagna C, Aurrand-Lions M, Imhof BA. Dual role of macrophages in tumor growth and angiogenesis. J. Leukoc. Biol. 2006;80:705–713. doi: 10.1189/jlb.1105656. [DOI] [PubMed] [Google Scholar]
- 8.Lambeau G, Gelb MH. Biochemistry and physiology of mammalian secreted phospholipases A2. Annu. Rev. Biochem. 2008;77:495–520. doi: 10.1146/annurev.biochem.76.062405.154007. [DOI] [PubMed] [Google Scholar]
- 9.Burke JE, Dennis EA. Phospholipase A2 structure/function, mechanism, and signaling. J. Lipid Res. 2009;50(Suppl):S237–S242. doi: 10.1194/jlr.R800033-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Triggiani M, Granata F, Giannattasio G, Marone G. Secretory phospholipases A2 in inflammatory and allergic diseases: not just enzymes. J. Allergy Clin. Immunol. 2005;116:1000–1006. doi: 10.1016/j.jaci.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 11.Granata F, Nardicchi V, Loffredo S, Frattini A, Ilaria Staiano R, Agostini C, Triggiani M. Secreted phospholipases A(2): A proinflammatory connection between macrophages and mast cells in the human lung. Immunobiology. 2009;214:811–821. doi: 10.1016/j.imbio.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 12.Abe T, Sakamoto K, Kamohara H, Hirano Y, Kuwahara N, Ogawa M. Group II phospholipase A2 is increased in peritoneal and pleural effusions in patients with various types of cancer. Int. J. Cancer. 1997;74:245–250. doi: 10.1002/(sici)1097-0215(19970620)74:3<245::aid-ijc2>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 13.Masuda S, Murakami M, Ishikawa Y, Ishii T, Kudo I. Diverse cellular localizations of secretory phospholipase A2 enzymes in several human tissues. Biochim. Biophys. Acta. 2005;1736:200–210. doi: 10.1016/j.bbalip.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 14.Mounier CM, Wendum D, Greenspan E, Fléjou JF, Rosenberg DW, Lambeau G. Distinct expression pattern of the full set of secreted phospholipases A2 in human colorectal adenocarcinomas: sPLA2-III as a biomarker candidate. Br. J. Cancer. 2008;98:587–595. doi: 10.1038/sj.bjc.6604184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sved P, Scott KF, McLeod D, King NJ, Singh J, Tsatralis T, Nikolov B, Boulas J, Nallan L, Gelb MH, et al. Oncogenic action of secreted phospholipase A2 in prostate cancer. Cancer Res. 2004;64:6934–6940. doi: 10.1158/0008-5472.CAN-03-3018. [DOI] [PubMed] [Google Scholar]
- 16.Tribler L, Jensen LT, Jørgensen K, Brünner N, Gelb MH, Nielsen HJ, Jensen SS. Increased expression and activity of group IIA and X secretory phospholipase A2 in peritumoral versus central colon carcinoma tissue. Anticancer Res. 2007;27(5A):3179–3185. [PubMed] [Google Scholar]
- 17.Furue S, Kuwabara K, Mikawa K, Nishina K, Shiga M, Maekawa N, Ueno M, Chikazawa Y, Ono T, Hori Y, et al. Crucial role of group IIA phospholipase A(2) in oleic acid-induced acute lung injury in rabbits. Am. J. Respir. Crit. Care Med. 1999;160:1292–1302. doi: 10.1164/ajrccm.160.4.9812042. [DOI] [PubMed] [Google Scholar]
- 18.Hallstrand TS, Chi EY, Singer AG, Gelb MH, Henderson WR., Jr. Secreted phospholipase A2 group X overexpression in asthma and bronchial hyperresponsiveness. Am. J. Respir. Crit. Care Med. 2007;176:1072–1078. doi: 10.1164/rccm.200707-1088OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henderson WR, Jr., Chi EY, Bollinger JG, Tien YT, Ye X, Castelli L, Rubtsov YP, Singer AG, Chiang GK, Nevalainen T, et al. Importance of group X-secreted phospholipase A2 in allergen-induced airway inflammation and remodeling in a mouse asthma model. J. Exp. Med. 2007;204:865–877. doi: 10.1084/jem.20070029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Masuda S, Murakami M, Mitsuishi M, Komiyama K, Ishikawa Y, Ishii T, Kudo I. Expression of secretory phospholipase A2 enzymes in lungs of humans with pneumonia and their potential prostaglandin-synthetic function in human lung-derived cells. Biochem. J. 2005;387:27–38. doi: 10.1042/BJ20041307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leung SY, Chen X, Chu KM, Yuen ST, Mathy J, Ji J, Chan AS, Li R, Law S, Troyanskaya OG, et al. Phospholipase A2 group IIA expression in gastric adenocarcinoma is associated with prolonged survival and less frequent metastasis. Proc. Natl. Acad. Sci. USA. 2002;99:16203–16208. doi: 10.1073/pnas.212646299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mannello F, Qin W, Zhu W, Fabbri L, Tonti GA, Sauter ER. Nipple aspirate fluids from women with breast cancer contain increased levels of group IIa secretory phospholipase A2. Breast Cancer Res. Treat. 2008;111:209–218. doi: 10.1007/s10549-007-9779-1. [DOI] [PubMed] [Google Scholar]
- 23.Morioka Y, Ikeda M, Saiga A, Fujii N, Ishimoto Y, Arita H, Hanasaki K. Potential role of group X secretory phospholipase A(2) in cyclooxygenase-2-dependent PGE(2) formation during colon tumorigenesis. FEBS Lett. 2000;487:262–266. doi: 10.1016/s0014-5793(00)02350-4. [DOI] [PubMed] [Google Scholar]
- 24.Surrel F, Jemel I, Boilard E, Bollinger JG, Payré C, Mounier CM, Talvinen KA, Laine VJ, Nevalainen TJ, Gelb MH, Lambeau G. Group X phospholipase A2 stimulates the proliferation of colon cancer cells by producing various lipid mediators. Mol. Pharmacol. 2009;76:778–790. doi: 10.1124/mol.108.053371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Triggiani M, Granata F, Frattini A, Marone G. Activation of human inflammatory cells by secreted phospholipases A2. Biochim. Biophys. Acta. 2006;1761:1289–1300. doi: 10.1016/j.bbalip.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 26.Dennis EA. Phospholipase A2 in eicosanoid generation. Am. J. Respir. Crit. Care Med. 2000;161:S32–S35. doi: 10.1164/ajrccm.161.supplement_1.ltta-7. [DOI] [PubMed] [Google Scholar]
- 27.Granata F, Frattini A, Loffredo S, Del Prete A, Sozzani S, Marone G, Triggiani M. Signaling events involved in cytokine and chemokine production induced by secretory phospholipase A2 in human lung macrophages. Eur. J. Immunol. 2006;36:1938–1950. doi: 10.1002/eji.200535567. [DOI] [PubMed] [Google Scholar]
- 28.Granata F, Petraroli A, Boilard E, Bezzine S, Bollinger J, Del Vecchio L, Gelb MH, Lambeau G, Marone G, Triggiani M. Activation of cytokine production by secreted phospholipase A2 in human lung macrophages expressing the M-type receptor. J. Immunol. 2005;174:464–474. doi: 10.4049/jimmunol.174.1.464. [DOI] [PubMed] [Google Scholar]
- 29.Park DW, Kim JR, Kim SY, Sonn JK, Bang OS, Kang SS, Kim JH, Baek SH. Akt as a mediator of secretory phospholipase A2 receptor-involved inducible nitric oxide synthase expression. J. Immunol. 2003;170:2093–2099. doi: 10.4049/jimmunol.170.4.2093. [DOI] [PubMed] [Google Scholar]
- 30.Saegusa J, Akakura N, Wu CY, Hoogland C, Ma Z, Lam KS, Liu FT, Takada YK, Takada Y. Pro-inflammatory secretory phospholipase A2 type IIA binds to integrins αvβ3 and α4β1 and induces proliferation of monocytic cells in an integrin-dependent manner. J. Biol. Chem. 2008;283:26107–26115. doi: 10.1074/jbc.M804835200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Triggiani M, Granata F, Oriente A, De Marino V, Gentile M, Calabrese C, Palumbo C, Marone G. Secretory phospholipases A2 induce β-glucuronidase release and IL-6 production from human lung macrophages. J. Immunol. 2000;164:4908–4915. doi: 10.4049/jimmunol.164.9.4908. [DOI] [PubMed] [Google Scholar]
- 32.Triggiani M, Granata F, Petraroli A, Loffredo S, Frattini A, Staiano RI, Monaco G, Marone G. Inhibition of secretory phospholipase A2-induced cytokine production in human lung macrophages by budesonide. Int. Arch. Allergy Immunol. 2009;150:144–155. doi: 10.1159/000218117. [DOI] [PubMed] [Google Scholar]
- 33.Oslund RC, Cermak N, Gelb MH. Highly specific and broadly potent inhibitors of mammalian secreted phospholipases A2. J. Med. Chem. 2008;51:4708–4714. doi: 10.1021/jm800422v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singer AG, Ghomashchi F, Le Calvez C, Bollinger J, Bezzine S, Rouault M, Sadilek M, Nguyen E, Lazdunski M, Lambeau G, Gelb MH. Interfacial kinetic and binding properties of the complete set of human and mouse groups I, II, V, X, and XII secreted phospholipases A2. J. Biol. Chem. 2002;277:48535–48549. doi: 10.1074/jbc.M205855200. [DOI] [PubMed] [Google Scholar]
- 35.Beck S, Lambeau G, Scholz-Pedretti K, Gelb MH, Janssen MJ, Edwards SH, Wilton DC, Pfeilschifter J, Kaszkin M. Potentiation of tumor necrosis factor alpha-induced secreted phospholipase A2 (sPLA2)-IIA expression in mesangial cells by an autocrine loop involving sPLA2 and peroxisome proliferator-activated receptor alpha activation. J. Biol. Chem. 2003;278:29799–29812. doi: 10.1074/jbc.M211763200. [DOI] [PubMed] [Google Scholar]
- 36.Detoraki A, Staiano RI, Granata F, Giannattasio G, Prevete N, de Paulis A, Ribatti D, Genovese A, Triggiani M, Marone G. Vascular endothelial growth factors synthesized by human lung mast cells exert angiogenic effects. J. Allergy Clin. Immunol. 2009;123:1142–1149. 1149, e1141–1145. doi: 10.1016/j.jaci.2009.01.044. [DOI] [PubMed] [Google Scholar]
- 37.Ruohola JK, Valve EM, Karkkainen MJ, Joukov V, Alitalo K, Härkönen PL. Vascular endothelial growth factors are differentially regulated by steroid hormones and antiestrogens in breast cancer cells. Mol. Cell. Endocrinol. 1999;149:29–40. doi: 10.1016/s0303-7207(99)00003-9. [DOI] [PubMed] [Google Scholar]
- 38.Joukov V, Sorsa T, Kumar V, Jeltsch M, Claesson-Welsh L, Cao Y, Saksela O, Kalkkinen N, Alitalo K. Proteolytic processing regulates receptor specificity and activity of VEGF-C. EMBO J. 1997;16:3898–3911. doi: 10.1093/emboj/16.13.3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stacker SA, Stenvers K, Caesar C, Vitali A, Domagala T, Nice E, Roufail S, Simpson RJ, Moritz R, Karpanen T, et al. Biosynthesis of vascular endothelial growth factor-D involves proteolytic processing which generates non-covalent homodimers. J. Biol. Chem. 1999;274:32127–32136. doi: 10.1074/jbc.274.45.32127. [DOI] [PubMed] [Google Scholar]
- 40.Iwata C, Kano MR, Komuro A, Oka M, Kiyono K, Johansson E, Morishita Y, Yashiro M, Hirakawa K, Kaminishi M, Miyazono K. Inhibition of cyclooxygenase-2 suppresses lymph node metastasis via reduction of lymphangiogenesis. Cancer Res. 2007;67:10181–10189. doi: 10.1158/0008-5472.CAN-07-2366. [DOI] [PubMed] [Google Scholar]
- 41.Malaguarnera L, Imbesi R, Di Rosa M, Scuto A, Castrogiovanni P, Messina A, Sanfilippo S. Action of prolactin, IFN-gamma, TNF-alpha and LPS on heme oxygenase-1 expression and VEGF release in human monocytes/macrophages. Int. Immunopharmacol. 2005;5:1458–1469. doi: 10.1016/j.intimp.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 42.Ohsaka A, Hirota-Komatsu S, Shibata M, Ezaki J, Shinohara F, Yoshida T. Specific association of increased vascular endothelial growth factor expression and its receptors with macrophage differentiation of HL-60 leukemia cells. Biochem. Biophys. Res. Commun. 2008;368:543–549. doi: 10.1016/j.bbrc.2008.01.129. [DOI] [PubMed] [Google Scholar]
- 43.Bas S, Neff L, Vuillet M, Spenato U, Seya T, Matsumoto M, Gabay C. The proinflammatory cytokine response to Chlamydia trachomatis elementary bodies in human macrophages is partly mediated by a lipoprotein, the macrophage infectivity potentiator, through TLR2/TLR1/TLR6 and CD14. J. Immunol. 2008;180:1158–1168. doi: 10.4049/jimmunol.180.2.1158. [DOI] [PubMed] [Google Scholar]
- 44.Janssen MJ, van de Wiel WA, Beiboer SH, van Kampen MD, Verheij HM, Slotboom AJ, Egmond MR. Catalytic role of the active site histidine of porcine pancreatic phospholipase A2 probed by the variants H48Q, H48N and H48K. Protein Eng. 1999;12:497–503. doi: 10.1093/protein/12.6.497. [DOI] [PubMed] [Google Scholar]
- 45.Rouault M, Rash LD, Escoubas P, Boilard E, Bollinger J, Lomonte B, Maurin T, Guillaume C, Canaan S, Deregnaucourt C, et al. Neurotoxicity and other pharmacological activities of the snake venom phospholipase A2 OS2: the N-terminal region is more important than enzymatic activity. Biochemistry. 2006;45:5800–5816. doi: 10.1021/bi060217r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boilard E, Rouault M, Surrel F, Le Calvez C, Bezzine S, Singer A, Gelb MH, Lambeau G. Secreted phospholipase A2 inhibitors are also potent blockers of binding to the M-type receptor. Biochemistry. 2006;45:13203–13218. doi: 10.1021/bi061376d. [DOI] [PubMed] [Google Scholar]
- 47.Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haskó G, Linden J, Cronstein B, Pacher P. Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat. Rev. Drug Discov. 2008;7:759–770. doi: 10.1038/nrd2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Caruso M, Holgate ST, Polosa R. Adenosine signalling in airways. Curr. Opin. Pharmacol. 2006;6:251–256. doi: 10.1016/j.coph.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 50.Spychala J. Tumor-promoting functions of adenosine. Pharmacol. Ther. 2000;87:161–173. doi: 10.1016/s0163-7258(00)00053-x. [DOI] [PubMed] [Google Scholar]
- 51.Leibovich SJ, Chen JF, Pinhal-Enfield G, Belem PC, Elson G, Rosania A, Ramanathan M, Montesinos C, Jacobson M, Schwarzschild MA, et al. Synergistic up-regulation of vascular endothelial growth factor expression in murine macrophages by adenosine A(2A) receptor agonists and endotoxin. Am. J. Pathol. 2002;160:2231–2244. doi: 10.1016/S0002-9440(10)61170-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pinhal-Enfield G, Ramanathan M, Hasko G, Vogel SN, Salzman AL, Boons GJ, Leibovich SJ. An angiogenic switch in macrophages involving synergy between Toll-like receptors 2, 4, 7, and 9 and adenosine A(2A) receptors. Am. J. Pathol. 2003;163:711–721. doi: 10.1016/S0002-9440(10)63698-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sajjadi FG, Takabayashi K, Foster AC, Domingo RC, Firestein GS. Inhibition of TNF-alpha expression by adenosine: role of A3 adenosine receptors. J. Immunol. 1996;156:3435–3442. [PubMed] [Google Scholar]
- 54.Jacobson KA, Gao ZG. Adenosine receptors as therapeutic targets. Nat. Rev. Drug Discov. 2006;5:247–264. doi: 10.1038/nrd1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nakano T, Nakashima Y, Yonemitsu Y, Sumiyoshi S, Chen YX, Akishima Y, Ishii T, Iida M, Sueishi K. Angiogenesis and lymphangiogenesis and expression of lymphangiogenic factors in the atherosclerotic intima of human coronary arteries. Hum. Pathol. 2005;36:330–340. doi: 10.1016/j.humpath.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 56.Rutanen J, Leppänen P, Tuomisto TT, Rissanen TT, Hiltunen MO, Vajanto I, Niemi M, Häkkinen T, Karkola K, Stacker SA. Vascular endothelial growth factor-D expression in human atherosclerotic lesions. Cardiovasc. Res. 2003;59:971–979. doi: 10.1016/s0008-6363(03)00518-2. [DOI] [PubMed] [Google Scholar]
- 57.Schoppmann SF, Birner P, Stöckl J, Kalt R, Ullrich R, Caucig C, Kriehuber E, Nagy K, Alitalo K, Kerjaschki D. Tumor-associated macrophages express lymphatic endothelial growth factors and are related to peritumoral lymphangiogenesis. Am. J. Pathol. 2002;161:947–956. doi: 10.1016/S0002-9440(10)64255-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barbera-Guillem E, Nyhus JK, Wolford CC, Friece CR, Sampsel JW. Vascular endothelial growth factor secretion by tumor-infiltrating macrophages essentially supports tumor angiogenesis, and IgG immune complexes potentiate the process. Cancer Res. 2002;62:7042–7049. [PubMed] [Google Scholar]
- 59.Cummings BS. Phospholipase A2 as targets for anti-cancer drugs. Biochem. Pharmacol. 2007;74:949–959. doi: 10.1016/j.bcp.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 60.Rizzo MT, Nguyen E, Aldo-Benson M, Lambeau G. Secreted phospholipase A(2) induces vascular endothelial cell migration. Blood. 2000;96:3809–3815. [PubMed] [Google Scholar]
- 61.Chen W, Li L, Zhu J, Liu J, Soria J, Soria C, Yedgar S. Control of angiogenesis by inhibitor of phospholipase A2. Chin. Med. Sci. J. 2004;19:6–12. [PubMed] [Google Scholar]
- 62.Itaya H, Imaizumi T, Yoshida H, Koyama M, Suzuki S, Satoh K. Expression of vascular endothelial growth factor in human monocyte/macrophages stimulated with lipopolysaccharide. Thromb. Haemost. 2001;85:171–176. [PubMed] [Google Scholar]
- 63.Salomonsson L, Pettersson S, Englund MC, Wiklund O, Ohlsson BG. Post-transcriptional regulation of VEGF expression by oxidised LDL in human macrophages. Eur. J. Clin. Invest. 2002;32:767–774. doi: 10.1046/j.1365-2362.2002.01072.x. [DOI] [PubMed] [Google Scholar]
- 64.Sato F, Imaizumi T, Sashinami H, Yoshida H, Kusumi T, Mori F, Wakabayashi K, Nakane A, Satoh K, Kijima H. Upregulation of vascular endothelial growth factor by heat-killed Listeria monocytogenes in macrophages. Biochem. Biophys. Res. Commun. 2007;354:608–612. doi: 10.1016/j.bbrc.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 65.Bello G, Cailotto F, Hanriot D, Kolopp-Sarda MN, Latger-Cannard V, Hess K, Zannad F, Longrois D, Ropars A. C-reactive protein (CRP) increases VEGF-A expression in monocytic cells via a PI3-kinase and ERK 1/2 signaling dependent pathway. Atherosclerosis. 2008;200:286–293. doi: 10.1016/j.atherosclerosis.2007.12.046. [DOI] [PubMed] [Google Scholar]