Abstract
Background
Alterations in pulmonary blood flow are often associated with the initiation and progression of pulmonary vascular disease. However, the cellular mechanisms involved in mediating flow effects in the pulmonary circulation remain unclear. Depending on the disease condition flow may be extremely low or high. We therefore examined effects of pathologically low and high flow on endothelial production of factors capable of affecting pulmonary vascular tone and structure and on potential underlying mechanisms.
Methods
Flow effects on pulmonary endothelial release of NO, PGF1a, ET-1 and TxB2, and on expression of total and phosphorylated eNOS and Akt, and VEGF were examined. Additionally, in a co-culture system, effects of flow-exposed endothelial cells on smooth muscle (SM) proliferation and contractile protein were studied.
Results
Compared to physiological flow, pathologically high and low flow attenuated endothelial release of NO, PGF1a, and enhanced release of ET-1. Physiological flow activated Akt/eNOS pathway while pathological flow depressed it. Pathologically high flow altered VE-cadherin expression. Pathologically high flow on the endothelium upregulated alpha-SM-actin and SM-MHC without affecting SM proliferation.
Conclusion
Physiological flow leads to production of mediators which favor vasodilation. Pathological flow alters the balance of mediator production which favors vasoconstriction.
Keywords: Pulmonary hypertension, Shear stress, Nitric Oxide, Endothelin, Vascular tone, Vascular remodeling
INTRODUCTION
Alterations in pulmonary blood flow are thought to be a critical factor in the initiation and exacerbation of pulmonary vascular diseases1–4. Changes in the flow magnitude are observed in both acute and chronic forms of pulmonary hypertension 3. Studies in animals and humans have shown that high blood flow can lead to the development of pulmonary hypertension5–7. Increases in blood flow through the creation of systemic-to-pulmonary shunts are often used to induce pulmonary hypertension in animals8. Significantly increased blood flow and flow shear stress is also found in patients with congenital heart diseases having a systemic-to-pulmonary communication such as patent ductus arteriosus or atrial and ventricular septal defects, conditions which often lead to pulmonary hypertension. Peak flow shear stresses in these conditions may reach levels far above physiological shear stress (> 80 dyne/cm2) 9. Additionally, reports have shown that patients with severe pulmonary hypertension are characterized by reduced blood flow and low shear stress (5–8 dyne/cm2) compared to normal flow and physiological shear stress (15–20 dyne/cm2) in normotensive patients 4. Thus, both increased and decreased flow may induce adverse responses in the pulmonary vasculature, as demonstrated in the banded left pulmonary artery model 10. However, despite these observations few studies have attempted to define the mechanisms underlying how the magnitude of flow regulates endothelial function in the pulmonary circulation.
Previous studies have established that shear stress is an important mechanical factor that regulates endothelial structure, function and growth. Numerous investigations, primarily in the systemic circulation, have focused on physiological shear stress (10 – 25 dyne/cm2) and relatively low shear stress (<5 dyne/cm2) as conditions relevant to development of atherosclerosis. Generally, it is believed that smooth, laminar, physiologic shear stress provides atheroprotective effects, while turbulent, oscillatory or low flow shear stress (−4 – 5 dynes/cm2) stimulates atherosclerosis formation 11. Physiological flow shear stress (10–25 dyne/cm2) serves as an important regulator of pathophysiologically relevant gene expression in endothelial cells12–15. Interestingly, despite the vast number of studies in endothelial mechanobiology, surprisingly little is known about the effect of pathologically high flow shear stress on the structure and function of the pulmonary vasculature despite the fact that shear stresses often above 70 dynes/cm2 levels have been observed in hypertension conditions11.
Given that the flow magnitude plays a role in the pathogenesis and progression of pulmonary diseases, we sought to investigate the effects of both pathologically high and low shear, compared to physiological shear, on pulmonary endothelial production of vasodilating and vasoconstricting factors and to examine effects of said changes on smooth muscle cell phenotype by examining contractile protein and proliferation changes in smooth muscle cells co-cultured with endothelial cells exposed to varying shear conditions. To test this, we have designed hemodynamic flow conditions to encompass both low and high end of pathological flow shear stresses ranging from 5 dyne/cm2 to 120 dyne/cm2 and studied their influences on pulmonary arterial endothelial cells (PAECs) and pulmonary arterial smooth muscle cells (PASMCs).
MATERIALS AND METHODS
Cell Culture
Bovine pulmonary artery endothelial cells (PAECs) and bovine pulmonary artery smooth muscle cells (PASMCs) were isolated from neonatal calves as previously described 16,17. PAECs were maintained in a growth medium (D-Valine MEM medium; Mediatech, Inc.; Herndon, VA) containing 20% fetal bovine serum (FBS, Gemini Bio-products; West Sacramento, CA), 2% L-glutamine (Invitrogen; Carlsbad, CA) and 5% gentamicin (Invitrogen/Gibco; Carlsbad, CA)]. PASMCs were maintained in Dulbecco’s modification of eagle’s medium (DMEM, Cellgro; Herndon, VA) containing 10% FBS and 5% gentamicin (MP Biomedicals, LLC; Solon, OH). Cells at passages 4–8 were used for all experiments.
Experimental protocols for flow shear stress studies
To examine the response of PAEC monolayer to flow conditions, plain microscope slides were coated with 25µg/ml fibronectin. Then, PAECs at a concentration of 4.0×105 per ml were seeded on the fibronectin-coated slides and grown to confluence. Monolayer of confluent PAECs was exposed to laminar shear stress with low pulsation (±10%) to mimic normal flow conditions in pulmonary arteries. The flow studies were carried out using a flow chamber connected to a variable speed flow pump (Control Company; Friendswood, TX). Physiological shear stresses in the arterial vascular network range from 10 to 70 dynes/cm2. Therefore, we chose 20 and 60 dynes/cm2 as representative mean values for shear stresses in the physiological range, 90 and 120 dynes/cm2 as representative mean values for pathologically high level of shear stresses and 5 dynes/cm2 as representative mean value for pathologically low level of shear stresses. PAEC grown in the absence of flow were also studied. Endothelial cells were exposed to the flow for 20 hours, and then they were collected and analyzed using either western blot assays or immuno-fluorescence analyses. A fixed amount of reservoir media (15ml) was used for the flow circulation to ensure that the concentrations of released substances were comparable between experiments. The media was collected and stored in −80°C until use. Two types of flow conditioning were tested: in one case, the flow was increased abruptly to the pre-defined shear stress level; in the other case, the flow was gradually increased to the pre-defined shear stress level by preconditioning with 20 dynes/cm2 for 2 hours. No significant differences were observed between these two conditions. Additionally, as pressure may also modulate pulmonary reactivity18, the pressure was measured and found to fall in a low pressure range (within the range of normal blood pressure in pulmonary vascular system, < 20mmHg). Thus, the pressure influence is not taken into consideration in this study.
To study the signaling pathway that regulates the shear stress induced eNOS expression and activation PAECs were incubated with 100nM phosphoinositide-3 kinase (PI3K) inhibitor wortmannin (Sigma; Saint Louis, MI) for 2hrs and then they were exposed to flow shear stress of 20 dynes/cm2 using medium with 100nM wortmannin.
To examine the response of co-cultured PAECs and PASMCs to various flow conditions, PASMCs were seeded on microscope slides that were coated with collagen matrix and PAECs were seeded on isopore polycarbonate membranes with 4µm pores (Millipore Corporate, Billerica, MA) coated with matrigel matrix, a mimetic basement membrane layer (1:10 dilution, BD Biosciences, Franklin Lakes, NJ). During experiments, membranes seeded with PAECs were brought into direct contact with slides seeded with PASMCs before PAECs were subject to flow.
Immunofluorescent confocal imaging
After cells were exposed to different shear stress for 20 hours, slides were fixed with 4% paraformaldehyde, permeablized with 0.1% triton, blocked with FBS, incubated with primary rabbit polyclonal anti-vascular endothelial cadherin (VE-cadherin) antibody (Alexis Biochemicals; San Diego, CA), followed by incubation with anti-rabbit IgG antibody conjugated with fluorescent dye (Cy3). Subsequently, slides were incubated with phalloidin conjugated with fluorescent dye (Alexa Fluor 488, Invitrogen; Carlsbad, CA) to detect F-actin stress fibers, and then mounted with DAPI SlowFade (Invitrogen; Carlsbad, CA) to detect cell nuclei. Fluorescently labeled cells were evaluated using an epifluorescent microscope and confocal microscope (Zeiss; Peabody, MA).
Western blot analysis
Western blot assays were carried out on PAECs after they were exposed to different shear stress conditions. Ten microgram of each PAEC protein extract were loaded, separated, and transferred to a nitrocellulose membrane. The membrane was incubated with polyclonal rabbit endothelial nitric oxide synthase (eNOS) antibody (Santa Cruz Biotechnology, INC.; Santa Cruz, CA; 1:5,000 dilution), polyclonal rabbit phospho-eNOSs1177 antibody (Cell Signaling; Danvers, MA; 1:1,000 dilution), polyclonal rabbit Akt antibody (Cell Signaling; Danvers, MA; 1:500 dilution), polyclonal rabbit phospho-AktSer473 antibody (Cell Signaling; Danvers, MA; 1:500 dilution), polyclonal rabbit prostaglandin I synthase (PGIS) antibody (Cayman Chemical; 1:5,000 dilution), or polyclonal rabbit anti- vascular endothelial growth factor (VEGF) antibody (Santa Cruz Biotechnology, INC.; Santa Cruz, CA; 1:100 dilution). The next day, the membrane was incubated with either anti-rabbit or anti-mouse IgG antibody (Amersham Biosciences; Piscataway, NJ), then incubated with electrochemiluminescence reagent (ECL, Amersham Biosciences; Piscataway, NJ), and finally exposed to hyperfilm (Amershame Biosciences; Piscataway, NJ) for visualization of protein expression.
For PASMC, blots were incubated with monoclonal mouse proliferating cell nuclear antigen (PCNA) antibody (Sigma; Saint Louis, MI; 1:1,000 dilution), monoclonal mouse smooth muscle α actin (α-SM-actin) antibody (Sigma; Saint Louis, MI; 1:1,000 dilution), or monoclonal mouse smooth muscle myosin heavy chain (SM-MHC) antibody (Sigma; Saint Louis, MI; 1:100 dilution). In the western blot assays for PAECs and PASMCs, β-actin or GAPDH was used as reference proteins.
ELISA assay
The ET-1 level in the media collected after flow experiments was determined using a commercial endothelin ELISA Kit (American Research Products, Inc.; Belmont, MA). In brief, a serial standard was made with standard cell culture medium. A detection antibody was added to all the samples and standard wells, and was incubated. The next day, the conjugates and substrate were added to all wells. Absorbance was determined using a plate reader at 450 nm using 620 nm as the reference. The results were corrected according to the protein weight and expressed as picomole per microgram of protein.
Enzyme immunoassay (EIA)
Prostacyclin and thromboxane A2 content in the media collected after flow experiment was measured as their stable metabolites, 6-keto-prostaglandin F1a (PGF1a) and thromboxane B2 (TxB2), using commercial PGF1α and TxB2 EIA Kit (Cayman; Ann Arbor, MI) following manufacture’s instruction.
Nitric oxide release assay
Total nitric oxide (NO) content in flow media was determined by measuring total nitrite concentration using Total Nitric Oxide Assay Kit (Thermo Scientific; Rockford, IL) following manufacture’s instruction.
Data analysis
All data were expressed as means ± SEM, and n indicates the number of sample studied. Comparisons among means of the experimental groups were made using One-Way ANOVA and if there was significant difference among groups then a Student’s t test was used to compare individual groups. A P value < 0.05 was considered significantly different.
RESULTS
Pathological shear stresses reduce vasodilator release and promote vasoconstrictor release
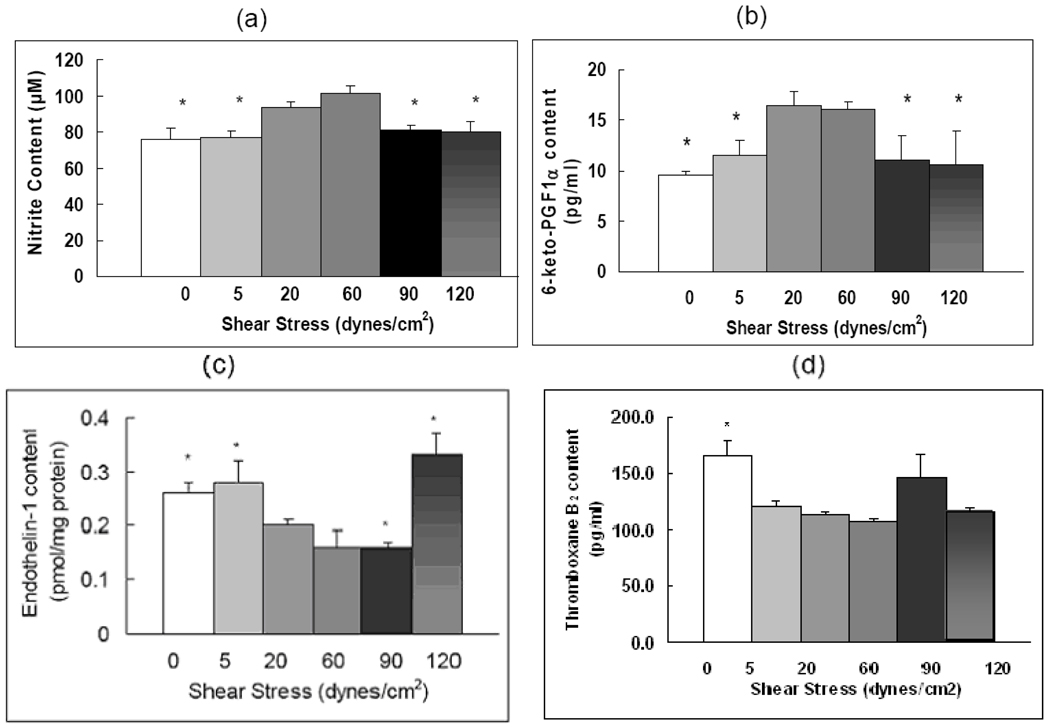
Because vasoconstriction is thought to play a major role in the pulmonary hypertension process, we examined the release of both vasodilators (i.e. nitric oxide, prostacyclin) and vasoconstrictors (i.e. endothelin, thromboxane) from the pulmonary arterial endothelium in response to various flow conditions. Figure 1a shows endothelial release of NO. Release was highest at physiological shear stresses, 20 and 60 dynes/cm2. Compared to 20 dynes/cm2, pathologically high shear stress reduced NO release by 14% at 90 dynes/cm2 and by 15% at 120 dynes/cm2 (P < 0.05). Compared to physiological shear stress of 20 dynes/cm2, a low shear stress (5 dynes/cm2) or no shear stress (0 dynes/cm2) also reduced NO release. Figure 1b shows release of prostacyclin, an endothelial-derived factor with potent vasodilatory effect. We assessed release of PGI2 in the media by measuring its stable metabolite PGF1α. PGF1α release was optimal at physiologic shear stresses (20 and 60 dynes/cm2). Compared to physiological shear stress of 20 dynes/cm2, pathologically high shear stress (90 and 120 dynes/cm2) decreased PGF1α release by 30–40% (P < 0.05) and a pathologically low shear stress (5 dynes/cm2) or no shear stress (0 dynes/cm2) also decreased PGF1α release by a similar percent (P < 0.05). Compared to NO and PGF1α, ET-1 release showed a different pattern in relation to flow shear stress. ET-1 production was low at 20, 60 and 90 dynes/cm2 (Figure 1c). Pathologically high shear stress increased ET-1 release by 65% at 120 dynes/cm2, compared to 20 dynes/cm2. ET-1 release at 5 dynes/cm2 was also increased 40% compared to 20 dynes/cm2. Thromboxane A2 is another important vasoconstrictor in pulmonary hypertension. We found that compared to the physiologic shear stress (20 dynes/cm2), the content of thromboxane B2, a stable metabolite of TxA2, in the medium was 46% higher at static condition (P < 0.05). Neither low (5 dynes/cm2) nor high pathological flow shear stresses (90 and 120 dynes/cm2) affected TxB2 concentrations compared to physiologic shear (20 dynes/cm2). (0.17 < P < 0.5, Figure 1d).
Figure 1. Pathological shear stresses reduce NO and PGF1α release and promote ET-1 release.
(a) Nitric oxide release as measured by the total content of nitrite in flow media. (b) EIA measurement results of the 6-keto-prostaglandin F1α (PGF1α) content in the flow media. (c) ELISA measurement results of the endothelin-1 (ET-1) content in the flow media; (d) EIA measurement results of the thromboxane B2 (TxB2) content in the flow media. The media was collected from the flow circulation after cells were exposed to different shear stresses. Data represent means ± SEM (n=3–6). The conditions marked with ‘*’ are significantly different from 20 dynes/cm2 shear stress (P ≤ 0.05).
Shear stresses affect NO release by altering expression and activation of eNOS through PI3K/Akt signaling pathway
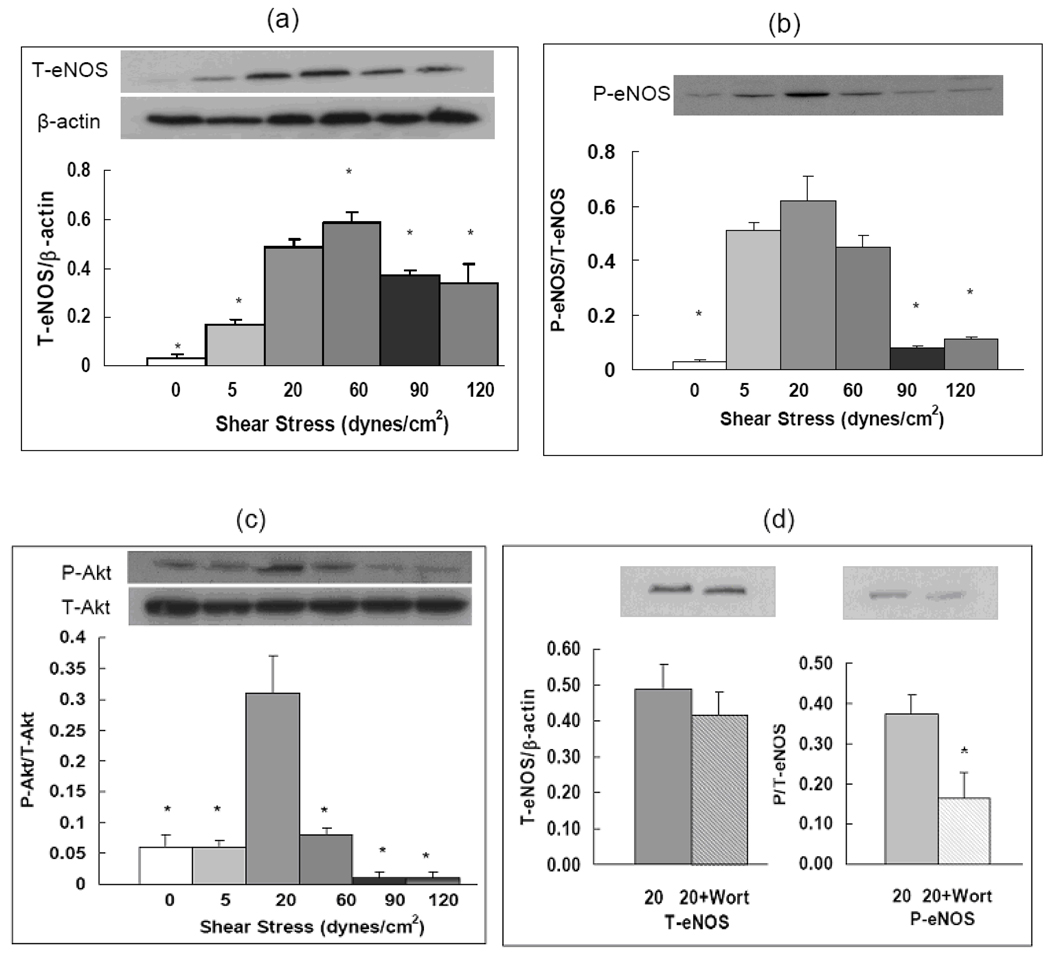
Because NO release was found to be significantly regulated by flow, we sought to determine mechanisms involved in this process. We examined changes in eNOS expression and activation in PAECs in response to shear stress and then studied the PI3K/Akt pathway in relation to changes in eNOS. Compared to physiological shear stress conditions total eNOS (T-eNOS) expression at shear stress above or below the physiological range was decreased (Figure 2a). It was decreased by 24% at 90 dynes/cm2 and by 31% at 120 dynes/cm2 (P < 0.05). Pathologically low shear stress (5 dynes/cm2) also decreased T-eNOS protein expression by 65%, compared to physiological shear stress of 20 dynes/cm2 (Figure 2a). We found that expression of T-eNOS at all flow conditions were higher than those under static conditions (0.03 ± 0.02), which is in good agreement with previous studies 19.
Figure 2. Shear stresses affect expression and activation of eNOS through PI3K/Akt signaling pathway.
Western blot images and quantitative measurements of proteins expressed by PAECs: (a) total eNOS (T-eNOS) expressed as the ratio of T-eNOS to β-actin; (b) phosphorylated eNOSer1177 (P-eNOS) expressed as the ratio of P-eNOS to T-eNOS; and (c) Phosphorylated AktSer473 (P-Akt) expressed as the ratio of P-Akt to total Akt (T-Akt). Protein expressions were measured with PAECs exposed to shear stresses of 0, 5, 20, 60, 90, 120 dynes/cm2. (d) Total and phosphorylated eNOS expression under flow of 20 dynes/cm2 with or without wortmannin (100nM). Data represent means ± SEM, n=4–6 for each group. The conditions marked with ‘*’ are significantly different from shear stress at 20 dynes/cm2 (P ≤ 0.05).
We also investigated shear-stress-mediated activation of eNOS by examining phosphorylation of eNOS. The expression of phosphorylated-eNOS (P-eNOS) at S1179, expressed as the ratio of P-eNOS to T-eNOS, was highest at 20 dynes/cm2 (0.62 ± 0.09). Phosphorylation of eNOS was dramatically decreased by 87% and 82% at 90 dynes/cm2 and 120 dynes/cm2 (0.08 ± 0.01, 0.11 ± 0.01 respectively; P < 0.05 compared to 20 dynes/cm2). Phosphorylation of eNOS at 5 dynes/cm2 (0.51 ± 0.03; P = 0.3608) was not statistically different from 20 dynes/cm2 (Figure 2b). However, because T-eNOS expression was far lower at 5 dynes/cm2 than that at 20 dynes/cm2, the amount of P-eNOS was actually lower at 5 dynes/cm2 as well. Phosphorylation of eNOS was dramatically reduced under static conditions (0.03 ± 0.01) compared to both 5 and 20 dynes/cm2.
Akt has been demonstrated as a critical signaling intermediate in the shear-dependent regulation of eNOS. Akt is a serine/threonine kinase activated by several phosphatidylinositol-dependent protein kinases. Phosphorylation of S473 on Akt is coincident with Akt activation in vivo and in vitro and has been used as a marker for Akt activity. Therefore, we examined phosphorylation of S473 on Akt in PAECs exposed to various flow conditions. As demonstrated in Figure 2c, phosphorylation of Akt, expressed as the ratio of phosphorylated Akt (P-Akt) to T-Akt, was highest at physiologic shear stress of 20 dynes/cm2 (0.31 ± 0.06). Phosphorylation of Akt was decreased by 74% at 60 dynes/cm2, and dramatically decreased by 97% at 90 dynes/cm2 and 120 dynes/cm2. Phosphorylation of Akt was also decreased by 81% at 5 dynes/cm2 (0.06 ± 0.01; P < 0.05) and static conditions (0.06 ± 0.02; P < 0.05). Our results showed that shear stress-mediated phosphorylation of Akt was generally consistent with changes of eNOS phophorylation in response to flow shear stresses.
To confirm the signaling pathway that regulates eNOS expression under flow stimulation we incubated PAECs with 100nM wortmannin, a PI3K inhibitor, and then exposed cells to flow shear stress of 20 dynes/cm2. We found that without flow shear stress, wortmannin had no effect on either T-eNOS expression or eNOS phosphorylation (data not shown). After exposure to shear stress, though expression of T-eNOS was not changed, phosphorylation of eNOS was significantly decreased by 54% (P < 0.05) in the presence of wortmannin (Figure 2d).
Effects of flow shear stresses on VEGF expression
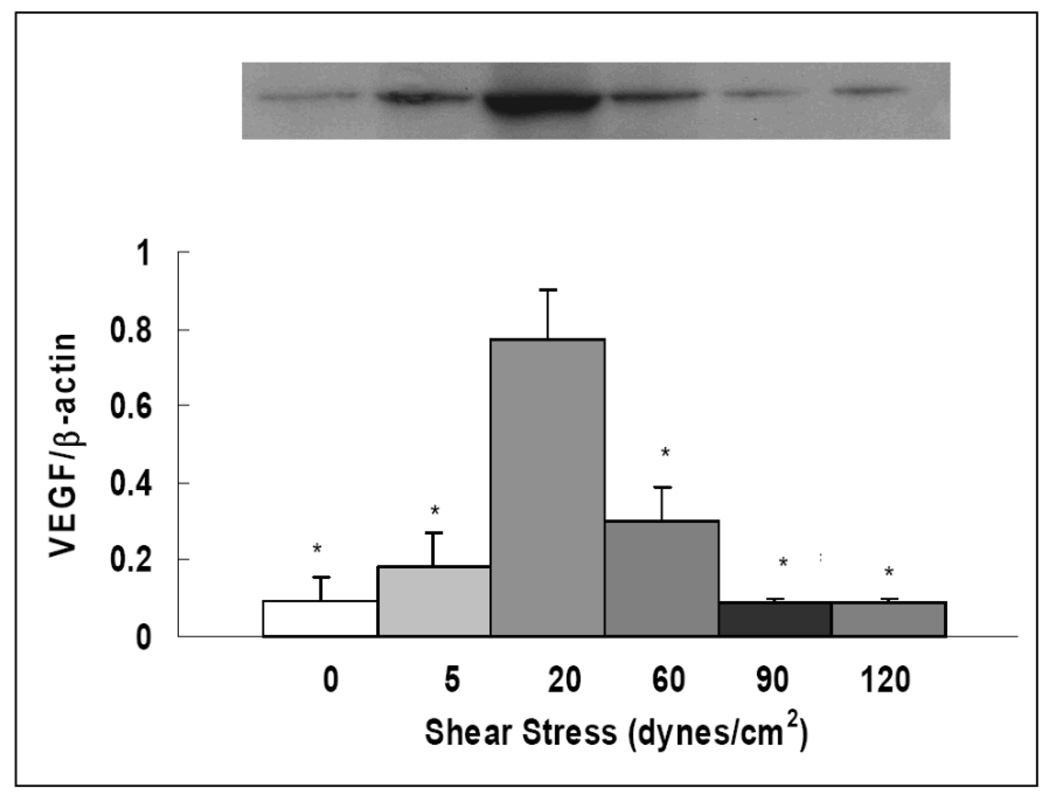
VEGF is an important growth factor and signaling protein that is associated with endothelial growth, vasculogenesis and angiogenesis, and it is essential in maintaining normal endothelial function. Thus, we explored changes in VEGF expression by PAECs in response to different flow conditions. As shown in Figure 3, VEGF protein expression, expressed as ratio of VEGF to β-actin, was highest at physiologic shear stress of 20 dynes/cm2 (0.77 ± 0.13). Its expression was decreased by more than 50% at 60 dynes/cm2. At the shear stresses of 90 and 120 dynes/cm2, VEGF expression was further decreased. Pathologically low shear stress at 5 dynes/cm2 also dramatically reduced VEGF protein expression. VEGF expression was extremely low under static conditions (0.09 ± 0.06).
Figure 3. Pathological shear stresses reduce VEGF expression.
Western blot images and quantitative measurements of VEGF expression. Results expressed as ratio of VEGF to β-actin. Data represent means ± SE (n=3–4). The conditions marked with ‘*’ are significantly different from 20 dynes/cm2 (P ≤ 0.05).
Effects of flow shear stresses on the endothelial structure
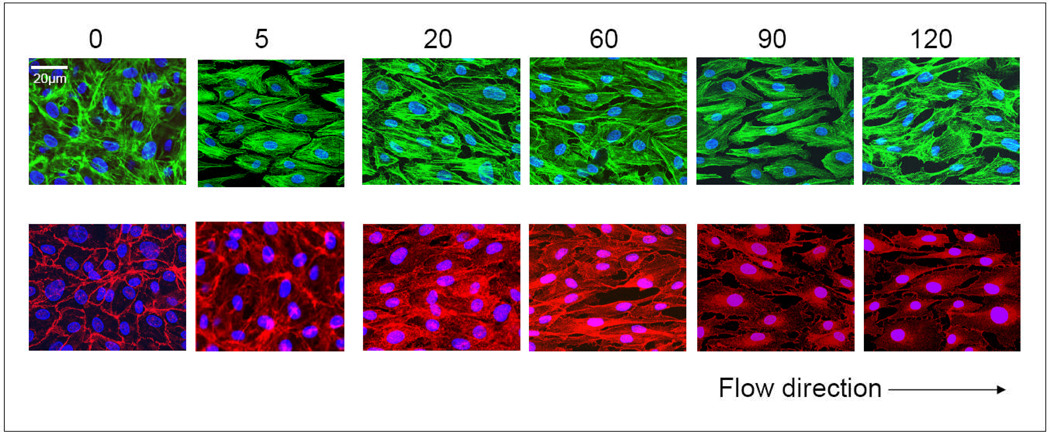
Endothelial cells sense flow conditions through mechanosensory complexes. Cytoskeletal proteins and VE-cadherin have been described as structural constituents of large mechanosensory complexes in endothelial cells12,20. A relationship between these structural molecules and Akt/eNOS module has been suggested in previous studies. Thus, we examined effects of flow on the distribution of F-actin and VE-cadherin (Figure 4). Staining with F-actin antibody showed that F-actin aligned in the direction of the flow. The brightness of staining and the pattern of F-actin stress fibers in endothelial cells were not significantly affected by the level of the shear stress though they were consistently different than those in endothelial cells cultured under static conditions. In contrast, the magnitude of shear stress regulated the brightness of staining and distribution of VE-cadherin. Under static or low flow conditions (5 dynes/cm2), endothelial cells displayed continuous VE-cadherin staining along the peripheral cell membrane. After exposure to physiological shear stress conditions with 20 and 60 dynes/cm2, endothelial cells still displayed continuous VE-cadherin staining along the peripheral cell membrane, while the staining in the center of basal and apical membranes was stronger and displayed punctuate accumulations. Discontinuous VE-cadherin was found on the cells exposed to shear stresses of 90 and 120 dynes/cm2. VE-cadherin staining along the peripheral cell membrane became intermittent, and predominantly exhibited small, distinct "dashes" rather than as the continuous linear staining. This suggested disruption of cell-cell junctions by pathologically high flow.
Figure 4. Shear stresses affect endothelial structure.
Subcellular structures of PAECs were stained with different colors: F-actin (green), VE-cadherin (red) and nuclei (blue). The numbers indicates the level of flow shear stresses (dynes/cm2) that cells were exposed to.
Effects of shear stresses on co-cultured PASMC proliferation and contractile protein expression
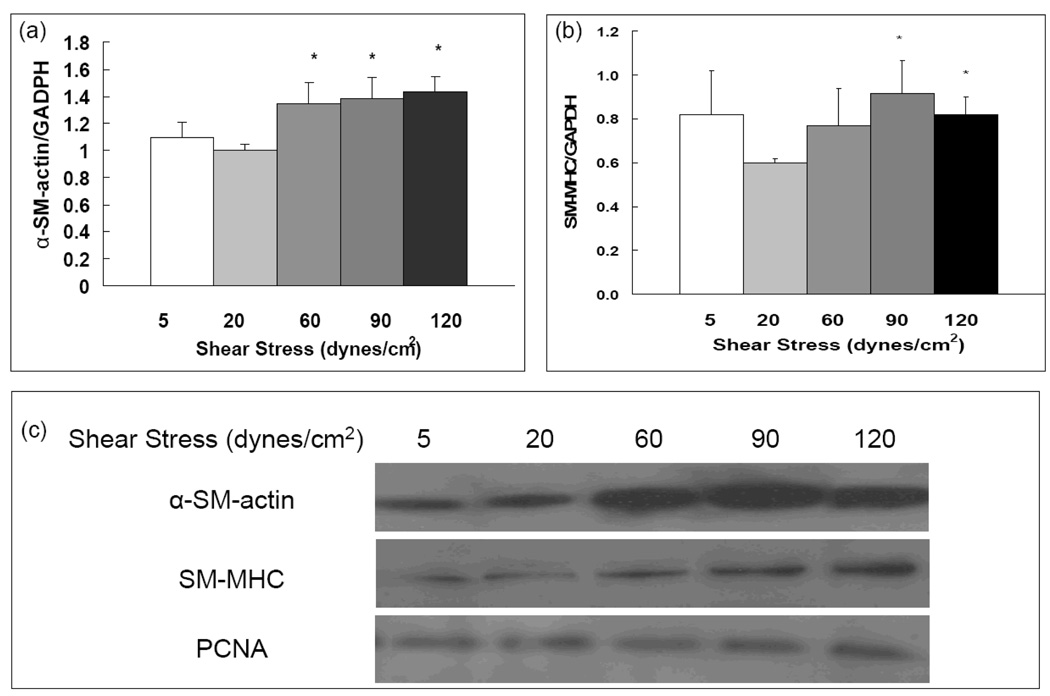
Endothelial cells serve as mechanical sensors that transduce mechanical signals to biochemical signals which can affect other cells or the blood vessel wall. Because pulmonary vascular diseases are often characterized by changes in SMC contractile activity and proliferation, we studied endothelial-mediated shear stress effects on PASMC contractile protein expression and proliferation in co-culture experiments. We examined two contractile proteins of smooth muscle cells, α-SM-actin and SM-MHC, and the proliferation marker PCNA. As shown in Figure 5, expression of α-SM-actin and SM-MHC was significantly increased at high shear stress level (90 and 120 dynes/cm2) compared to that at physiological shear stress (20 dynes/cm2). Shear stress did not have significant effects on smooth muscle proliferation as assessed by PCNA (Figure 5c).
Figure 5. High shear stresses increase α-SM-actin and SM-MHC expression, but not DNA synthesis in co-cultured PASMC.
Quantitative measurements of α-SM-actin (a), and SM-MHC (b) in PASMCs co-cultured with PAECs. (c) Western blot images of α-SM-actin, SM-MHC, and PCNA expressions in PASMCs co-cultured with PAECs. Data represent means ± SE (n=3–4). The conditions marked with ‘*’ are significantly different from 20 dynes/cm2 (P ≤ 0.05).
DISCUSSION
Many forms of pulmonary hypertension are associated with significant increases and/or decreases in blood flow and shear stress in the pulmonary circulation. To better understand the effects of shear on endothelial production of vasoactive mediators and growth factors in the pulmonary circulation, in the context of their potential contribution to the hypertensive process, we devised an artificial flow system in which the effects of a wide range of shear stresses on endothelium and endothelium-SMC interactions could be assessed. Our results reveal that: 1) compared to physiological flow shear stresses (20 and 60 dyne/cm2), pathologically high (90 and 120 dyne/cm2) and pathologically low (5 dyne/cm2) flow shear stresses attenuated PAEC production of vasodilation- and angiogenesis-related molecules such as NO, PGI2, and VEGF, and enhanced release of vasoconstrictor ET-1, 2) physiological flow activated Akt/eNOS pathway while pathologically flow depressed this signaling pathway, 3) pathologically high flow redistributed and weakened the staining of peripheral VE-cadherin junctions, 4) pathologically high and low flow conditions imposed on endothelial cells upregulated α-SM-actin and SM-MHC expression in the underlying smooth muscle cells, but did not change smooth muscle proliferation. Collectively, these findings are consistent with the high vascular resistance observed in patients and animals with high flow.
While the effects of hemodynamic shear stresses on the vascular endothelium have been extensively studied and the resulting findings associated with pathogenesis of atherosclerosis and coronary heart diseases in the systemic circulation, few studies have examined whether and how shear stresses play a role in the endothelial dysfunction which contributes to pulmonary vascular diseases 2. Results from atherosclerosis-related flow studies in the systemic circulation might not be directly related to hemodynamic effects in the pulmonary circulation since it is increasingly appreciated that endothelial phenotypes and functional responses are organ-specific 21. The flow conditions and primary cells used in the present study are specific to the pulmonary circulation, and the results are consistent with a potentially dual contribution of changes in flow magnitude to the pathogenesis of pulmonary vascular diseases10. Pathologically high flow conditions were studied because acute increase of pulmonary blood flow is an integral part of many congenital heart defects which often lead to endothelial dysfunction and pulmonary vascular diseases 22. Pathologically low flow conditions were studied because chronic reduction of blood flow and flow shear stress have also been observed in certain conditions and might have long-term effects on pulmonary vascular disease 23. Assays on the molecules related to key vascular tone mediators such as NO, PGI2, ET-1 and TxA2 were studied because they are associated with regulation of vascular tone in the disease conditions. Co-culture studies were performed to better understand endothelial-SMC interactions under varying flow conditions.
With regard to existing knowledge about flow-mediated effects on the endothelium, several findings in the present study merit discussion. First, prior work has established that flow-induced relaxation is an endothelial-mediated response. We also found that physiologically normal shear maximized vasodilator release and that pathologically low flow reduced endothelial release of vasodilators. The unique finding of the present study was that the production of NO and PGI2 did not increase monotonically and the ET-1 and TxA2 release did not decrease monotonically with the increase of flow. Our results suggested that both pathologically high and low shear stresses lead to an increased vasoconstrictor (i.e. ET-1 and TxA2) to vasodilator (i.e. NO, PGI2) ratio. NO, PGI2, ET-1 and TxA2 are important vasoactive substances and the balance among them largely determines vascular tone and platelet aggregation. The flow-mediated effects demonstrated here under pathological shear stress conditions may partly contribute to prolonged vasoconstriction and enhanced vascular resistance observed in flow-induced pulmonary hypertension. This result is consistent with previous study which shows that eNOS expression is decreased in pulmonary arterial endothelial cells isolated from animals with high flow pulmonary shunts 6.
Though it has been established that flow modulates eNOS expression, the underlying signaling pathways involved remain unclear and controversial. Various signaling pathways can promote NO release. There have been conflicting results as to which protein kinases—protein kinase A, protein kinase B (Akt), other Ser/Thr protein kinases, or tyrosine kinases—are responsible for shear-dependent eNOS regulation 24. Several in vitro studies showed that positive-mean pulsatile flow shear stress induced eNOS and Akt activity in several endothelial cell lines 25,26. In contrast, Boo et al. showed that laminar shear stress stimulates phosphorylation of eNOS by protein kinase A but not Akt 27. Our results in bovine PAECs demonstrate that the Akt/eNOS pathway is involved in flow mediated eNOS expression and that its activation is dependent on the flow shear stress magnitude. However, the fold changes of reduction at 5 and 60 dynes/cm2 compared to 20 dynes/cm2 were much higher for P-Akt than for P-eNOS. This suggests that though the Akt/eNOS module is a major signaling pathway that contributes to flow activated eNOS expression, other pathways are likely involved in eNOS activation, particularly in the range of low and physiological flow shear stresses. ERK-1 and MAPK, for example, have been demonstrated to be involved in the flow-mediated eNOS activation. The role of signaling pathways in regulating eNOS needs further evaluation.
The demonstration of structural changes in PAEC might partly explain the functional changes observed. It shows that different sensing mechanisms might be involved in pulmonary vascular endothelial responses to the two types of pathological flow conditions (low and high flow shear stresses) studied. The important questions raised by these observations are interesting and deserve in-depth exploration in the future. The vascular endothelial cells serve as an integrated barrier between blood and underlying smooth muscle cells. They also act as important mechanosensory complexes to sense hemodynamic forces and translate mechanical signals to molecular signals. As demonstrated in previous studies20,28,29, both F-actin and VE-cadherin can act as structural constituents of large mechanosensory complexes, and involve the PI3K/Akt pathway in endothelial cells. VE-cadherin is part of multi-component mechanosensory complexes which transmit forces and activate signaling pathways 20,28. The redistribution of VE-cadherin under flow conditions was previously reported in cell culture and in vivo. Additionally, VE-cadherin was shown to be involved in the activation of Akt by flow 28. In agreement with these studies, we showed that flow induced redistribution of VE-caderin. Our data showed an interesting and unique finding that compared to physiological flow conditions, no flow or pathologically low flow exerts effects on the cell cytoskeleton (F-actin) while pathologically high flow conditions exert more influence on the cell-cell junctions. Discontinuous VE-cadherin expression was found on the cells exposed to shear stresses of 90 and 120 dynes/cm2. This suggests that VE-cadherin expression was deleteriously affected by these high flow conditions and might contribute to the altered protein expression observed. Our data also support the involvement of VE-cadherin in the activation of Akt by showing phosphorylation of Akt decreased along with disruption of VE-cadherin junction under high flow conditions.
Previous studies have suggested that the phenotypic changes in PASMCs observed in high flow pulmonary hypertension models were induced by factors released by the endothelium. Decrease in vasodilator release and increase in vasoconstrictor release by PAECs under pathological flow conditions will likely increase vascular tone. Our results showing that high shear stress lead to changes in the production of these endothelial factors that increase PASMC expression of the contractile proteins α-SM-actin and SM-MHC would provide an explanation of increased tone and contractile responses in vessels exposed to prolonged pathophysiologic flow conditions. In contrast to other observations30, we found that the flow magnitude did not influence PASMC proliferation when cultured with PAECs exposed to pathological flow conditions. This is somewhat surprising since the NO and PGI2 release decreased and the ET-1 release increased at high shear stress. It is known that prostacyclin and NO suppresses smooth muscle proliferation and endothelin can act as a mitogen under certain conditions. But as shown in a recent paper by Bakker, altered blood flow may trigger an inflammatory response that facilitates vessel remodeling, and vascular tone is a crucial determinant of the direction of the remodeling response31. In the model system used, pathologic flow on endothelial cells did not result in production of factor whose net effect was to increase SMC proliferation but rather in production of a factor(s) that increased contractile protein expression in SMC. The identity of this factor(s) remains to be determined.
Several limitations of the study are acknowledged. First, the particular values of flow shear stress chosen in this study (5, 20, 60, 90 and 120 dynes/cm2) are not absolutely representative of low-pathological, normal and high-pathological shear values seen in the pulmonary vasculature. These values, especially the larger ones, were chosen based on patient-specific computational modeling studies of the pulmonary vasculature in children with pulmonary arterial hypertension, but certainly may not represent the entire range of normal or high shear values that may be seen in this highly heterogeneous population. However, the particular patterns of cell response between lower and higher values of shear suggest strong correlations between pathologically high shear and abnormal cell response; these will undoubtedly be clarified by additional studies linking patient-specific measurements of pulmonary vascular hemodynamics and in vitro shear studies. Also, in our studies endothelial cells were cultured on a fibronectin-coated stiff substrate. The rigidity of the system is different than the actual interactions between vascular endothelial cells and the basement membrane in vivo. Further, the effect of vascular smooth muscle cells on vascular endothelial cell responses was not considered in this study because in our co-culture studies they were separated by a polycarbonate membrane and endothelial-SMC contact did not reflect that in vivo.
In conclusion, present studies show that physiological flow shear stress activates Akt/eNOS pathway, favors the release of vasodilators and endothelial growth factors, and inhibits the release of vasoconstrictors from pulmonary arterial endothelial cells. In contrast, either high or low pathological flow shear stress suppress Akt/eNOS pathway, decrease the ratio of vasodilators to vasoconstrictors produced from endothelial cells, and enhance expression of contractile proteins in underlying smooth muscle cells. These findings suggest flow is a critical factor in the regulation of pulmonary vascular cell physiology and pathology.
ACKNOWLEDGEMENTS
The authors would like to acknowledge Sandra Walchak from CVP group for providing bovine PAECs and PASMCs in this study. Also, we would like to thank Lila Sisbarro and Aaron Richman for their help in the study, and thank Craig Lanning and Dr Kendall Hunter for their insightful input into the study.
This study was funded in part by a grant from the NIH (HL 072738 to R.S., HL 084923 to K.R.S., HL 14985-35 to K.R.S.).
References
- 1.Dakshinamurti S. Pathophysiologic mechanisms of persistent pulmonary hypertension of the newborn. Pediatr Pulmonol. 2005;39:492–503. doi: 10.1002/ppul.20201. [DOI] [PubMed] [Google Scholar]
- 2.Cool CD, Groshong SD, Oakey J, Voelkel NF. Pulmonary hypertension: cellular and molecular mechanisms. Chest. 2005;128:565S–571S. doi: 10.1378/chest.128.6_suppl.565S. [DOI] [PubMed] [Google Scholar]
- 3.Botney MD. Role of hemodynamics in pulmonary vascular remodeling: implications for primary pulmonary hypertension. Am J Respir Crit Care Med. 1999;159:361–364. doi: 10.1164/ajrccm.159.2.9805075. [DOI] [PubMed] [Google Scholar]
- 4.Ben Driss A, Devaux C, Henrion D, Duriez M, Thuillez C, Levy BI, Michel JB. Hemodynamic stresses induce endothelial dysfunction and remodeling of pulmonary artery in experimental compensated heart failure. Circulation. 2000;101:2764–2770. doi: 10.1161/01.cir.101.23.2764. [DOI] [PubMed] [Google Scholar]
- 5.Storme L, Parker TA, Kinsella JP, Rairigh RL, Abman SH. Chronic hypertension impairs flow-induced vasodilation and augments the myogenic response in fetal lung. Am J Physiol Lung Cell Mol Physiol. 2002;282:L56–L66. doi: 10.1152/ajplung.2002.282.1.L56. [DOI] [PubMed] [Google Scholar]
- 6.Ross GA, Oishi P, Azakie A, Fratz S, Fitzgerald RK, Johengen MJ, Harmon C, Hendricks-Munoz K, Xu J, Black SM, Fineman JR. Endothelial alterations during inhaled NO in lambs with pulmonary hypertension: implications for rebound hypertension. Am J Physiol Lung Cell Mol Physiol. 2005;288:L27–L35. doi: 10.1152/ajplung.00144.2004. [DOI] [PubMed] [Google Scholar]
- 7.Rondelet B, Kerbaul F, Van Beneden R, Hubloue I, Huez S, Fesler P, Remmelink M, Brimioulle S, Salmon I, Naeije R. Prevention of pulmonary vascular remodeling and of decreased BMPR-2 expression by losartan therapy in shunt-induced pulmonary hypertension. Am J Physiol Heart Circ Physiol. 2005;289:H2319–H2324. doi: 10.1152/ajpheart.00518.2005. [DOI] [PubMed] [Google Scholar]
- 8.Steinhorn R, Russell J, Lakshminrusimha S, Gugino S, Black S, Fineman J. Altered endothelium-dependent relaxations in lambs with high pulmonary blood flow and pulmonary hypertension. Am J Physiol Heart Circ Physiol. 2001;280:H311–H317. doi: 10.1152/ajpheart.2001.280.1.H311. [DOI] [PubMed] [Google Scholar]
- 9.Hunter K, Lanning C, Zhang Y, Garg R, Ivy D, Shandas R. Simulations of Congenital Defect Closure and Drug Reactivity Testing in Patient-Specific Models of the Pediatric Pulmonary Vasculature: A 3-D Numerical Study with Fluid-Structure Interaction. J. Biomech Engr. 2006;128:564–572. doi: 10.1115/1.2206202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rabinovitch M, Konstam MA, Gamble WJ, Papanicolaou N, Aronovitz MJ, Treves S, Reid L. Changes in pulmonary blood flow affect vascular response to chronic hypoxia in rats. Circ Res. 1983;52:432–441. doi: 10.1161/01.res.52.4.432. [DOI] [PubMed] [Google Scholar]
- 11.Malek AM, Alper SL, Izumo S. Hemodynamic shear stress and its role in atherosclerosis. JAMA. 1999;282:2035–2042. doi: 10.1001/jama.282.21.2035. [DOI] [PubMed] [Google Scholar]
- 12.Davies PF, Spaan JA, Krams R. Shear stress biology of the endothelium. Ann Biomed Eng. 2005;33:1714–1718. doi: 10.1007/s10439-005-8774-0. [DOI] [PubMed] [Google Scholar]
- 13.Helmlinger G, Geiger RV, Schreck S, Nerem RM. Effects of Pulsatile Flow on Cultured Vascular Endothelial Cell Morphology. J. Biomech. Eng. 1991;113:123–131. doi: 10.1115/1.2891226. [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Cardena G, Comander J, Anderson KR, Blackman BR, Gimbrone MA., Jr Biomechanical activation of vascular endothelium as a determinant of its functional phenotype. Proc Natl Acad Sci U S A. 2001;98:4478–4485. doi: 10.1073/pnas.071052598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.White C, Frangos J. The shear stress of it all: the cell membrane and mechanochemical transduction. Philos Trans R Soc Lond B Biol Sci. 2007 doi: 10.1098/rstb.2007.2128. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stiebellehner L, Frid M, Reeves J, Low R, Gnanasekharan M, Stenmark K. Bovine distal pulmonary arterial media is composed of a uniform population of well-differentiated smooth muscle cells with low proliferative capabilities. Am J Physiol Lung Cell Mol Physiol. 2003;285:819–828. doi: 10.1152/ajplung.00062.2003. [DOI] [PubMed] [Google Scholar]
- 17.Frid M, Kale V, Stenmark K. Endothelial-Mesenchymal Transdifferentiation: In Vitro Analysis Mature Vascular Endothelium Can Give Rise to Smooth Muscle Cells. Circ. Res. 2002;90:1189–1196. doi: 10.1161/01.res.0000021432.70309.28. [DOI] [PubMed] [Google Scholar]
- 18.Tourneux P, Chester M, Grover T, Abman SH. Fasudil inhibits the myogenic response in the fetal pulmonary circulation. Am J Physiol Heart Circ Physiol. 2008;295:H1505–H1513. doi: 10.1152/ajpheart.00490.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wedgwood S, Mitchell CJ, Fineman JR, Black SM. Developmental differences in the shear stress-induced expression of endothelial NO synthase: changing role of AP-1. Am J Physiol Lung Cell Mol Physiol. 2003;284:L650–L662. doi: 10.1152/ajplung.00252.2002. [DOI] [PubMed] [Google Scholar]
- 20.Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, Cao G, DeLisser H, Schwartz MA. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 2005;437:426–431. doi: 10.1038/nature03952. [DOI] [PubMed] [Google Scholar]
- 21.Aird W. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ Res. 2007;100:158–173. doi: 10.1161/01.RES.0000255691.76142.4a. [DOI] [PubMed] [Google Scholar]
- 22.Steinhorn RH, Russell JA, Lakshminrusimha S, Gugino SF, Black SM, Fineman JR. Altered endothelium-dependent relaxations in lambs with high pulmonary blood flow and pulmonary hypertension. Am J Physiol Heart Circ Physiol. 2001;280:H311–H317. doi: 10.1152/ajpheart.2001.280.1.H311. [DOI] [PubMed] [Google Scholar]
- 23.Rosenberg AA, Kinsella JP, Abman SH. Cerebral hemodynamics and distribution of left ventricular output during inhalation of nitric oxide. Crit Care Med. 1995;23:1391–1397. doi: 10.1097/00003246-199508000-00013. [DOI] [PubMed] [Google Scholar]
- 24.Boo YC, Jo H. Flow-dependent regulation of endothelial nitric oxide synthase: role of protein kinases. Am J Physiol Cell Physiol. 2003;285:C499–C508. doi: 10.1152/ajpcell.00122.2003. [DOI] [PubMed] [Google Scholar]
- 25.Kadohama T, Nishimura K, Hoshino Y, Sasajima T, Sumpio BE. Effects of different types of fluid shear stress on endothelial cell proliferation and survival. J Cell Physiol. 2007;212:244–251. doi: 10.1002/jcp.21024. [DOI] [PubMed] [Google Scholar]
- 26.Ziegler T, Bouzourene K, Harrison VJ, Brunner HR, Hayoz D. Influence of oscillatory and unidirectional flow environments on the expression of endothelin and nitric oxide synthase in cultured endothelial cells. Arterioscler Thromb Vasc Biol. 1998;18:686–692. doi: 10.1161/01.atv.18.5.686. [DOI] [PubMed] [Google Scholar]
- 27.Boo YC. Shear stress stimulates phosphorylation of protein kinase A substrate proteins including endothelial nitric oxide synthase in endothelial cells. Exp Mol Med. 2006;38:63–71. doi: 10.1038/emm.2006.8. [DOI] [PubMed] [Google Scholar]
- 28.Shay-Salit A, Shushy M, Wolfovitz E, Yahav H, Breviario F, Dejana E, Resnick N. VEGF receptor 2 and the adherens junction as a mechanical transducer in vascular endothelial cells. Proc Natl Acad Sci U S A. 2002;99:9462–9467. doi: 10.1073/pnas.142224299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao M, Bai H, Wang E, Forrester J, McCaig C. Electrical stimulation directly induces pre-angiogenic responses in vascular endothelial cells by signaling through VEGF receptors. J Cell Sci. 2004;117:397–405. doi: 10.1242/jcs.00868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buus CL, Pourageaud F, Fazzi GE, Janssen G, Mulvany MJ, De Mey JG. Smooth muscle cell changes during flow-related remodeling of rat mesenteric resistance arteries. Circ Res. 2001;89:180–186. doi: 10.1161/hh1401.093575. [DOI] [PubMed] [Google Scholar]
- 31.Bakker ENTP, Matlung HL, Bonta P, Vries CJ, Rooijen N, VanBavel E. Blood flow-dependent arterial remodelling is facilitated by inflammation but directed by vascular tone. Cardiovas Res. 2008;78:341–348. doi: 10.1093/cvr/cvn050. [DOI] [PubMed] [Google Scholar]