Abstract
OBJECTIVE
To examine the cross-sectional associations between activity of daily living (ADL) limitation stage and specific physical and mental conditions, global perceived health, and unmet needs for home accessibility features of community-dwelling adults aged 70 and older.
DESIGN
Cross-sectional.
SETTING
Community.
PARTICIPANTS
Nine thousand four hundred forty-seven community-dwelling persons interviewed through the Second Longitudinal Study of Aging (LSOA II).
MEASUREMENTS
Six ADLs organized into five stages ranging from no difficulty (0) to unable (IV).
RESULTS
ADL stage showed strong ordered associations with perceived health, dementia severe enough to require proxy use, and history of stroke. For example, the relative risks (RRs) defined as risk of being at Stages I, II, III, or IV divided by risk of being at Stage 0 for those with dementia ranged from 3.2 (95% confidence interval (CI) = 2.4–4.4) to 41.9 (95% CI = 19.6–89.6) times the RRs for those without dementia. The RR ratios (RRR) comparing respondents who perceived unmet need for accessibility features in the home to those without these perceptions peaked at Stage III (RRR = 17.8, 95% CI = 13.0–24.5) and then declined at Stage IV. All models were adjusted for age, sex, and race.
CONCLUSIONS
ADL stages showed clinically logical associations with other health-related concepts, supporting external validity. Findings suggest that specificity of chronic conditions will be important in developing strategies for disability reduction. People with partial rather than complete ADL limitation appeared most vulnerable to unmet needs for home accessibility features.
Keywords: activities of daily living, staging, chronic disease, environment, biopsycho-ecological framework
With the number of people aged 65 and older living in the United States projected to grow to 71.5 million by 2030, medicine's challenges will shift from prolonging to enhancing the quality of survival over the 21st century.1–3 Estimates from the Medicare Current Beneficiary Survey (MCBS) indicated that, in 2005, 18% of persons had one or two, 5% had three or four, and 3% had five or six activity of daily living (ADL) limitations.1,4 Counts do not indicate which activities were affected, making it difficult to project specific service needs.
Stages of ADL limitation were defined to better express the nature of disability in response to recent calls for better disability measurement in population surveys of older adults.1,4,5 Applying the International Classification of Functioning, Disability and Health (ICF),6 stages define clinically important thresholds of functioning reflecting the relative probabilities that people will describe difficulty with each ADL. Established to mirror the hierarchical sequence of activities within the index of ADLs,7 ADL stage thresholds were derived adopting methods from Functional Independence Staging (FIS)8 that predict a variety of patient outcomes.8–12 Intended to reflect the needs as perceived by community-dwelling older people or their caregivers, ADL stages differ from the index of ADLs and FIS by applying self- or proxy-reported rather than clinician-rated functional information.
Stages reflect the five ICF performance levels, beginning with “no difficulty” (Stage 0) and ending with “unable” (Stage IV), and distinguish five strata of people according to increasing severity. Expressing the type and severity of limitations through hierarchical thresholds of retained ability, stages are intended to capture the difficulties that people have with self-care and thus the nature of support needed to remain living in their homes.
The biopsycho-ecological framework of Health Environmental Integration (HEI),13 which acknowledges intraand extra-individual determinants of disability, informed this study. Intra-individual determinants are the combined effects of an individual's physical and mental health conditions. Extra-individual determinants are represented as barriers and facilitators in the man-made and natural environments. Stage of ADLs is presumed to result from interactions between illness burden (severity and type(s) of chronic physical and mental health conditions) and particular architectural features.14 The vast majority of measures and studies relate chronic health conditions to risks of mortality,15–17 yet chronic health conditions are not related to disabilities and mortality in the same ways.18 Also, disabilities create environmental vulnerability,19 with home architectural features being barriers or facilitators6 depending on individuals’ health conditions. Previous studies found the perception of unmet need for accessibility features in the home to be associated with greater likelihood of ADL limitation; conversely, elderly people with accessibility features in their homes were less likely to experience decline in ADLs.20,21 Knowledge of linkages between particular health conditions and stage and between unmet needs for home accessibility features and stage in elderly persons in the U.S. population could help project population needs.
Cross-sectional associations between ADL stage and global perceived health status, type of physical and mental conditions, and perceived home inaccessibility were examined, and the degree to which traditional medical concepts of illness and the perception of extrinsic home environmental barriers relate to stage of ADL disability was documented. Although there is growing consensus that multiple chronic conditions, frailty, and disability are causally related, it is essential to understand the distinct characteristics of each concept when establishing care strategies.22–24 Recognizing likely cumulative effects, the first hypothesis was that people at stages of greater ADL severity will perceive poorer health. The second hypothesis was that people with stroke and those with Alzheimer's disease and other types of severe dementia would cluster at the most-limited ADL stages; people with conditions such as arthritis, major mental illness, and diabetes mellitus would cluster at the intermediate ADL stages; and those with hypertension would cluster at the least-limited stages.25 The third hypothesis was that people at stages of greater ADL severity would be more likely to experience unmet needs for home accessibility features.
METHODS
The institutional review board at the University of Pennsylvania approved this study.
Data
Data were originally collected under the Second Longitudinal Study of Aging II (LSOA II),26 a prospective study of a representative U.S. sample of 9,447 community-dwelling persons aged 70 and older. Respondents were drawn from all sample persons (SPs) interviewed during the 1994 National Health Interview Survey (NHIS) core and were 70 at the time of the LSOA II baseline interview. The NHIS core was linked to the disability supplement follow-back survey (NHIS-D)27 that re-interviewed people with impairments or disabilities between 6 months and 1.5 years after the core. Many government agencies worked together to develop the NHIS-D follow-back survey after passage of the Americans with Disabilities Act (ADA), when it was recognized that there was little information available to help guide national disability policy. The NHIS-D included questionnaires that addressed the administrative, social, and medical aspects of disability and the problems and environmental barriers that individuals faced. The data used for this manuscript were primarily from the baseline LSOA II, with additional context-rich information about disability drawn from the NHIS-D follow-back survey occurring at the same time. The LSOA II was intended to provide information on the causes and correlations of changes in health and functioning. Although attempts were made to interview the SP, close proxy respondents (living in the same household) provided information when the SP could not be interviewed.
Variables
Demographics included age, sex, and race (white, black or African American, and other).
ADL stages reflect patterns of self- or proxy-reported difficulties in eating, using the toilet (including getting to the toilet), dressing, transferring (getting in and out of bed and chairs), walking in the home, and bathing. Each ADL has four levels: no difficulty (0), some difficulty (1), a lot of difficulty (2), and unable (3).6 Previous confirmatory factor analyses of a core set of 20 activities validated these six activities as a distinct ADL concept, distinguished from basic mobility and the instrumental activities of daily living.28 ADL stages were developed by observing patterns of item responses in the LOSA II baseline data using methods described previously8 to express sequences of known relative item difficulty, reflective of the index of ADLs and other measures.7,8,29
Stages 0 through IV indicate groups of people with increasing difficulties with ADLs (Table 1). People are staged according to responses to the simple questions shown. People at Stage 0 experience no ADL difficulties. At Stage I or II, the person (or close proxy) must report no more than the defined threshold amounts of difficulty performing each ADL. Individuals at Stage III describe less severe limitations than a Stage IV, but fall below the thresholds necessary for Stage II assignment. At Stage IV, individuals are unable to perform any ADLs. The empirically derived threshold definitions across increasingly severe ADL stages follow the well-established functional hierarchy documented in the literature.7,8,29
Table 1.
Activity of Daily Living (ADL) Stage Threshold Definitions and Weighted Prevalence
| ADL Stage | Threshold Definition | Weighted Prevalence % |
| 0 = No difficulty: none, absent, or negligible ADL limitation | Is the individual able to eat, toilet, dress, transfer, bathe, and walk without difficulty (all = 0)? | 71.6 |
| I = Mild difficulty: slight or low level ADL limitation | Is the individual able to eat and toilet without difficulty (0), dress and transfer with no more than some difficulty (≤1), and bathe and walk with no more than a lot of difficulty (≤2)? | 16.3 |
| II = Moderate difficulty: medium or fair ADL limitation | Is the individual able to eat without difficulty (0); use the toilet, dress, and transfer with no more than a lot of difficulty (≤2); and possibly unable to bathe and walk (≤3)? | 7.1 |
| III = Severe difficulty: high or extreme ADL limitation | Is the individual able to perform at least one ADL (eat, toilet, dress, transfer, bathe or walk) with or without assistance but not able to meet the defined threshold for stage II? | 4.5 |
| IV = Complete difficulty: total ADL limitation | Is the individual unable to eat, toilet, dress, transfer, bathe, and walk (all = 3)? | 0.5 |
Information about cardiopulmonary disorders (heart attack, myocardial infarction, angina pectoris, other heart disease, bronchitis, emphysema, or asthma), stroke, osteoporosis, diabetes mellitus, arthritis, hypertension, and cancer were captured by asking respondents whether a doctor had ever told the SPs that they had the condition. SPs were considered to have history of a major mental illness if, during the past 12 months, they had schizophrenia, paranoid disorder, bipolar disorder, or major depression lasting 2 or more weeks. An individual was considered to have serious dementia if survey information was reported by proxy because of poor memory or Alzheimer's disease. Each type of condition formed a separate variable.
Respondents reported global perceived health status as excellent, very good, good, fair, or poor.
Perceived home inaccessibility was reported as the respondent's perception of unmet needs for one or more accessibility features in their homes, including widened doorways, ramps, kitchen modifications, railings, easy-open doors, accessible parking or drop-off sites, elevators or stair glides, alerting devices, or other special features.
Analysis
The LSOA II uses a multistage sample design. To obtain the correct variance estimates, clustering, sample weights, and stratification were taken into account in all analyses. The prevalence of stage was calculated as weighted proportions from the LSOA II data.
For unadjusted associations between variables used in this study and ADL stages, demographics, illness burden (presence of particular mental or physical health conditions and perceived health status), and perception of unmet need for accessibility features in the home were first described according to ADL stage. Unweighted sample sizes and weighted proportions were reported.
Because stages have multiple levels, multinomial logistic regression models were fit on the ADL stages. Several models were used to examine the associations between ADL stage and predictors such as chronic illness burden, perceived health status, and perception of unmet need for home accessibility features. To determine adjusted associations between each concept and stage, models were fit adjusting for demographics because of known associations of age, sex, and race with functioning and other health constructs.30 Other predictor(s) included in each model were:
Model 1: Global perceived health status expressed as 5—levels as described above.
Model 2: Type of chronic conditions expressed as dichotomous indicators, including the presence versus absence of eight conditions in a single model.
Model 3: Perception of need for accessibility features in the home (yes/no).
These models were fit separately because of intercorrelation between the concepts and to avoid overadjusting.
All statistical analyses were performed on Stata/MP version 11.0 (StataCorp, College Station, TX), using the proper statements to account for the complex sampling, including weight, clustering, and stratification. For the three models above, the ratio of the probability of being at another stage (e.g., Stage I) to the probability of being at the reference stage (e.g., Stage 0) was modeled. If there were only two levels (e.g., Stages 0 and I) in the outcome, the ratio that would have been modeled was the odds of being at Stage I. When there were multiple stages, the ratio was called relative risk (RR) of being at Stage I (e.g., relative to Stage 0). Relative risk ratios (RRRs) (similar to odds ratios in logistic regression models) and their 95% confidence intervals (CIs) were reported. All P-values presented are two-tailed; P < .05 was considered statistically significant.
RESULTS
Of the 9,447 persons in the LSOA II baseline data, 130 (1.4%) were missing one or more ADLs and were excluded. Of the 9,317 persons left in the analyses, information was self-reported 81.3%, reported partially by proxy 16.5%, and reported fully by proxy 2.2% of the time. Proxy reports were primarily because of illness, sensory loss, dementia, or communication difficulties. Global perceived health status was not rated for 54 persons, so information from 9,263 persons was used in only that analysis.
The mean ± standard deviation age of the overall sample was 76.3 ± 7.1. For the individual stages, ages were 75.5 ± 5.8, 77.3 ± 5.3, 79.3 ± 6.3, 79.0 ± 6.5 and 81.6 ± 7.0 for stages 0, I, II, III, and IV, respectively. Table 2 shows frequencies and unadjusted associations according to ADL stage. Arthritis, hypertension, and cardiopulmonary disorders were the most common conditions, described in 46.5%, 43.0%, and 33.6% of the population, respectively. More than half of those at Stage IV (65.2%) had dementia of sufficient severity that a proxy respondent was required, and 39.1% had a history of stroke, compared with 2.0% and 4.9%, respectively, at Stage 0. Those with cardiopulmonary disorders, osteoporosis, arthritis, hypertension, and cancer tended to cluster at the intermediate stages. There was not a consistent pattern with diabetes mellitus and stage. Global perceived health status was rated as excellent in 17.8% of SPs at Stage 0 and 2.2% at Stage IV and as poor in 3.3% of SPs at Stage 0 and 54.3% at Stage IV. The proportions of SPs perceiving unmet need for environmental accessibility features peaked at Stage III (27.8%).
Table 2.
Unadjusted Associations Between Activity of Daily Living Stage and Other Person- and Environment-Related Factors
| N (%) |
% |
|||||
|---|---|---|---|---|---|---|
| Factor | Total N = 9,317 | Stage 0 n = 6,675 | Stage I n = 1,514 | Stage II n = 666 | Stage III n = 416 | Stage IV n = 46 |
| Age (P<.001) | ||||||
| 70–75 | 4,290 (46.7) | 50.7 | 42.1 | 27.8 | 31.0 | 15.2 |
| 76–80 | 2,512 (27.0) | 28.0 | 23.4 | 26.0 | 25.7 | 21.6 |
| 81–85 | 1,565 (16.4) | 14.6 | 19.7 | 23.3 | 20.5 | 29.9 |
| > 85 | 950 (9.9) | 6.7 | 14.7 | 22.9 | 22.8 | 33.4 |
| Sex (P<.001) | ||||||
| Male | 3,698 (40.2) | 42.8 | 33.0 | 29.8 | 39.5 | 39.3 |
| Female | 5,619 (59.8) | 57.2 | 67.0 | 70.2 | 60.5 | 60.7 |
| Race (P<.001) | ||||||
| White | 7,965 (88.3) | 89.3 | 86.1 | 84.3 | 86.7 | 79.7 |
| Black | 988 (7.6) | 6.7 | 9.3 | 11.5 | 10.4 | 13.8 |
| Other | 364 (4.1) | 4.0 | 4.6 | 4.3 | 2.9 | 6.5 |
| Stroke (P<.001) | ||||||
| Yes | 768 (8.2) | 4.9 | 12.6 | 16.8 | 28.4 | 39.1 |
| No | 8,549 (91.8) | 95.1 | 87.4 | 83.2 | 71.6 | 60.9 |
| Hypertension (P<.001) | ||||||
| Yes | 4,062 (43.0) | 40.0 | 50.5 | 54.4 | 46.0 | 40.9 |
| No | 5,255 (57.0) | 60.0 | 49.5 | 45.6 | 54.0 | 59.1 |
| Cardiopulmonary condition (P<.001) | ||||||
| Yes | 3,132 (33.6) | 28.5 | 44.8 | 48.5 | 50.7 | 34.8 |
| No | 6,185 (66.4) | 71.5 | 55.2 | 51.5 | 49.3 | 65.2 |
| Osteoporosis (P<.001) | ||||||
| Yes | 656 (7.0) | 4.9 | 12.2 | 12.9 | 13.0 | 10.9 |
| No | 8,661 (93.0) | 95.1 | 87.8 | 87.1 | 87.0 | 89.1 |
| Arthritis (P<.001) | ||||||
| Yes | 4,335 (46.5) | 39.2 | 66.7 | 66.4 | 59.6 | 41.3 |
| No | 4,982 (53.5) | 60.8 | 33.3 | 33.6 | 40.4 | 58.7 |
| Diabetes mellitus (P<.001) | ||||||
| Yes | 1,104 (11.8) | 9.4 | 16.4 | 20.6 | 19.0 | 32.6 |
| No | 8,213 (88.2) | 90.6 | 83.6 | 79.4 | 81.0 | 67.4 |
| Cancer (P<.001) | ||||||
| Yes | 1,565 (16.8) | 15.9 | 19.9 | 18.2 | 18.8 | 15.2 |
| No | 7,752 (83.2) | 84.1 | 80.1 | 81.8 | 81.3 | 84.8 |
| Major mental illness (P<.001) | ||||||
| Yes | 94 (1.0) | 0.6 | 1.8 | 2.3 | 1.9 | 4.3 |
| No | 9,223 (99.0) | 99.4 | 98.2 | 97.7 | 98.1 | 95.7 |
| Dementia (P<.001) | ||||||
| Yes | 495 (5.3) | 2.0 | 6.9 | 14.7 | 30.5 | 65.2 |
| No | 8,822 (94.7) | 98.0 | 93.1 | 85.3 | 69.5 | 34.8 |
| Perceived health status* (P<.001) | ||||||
| Excellent | 1,326 (14.3) | 17.8 | 6.7 | 3.3 | 4.3 | 2.2 |
| Very good | 2,126 (23.0) | 27.1 | 14.8 | 10.1 | 8.9 | 2.2 |
| Good | 3,206 (34.6) | 36.8 | 31.8 | 27.4 | 22.5 | 21.7 |
| Fair | 1,785 (19.3) | 15.0 | 30.6 | 30.4 | 29.5 | 19.6 |
| Poor | 820 (8.9) | 3.3 | 16.1 | 28.7 | 34.8 | 54.3 |
| Perceived unmet home feature(s) (P<.001) | ||||||
| Yes | 597 (6.3) | 2.1 | 10.5 | 25.3 | 27.8 | 17.2 |
| No | 8,720 (93.8) | 97.9 | 89.5 | 74.7 | 72.2 | 82.8 |
Perceived health status was not rated for 54 sample persons, so their categories do not add up to n = 9,317, as is the case for all the other variables. SD = standard deviation.
Model 1: After removing the effects of demographics, there were strong and ordered associations between perceptions of greater illness and being at a more limited stage (Table 3). For example, the RR of being at ADL Stage I (risk of being at Stage I divided by risk of being at Stage 0) for a patient with perceptions of very good health was 1.5 times the RR for a patient with excellent health, after adjusting for age, sex, and race. The RRR increased from 1.5 to 2.4, 5.7, and 14.1 for those with perceptions of health that were very good, good, fair, and poor, respectively, for those at Stage I. When comparing across stages, the RRR for perception of health showed a strong ordered increase with stage of increasing limitation.
Table 3.
Age-, Sex-, and Race-Adjusted Associations Between Activity of Daily Living Stage and Other Person- and Environment-Related Factors
| Relative Risk Ratio (95% Confidence Interval) |
||||
|---|---|---|---|---|
| Model | Stage I | Stage II | Stage III | Stage IV |
| Model 1: Global perceived health status (reference: excellent) | ||||
| Very good | 1.5 (1.2–2.0) | 2.0 (1.2–3.5) | 1.4 (0.7–2.5) | 0.5 (0.0–8.0) |
| Good | 2.4 (1.9–3.1) | 3.8 (2.4–6.1) | 2.7 (1.6–4.7) | 5.7 (0.7–45.1) |
| Fair | 5.7 (4.4–7.4) | 10.3 (6.3–16.9) | 8.1 (4.7–14.0) | 12.1 (1.5–95.5) |
| Poor | 14.1 (10.8–18.2) | 47.9 (29.2–78.8) | 48.1 (27.3–84.6) | 202.3 (26.6–1,538.1) |
| Model 2: Presence of particular conditions (reference: no for each condition in single model) | ||||
| Stroke | 2.2 (1.8–2.8) | 2.8 (2.1–3.6) | 4.8 (3.6–6.3) | 6.9 (3.5–13.5) |
| Hypertension | 1.1 (1.0–1.3) | 1.2 (1.0–1.4) | 0.8 (0.7–1.1) | 0.7 (0.4–1.4) |
| Cardiopulmonary | 1.6 (1.4–1.9) | 1.9 (1.6–2.3) | 2.1 (1.7–2.5) | 1.1 (0.5–2.5) |
| Osteoporosis | 2.0 (1.6–2.4) | 2.0 (1.5–2.6) | 2.6 (1.8–3.7) | 1.9 (0.7–5.4) |
| Diabetes mellitus | 1.6 (1.3–1.9) | 2.2 (1.7–2.7) | 2.0 (1.5–2.6) | 4.9 (2.1–11.3) |
| Cancer | 2.6 (2.3–3.0) | 2.6 (2.2–3.1) | 2.1 (1.7–2.7) | 1.2 (0.6–2.2) |
| Major mental | 1.3 (1.1–1.5) | 1.1 (0.9–1.4) | 1.2 (0.9–1.6) | 1.0 (0.4–2.9) |
| Dementia | 3.2 (2.4–4.4) | 5.9 (4.3–8.2) | 15.3 (11.1–21.1) | 41.9 (19.6–89.6) |
| Model 3: Unmet needs for accessibility features in the residence (reference: no) | ||||
| ≥1 | 5.3 (4.0–7.0) | 14.9 (11.0–20.3) | 17.8 (13.0–24.5) | 8.7 (4.4–17.0) |
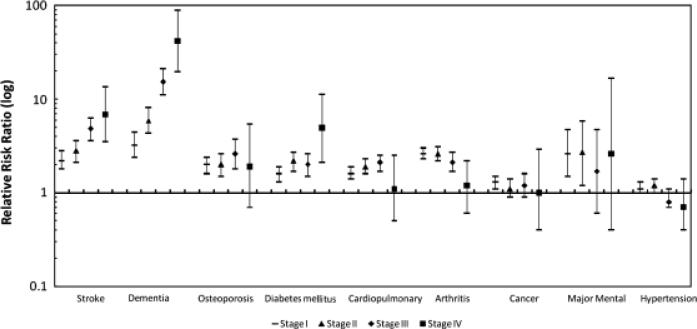
Model 2: Dementia of sufficient severity to warrant proxy responses and stroke were the conditions most strongly associated with stage after adjusting for demographics and all the other conditions. Of those requiring a proxy for dementia, the RRRs for Stages I, II, III, and IV were 3.2, 5.9, 15.3, and 41.9, respectively, comparing those with dementia with those without. For stroke, the RRRs for Stages I, II, III, and IV were 2.2, 2.8, 4.8, and 6.9, respectively, comparing those with a history of stroke with those without. The patterns between other types of conditions across the stages were not ordered. For most of the other conditions, the RRR was higher comparing Stage I with 0, remained relatively constant comparing Stages II and III with 0, and then became insignificant comparing Stage IV with 0. Figure 1 shows the adjusted patterns of associations across each of the conditions and the stages.
Figure 1.
Patterns of association between activity of daily living stage across different type(s) of mental and physical chronic health conditions. A multinomial logistic regression addressed condition type as the history of cardiopulmonary disorders (heart attack, myocardial infarction, angina pectoris, other heart disease, bronchitis, emphysema, or asthma), stroke, osteoporosis, diabetes mellitus, arthritis, hypertension, cancer, major mental illness (schizophrenia, paranoid disorder, bipolar disorder, or major depression), or serious dementia (need for proxy because of poor memory or Alzheimer's disease). The reference category in each condition comparison was absence of the condition or other types of conditions. Each plotted association was adjusted for age, sex, and race and for all other conditions. Points indicate relative risk ratios, and vertical lines represent 95% confidence intervals. All relative risk ratio estimates were weighted using 1994 or 1995 sample weights.
Model 3: The adjusted RRR for Stages I, II, III, and IV comparing persons who perceived unmet needs for home accessibility features with those without these perceptions were 5.3, 14.9, 17.8, and 8.7, respectively (Table 3).
DISCUSSION
To prepare the U.S. healthcare workforce to meet forthcoming challenges of population aging, it is essential to understand linkages between ADL limitations and other health concepts. In this nationally representative sample of community-dwelling people aged 70 and older, global perceived health status, types of physical and mental conditions, and perceptions of home environmental barriers were all associated with ADL stage in complex ways, consistent with the HEI13 framework. Concordance with other studies and clinical plausibility of the relationships support the internal validity of the stages.20,24,30–34 An enhanced understanding of associations between ADL stage and other health-related concepts has the potential to improve the care of populations and persons with disabilities in several ways. First, knowledge of patterns of association across explicitly defined ADL stages can help direct research toward identifying the contributing factors behind the associations. Second, policy-makers and clinicians may be better able to plan and project disability-related needs for persons with chronic health conditions as the population ages.
Consistent with a review of 24 studies demonstrating that the combination of chronic conditions and the overall severity of conditions were associated with poorer functioning or quality of life,24 the findings of the current study suggest that knowledge of global perceived health, along with the type(s) of mental and physical condition(s), is essential to understanding linkages between illness and disability. The association between perceptions of poorer health and stage was strongly ordered, suggesting that self-reported global health assessments are closely related to functional stage.34 Although less is known about associations with function, global perceived health status is known to predict mortality with greater precision than even physician ratings, suggesting that people may have important self-knowledge that physicians do not.35,36 Lack of a one-to-one correspondence between perceptions of health and ADL stage highlights that severe illness is not always associated with severe disability. A small proportion of persons (4.4%) whose health was rated as very good or excellent were at the most disabled stage. Conversely, 18.3% of those whose health was rated as fair or poor had no ADL difficulty, highlighting the importance of recognizing disability and illness as related but distinct.
All types of mental and physical health conditions studied were independently associated with stage, after removing the effects of demographic differences and other conditions, but the magnitude of effects and patterns of effects across the stages differed markedly and in expected ways. Stroke and need for proxy use because of dementia were the most strongly associated conditions. The likelihood of being at each more-limited stage increased from Stage 0 in a dramatically ordered fashion. The proportions of elderly people with stroke and dementia can be expected to increase sharply with stage, becoming highest at the complete ADL difficulty Stage IV. Diabetes mellitus showed a similar pattern but was less ordered. Osteoporosis, card-iopulmonary disorders, and arthritis were moderately associated with stage. People with these conditions have much higher probabilities of being at Stages I, II, or III than Stage 0 but not of being at Stage IV. People with major mental illness showed a similar pattern for Stages I and II compared with Stage 0. Hypertension and cancer were not strongly associated with stage. Although stroke and dementia are often catastrophically disabling, the findings highlighted that most chronic conditions can be expected to manifest in partial rather than complete functional loss (cluster at intermediate ADL stages).
Results indicate that perception of unmet needs for home accessibility features can be expected in a small percentage of elderly persons living in the community even before onset of ADL limitation at Stage 0, to increase progressively in Stage I and II, peak in Stage III, and then decline at Stage IV. Reasons for relative decline at Stage IV are uncertain, but it may be because people are so dependent they ignore home accessibility concerns. Alternatively, at the stage of total dependency, an individual's abilities to interact with the environment becomes so limited that further accessibility features may not seem helpful. Recent longitudinal evidence supports the assumption that environmental modifications are most effective when supplied early in the aging process.14 Applying propensity score methods, one study recently demonstrated that older people with accessibility features in their homes were less likely to decline in ADL limitation. It further noted that the benefit of having residential modifications may differ according to subgroup, suggesting that people may pass a functional threshold when these modifications are no longer beneficial.20
The prevalence of disability in community-dwelling people aged 70 and older has decreased over the past decades in the United States,37 although the decline occurred primarily in the more-complex instrumental ADLs than in basic ADLs.38 Rates of ADL limitation remained remarkably constant from 1983 to 2005. Assuming that these trends continue and distributions of persons according to stage remain constant, based on current census data,39 we estimate that the numbers of people living in the community at the heaviest care Stages III and IV nationwide will jump from 5.5 million in 2010 to approximately 7.1 million by 2020, representing an increasing burden on families and society.
Impending morbidity of the aging baby boomer generation could overwhelm the healthcare system unless high-quality, cost-effective chronic care strategies are established.40
Reductions in the proportion of people with disabilities living in congregate care facilities reflect proposed Healthy People 2020 objectives.41 The optimal matching of resources to personal and environmental needs will become increasingly difficult to achieve with the declining proportion of younger persons in the population that are potentially available to provide care for older adults.4
Stages capture the severity and type(s) of difficulties people experience with self-care, thus indicating the nature of support that people need to remain living in their homes. The sequence of increasing difficulties from less- to more-severe stages of limitation follow the well-established ADL hierarchy, defining thresholds of retained functional ability that are clinically significant.7,8,29 People at Stage I, for example, are by definition able to eat and toilet without difficulty but can be expected to have some problems with the more-complex ADLs such as dressing. Although people at Stage I are still able to perform all of the ADLs but with difficulty, their status is in sharp contrast to those at Stage IV, at which people are unable to perform any of the ADLs and require total care. ADL stages as aggregated measures of functioning might, in future efforts, be shown to be appropriate for population surveillance or screening in clinical settings where periodic self-reports (or proxy reports) of functioning are obtained. A reduction in ADL status to a lower stage could trigger system responses and a more-detailed needs assessment. It is hoped that, by simultaneously expressing the types and severity of ADL limitations, ADL stages will help clinicians, healthcare managers, and policymakers address functional needs with greater specificity than typical score- or count-based indicators that obscure the nature of disability.
This study has several limitations. First, self-reported status and functioning could be biased. The major National Institutes of Health Patient Reported Outcomes Measurement Information System initiative which is developing standard self-report batteries of functional measures for trials, however, supports a general movement toward the reporting of self-reported functioning.42 Self- or close proxy–reported functioning is particularly valuable when the objective is to understand the challenges that people living in the community face. Second, approximately one-third of responses in the LSOA II were by proxy. Proxy informants may not always reflect self-perceptions and could generate systematic bias in population prevalence estimates,43 although correlations between self-administered and proxy-reported ADL questions have been shown to be high and were demonstrated to be from moderate to good even in people with strokes.44 When self-reported and caregiver ratings were compared with physical therapist ratings, self-reported function more closely correlated with therapist ratings than that caregiver-reported function, although all three (self, caregiver, and therapist) ratings of function were highly correlated.45 In contrast, self- and caregiver-reported psychosocial status were not highly correlated.46 Consequently, ratings of psychological well-being were not included. Previous analyses of NHIS-D data found large differences in prevalence of ADL limitations in people who report for themselves and those for whom proxies respond. Consequently, it was believed that inclusion of proxy reports would result in less-biased prevalence estimations than excluding them, so proxy information was included to avoid eliminating the most-vulnerable segments of the population.47
Third, the age of the LSOA II data is troubling, but the study would have been impossible without these data because no other source as rich in disability-related information could be found. Even though data were used from the mid-1990s, the findings are still valid because ADL limitation prevalence in the elderly population has remained remarkably stable for more than 2 decades.38
Although environmental details have not been re-collected in a linkable format, the ongoing MCBS includes the same ADL questions.48 The prevalence estimates from the LSOA II were concordant with later estimates from the MCBS. Although it was estimated that 28.4% of the U.S. population aged 70 and older had ADL limitations in 1995, the MCBS estimate was 26% of those aged 65 and older a decade later.1
Fourth, a cross-sectional design of association cannot be considered causal. It is impossible to know which conditions most explained the older people's distribution across the various stages. Future efforts will need to address longitudinal associations between stage and a variety of outcomes. Finally, prevalence estimates according to stage can be expected to generalize only to community-dwelling older persons. People in nursing homes were not included in the LSOA II.
This study presents three important findings. First, individuals’ intrinsic mental and physical conditions, along with external environmental factors, recognized in the HEI theoretical model as co-determinants of disability, were correlated with SPs’ stages of ADL limitation. Second, when addressing linkages between chronic illness and disability, it appears essential to measure global illness severity, as well as the specific type(s) of mental and physical condition(s) the individual is experiencing. Third, there are striking population-level associations between ADL stage and perceptions of unmet need for home accessibility features, with vulnerability appearing greatest at intermediate stages of severity. It is hoped that future work will establish ways of using staging to help identify integrative healthcare strategies that incorporate the most effective and achievable means of improving health status and reducing the incidence or progression of preventable disabilities. Staging might prove helpful in projecting the future needs and challenges associated with disabilities, encouraging much-needed shifts in the national health agenda toward life enrichment.
ACKNOWLEDGMENTS
The research for this manuscript was supported by the National Institutes of Health (AG032420-01A1). There are no personal conflicts of interest of any of the authors, and no authors reported disclosures beyond the funding source. The opinions and conclusions of the authors are not necessarily those of the sponsoring agency or of the NCHS, which was responsible only for provision of the data.
Sponsor's Role: The National Institute on Aging played no role in the design or conduct of the study; in the analysis and interpretation of the data; or in the preparation, review, or approval of the manuscript.
Footnotes
Author Contributions: Margaret Stineman: manuscript writing, study concept and design, analysis and statistical interpretation of data, and acquisition of data. Dawei Xie: study concept and design, analysis and statistical interpretation of data, and critical revision of the manuscript for important intellectual content. Qiang Pan: analysis and statistical interpretation of data. Jibby E. Kurichi and Joel Streim: critical revision of the manuscript for important intellectual content and acquisition of data. Debra Saliba: critical revision of the manuscript for important intellectual content.
Conflict of Interest: The editor in chief has reviewed the conflict of interest checklist provided by the authors and has determined that the authors have no financial or any other kind of personal conflicts with this paper.
REFERENCES
- 1.Federal Interagency Forum on Aging Related Statistics Older Americans 2008: Key indicators of well-being [on-line] March 22; Available at http://www.agingstats.gov/agingstatsdotnet/Main_Site/Data/2008_Documents/Health_Status.pdf. 2010.
- 2.Population Division DESA, United Nations, World Population Ageing 1950–2050: Executive Summary [on-line] February 24; Available at http://www.un.org/esa/population/publications/worldageing19502050/pdf/90chapteriv.pdf. 2010.
- 3.Winker MA, DeAngelis CD. Caring for an aging population: Call for papers. JAMA. 2010;303:455–456. [Google Scholar]
- 4.National Academy on an Aging Society Caregiving: Helping the Elderly with Activity Limitations [on-line] March 27; Available at http://www.agingsociety.org/agingsociety/pdf/Caregiving.pdf. 2010.
- 5.Committee on Disability in America . In: The Future of Disability in America. 1st Ed. Field MJJA, editor. National Academies Press; Washington, DC: 2007. p. 618. [PubMed] [Google Scholar]
- 6.World Health Organization . International Classification of Functioning, Disability and Health: ICF. World Health Organization; Geneva, Switzerland: 2001. [Google Scholar]
- 7.Katz S, Ford AB, Moskowitz RW, et al. Studies of illness in the aged. The Index of ADL: A standardized measure of biological and psychosocial function. JAMA. 1963;185:914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 8.Stineman MG, Ross RN, Fiedler R, et al. Functional independence staging: Conceptual foundation, face validity, and empirical derivation. Arch Phys Med Rehabil. 2003;84:29–37. doi: 10.1053/apmr.2003.50061. [DOI] [PubMed] [Google Scholar]
- 9.Jette DU, Warren RL, Wirtalla C. Validity of functional independence staging in patients receiving rehabilitation in skilled nursing facilities. Arch Phys Med Rehabil. 2005;86:1095–1101. doi: 10.1016/j.apmr.2004.11.035. [DOI] [PubMed] [Google Scholar]
- 10.Jette DU, Warren RL, Wirtalla C. The relation between therapy intensity and outcomes of rehabilitation in skilled nursing facilities. Arch Phys Med Rehabil. 2005;86:373–379. doi: 10.1016/j.apmr.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 11.Stineman MG, Kurichi JE, Kwong PL, et al. Survival analysis in amputees based on physical independence grade achievement. Arch Surg. 2009;144:543–551. doi: 10.1001/archsurg.2009.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stineman MG, Ross RN, Granger CV, et al. Predicting the achievement of 6 grades of physical independence from data routinely collected at admission to rehabilitation. Arch Phys Med Rehabil. 2003;84:1647–1656. doi: 10.1053/s0003-9993(03)00317-4. [DOI] [PubMed] [Google Scholar]
- 13.Stineman MG, Streim JE. The biopsycho-ecological paradigm: A foundational theory for medicine. PM&R. 2010;2:1035–1045. doi: 10.1016/j.pmrj.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Werngren-Elgstrom M, Carlsson G, Iwarsson S. A 10-year follow-up study on subjective well-being and relationships to person-environment (P-E) fit and activity of daily living (ADL) dependence of older Swedish adults. Arch Gerontol Geriatr. 2009;49:e16–e22. doi: 10.1016/j.archger.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 15.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 16.Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 17.McGee D, Cooper R, Liao Y, et al. Patterns of comorbidity and mortality risk in blacks and whites. Ann Epidemiol. 1996;6:381–385. doi: 10.1016/s1047-2797(96)00058-0. [DOI] [PubMed] [Google Scholar]
- 18.Stineman MG, Ross RN, Williams SV, et al. A functional diagnostic complexity index for rehabilitation medicine: Measuring the influence of many diagnoses on functional independence and resource use. Arch Phys Med Rehabil. 2000;81:549–557. doi: 10.1016/s0003-9993(00)90033-9. [DOI] [PubMed] [Google Scholar]
- 19.Lawton MP, Simon B. The ecology of social relationships in housing for the elderly. Gerontologist. 1968;8:108–115. doi: 10.1093/geront/8.2.108. [DOI] [PubMed] [Google Scholar]
- 20.Liu SY, Lapane KL. Residential modifications and decline in physical function among community-dwelling older adults. Gerontologist. 2009;49:344–354. doi: 10.1093/geront/gnp033. [DOI] [PubMed] [Google Scholar]
- 21.Stineman MG, Ross RN, Maislin G, et al. Population-based study of home accessibility features and the activities of daily living: Clinical and policy implications. Disabil Rehabil. 2007;29:1165–1175. doi: 10.1080/09638280600976145. [DOI] [PubMed] [Google Scholar]
- 22.Covinsky KE, Yaffe K, Lindquist K, et al. Depressive symptoms in middle age and the development of later-life functional limitations: The long-term effect of depressive symptoms. J Am Geriatr Soc. 2010;58:551–556. doi: 10.1111/j.1532-5415.2010.02723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fried LP, Ferrucci L, Darer J, et al. Untangling the concepts of disability, frailty, and comorbidity: Implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59A:255–263. doi: 10.1093/gerona/59.3.m255. [DOI] [PubMed] [Google Scholar]
- 24.Gijsen R, Hoeymans N, Schellevis FG, et al. Causes and consequences of comorbidity: A review. J Clin Epidemiol. 2001;54:661–674. doi: 10.1016/s0895-4356(00)00363-2. [DOI] [PubMed] [Google Scholar]
- 25.Tabbarah M, Silverstein M, Seeman T. A health and demographic profile of noninstitutionalized older Americans residing in environments with home modifications. J Aging Health. 2000;12:204–228. doi: 10.1177/089826430001200204. [DOI] [PubMed] [Google Scholar]
- 26.National Center for Health Statistics . Data File Documentation-National Health Interview Second Supplement on Aging-1994 (Machine Readable Data File and Documentation) National Center for Health Statistics; Hyattsville, MD: 1998. 1998. [Google Scholar]
- 27.National Center for Health Statistics The Second Longitudinal Study on Aging, 1994–2000 [on-line] December 9; Available at http://www.cdc.gov/nchs/lsoa/lsoa2.htm. 2009.
- 28.Stineman MG, Ross RN, Maislin G. Functional status measures for integrating medical and social care. Int J Integr Care. 2005;5:e07. doi: 10.5334/ijic.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Linacre JM, Heinemann AW, Wright BD, et al. The structure and stability of the Functional Independence Measure. Arch Phys Med Rehabil. 1994;75:127–132. [PubMed] [Google Scholar]
- 30.Boyington JE, Howard DL, Holmes DN. Self-rated health, activities of daily living, and mobility limitations among black and white stroke survivors. J Aging Health. 2008;20:920–939. doi: 10.1177/0898264308324643. [DOI] [PubMed] [Google Scholar]
- 31.Carod-Artal FJ, Egido JA. Quality of life after stroke: The importance of a good recovery. Cerebrovasc Dis. 2009;27(Suppl 1):204–214. doi: 10.1159/000200461. [DOI] [PubMed] [Google Scholar]
- 32.Herman DR, Solomons NW, Mendoza I, et al. Self-rated health and its relationship to functional status and well-being in a group of elderly Guatemalan subjects. Asia Pac J Clin Nutr. 2001;10:176–182. doi: 10.1046/j.1440-6047.2001.00245.x. [DOI] [PubMed] [Google Scholar]
- 33.Lee Y. The predictive value of self assessed general, physical, and mental health on functional decline and mortality in older adults. J Epidemiol Commun Health. 2000;54:123–129. doi: 10.1136/jech.54.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leinonen R, Heikkinen E, Jylha M. A path analysis model of self-rated health among older people. Aging (Milano) 1999;11:209–220. doi: 10.1007/BF03339661. [DOI] [PubMed] [Google Scholar]
- 35.Maddox GL, Douglass EB. Self-assessment of health: A longitudinal study of elderly subjects. J Health Soc Behav. 1973;14:87–93. [PubMed] [Google Scholar]
- 36.Winter L, Lawton MP, Langston CA, et al. Symptoms, affects, and self-rated health: Evidence for a subjective trajectory of health. J Aging Health. 2007;19:453–469. doi: 10.1177/0898264307300167. [DOI] [PubMed] [Google Scholar]
- 37.Freedman VA, Martin LG, Schoeni RF. Recent trends in disability and functioning among older adults in the United States: A systematic review. JAMA. 2002;288:3137–3146. doi: 10.1001/jama.288.24.3137. [DOI] [PubMed] [Google Scholar]
- 38.Schoeni RF, Freedman VA, Martin LG. Why is late-life disability declining? Milbank Q. 2008;86:47–89. doi: 10.1111/j.1468-0009.2007.00513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Table 12. Projections of the Population by Age and Sex for the United States: 2010 to 2050 (NP2008-T12) [on-line] June 14; Available at http://wwew.cnsus.gov/population/www/projections/summarytables.htm. 2010.
- 40.Boult C, Christmas C, Durso SC, et al. Perspective: Transforming chronic care for older persons. Acad Med. 2008;83:627–631. doi: 10.1097/ACM.0b013e3181782b14. [DOI] [PubMed] [Google Scholar]
- 41.US Department of Health and Human Services Office of Disease prevention & Health Promotion, Healthy people 2020 proposed objectives [on-line] 2009 October 30 23;March 30 23; Available at http://www.healthypeople.gov/hp2020/objectives/TopicArea.aspx?id=17&TopicArea=Disability+and+Secondary+Conditions. 2010.
- 42.Patient Reported Outcomes Measurement Information System (PROMIS) Dynamic Tools to Measure Health from the Patient Perspective [on-line] January 15; Available at http://www.nihpromis.org/default.aspx. 2010.
- 43.Todorov A, Kirchner C. Bias in proxies’ reports of disability: Data from the National Health Interview Survey on disability. Am J Public Health. 2000;90:1248–1253. doi: 10.2105/ajph.90.8.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen MH, Hsieh CL, Mao HF, et al. Differences between patient and proxy reports in the assessment of disability after stroke. Clin Rehabil. 2007;21:351–356. doi: 10.1177/0269215507072544. [DOI] [PubMed] [Google Scholar]
- 45.Dorevitch MI, Cossar RM, Bailey FJ, et al. The accuracy of self and informant ratings of physical functional capacity in the elderly. J Clin Epidemiol. 1992;45:791–798. doi: 10.1016/0895-4356(92)90057-t. [DOI] [PubMed] [Google Scholar]
- 46.Rothman ML, Hedrick SC, Bulcroft KA, et al. The validity of proxy-generated scores as measures of patient health status. Med Care. 1991;29:115–124. doi: 10.1097/00005650-199102000-00004. [DOI] [PubMed] [Google Scholar]
- 47.Stineman MG, Ross RN, Maislin G, et al. Estimating health-related quality of life in populations through cross-sectional surveys. Med Care. 2004;42:569–578. doi: 10.1097/01.mlr.0000128004.19741.81. [DOI] [PubMed] [Google Scholar]
- 48.US Department of Health & Human Services Medicare Current Beneficiary Survey (MCBS) [on-line] March 26; Available at http://www.cms.hhs.gov/MCBS/ 2010.