Abstract
Environmental tobacco smoke (ETS) and ambient air fine particulate matter (PM2.5) are both complex mixtures that have important adverse effects on the cardiovascular system. Although exposures to these complex mixtures have been studied individually, direct comparisons between the two has not been performed. In this study, the authors employed a novel, noninvasive ultrasound biomicroscopy method (UBM) to assess the effects of long-term, low-concentration inhalations of side-stream smoke (SS) and concentrated ambient PM2.5 (CAPs) on plaque progression. ApoE−/− mice (n = 8/group) on high-fat chow (HFC), or normal chow (NC), were exposed to SS (PM = 450 μg/m3) or filtered air (FA) for 6 h/day, 5 days/week, for 6 months; CAPs exposure was at 134 μg/m3 (NC only). Mortality during the SS exposure was greater in the HFC than in the NC, and SS significantly enhanced the effects of diet. No mortality was observed in CAPs-exposed mice. At 4 and 6 months, SS produced the greatest change in plaque area in the left common carotid artery (CCA) in HFC as compared to FA or NC, but not in the brachiocephalic artery. In contrast, CAPs exposure significantly enhanced plaque areas in brachiocephalic and left CCA at 3 and 6 months of exposure. The effect of SS was comparable in magnitude to that produced by CAPs at an average PM2.5 mass concentration that was only 30% as high. In light of the employment of the same animal model, uniform inhalation exposure protocols, time schedules, a noninvasive monitoring protocol, and a parallel study design, these findings have broad applicability.
Keywords: Concentrated ambient particles, environmental tobacco smoke, cardiovascular disease, atherosclerosis ultrasound imaging
Introduction
Epidemiological data from prospective and case-control studies conducted in diverse populations, including males and females in both eastern and western countries, are supportive of a causal association between environmental tobacco smoke (ETS) exposure and coronary heart disease (CHD) mortality and morbidity among nonsmokers (California EPA, 1997, 2004). The American Heart Association (AHA) listed ETS as a major pollutant and cardiovascular toxicant (Taylor et al., 1992). According to California EPA (California EPA, 2004), for nonsmokers exposed to spousal ETS, compared to nonsmokers not so exposed, the risk of CHD mortality is increased by a factor of 1.3. Long-term exposure to ETS has been estimated to cause 40,000–60,000 excess heart disease deaths yearly in the US (Steenland, 1992; Wells, 1994). Vulnerable plaque rupture, ventricular arrhythmias, and chronic progression of atherosclerosis have been proposed as mechanisms by which tobacco smoke contributes to the clinical manifestations of coronary vascular disease (CVD) (Jousilahti et al., 2002). Depending on the mechanism and the duration of exposure, the effects of tobacco smoke on CVD risk are both acute and cumulative (Jousilahti et al., 2002).
Using serial quantitative coronary arteriography, smoking was shown to accelerate coronary lesion progression and new lesion formation (Waters et al., 1996). It also produces a more atherogenic serum lipid profile, and these effects are generally nonreversible (Jousilahti et al., 2002). Moreover, carotid intima-media thickness (IMT), an important surrogate predictor for future acute cardioand cerebrovascular events (Chambless et al., 1997), is increased more among people exposed to ETS than among unexposed subjects (Jousilahti et al., 2002). In previous inhalation studies at New York University (NYU), relatively low levels of ETS had been shown to cause endothelial damage and arterial plaque formation in a cockerel model (Penn et al., 1994; Penn and Snyder, 1996), even with exposure to secondhand smoke of just 1 cigarette per day, in which the PM mass concentration was approximately 3.5 mg/m3 (Penn et al., 1994).
There are many similarities in the nature and extent of the risks associated with the inhalation of ETS and ambient air fine particulate matter (PM2.5). Population-based risk estimates of chronic exposures are significantly elevated for both of these complex mixtures when one examines clinically relevant endpoints, such as attenuation of lung function and lung growth in children, and propensity for cardiovascular disease and lung cancer in adults. There have been some studies that have tested whether PM accelerates atherosclerosis in animal models. For example, Suwa et al. (2002) reported that repeated lung instillation of urban PM twice a week for 4 weeks caused adverse cellular changes in atherosclerotic plaques in hyperlipidemic rabbits. We have examined this issue using mouse models of hyperlipidemic atherosclerosis exposed to concentrated ambient PM (CAPs) and found that subchronic exposure to CAPs in mice prone to develop atherosclerotic lesions had a significant adverse effect on the size, severity, as well as composition of plaque, providing a biologically plausible mechanism linking excess cardiovascular mortality to chronic effects of ambient air PM2.5 exposure.(Chen and Nadziejko, 2005; Sun et al., 2005).
This study expanded upon our initial ambient PM2.5 study by conducting subchronic inhalation studies with side-stream cigarette smoke (SS). In it, we compared SS-induced atherosclerotic effects with the effects of ambient PM2.5, at exposure concentrations of both SS and ambient air PM2.5 that are about 10-fold those found in the homes and workplaces of smokers, i.e., 450 μg/m3 for SS and 134 μg/m3 for PM2.5 CAPs. For both exposures, we used the same exposure systems, effects assays, and mouse models that we used in our initial subchronic ambient PM2.5 studies (Nadziejko et al., 2005).
Materials and methods
Animals
Mice, C57BL/6JBom-Apoetm1Unc (apoliprotein E deficient [ApoE−/−]), 6 weeks old, were obtained from M&B Taconic (Germantown, NY), and housed two to a cage in our ALAAC (Association for Assessment and Accreditation of Laboratory Animal Care International)-accredited animal housing facility at Tuxedo, NY. In SS study, 96 male ApoE−/− mice (8 mice/exposure group/diet/time point, three time points, two exposure groups, two diets) were used for atherosclerosis endpoints. Half of these mice were fed with regular rodent chow (NC; 4% fat) and the other half were fed with high-fat chow (HFC; 21% fat; Harlan Adjusted Calories Diet TD88137; Madison, WI). Animals were kept on normal 12-h light/dark cycles and received food and water ad libitum, except during exposure. The mice had subcutaneously implanted ID chips (that are inserted through a needle without anesthesia) that were read by a special wand (DAS-5006; BMDS, Seaford, DE) connected to a computer. All procedures were approved by the New York University School of Medicine’s Animal Care and Use Committee.
Side stream (SS) cigarette smoke exposure
Except for the aerosol generation systems, identical exposure protocols and equipment were used to expose mice to SS, as a surrogate for ETS, and to CAPs. Realistic exposure regimens were employed. Because ETS concentrations at home or workplace do not vary greatly from day-to-day, we used a constant concentration of SS for the mouse exposures in this study. Conversely, there are considerable daily differences in ambient PM concentrations due to meteorological and seasonal variations. We exposed mice to CAPs that were 10 times the daily ambient PM concentrations.
As reported by California EPA, indoor ETS concentration has been found to range from 0.5 to 6.0 μg/m3 in the home environment and up to 76 μg/m3 in certain workplaces such as bingo parlors. In this study, mice were exposed to 480 μg/m3 ETS for 6 h/day, 5 days/week, for up to 6 months. Although this concentration is considerably higher than those measured in homes, it is only ~10 times those found in many workplaces.
SS, which constitutes 85–90% of ETS, was used for animal exposure in this study. The cigarette smoke was generated with an automated cigarette-smoking machine (CH Technologies, New Jersey), using Tobacco and Health Research Institute’s 2R4F cigarettes (stored, as suggested by the Institute, at 24°C and 60% relative humidity [RH]). One cigarette was lighted at a time to produce mainstream smoke with an automatically regulated piston pump, using a 2-s puff of 35-ml volume in a bell-shaped profile as specified in ISO 3308 (Routine Analytical Cigarette-Smoking Machine, International Organization for Standards, Geneva, Switzerland), once per minute. The SS was generated by diluting the mainstream smoke with filtered air, and was introduced into a 1.3-m3 stainless steel chamber for animal exposure. Mice were exposed, whole body, in their own cages, with food and water removed during the 6-h daily exposure period. Control mice were housed in identical exposure chambers using the same chamber flow characteristics except that the mice were exposed to charcoal and high-efficiency particulate arresting (HEPA)-filtered air.
The exposure to SS was monitored for both the particulate and gas phases. Carbon monoxide (CO) values were determined on an hourly basis using a CO Monitor (Langan Products, San Francisco, CA). A real-time aerosol monitor (MIE DataRAM; Thermo Electron, Franklin, MA) provided continuous monitoring of the particle exposure. Daily average particle exposure levels were measured gravimetrically by collecting SS PM2.5 samples on Teflon filters (Teflo, 2.5 μm pore size; Pall, East Hills, NY), weighed before and after collection using a microbalance (MT-5; Mettler-Toledo, Columbus, OH) located within a temperature- and humidity-controlled environment (30% ± 5% RH, 22°C ± 5%). Particle size distributions were measured with a wide-range particle spectrometer (WPS; MSP, St. Paul, MN) on a daily basis.
Concentrated ambient particles (CAPs) exposures
Details of the CAPs exposure were described previously (Maciejczyk et al., 2005; Sun et al., 2005). Briefly, between May 27 and November 12, 2007, mice were exposed to filtered air (FA) or CAPs, in our Sterling Forest (SF) laboratory at Tuxedo, NY, whole body, for 6 h/day, 5 days per week, for a maximum of 6 months. At 3 and 6 months of exposure, a noninvasive ultrasound biomicroscopy (UBM) was used to evaluate the atherosclerotic plaques before they were euthanized for other endpoints of lung and heart tissues (n = 15 and 10 for the 3- and 6-month endpoints, respectively). Daily average exposure concentrations were measured gravimetrically. Wipe samples of the exposure chamber surface as well as mouse skin in another subchronic inhalation study (using nickel nanoparticles) have shown that surface contamination was below the analytical detection limit and, therefore, would not contribute significantly to the exposure dose.
Quantification of plaque by ultrasound biomicroscopy (UBM) imaging
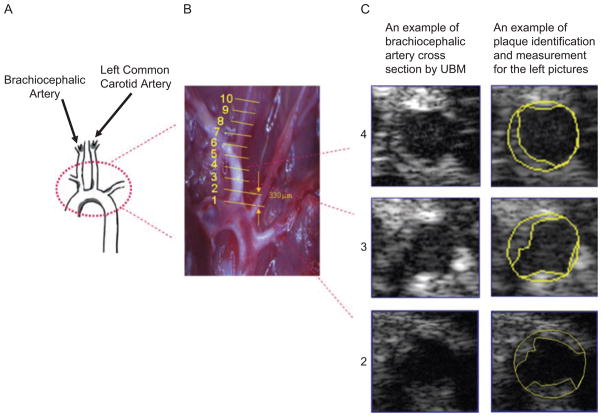
At 2, 4, and 6 months of SS and 3 and 6 months of CAPs exposures, UBM measurements were performed to measure plaque progression. The ultrasound biomicroscope used in this study was a Vevo 770 (VisualSonics, Toronto, Ontario, Canada), with a single-crystal mechanical transducer (704) that has a central frequency of 40 MHz. Each mouse was anesthetized using continuous 1.5% isoflurane mixed in pure oxygen (EZ-Anesthesia; Euthanex, Palmer, PA), and was then placed supine on a physiological platform. Hairs were removed from the chest using Nair (Cater-Horner, Mississauga, Ontario, Canada), and then a layer of prewarmed sterile ultrasound gel (Aquasonic 100; Parker Lab, Orange, NJ) was spread over the chest. Ten B-Mode movies (in cross-sectional position) were obtained along the brachiocephalic artery at around 333-μm intervals (Figure 1). Three pictures were selected from each movie based on following criteria: pictures were clear and representative of the entire movie, and all pictures selected were electroncardiogram (ECG)-synchronized. Plaque area was measured using National institutes of Health (NIH) ImageJ freehand drawing in each picture, and expressed as percentage of plaque area relative to the cross-sectional vessel cavity area. For each artery, the percent area in each of the 10 locations was averaged; the averaged value was expressed as the percent area of the plaque of each artery. All aorta capture and plaque quantification steps were performed in a blinded fashion.
Figure 1.
Ultrasound-based plaque quantification method. (A) Sketch of aorta adopted from Vanderlaan et al. (2004). (B) Image of aorta arch and brachiocephalic and left common carotid arteries as they attached to the heart in the thoracic cavity. White opaque areas were atherosclerotic plaques. Lines denote where ultrasound images were taken for analysis. (C) B-mode images of sections 2–4 of the brachiocephalic artery depicted in B, and the outlines of the cross-section and plaque areas.
Validation method for ultrasound method used in plaque quantification
Before the above ECG-synchronized three-dimensional (3D) ultrasound method was used to quantify the plaque area in this study, a pilot study was conducted to validate this ultrasound-based plaque qualification method. Two well-known risk factors of atherosclerosis, i.e., old age or high-fat diet, were tested. ApoE−/− mice, either 1 or 10 months old, were selected to represent young (Y) and old (O) animals, respectively. These mice were then fed with either HFC or NC for 9 months. Five mice comprised each of the following groups: young mice fed normal chow (Y-NC), young mice fed high-fat diet (Y-HFC), old mice fed normal chow (O-NC), and old mice fed high-fat diet (O-HFC).
The above-developed ultrasound scanning and quantification method was then used to follow plaque progression in the brachiocephalic and left common carotid (LCC) arteries in all mice at the following time points: before subjection to the different diets, and after 2, 4, 6, and 9 months of the different diets. At the end of the 9-month dietary period, all animals that survived the experimental period were sacrificed and tissues were isolated. En face Sudan IV staining for identifying plaques in the thoracic and abdominal aorta were conducted. The plaque quantification results obtained by Ultrasound method at the 9-month time point were then compared with the plaque quantification results from en face Sudan IV staining. Pearson correlation coefficients (r) were calculated between the en ace staining results and those obtained using UBM at each time points.
En face Sudan IV stain for plaque in whole aorta
The aorta (from aortic valve to iliac bifurcation, including three branching arteries at aortic arch) was dissected away intact from the dorsal wall, immersed immediately in 2% glutaraldehyde, and held at room temperature until analysis. Any adventitial and adipose tissue was removed carefully with the use of a dissecting microscope (Model Stemi 2000-C; Zeiss Axioskop, Jena, Germany). The aorta was then cut open longitudinally, splayed, and pinned flat on a black wax surface. The opened aorta was briefly rinsed with 70% ethanol for 5 min, then was immersed for 8 min with filtered Sudan IV stain solution (a fat-soluble dye that stains triglycerides and protein-bound lipids red) containing 0.5% Sudan IV (Sigma), 35% ethanol, 50% acetone, and 15% water, then was de-stained in 80% ethanol for 5 min to eliminate background staining (Palinski et al., 1994; Chen and Nadziejko, 2005). The stained aorta was then immersed in phosphate-buffered saline (PBS) and images were captured through a microscope using a Canon S500 digital camera with Scopetronix microscope adapter. The total Sudan IV–stained lesion area was quantified using ImageJ software, version 1.38X (National Institutes of Health, Bethesda, MD), by manual drawing with polygon selection. The final data were expressed as percentage positive-staining area relative to total aortic area.
Hematoxylin and eosin (H&E) stain for plaque in brachiocephalic artery cross-sections
At 2, 4, and 6 months of SS and 3 and 6 months of CAPs exposures, H&E stain for plaques in brachiocephalic and carotid artery cross sections were also performed. Brachiocephalic and left common carotid arteries were carefully isolated and embedded perpendicularly in optimal cutting temperature (OCT; Tissue-Tek; Sakura Finetek USA, Torrance, CA) compound, and were then snap frozen and stored in −80°C. Serial cross-sections were taken using a calibrated Microm HM500M cryostat (Microm, Walldorf, Germany). Three successive sections (8 μm thick) were collected onto Fisher Superfrost Plus–coated slides, and 10 slides were collected for each mouse. For each mouse, the 1st (near entrance of artery) and 10th slides (around 300 μm away from entrance) were stained with H&E. Each image was digitized under a research microscope (Olympus, Model BH2) with a digital camera (SONY 3CCD color video camera; Model DXC-390). Plaque area was quantified with NIH ImageJ software by manual drawing with polygon selection. Plaque areas were adjusted for the cross-sectional vessel cavity area and expressed as percentage plaque area relative to total cross-sectional vessel cavity area. The mean percent atherosclerotic plaque area was determined by averaging the plaque area in two stained slides.
Statistical data analyses
Two-way analyses of variance (ANOVAs) were used to assess any interactions between SS exposure and diet (NC and HFC) as two independent factors. Grubbs and Dixon tests were used to identify any outliers in a given data set. One-way ANOVAs, followed by Dunnett’s test, were used to assess differences in the effects between each different exposure as compared to FA. The t test was used to test the difference in effects between any two exposure groups at a fixed time point, and to test the differences within a given group at different time points. All p values were two-tailed; and statistical significance was set at p < .05. For endpoints that had a limited sample size (e.g., n = 3), p < .1 was also examined. All statistical analyses were performed using Graph-Pad Prism software version 4 (GraphPad Software, San Diego, CA).
Results
Exposure parameters
The average SS concentration during the entire exposure period was 450 (± 48) μg/m3. The number and volume median particle sizes of the SS, as measured using the WPS system, were 55 (GSD 1.82) and 179 (GSD 1.93) nm, respectively. Average CO concentration was 0.7 ± 0.1 ppm (mean ± SD). For the CAPs exposure, the average PM2.5 mass concentration was 134 ± 110 μg/m3 (mean ± SD). Detailed characterizations of the exposure matrix and the results of other biological endpoints of the CAPs exposure will be reported elsewhere.
Mortality and body weight
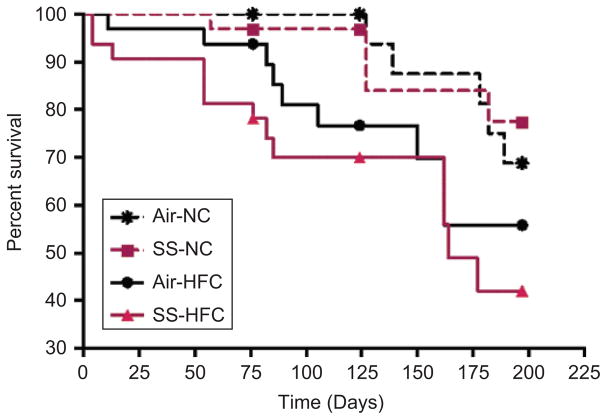
During the course of the exposure, several mice died in both the SS- and FA-exposed groups. Survival analysis showed that the diet, but not SS, had a significant effects on survival (p = .01) and that the life span was significantly reduced (p = .04) in SS-exposed and HFC-fed mice as compared to SS-exposed and NC-fed mice (Figure 2). In contrast, no deaths were observed in mice undergoing CAPs exposure that were fed a normal chow diet. Mice were weighed once a month. As expected, HFC groups were heavier than NC groups, but there was no significant exposure effect in either diet group (−1.1 g in SS-NC and −2.0 g in SS-HFC).
Figure 2.
Percent survival of mice during the exposure period.
Validation of ultrasound-based plaque quantification method
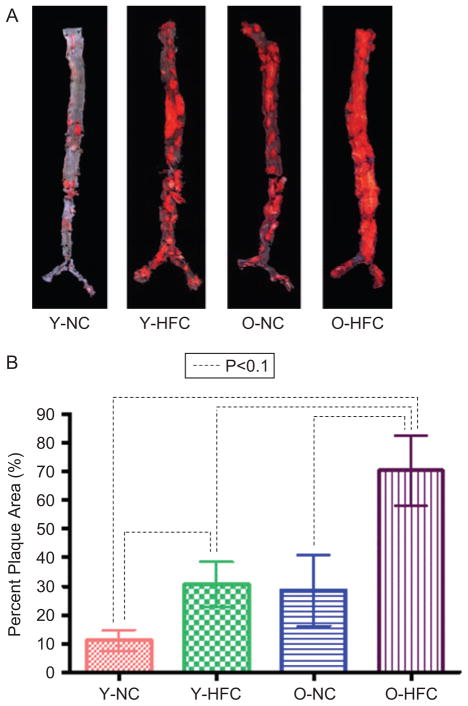
To validate the ultrasound-based plaque quantification method, a pilot study was designed as described previously. Figure 3A shows the images of plaque on aorta surfaces obtained from en face Sudan IV staining, which were consistent with what was expected. Specifically, the older mice developed more plaques than the younger mice, and the mice fed high-fat chow developed more plaques than mice fed normal chow. Figure 3B shows the plaque quantification results obtained using the en face Sudan IV method.
Figure 3.
For ultrasound method validation, plaque quantification by en face Sudan IV staining in abdominal aorta of young/old mice fed with normal chow/high-fat chow at the end of the 9-month follow-up. (At the time of analyses, young mice was 10 months old, and the old mice was 19 months old). (A) En face Sudan IV staining; (B) quantification of plaque area in the brachiocephalic artery using UBM.
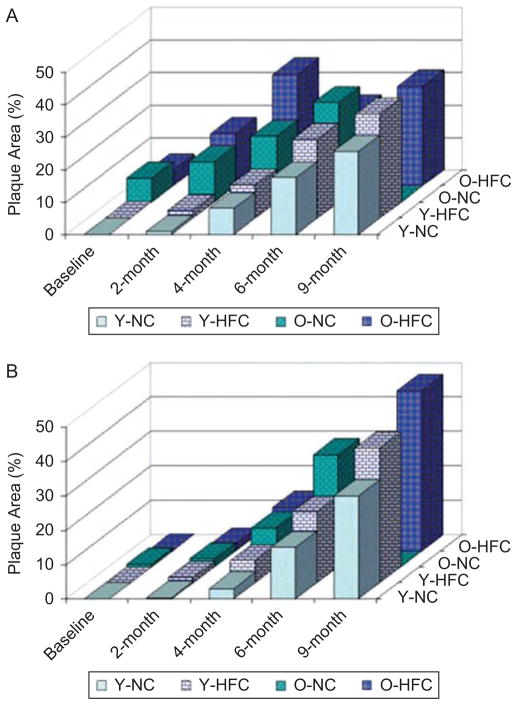
As shown in Figure 4A and B, at 2- and 4-month follow-up, plaque development among the four groups agreed with what was expected. Because mice in the O-HFC group tended to have larger and more advanced plaques, and therefore were likely to die earlier, any surviving O-HFC mice tended to be those that were not very susceptible to develop plaque (healthy survival effects). Therefore, at the 6-month follow-up time point, less plaques were detected (by ultrasound) in both the brachiocephalic and left common carotid (LCC) arteries in the O-HFC group than that in O-NC group, and than that in the O-HFC group at the 4-month time point. By the end of the 9-month follow-up, mice in the O-NC group had all died; therefore no data by ultrasound method for this group were available.
Figure 4.
For ultrasound method validation, plaque quantification by ultrasound-based method in young/old mice fed with normal chow/high-fat chow during the 9-month period at (A) brachiocephalic artery and (B) left common carotid artery.
As shown in Table 1, UBM measurements in both arteries correlated very well (correlation coefficients ranged from .60 to .96) with the en face Sudan IV methods, with the exception of the 6-month measurements at which time the number of animals were small (n = 2). Overall, at each time point, plaque development among the four groups (O-HFC, O-NC, Y-HFC, and Y-NC) detected by the ultrasound-based method were consistent with what had been expected and with what had been detected by en face Sudan IV staining. In addition, the ultrasound-based method was able to follow plaque progression in each individual animal. Thus, it was concluded that this ultrasound-based plaque quantification method was valid, and had the sensitivity needed to detect in vivo plaque development among mice in the five different treatment groups over the 6-month exposure time frame.
Table 1.
Correlation of en face and UBM measurements.
| En face*,† |
UBM*,¥ |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Aorta |
Brachiocephlic artery |
Left common carotid artery |
|||||||
| 9-Month | 2-Month | 4-Month | 6-Month | 9-Month | 2-Month | 4-Month | 6-Month | 9-Month | |
| Y-NC | 11.08 | 1.01 | 7.99 | 17.51 | 25.60 | 0.51 | 3.06 | 15.03 | 30.01 |
| Y-HFC | 30.47 | 2.44 | 10.29 | 24.32 | 32.10 | 1.58 | 6.41 | 20.57 | 39.29 |
| O-NC | 28.50 | 12.47 | 20.27 | 30.77 | ND | 2.27 | 11.33 | 32.58 | ND |
| O-HFC | 70.30 | 16.15 | 34.46 | 23.15 | 30.69 | 1.96 | 12.61 | 12.45 | 46.56 |
| r | .81 | .93 | .19 | .60 | .60 | .80 | −.34 | .96 | |
Percentage of plaque area.
Graphic shown in Figure 3.
Shown in Figure 4A and B.
r: Pearson correlation coefficient.
ND: not done, no animal in O-NC group survived at 9 months.
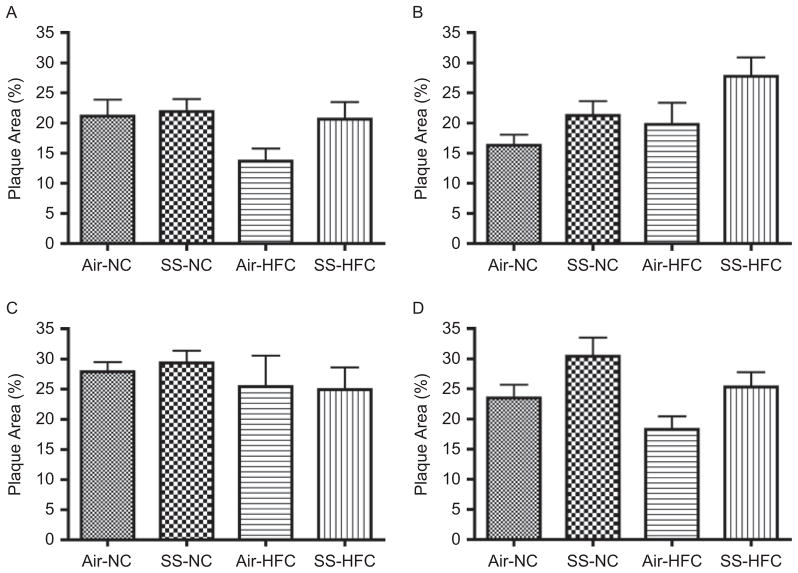
Effects of SS on the progression of atherosclerotic lesions
Figure 5A–D show the progression of atherosclerosis in the brachiocephalic and left common carotid artery in mice exposed to air and SS using UBM. No significant change in plaque area was observed in the brachiocephalic artery regardless of diet or exposure (Figure 5A, C). In the LCC (Figure 5B), compared to filtered air (FA) control, 4 months of SS exposure significantly increased the plaque area by 30% and 37% in mice fed with NC and HFC, respectively (p = .02). The effect of diet was approaching significance (p = .07). Although SS effects were still significant, the effect on plaque area did not increase when SS exposure was extended to 6 months in the LCC (increase of 25% and 39% in SS-NC and SS-HFC, respectively, p = .02; Figure 5D). At 6 months, the plaque area was smaller in HFC-fed mice, compared to NC-fed animals, in both arteries regardless of the exposure. Because more HFC mice died during the course of the experiment than NC mice, it is likely that those surviving mice were less susceptible to HFC-induced atherosclerosis. In the surviving mice, plaque area was increased by 6 months of SS-NC mice, but not in SS-HFC mice.
Figure 5.
Progression of atherosclerosis in brachiocephalic and left common carotid arteries as measured using UBM in SS-exposed animals. (A) Brachiocephalic artery at 4 months of SS exposure; p = .09 and .13 for chow and SS effects, respectively. (B) Left common carotid artery at 4 months of SS exposure; p = .07 and .02 for chow and SS effects, respectively. (C) Brachiocephalic artery at 6 months of SS exposure; p = .24 and .86 for chow and SS effects, respectively. (D) Left common carotid artery at 6 months of SS exposure; p = .07 and .02 for chow and SS effects, respectively. There was no interaction in any of these measurements.
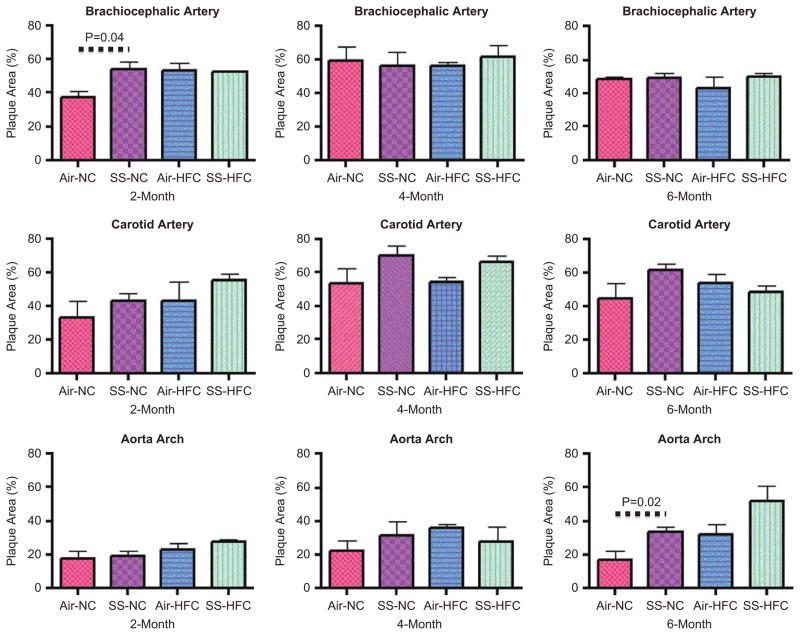
We also examined the atherosclerotic lesion size using H&E staining of the cross-sections of aorta arch, brachiocephalic and left common carotid arteries. As shown in Figure 6, there was a trend for SS-enhanced lesion size, but only two of these measurements were statistically significant, and no consistent pattern was observed. In NC animals, SS exposure caused 44 (p = .04) and 93% (p = .02) increase in plaque size at the brachiocephalic artery at 2 months and aorta arch at 6 months, respectively. Although there were exposure (SS) and diet effects, there was no interaction between these two risk factors for any of the endpoints measured in this study.
Figure 6.
Plaque progression in aorta archand brachiocephalic and left common carotid arteries in H&E-stained sections.
Effects of CAPs on the progression of atherosclerotic lesions
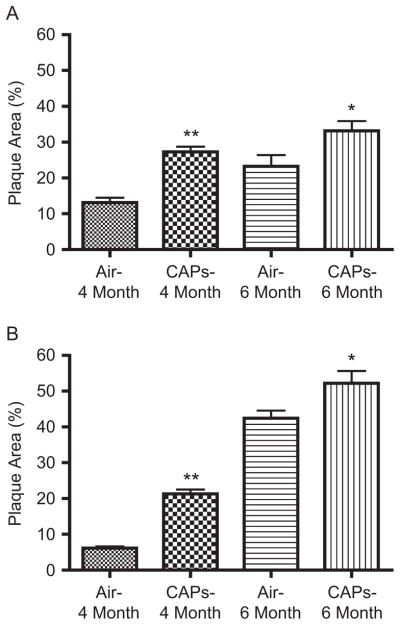
The effects of CAPs on atherosclerotic lesions are shown in Figure 7A and B. In the brachiocephalic artery, plaque area doubled at 3 months of CAPs exposure as compared to FA controls (27.3% ± 8.6% versus 13.1% ± 7.0% for CAPs and FA, respectively; p < .001). The plaque area increased at 6 months of exposure, but the difference between CAPs and FA was reduced to 43% (33.2% ± 9.0% versus 23.3% ± 8.8% for CAPs and FA, respectively; p = .03). Similar results were seen in the LCC. At 3 months, plaque area was 21.3% ± 1.2% in the LCC of CAPs-exposed animals. However, because plaques were found in only one animal in the FA group, no statistical analysis was performed for this period. At 6 months of exposure, CAPs enhanced the plaque area by 23% in the LCC (52.2% ± 10.7% versus 42.5% ± 6.5% for CAPs and FA, respectively; p = .03). Although the intermediate UBM measurements were performed at different time points (3 months for CAPs, and at 2 and 4 months for SS), we compared the plaque areas in these arteries of CAPs and SS exposure at 3 and 4 months of exposure, respectively. As shown in Table 2, CAPs produced greater plaque area than SS in both arteries, with exacerbation in the brachiocephalic artery at the earlier time point of the exposure (27.3% versus 21.9%; p = .04), whereas the exacerbation occurred in the LCC at a later time point (52.2% versus 30.4%; p = .0001).
Figure 7.
Progression of atherosclerosis in brachiocephalic and left common carotid arteries as measured using UBM in CAPs-exposed animals. (A) Brachiocephalic artery at 4 and 6 months of CAPs exposure. (B) Left common carotid artery at 4 and 6 months of CAPs exposure. *p < .05; **p < .01.
Table 2.
Comparisons of the effects of SS and CAPs on atherosclerosis in normal chow fed mice.
| Vessel | Exposure | Duration | Plaque area (%)a |
|---|---|---|---|
| Brachiocephalic artery | SS | 4 Months | 21.9 ± 2.1 |
| CAPs | 3 Months | 27.3 ± 1.4 | |
| SS | 6 Months | 29.3 ± 2.0 | |
| CAPs | 6 Months | 33.2 ± 2.7 | |
| Left common carotid artery | SS | 4 Months | 21.3 ± 2.4 |
| CAPs | 3 Months | 21.3 ± 1.2 | |
| SS | 6 Months | 30.4 ± 3.1 | |
| CAPs | 6 Months | 52.2 ± 3.4 |
Mean ± SE.
Discussion
The main contribution of this work is the direct comparison of the magnitude of effects seen with ambient air PM2.5 when compared to tobacco smoke, a risk factor strongly linked with the progression and complications associated with atherosclerosis. The magnitude of SS-induced atherosclerosis exacerbation seen in this study was comparable with that reported by others, but notably in this study it occurred at a much lower exposure concentration (Gairola et al., 2001; Yuan et al., 2007). Conversely the progression noted with SS in our study occurred at a much lower concentration (450 μg/m3), which was ≈55 times lower than the concentration (25 mg/m3) used in these previous studies. In prior work by Garirola et al. (2001), 33% of the aorta surface was covered with plaque (en face staining) as compared to FA control group (10%) after 14 weeks of daily 6 h, 5 days/week, SS exposure in ApoE−/− mice on a HFC diet. In another previous study (Yuan et al., 2007), the lesion area in aortic cross sections was 49%, compared to 24% in the FA control group. That study used ApoB100 transgenic mice, on high-fat diet, exposed to SS at 25 ± 2 mg/m3 for 6 h/day, 5 days/week, for 1 year. The use of the more sensitive and comprehensive UBM assay in our studies could have contributed to our ability of our analysis to show effects at the much lower exposure level. Most interestingly, the exacerbations of plaque lesion due to a subchronic CAPs inhalation exposure were similar or greater in magnitude than that due to the SS exposure in this study (Table 2), although the average CAPs concentration was less than one third of the average SS concentration, and the lesion growth was measured at 3 months of CAPs exposure rather than the 4-month time point for the SS exposure. The difference between SS and CAPs effects in terms of plaque progression was greatest in the left common carotid artery at 6 months of exposure. The underlying mechanisms of the atherogenic potency of SS and CAPs are still under investigation.
A novel aspect of this study was the emphasis on longitudinal changes using a noninvasive approach that allowed us unparalleled ability to follow progression rates with exposures to SS and PM2.5. Undoubtedly the use of this approach in our studies may have contributed to our ability to demonstrate effects at the much lower exposure levels. For this study, we had to adapt an existing noninvasive method to measure plaque area in arteries, such as the brachiocephalic and left common carotid arteries, using UBM. We verified the utility and reliability of UBM measurements by comparing these measurements to those of conventional en face analysis of the abdominal aorta (Figure 3A and B). We used the UBM method to track the plaque progression in the brachiocephalic and common carotid arteries, in young and old mice fed with either a normal or a high-fat diet (Figure 4A and B) and these measurements correlated very well with those observed in the aorta (Table 1). This ability to make noninvasive measurements in the same mice at different time points in these areas is very important for assessing the progression of atherosclerosis. In ApoE−/− mice, the atherosclerotic plaques often occur in aortic sinuses, along the lesser curvature of the aortic arch, in the brachiocephalic artery, and in the proximal part of the common carotid arteries (Rosenfeld et al., 2000), but with the brachiocephalic artery developing the most advanced lesions (Rosenfeld et al., 2008). Although the lesions in these arteries are not identical to those occurred in the coronary artery in humans, the underlying process of the plaque development is considered a good model for some of the critical processes leading to the development and breakdown of human atherosclerotic plaque (Rosenfeld et al., 2000). Another motivation to use the brachiocephalic and carotid arteries is that these vessels often develop characteristics associated with vulnerability seen in humans such as intraplaque hemorrhage.
Using the UBM technique, we showed that a relatively low level of subchronic SS exposure significantly increased the plaque area in the left common carotid artery after 6 months of exposure in mice fed with HFC. In contrast, no statistical significant change was observed when a considerably more spatially restricted morphometric technique was used. We also noted diet-independent effects with exposure, especially when extended from 4 to 6 months with SS exposure, and from 3 to 6 months for the CAPs exposure. For SS, the enhanced lesion area at 4 months approached significance in the HFC group (p = .06 and .10 for the brachiocephalic and LCC, respectively). In addition, as seen in H&E staining (Figure 6), SS-enhanced plaque size was evident in the brachiocephalic artery in the NC group at 2 months of observation (p = .04), suggesting that SS exposure could have exacerbated plaque lesion in as little as 2 months of exposure. Unfortunately, UBM was not performed at 2 months into the SS exposure because the UBM instrument was not yet available at that time point.
In addition to plaque progression, the SS exposure was associated with excess mortality, most notably in the HFC-exposed group. As shown in Figure 2, high-fat diet had a substantial negative impact on survival with SS exposure, with the survival of the SS-exposed, HFC-fed mice being considerably lower than for the HFC-fed mice exposed to FA. Even though SS inhalation per se did not reduce life span, this interactive effect with diet provides some limited support for the hypothesis that excess mortality reported in humans exposed to ETS is often in the setting of preexistent risk factors such as diet. In contrast, the CAPs exposure, although more atherogenic, was not associated with mortality. It is possible that organic components of the SS smoke and/or the other associated smoke stream gases and vapors that were not present in the CAPs exposures may have contributed to differential effects on mortality.
Although the precise components in PM2.5 and/or SS defy easy characterization, we do believe that the presence of higher concentrations of trace transition metals in CAPs, such as vanadium, nickel, chromium, zinc, and iron, which were generally absent in SS, may have accounted for the greater potency of PM2.5 in exacerbating atherosclerotic progression (Chen and Nadziejko, 2005; Lippmann et al., 2005; Maciejczyk and Chen, 2005). Daily 6-h PM2.5 air samples were also collected and analyzed by x-ray fluorescence (XRF), permitting attribution to major PM2.5 source categories (secondary SO42−, suspended soil, residual oil combustion, and a remainder category, which was largely due to long-range transported pollutants associated with motor vehicle traffic). These previous studies examined associations between the PM2.5 components and both heart rate (HR) and hear rate variability (HRV), and found major contributions from secondary sulfate, residual oil combustion (predominantly V and Ni), suspended soil (predominantly Si, Al, Ca), and traffic (Br, Fe, elemental carbon) to the observed changes. Whether similar components are responsible for the atherosclerosis exacerbation observed in this study is not known at this time. The fact that transition metal components are highly reactive, mostly soluble, and have access to cells such as macrophages in the lung, besides participating in free radical reactions in a facile manner, may translate into their propensity to exert local and systemic effects on the cardiovascular system.
The results of the current study with CAPs exposure were consistent with those of a previous CAPs exposure studies that compared the mice in the CAPs-exposed subgroup on a high-fat diet (HF) with those exposed to filtered air (FA) (Sun et al., 2005). In that study, similar to the observations of SS exposure of the current study, both diet and CAPs exposure synergistically potentiated plaque progression. CAPs exposure also had marked increases in macrophage infiltration, reactive oxygen species (ROS) generation, inducible NO synthase (iNOS), and immunostaining for 3-nitrotyrosine. In subsequent experiments to test the hypothesis that exposure to CAPs enhances atherosclerosis through induction of vascular reactive oxygen and nitrogen species, we exposed ApoE−/− mice on a high-fat diet to FA or PM2.5 CAPs for 6 h/day, 5 days/week, for 4 months in northern Manhattan at a mean concentration of 173 μg/m3 (Ying et al., 2009). Analysis of vascular responses revealed significantly decreased phenylephrine constriction in CAPs-exposed mice in conjunction with abundant expression of iNOS. These experiments confirmed the fact that CAPs exposure was accompanied by increased aortic expression of NADPH oxidase subunits (p47phox and rac1) and iNOS, paralleled by increases in superoxide generation and extensive protein nitration in the aorta. Vascular relaxation to A23187, but not acetylcholine, was attenuated in CAPs-exposed mice, whereas responses to phenylephrine were potentiated. Interestingly, the vascular responses to phenylephrine were restored by a soluble guanine cyclase inhibitor (ODQ). The composite plaque area of the thoracic aorta was also significantly increased, with pronounced macrophage infiltration and lipid deposition in the CAPs-exposed mice. Thus, CAPs exposure alters vasomotor tone and inflammatory pathways, presumably through the involvement of ROS mechanisms.
Conclusions
Our results showed that long-term inhalation exposure to a relatively low concentration of SS significantly exacerbated lesion progression in atherosclerosis and was associated with premature mortality. On a mass basis, ambient PM2.5 concentration (134 μg/m3), although substantially lower than mass concentration of the SS PM (an average PM2.5 concentration only 30% as high as SS), had very similar effects in promoting atherosclerosis in a susceptible animal model, albeit without causing premature mortality. The use of identical protocols and animal model and, with minor deviations, following the same exposure protocols and time schedules make it likely that differences in chemical composition between SS and CAPs contributed to the differential effects on mortality and the effect of exposure on plaque progression. Because both of these complex mixtures are likely to affect plaque progression through their capacity to generate ROS and induce other inflammatory responses, and because the health risks of both are commonly characterized in terms of PM mass concentration, it is reasonable to compare the effect that they have in terms of their PM mass concentrations. In this context, we report that the effect of SS was comparable in kind and magnitude to that produced by CAPs at an average PM2.5 concentration only 30% as high.
These experimental findings go a long way toward establishing greater biological plausibility for the epidemiological reports of atherosclerotic disease associated with these two complex environmental mixtures. There was a significant reduction in survival among those mice on a HFC diet associated with SS exposure compared to that for those on a NC diet, and is consistent with the excess mortality that has been associated with ETS exposure in homes and workplaces at comparable concentrations. Further investigations to identify and understand the influence of specific components that may mediate these responses to SS and CAPs are needed.
Acknowledgments
The authors are also grateful to Dr. Lippmann for his review of the manuscript and to Ken Magar for his technical assistance.
Footnotes
Declaration of interest
The authors acknowledge the support they received from a NIEHS Center Grant ES 00260 (Lippmann and Chen), a NIEHS research grant R01ES015495 (Chen), a research grant supporting the CAPs exposures from the Health Effects Institute NPACT Initiative (Lippmann and Chen), and a research grant from the Philip Morris External Research Program that supported the SS smoke exposures. Dr. Sun was supported by NIEHS grant ES016588.
References
- California EPA. Health effects of exposure to environmental tobacco smoke. Sacramento, CA: Office of Environmental Health Hazard Assessment; 1997. [Google Scholar]
- California EPA. Proposed identification of environmental tobacco smoke as a toxic air contaminant. 2004. [Google Scholar]
- Chambless LE, Heiss G, Folsom AR, Rosamond W, Szklo M, Sharrett AR, Clegg LX. Association of coronary heart disease incidence with carotid arterial wall thickness and major risk factors: The Atherosclerosis Risk in Communities (ARIC) Study, 1987–1993. Am J Epidemiol. 1997;146:483–494. doi: 10.1093/oxfordjournals.aje.a009302. [DOI] [PubMed] [Google Scholar]
- Chen LC, Nadziejko CN. Effects of subchronic exposures to concentrated ambient particles (CAPs) in mice: V. CAPs exacerbate aortic plaque development in hyperlipidemic mice. Inhal Toxicol. 2005;17:217–224. doi: 10.1080/08958370590912815. [DOI] [PubMed] [Google Scholar]
- Gairola CG, Drawdy ML, Block AE, Daugherty A. Sidestream cigarette smoke accelerates atherogenesis in apolipoprotein E−/− mice. Atherosclerosis. 2001;156:49–55. doi: 10.1016/s0021-9150(00)00621-3. [DOI] [PubMed] [Google Scholar]
- Goto Y, Hogg JC, Shih CH, Ishii H, Vincent R, van Eeden SF. Exposure to ambient particles accelerates monocyte release from bone marrow in atherosclerotic rabbits. Am J Physiol Lung Cell Mol Physiol. 2004;287:L79–L85. doi: 10.1152/ajplung.00425.2003. [DOI] [PubMed] [Google Scholar]
- Jousilahti P, Patja K, Salomaa V. Environmental tobacco smoke and the risk of cardiovascular disease. Scand J Work Environ Health. 2002;28:41–51. [PubMed] [Google Scholar]
- Libby P. Vascular biology of athersclerosis: Overview and state of the art. Am J Cardiol. 2003;91:3A–6A. doi: 10.1016/s0002-9149(02)03143-0. [DOI] [PubMed] [Google Scholar]
- Libby P, Aikawa M. Stabilization of atherosclerosis plaques: New mechanisms and clinical targets. Nat Med. 2002;8:1257–1262. doi: 10.1038/nm1102-1257. [DOI] [PubMed] [Google Scholar]
- Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- Lippmann M, Gordon T, Chen LC. Effects of subchronic exposures to concentrated ambient particles in mice. IX. Integral assessment and human health implications of subchronic exposures of mice to CAPs. Inhal Toxicol. 2005;17:255–261. doi: 10.1080/08958370590912941. [DOI] [PubMed] [Google Scholar]
- Lippmann M, Ito K, Hwang JS, Maciejczyk P, Chen LC. Cardiovascular effects of nickel in ambient air. Environ Health Perspect. 2006;114:1662–1669. doi: 10.1289/ehp.9150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciejczyk P, Chen LC. Effects of subchronic exposures to concentrated ambient particles (CAPs) in mice. VIII. Source-related daily variations in in vitro responses to CAPs. Inhal Toxicol. 2005;17:243–253. doi: 10.1080/08958370590912914. [DOI] [PubMed] [Google Scholar]
- Maciejczyk P, Zhong M, Li Q, Xiong J, Nadziejko C, Chen LC. Effects of subchronic exposures to concentrated ambient particles (CAPs) in mice. II. The design of a CAPs exposure system for biometric telemetry monitoring. Inhal Toxicol. 2005;17:189–197. doi: 10.1080/08958370590912743. [DOI] [PubMed] [Google Scholar]
- Mukae H, Vincent R, Quinlan K, English D, Hards J, Hogg JC, van Eeden SF. The Effect of repeated exposure to particulate air pollution (PM10) on the bone marrow. Am J Respir Crit Care Med. 2001;163:201–209. doi: 10.1164/ajrccm.163.1.2002039. [DOI] [PubMed] [Google Scholar]
- Palinski W, Ord VA, Plump AS, Breslow JL, Steinberg D, Witztum JL. ApoE-deficient mice are a model of lipoprotein oxidation in atherogenesis. Demonstration of oxidation-specific epitopes in lesions and high titers of autoantibodies to malondialdehyde-lysine in serum. Arterioscler Thromb. 1994;14:605–616. doi: 10.1161/01.atv.14.4.605. [DOI] [PubMed] [Google Scholar]
- Penn A, Chen LC, Snyder CA. Inhalation of steady-state sidestream smoke from one cigarette promotes arteriosclerotic plaque development. Circulation. 1994;90:1363–1367. doi: 10.1161/01.cir.90.3.1363. [DOI] [PubMed] [Google Scholar]
- Penn A, Snyder CA. 1,3-Butadiene, a vapor phase component of environmental tobacco smoke, accelerates arteriosclerotic plaque development. Circulation. 1996;93:552–557. doi: 10.1161/01.cir.93.3.552. [DOI] [PubMed] [Google Scholar]
- Pope CA, III, Burnett RT, Thurston GD, Thun MJ, Calle EE, Krewski D, Godleski JJ. Cardiovascular mortality and long-term exposure to particulate air pollution. Epidemiological evidence of general pathophysiological pathways of disease. Circulation. 2004;109:71–77. doi: 10.1161/01.CIR.0000108927.80044.7F. [DOI] [PubMed] [Google Scholar]
- Rosenfeld ME, Averill MM, Bennett BJ, Schwartz SM. Progression and disruption of advanced atherosclerotic plaques in murine models. Curr Drug Targets. 2008;9:210–216. doi: 10.2174/138945008783755575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld ME, Polinsky P, Virmani R, Kauser K, Rubanyi G, Schwartz SM. Advanced atherosclerotic lesions in the innominate artery of the ApoE knockout mouse. Arterioscler Thromb Vasc Biol. 2000;20:2587–2592. doi: 10.1161/01.atv.20.12.2587. [DOI] [PubMed] [Google Scholar]
- Steenland K. Passive smoking and the risk of heart disease. JAMA. 1992;267:94–99. [PubMed] [Google Scholar]
- Sun Q, Wang A, Jin X, Natanzon A, Duquaine D, Brook RD, Aguinaldo JG, Fayad ZA, Fuster V, Lippmann M, Chen LC, Rajagopalan S. Long-term air pollution exposure and acceleration of atherosclerosis and vascular inflammation in an animal model. JAMA. 2005;294:3003–3010. doi: 10.1001/jama.294.23.3003. [DOI] [PubMed] [Google Scholar]
- Sun Q, Yue P, Deiuliis JA, Lumeng CN, Kampfrath T, Mikolaj MB, Cai Y, Ostrowski MC, Lu B, Parthasarathy S, Brook RD, Moffatt-Bruce SD, Chen LC, Rajagopalan S. Ambient air pollution exaggerates adipose inflammation and insulin resistance in a mouse model of diet-induced obesity. Circulation. 2009;119:538–546. doi: 10.1161/CIRCULATIONAHA.108.799015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwa T, Hogg JC, Quinlan KB, Ohgami A, Vincent R, van Eeden SF. Particulate air pollution induces progression of atherosclerosis. J Am Coll Cardiol. 2002;39:935–942. doi: 10.1016/s0735-1097(02)01715-1. [DOI] [PubMed] [Google Scholar]
- Taylor A, Johnson D, Kazemi H. Environmental tobacco smoke and cardiovascular disease. Circulation. 1992;88:699–702. doi: 10.1161/01.cir.86.2.699. [DOI] [PubMed] [Google Scholar]
- Waters D, Lesperance J, Gladstone P, Boccuzzi SJ, Cook T, Hudgin R, Krip G, Higginson L. Effects of cigarette smoking on the angiographic evolution of coronary atherosclerosis: A Canadian Coronary Atherosclerosis Intervention Trial (CCAIT) substudy. Circulation. 1996;94:614–621. doi: 10.1161/01.cir.94.4.614. [DOI] [PubMed] [Google Scholar]
- Wells AJ. Passive smoking as a cause of heart disease. J Am Coll Cardiol. 1994;24:546–554. doi: 10.1016/0735-1097(94)90315-8. [DOI] [PubMed] [Google Scholar]
- Ying Z, Kampfrath T, Thurston G, Farrar B, Lippmann M, Wang A, Sun Q, Chen LC, Rajagopalan S. Ambient particulates alter vascular function through induction of reactive oxygen and nitrogen species. Toxicol Sci. 2009;111(1):80–8. doi: 10.1093/toxsci/kfp004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J, Libby P, Schonbeck U. Cytokines in the pathogensis of atheroclerosis. Thromb Haemost. 2002;88:554–567. [PubMed] [Google Scholar]
- Yuan H, Wong LS, Bhattacharya M, Ma C, Zafarani M, Yao M, Schneider M, Pitas RE, Martins-Green M. The effects of second-hand smoke on biological processes important in atherogenesis. BMC Cardiovasc 74 Disord. 2007;7:1–16. doi: 10.1186/1471-2261-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]