Abstract
The plasma membrane delimits the cell and controls material and information exchange between itself and the environment. How different plasma-membrane processes are coordinated and how the relative abundance of plasma-membrane lipids and proteins is homeostatically maintained are not yet understood. Here, we used a quantitative genetic interaction map, or E-MAP, to functionally interrogate a set of ~400 genes involved in various aspects of plasma-membrane biology, including endocytosis, signaling, lipid metabolism and eisosome function. From this E-MAP, we derived a set of 57,799 individual interactions between genes functioning in these various processes. Using triplet genetic motif analysis, we identified a new component of the eisosome, Eis1, and linked the poorly characterized gene EMP70 to endocytic and eisosome function. Finally, we implicated Rom2, a GDP/GTP exchange factor for Rho1 and Rho2, in the regulation of sphingolipid metabolism.
The plasma membrane is the defining feature of the cell, separating its interior from the exterior space. It controls exchange and communication processes between the cell and its environment. The delivery of cellular material to the plasma membrane or cell exterior is mediated by exocytosis. Conversely, endocytosis is used to take up plasma membrane and external components. In addition, many signaling processes occur at the plasma membrane simultaneously and are often regulated by the endocytosis of receptors or delivery of messenger molecules. To coordinate these processes and maintain cell integrity under changing conditions, both plasma-membrane protein and lipid composition are regulated and adjusted to external conditions. Despite impressive advances in our understanding of these individual processes, it is not well understood how they are coordinated.
To accommodate its many functions, the plasma membrane is highly organized, both spatially and temporally. In Saccharomyces cerevisiae, several plasma-membrane domains of different composition are distinguishable by light microscopy. This organization is mediated, at least in part, by eisosomes, large protein complexes that underlie one of the domains, named MCC after the marker protein Can1 found there. When PIL1, encoding a major eisosome component, is deleted, cells have abnormal plasma-membrane structure with large invaginations and loss of MCC protein organization1,2. In addition, the endocytosis of several plasma-membrane proteins is either accelerated or delayed2,3. The molecular function of eisosomes is still unknown, but recent data show that they interact with sphingolipid-regulated Pkh-kinases, which phosphorylate their core components and are required for efficient endocytosis4–6. In addition to Pkh-kinases, Tor kinase complex 2 (TORC2) is implicated in sphingolipid metabolism regulation7. However, it is unclear how these different signaling pathways are controlled and coordinated as well as what their downstream effects are. Experimental evidence supports a model in which regulation of sphingolipid, sterol and glycerophospholipid levels in the plasma membrane are coordinated, but mechanistic insights as to how this is achieved are currently lacking8,9. To reveal functional links between the different processes, we generated a quantitative genetic-interaction map targeting a large set of genes implicated in plasma-membrane function.
Genetic interactions have long been used to dissect functional relationships between genes. Classically, researchers have looked for qualitative differences between observed phenotypes of double mutants and the phenotypes of the two related single mutants. More recently, we employed the epistatic miniarray profile (E-MAP) approach, a variation on synthetic genetic arrays10. This allows for the quantitative analysis of genetic interactions, including negative (for example, synthetic sick or lethal) as well as positive ones (for example, suppression)11. For this approach, a comprehensive set of double mutants is generated and their growth is measured. To determine individual genetic interactions, deviations of growth rates from the medians of all combinations with one particular gene are calculated for each combination as a quantitative interaction score (or S-score)12,13. Each mutation has a genetic-interaction profile, or phenotypic signature, consisting of all its S-scores with all other genes in the E-MAP. A particularly useful parameter to judge the similarities of profiles is to compare correlations of two genes’ interactions with all other genes in the set. In addition, bioinformatic extraction based on mathematical models can be applied to yield functional modules in an unbiased fashion from E-MAP datasets, and correlations and S-scores can be used to reveal their connections14,15. The E-MAP approach has been previously used to functionally interrogate several processes, and the dissection of genetic interactions from these E-MAPs has led to a deluge of biological insights in a variety of processes11,16–18.
Here we report an E-MAP targeting plasma-membrane functions to generate previously unknown biological insight relating to plasma-membrane functions. Using this E-MAP, we have linked two new genes (EMP70 and EIS1) to eisosome function and uncovered a link between GDP/GTP exchange protein Rom2 signaling and sphingolipid metabolism.
RESULTS
Overview of the plasma-membrane E-MAP
To address functional relationships between plasma-membrane processes, we systematically determined the genetic interactions among a set of 374 genes involved in plasma-membrane biology. We selected candidate genes encoding proteins functioning in membrane transport and organization, especially eisosomes, actin patches, endocytosis and exocytosis. In addition, we picked genes involved in ergosterol and sphingolipid metabolism, as these lipids are implicated in many plasma-membrane processes. Our selection criteria were based on available functional annotation (gene ontology terms) and a literature survey. We also included a diverse set of genes whose products localize to the plasma membrane and/or interact genetically or physically with previously characterized plasma-membrane genes/proteins. The selected genes were categorized into the functional groups presented in Figure 1a and Supplementary Table 1. We included a number of genes analyzed in previous systematic genetic studies to facilitate comparison between datasets11,16,17. From this set, we quantitated a total of 57,799 genetic interactions using the E-MAP approach (~83% of the possible interactions).
Figure 1.
Composition of the plasma membrane E-MAP. (a) Genes selected for the plasma membrane E-MAP are classified according to their biological function. (b,c) Genes encoding proteins interacting with each other are more likely to show positive genetic interactions (b) and correlated genetic interaction profiles (c). Green, interaction and correlation scores of gene pairs known to encode interacting proteins; black, the remainder of gene pairs.
Previously, we found that gene pairs encoding physically interacting proteins are enriched for positive genetic interactions and show a higher propensity for having highly correlated genetic-interaction profiles11,16,17. To assess the richness and quality of the genetic-interaction data of the plasma-membrane E-MAP, we compared the pairwise correlation of genetic-interaction profiles to a high-quality set of protein-protein interactions (PPIs)19 and found that the power of the genetic map to predict PPIs is comparable to that of previously published E-MAPs (Supplementary Fig. 1). Furthermore, comparison of interaction scores or correlation coefficients of gene pairs encoding physically interacting proteins19–21 (see Supplementary Table 2) among all plasma-membrane E-MAP gene pairs revealed that they have a higher likelihood to interact positively and to have correlated genetic-interaction profiles (Fig. 1b,c, yellow area under the green graph). Conversely, gene pairs with highly correlated interaction profiles and positive interactions are likely to physically interact.
To better visualize groups of interacting genes and their relationships, we used a previously developed algorithm that defines functional modules from quantitative genetic and PPI data14 (Supplementary Fig. 2). This method identified 18 modules encompassing 53 genes (Supplementary Fig. 2 and Supplementary Table 3). Genes in each module have similar genetic-interaction profiles and form a connected subnetwork in the PPI network. These modules corresponded to known protein complexes, such as the F-actin capping protein complex and the AP-3 adaptor, or to known pathways, such as sphingolipid metabolism, the HOG osmosensory pathway and ergosterol biosynthesis (Supplementary Fig. 2). To identify modules for which PPI data is not available, we performed the modular analysis without requiring PPI connectivity (Supplementary Fig. 3). This identified 29 modules encompassing 190 genes (Supplementary Table 4 and http://acgt.cs.tau.ac.il/pmemap). This analysis yielded similar amounts of modules for the plasma membrane and the previously reported E-MAP on the early secretory pathway11 (Supplementary Table 5). Additional information can be extracted by considering interactions of single genes with modules (data not shown).
Insights from hierarchical clustering of the genetic-interaction data
Each mutant engenders a genetic-interaction profile, or phenotypic signature, representing how it genetically interacts with all other mutants tested. Comparison of these profiles using hierarchical clustering (Fig. 2, Supplementary Data and http://interactome-cmp.ucsf.edu/plasma_membrane/) is a powerful and unbiased approach to identify genes of the same pathway. In the following, we provide a brief summary of several functional connections revealed by such gene clustering.
Figure 2.
Overview of the clustergram of the plasma membrane E-MAP. Top, selected areas are marked in the overview and highlighted as inserts 1–4. Yellow, positive genetic interactions; blue, negative genetic interactions. Bottom, genes with correlating genetic profiles are shared between RTG1 and MKS1. Pairwise correlations between RTG1 and MKS1 and all other genes in the plasma membrane E-MAP were calculated and plotted against each other.
RVS161 and RVS167 encode proteins that operate together in membrane remodeling during endocytosis22. As expected from their overlapping functions, rvs161Δ and rvs167Δ clustered together with high correlation (correlation = 0.54; Fig. 2, inserts 2). Consistent with previous reports, both share positive genetic interactions with a number of genes involved in fatty-acid elongation for sphingolipid synthesis, such as FEN1 and SUR4 (ref. 23) (Fig. 2, insert 2d). Notably, we observed positive interactions with genes encoding components of the Hog1 MAP-kinase cascade and the ergosterol biosynthesis pathway (erg3Δ, erg5Δ, erg6Δ, Fig. 2, inserts 2). In additions to changes in their sterols, these erg mutants have altered sphingolipid composition8. Thus, defects resulting from deletion of RVS genes could be compensated by erg mutants via changes in sphingolipids. Also in line with previous work, both rvs161Δ and rvs167Δ show negative interactions with actin cytoskeleton genes, such as BBC1, JSN1 and BZZ1 (refs. 10,24–26) (Fig. 2, insert 2a). In addition, we found several previously unrecognized relationships, including negative interactions between the RVS genes and ire1Δ and hac1Δ, two mediators of the unfolded protein response (UPR) control system for endoplasmic reticulum function. Possibly, cells react to Rvs deficiency by altering lipid synthesis or transport, which in turn activates the UPR. Cells lacking the UPR in addition to the Rvs proteins could have decreased fitness. Consistent with this notion, a recent genome-wide study found the UPR activated in rvsΔ cells27.
We also detected many genetic interactions and highly correlated profiles between genes encoding actin-patch components. For example, sla1Δ and ede1Δ, which function in endocytosis, are highly correlated (correlation = 0.64, Fig. 2, insert 1) and show a negative genetic interaction (interaction score = -7.7). Unexpectedly, given its function in exocytosis rather than endocytosis, we also found chs6Δ to be highly correlated with sla1Δ and ede1Δ (correlations ede1Δ-chs6Δ = 0.53 and sla1Δ-chs6Δ = 0.43; Fig. 2, insert 1). Furthermore, these three genes all result in negative genetic interactions when any two of them are combined. Collectively, this indicates that Chs6 might function in coordinating exo- and endocytosis, perhaps by delivering a subset of cargos to the plasma membrane28. In this scenario, chs6Δ would lead to the depletion of an endocytic factor from the plasma membrane and, as a consequence, a decrease in endocytosis efficiency. Combination with mutants defective in this process would further decrease the fitness of the resulting strains.
We also observed many strong genetic interactions between trafficking complexes. Genes encoding the retromer complex (VPS17, VPS29, VPS35, PEP8), the COG complex (COG5, COG6, COG7, COG8) or the AP3 complex (APM3, APL5) all formed highly correlated clusters in the plasma-membrane E-MAP (Fig. 2, insert 4). In addition, potential new connections between these complexes and heretofore poorly characterized components of the endocytic machinery are apparent in these clusters. As an example, the retromer complex coclusters with deletion of MON2 (correlation = 0.48), a gene encoding an evolutionarily conserved scaffolding protein functioning in endosome-to-Golgi trafficking29. Our data suggest that Mon2 acts together with the retromer in this process.
Many genes encoding members of signaling cascades showed strong genetic relationships. For example, two kinases of the cell integrity MAP kinase signaling module, Slt2 (the MAP kinase) and Bck1 (the MAP kinase kinase kinase)30, showed one of the highest correlations (0.75). Similarly, genes encoding components of retrograde signaling (RTG1, RTG2, RTG3 and MKS1) all cluster together (correlation = 0.44) indicating that all pairs have high correlation coefficients (for example, MKS1/RTG1 correlation coefficient = 0.59; Fig. 2, bottom).
Functional links involving eisosomes
Although the eisosome has been linked to endocytosis regulation, details regarding its biological roles remain unresolved. To understand eisosome function in vivo, we genetically analyzed its core components, PIL1 and LSP1. As the encoded proteins are >70% identical and are stoichiometric components of the eisosome, we expected very similar genetic profiles for them. Unexpectedly, PIL1 and LSP1 showed very different genetic interactions and, accordingly, cluster in different regions of the E-MAP (correlation = 0.038; Fig. 2, insert 4). This parallels the cell-biological observation that deletion of PIL1 but not LSP1 results in strong effects on plasma-membrane organization and protein turnover.
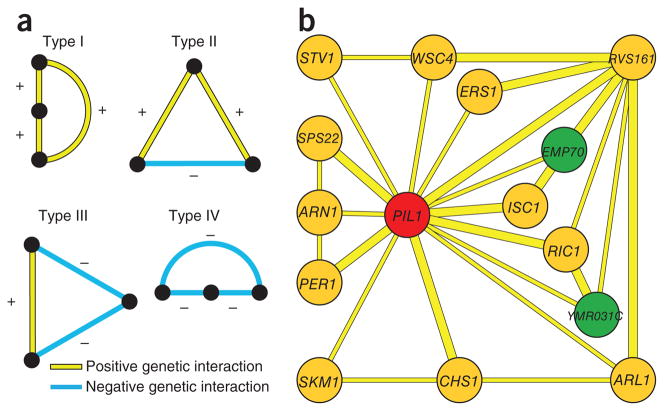
To gain further insight into eisosome function, we analyzed the triplet genetic motifs (TGMs) in which pil1Δ participates17. TGMs are the simplest motifs apart from binary interactions and can exist in four forms: type I (all three genes showing positive genetic interactions), type II (two positive and one negative), type III (two negative and one positive) and type IV (three negative interactions) (Fig. 3a). We have previously shown that genes with all positive genetic interactions (type I TGM) are enriched for functioning in the same pathway17. We therefore assembled a complete map of type I TGMs found in the plasma-membrane E-MAP (Supplementary Fig. 4). Because Pil1 has a more prominent role than Lsp1 in eisosome and plasma-membrane function, we extracted all type I TGMs involving pil1Δ (Fig. 3b). In this representation, we highlighted genes that are important for eisosome localization or are closely related to such genes (YMR031c and EMP70, respectively31; green nodes in Fig. 3b) and characterized them further.
Figure 3.
TGMs of the plasma membrane E-MAP. (a) All four potential TGMs are shown. Nodes in vertical order represent involvement in the same pathway; horizontal orientation indicates possible parallel pathways. (b) Type I TGMs that have PIL1 as a node. Nodes in green represent a gene important for Pil1-GFP localization (YMR031C) or a homolog of such a gene (EMP70)31.
EIS1/YMR031c encodes a novel eisosome component
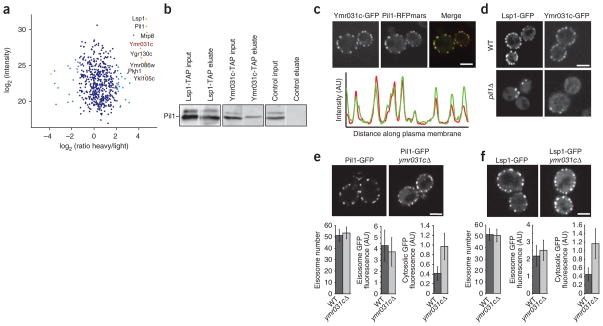
Because ymr031cΔ and pil1Δ have a positive genetic interaction and a correlated interaction profile (Fig. 3b), we tested whether the corresponding proteins physically associate. To this end, we fused the sequence encoding the green fluorescent protein (GFP) tag to PIL1 at its endogenous location in the yeast genome and immune-purified the expressed Pil1-GFP from a yeast culture that was metabolically labeled with heavy, nonradioactive lysine (SILAC)32. In parallel, we performed a mock purification from control, light-labeled wild-type cells. We identified 533 proteins present over a 10,000-fold dynamic range in the mixed eluates from both purifications. As expected, we found Pil1 and Lsp1 as well as the recently identified eisosomes binding protein Mrp8 to be significant outliers, with a high ratio of labeled to nonlabeled protein, indicating that they are specific interactors2,33 (P < 0.0001; Fig. 4a). In addition, we found a number of other specific interactors, including Ymr031c, which is consistent with a recent report34. To independently confirm this observation, we performed immunoprecipitations of TAP-tagged Ymr031c and, as a control, Lsp1, and we found that both specifically precipitated Pil1 (Fig. 4b). To test whether Ymr031c colocalizes with Pil1, we fluorescently tagged both proteins. The signal from Pil1 and Ymr031c perfectly overlapped at eisosomes (Fig. 4c, upper panel; Pearson correlation = 0.81 ± 0.06). Consistent with these data, Ymr031c was recently detected at MCCs3. One prediction for a genuine eisosome component is that it relocalizes to eisosome remnants in a PIL1 deletion strain2. We therefore investigated Ymr031c-GFP localization in pil1Δ cells and found that both Ymr031c and the eisosome component Lsp1 localized to one or a few eisosome remnants in the cell periphery (Fig. 4d). To investigate whether YMR031c has a role in eisosome architecture or assembly, we deleted it and analyzed the localization of eisosome core components in the resulting strain. For both Pil1 and Lsp1-GFP, we observed substantially increased cytosolic fluorescence in ymr031cΔ cells (Fig. 4e,f). Collectively, these data show that Ymr031c is physically associated with eisosomes and is required for their normal formation. We have therefore named this gene EIS1.
Figure 4.
YMR031C/EIS1 encodes an eisosome component. (a) Affinity purification and MS analysis of heavy labeled cells expressing GFP-tagged Pil1 and untagged control cells. Averaged peptide intensities are plotted against heavy/light SILAC ratios. Significant outliers (P < 0.0001) are colored in orange or light blue (P < 0.05); other identified proteins are shown in dark blue. (b) Pulldown purification from cells expressing tandem affinity-tagged Lsp1, Ymr031c or untagged control cells. Inputs and eluates from the pulldown were blotted and probed with antibodies against Pil1. (c) Colocalization of GFP-tagged Ymr031c with RFPmars-tagged Pil1. Representative confocal midsections are shown. The graph shows the intensity profiles for both channels along the perimeter of the cell. (d) PIL1 is required for normal localization of Ymr031c. Ymr031c-GFP or Lsp1-GFP was expressed and imaged either in WT or pil1Δ cells. Representative confocal midsections are shown. (e,f) Ymr031c is required for normal eisosome formation. Pil1-GFP (e) or Lsp1-GFP (f) was expressed in ymr031cΔ or control cells. Representative midsections are shown. For each experiment, the number of eisosomes per cell, the GFP fluorescence per eisosome and the cytosolic GFP fluorescence were quantified from at least 100 cells and are shown below the images. Scale bars, 2.5 μm.
EMP70 is an early endosomal and vacuolar protein
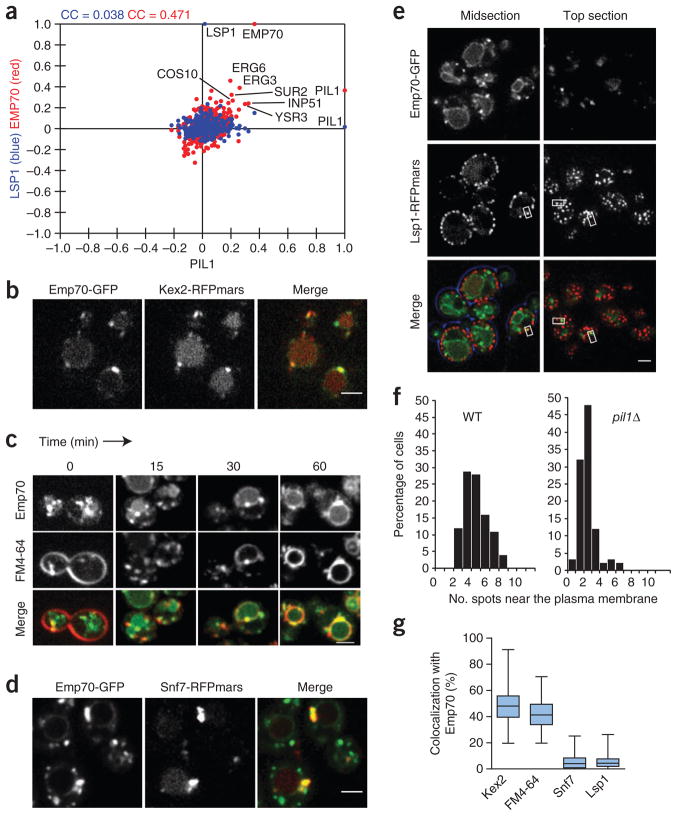
In the genetic network of the plasma-membrane E-MAP, EMP70 is the strongest candidate for a functional relationship with PIL1 because (i) the two genes have highly correlated genetic profiles (correlation of PIL1 and EMP70 = 0.37 (EMP70 has the most similar profile to PIL1 of all the E-MAP genes); Fig. 5a); (ii) the two genes participate in two type I TGMs (Fig. 3b); and (iii) the Emp70 homolog Tmn2 is required for normal Pil1-GFP localization31. In addition, our modular analysis identified EMP70 and PIL1 as part of the same six-gene module (Supplementary Fig. 3; S-score between PIL1 and EMP70 = 1.78; Supplementary Table 4).
Figure 5.
The eisosome-linked Emp70 is an early endosomal protein. (a) Genes with correlating genetic profiles are shared between PIL1 and EMP70 but not PIL1 and LSP1. Correlation coefficients between the genetic profile of PIL1 and each of the other 373 profiles in the E-MAP are plotted on the x axis against, on the y axis, either the similar set of values for the LSP1 profile with all other profiles (blue) or those for EMP70 with all other profiles (red). Labeled points indicate some genes with profiles that are positively correlated with both the profile of PIL1 and that of EMP70. CC values in blue and red indicate the correlation coefficients for the full set of blue or red points plotted. (b) Emp70 colocalizes with Kex2. Emp70-GFP and Kex2-RFPmars were coexpressed and imaged. Representative confocal midsections are shown. (c) Emp70 localizes to an FM4-64 marked endocytic compartment. Cells expressing Emp70-GFP (green) were pulse labeled with FM4-64 (red) and imaged for 1 h. Images of midsections of cells at selected time are shown as indicated. (d) Emp70 localizes to the class E compartment in SNF7 mutants. GFP-tagged Emp70 was expressed in cells harboring nonfunctional Snf7-RFPmars, resulting in the clustering of endosomal proteins in the class E compartment. Representative confocal midsections are shown. (e) Emp70-GFP foci localize to the cell periphery. Emp70-GFP (green) was expressed in cells harboring the fluorescent eisosomes marker Lsp1-MARS. Representative mid- (left) and top sections (right) are shown. Boxes highlight selected areas of colocalization. (f) PIL1 is required for normal Emp70 localization to the cell periphery. Emp70-GFP was expressed in cells expressing the plasma membrane marker Ylr413w-RFPmars, and foci overlaying this marker were counted in more than 100 WT and pil1Δ cells. Results are shown as a histogram of number of spots opposed to the plasma membrane in each cell. (g) Quantitation of the organelle distribution of Emp70. Emp70-GFP was imaged in live cells and analyzed for colocalization with Kex2-RFPmars (n = 100), vacuolar FM4-64 (n = 91), Snf7-RFPmars (n = 93, diploid strain expressing one tagged Snf7 allele) and Lsp1-Cherry (n = 107). The relative area of overlap between signals was quantified as a percentage of total area occupied by Emp70 signal. Box plots representing maxima, 75th percentile, median, 25th percentile and minima are shown for the colocalization with each marker. Scale bars, 2.5 μm.
These genetic links prompted us to investigate EMP70 in more detail (Fig. 5). We fluorescently tagged Emp70 with GFP and found that it localizes in a complex pattern consisting of a central ring reminiscent of vacuoles and several bright foci in the cytoplasm that often seem connected to the vacuole (Fig. 5b and Supplementary Video 1). Emp70 was previously found in an endosomal membrane fraction35. We therefore tested whether cytosolic Emp70 foci represent endosomes. We used a number of endosomal markers and found Emp70-GFP foci to colocalize with Kex2, marking the early endosome, which in yeast is functionally continuous with the trans-Golgi network. In contrast, Emp70 localization did not overlap with the late endosomal/prevacuolar marker Vps5 (Fig. 5b and Supplementary Fig. 5a).
To test whether the Emp70-labeled compartments are part of the endocytic route, we used the endocytosis tracer FM4-64. This lipid dye is incorporated in the plasma membrane, taken up by endocytosis and trafficked through the endosomal system to the vacuole36. We found in pulse-chase experiments that early FM4-64 intermediates colocalize with Emp70 foci (Fig. 5c, 0 min). As the dye migrated through the endocytic system, it also colocalized with a subset of Emp70-positive foci toward the end of the reaction but markedly less at intermediate time points (Fig. 5c, 30 min). At the final time point, FM4-64 clearly labeled the vacuole delimiting membrane where it colocalized with the Emp70-GFP ring staining. Trafficking from early endosomes can be blocked by incubation of cells at 16 °C, which leads to the accumulation of FM4-64 (ref. 37). Emp70-GFP almost perfectly colocalized with FM4-64 when the latter was accumulated in such a ‘16 °C compartment’, further arguing that Emp70 localizes to early endosomes (Supplementary Fig. 5b). Strains harboring a deleted or C-terminally tagged SNF7 (an ESCRT-III gene) show a ‘class E’ vacuolar protein sorting defect. This is characterized by collapse of endosomes to one or a few large class E compartments38,39. Under these conditions, Emp70-GFP formed fewer, very large clusters that colocalized with Snf7-RFPmars marked class E compartments and showed reduced vacuolar membrane staining (Fig. 5d). From these data, we conclude that Emp70 localizes to early endosomes and the vacuole. To better characterize the localization of Emp70 in these two pools, we quantitated the relative amount of Emp70 colocalizing with markers for each organelle and found 48% of Emp70 to localize in the TGN/endosomal compartment and 41% at the vacuolar membrane (Fig. 5g).
During our localization studies, we often observed early endosomal foci marked by FM4-64 dynamically associating with the plasma membrane. To test whether the genetic link of EMP70 with PIL1 was reflected in the recruitment of Emp70-GFP foci to eisosomes, we investigated the Emp70-GFP localization with respect to fluorescently tagged eisosomes. Strikingly, we found many spots of Emp70-GFP at eisosomes (Fig. 5e). In any given cell, 4% of the total Emp70-GFP signal colocalized with an eisosome marker (Fig. 5g and Supplementary Video 2). To test whether this association has functional relevance, we investigated the Emp70 localization in pil1Δ cells and found a marked reduction of foci close to the plasma membrane (Fig. 5f).
EMP70 proteins are required for normal endosomal sorting
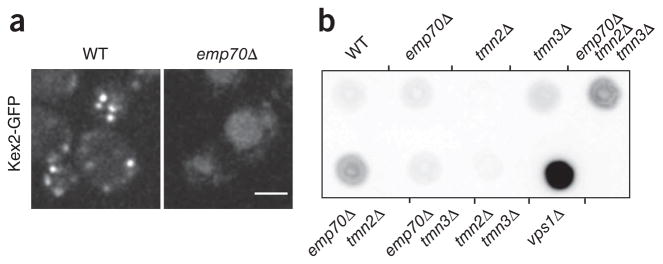
To test whether Emp70 is important for early endosome-to-vacuole trafficking, we analyzed Kex2-GFP localization in an emp70Δ strain and found a substantial Kex2 relocalization from early endosomes to the vacuole (Fig. 6a). Kex2 steady-state localization depends on signals that send it to early endosomes, which subsequently mature into late endosomes, from which Kex2 is actively retrieved40,41. It is possible that vacuolar mislocalization of Kex2 in emp70Δ cells results from a defect in retrieval from the late endosome or a complex-trafficking problem affecting early endosome function. Normally, if retrieval is compromised, vacuolar sorted carboxypeptidase Y (CPY) is secreted. We tested this and found that, in contrast to the control vps1Δ, emp70Δ alone does not lead to CPY secretion42,43. EMP70 has two homologs in the genome, TMN2 and TMN3. To address whether they could compensate for Emp70 function in its absence, we tested CPY secretion in strains with different combinations of the family members deleted. TMN2 deletion alone had no effect, and TMN3 deletion alone only a weak effect, on CPY sorting (Fig. 6b). In contrast, combining emp70Δ with either tmn2Δ or tmn2Δ tmn3Δ resulted in CPY secretion, showing that Emp70 is functionally redundant with Tmn2 in vacuolar protein sorting and that the Emp70 protein family is required for normal endosomal function.
Figure 6.
Emp70 is required for normal endosome function. (a) EMP70 is required for normal localization of Kex2-GFP. Kex2-GFP was expressed in either WT or emp70Δ cells, and representative confocal midsections are shown. (b) Emp70 family members are required for late endosomal protein retrieval. Mutants of EMP70, TMN2 or TMN3 were tested alone or in combination for CPY secretion. A representative colony blot is shown. Scale bar, 2.5 μm.
Sphingolipid metabolism and its regulation
The plasma-membrane E-MAP interrogates relationships within metabolic networks that are important for plasma-membrane function, including sphingolipid metabolism (Fig. 2, inserts 3a and 3b, and Fig. 7a). Consistent with their common function, many of the sphingolipid pathway genes showed high correlation (>0.2). Figure 7b shows the distance of the action of enzymes in the pathway plotted against the correlation coefficient of the corresponding genes. The linear best fit on all data points revealed that genes encoding enzymes catalyzing subsequent steps are more highly correlated than genes further away in the metabolic network. Moreover, whereas most mutations in genes encoding enzymes catalyzing early steps of sphingolipid synthesis have negative genetic interactions with each other (Fig. 2, insert 3b, and Fig. 7a), they show positive genetic interactions when combined with mutations in genes acting late in complex sphingolipid formation (Fig. 2, insert 3a, and Fig. 7a). This might indicate that deficiency in late-acting enzymes leads to a buildup of toxic intermediates, which can be suppressed by deleting genes encoding upstream-acting enzymes. Precedence for this includes inhibition of Aur1, which converts ceramide to inositolphosphoceramide by aureobasidin A, leading to complex sphingolipids depletion and a concomitant accumulation of ceramide, which both contribute to toxicity44.
Figure 7.
Genetic interactions of sphingolipid metabolism. (a) Graphic representation of the sphingolipid synthesis pathway. Blue, negative genetic interactions; yellow, positive interactions. (b) Genes encoding enzymes acting in succession in sphingolipid synthesis show higher correlation than genes further apart in the metabolic network. For each gene pair in sphingolipid synthesis, the pathway distance of genes (that is, the number of metabolic intermediates between the catalyzed reactions) is plotted against the correlation coefficient of the gene pairs. The red line is a best-fit linear regression line fitted for all the data points on the graph.
The plasma-membrane E-MAP also revealed that ROM2, encoding a Rho1 GTPase exchange factor, has strong genetic connections to sphingolipid synthesis genes. For example, ROM2 has correlated genetic profiles with FEN1, SUR2, LCB3 and SUR4, all acting early, but shows negative correlation with CSG2 acting late in sphingolipid synthesis (Fig. 8a). In addition, a ROM2 deletion mutation results in a strong synthetic sick phenotype with lcb3Δ, sur2Δ dpl1Δ and ysr3Δ, all genes encoding enzymes catalyzing different steps of sphingolipid metabolism (interaction score < -2; see Fig. 2, insert 3b, and data not shown). Together, this suggests that Rom2 is an activator of sphingolipid metabolism. To test this model, we profiled the lipidome of rom2Δ and several other mutants in the sphingolipid pathway by ‘shotgun’ lipidomics45. ROM2 deletion resulted in a lipid phenotype similar to that of sur2Δ or sur4Δ cells (Fig. 8b and Supplementary Table 6). Particularly, ROM2 deletion led primarily to accumulation of long chain bases and a small decrease of ceramides. This argues that Rom2 activates sphingolipid synthesis by regulating the conversion of long chain bases to ceramides.
Figure 8.
Rom2 interacts with sphingolipid metabolism. (a) Genes with correlating genetic profiles are shared between SUR4 and ROM2 but not between CSG2 and ROM2. Correlation coefficients between the genetic profile of ROM2 and each of the other 373 profiles in the E-MAP are plotted on the x axis against, on the y axis, either the similar set of values for the SUR4 profile with all other profiles (blue) or those for CSG2 with all other profiles (red). Labeled points indicate genes with profiles that are positively correlated with the profile of ROM2. CC values in blue and red indicate the correlation coefficients for the full set of blue or red points plotted. (b) Lipidome profiling of rom2Δ and selected sphingolipid metabolism mutants. Lipid class abundances were normalized to WT levels. Sterol esters (SE), phosphatidic acid (PA), triacylglycerol (TAG), long chain base (LCB) mannosylinositol phosphoceramide (MIPC), phosphatidylethanolamine (PE), diacylglycerol (DAG), phoshphatidylcholine (PC), phoshphatidylinositol (PI), ceramide (Cer), phosphatidylserine (PS) mannosylinositol-2-phosphoceramide (M(IP)2C) and inositol phosphoceramide (IPC) levels are shown.
DISCUSSION
The plasma-membrane E-MAP quantitatively describes interactions between genes involved in plasma-membrane processes. Together with previous studies, it shows that the E-MAP technology can be used to detect protein interactions and signaling pathways as well as to uncover complex biological connections. Here, we highlighted several examples of novel insights into plasma-membrane function derived from the E-MAP, focusing on its spatial organization and homeostasis. As an example of a physical interaction revealed from the E-MAP data, we investigated Eis1/Ymr031c and defined it as an eisosome component. Based on its much lower abundance compared to the eisosome core components, it might have a special architectural or regulatory role there. This is also a case where we combined data from the plasma-membrane E-MAP with our visual screen for genes affecting Pil1-GFP localization31, which provides an example how the combination of different high-throughput datasets helps to uncover previously unrecognized relationships.
Mining of the plasma-membrane E-MAP also yielded information on more functional interactions not reflected in physical associations. The transmembrane protein Emp70 has a fascinatingly complex localization and genetically interacts with eisosome components. Particularly intriguing is the Emp70 pool localized in endosomal structures that often appear connected with the vacuolar membrane. This observation raises the possibility that endosomes reach out to the plasma membrane and pick up their cargo. It also suggests that at least parts of the endosomal membrane system might be a tubular network connected to the vacuole, but further detailed cell-biological studies will have to clarify this point.
We also used the plasma-membrane E-MAP to interrogate metabolic networks and their regulation. The strong correlation profiles of sphingolipid synthesis genes argues that novel functionally related genes could be found by using the genetic profiles from the plasma-membrane E-MAP. For example, genes that function in sphingolipid metabolism or are involved in its regulation would be expected to cluster with known sphingolipid synthesis genes. Using this logic, we identified Rom2 as a regulator of sphingolipid metabolism. Mechanistically, its activator function could occur either through ceramide synthesis activation by Rom2 or through negative regulation of ceramidase. Between these two hypotheses, we consider the first one more likely, as rom2Δ clusters with genes encoding ceramide synthase (lag1Δ lac1Δ) but not ydc1Δ, which encodes ceramidase (Fig. 2, insert 3b). This is consistent with previous findings that connect the Tor2 kinase pathway with Rho1-signaling via Rom2 as well as recent findings that TORC2 is required for ceramide biosynthesis7,46. This previous study7 implicated an alternative branch of TORC2 signaling through the Ypk2 kinase in regulation of ceramide biosynthesis but did not rule out involvement of Rom2. The effect of ROM2 deletion could either be directly on ceramide synthase or, alternatively, could block the synthesis of its substrate, long chain fatty acid–CoA. In the latter model, the depletion of long chain fatty acids would slow ceramide synthesis and would therefore lead to the accumulation of long chain bases, the second substrate of ceramide synthase. In either scenario, Rom2 has a stimulatory function in sphingolipid synthesis at the step converting long chain bases to ceramides. Consistent with this notion, the inhibition of sphingolipid synthesis by the antifungal drug myriocin leads to a relocalization of Rom2 from the plasma membrane47. Rom2 is recruited to the plasma membrane through the binding of phosphoinositol-(4,5)-bisphosphate (PI(4,5)P2) by its pleckstrin homology domain, and reduction of PI(4,5)P2 also relocalizes Rom2 (ref. 48). This raises the possibility that Rom2 serves to connect phosphoinositide and sphingolipid signaling pathways. The details of this regulation of sphingolipid metabolism remain to be worked out, but it shows how genetic interactions in the plasma-membrane E-MAP yield novel insights into metabolic networks and their regulation.
We anticipate that this dataset will fuel many more mechanistic studies. In particular, integration with other data from lipidomics, interaction proteomics or systematic visuals screens are likely to reveal novel insights into the regulation of plasma-membrane processes. In addition, many antifungal drugs target functions connected to the plasma membrane, such as cell-wall and ergosterol synthesis. Probing the set of genes on the E-MAP presented here with a battery of drugs and comparing of the resulting drug profiles to the mutant profiles is an effective way to identify putative drug targets. This would facilitate the identification of compounds impinging on these various processes and could potentially have therapeutic value.
METHODS
Methods and any associated references are available in the online version of the paper at http://www.nature.com/nsmb/.
Supplementary Material
Acknowledgments
We thank members of the Walther and Krogan laboratory for critical reading and comments, J. Brickner for suggestions, Ulrike Laabs for excellent technical assistance, O. Nørregaard Jensen (University of Southern Denmark) for providing access to the Nanomate Triversa used for the lipidomic experiments and P. Kemmeren for establishing the database. This work was supported by the Max Planck Society (T.C.W.), the German Research Foundation (DFG; T.C.W.), the German-Israeli Foundation (T.C.W.), the US National Institutes of Health, the Searle, Sandler and Keck Foundations (N.J.K.), the International Human Frontier Science Program (HFSP; T.C.W.), the Israel Science Foundation (grant no. 802/08; R.S.), the Edmond J. Safra Bioinformatics program at Tel Aviv University (I.U.), the Programa de Apoyo Sectorial a la Estrategia Nacional de Innovación (INNOVA URUGUAY, DCI-ALA/2007/19.040, P.S.A.), the Agencia Nacional de Investigación e Innovación (ANII, A.O.-C.), the Danish Council for Independent Research (DOK1155860, C.S.E.) and Lundbeckfonden (R45-A4342, C.S.E.).
Footnotes
AUTHOR CONTRIBUTIONS
All authors contributed to every aspect of this work.
COMPETING FINANCIAl INTERESTS
The authors declare no competing financial interests.
Reprints and permissions information is available online at http://npg.nature.com/reprintsandpermissions/.
Note: Supplementary information is available on the Nature Structural & Molecular Biology website.
References
- 1.Grossmann G, Opekarova M, Malinsky J, Weig-Meckl I, Tanner W. Membrane potential governs lateral segregation of plasma membrane proteins and lipids in yeast. EMBO J. 2007;26:1–8. doi: 10.1038/sj.emboj.7601466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walther TC, et al. Eisosomes mark static sites of endocytosis. Nature. 2006;439:998–1003. doi: 10.1038/nature04472. [DOI] [PubMed] [Google Scholar]
- 3.Grossmann G, et al. Plasma membrane microdomains regulate turnover of transport proteins in yeast. J Cell Biol. 2008;183:1075–1088. doi: 10.1083/jcb.200806035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.deHart AK, Schnell JD, Allen DA, Hicke L. The conserved Pkh-Ypk kinase cascade is required for endocytosis in yeast. J Cell Biol. 2002;156:241–248. doi: 10.1083/jcb.200107135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friant S, Lombardi R, Schmelzle T, Hall MN, Riezman H. Sphingoid base signaling via Pkh kinases is required for endocytosis in yeast. EMBO J. 2001;20:6783–6792. doi: 10.1093/emboj/20.23.6783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walther TC, et al. Pkh-kinases control eisosome assembly and organization. EMBO J. 2007;26:4946–4955. doi: 10.1038/sj.emboj.7601933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aronova S, et al. Regulation of ceramide biosynthesis by TOR complex 2. Cell Metab. 2008;7:148–158. doi: 10.1016/j.cmet.2007.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guan XL, et al. Functional interactions between sphingolipids and sterols in biological membranes regulating cell physiology. Mol Biol Cell. 2009;20:2083–2095. doi: 10.1091/mbc.E08-11-1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tabuchi M, Audhya A, Parsons AB, Boone C, Emr SD. The phosphatidylinositol 4,5-biphosphate and TORC2 binding proteins Slm1 and Slm2 function in sphingolipid regulation. Mol Cell Biol. 2006;26:5861–5875. doi: 10.1128/MCB.02403-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tong AH, et al. Global mapping of the yeast genetic interaction network. Science. 2004;303:808–813. doi: 10.1126/science.1091317. [DOI] [PubMed] [Google Scholar]
- 11.Schuldiner M, et al. Exploration of the function and organization of the yeast early secretory pathway through an epistatic miniarray profile. Cell. 2005;123:507–519. doi: 10.1016/j.cell.2005.08.031. [DOI] [PubMed] [Google Scholar]
- 12.Collins SR, Schuldiner M, Krogan NJ, Weissman JS. A strategy for extracting and analyzing large-scale quantitative epistatic interaction data. Genome Biol. 2006;7:R63. doi: 10.1186/gb-2006-7-7-r63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schuldiner M, Collins SR, Weissman JS, Krogan NJ. Quantitative genetic analysis in Saccharomyces cerevisiae using epistatic miniarray profiles (E-MAPs) and its application to chromatin functions. Methods. 2006;40:344–352. doi: 10.1016/j.ymeth.2006.07.034. [DOI] [PubMed] [Google Scholar]
- 14.Ulitsky I, Shlomi T, Kupiec M, Shamir R. From E-MAPs to module maps: dissecting quantitative genetic interactions using physical interactions. Mol Syst Biol. 2008;4:209. doi: 10.1038/msb.2008.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bandyopadhyay S, Kelley R, Krogan NJ, Ideker T. Functional maps of protein complexes from quantitative genetic interaction data. PLOS Comput Biol. 2008;4:e1000065. doi: 10.1371/journal.pcbi.1000065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Collins SR, et al. Functional dissection of protein complexes involved in yeast chromosome biology using a genetic interaction map. Nature. 2007;446:806–810. doi: 10.1038/nature05649. [DOI] [PubMed] [Google Scholar]
- 17.Fiedler D, et al. Functional organization of the S. cerevisiae phosphorylation network. Cell. 2009;136:952–963. doi: 10.1016/j.cell.2008.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilmes GM, et al. A genetic interaction map of RNA-processing factors reveals links between Sem1/Dss1-containing complexes and mRNA export and splicing. Mol Cell. 2008;32:735–746. doi: 10.1016/j.molcel.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collins SR, et al. Toward a comprehensive atlas of the physical interactome of Saccharomyces cerevisiae. Mol Cell Proteomics. 2007;6:439–450. doi: 10.1074/mcp.M600381-MCP200. [DOI] [PubMed] [Google Scholar]
- 20.Gavin AC, et al. Proteome survey reveals modularity of the yeast cell machinery. Nature. 2006;440:631–636. doi: 10.1038/nature04532. [DOI] [PubMed] [Google Scholar]
- 21.Krogan NJ, et al. Global landscape of protein complexes in the yeast Saccharomyces cerevisiae. Nature. 2006;440:637–643. doi: 10.1038/nature04670. [DOI] [PubMed] [Google Scholar]
- 22.Ren G, Vajjhala P, Lee JS, Winsor B, Munn AL. The BAR domain proteins: molding membranes in fission, fusion, and phagy. Microbiol Mol Biol Rev. 2006;70:37–120. doi: 10.1128/MMBR.70.1.37-120.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Revardel E, Bonneau M, Durrens P, Aigle M. Characterization of a new gene family developing pleiotropic phenotypes upon mutation in Saccharomyces cerevisiae. Biochim Biophys Acta. 1995;1263:261–265. doi: 10.1016/0167-4781(95)00124-y. [DOI] [PubMed] [Google Scholar]
- 24.Breton AM, Aigle M. Genetic and functional relationship between Rvsp, myosin and actin in Saccharomyces cerevisiae. Curr Genet. 1998;34:280–286. doi: 10.1007/s002940050397. [DOI] [PubMed] [Google Scholar]
- 25.Brizzio V, Gammie AE, Rose MD. Rvs161p interacts with Fus2p to promote cell fusion in Saccharomyces cerevisiae. J Cell Biol. 1998;141:567–584. doi: 10.1083/jcb.141.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Friesen H, et al. Characterization of the yeast amphiphysins Rvs161p and Rvs167p reveals roles for the Rvs heterodimer in vivo. Mol Biol Cell. 2006;17:1306–1321. doi: 10.1091/mbc.E05-06-0476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jonikas MC, et al. Comprehensive characterization of genes required for protein folding in the endoplasmic reticulum. Science. 2009;323:1693–1697. doi: 10.1126/science.1167983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Valdivia RH, Baggott D, Chuang JS, Schekman RW. The yeast clathrin adaptor protein complex 1 is required for the efficient retention of a subset of late Golgi membrane proteins. Dev Cell. 2002;2:283–294. doi: 10.1016/s1534-5807(02)00127-2. [DOI] [PubMed] [Google Scholar]
- 29.Efe JA, et al. Yeast Mon2p is a highly conserved protein that functions in the cytoplasm-to-vacuole transport pathway and is required for Golgi homeostasis. J Cell Sci. 2005;118:4751–4764. doi: 10.1242/jcs.02599. [DOI] [PubMed] [Google Scholar]
- 30.Levin DE. Cell wall integrity signaling in Saccharomyces cerevisiae. Microbiol Mol Biol Rev. 2005;69:262–291. doi: 10.1128/MMBR.69.2.262-291.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frohlich F, et al. A genome-wide screen for genes affecting eisosomes reveals Nce102 function in sphingolipid signaling. J Cell Biol. 2009;185:1227–1242. doi: 10.1083/jcb.200811081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ong SE, et al. Stable isotope labeling by amino acids in cell culture, SILAC, as a simple and accurate approach to expression proteomics. Mol Cell Proteomics. 2002;1:376–386. doi: 10.1074/mcp.m200025-mcp200. [DOI] [PubMed] [Google Scholar]
- 33.Wang H, et al. A complex-based reconstruction of the Saccharomyces cerevisiae interactome. Mol Cell Proteomics. 2009;8:1361–1381. doi: 10.1074/mcp.M800490-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deng C, Xiong X, Krutchinsky AN. Unifying fluorescence microscopy and mass spectrometry for studying protein complexes in cells. Mol Cell Proteomics. 2009;8:1413–1423. doi: 10.1074/mcp.M800397-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schimmoller F, Diaz E, Muhlbauer B, Pfeffer SR. Characterization of a 76 kDa endosomal, multispanning membrane protein that is highly conserved throughout evolution. Gene. 1998;216:311–318. doi: 10.1016/s0378-1119(98)00349-7. [DOI] [PubMed] [Google Scholar]
- 36.Vida TA, Emr SD. A new vital stain for visualizing vacuolar membrane dynamics and endocytosis in yeast. J Cell Biol. 1995;128:779–792. doi: 10.1083/jcb.128.5.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zheng B, Wu JN, Schober W, Lewis DE, Vida T. Isolation of yeast mutants defective for localization of vacuolar vital dyes. Proc Natl Acad Sci USA. 1998;95:11721–11726. doi: 10.1073/pnas.95.20.11721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Babst M, Wendland B, Estepa EJ, Emr SD. The Vps4p AAA ATPase regulates membrane association of a Vps protein complex required for normal endosome function. EMBO J. 1998;17:2982–2993. doi: 10.1093/emboj/17.11.2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Teis D, Saksena S, Emr SD. Ordered assembly of the ESCRT-III complex on endosomes is required to sequester cargo during MVB formation. Dev Cell. 2008;15:578–589. doi: 10.1016/j.devcel.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 40.Brickner JH, Fuller RS. SOI1 encodes a novel, conserved protein that promotes TGN-endosomal cycling of Kex2p and other membrane proteins by modulating the function of two TGN localization signals. J Cell Biol. 1997;139:23–36. doi: 10.1083/jcb.139.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sipos G, et al. Soi3p/Rav1p functions at the early endosome to regulate endocytic trafficking to the vacuole and localization of trans-Golgi network transmembrane proteins. Mol Biol Cell. 2004;15:3196–3209. doi: 10.1091/mbc.E03-10-0755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Robinson JS, Klionsky DJ, Banta LM, Emr SD. Protein sorting in Saccharomyces cerevisiae: isolation of mutants defective in the delivery and processing of multiple vacuolar hydrolases. Mol Cell Biol. 1988;8:4936–4948. doi: 10.1128/mcb.8.11.4936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rothman JH, Howald I, Stevens TH. Characterization of genes required for protein sorting and vacuolar function in the yeast Saccharomyces cerevisiae. EMBO J. 1989;8:2057–2065. doi: 10.1002/j.1460-2075.1989.tb03614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cerantola V, et al. Aureobasidin A arrests growth of yeast cells through both ceramide intoxication and deprivation of essential inositolphosphorylceramides. Mol Microbiol. 2009;71:1523–1537. doi: 10.1111/j.1365-2958.2009.06628.x. [DOI] [PubMed] [Google Scholar]
- 45.Ejsing CS, et al. Global analysis of the yeast lipidome by quantitative shotgun mass spectrometry. Proc Natl Acad Sci USA. 2009;106:2136–2141. doi: 10.1073/pnas.0811700106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmidt A, Bickle M, Beck T, Hall MN. The yeast phosphatidylinositol kinase homolog TOR2 activates RHO1 and RHO2 via the exchange factor ROM2. Cell. 1997;88:531–542. doi: 10.1016/s0092-8674(00)81893-0. [DOI] [PubMed] [Google Scholar]
- 47.Kobayashi T, Takematsu H, Yamaji T, Hiramoto S, Kozutsumi Y. Disturbance of sphingolipid biosynthesis abrogates the signaling of Mss4, phosphatidylinositol-4-phosphate 5-kinase, in yeast. J Biol Chem. 2005;280:18087–18094. doi: 10.1074/jbc.M414138200. [DOI] [PubMed] [Google Scholar]
- 48.Audhya A, Emr SD. Stt4 PI 4-kinase localizes to the plasma membrane and functions in the Pkc1-mediated MAP kinase cascade. Dev Cell. 2002;2:593–605. doi: 10.1016/s1534-5807(02)00168-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.