Peripheral Facial Neuropathy
Every year in the US, millions of people suffer from peripheral neuropathy caused by accidental, compressive, or iatrogenic, e.g. surgically associated, injury to the peripheral nervous system (PNS). Virtually all of the peripheral nerve injuries to the face occur as a result of nerve compression, stretching, or inflammation of the trigeminal nerve. Elucidation of the mechanisms that influence the rate of peripheral nerve repair after injury is of particular importance for the development of treatments for patients who, after an iatrogenic or other traumatic injury to a peripheral nerve, experience suboptimal recovery of sensory function or the development of neuropathic pain (1,2). Sensory peripheral nerve injury can result in symptoms that range from a complete or partial loss of sensation (anesthesia or hypoesthesia); to nonpainful tingling sensations (paresthesia); to increased sensitivity to touch or pressure with or without numbness or pain (hyperesthesia or dysesthesia) and numbness. (3,4,5). The extent of sensory impairment as indicated by stimulus testing measures has been shown to be reflected in the word descriptors that patients choose to describe their symptoms of altered sensation. (6)
Most trigeminal nerve injuries are associated with fracture of the mandible or maxilla. For example, the incidence of somatosensory deficits following facial injuries has been reported as 54.5% in nondisplaced fractures, 88.2% in dislocated fractures, and 100% in fractures with a direct nerve injury. (7) Indeed, following bilateral sagittal split osteotomy, the incidence of nerve injury (8,9,10) approaches 100%. Using nerve conduction recording methods, the gold standard for assessing the structural integrity of a nerve, one study of 38 trigeminal nerves recorded intraoperatively found that 21 nerves experienced demyelinating injury and 15 axonal damage during the surgery (8). These injuries result in somatosensory deficit and associated symptoms that most often vary over time and can be unpleasant or painful(4). Moreover, persistent altered orofacial sensations following a peripheral trigeminal nerve injury often negatively impact patients’ lives (11,12,13). Those patients who report dysesthetic altered sensations or pain experience the most interference or associated burden in their lives (11,15).
Soft tissue injury and inflammation generally resolves in the first post-operative month after surgery but the sensory sequelae of the nerve injury may persist for at least 2 years after surgery – the longest duration that most studies have followed patients after treatment (11,12,13,14,15,16,17). Greater than 60% percent of BSSO patients report persistent altered sensation six months after surgery and approximately 20% use descriptors suggestive of unpleasant sensations (dysesthesia) including pain (4,6,11).
Afferent Nerve Recovery and Cortical Remodeling after Nerve Injury
Following any degree of peripheral nerve injury, a complex of cellular and molecular signaling alterations is immediately initiated, and the quality of functional recovery tightly correlates to the molecular responses that attempt to repair and restore the nerve to its pre-injury state. After resolution of inflammation and edema, the sensory deficits can be attributed to anatomical or functional changes within the peripheral nerve or to changes induced in the central nervous system by the nerve injury (18,19) In general, three often temporally overlapping phases may be used to describe this biological response: the fate determination of the cell body; the active restoration of any loss in the continuity of the proximal and distal segments of the axon and/or reconstitution of axonal diameter and myelination; and the remodeling of the cortical representation of tissues innervated by the damaged axon (20).
Virtually all of the recovery pathway data is derived from transectional or crush injuries in animal models, in which case axonal regrowth, reconstitution, and remyelination are essential, but it’s reasonable to assume that non-transecting injuries activate similar pathways (21). Axonal damage is often severe even without transection, requiring reconnection of axonal sprouts to target tissues, reconstitution of axonal damage, and remyelination of myelinated afferents (8) Once the fate of the injured neuron is set, the surviving cell body actively intensifies its transcriptional machinery for the heightened synthesis of structural proteins for axonal repair and regeneration, if required, and restoration of electrical conduction from the tissues. (22,23,24,25,26).
Finally, injury-associated alterations in the peripheral nerve induce changes in neural substrates at subcortical and cortical levels within the CNS (27,28). The underlying mechanisms of this central plasticity are largely unknown but a heightened excitability is often observed in cortical regions that remodel in response to nerve injury (20). In a sense neuroplasticity reflects the competition between afferent inputs for connections in the sensory cortex. Microelectrodes implanted in the cortex and subcortical relay stations on the sensory path between the face and the cortex in rats showed new responses to other facial areas within minutes of the deactivation of their usual sensory input (29)
This cortical reorganization is reflected in the altered symptoms that are experienced by individuals after sensory nerve injuries. In the normal state, stimulation of the face or lips by contact with the external environment stimulates the sensory receptors and a profile of neural impulses is elicited. These impulses impact upon the sensory cortex and are associated with previous memory of experiences. After a nerve injury, the same contact (the same stimulus) with the external world elicits a different, altered profile of neural impulses (30)
Sensory Re-training Background
Sensory re-training (also referred to as sensory re-education) is a cognitive behavioral therapy technique that helps the patient with a nerve injury to meaningfully interpret the altered profile or neural impulses reaching his conscious level after the altered sensation area has been stimulated (30). Moreover, the repetitive neural input from sensory re-training exercises can produce plastic changes in the somatosensory cortex via the same mechanisms underlying those evoked by altered input from the nerve damage. This reorganization through re-training can compensate, in part, for some of the impairments associated with nerve injury (31,32,33,34,35,36,37).
Animal studies have shown that behavioral sensory training alters the central neural representation of the involved skin sites, alters the response of individual somatosensory cortical cells to tactile stimulation, and increases synapse to neuron ratios and improves behavioral function after induced brain damage more than simple repetitive exercise (38,39,40,41,42,43,44). Neuroimaging studies indicate that similar changes occur in human subjects following sensory denervation and sensory training (45). Sensory experience or retraining results in somatosensory cortical maps that exhibit higher sensory resolution and greater topographical organization which facilitate better interpretation of sensory inputs. In contrast to the central neural changes, sensory re-training does not alter the course of nerve regeneration or the absolute thresholds to touch (39,46,47,48) but does improve both the patient’s cognitive and adaptive response to stimulation of the affected skin region. (12,30,49,50,51).
Although improvement has been reported when re-training isn’t initiated soon after the injury, reorganization of the cortex after changes in peripheral input happens quite quickly. Persistent chronic altered sensation may result in irreversible cortical changes. One of the goals of re-training is to avoid, minimize or modulate the central functional re-organization (52)
The process of sensory re-training can be likened to the brain learning a new language in progressive phases of difficulty. Initially, use of the words is slow, challenging and error prone. With time and practice, verbal fluency may be acquired. Unfortunately, no research has been conducted to determine the optimal number of phases or the exercises required to obtain the maximum benefit to patients with orofacial nerve injuries.
Historically, in the early phase of sensory retraining (Table 1), the intent is to re-educate constant vs moving touch perceptions. That is, a patient must re-learn what constant touch feels like compared to moving touch and where on the skin the touch is actually occurring. In the early phase, a greater stimulus intensity may be necessary for the patient to differentiate constant from moving touch but the intensity should never be so great as to evoke pain. If hyperesthesia or dysesthesia occurs, desensitization with gentle stroking using different textures or gentle tapping is recommended(53,54,55,56). In the late phase of retraining (Table 1), the intent is to re-educate the directionality of movement perceptions of the patient. For example, is the movement of an external object across the skin from left to right or right to left?
Table 1.
General Concepts of Sensory Re-training
| Two Phases: |
| Early Phase: Constant vs Moving Touch |
| Late Phase: Directionality |
| Frequency: 3 or 4 times a day for a couple of minutes |
| General Strategies: |
| Quiet surroundings. Concentration is important. |
| Use stimulus (cloth, cosmetic brush, cotton swab) not finger. Using a finger would create two sets of sensory information for the patient which would confuse the all ready distorted sensory picture. |
Components of Re-training
|
For orofacial sensory retraining, an important component of the retraining exercises is the visual feedback provided by performing the exercises in front of a mirror. This elicits two different sensory events, the sensation of the brush on the facial skin and the sight of the brush on the face. Recent experimental studies have shown that viewing a body surface can directly enhance tactile perception and detection (57,58) even when the “touch” is not physical but a mirrored reflection.(59,60). The frequency with which the exercises are performed each day is much more important than the length of time spent at any given time. It may be that encouraging patients to perform orofacial sensory retraining exercises with a small handheld mirror for a short period of time, perhaps 1–2 minutes, 4 to 6 times per day would be as or more effective than a longer less frequent protocol.
Both the potential for acquiring the “second language” of sensory retraining and its effectiveness decreases with age (49,50,61), varies with the verbal learning capacity and visuo-spatial cognitive skills of the patient, and depends on motivation and positive reinforcement (45).
Sensory re-training as a rehabilitative approach has been used extensively over the past several decades for patients who had nerve injuries affecting the hand. The emphasis of the sensory re-training exercises for hand injury and stroke patients has been to teach the patient to interpret the percepts of objects manipulated by the fingers in a meaningful and functional way (30,53,62,63,64). Hand injury patients learn to recognize and to discriminate the shapes of small objects (various buttons, coins and keys). Patients gain the ability to button their own shirt and to identify shapes without visual cues (ex. a key versus a coin). Although the touch percepts produced by the objects remain abnormal after re-training, patients become more comfortable with, and accepting of, the situation since the percepts are no longer functionally disabling.
The same therapeutic approach, incorporating meaningful and graded stimuli, active participation, and accurate feedback, has successfully been used to improve tactile and proprioceptive discrimination following a stroke (65,66) and recovery of function in people with brain damage (67). An adaptation of sensory re-education, mirror box therapy, has successfully been used with patients with phantom limb pain (68), hemiparesis after stroke (69), and complex regional pain syndrome type I (70). Patients have regained functionality and mobility with reduced pain and evidence of cortical reorganization of the primary somatosensory cortex that paralleled their clinical improvement (71).
Sensory Retraining for Altered Orofacial Sensation
The question of whether sensory retraining exercises could be used effectively with patients with altered orofacial sensation was first raised in the literature by Gregg in 1992(72) In 2001, Meyer and Rath presented a retrospective review of 372 patients who had had a microsurgical repair for a nerve injury after 1981 and for whom at least an 18 month postsurgical followup was available. A non-random sample of patients had been given facial sensory exercise instructions that incorporated some of the early stage components of sensory re-training with the expectation that sensory retraining would help patients with altered oral-facial sensation following nerve injury by i) improving patients’ ability to interpret lip/chin sensations and movements, ii) improving perioral motor function subjectively and objectively, and iii) lessening the objectionable impression of numb/paresthetic sensations in the lip and chin by decreasing the subjective differences between affected and unaffected skin areas.(53) The percentage of patients who achieved a useful sensory recovery on the Medical Research Council Scale, a clinical assessment, did not differ for those who did and did not receive instructions regarding facial sensory exercises. However, those patients who received instructions reached their final level of sensory recovery much sooner, on average 3 months earlier (53).
In order to assess the efficacy of sensory re-training for facial altered sensation, a multi-center double blind parallel two-arm stratified block randomized clinical trial (RCT) was conducted at an academic center and a community-based center with enrollment of 191 subjects. The intent was to assess whether the magnitude and duration of patient-reported burden from altered sensation was lessened when facial sensory retraining exercises were performed in conjunction with standard opening exercises than when the opening exercises were performed alone. The subjects were patients with a developmental disharmony who were scheduled for a bilateral sagittal split osteotomy with or without a maxillary osteotomy. Just as third molar extraction is an excellent model for analgesic pain studies, candidates for orthognathic surgery constitute an ideal subject group for the investigation of novel putative therapies for nerve injury associated altered sensation. Baseline data can be obtained before altered sensation develops, i.e. presurgically, and these baseline responses can be compared subsequently to those obtained immediately after nervy injury and during the recovery process. Because the surgery is elective, patients are typically healthy young adults without pre-existing conditions or complications that can make interpretation of therapeutic effect more difficult.
The emphasis in the RCT on patient-report was motivated by two factors: 1) the assumption that sensory retraining would not affect nerve recovery and therefore basic sensory testing measures of nerve function and 2) the recognition of the different functions of the sensory innervations to the facial versus digital skin. The terminal distribution of the inferior alveolar nerve, i.e., the mental nerve, innervates skin functionally more like the back of the hand (radial nerve) than the palm side of the hand (median and ulnar nerves).(73) Thus, the skin of the hairy lower lip/chin of the face deform in response to movements during function, and as such, the evoked neural discharge serves a proprioceptive role including a conscious awareness of facial expressions(74,75).
The sensory re-training protocol in the RCT had three, time-dependent levels of instructions that were given to patients at 1 week, 1 month (4 to 6 weeks), and 3 months after surgery. The time points were selected based on their use in clinical studies of the impact of sensory reeducation in patients with an injured median or ulnar nerve (64) and in clinical studies of sensory impairment in patients following orthognathic surgery. (76,77,78,79) The three levels of sensory retraining were designed to increasingly challenge patients congruent with the early and late phases of sensory education used for the hand: constant vs moving touch; orientation of moving touch; and direction of moving touch (Figure 1 and Table 2). (Three videos demonstrating each exercise at each level are available online within this article at: www.oralmaxsurgeryatlas.theclinics.com, March 2011 issue. The videos were produced by Video Services of the Center for Instructional Technology at the University of North Carolina. Written instructions provided to subjects and copies of the instructional tapes are available from the corresponding author upon request.)
Figure 1.

Screen shot of sensory retraining exercise instruction: simple touch and stroke with cosmetic brush (motion training) and mirror. Three videos demonstrating each exercise at each level are available online within this article at: www.oralmaxsurgeryatlas.theclinics.com, March 2011 issue. (The screen shot and videos are © Video Services of the Center for Instructional Technology at the University of North Carolina, Chapel Hill, NC).
Table 2.
Synopsis of instructions given to the opening-only exercise group and the sensory-retraining group at each of the 3 training sessions. Subjects in the sensory-retraining group also were instructed and ask to perform the opening exercises.
| Visit | Opening Exercises (3x/day) | Sensory Retraining Exercises (2x/day) |
|---|---|---|
| 1 Week |
|
|
| 1 Month |
|
|
| 3 Months |
|
|
From Phillips C, Essick G, Preisser JS, Turvey TA, Tucker M, Lin D. Sensory Retraining following orthognathic surgery: effect on patient perception of altered sensation. J Oral Maxillofac Surg 65:1162–73, 2007.
Consistent with the anecdotal reports, the patients in this clinical trial who received the sensory retraining exercise instruction were less likely to report a problem related to unusual feelings on the face, loss of lip sensitivity, or numbness at 3 and 6 months after surgery than subjects who received standard opening exercises only (12,80). At 6 months, subjects in the opening-only exercise group were almost twice as likely as those in the sensory retraining group to report a problem with altered sensation. (12,80). In addition to patient-reported outcomes, two-point perception, two – point discrimination, and contact detection thresholds were measured as secondary outcomes. The sensory re-training patients were more adept at perceiving touch (Figure 2), indicating accommodation, even though there was no improvement in the ability to discriminate two distinct points of contact from one (nerve recovery) (46).
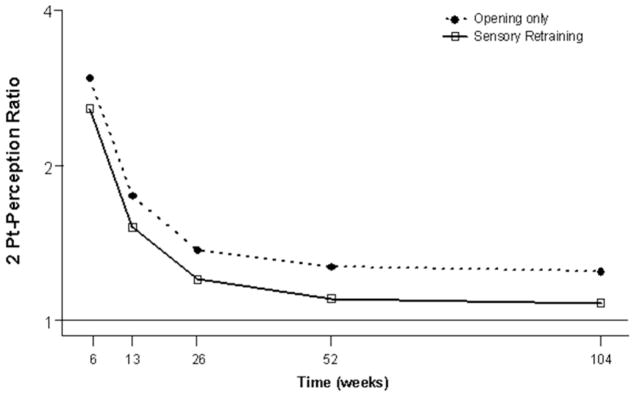
Figure 2.
Estimates of the adjusted mean impairment ratio in two point perception for subjects who did and did not receive sensory retraining exercises. The lower 2-point perception impairment ratio, on average, for the sensory retraining(SR) group indicates that this group was able to report two distinct points at shorter separations than the opening only group. The Y-axis is scaled logarithmically. A value of 1 indicates a return to pre-surgical value.
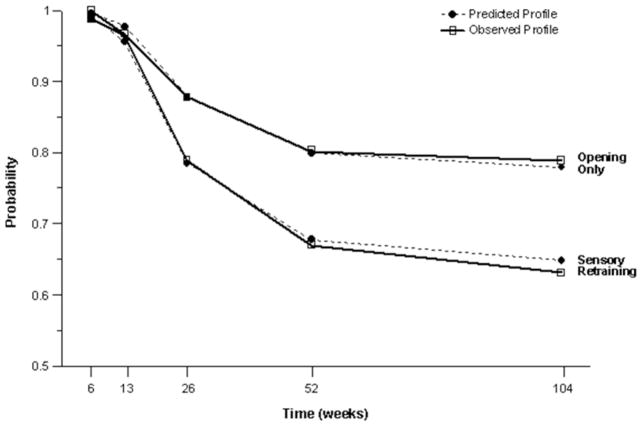
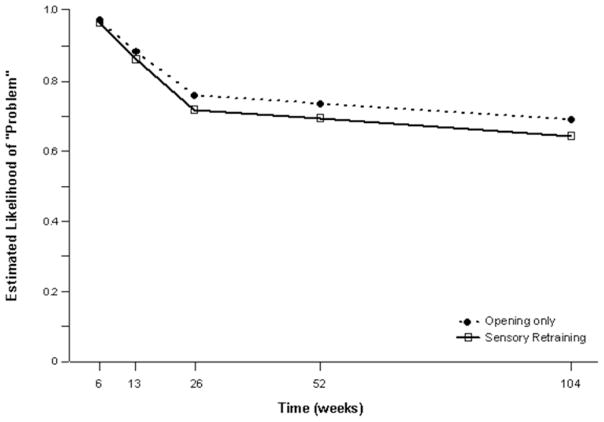
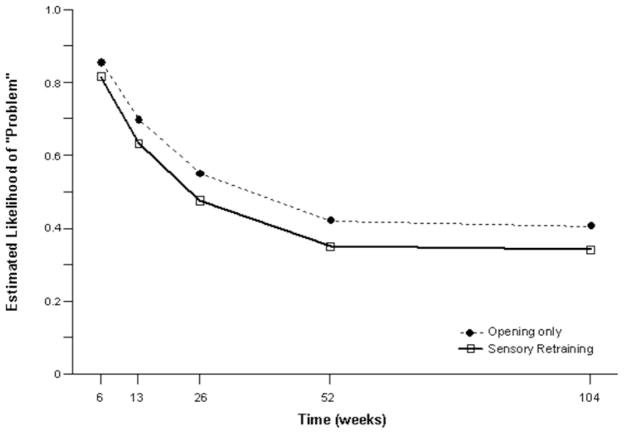
The positive effect of the sensory retraining persisted even after the exercise protocol was completed. Although the likelihood that a subject would report altered sensation steadily decreased in both groups over a two-year follow-up, the difference between the groups was relatively consistent. Even at two years after surgery, patients who received only the opening exercises were about 2 times more likely to report an altered sensation than patients who used the sensory retraining exercises after surgery(Figure 3).(49) And patients in the sensory retraining group were less likely to report interference in daily life activities from numbness or loss of lip sensitivity (Figure 4a,b) (50) This difference between the two exercise groups appears to be related to the difference in how the “retrained” individual experienced or interpreted tactile stimuli rather than any difference in nerve recovery or repair (46,47).
Figure 3.
Estimated and observed likelihood of the presence of altered sensations for subjects who did and did not receive sensory retraining exercises after controlling for psychological distress and age. (From Phillips C, Kim S, Essick G, Tucker M, Turvey TA. Sensory retraining following orthognathic surgery: Effect on Patient Report of the Presence of Altered Sensation. Am J Ortho Dentofac Orthop, 136:788–794, 2009.)
Figure 4.
Estimated likelihood of a subject reporting at least some problem or interference in daily life after controlling for psychological distress and age for subjects who did and did not receive sensory re-training exercises A) Problem associated with Numbness. B) Problem associated with loss of lip sensitivity.
The primary efficacy results at 6 months and the longer term recovery analyses at 24 months indicate that for patients who experience an acute nerve injury, as is highly likely during a mandibular osteotomy, the simple, non-invasive sensory retraining facial exercises, which require only an inexpensive cosmetic brush and a mirror, are an effective cognitive behavioral therapy to promote accommodation to a sensory deficit on the face. Perhaps the desired outcome for “re-trained” patients was best stated by Callahan, “If sensory re-education results in a person’s increased ability to better enjoy the tactile sensations of everyday living, then reeducation has been meaningful and successful.”(54)
Conclusions
Sensory retraining teaches the patient to ignore or blot out post-injury unpleasant orofacial sensations to optimally tune into and decipher the weakened and damaged signals from the tissues.
Sensory retraining is a simple, inexpensive, noninvasive exercise program, which initiated shortly after injury, can lessen the objectionable impression of orofacial altered sensations.
Sensory retraining exercises are most effective on decreasing the perceived burden associated with hypoesthetic orofacial altered sensations.
Acknowledgments
We wish to thank Dr. Myron Tucker and the staff at Oral and Maxillofacial Surgery in Charlotte, NC, for their participation in the RCT.
This project was supported in part by National Institute of Health grants R01-DE013967 and R01-DE005215.
Footnotes
The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kawasaki Y, Xu Z-Z, Wang X, et al. Distinct roles of matrix metalloproteases in the early- and late-phase development of neuropathic pain. Nat Med. 2008;14:331–336. doi: 10.1038/nm1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Treede RD, Jensen TS, Campbell JN, et al. Neuropathic pain. Redefinition and a grading system for clinical and research purposes. Neurology. 2008;70:1630–1635. doi: 10.1212/01.wnl.0000282763.29778.59. [DOI] [PubMed] [Google Scholar]
- 3. [Accessed 9/29/2008]; http://www.neuropathy.org/site/PageServer?pagename=About_Facts.
- 4.Phillips C, Essick G, Zuniga J, Tucker M, Blakey GH., III Qualitative descriptors used by patients following orthognathic surgery to portray altered sensation. J Oral Maxillofac Surg. 2006;64:1751–1760. doi: 10.1016/j.joms.2005.11.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meyer-Rosberg K, Kvarnstrom A, Kinnman E, et al. Peripheral neuropathic pain-a multidimensional burden for patients. Europ J Pain. 2001;5:3779–389. doi: 10.1053/eujp.2001.0259. [DOI] [PubMed] [Google Scholar]
- 6.Essick GK, Phillips C, Turvey TA, Tucker M. Facial altered sensation and sensory impairment after orthognathic surgery. Int J Oral Maxillofac Surg. 2007;36:577–582. doi: 10.1016/j.ijom.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Renzi G, Carboni A, Perugini M, Giovannetti F, Becelli R. Posttraumatic trigeminal nerve impairment: a prospective analysis of recovery patterns in a series of 103 consecutive facial fractures. J Oral Maxillofac Surg. 2004;62:1341–1346. doi: 10.1016/j.joms.2004.05.212. [DOI] [PubMed] [Google Scholar]
- 8.Jääskeläinen SK, Teerijoki-Oksa T, Virtanen A, Tenovuo O, Forssell K, Forssell H. Sensory regeneration following intraoperatively verified trigeminal nerve injury. Neurology. 2004;62:1951–1957. doi: 10.1212/01.wnl.0000129490.67954.c2. [DOI] [PubMed] [Google Scholar]
- 9.Nakagawa K, Ueki K, Takatsuka S, Takazakura D, Yamamoto E. Somatosensory-evoked potential to evaluate the trigeminal nerve after sagittal split osteotomy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91:146–152. doi: 10.1067/moe.2001.112331. [DOI] [PubMed] [Google Scholar]
- 10.Jones DL, Wolford LM. Intraoperative recording of trigeminal evoked potentials during orthognathic surgery. Int J Adult Orthodont Orthognath Surg. 1990;5:167–174. [PubMed] [Google Scholar]
- 11.Phillips C, Essick G, Blakey G, 3rd, Tucker M. Relationship between patients’ perceptions of postsurgical sequelae and altered sensations after bilateral sagittal split osteotomy. J Oral Maxillofac Surg. 2007a;65:597–607. doi: 10.1016/j.joms.2005.12.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phillips C, Essick G, Preisser JS, Turvey TA, Tucker M, Lin D. Sensory retraining after orthognathic surgery: effect on patients’ perception of altered sensation. J Oral Maxillofac Surg. 2007b;65:1162–1173. doi: 10.1016/j.joms.2006.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Phillips C, Proffitt WR. Psychosocial aspects of dentofacial deformity and its treatment. In: Proffit WR, White RP Jr, Sarver DM, editors. Contemporary treatment of dentofacial deformity. New York: Mosby; 2003. pp. 69–90. [Google Scholar]
- 14.Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367:L1618–L1825. doi: 10.1016/S0140-6736(06)68700-X. [DOI] [PubMed] [Google Scholar]
- 15.Harvey WS, Phillips CL, Essick GK. Neurosensory impairment and patient perception of recovery following orthognathic surgery. J Dent Res. 2001;80 (Special Issue):187. [Google Scholar]
- 16.Westermark A, Bystedt H, von Konow L. Inferior alveolar nerve function after mandibular osteotomies. Br J Oral Maxillofac Surg. 1998;36:425–8. doi: 10.1016/s0266-4356(98)90457-0. [DOI] [PubMed] [Google Scholar]
- 17.Westermark A, Englesson L, Bongenhielm U. Neurosensory function after sagittal split osteotomy of the mandible: A comparison between subjective evaluation and objective assessment. Int J Adult Orthod Orthognath Surg. 1999;14:268–275. [PubMed] [Google Scholar]
- 18.Essick G. Psychophysical assessment of patients with posttraumatic neuropathic trigeminal pain. J Orofac Pain. 2004;18:345–354. [PubMed] [Google Scholar]
- 19.Becerra L, Morris S, Bazes S, Gostic R, et al. Trigeminal neuropathic pain alters responses in CNS circuits to mechanical (brush) and thermal (cold and heat) stimuli. J Neurosci. 2006;26:10646–10657. doi: 10.1523/JNEUROSCI.2305-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Navarro X, Vivó M, Valero-Cabré A. Neural plasticity after peripheral nerve injury and regeneration. Prog Neurobiol. 2007;82:163–201. doi: 10.1016/j.pneurobio.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 21.Atusmi Y, Imai T, Matsumoto K, Sakuda M, Maeda T, Kurisu K, Wakisaka S. Effects of different types of injury to the inferior alveolar nerve on the behavior of Schwann cells during the regeneration of periodontal nerve fibers of rat incisor. Arch Histol Cytol. 2000;63:43–54. doi: 10.1679/aohc.63.43. [DOI] [PubMed] [Google Scholar]
- 22.Abe N, Cavalli V. Nerve injury signaling. Curr Opin Neurobiol. 2008;18:276–283. doi: 10.1016/j.conb.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanz S, Fainzilber M. Retrograde signalling in injured nerve-the axon reaction revisited. J Neurochem. 2006;99:13–19. doi: 10.1111/j.1471-4159.2006.04089.x. [DOI] [PubMed] [Google Scholar]
- 24.Mandolesi G, Madeddu F, Bozzi Y, Maffei L, Ratto GM. Acute physiological response of mammalian central neurons to axotomy: ionic regulation and electrical activity. Faseb J. 2004;18:1934–1936. doi: 10.1096/fj.04-1805fje. [DOI] [PubMed] [Google Scholar]
- 25.Boyd JG, Gordon T. Neurotrophic factors and their receptors in axonal regeneration and functional recovery after peripheral nerve injury. Mol Neurobiol. 2003;27:277–324. doi: 10.1385/MN:27:3:277. [DOI] [PubMed] [Google Scholar]
- 26.Herdegen T, Skene P, Bahr M. The c-Jun transcription factor--bipotential mediator of neuronal death, survival and regeneration. Trends Neurosci. 1997;20:227–231. doi: 10.1016/s0166-2236(96)01000-4. [DOI] [PubMed] [Google Scholar]
- 27.Kaas JH, Collins CE. Anatomic and functional reorganization of somatosensory cortex in mature primates after peripheral nerve and spinal cord injury. Adv Neurol. 2003;93:87–95. [PubMed] [Google Scholar]
- 28.Wall JT, Xu J, Wang X. Human brain plasticity: an emerging view of the multiple substrates and mechanisms that cause cortical changes and related sensory dysfunctions after injuries of sensory inputs from the body. Brain Res Rev. 2002;39:181–215. doi: 10.1016/s0165-0173(02)00192-3. [DOI] [PubMed] [Google Scholar]
- 29.Faggin BM, Nguyen KT, Nicolelis MAL. Immediate and simultandous sensory reorganization at cortical and subcortical levels of the somatosensory system. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:9428–9233. doi: 10.1073/pnas.94.17.9428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dellon AL. Re-education of sensation. In: Dellon AL, editor. Evaluation of Sensibility and Re-Education of Sensation in the Hand. Baltimore, MD: John D Lucas; 1988. pp. 203–246. [Google Scholar]
- 31.Dubner R, Ruda MA. Activity-dependent neuronal plasticity following tissue injury and inflammation. [Review]] Trends in Neurosciences. 1992;15(3):96–103. doi: 10.1016/0166-2236(92)90019-5. [DOI] [PubMed] [Google Scholar]
- 32.Essick GK. Comprehensive clinical evaluation of perioral sensory function. Oral Maxillofac Surg Clin North Am. 1992;4(2):503–26. [Google Scholar]
- 33.Woolf CJ, Walters ET. Common patterns of plasticity contributing to nociceptive sensitization in mammals and Aplysia [Review] [60 refs] Trends in Neurosciences. 1991;14(2):74–8. doi: 10.1016/0166-2236(91)90024-o. [DOI] [PubMed] [Google Scholar]
- 34.Gregg JM. Studies of traumatic neuralgias in the maxillofacial region: surgical pathology and neural mechanisms. J Oral & Maxillofac Surg. 48(s):228–237. doi: 10.1016/0278-2391(90)90385-f. discussion, 1990. [DOI] [PubMed] [Google Scholar]
- 35.Cusick CG, Wall JT, Whiting JHJ, Wiley RG. Temporal progression of cortical reorganization following nerve injury. Brain Research. 1990;537(1–2):355–8. doi: 10.1016/0006-8993(90)90385-o. [DOI] [PubMed] [Google Scholar]
- 36.Merzenich MM, Recanzone G, Jenkins WM, Allard TT, Nudo RJ. Cortical representational plasticity. In: Rakic P, Singer W, editors. Neurobiology of Neocortex. John Wiley & Sons; 1988. [Google Scholar]
- 37.Wall JT, Kaas JH, Sur M, Nelson RJ, Felleman DF, Merzenich MM. Functional reorganization in somatosensory cortical areas 3b and 1 of adult monkeys after median nerve repair: possible relationships to sensory recovery in humans. J Neuroscience. 1986;6:218–223. doi: 10.1523/JNEUROSCI.06-01-00218.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Florence SL, Boydston LA, Hackett TA, Taub Lachoff H, Strata F, Niblock MM. Sensory enrighment after peripheral nerve injury restores cortical, not thalamic, receptive field organization. European Journal of Neuroscience. 2001;13:1755–1766. doi: 10.1046/j.0953-816x.2001.01555.x. [DOI] [PubMed] [Google Scholar]
- 39.Jones TA, Ghu CJ, Grande LA, Gregory AD. Motor Skills Training Enhances Lesion-Induced Structural Plasticity in the Motor Cortex of Adult Rats. J Neuroscience. 1999;19(22):10153–10163. doi: 10.1523/JNEUROSCI.19-22-10153.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jones TA, Hawrylak N, Klintsova AY, Greenough WT. Brain damage, behavior, rehabilitation, recovery, and brain plasticity. Ment Retard Dev Disabil Res Rev. 1998;4:231–237. [Google Scholar]
- 41.Recanzone GH, Jenkins WM, Hradek GT, Merzenich MM. Progressive improvement in discriminative abilities in adult owl monkeys performing a tactile frequency discrimination task. J Neurophysiol. 1992a;67:1015–1030. doi: 10.1152/jn.1992.67.5.1015. [DOI] [PubMed] [Google Scholar]
- 42.Recanzone GH, Merzenich MM, Jenkins WM, Grajski KA, Dinse HR. Topographic reorganization of the hand representation in cortical area 3b of owl monkeys trained in a frequency-discrimination task. J Neurophysiol. 1992b;67:1031–1055. doi: 10.1152/jn.1992.67.5.1031. [DOI] [PubMed] [Google Scholar]
- 43.Recanzone GH, Merzenich MM, Schreiner CE. Changes in the distributed temporal response properties of SI cortical neurons reflect improvements in performance on a temporally based tactile discrimination task. J Neurophysiol. 1992c;67:1071–1092. doi: 10.1152/jn.1992.67.5.1071. [DOI] [PubMed] [Google Scholar]
- 44.Jenkins WM, Merzenich MM, Ochs MT, Allard T, Guic-Robles E. Functional reorganization of primary somatosensory cortex in adult owl monkeys after behaviorally controlled tactile stimulation. J Neurophysiol. 1990;63:82–104. doi: 10.1152/jn.1990.63.1.82. [DOI] [PubMed] [Google Scholar]
- 45.Lundborg G. Nerve injury and repair: A challenge to the plastic brain. J Peripher Nerv Syst. 2003;8:209. doi: 10.1111/j.1085-9489.2003.03027.x. [DOI] [PubMed] [Google Scholar]
- 46.Essick GK, Phillips C, Zuniga J. Effect of facial sensory retraining on sensory thresholds. J Dent Res. 2007;86:571–575. doi: 10.1177/154405910708600616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Essick GK, Phillips C, Kim SK, Zuniga J. Sensory retraining following orthognathic surgery: Effect on threshold measures of sensory function. J Oral Rehabilitation. 2009 Jun;36(6):415–26. doi: 10.1111/j.1365-2842.2009.01954.x. Epub 2009 Apr 28. [DOI] [PubMed] [Google Scholar]
- 48.Bell-Krotoski J, Weinstein S, Weinstein C. Testing sensibility, including tough-pressure, two-point discrimination, point localization, and vibration. J Hand Therapy. 1993 Apr–Jun;:114–123. doi: 10.1016/s0894-1130(12)80292-4. [DOI] [PubMed] [Google Scholar]
- 49.Phillips C, Kim S, Essick G, Tucker M, Turvey TA. Sensory retraining following orthognathic surgery: Effect on Patient Report of the Presence of Altered Sensation. Am J Ortho Dentofac Orthop. 2009;136(6):788–94. doi: 10.1016/j.ajodo.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 50.Phillips C, Kim S, Tucker M, Turvey TA. Sensory retraining: burden in daily life related to altered sensation after orthognathic surgery, a randomized clinical trial. Orthodontics and Craniofacial Research. 2010 Aug;13(3):169–78. doi: 10.1111/j.1601-6343.2010.01493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Daniele HR, Aguado L. Early compensatory sensory re-education. Journal of Reconstructive Microsurgery. 2003;19:107–110. doi: 10.1055/s-2003-37815. [DOI] [PubMed] [Google Scholar]
- 52.Rosen B, Lundborg G. Sensory re-education after nerve repair: Aspects of timing. Handchir Mikrochir Plast Chir. 2004;36:8–12. doi: 10.1055/s-2004-815808. [DOI] [PubMed] [Google Scholar]
- 53.Meyer RA, Rath EM. Sensory rehabilitation after trigeminal nerve injury or nerve repair. Atlas Oral Maxillofac Surg Clin North Am. 2001;13:365. [Google Scholar]
- 54.Callahan AD. Methods of compensation and re-education for sensory dysfunction. In: Hunter JM, Mackin EJ, Callahan AD, editors. Rehabilitation of the Hand. St Louis, MO: Mosby; 1995. [Google Scholar]
- 55.Imai H, Tajima T, Natsumi Y. Successful reeducation of functional sensibility after median nerve repair at the wrist. J Hand Surg. 1991;16:60. doi: 10.1016/s0363-5023(10)80014-0. [DOI] [PubMed] [Google Scholar]
- 56.Waylett-Rendall J. Sensibility evaluation and rehabilitation. Orthop Clin North Am. 1988;19:43. [PubMed] [Google Scholar]
- 57.Fiorio M, Haggard P. Viewing the body prepares the brain for touch: effects of TMS over somatosensory cortex. European Journal of Neuroscience. 2005;22:773–777. doi: 10.1111/j.1460-9568.2005.04267.x. [DOI] [PubMed] [Google Scholar]
- 58.Taylor-Clarke M, Kennett S, Haggard P. Vision modulates somatosensory cortical processing. Current Biology. 2002;12:233–236. doi: 10.1016/s0960-9822(01)00681-9. [DOI] [PubMed] [Google Scholar]
- 59.Ro T, Wallace R, Hagedorn J, Farne A, Pienkos E. Visual enhancing of tactile perception in the posterior parietal cortex. J Cogn Neurosci. 2004;16:24–30. doi: 10.1162/089892904322755520. [DOI] [PubMed] [Google Scholar]
- 60.Sathian K, Greenspan AI, Wolf SL. Doing it with mirrors: a case study of a novel approach to neurorehabilitation. Neurorehabil and Neural Repair. 2000;14:73–76. doi: 10.1177/154596830001400109. [DOI] [PubMed] [Google Scholar]
- 61.Lundborg G, Rosen B. Sensory relearning after nerve repair. Lancet. 2001;358:809–810. doi: 10.1016/S0140-6736(01)06001-9. [DOI] [PubMed] [Google Scholar]
- 62.Shieh S-J, Chiu HOY, Lee J-W, Hsu H-Y. Evaluation of the effectiveness of sensory reeducation following digital replantation and revascularization. Microsurgery. 1995;16:578–582. doi: 10.1002/micr.1920160813. [DOI] [PubMed] [Google Scholar]
- 63.Wei F-C, Ma H-S. Delayed Sensory Reeducation after Toe-to-Hand Transfer. Microsurgery. 1995;16:583–585. doi: 10.1002/micr.1920160814. [DOI] [PubMed] [Google Scholar]
- 64.Wynn Parry CB, Slater M. Sensory re-education after median nerve lesions. The Hand. 1976;8:250–257. doi: 10.1016/0072-968x(76)90010-3. [DOI] [PubMed] [Google Scholar]
- 65.Carey LM, Matyas TA, Oke LE. Sensory loss in stroke patients: effective training of tactile and proprioceptive discrimination. Arch Phys Med Rehabil. 1993;74:602–611. doi: 10.1016/0003-9993(93)90158-7. [DOI] [PubMed] [Google Scholar]
- 66.Yekutiel M. Sensory Re-education of the hand after stroke. Whurr Pub; Phila Pa: 2005. Sensory loss in stroke; pp. 14–28. [Google Scholar]
- 67.Bach-y-Rita B. Brain plasticity as a basis for therapeutic procedures. In: Bach-y-Rita P, editor. Recovery of function: theoretical considerations for brain injury rehabilitation. Vienna: Hans Huber; 1980. pp. 225–263. [Google Scholar]
- 68.Ramachandran VS. Phantom limbs and neural plasticity. Arch Neurol. 2000;57:317–320. doi: 10.1001/archneur.57.3.317. [DOI] [PubMed] [Google Scholar]
- 69.Altschuler E, Wisdom SB, Stone L, Foster C, Galasko D, Llewellyn DM, et al. Rehabilitation of hemiparesis alfter stroke with a mirror. Lancet. 1999;353:235. doi: 10.1016/s0140-6736(99)00920-4. [DOI] [PubMed] [Google Scholar]
- 70.McCabe CS, Haigh RC, Ring EFJ, Halligan PW, Wall PD, Blake DR. A controlled pilot study of the utility of mirror visual feedback in the treatment of complex regional pain syndrome (type 1) Rheumatology. 2003;42:971–101. doi: 10.1093/rheumatology/keg041. [DOI] [PubMed] [Google Scholar]
- 71.Mailhofner C, Handwerker HO, Neundorfe B, Birklein F, et al. Cortical reorganization during recovery from complex regional pain syndrome. Neurology. 2004;63:693–701. doi: 10.1212/01.wnl.0000134661.46658.b0. [DOI] [PubMed] [Google Scholar]
- 72.Gregg JM. Nonsurgical management of traumatic trigeminal neuralgias and sensory neuropathies. Oral Maxillo Surg Clinics of North America. 1992;4:375–392. [Google Scholar]
- 73.Trulsson M, Essick GK. Low-threshold mechanoreceptive afferents in the human lingual nerve. J Neurophysiol. 1997;77:737. doi: 10.1152/jn.1997.77.2.737. [DOI] [PubMed] [Google Scholar]
- 74.Edin BB, Johansson N. Skin strain patterns provide kinaesthetic information to the human central nervous system. J Physiol (Lond) 1995;1:243. doi: 10.1113/jphysiol.1995.sp020875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gandevia SC, Phegan CML. Perceptual distortions of the human body image produced by local anaesthesia, pain and cutaneous stimulation. J Physiol (Lond) 1999;2:609. doi: 10.1111/j.1469-7793.1999.609ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yoshida T, Nagamine T, Kobayashi T, et al. Impairment of the inferior alveolar nerve after sagittal split osteotomy. J Craniomaxillofac Surg. 1989;17:271. doi: 10.1016/s1010-5182(89)80095-2. [DOI] [PubMed] [Google Scholar]
- 77.Karas ND, Boyd SB, Sinn DP. Recovery of neurosensory function following orthognathic surgery. J Oral Maxillofac Surg. 1990;48:124. doi: 10.1016/s0278-2391(10)80199-5. [DOI] [PubMed] [Google Scholar]
- 78.Van Boven RW, Johnson KO. A psychophysical study of the mechanisms of sensory recovery following nerve injury in humans. Brain. 1994;117:149. doi: 10.1093/brain/117.1.149. [DOI] [PubMed] [Google Scholar]
- 79.Fridrich KL, Holton TJ, Pansegrau KJ, et al. Neurosensory recovery following the mandibular bilateral sagittal split osteotomy. J Oral Maxillofac Surg. 1995;53:1300. doi: 10.1016/0278-2391(95)90588-x. [DOI] [PubMed] [Google Scholar]
- 80.Preisser JS, Phillips C, Perin J, Schwartz TA. Regression Models for Patient-Reported Measures having Ordered Categories Recorded on Multiple Occasions. Community Dentistry Oral Epidemiology. 2010 doi: 10.1111/j.1600-0528.2010.00583.x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]