Abstract
Background
Quantum Dots (QD) are fluorescent nanocrystals that are highly useful in imaging and flow cytometric analyses. During routine use of monoclonal antibody conjugates of QD, we have occasionally seen partial or total loss of fluorescence when using certain lots of fixative solutions. We hypothesized that a low level contamination with heavy metal cations was responsible.
Methods
QD reagent fluorescence was quantified using different lots of fixative solutions that were known to perform well or poorly. The role of heavy metal cation contamination was evaluated by addition of chelators or addition of metal cation salt solutions. Concentrations of metals in solution was determined by mass spectroscopy.
Results
Millimolar concentrations of ferrous and zinc ions, and as low as 50 nanomolar cupric ions, permanently eliminated QD fluorescence. The commercial lots of fixative that led to poor QD fluorescence patterns, but not those that preserved QD fluorescence, had concentrations of copper that far exceeded this threshold. The elimination of QD fluorescence is irreversible; cells labeled with QD, and are highly fluorescent, can be rendered nonfluorescent by the addition of cupric sulfate. These cells can then be subsequently successfully stained with other QD reagents with no fluorescence arising from the first staining.
Conclusion
Low level metal contaminants are not uncommon in formalin solutions; this can lead to highly variable performance of QD reagents. Addition of EDTA can prevent this; solutions can also be pre-tested to ensure best performance. This property of QD can also be exploited in cases where fluorescently-stained cells must be rendered non-fluorescent for experimental reasons.
Keywords: Immunophenotyping, formalin, fluorescence intensity, quality control
Introduction
The use of fluorescent quantum dots (QD) for immunophenotyping has become prevalent in recent years with the advent of commercially-available conjugates. The unique spectral properties of QD lend themselves to flow cytometry and imaging, allowing for simple multiplexing and excellent resolution of differentially expressed cellular proteins (1). Most QD are made from similar materials, with similar conjugation chemistry as well as performance in immunophenotyping experiments, yet have significantly different emission spectra.
As with any fluorescence reagent, consistent staining in terms of background binding and intensity of positive cells is important. Over the past years, we noticed occasional partial or complete loss of QD fluorescence following staining. We carefully investigated the reasons for this, by a thorough analysis of procedures, staining conditions and reagents (such as fixatives) to determine “ideal” conditions for staining with QD-conjugated monoclonal antibodies.
It has previously been reported that metal cations can quench QD fluorescence in solution, albeit incompletely (2–6). Here we show that very low concentrations of cupric ions are sufficient to eliminate QD fluorescence in immunofluorescence experiments. The contamination of some reagents with copper leads to variable results, particularly when using reagents that might chelate the copper, such as serum, EDTA, or even hemoglobin from red blood cells. We found that some commercial lots of fixatives had sufficient levels of copper ions to affect QD staining, accounting for variability in QD performance seen with different laboratories even when using the same QD monoclonal antibody. We provide a set of recommendations for avoiding this problem, and achieved consistent staining with QD reagents even in the presence of contaminating cupric ions. Finally, we note that this property of QD can be exploited: treating stained cells with cupric ions can completely eliminate all cell-associated QD fluorescence, and the cells can then be stained with different QD reagents.
Materials and Methods
Cells and Immunophenotyping
Peripheral Blood Mononuclear Cells (PBMCs) were stained for analysis by flow cytometry using multiple different media. Phosphate-buffered saline (PBS; GIBCO, Grand Island, NY), RPMI-1640 (GIBCO, Grand Island, NY), and R10 were each selected for their differing properties and common use in cell staining. R10 is comprised of RPMI-1640, 10% FBS (GIBCO, Grand Island, NY), and 0.02% azide (Sigma, St. Louis, MO). PBMCs were washed twice (400×g for 3 min) in a 12×75mm test tube and stained for 20 minutes with CD8 QD655 (manufactured in-house using conjugatable QD655 from Invitrogen) at room temperature in their respective media. They were subsequently washed twice more before the addition of 1% formalin, (Formaldehyde + PBS, Electron Microscopy Sciences, Hatfield, PA). As noted in some experiments, EDTA to a final concentration of 1 mM was added at various steps in the procedure. A flow cytometer (LSR II -SORP, Becton Dickinson, San Jose, CA) was used to performed phenotyping, and analysis was done using the Flowjo software (Tree Star Inc., Ashland, OR).
Analysis of heavy metals
Three different lots of 1% formalin were compared in order to examine potential lot specific differences. Aliquots of two of these lots were analyzed by mass spectrometry in order to quantify heavy metal concentrations in the different solutions. Mass spectrometry was performed by the Analytical Chemistry Division, National Institute of Standards and Technology.
Analysis of the effects of four heavy metals on QD fluorescence was performed by titration of different salt solutions on singly-stained compensation. Solutions of Zn2+, Fe2+, Fe3+, Cu2+ ranging from 1.0 nM to 1.0 mM were created by dissolving Zinc Chloride, Iron Sulfate, Iron Citrate, and Copper Sulfate (Sigma, St. Louis, MO) in PBS. Compensation beads were stained with CD8 QD655 in PBS, and resuspended in 300 µl of the metallic solution or PBS alone. The beads were incubated at room temperature for 20 min before being analyzed on the LSR.
Results
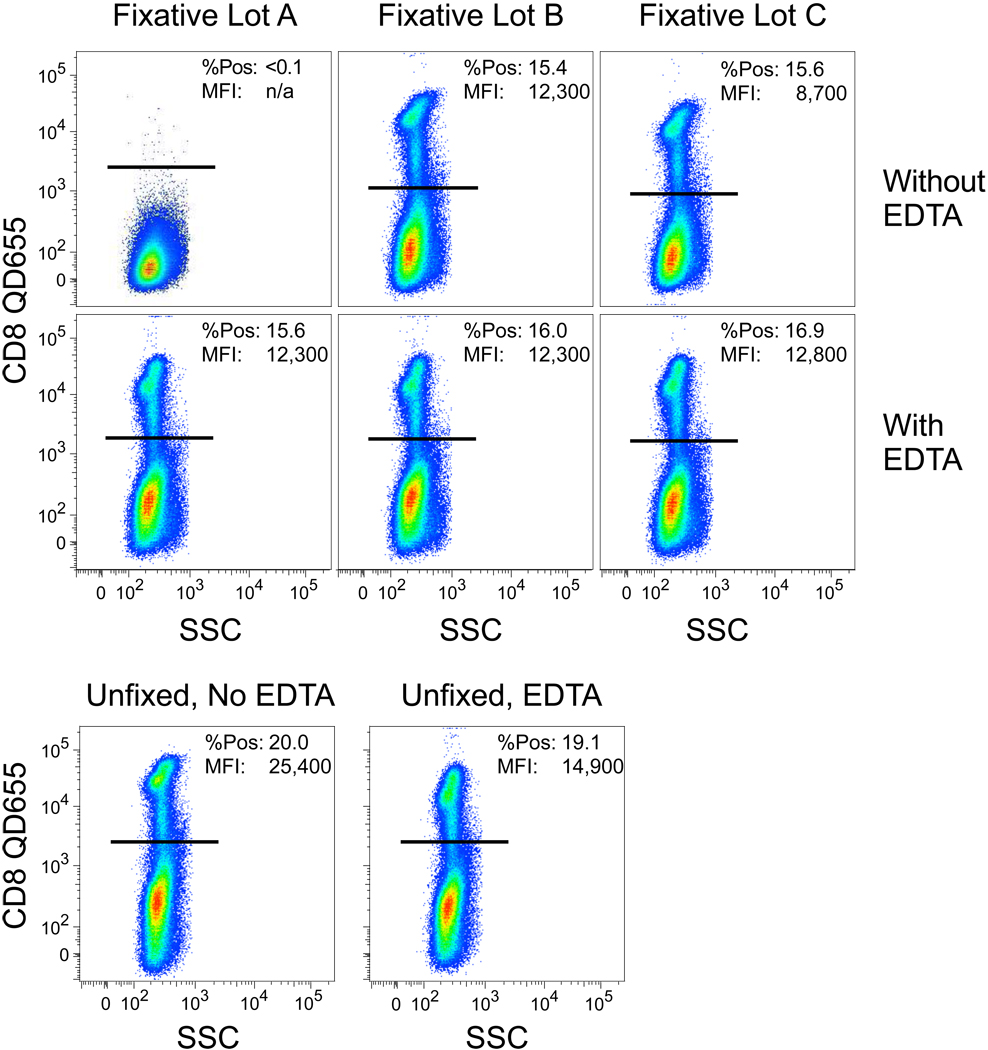
We had observed occasional, variable performance of QD staining that was eventually traced to certain staining conditions when using particular lots of fixative. As shown in Fig 1, when cells were stained with CD8 QD655, followed by fixation in formalin, there was a complete loss of fluorescence when using lot “A” compared to the other lots. Lot “C” exhibited partial loss of fluorescence. Based on previous reports of heavy metal poisoning of QD luminescence (4,6), we reasoned that addition of EDTA during the fixation might reverse the inhibition. As shown in Fig 1, this was the case; the fluorescence resulting after fixation in the presence of 1 mM EDTA could completely protect the QD fluorescence. Notably, addition of EDTA alone, in the absence of fixative, marginally reduced QD fluorescence.
Figure 1. Variable quenching of QD fluorescence by different lots of fixative.
Cells were stained with CD8 QD655 and then subjected to fixation with one of three different lots of fixative per standard protocols (top). In the second row, 1 mM EDTA was added to the fixative prior to incubation with cells. In the bottom row, cells were stained with or without 1 mM EDTA, but not fixed. In each graphic, the percentage of CD8+ cells is shown, together with the median fluorescence intensity (MFI) of the QD655 fluorescence on the positively gated cells.
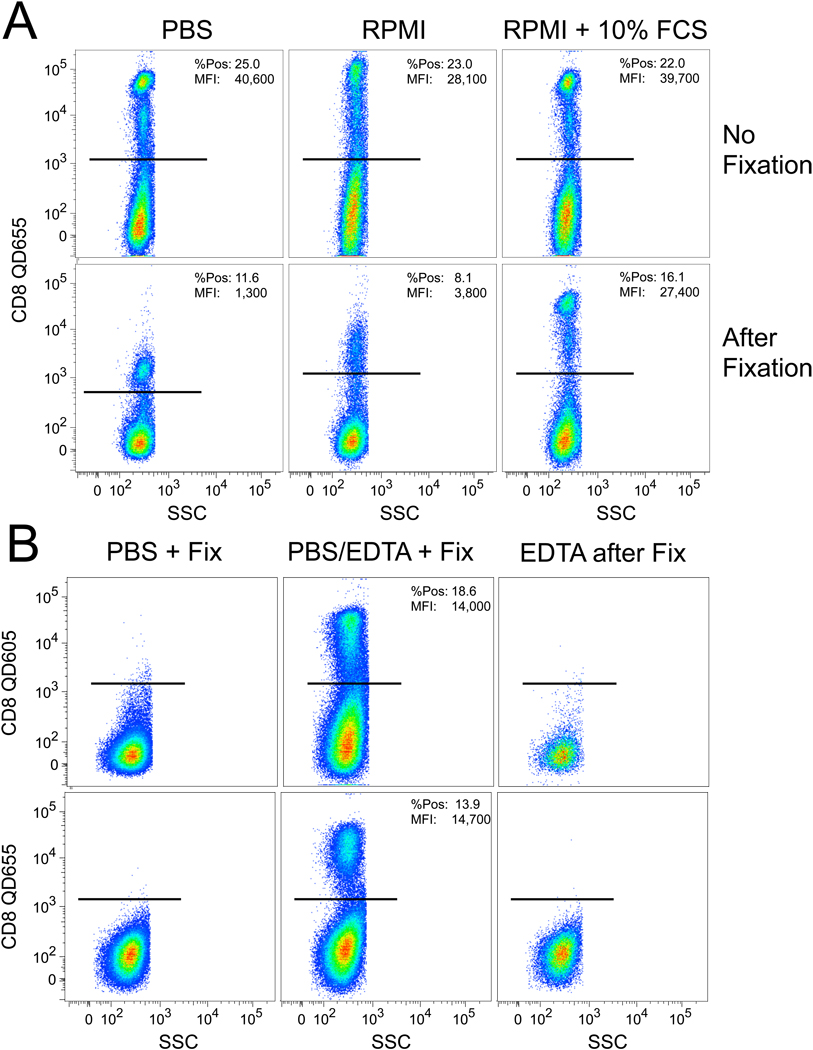
As shown in Fig 2A, the use of different staining media in the absence of fixation did not affect QD fluorescence. However, after adding 1% formalin from lot “A” we observed a dramatic loss of QD fluorescence in the samples stained with PBS or RPMI but not with the R10 medium. This suggests that the contaminant in the formalin quenched the QD fluorescence in a manner that was inhibited by the additional serum present in R10 medium; certainly, there could be sufficient metal-binding proteins in serum to chelate the contaminant. Finally, as shown in Fig 2B, addition of 1 mM EDTA could inhibit the effect of the contaminant in the formalin – but only if the EDTA was present concurrent with the formalin. Adding EDTA subsequent to the formalin did not reverse the inhibition of fluorescence. These data show that the contaminant was likely a chelatable heavy metal cation that, once intercalated into the QD, could not be removed. Finally, Fig 2B illustrates that the elimination of fluorescence occurs equally well with another QD reagent, QD605.
Figure 2. Different media can alter the QD fluorescence quenching by the contaminated fixative.
(A) Separate tubes were washed and stained in the specified media, and analyzed unfixed immediately after staining. The remaining cells in each tube were then fixed in 1% formalin (lot “A” from Figure 1) and then analyzed. (B) 1mM of EDTA was included at various time points of the staining protocol in order to examine if the chelating agent could prevent loss of QD fluorescence. In this experiment, cells were stained either with QD655 or QD605 conjugated to anti-CD8.
In order to determine which heavy metals might be the source of the inhibitory activity, mass spectrometric analysis of the different lots of fixative were performed to quantify a number of possible cations. As shown in Table 1, the analysis revealed measurable levels of copper and iron present in lot “A”, suggesting that one of these is likely the root cause.
Table 1.
Mass spectrographic quantification of total copper and iron in staining solutions
| Sample | Total Copper | Total Iron | ||
|---|---|---|---|---|
| Concentration (nM) | Uncertainty (nM) | Concentration (nM) | Uncertainty (nM) | |
| PBS in Water | 20.5 | 7.9 | 462. | 140. |
| Formalin + PBS (“A”, lot #070806) | 258. | 11.0 | 634. | 66.0 |
| Formalin + PBS (“B”, lot #080822) | 33.0 | 7.9 | 337.0 | 72.0 |
Note: Total metal concentrations include all ionic forms. Lot “A” and “B” refer to the designations shown in Figure 1.
To determine the precise effect of various metal cations, compensation beads were stained with CD8 QD655 and treated with various levels of several heavy metal cations: Zn+2, Fe+2, Fe+3 and Cu+2 (Fig 3). As shown, all but ferrous ions could inhibit fluorescence at some concentration below 1 mM, although ferrous and zinc ions inhibited at much higher concentrations, i.e., 0.1 and 0.5 mM respectively. Notably, cupric ions showed a half-maximal inhibitory concentration that is very low, 20 nM.
Figure 3. Concentration-dependent inhibition of QD fluorescence by heavy metal cations.
Compensation beads stained with CD8 QD655 were incubated with a range of concentrations of the indicated cations. The relative fluorescence (compared to absence of cations) is shown.
In other experiments, we found that 30 µM cupric ions can eliminate the fluorescence of at least six different QDs, including QD545, QD565, QD605, QD655, QD705, and QD800 (data not shown).
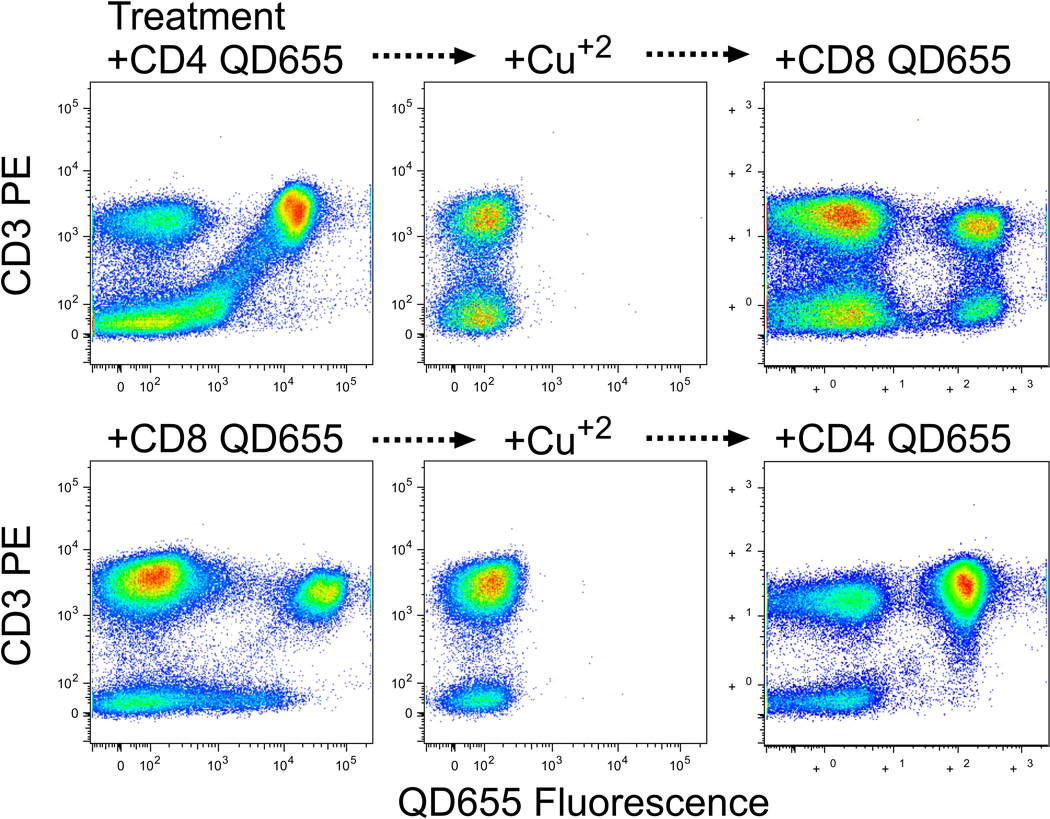
Finally, we demonstrate that this property of QD can be used to re-stain cells with different reagents of the same fluorescence. In Fig 4, fluorescence distributions are shown for PBMC that were stained with QD655 conjugated to anti-CD4 or anti-CD8. After treatment with cupric sulfate, the cells are rendered completely non-fluorescent for the QD reagents (with the antibodies presumably still bound). After thorough washing with 1 mM EDTA, the cells were successfully re-stained with QD655 conjugated to the other antibody. We observed slightly less staining in the second step compared to the first step, which may be attributable to incomplete removal of the cupric sulfate.
Figure 4. Potential utility of the elimination of fluorescence.
PBMC were stained with PE-anti-CD3 and QD655 conjugated to either anti-CD4 (top) or anti-CD8 (bottom). After analysis (left), cells were treated for 30 min with 30 µM cupric sulfate and reanalyzed (middle). Finally, cells were incubated for 30 min with 1 mM EDTA and washed three times, then re-stained with QD655 conjugated to either anti-CD8 (top) or anti-CD4 (bottom).
Discussion
Anecdotal reports of variable performance of QD have been related to us over the years, both from outside laboratories and from our own laboratories. We had been unable to pin down precise conditions leading to this problem until recently; one clue was an observation by one of our researchers that there was much better QD fluorescence in a given experiment for those PBMC samples with more contaminating erythrocytes. We reasoned that a low level contaminant was being removed by the hemoglobin (or other proteins) released by lysed red blood cells.
Various heavy metal cations have been shown to impair QD fluorescence, presumably through their incorporation into the nanocrystal (2–6). As we show here, the QD commonly used for conjugating monoclonal antibodies are susceptible to this. The inhibitory concentrations of most metal cations are too high to be a concern as contaminants in typical staining solutions. However, very low levels of cupric ions can completely quench QD fluorescence, and may be below typical quality control criteria.
The origin of the contaminating copper ions has not been determined. It is possible that it may have arisen from the container that the fixative is supplied in (the dark-colored plastic may contain copper); it is also possible that it is carry-over from the manufacturing stage (notably, both copper and iron are used in the manufacture of formalin). We routinely test all lots of reagents now for this inhibitory activity; it is only rare lots that we must discard.
We found that addition of 1 mM EDTA could completely inhibit the deleterious effect of the contaminating copper. Inclusion of 1 mM EDTA into all staining media could represent one solution to avoid the QD quenching problem. However, we found minor quenching of QD fluorescence (<2-fold) with EDTA as well as a minor decrease in cell viability in some treated samples. Thus, we prefer to simply test all media and solutions that will be used with QD staining to ascertain if they show this activity, and to discard them (or add EDTA only to that solution) if so. Any media or reagent containing formaldehyde should be quality controlled using compensation beads stained with QD antibody conjugates. It should be kept in mind that some “fix/perm” reagents are typically not included in compensation tube staining but only for cell samples; these reagents must also be tested.
This property of QD may be exploited in experiments. In some cases, it may be useful to completely eliminate the cell-associate fluorescence after analysis. Incubation with 30 µM cupric sulfate accomplishes this, leaving cells as non-fluorescent as unstained cells. These cells can then be re-stained with other QD reagents for further analysis. This might be useful in certain experimental setups (for example, adoptive transfer of stained, sorted cells), in which subsequent analysis requires no contaminating fluorescence from the first stain.
In summary, we demonstrate that low levels of certain heavy metal cations can permanently and completely eliminate QD fluorescence. While this can be used productively in some experimental situations, in most cases it is unwanted. The problem can be avoided by careful assessment of solutions to ensure lack of contaminating metals, or eliminated by inclusion of 1 mM EDTA during and following all QD staining steps.
Acknowledgments
We thank the members of the Vaccine Research Center laboratories for their diligent reporting and analysis of sporadic staining issues with QD that led us to our conclusions, and Joanne Yu for manufacturing and qualifying custom conjugates used in our research. This work was supported by the Intramural Research Program of the National Institute for Allergy and Infectious Diseases of the National Institutes of Health.
References
- 1.Chattopadhyay PK, Price DA, Harper TF, Betts MR, Yu J, Gostick E, Perfetto SP, Goepfert P, Koup RA, De Rosa SC, et al. Quantum dot semiconductor nanocrystals for immunophenotyping by polychromatic flow cytometry. Nat Med. 2006;12(8):972–977. doi: 10.1038/nm1371. [DOI] [PubMed] [Google Scholar]
- 2.Chen Y, Rosenzweig Z. Luminescent CdS quantum dots as selective ion probes. Anal Chem. 2002;74(19):5132–5138. doi: 10.1021/ac0258251. [DOI] [PubMed] [Google Scholar]
- 3.Frigoli M, Ouadahi K, Larpent C. A cascade FRET-mediated ratiometric sensor for Cu2+ ions based on dual fluorescent ligand-coated polymer nanoparticles. Chemistry. 2009;15(33):8319–8330. doi: 10.1002/chem.200900475. [DOI] [PubMed] [Google Scholar]
- 4.GouanvÈ F, Schuster T, Allard E, MÈallet-Renault R, Larpent C. Fluorescence Quenching upon Binding of Copper Ions in Dye-Doped and Ligand-Capped Polymer Nanoparticles: A Simple Way to Probe the Dye Accessibility in Nano-Sized Templates. Advanced Functional Materials. 2007;17(15):2746–2756. [Google Scholar]
- 5.Meallet-Renault R, Herault A, Vachon JJ, Pansu RB, Amigoni-Gerbier S, Larpent C. Fluorescent nanoparticles as selective Cu(II) sensors. Photochem Photobiol Sci. 2006;5(3):300–310. doi: 10.1039/b513215k. [DOI] [PubMed] [Google Scholar]
- 6.Meallet-Renault R, Pansu R, Amigoni-Gerbier S, Larpent C. Metal-chelating nanoparticles as selective fluorescent sensor for Cu2+ Chem Commun (Camb) 2004;(20):2344–2345. doi: 10.1039/b407766k. [DOI] [PubMed] [Google Scholar]