Abstract
Amyloid cascades leading to peptide β-sheet fibrils and plaques are central to many important diseases. Recently, intermediate assemblies of these cascades were identified as the toxic agents that interact with the cellular machinery. The location and cause of the transformation from natively unstructured assembly to the beta-sheet oligomers found in all fibrils is important in understanding disease onset and the development of therapeutic agents. Research on this early oligomeric region has largely been unsuccessful since all traditional techniques measure only ensemble average oligomer properties. Here, ion mobility methods are utilized to deduce the peptide self-assembly mechanism. We look at a series of amyloid forming peptides clipped from larger peptides or proteins associated with disease. We provide unambiguous evidence for structural transitions in each of these fibril forming peptide systems establishing the potential of this method for the development of therapeutic agents and drug evaluation.
Highly organized tubular and fibrillar assemblies have been characterized for a number of organic1,2, bioorganic 3,4 and biological materials 3,5-11. Of particular interest are the fibrillar assemblies based on the generic cross-β spine reported for peptides and proteins with a high degree of variability in sequence, structure, and function 8-10,12,13. Despite extensive knowledge about the insoluble, mature peptide fibril 3,9,10,12,14,15, the principles governing self-assembly of soluble oligomers have yet to be addressed 13,16-20. This substantial gap in understanding takes on increased importance since soluble, prefibrillar peptide oligomers are the primary pathogenic species implicated in amyloid diseases including Alzheimer’s and Diabetes Type 2 13,17-19,21-26. Understanding the fundamental forces that relate aggregation, morphology and biochemistry of soluble peptide aggregates is central to developing diagnostic and therapeutic strategies for amyloid diseases 15,26. So far, these processes have resisted detailed characterization due to their transient and dynamic nature. Of key relevance to disease pathogenesis13,17,19-21,23-25 is a postulated transition of soluble oligomers from globular conformations into β-strand structures 13,17,19,20,23,26-28, which has never been directly observed. Mass spectrometry has been widely applied to the formation of non-covalent complexes including peptide/protein aggregation29-35. However, here we present the first observation of the globular – β-sheet transformation in an aggregating peptide system using ion mobility spectrometry - mass spectrometry (IMS-MS) 23,27,36-39. This is achieved by successively mass-extracting a specific aggregation state from a distribution of oligomers and subsequent determination of its collision cross section, enabling us to deduce the peptide self-assembly mechanism (see Fig.1). Our results show transitions of early, soluble peptide oligomers from a globular to a fibrillar conformation, including the “steric zippers” 9,10,14 motif of two interdigitated β-strands. Atomic force microscopy (AFM) and x-ray data of macroscopic aggregates clearly support these findings on the early oligomers, demonstrating that IMS-MS is capable of elucidating the structural processes involved during onset of amyloid formation. IMS-MS therefore has the potential to open new avenues for investigating the pathogenic mechanisms of amyloid diseases, their early diagnosis, and eventual treatment.
Figure 1. Processes proposed to occur during the soluble stage of peptide self-assembly in the current amyloid formation paradigm.
(a) Peptide monomers self-assemble into mature, insoluble β -sheet fibrils via an intermediate phase of soluble oligomers. A pronounced transition of soluble oligomers from globular conformations into β -strand structures occurs during this phase. Soluble oligomers are the primary cytotoxic species implicated in amyloid diseases. (b) Self-assembly starts at the folded monomer (left) and proceeds to soluble peptide assemblies of increasing mass (right). Soluble peptide oligomers with identical mass (i.e. number of monomer units n) can assume different conformations such as globular (bottom) or β-strand conformations (top) with different collision cross sections. Successively mass-extracting a specific aggregation state from the solution-phase distribution and subsequent determination of its collision cross section reveals the self-assembly pathway taking place in solution (see arrow).
Results and Discussion
The peptides studied include H-Asn-Asn-Gln-Gln-Asn-Tyr-OH (NNQQNY), H-Val-Glu-Ala-Leu-Tyr-Leu-O H (VEALYL), and H-Ser-Ser-Thr-Asn-Val-Gly-OH (SSTNVG), important segments from amyloid forming yeast prion protein Sup35, human insulin and human islet amyloid polypeptide (IAPP, diabetes type 2), respectively. Fibril structures for NNQQNY, VEALYL, and SSTNVG were recently reported by Eisenberg and co-workers at atomic resolution 9,10,14. For comparison we include the hormone Leu-Enkephalin (H-Tyr-Gly-Gly-Phe-Leu-OH, YGGFL), which forms an isotropic crystal lattice instead of amyloid fibrils 40,41.
All peptides show pronounced oligomerization under the IMS-MS conditions employed. Typical mass spectra for YGGFL and VEALYL are shown in Fig. 2, and those for NNQQNY and SSTNVG in Supplementary Fig. S7. The mass spectrum of VEALYL shows formation of oligomers up to the tetradecamer (n=14) which can be unambiguously identified based on their mass-to-charge (m/z) ratio. The singly protonated momoner (oligomer number n = 1, charge z = 1, m/z = 707.4) is the most abundant peak. However, larger oligomers, such as the triply protonated pentamer (n/z=5/3; m/z = 1178.3) or the triply protonated heptamer (n/z=7/3; m/z = 1649.3), are of significant abundance. For YGGFL the largest oligomer identified unambiguously is the six-fold protonated nonadecamer (n/z = 19/6; m/z = 1759.4). Mass-selected arrival time distributions (ATDs) (see Supplementary Figs. S8, S9, S11 and S14) allow the determination of accurate collision cross sections for each of these species and thus provide detailed structural information on the individual aggregation states. Mass spectra and collision cross sections where obtained over the time course of several weeks, and are consistent with a rapidly established steady-state among the soluble peptide oligomers (see section S5 in the Supplementary Information).
Figure 2. Peptide aggregation observed by IMS-MS for the peptides YGGFL (a) and VEALYL (b).
ESI-Q mass spectra (positive ion mode) show extensive aggregation for the peptides YGGFL (a) and VEALYL (b). The peaks are annotated by the number of monomers n over the charge z (n/z). Large oligomers (n/z=19/6; n/z=11/5) can be unambiguously identified in the spectra.
Aliquots for AFM imaging (see Fig. 3 and Supplementary Figs. S3-S6) were taken from the same samples that were used for IMS-MS analysis and imaged as described in the Methods section. AFM images reveal the existence of macroscopic peptide fibrils for NNQQNY (Supplementary Fig. S4), VEALYL (Fig. 3b and Supplementary Fig. S5) and SSTNVG (Supplementary Fig. S6). This is in line with previous reports by Eisenberg and co-workers on the same peptides 9,10,14. Under the conditions applied in the current work, exclusive formation of fibril structures is observed for VEALYL only, while for NNQQNY and SSTNVG macroscopic granular aggregates are observed in addition to peptide fibrils (Supplementary Figs. S5 and S6). The hormone YGGFL exclusively forms granular structures and no fibrils (Figs. 3b and Supplementary Fig. S6), in line with previous reports based on x-ray diffraction methods 40,41. These results indicate a significant conformational transition from isotropic oligomers to β-strand structures (see Fig.1) among soluble oligomers of the three fibril forming peptides peptides NNQQNY, VEALYL, SSTNVG, but not YGGFL under the conditions applied in the current work.
Figure 3. AFM imaging for the peptides YGGFL (a) and VEALYL (b).
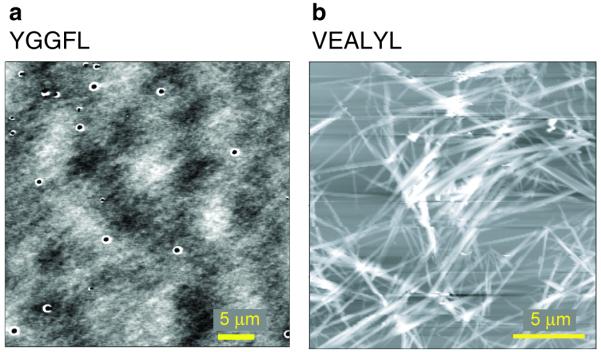
Aliquots taken from the same samples used to obtain the mass spectra were imaged by AFM. For YGGFL (a) isotropic aggregates are exclusively observed, while VEALYL (b) shows exclusive formation of fibril structures.
While granular aggregates ultimately result from spatially isotropic growth, fibril structures imply a spatially strongly directed self-assembly of soluble oligomers. These two modes of self-assembly reveal significantly different signatures when the structural evolution of peptide oligomers is determined by IMS-MS (Fig.1b, Supplementary Information section S1) .
In isotropic growth the collision cross section σ is related to the oligomeric state n by , where σmon is the monomer cross section. This type of self-assembly is easily distinguished from idealized fiber assembly. As oligomers grow specifically in one direction the collision cross section increases linearly with oligomer number (σ = a · n + k, see Supplementary Information section S1.2). Here, the slope a describes the fibril shell area per monomer unit and k is related to the fibril base given by the fibril diameter, yielding detailed physical parameters of the fibril growing in solution. For instance, the base area (given by k) of a growing double stranded fibril (“steric zipper” 9,10) would be approximately twice that of a single strand.
Fig.4a depicts the structural evolution of YGGFL oligomers as a function of the oligomer number n from n = 1 to n = 17 deduced from IMS-MS experiments. In these experiments, oligomers from a YGGFL sample solution are electrosprayed, mass-selected, and characterized by their collision cross sections measured in a drift tube filled with helium. Only one major conformational family is observed for each YGGFL oligomer. The YGGFL collision cross sections (Supplementary Information section S4.3) are in excellent agreement with fully isotropic self-assembly. Consequently, the self-assembly mechanism (Fig.5) is consistent with formation of granular, isotropic insoluble macroscopic aggregates. AFM imaging of aliquots taken from the same sample that was used for IMS-MS analysis shows exclusive formation of granular macroscopic aggregates for YGGFL (Fig. 3), consistent with isotropic assembly in solution. This observation is further supported by the excellent agreement of the measured monomer collision cross section of YGGFL (160 Å2) with that obtained for the x-ray geometry (162 Å2) 40.
Figure 4. Measured collision cross sections as a function of the oligomer number n for YGGFL (a), NNQQNY (b), VEALYL (c) and SSTNVG (d).
Dominant features in each arrival time distribution (ATD) are given as dark red squares, minor features as transparent squares. Lines and symbols for all panels are defined in panel d. (a) YGGFL self-assembles isotropically with cross sections increasing as n2/3 (green line). (b) NNQQNY follows an isotropic assembly up to the octamer. Fibril-like β-sheet conformations emerge at the nonamer, and become prevalent at the nonadecamer. (c) VEALYL grows as a single fibril from the dimer to the pentamer (solid line) and then continues to grow as a dimeric β-sheet “steric zipper” 9. d) SSTNVG oligomers grow isotropically over the entire range investigated. However, minor features in good agreement with dimeric β-sheet “steric zipper” growth (dashed line) emerge at the undecamer. We note a very good agreement between the physical dimensions of fibrils measured by IMS-MS and those deduced from x-ray studies for b), c) and d).
Figure 5. Proposed self-assembly pathways of YGGFL, NNQQNY, VEALYL, and SSTNVG.
Aggregation is fully isotropic for all oligomeric states observed of YGGFL. For NNQQNY self-assembly initially proceeds via spatially isotropic clusters until ca. the nonamer (n = 9). Between n = 9 and n = 12, oligomers with single-strand fibril structures coexist with isotropic peptide clusters. Fibrillar β-sheet structures become prevalent for n > 16, indicative of a conformational transition from globular to β-sheet fibrillar oligomers. VEALYL exhibits growth as a single fiber strand from n = 2 to n = 5, and from n = 5 onwards is found to self-assemble via a “steric zipper” pathway. SSTNVG self-assembles as orientationally isotropic compact oligomers over all oligomeric states examined. However, “steric zippers” emerge for n > 11.
The structural evolution of YGGFL oligomers is in strong contrast with that observed for NNQQNY (Fig.4b and Supplementary Information section S4.4). Here, the lower curve is that expected for isotropic growth as observed for YGGFL, and the upper straight line represents single-strand fibril growth deduced from the x-ray data of Eisenberg and coworkers 9,10. Clearly, oligomer growth proceeds isotropically up to about the nonamer; larger structures then emerge which cannot be explained by isotropic self-assembly. The presence of multiple conformations for one particular oligomer size is evident by IMS as multiple peaks in the ATD (see Supplementary Information section S4). Species with a large cross section arrive at the detector later due to a longer drift time in the IMS tube. The most extended conformation of a particular oligomer is commonly significantly larger (e.g. ca. 20% for n = 12 and ca. 40% for n = 16) than the corresponding isotropic one (green line) and better agreement with single strand fiber growth for these more extended oligomers is observed. These fibril-like structures continue to compete with isotropic assemblies, until at the nonadecamer and eicosamer only a single fibril-like oligomer conformation remains. Both isotropic as well as fibrillar macroscopic aggregates are observed by AFM of the same sample (Supplementary Fig. S4). This suggests that the structural polymorphism revealed in Fig.4b persists for larger oligomers. IMS-MS analysis yields a fiber base of 122 Å2 and a shell per monomer unit of 90 Å2, respectively (Supplementary Information section S4.4). These physical dimensions of the fibril growing in solution observed by IMS-MS are in very good agreement with those for single-strand fibrils deduced from available x-ray structures (base and shell of 130 and 99 Å2, respectively) 9,10. The IMS-MS data in Fig.4b thus presents the first unequivocal evidence of globular peptide oligomers converting into peptide fibrils rich in β-sheet content. This type of transition from a globular to a β-strand conformation of peptide oligomers has been strongly implicated in a number of amyloid systems 13,14,16,17,19-21,23-28 but has never been directly observed. The difficulty has been in isolating oligomers as a function of size and determining their structures using traditional methods due to their transient and dynamic nature 16. The IMS-MS technique 23,27,37,38 used here is the first to allow explicit examination of the onset of fibril formation. A summary of the NNQQNY oligomerization mechanism is given in Fig.5.
The collision cross sections of VEALYL oligomers in Fig.4c reveal two distinct regions with linear growth. One segment extends from n = 2 to n = 5 and the second from n = 5 to n = 14, the largest oligomer for which we have reliable cross sections (Supplementary Information section S4.5). Significant differences are observed in the fibril shells (91 and 62 Å2) and end caps (118 and 265 Å2) of the two segments, indicating that the physical dimensions of the growing fibrils are clearly different in the two regions. It is of note that the physical dimensions of the first segment from n = 2 to n = 5 (shell = 91 Å2, cap = 118 Å2) are in close agreement to those observed for the single strand fiber growth observed for NNQQNY from n = 10 to n = 20 (shell = 90 Å2, cap = 122 Å2). As single fiber strands from two hexapeptides should have similar physical dimensions, this result suggests that the early oligomers of VEALYL from the dimer to the pentamer grow as single-strand fibers. However, more important is the fact that the fiber end cap of the second segment from n = 5 to n = 14 (265 Å2) is about twice that of the single-strand fiber growth observed in the first (118 Å2). This clearly indicates a significant structural rearrangement of the growing fiber occurring at the pentamer, with the resulting fiber having about twice the diameter of the initial one, consistent with a “steric zipper” for this segment. From these observations, we therefore conclude that single strand fibril growth occurs initially from the dimer to the pentamer, where a structural rearrangement to a growing “steric zipper” occurs. A summary of the VEALYL oligomerization mechanism is given in Fig.5. Eisenberg’s x-ray data 9 yield fiber shells of 96 and 71 Å2 and fibril end caps of 118 and 265 Å2 for the single- and double-strand fiber growth, respectively, in very good numerical agreement with our IMS results (91 and 62 Å2 and 118 and 265 Å2, respectively). This agreement provides strong support for the self-assembly mechanism depicted for VEALYL in Fig.5. It is also of importance that only fibers – and no granular macroscopic aggregates – are observed by AFM (see Fig.3) of the same sample used for IMS-MS, consistent with this mechanism.
The cross section data of SSTNVG are given in Fig.4d as are the three limiting model predictions. Only a single family of structures is observed until n = 12 at which point weak new families emerge. The dominant family closely follows the isotropic growth pattern through n = 14 but the weaker families approach the steric zipper line beginning at n = 12. This process is schematically shown in Fig.5. Fibrils are formed from our experimental solution, however, as indicated by AFM. These fibril structures coexist with macroscopic granular aggregates (Supplementary Fig.S6).
On the basis of the collision cross section data (Fig.4) for YGGFL, NNQQNY, VEALYL and SSTNVG we conclude that highly significant differences are observed in the soluble self-assembly phase of all peptides, but especially between the fibril (NNQQNY, VEALYL and SSTNVG) and the crystal lattice forming (YGGFL) classes of peptides. YGGFL self-assembles isotropically, while this growth mechanism is observed only for the earlier oligomeric states of the fibril forming peptides. For these latter peptides fibrillar cooperative β-sheets prevail for large oligomers. Fibril growth in a β-sheet conformation is observed both as a single-strand fiber (NNQQNY, VEALYL) as well as in the two-strand fiber “steric zipper” model (VEALYL, SSTNVG) proposed by Eisenberg 9,10. Also of importance is the fact that NNQQNY undergoes a transition from a natively unstructured assembly to a highly ordered β-sheet assembly beginning near the ocatamer. While this type of transition has long been postulated in amyloid peptides and proteins 13,14,16,17,19-21,23-28, here we present the first unambiguous observation of this phenomenon. Of direct relevance to amyloid disease research is the observation of conformational transitions to β-sheet prevalent peptide assemblies before the formation of the eicosamer, because this is the regime of soluble peptide oligomers in which conformational polymorphism has been implicated with cytotoxicity 26. The cross section data presented in Fig.4 for oligomers are well correlated with the AFM data on macroscopic assemblies obtained from the same samples that were used for IMS-MS analysis. The self-assembly mechanism of YGGFL observed by IMS-MS is consistent with the AFM data in that no peptide fibrils and exclusively granular macroscopic aggregates are formed. However, the cross section data for NNQQNY, and especially SSTNVG, indicate both fibrils and spatially isotropic (i.e. granular) macroscopic aggregates may coexist for these systems. These observations are completely consistent with the corresponding AFM data. At the same time AFM imaging shows that no granular structures are formed by VEALYL, again confirming the self-assembly mechanism observed by IMS-MS for this peptide. This strong correlation is further highlighted by the almost exact agreement with x-ray data obtained by Eisenberg and coworkers 9,10 for the physical dimensions of the growing fibrils of NNQQNY, VEALYL and SSTNVG.
We conclude that the IMS-MS technique enables us to study the structural evolution of soluble peptide oligomers one monomer at a time. IMS-MS analysis of peptide self-assembly thus opens new avenues for the investigation, detection and eventual treatment of pathogenic processes in amyloid diseases implicated by soluble peptide or protein oligomers.
Methods
A full description of methods is given in Supplementary Information. Briefly, for IMS-MS samples were dissolved in Water:Methanol to the desired concentration (100μM - 1mM) and loaded into gold coated nanoESI capillaries and electrosprayed on home-built instruments. Ions are focused, stored in an ion funnel and pulsed into a drift tube filled with 13 torr of He. They are pulled through the drift tube under the influence of a weak electric field. At the end of the drift tube ions of a particular oligomeric state are mass selected and their arrival time distribution (ATD) is recorded. The arrival time is related to the collision cross-section of the ion (see Supplementary Information). Theoretical cross sections were computed as described in the Supplementary Information. For AFM images aliquots of the same peptide sample solutions were drop cast onto freshly cleaved mica slides and imaged on a MFP-3D-BIO instrument (AsylumResearch, Santa Barbara).
Supplementary Material
Acknowledgements
This research was supported by the National Science Foundation and the National Institutes of Health. A prototype Synapt instrument was graciously provided by the Waters Corporation. C. B. is grateful to the Alexander-von-Humboldt-Foundation for a Feodor-Lynen-Fellowship. The authors are grateful to James O’Dea for obtaining the AFM images. We thank R. Gleiter and D.B. Werz for useful discussions.
References
- 1.Gleiter R, Werz DB, Rausch BJ. A world beyond hydrogen bonds? - Chalcogen-Chalcogen interactions yielding tubular structures. Chem. Eur. J. 2003;9:2676–2683. doi: 10.1002/chem.200204684. [DOI] [PubMed] [Google Scholar]
- 2.Bong DT, Clark TD, Granja JR, Ghadiri MR. Self-Assembling Organic Nanotubes. Angew. Chem. Int. Ed. 2001;40:988–1011. [PubMed] [Google Scholar]
- 3.Hamley IW. Peptide fibrillization. Angew. Chem. Int. Ed. 2007;46:8128–8147. doi: 10.1002/anie.200700861. [DOI] [PubMed] [Google Scholar]
- 4.Couet J, Samuel JD, Kopyshev A, Santer S, Biesalski M. Peptide-Polymer Hybrid Nanotubes. Angew. Chem. Int. Ed. 2005;44:3297–3301. doi: 10.1002/anie.200462993. [DOI] [PubMed] [Google Scholar]
- 5.Unwin N. Acetylcholine receptor channel imaged in the open state. Nature. 1995;373:37–43. doi: 10.1038/373037a0. [DOI] [PubMed] [Google Scholar]
- 6.Ghadiri MR, Granja JR, Milligan RA, McRee DE, Khazanovich N. Self-assembling organic nanotubes based on a cyclic peptide architecture. Nature. 1993;366:324–327. doi: 10.1038/366324a0. [DOI] [PubMed] [Google Scholar]
- 7.Lear JD, Wasserman ZR, DeGrado WF. Synthetic amphiphilic peptide models for protein ion channels. Science. 1988;240:1177–1181. doi: 10.1126/science.2453923. [DOI] [PubMed] [Google Scholar]
- 8.Fowler DM, Koulov AV, Balch WE, Kelly JW. Functional amyloid – from bacteria to humans. Trends Biochem. Sci. 2007;32:217–224. doi: 10.1016/j.tibs.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 9.Sawaya MR, et al. Atomic structures of amyloid cross-β spines reveal varied steric zippers. Nature. 2007;447:453–457. doi: 10.1038/nature05695. [DOI] [PubMed] [Google Scholar]
- 10.Nelson R, et al. Structure of the cross-β spine of amyloid-like fibrils. Nature. 2005;435:773–778. doi: 10.1038/nature03680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hartgerink JD, Clark TD, Ghadiri MR. Peptide nanotubes and beyond. Chem. Eur. J. 1998;4:1367–1372. [Google Scholar]
- 12.Sipe JD, Cohen AS. Review: History of the Amyloid Fibril. J. Struct. Biol. 2000;130:88–98. doi: 10.1006/jsbi.2000.4221. [DOI] [PubMed] [Google Scholar]
- 13.Chiti F, Dobson CM. Amyloid formation by globular proteins under native conditions. Nat. Chem. Biol. 2008;5:15–21. doi: 10.1038/nchembio.131. [DOI] [PubMed] [Google Scholar]
- 14.Nelson R, Eisenberg D. Recent atomic models of amyloid fibril structure. Curr. Opin. Struct. Biol. 2006;16:260–265. doi: 10.1016/j.sbi.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 15.Luhrs T, et al. 3D structure of Alzheimer’s amyloid-beta(1-42) fibrils. Proc. Natl. Acad. Sci. USA. 2005;102(48):17342–17347. doi: 10.1073/pnas.0506723102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morris AM, Watzky MA, Finke RG. Protein aggregation kinetics, mechanism, and curve-fitting: A review of the literature. Biochim. Biophys. Acta. 2009;1794:375–397. doi: 10.1016/j.bbapap.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 17.Eisenberg D, et al. The structural biology of protein aggregation diseases: Fundamental questions and some answers. Acc. Chem. Res. 2006;39(9):568–575. doi: 10.1021/ar0500618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid β-peptide. Nat. Rev. Mol. Cell Biol. 2007;8:101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- 19.Ehrnhoefer DE, et al. EGCG redirects amyloidogenic polypeptides into unstructured, off-pathway oligomers. Nat. Struct. Bio. 2008;15:558–566. doi: 10.1038/nsmb.1437. [DOI] [PubMed] [Google Scholar]
- 20.Kodali R, Wetzel R. Polymorphism in the intermediates and products of amyloid assembly. Curr. Opin. Struct. Biol. 2007;17:48–57. doi: 10.1016/j.sbi.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 21.Lambert MP, et al. Diffusible, nonfibrillar ligands derived from Aβ1-42 are potent central nervous system neurotoxins. Proc. Natl. Acad. Sci. USA. 1998;95:6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kirkitadze MD, Bitan G, Teplow DB. Paradigm shifts in Alzheimer’s disease and other neurodegenerative disorders: The emerging role of oligomeric assemblies. J. Neurosci. Res. 2002;69:567–577. doi: 10.1002/jnr.10328. [DOI] [PubMed] [Google Scholar]
- 23.Bernstein SL, et al. Amyloid-β protein oligomerization and the importance of tetramers and dodecamers in the aetiology of Alzheimer’s disease. Nat. Chem. 2009;1:326–331. doi: 10.1038/nchem.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kayed R, et al. Common Structure of Soluble Amyloid Oligomers Implies Common Mechanism of Pathogenesis. Science. 2003;300:486–489. doi: 10.1126/science.1079469. [DOI] [PubMed] [Google Scholar]
- 25.Bucciantini M, et al. Inherent toxicity of aggregates implies a common mechanism for protein misfolding diseases. Nature. 2002;416:507–511. doi: 10.1038/416507a. [DOI] [PubMed] [Google Scholar]
- 26.Glabe CG. Conformation-dependent antibodies target diseases of protein misfolding. Trends Biochem. Sci. 2004;29:542–547. doi: 10.1016/j.tibs.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 27.Dupuis NF, Wu C, Shea JE, Bowers MT. Human Islet Amyloid Polypeptide Monomers Form Ordered β-hairpins: A Possible Amyloidogenic Conformation. J. Am. Chem. Soc. 2009;131:18283–18292. doi: 10.1021/ja903814q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kayed R, et al. Fibril specific, conformation dependent antibodies recognize a generic epitope common to amyloid fibrils and fibrillar oligomers that is absent in prefibrillar oligomers. Mol. Neurodegen. 2007;2(18) doi: 10.1186/1750-1326-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Larson JL, Ko E, Miranker AD. Direct measurement of islet amyloid polypeptide fibrillogenesis by mass spectrometry. Protein Sci. 2000;9(2):427–431. doi: 10.1110/ps.9.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith RD, Lightwahl KJ, Winger BE, Loo JA. Preservation of Noncovalent Associations in Electrospray Ionization Mass-Spectrometry - Multiply Charged Polypeptide and Protein Dimers. Org Mass Spectrom. 1992;27(7):811–821. [Google Scholar]
- 31.Lightwahl KJ, Schwartz BL, Smith RD. Observation of the Noncovalent Quaternary Associations of Proteins by Electrospray-Ionization Mass-Spectrometry. J. Am. Chem. Soc. 1994;116(12):5271–5278. [Google Scholar]
- 32.Smith RD, Lightwahl KJ. The Observation of Noncovalent Interactions in Solution by Electrospray-Ionization Mass-Spectrometry - Promise, Pitfalls and Prognosis. Biol Mass Spectrom. 1993;22(9):493–501. [Google Scholar]
- 33.Nettleton EJ, et al. Characterization of the oligomeric states of insulin in self-assembly and amyloid formation by mass spectrometry. Biophys. J. 2000;79:1053–1065. doi: 10.1016/S0006-3495(00)76359-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caddy GL, Robinson CV. Insights into amyloid fibril formation from mass spectrometry. Protein Pept. Lett. 2006;13:255–260. doi: 10.2174/092986606775338416. [DOI] [PubMed] [Google Scholar]
- 35.Loo JA. Studying noncovalent protein complexes by electrospray ionization mass spectrometry. Mass Spectrom Rev. 1997;16(1):1–23. doi: 10.1002/(SICI)1098-2787(1997)16:1<1::AID-MAS1>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 36.von Helden G, Gotts NG, Bowers MT. Experimental Evidence for the Formation of Fullerenes by Collisional Heating of Carbon Rings in the Gas Phase. Nature. 1993;363:60–63. [Google Scholar]
- 37.von Helden G, Wyttenbach T, Bowers MT. Conformation of Macromolecules in the Gas Phase: Use of Matrix-Assisted Laser Desorption Methods in Ion Chromatography. Science. 1995;267:1483–1485. doi: 10.1126/science.267.5203.1483. [DOI] [PubMed] [Google Scholar]
- 38.Bowers MT, Kemper PR, von Helden G, van Koppen PAM. Gas-Phase Ion Chromatography - Transition-Metal State Selection and Carbon Cluster Formation. Science. 1993;260(5113):1446–1451. doi: 10.1126/science.260.5113.1446. [DOI] [PubMed] [Google Scholar]
- 39.Ruotolo BT, et al. Evidence for Macromolecular Protein Rings in the absence of bulk water. Science. 2005;310:1658–1661. doi: 10.1126/science.1120177. [DOI] [PubMed] [Google Scholar]
- 40.Smith GD, Griffin JF. Conformation of [Leu5] Enkephalin from X-ray Diffraction: Features Important for Recognition at Opiate Receptor. Science. 1978;199:1214–1216. doi: 10.1126/science.204006. [DOI] [PubMed] [Google Scholar]
- 41.Deschamps JR, George C, Flippen-Anderson JL. Structural studies of opioid peptides: A review of recent progress in x-ray diffraction studies. Biopolymers. 1996;40:121–139. doi: 10.1002/bip.360400102. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.






