Abstract
1 Cytochrome P450 2B4 is a microsomal protein with a multi-step reaction cycle similar to that observed in the majority of other cytochromes P450. The cytochrome P450 2B4-substrate complex is reduced from the ferric to the ferrous form by cytochrome P450 reductase. After binding oxygen, the oxyferrous protein accepts a second electron which is provided by either cytochrome P450 reductase or cytochrome b5. In both instances, product formation occurs. When the second electron is donated by cytochrome b5, catalysis (product formation) is ∼ 10 to 100-fold faster than in the presence of cytochrome P450 reductase. This allows less time for side product formation (hydrogen peroxide and superoxide) and improves by ∼ 15% the coupling of NADPH consumption to product formation. Cytochrome b5 has also been shown to compete with cytochrome P450 reductase for a binding site on the proximal surface of cytochrome P450 2B4. These two different effects of cytochrome b5 on cytochrome P450 2B4 reactivity can explain how cytochrome b5 is able to stimulate, inhibit, or have no effect on cytochrome P450 2B4 activity. At low molar ratios (<1) of cytochrome b5 to cytochrome P450 reductase, the more rapid catalysis results in enhanced substrate metabolism. In contrast, at high molar ratios (>1) of cytochome b5 to cytochrome P450 reductase, cytochrome b5 inhibits activity by binding to the proximal surface of cytochrome P450 and preventing the reductase from reducing ferric cytochrome P450 to the ferrous protein, thereby aborting the catalytic reaction cycle. When the stimulatory and inhibitory effects of cytochrome b5 are equal, it will appear to have no effect on the enzymatic activity. It is hypothesized that cytochrome b5 stimulates catalysis by causing a conformational change in the active site, which allows the active oxidizing oxyferryl species of cytochrome P450 to be formed more rapidly than in the presence of reductase.
Keywords: cytochrome P450, cytochrome b5, cytochrome P450 reductase, redox partner, enzyme kinetics, stoichiometry
1. Introduction
The cytochromes P450 are a ubiquitous superfamily of mixed function oxidases found in all kingdoms of life. They are one of the most extensively studied proteins. A recent search in PubMed for cytochrome P450 yielded ∼ 65,000 articles describing more than 11,000 cytochrome P450 family members. Chemists and biochemists have been intrigued by these enzymes for over half a century since they are able to deftly activate the stable carbon hydrogen bond of alkanes, hence the sobriquet “Mother Nature's Blowtorch”. It is anticipated that an understanding of the mechanism of action of these proteins will lead to the development of novel, selective catalysts and compounds, including drugs, that could be of considerable socio-economic value. Many excellent reviews and a book on numerous aspects of cytochrome P450 have been written [1,2,3]. The focus of this review article will be on the interaction of a full-length liver microsomal cytochrome P450, 2B4, with its full-length redox partners, cytochrome P450 reductase and cytochrome b5, with emphasis on relatively new findings and conclusions from work in my laboratory. This topic has been comprehensively reviewed [4,5,6,7].
For decades investigators have been fascinated by the observation that cytochrome b5 can inhibit, stimulate, or not affect the catalytic activity of microsomal cytochromes P450. To add to the intrigue, the effect was noted to be dependent on the substrate and the cytochrome P450 isozyme [8. Our interest was piqued by the observation that the volatile anesthetic, methoxyflurane (a methyl ethyl ether) was not significantly metabolized by pure rabbit phenobarbital inducible cytochrome P450 2B4 even though its metabolism was enhanced in hepatic microsomes from phenobarbital-treated rabbits. Cytochrome P450 2B4 was the major cytochrome P450 isozyme induced by phenobarbital. It was, therefore, expected that the pure protein would metabolize the anesthetic. Eventually it was discovered that the addition of pure cytochrome b5 to the reconstituted, purified reaction mixture containing cytochrome P450 2B4 and cytochrome P450 reductase resulted in marked enhancement of the metabolism of methoxyflurane, whereas the metabolism of the model substrate, benzphetamine, was minimally, if at all, enhanced by cytochrome b5 [9]. These observations were not understood at the time in view of the limited 1980's knowledge of cytochrome P450. To add to the already considerable confusion about the role of cytochrome b5 in cytochrome P450 catalysis, it could be demonstrated that the effect of cytochrome b5 depended on the sequence of addition of the reactants to the assay mixture [10,11]. In view of such variability of the effect of cytochrome b5 in vitro, one wonders whether cytochrome b5 influences cytochrome P450 catalysis in vivo. Recent publications by Wolf and co-workers demonstrated unambiguously that cytochrome b5 modulates the rate of cytochrome P450 monoxygenation reactions in vivo [12,13]. They generated a conditional deletion of microsomal cytochrome b5 in mouse liver to create a hepatic microsomal cytochrome b5 null mouse. These mice had no overt phenotype. Nevertheless, the in vivo activity of cytochromes P450 in the 3A and 2C families was diminished as measured by decreased metabolism of midazolam, metoprolol, and tolbutamide. However, elimination of one drug, chlorzoxazone, was unchanged, indicating that there is specificity to the effects of cytochrome b5 in vivo as well as in vitro. Decreased drug metabolism was also observed in microsomes isolated from these genetically-engineered mice. Cytochrome b5 has also been knocked out in all mouse tissues with a profound reduction in metabolism of some substrates particularly in lung, kidney, and small intestine. Testicular cytochrome P450 17 α-hydroxylase/17,20-lyase activity was also significantly reduced by the cytochrome b5 deletion [13].
In summary, the studies just described demonstrate that microsomal cytochrome b5 can play a major role in mice, and presumably humans, in the in vivo metabolism of selected drugs in a cytochrome P450 and substrate dependent manner. Future studies of the role of cytochrome P450 in cytochrome b5 catalysis should attempt to understand how these proteins interact in the endoplasmic reticulum of cells with state-of-the-art microscopy techniques. Cytochrome b5 has also been observed to modify the activity of microsomal drug metabolizing cytochrome P450 in heterologous expression systems (vaccinia virus, baculovirus, COS cells, E. coli, and yeast) indicating that this mode of regulating cytochrome P450 activity is prevalent in a large and diverse number of organisms [14,15,16]. Antibodies to cytochrome b5 can also inhibit the activity of human and rabbit cytochromes P450 [17,18]. An additional factor in favor of an in vivo role for cytochrome b5 is the presence of approximately equal amounts of cytochrome b5 and cytochrome P450 in hepatic microsomes whereas reductase only occurs at one tenth the concentration of cytochrome P450.
Besides influencing drug metabolism by hepatic cytochromes P450, cytochrome b5 is required by a testicular and adrenal microsomal cytochrome P450, pregnenolone 17 α-hydroxylase/17,20-lyase, for human testosterone biosynthesis [19]. The dual function 17 α-hydroxylase/17,20 lyase requires cytochrome b5 to form dehydroepiandrosterone from the first product, 17 α-hydroxy pregnenolone, of the dual function enzyme. Dehydroepiandrosterone is a testosterone precursor. Presumably cytochrome b5 dictates which product is produced by causing a conformational change in the active site. An individual lacking cytochrome b5 was a pseudohermaphrodite (a chromosomal male with female-like genitalia) [20,21].
2. Stoichiometry of the metabolism of cytochrome P450 with and without cytochrome b5
In view of the variable effects of cytochrome b5, one of our first experiments was designed to determine the stoichiometry of the system we were studying, since this information is an essential first step in understanding a catalytic mechanism. The long-term plan was to understand the role of cytochrome b5 in catalysis by cytochrome P450 2B4, using the model substrates, methoxyflurane, whose metabolism was stimulated by approximately 5 to 10-fold, and benzphetamine, whose metabolism was not significantly altered by addition of cytochrome b5 to the purified reconstituted system. Methoxyflurane was a poor substrate, only ∼ 2-3% coupled, while benzphetamine was a good substrate, which coupled NADPH to product formation with 50% efficiency. It was rationalized that once the role of cytochrome b5 was understood in a particular system, this knowledge would enable us to explore its function in a more rational manner in other systems. In hindsight, this has turned out to be the situation. As expected from an experiment designed to measure the stoichiometry of a reaction, the reactants consumed, NADPH and oxygen, and the products (metabolites, hydrogen peroxide, superoxide) generated were quantified [11]. Surprisingly, it was observed that cytochrome b5 improved the efficiency of NADPH utilization for product formation for both substrates, methoxyflurane and benzphetamine, approximately 6-15% at the expense of the side product, superoxide. Presumably the superoxide resulted from the autoxidation of oxyferrous cytochrome P450 2B4 (See cytochrome P450 reaction cycle, Figure 1). The mechanism by which cytochrome b5 enhances product formation at the expense of superoxide is still a puzzle, since it is known that cytochrome b5 and reductase both reduce oxyferrous cytochrome P450 2B4 at the same rate [22].
Figure 1.
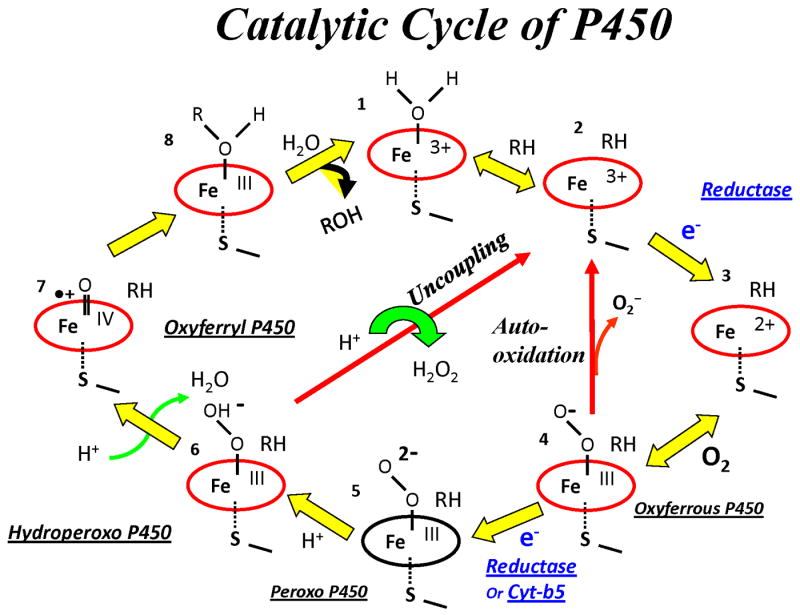
Catalytic Cycle of Cytochrome P450
Another interesting observation was recorded during the stoichiometry experiments. When cytochrome b5 was added to the reaction mixture before the reductase, it was observed by us and others, that cytochrome b5 inhibited NADPH consumption and product formation while cytochrome b5 invariably enhanced the utilization of NADPH for product formation by 6-15% [10,11]. Of note, the inhibitory action of cytochrome b5 could be reversed by incubation of the reaction mixture overnight at 4 °C. At the time these experiments were performed, their significance was not understood. Presently, they are interpreted to support the hypothesis that cytochrome b5 and cytochrome P450 reductase compete for a binding site on cytochrome P450 2B4. Since reductase displaced cytochrome b5, it was concluded that cytochome P450 reductase has a greater affinity for cytochrome P450 2B4 than cytochrome b5, in agreement with other studies [5, 23]. In conclusion, determination of the stoichiometry of the metabolism of the poor substrate, methoxyflurane, and the good substrate, benzphetamine, in the presence and absence of cytochrome b5 revealed that cytochrome b5 increased the efficiency of NADPH utilization for product formation but could also inhibit NADPH consumption and product formation under certain circumstances. With the poor substrate, methoxyflurane, (∼2% coupled) the stimulatory effects of cytochrome b5 (6-15%) were greater than its inhibitory effects. In contrast, under certain conditions, the inhibitory effects of cytochrome b5 were able to overcome its stimulatory effects with the good substrate, benzphetamine (50% coupled). The circumstances which underlay the variable effects of cytochrome b5 will be discussed in Section 6, following the presentation of additional data which has assisted in clarifying the mechanism of action of cytochrome b5.
3. Characterization of the binding of cytochrome P450 2B4 to cytochrome b5 and cytochrome P450 reductase, including identification of the binding site on cytochrome P450 2B4 for its redox partners
3.1 Cytochrome b5 and cytochrome P450 reductase bind to the basic, positively-charged, proximal surface of cytochrome P450 2B4 on unique but overlapping sites
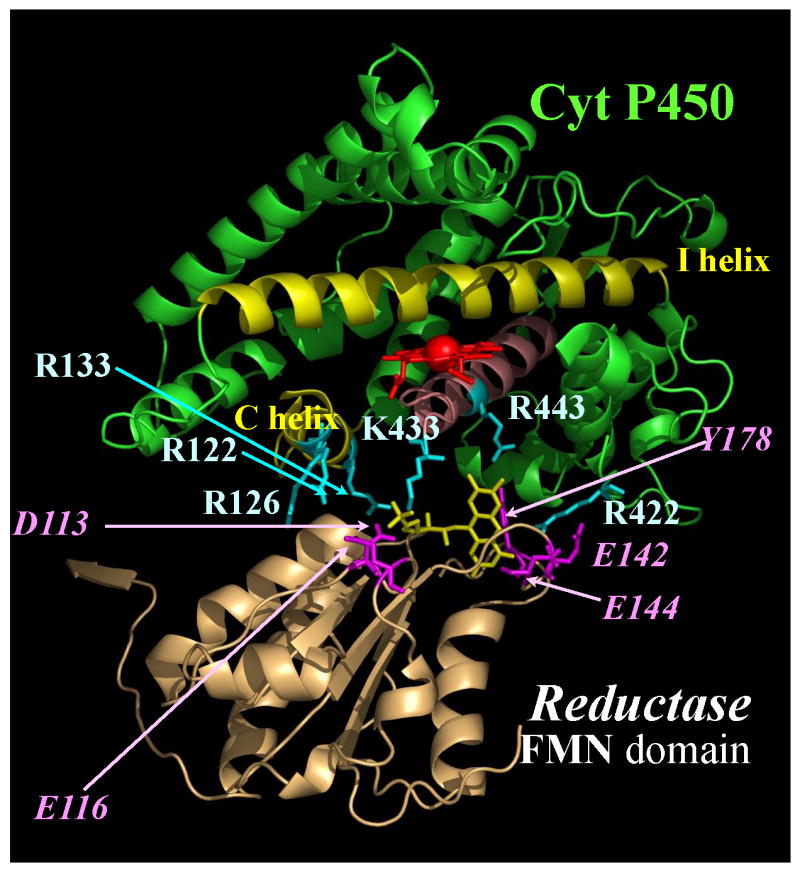
The heme of cytochromes P450 is buried and not exposed to solvent. It is bound to the protein via a thiolate ligand located on the basic, concave surface of the protein. It was predicted that the basic residues on the proximal face of cytochrome P450 where the heme comes closest to the surface would bind to its redox partners, which have acidic convex surfaces. In general, redox proteins interact via interfaces which are complementary in charge and shape. To investigate where cytochrome P450 2B4 bound its redox partners, 25 amino acids distributed over the entire surface of cytochrome P450 2B4 were mutated to alanine to investigate the function of the mutated side chain distal to the β-carbon. The mutant proteins were then characterized with respect to their ability to bind cytochrome b5 and cytochrome P450 reductase and support substrate oxidation [23]. Both hydrophobic and basic amino acids were mutated on the proximal surface. The arginine and lysine residues were expected to pre-orient the proteins and form an encounter complex, which subsequently explores conformations suitable for facile electron transfer. Hydrophobic and polar amino acids are typically prominent in the actual electron transfer complex [24, 25]. Of the 25 mutated amino acids, only seven (R122, R126, R133, F135, M137, K139, and K433) proved to be important in binding cytochrome b5. All were in the mobile C-helix on the proximal surface of the protein, except for K433, which is located in the β-bulge three residues upstream of the axial cysteine, 436 (See [26] for nomenclature, Figure 2).
Figure 2.
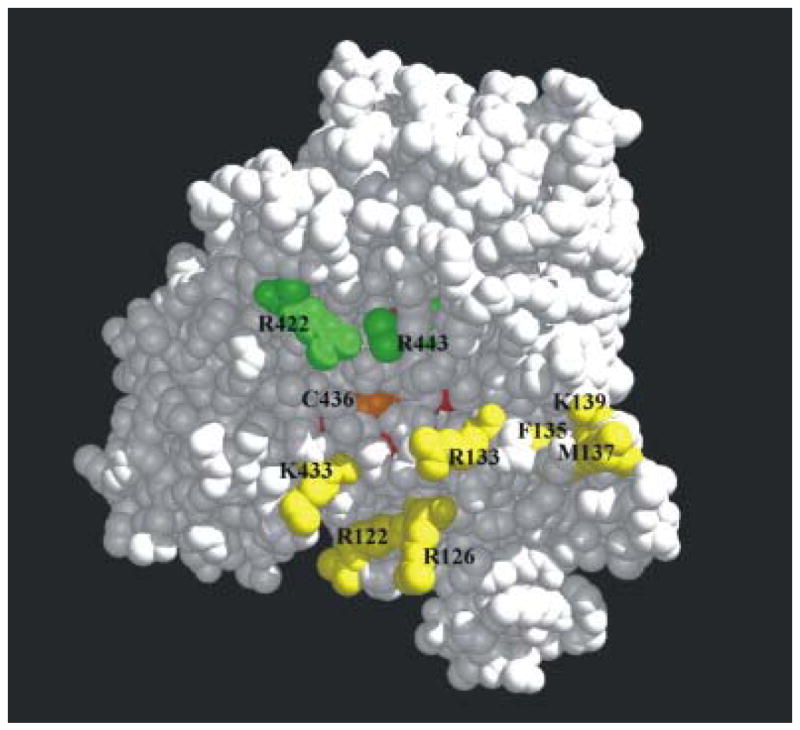
The binding site for cyt b5 and cyt P450 reductase on the proximal surface of cyt P450 2B4. The largely buried heme is in red; residues R422 and 443, involved in binding only the reductase, are illustrated in green; residues which participate in binding both cyt b5 and reductase in and near the C-helix (R122-K139) and K433 are shown in yellow. The figure was generated using the Midas Plus software system from the Computer Graphics Laboratory, University of California, San Francisco, [53] (Reprinted with permission of Elsevier, Biochem Biophys Res Commun 338 (2005) 499-506)
The mutant proteins harboring these mutations exhibited a decreased ability to stimulate the metabolism of methoxyflurane in the presence of cytochrome b5 and an increase in the dissociation constant between the two proteins (Table 1). Substrates bound normally to the seven mutants proteins. Amazingly, the same seven mutants also bound cytochrome P450 reductase poorly with an apparent increase in Kd ranging from 7-to-60-fold. However, upon addition of excess reductase to the reaction mixture, the Vmax was determined to be normal, indicating that the mutations did not affect the catalytic mechanism, only binding of the redox partners. In addition to the seven mutations which altered binding to both cytochrome b5 and reductase, Arg 443 in the L helix and Arg 422, critically located between the β-bulge and meander, also had a decreased ability to bind cytochrome P450 reductase with an essentially normal or slight decreased Vmax with benzphetamine, demonstrating that the cytochrome P450 catalytic mechanism was intact. Figure 2 demonstrates that the binding sites for cytochrome b5 and reductase overlap on the proximal surface of cytochrome P450 2B4, but they are unique because reductase binding involves at least two additional amino acids. The binding site for cytochrome b5 is located primarily on the C-helix and β-bulge (K433), on the edge of the concavity on the proximal surface of cytochrome P450 2B4 (Figure 2). Note that Cys 436, the axial ligand, is at the bottom of the concavity. It is not unusual for electron transfer proteins with multiple partners to possess overlapping but unique binding sites for its redox partners. These data also predict that cytochrome b5 and reductase cannot both occupy their binding sites simultaneously. Their binding will be mutually exclusive and will depend on the relative affinity of each redox partner for cytochrome P450 2B4.
Table 1. Characterization of the binding site mutants of cytochrome P450 2B4.
| P450 residues and location in 2° structure | Kd P450-b5 complex (μM ± SD) | Ratio of MF metabolism ± cyt b5 | Kd (app) P450-reductase complex (μM ± SD) |
|---|---|---|---|
| Group A | |||
| WT | 0.2 ± 0.16 | 4.9 | 0.02 ± 0.02 |
| R422A (m-β) | 0.3 ± 0.04 | 5.3 | 0.28 ± 0.13 |
| R443A (L) | 0.2 ± 0.15 | 3.8 | 0.46 ± 0.16 |
| Group B | |||
| R122A (B-C) | 1.2 ± 0.16 | 2.9 | 0.23 ± 0.08 |
| F135A (C*) | 1.6 ± 0.56 | 2.0 | 0.14 ± 0.04 |
| M137A (C*) | 2.8 ± 1.2 | 1.1 | 0.17 ± 0.02 |
| K139A (C*-D) | 3.0 ± 1.1 | 1.5 | 0.46 ± 0.11 |
| K433A (β) | 3.6 ± 0.77 | 0.3 | 1.1 ± 0.81 |
| R126A (C) | 4.9 ± 4.2 | 0.7 | 0.27 ± 0.08 |
| R133A (C-C*) | ➢ 10 | 1.2 | 1.2 ± 0.57 |
The secondary structure nomenclature and data are from [23].
Our mutagenesis experiments are not in agreement with conclusions reach by Schenkman and coworkers, who characterized the activity of a covalently crosslinked cytochrome P450 2B4-cytochrome b5 [5,27]. They concluded that reductase and cytochrome b5 had separate non-overlapping binding sites on cytochrome P450 2B4. However, residues participating in the crosslinking were not identified so it has not been possible to directly compare the identity of the binding sites on the crosslinking and mutagenesis experiments. At present, we do not understand the reason for the different results. Subsequent studies in our laboratory which varied the concentrations of cytochrome b5 and reductase are consistent with the conclusion that these two redox partners compete for a binding site on cytochrome P450 2B4[28,29].
3.2. Evidence that the binding of cytochrome b5 and cytochome P450 reductase to cytochrome P450 2B4 produce different conformational changes
When cytochrome b5 binds to cytochrome P450 2B4, it causes a significant change in the spin state from the low spin form in which water is the sixth ligand to the high spin form which is pentacoordinate[23,30]. This result has been interpreted to indicate that cytochrome b5 produces a conformational change on the distal side of the heme, which displaces water as a ligand to the heme. Whether it does this by creating a better binding site for water elsewhere in the active site or whether it completely displaces water from the active site because of steric interference is not known. The binding of substrate to cytochrome P450 BM-3 causes a conformational change in the I-helix that creates a better binding site for water in the active site. Substrate does not sterically displace the water as it does in cytochrome P450 camphor and cytochrome P450 2B4 [31,32]. In contrast, the fact that binding of cytochrome P450 reductase to cytochrome P450 2B4 causes minimal, if any, change in spin state provides indirect evidence that the different redox partners do not affect the active site structure in an identical manner [23,30].
In an effort to obtain more substantial evidence about the effect of cytochrome b5 and reductase on the structure of heme and the active site, resonance Raman studies were conducted on cytochrome P450 2B4 in the presence and absence of its redox partners [33]. In order to avoid spectroscopic interference between the hemes of cytochrome P450 and cytochrome b5, cytochrome b5 containing manganese protoporphyrin IX, instead of the usual iron porphyrin, was employed. Manganese cytochrome b5 has the same structure as the wild-type so it is an excellent substitute [34]. This was the first time that resonance Raman spectroscopy was employed to interrogate structural changes in the active site induced by the binding of cytochrome b5 and cytochrome P450 reductase to a full-length wild-type cytochrome P450 2B4. Except for a low to high spin state conversion, substrate (benzphetamine and butylated hydroxytoluene) had minimal effects on heme structure and the iron-carbon monoxy derivative, indicating that the protein was flexible since its binding did not exert significant strain on the peripheral groups of the heme. In contrast, substrate binding produces substantial effects on the heme peripheral groups of cytochrome P450cam which binds its substrate tightly versus the usually weak binding of substrates to microsomal drug- metabolizing enzymes [35]. Although substrate binding had minimal effects on the ferric protein, cytochrome b5 and reductase demonstrated effects consistent with their effect on the spin state of the protein. Mn cytochrome b5 lengthened the Fe+3-S bond, reflecting the enhancement of the high spin state, whereas the reductase did not perturb the Fe+3-S bond. The ferrous carbon monoxide form of cytochrome P450 was differentially modified by the redox partners, suggesting that they will likely exhibit different effects in the physiologically relevant oxyferrous species. Mn cytochrome b5 caused the displacement of the peripheral vinyl groups toward planarity whereas the reductase displaced the vinyl groups away from planarity, decreasing the ability of the vinyl π electrons to conjugate with the heme π electrons. More planar vinyl groups stabilize the ferrous states and are expected to increase the reduction potential due to their ability to withdraw electrons from the aromatic heme ring.
Cytochrome b5 is a membrane-bound protein that is inserted into the membrane via an α-helix at its carboxy terminus. A random-coil linker of 15 amino acids connects the acidic soluble cytosolic heme domain to the membrane anchor. Site-directed mutagenesis was employed to investigate whether the length or the sequence of the linker influences the ability of cytochrome b5 to bind to cytochrome P450 and support catalytic activity [36]. The linker was doubled in length to 30 amino acids; the amino acid sequence was modified by reversing the amino acid sequence of the linker; and conserved amino acids in the linker were mutated. None of these mutations affected the ability of the mutant proteins to bind cytochrome P450 2B4 and support catalysis. However, when the linker was shortened by two residue increments to generate mutants shortened by 2, 4, 6, 8, 10, 12, and 15 residues, it was determined that at least a 7 amino acid linker is required to enable complex formation and electron transfer between the two cytochromes. A random coil 7 amino acid linker would span 24 Å if maximally extended but only 10.5 Å if folded as an α-helix. A length of 24 Å is just sufficient to allow the two heme domains to interact in a physiologically relevant complex when bound to a membrane (Figure 3). Cytochrome P450 2B4 is modeled in the membrane as described [37]. It is hypothesized that the heme domain of cytochrome P450 is fixed with respect to the membrane via a series of basic residues which are located just downstream of the membrane anchor. Cytochrome b5 and reductase each have multiple redox partners and a flexible linker connecting their membrane-bound and soluble cytosolic domains. The functioning of this flexible linker is presumably to allow the cytosolic domains the necessary freedom of motion to complex with their various physiologic partners. Experiments are planned to study the role of the analogous linker in reductase.
Figure 3.
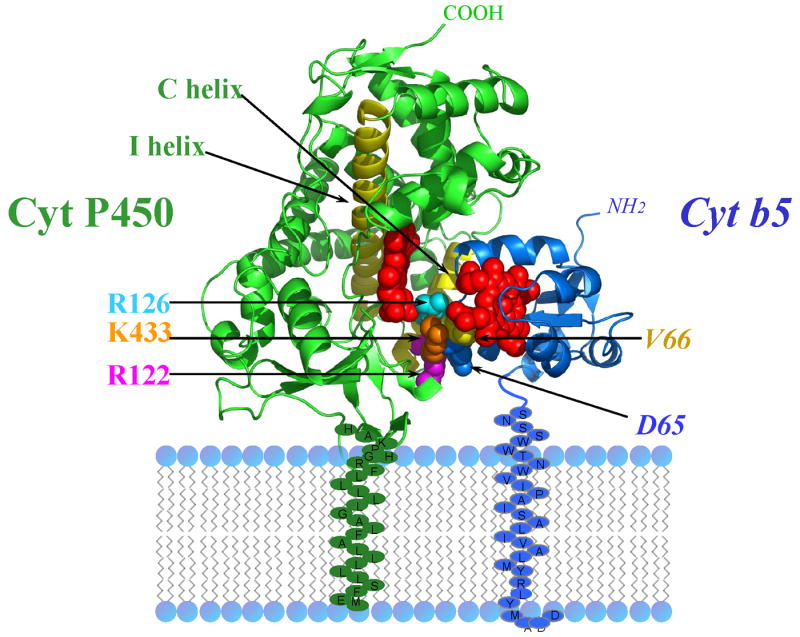
Cytochrome P450 2B4-cytochrome b5 model complex bound to a membrane. Residues V66 and D65 of cytochrome b5 are in contact with R126, K433, R122 of cytochrome P450 2B4. (PDB code of 1SUO).
The membrane anchor of cytochrome b5 is required for binding to cytochrome P450 [9,38,39]. A mutant, which has been deleted by 19 amino acids at its COOH-terminus, is only 20% active with cytochrome P450 2B4, indicating that the COOH terminal half of the membrane anchor is most important for the cytochrome P450 interaction. The length of the membrane anchor is important for targeting to intracellular organelles. Deletion of 11 amino acids yielded a cytosolic protein that did not bind to the endoplasmic reticulum, whereas lengthening the membrane anchor by 5 amino acids caused the protein to be transported to the plasma membrane of COS cells [40,41]. In vitro the α-helical membrane spanning domain has been demonstrated to interact with cytochrome P450 2B4 via non-specific interactions, not via a specific stereochemical knobs in socket fit [42].
Alanine was inserted by site-directed mutagenesis in the transmembrane domain at six positions to “frame shift” the amino acids carboxy terminus to it. Insertion of a residue to an α-helix causes all downstream residues to be rotated by ∼ 100°. All mutants were as active as the wild-type protein. Whether the membrane anchors of the two heme proteins interact in a membrane bilayer is not known. It would depend on the relative affinity of the membrane anchors for one another compared to their affinity for the hydrophobic lipids of the bilayer.
A model of the cytochrome P450 2B4-cytochrome b5 complex is presented in Figures 3 and 4, which accounts for the mutagenesis data on both proteins. The cytochrome P450 2B4 binding site for cytochrome b5 has been identified on the proximal surface and described in Section 3.1. It was expected that acidic residues surrounding the heme of cytochrome b5 would be involved in the interaction with cytochrome P450. Amino acids surrounding the heme have been mutated and tested for their ability to bind and support the activity of cytochrome P450 2B4 (manuscript in preparation). In order to determine which residues from the two proteins were in contact, a double mutant cycle experiment was conducted with all possible combinations of wild-type and mutant proteins. In such cycles, the sum of free energy loss by the two single mutations is compared to the free energy loss by the double mutation. When the loss by the complex with the two mutant proteins is greater, the residues are considered to be in contact in the complex [43]. This experiment revealed that Asp64 and Val65 of cytochrome b5 are in contact with Arg 122, R126 and Lys 433 of cytochrome P450 2B4 (Figures 3 and 4).
Figure 4.
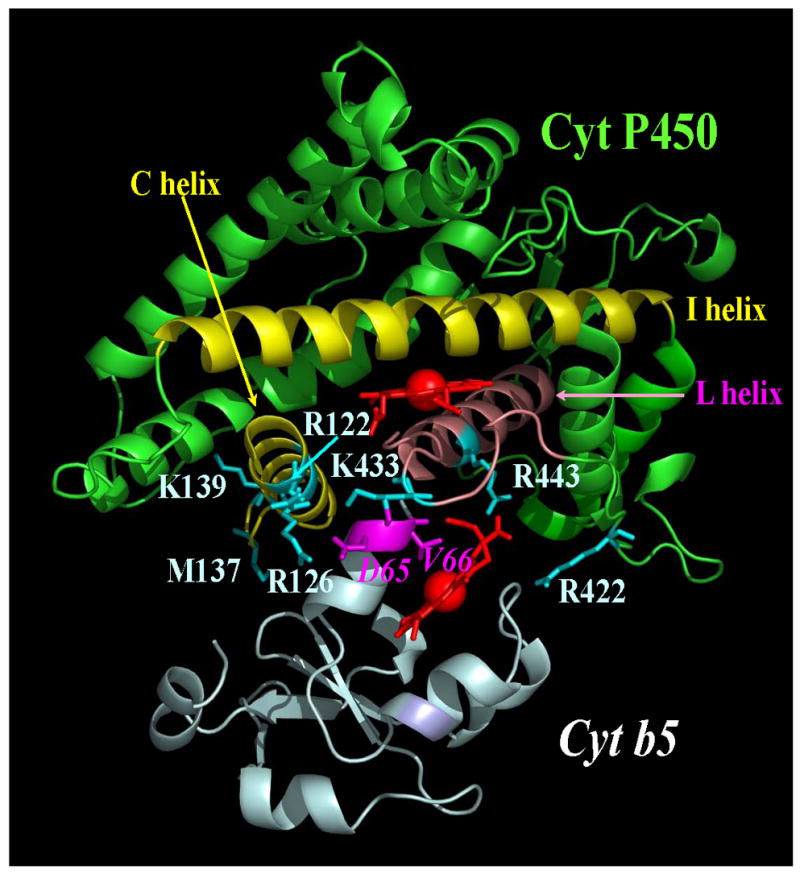
Cytochrome P450-cytochrome b5 model complex. The complex was generated with pymol and accounts for the available mutagenesis data. PDB code of P450 2B4 1SUO.
3.3 Interaction of Cytochrome P450 2B4 with cytochrome P450 reductase
Cytochrome P450 reductase is a membrane-bound diflavin protein, which transfers two electrons sequentially from NADPH through FAD to the FMN cofactor, which is the ultimate donor of electrons to cytochrome P450 and other acceptor proteins [4]. The reductase is bound to the membrane by an amino terminal α-helix. A linker of approximately 15 amino acids connects the membrane-bound domain to the soluble flavin containing domain. A crystal structure of the reductase has been determined in a conformation appropriate for interflavin electron transfer, in which the two flavin rings are in contact [44]. However, cytochrome P450 cannot be docked to this conformation of the reductase in a manner that would allow physiologic electron transfer between the proteins. Therefore, it has been proposed that the two flavin domains would separate in order to allow the FMN domain to dock with cytochrome P450. The structure of a mutant with a 4-amino-acid deletion in a hinge connecting the FMN domain to the rest of the protein has been determined in three markedly extended conformations in which the flavins are separated by 30-60 Å [45]. These three very different conformations demonstrate that the enzyme is able to undergo a structural rearrangement that separates the FAD and FMN domains, which allows the FMN to dock with its redox partners. It is proposed that the wild-type enzyme undergoes an analogous conformational change in the course of shuttling electrons from FAD to acceptor proteins. To visualize a plausible movement, the FMN domain would undergo to transfer electrons from FAD to cytochrome P450 2B4, see http://www.molmovdb.org/cgi-bin/morph.cgi?ID=234385-8941. The link shows a movie of energy minimized conformational changes the FMN domain would experience in translating from its position in the wild-type protein to its position in molecule A, the least open conformation of the three conformations [46]. Figures 5 and 6 show a model of the complex.
Figure 5.
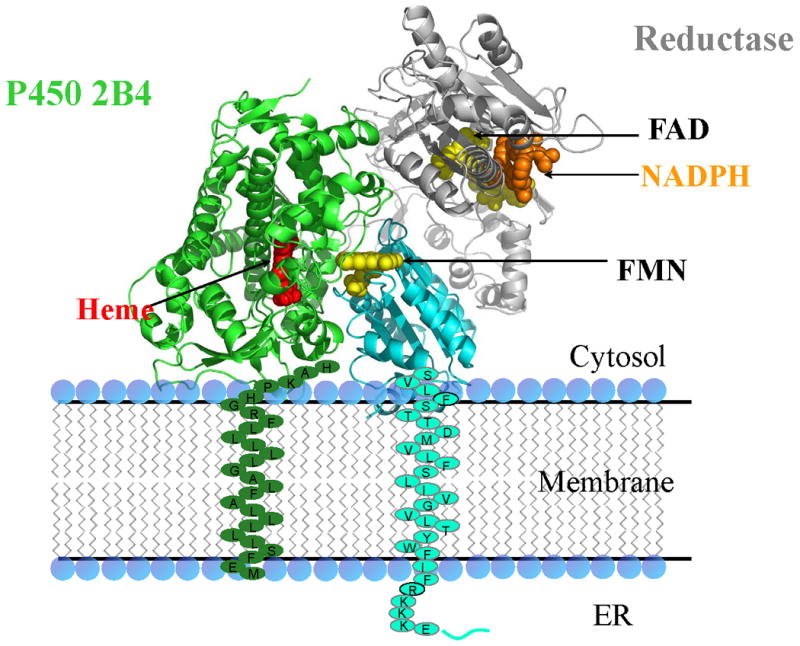
Cytochrome P450-reductase model complex bound to a membrane. The reductase is Molecule A (PDB Code 3ES9) of the open conformation of reductase.
Figure 6.
Cytochrome P450-reductase model complex. The complex was generated with pymol and accounts for available mutagenesis data. PDB code of P450 2B4 1SUO and Molecule A PDB code 3ES9.
When provided with sufficient electrons, the mutant protein can reduce both the ferric and oxyferrous proteins at the same rate as the wild-type protein. Reduction of the oxyferrous protein results in product formation demonstrating that the open conformation of reductase is functional. In view of the great distance separating the FMN and FAD domains in the open conformation, it is not unexpected that electron transfer between the FAD and FMN domains would be dramatically slower (410-fold) compared to the wild-type enzyme.
4. Comparison of the kinetics of the reduction of oxyferrous cytochrome P450 and its decay to the ferric protein in the presence of cytochrome b5 and cytochrome P450 reductase
There were two reasons we were prompted to measure the rate of delivery of the second electron to cytochrome P450. The first was the observation that cytochrome b5 increased the efficiency of catalysis in a reconstituted system under steady-state conditions by decreasing superoxide formation [8,11,47]. The second was the suggestion that cytochrome b5 mediated its effect by reducing oxyferrous cytochrome P450 faster than cytochrome P450 reductase, allowing less time for autoxidation and superoxide formation. Examination of the cytochrome P450 reaction cycle (Figure 1) demonstrates that oxidation of substrate requires two electrons and two protons. The first electron, which reduces the ferric protein, is delivered by cytochrome P450 reductase. Cytochrome b5 cannot deliver the first electron because of its high potential (+25 mV versus approximately -245 mV for benzphetamine bound enzyme) [22]. The second electron, which reduces oxyferrous cytochrome P450, can be delivered by either cytochrome b5 or cytochrome P450 reductase. Cytochrome b5 is able to donate the second electron because the potential of the oxyferrous enzyme has increased to about +20 mV [39]. It was possible to directly measure both the rate of reduction of oxyferrous cytochrome P450 2B4 and its rate of oxidation of reductase by utilizing the 5-deaza FAD T491V cytochrome P450 reductase instead of the wild-type protein. The advantage of employing the 5-deaza FAD reductase was that it underwent a single redox reaction, oxidation of the FMN hydroquinone to a semiquinone, whose spectral changes were readily interpretable [22,48]. Because the T491V reductase mutant bound FAD less tightly than the wild-type protein, it was possible to exchange FAD for 5-deaza FAD, which does not undergo a change in redox state under our experimental conditions. The redox active critical N5 of FAD is replaced by a carbon atom which renders the 5-deaza FAD essentially redox inactive but still able to bind to and maintain the structural integrity of the reductase. It was necessary to replace the FAD with a redox silent analogue in order to be able to unambiguously interpret the spectral changes that occur when the two electron reduced reductase transferred an electron to oxyferrous cytochrome P450. Since the distribution of electrons in this diflavin protein is governed solely by the reduction potentials of its cofactors, there are nine different ways electrons can be distributed. Hence, nine possible unique forms of the protein exist. At any given level of oxidation, other than complete oxidation or total reduction, more than one species of reductase will exist. Stoichiometric addition of two electron equivalents to the 5-deaza FAD reductase by dithionite produced a two electron reduced reductase capable of transferring only a single electron from its FMN domain to its redox partners. The FMN semiquinone is stable and does not donate an electron to acceptor proteins.
A brief description of the experimental protocol employed to measure the reduction of cytochrome P450 2B4 by 5-deaza FAD T491V reductase under single turnover conditions follows. A similar protocol was also employed in subsequent experiments where the wild-type reductase was utilized. Single turnover conditions refer to the fact that the experiments were conducted under conditions in which a molecule of cytochrome P450 can generate, at most, a single molecule of product. The strategy of this experiment is to bypass the first electron transfer step by reducing the proteins with stoichiometric amounts of dithionite so that the reaction can be initiated by mixing an oxygen-containing solution with a solution of the prereduced 1:1 cytochrome P450-redox partner complex. Cytochrome P450, its redox partner – either cytochrome b5 or reductase – along with substrate, phospholipid and buffer are mixed together and incubated in a glovebox to preform the desired complex and remove oxygen. The preformed complex, 15-30 μM, consisting of equal amounts of cytochrome P450 and its redox partner is stoichiometrically reduced with dithionite. In the case of the cytochrome b5 - cytochrome P450 complex, both proteins are reduced to the ferrous state. When reductase is the redox partner, it is reduced to the 2-electron state, which can transfer only a single electron to cytochrome P450 since the one electron reduced FMN semiquinone is stable and not capable of donating an electron.
The prereduced complex is placed in one syringe of the UV-visible stopped-flow spectrophotometer, which is housed in an anaerobic glove box, and subsequently rapidly mixed with the oxygen-containing buffer from a second syringe. The kinetics of the reaction are followed spectrophotometrically by observing the spectral changes that occur as a result of changes in the redox state of the interacting proteins. Within the dead time of the instrument, oxygen rapidly binds to the ferrous cytochrome P450. The oxyferrous cytochrome P450 immediately accepts an electron (the second electron required for catalysis; recall the first electron was provided by dithionite) from the redox partner and undergoes catalysis resulting in product formation and regeneration of the ferric protein (Figure 1). Alternatively, uncoupling of the reaction will occur with hydrogen peroxide and ferric protein formation. The more rapid formation of the ferric cytochrome P450 in the presence of a competent redox partner was considered to represent the rate of product formation. Control studies determined the rate of autoxidation of each protein in the absence of a redox partner. Meanwhile the redox partner has undergone oxidation after donating the electron. Cytochrome b5 reverts to the ferric protein and the reductase becomes the semiquinone form, neither of which can reduce the newly formed ferric cytochrome P450. Hence, the cytochrome P450 turns over only once. A similar protocol was also employed in rapid chemical quench studies and freeze quench EPR studies to be described later in this article [28,29].
Figure 7 compares the kinetics of formation of the ferric cytochrome P450 (followed at 438 nm) from the oxyferrous species in the presence of cytochrome b5 (k=11s-1) and cytochrome P450 reductase (k= 0.09s-1). Remarkably, oxyferrous cytochrome P450 decayed to the ferric enzyme approximately 100-fold faster in the presence of cytochrome b5. Figure and Table 2 demonstrate that cytochrome b5 (k= 9.3s-1) and cytochrome P450 (k= 10.5s-1) oxidize simultaneously to the ferric proteins. In contrast, Figure 7 and Table 2 demonstrate that reductase oxidizes with a rate constant of 8.4 s-1 while cytochrome P450 forms ferric cytochrome P450 approximately 100-fold more slowly, with a rate constant of 0.09 s-1 which is the rate of catalysis under steady-state conditions (kcat = 0.08 s-1) at 15°C, the experimental temperature. Note that the reductase and cytochrome b5 undergo spectral changes which reflect donation of an electron to cytochrome P450 at essentially the same rate. Surprisingly, cytochrome P450 behaves differently in the presence of its two redox partners. These data were interpreted to indicate that in the presence of cytochrome P450 reductase catalysis by cytochrome P450 occurs via a long-lived intermediate. Global analysis of the spectral data obtained with the photodiode array detector was unable to detect a new spectral intermediate. Quantitative analysis of the reaction mixture by LC-MS/MS revealed that the product, norbenzphetamine, was formed with a coupling efficiency of 52% with cytochrome b5 versus 32% with the reductase. Considered as a whole, the results indicate that in the presence of reductase, a relatively stable, reduced oxyferrous cytochrome P450 intermediate is formed and that the rate-limiting step in catalysis is not introduction of the second electron but rather a later step in the catalytic cycle. It is hypothesized that cytochrome b5 and reductase are effectors of cytochrome P450 that induce different conformational changes in the active site upon binding. In view of the instability of the known reduced oxyferrous species (peroxo and hydroperoxo in Figure 1), it is remarkable that one of them is stabilized under ambient conditions. It is also possible that proton delivery is slower because of suboptimal organization and function of the essential proton delivery machinery.
Figure 7.

Comparison of the kinetics of the decay of oxyferrous cytochrome P450 to the ferric species and of norbenzphetamine formation in the presence of substrate, benzphetamine. The ΔA438nm represents the decay of the oxyferrous P450 2B4 to the ferric protein [22. The data for product formation are from [28].
 , product formation by P450-b5; , product formation by P450-b5;
|
 , product formation by P450-CPR; , product formation by P450-CPR;
|
 , ΔA438nm for P450-b5; , ΔA438nm for P450-b5;
|
 , ΔA438nm for P450-CPR. , ΔA438nm for P450-CPR.
|
Table 2. Summary of Rate Constants and Amplitudes for Autoxidation and Redox Reactions of cyt P450, cyt b5, and 5-DeazaFAD reductase in the absence and presence of their redox partners.
| Syringe 1 | Syringe 2 | λ(nm) obsa | Species obsa | Phase 1 | Phase 2 | Phase 3 | |||
|---|---|---|---|---|---|---|---|---|---|
| A (%) | k1(s-1) | A (%) | k2(s-1) | A (%) | k3(s-1) | ||||
| 2e-reduced 5-deazaFAD reductasec | O2 | 450 | reductase | 100 ± 11 | 0.007 ± 0.001 | ||||
| 585 | reductase | 100 ± 8 | 0.007 ± 0.001 | ||||||
| cyt b52+ | O2 | 422 | cyt b5 | 97 ± 5 | 0.005 ± 0.0003 | ||||
| P4502+ | O2 | 438 | P450 | 25 ± 3 | 0.96 ± 0.2 | 34 ± 6 | 0.13 ± 0.04 | 41 ± 7 | 0.016 ± 0.005 |
| P4502+ + BPb | O2 + BP | 438 | P450 | 40 ± 4 | 0.13 ± 0.05 | 60 ± 7 | 0.0480±00.004 | ||
| 2e-reduced 5-deazaFAD reductase | cyt b52+ | 422 | cyt b5 | 98 ± 10 | 0.002 ± 0.0002 | ||||
| 567 | reductase | 100 ± 12 | 0.002 ± 0.0004 | ||||||
| P4502+-cyt b52+ + BP | O2 + BP | 422 | cyt b5 | 50 ± 6 | 9.3 ± 0.7 | 4 ± 0.1 | 0.43 ± 0.21 | 46 ± 7 | 0.005 ± 0.0003 |
| 438 | P450 | 62 ± 7 | 10.5 ± 1.5 | 18 ± 1.1 | 0.83 ± 0.18 | 20 ± 3 | 0.005 ± 0.001 | ||
| P4502+ - 2e-reduced 5-deazaFAD reductase + BP | O2 + BP | 598 | reductase | 31 ± 6 | 8.4 ± 1.5 | 52 ± 6 | 0.37 ± 0.06 | 17 ± 0.7 | 0.041 ± 0.005 |
| 438 | P450 | 86 ± 10 | 0.090 ± 0.01 | 14 ± 0. 5 | 0.0012 ± 0.002 | ||||
5. Catalysis is faster in the presence of cytochrome b5 under single turnover conditions
Because the spectrally measured rate of decay of oxyferrous cytochrome P450 to the ferric protein in the presence of its redox partners was multiphasic, it was not possible to unambiguously determine when product was formed. For this reason the rate of product formation was measured directly using a rapid chemical quench technique [28]. The reaction was conducted as described for the spectrophotometric studies except that the reaction was quenched at designated times and product formation measured by a sensitive LC-MS/MS assay capable of detecting low picomole quantities of the product, norbenzphetamine. A prereduced complex of equal amounts of cytochrome P450 with either cytochrome b5 or reductase was mixed with an oxygen-containing buffer. The reaction was allowed to proceed and samples were collected as a function of time. Figure 7 demonstrates that the rate constant for formation of norbenzphetamine (product of benzphetamine metabolism) was within experimental error identical to the rate constant of the formation of the ferric cytochrome P450 in spectrophotometric experiments. The results also establish that benzphetamine is metabolized approximately 100-fold more quickly in the presence of cytochrome b5 than in the presence of reductase. Conversely, metabolism is 100-fold slower in the presence of reductase than cytochrome b5. Metabolism of a second substrate, cyclohexane, is also slower and less efficient in the presence of reductase, demonstrating the different effects of the redox partners on cytochrome P450 2B4 activity is not dependent on the substrate. In aggregate, our experiments under single turnover conditions with preformed reduced complexes of cytochrome P450 2B4 showed that cytochrome b5 and reductase catalyze product formation monophasically, with easily distinguishable rate constants for two substrates, benzphetamine and cyclohexane.
Why catalysis is slower when the second electron is provided by cytochrome P450 reductase is not understood at the present time. Understanding the biochemical mechanism by which the drug metabolizing cytochromes P450 catalytic rate and efficiency is modulated by its redox partners should provide insights which will assist in harnessing its strong oxidative power for a number of practical applications.
The observation that the two redox partners support catalysis at such markedly different rates raises an intriguing question: what is the basis of this distinctive behavior of oxyferrous cytochrome P450 in the presence of its two electron donors? Examination of the cytochrome P450 catalytic cycle (Figure 1) reveals that, in theory, either the peroxo species (Fe3+OO)2 −, its protonated form, the hydroperoxo intermediate (Fe3+OOH)−, an oxidized substrate-heme complex or a previously unidentified ephemeral compound could be the reduced oxyferrous transient species. A hydroperoxo species would be more stable than the very nucleophilic peroxo species which is protonated at 70K but not 4K in cytochrome P450cam [49]. In fact, preliminary freeze quench EPR data has been obtained indicating that a hydroperoxo intermediate accumulates under single turnover conditions in the presence of reductase. If this is true, it follows that delivery of the second proton to the hydroperoxo species is delayed, presumably by a conformational change induced in the active site by reductase binding on the proximal surface of cytochrome P450 (Figure 5). In some manner, the proton delivery machinery has been temporarily disrupted.
6. Under single and multiple-turnover conditions, cytochrome b5 and reductase compete to provide the second electron required for cytochrome P450 catalysis
Establishing distinctive rates at which cytochrome b5 and reductase support catalysis under single turnover conditions has enabled us to determine whether cytochrome b5 or reductase mediates catalysis when both redox partners are present and to gain a better understanding of how cytochrome b5 influences catalysis under steady-state, multiple-turnover conditions [28]. By varying the ratio of cytochrome b5 to reductase present in the reaction mixture, it has been possible to demonstrate that these two redox partners compete for the binding site on the proximal surface of cytochrome P450 2B4. In the presence of equimolar concentrations of cytochrome P450, cytochrome b5, and reductase, the formation of product was biphasic and occurred with fast and slow rate constants diagnostic of catalysis by cytochrome b5 and reductase respectively. At the 1:1:1 molar ratio, 32% of the product can be attributed to cytochrome b5 whereas 68% represents product formation by reductase. Because of a lengthy overnight preincubation of the three proteins prior to the experiment, it was concluded that under our experimental conditions reductase had a higher affinity for cytochrome P450 than cytochrome b5. A higher molar ratio of cytochrome b5 enhanced the amount of product generated by cytochrome b5 and decreased the quantity of product formed by reductase. At a three-fold molar excess, cytochrome b5 generated 74% of the product, while the remainder was contributed by the reductase.
Recall that under single turnover conditions the first electron is provided by dithionite, not reductase. Under steady-state conditions the situation is more complicated, due to the fact that only reductase possesses a redox potential capable of delivering an electron to the ferric cytochrome P450. Cytochrome b5 cannot provide the first electron, only the second. Using published data from steady-state experiments performed in our laboratory, Table 3 demonstrates the effect of increasing the molar ratio of cytochrome b5 on NADPH consumption and formaldehyde formation from the N-demethylation of the substrate, benzphetamine [28]. At molar ratios equal to or greater than 1, cytochrome b5 inhibits NADPH consumption and product formation. However, it stimulates product formation and enhances reaction efficiency at molar ratios less than 1. Thus at molar ratios ≤ 1, cytochrome b5 enhances substrate metabolism but progressively inhibits NADPH consumption and metabolism at higher cytochrome b5 :reductase molar ratios. The systematic variation of the molar ratio of cytochrome b5 on the activity of the purified reconstituted cytochrome P450 2B4 system leads to the proposal that the stimulatory effect of cytochrome b5 on catalysis is due to its ability to increase the catalytic rate and efficiency of NADPH coupling to product formation. The inhibitory properties of cytochrome b5 are a consequence of its ability to occupy the reductase binding site on the proximal surface of cytochrome P450, thereby preventing the reductase from binding and providing the first electron to ferric cytochrome P450. Formation of ferrous cytochrome P450 is an early critical step in the reaction cycle.
Table 3. Effect of cyt b5 on the rate of NADPH consumption and benzphetamine metabolism by cyt P450 2B4 under steady-state conditions at 30° C.
| Molar ratio P450:CPR:b5 |
NADPH | Formaldehyde |
|---|---|---|
| nmol/min/nmol of cyt P450 | nmol/min/mol of cyt P450 | |
| 1:1:0 | 83 ± 5.5 | 47 ± 3.3 |
| 1:1:0.5 | 81 ± 2.1 | 56 ± 0.8 |
| 1:1:1 | 66 ± 3.2 | 48 ± 1.0 |
| 1:1:3 | 36 ± 6.3 | 30 ± 3.8 |
| 1:1:5 | 25 ± 1.3 | 21 ± 1.8 |
Data from: [28].
7. Cytochrome b5 inhibits reduction of ferric cytochrome P450 2B4
To examine our hypothesis that, under steady state conditions, cytochrome b5 attenuated NADPH utilization and the activity of cytochrome P450 2B4 by competing with reductase for its cytochrome P450 binding site and preventing reduction of ferric cytochrome P450, the rate of electron transfer was directly measured in the presence of varying concentrations of cytochrome b5 and Mn cytochrome b5. The reduction of ferric cytochrome P450 2B4 was measured by determining the rate of formation of the ferrous carbon monoxide bound protein [29]. Both cytochrome b5 and Mn cytochrome b5 inhibited reduction of ferric cytochrome P450 2B4 in a concentration dependent manner. At a 5:1 molar ratio of Mn cytochrome b5 to reductase, the rate of reduction was attenuated by 10-fold and, after 20 minutes, only 30% of the enzyme had been reduced, whereas 70% remained oxidized. As a control, the ability of reductase to reduce cytochrome c was measured in the presence of Mn cytochrome b5. The purpose of the control was to eliminate the theoretical possibility that Mn cytochrome b5 was inhibitory due to an interaction with reductase. As predicted, reductase reduced cytochrome c normally in the presence of a 1-to-5-fold excess of Mn cytochrome b5.
What happens when Mn cytochrome b5 is added to a reconstituted monooxygenase system under steady-state conditions? In the preceding section, it was proposed that cytochrome b5 stimulates activity because it enhances the rate of catalysis and increases catalytic efficiency. Mn cytochrome b5 lacks the ability to reduce oxyferrous cytochrome P450 and, therefore, should not stimulate catalysis. However, Mn cytochrome b5 is predicted to be capable of inhibiting catalysis at both low and high concentrations since it, nevertheless, binds cytochrome P450. Experiments under steady-state conditions demonstrated that, as expected, even at low molar ratios of 0.5:1 of Mn cytochrome b5 to reductase, the rate of NADPH consumption was inhibited by ∼ 10%, while activity was unchanged. At equimolar concentrations of the two proteins, the rate of NADPH oxidation was decreased 44% and activity was inhibited by a similar amount, 38%. At a 5-fold molar excess of Mn cytochrome b5, activity and NADPH utilization were attenuated by ∼ 90%!
The mechanism by which cytochrome b5 modulates the activity of cytochrome P450 2B4 has been elucidated. It remains to be determined to what extent these results apply to other microsomal cytochrome P450 isozymes. Nonetheless, the studies with cytochrome P450 2B4 will guide our experiments and focus our efforts on attempting to understand how cytochrome b5 influences the catalytic mechanism and activity of other cytochromes P450. Ultimately our interest lies in understanding the interaction in vivo, an achievable goal because better tools are becoming available to study proteins in vivo.
8. Discussion
One of the original goals of this project was to characterize the cytochrome b5-cytochrome P450 interaction in enough detail to be able to understand the substrate dependent effects of cytochrome b5. Or stated in an alternative way: why does cytochrome b5 stimulate the metabolism of some substrates more than others?
Table 4 illustrates our current explanation for this phenomenon. First of all, it should be emphasized that this summary is based on semiquantitative results, due in large part to the heterogeneous aggregation of the three membrane proteins. The assumption that NADPH consumption is constant is not strictly accurate. This value is subject to moderate variation while the increase in catalytic efficiency observed with all substrates with cytochrome b5 is less variable and has been observed in several laboratories [23,50]. In other words, there is a significant standard deviation in NADPH consumption and the increased efficiency/coupling of NADPH utilization. In spite of this caveat, the conclusions drawn from Table 4 appear to be robust. Let's assume NADPH consumption is 100 nmole/nmole P450/min and cytochrome b5 increases the catalytic efficiency by 15%. The absolute activity increase induced by cytochrome b5 for each of three substrates is compared: a poor substrate, methoxyflurane, which only produces 2 nmoles product/nmole P450/min without cytochrome b5; a moderately good substrate, cyclohexane, and a good substrate, benzphetamine, which yield 13 and 50 nmoles of product/nmole P450/min, respectively, in the absence of cytochrome b5. If the addition of cytochrome b5 stimulates product formation by all three substrates by 15 nmoles/nmoleP450/min, then the absolute activity for the poor substrate, methoxyflurane, will have increased from 2 to 17, an 8.5-fold increase. Product formation from the good substrate, benzphetamine, will increase from 50 to 65 nmoles/nmole P450/min. Cyclohexane is intermediate. This modest stimulation is within the experimental error of the measurement and an investigator might conclude that cytochrome b5 did not have an effect on the metabolism of benzphetamine unless a careful measurement of side product formation, H2O2, was made. Another complication of studying the stoichiometry of microsomal cytochrome P450 reactions is that excess NADPH and oxygen may be consumed in a 2:1 ratio to form “non-enzymatic water” [11,51,52].
Table 4. Substrate-dependent effect of cytochrome b5 at a 1:1:1 molar ratio of P450:Reductase:b5.
To recap, cytochrome b5 appears to stimulate the metabolism of some substrates more than others because NADPH consumption and the absolute amount of additional product produced by cytochrome b5 remains approximately constant. If product formation is minimal without cytochrome b5, a poor substrate, then the increased product generated by cytochrome b5 can be large by comparison. With a good substrate forming a generous amount of product, the additional product contributed by cytochrome b5 can appear to be small and/or noncontributory.
9. Summary
The two stated questions which prompted all of the previously presented experiments are: 1) what is the biochemical basis of the ability of cytochrome b5 to stimulate, inhibit, or not effect microsomal cytochrome P450 catalysis? and 2) what accounts for the fact that the effect of cytochrome b5 is dependent on the substrate and cytochrome P450 isozyme? Our current understanding of the effect of cytochrome b5 on cytochrome P450 2B4 activity will be briefly summarized and a hypothesis will be proposed for why the effect of cytochrome b5 is enzyme dependent. Cytochrome b5 has both a stimulatory and inhibitory effect on cytochrome P450 activity. When the two opposite effects are equal, it will appear as though cytochrome b5 does not affect cytochrome P450 activity. The effect of cytochrome b5 in the purified reconstituted system depends on the cytochrome b5:cytochrome P450 reductase molar ratio. At low concentrations, molar ratios ≤ 1, cytochrome b5 enhances activity but at high concentrations of molar ratio ≥ 1 inhibits activity. Cytochrome b5 stimulates activity by virtue of its ability to increase the rate of catalysis and thereby enhance the efficiency of the reaction (more NADPH is converted to product at the expense of the side products, hydrogen peroxide and superoxide) by approximately 15 ± 5%, regardless of the substrate. Cytochrome b5 inhibits activity due to its ability to inhibit cytochrome P450 reductase from reducing ferric cytochrome P450, a critical early step in the catalytic cycle. Cytochrome b5 does this by binding to the proximal surface of cytochrome P450 2B4, which prevents reductase from occupying its overlapping binding site on the proximal cytochrome P450 surface. To further complicate matters, the effect of cytochrome b5 also depends on whether the metabolism of the substrate is highly-coupled (efficient use of NADPH for product formation) or poorly coupled (NADPH produces copious amounts of side products rather than product). In general, highly coupled (50%) substrates are metabolized extensively and are relatively insensitive to cytochrome b5, while the oxidation of poorly coupled (2%) substrates that undergo minimal metabolism is markedly stimulated. This situation arises because in a purified reconstituted system, NADPH consumption and the ∼ 15% enhancement of catalytic efficiency by cytochrome b5 are approximately constant and are not a function of the substrate. For example, a 15% increase in catalytic efficiency for a substrate that is 50% coupled (benzphetamine) only enhances the reaction by 15%, which is well within the error of the measurement of product formation. In contrast, a 15% increase in catalytic efficiency for a substrate that is only 2% coupled (methoxyflurane) will be markedly enhanced because NADPH consumption with both substrates is similar. Presently, there are biochemically-sound explanations for the stimulatory, inhibitory, and substrate-dependent effects of cytochrome b5. Lacking is an understanding of the molecular basis of the isozyme-dependent effects of cytochrome b5. In view of what is known about cytochrome P450 2B4, our hypothesis is that cytochrome b5 does not stimulate the activity of selected isozymes such as cytochrome P450 2D6 because it does not cause a conformational change in the active site, allowing for rapid delivery of the second proton to the hydroperoxo intermediate and subsequent rapid formation of the active oxidizing, oxyferryl, cytochrome P450 intermediate. At high concentrations/molar ratios, the acidic, convex surface of cytochrome b5 is predicted to bind to the basic concave surface of microsomal cytochrome P450 where the reductase normally binds and thereby inhibit the binding of reductase and reduction of ferric cytochrome P450. Although recent experiments have led to a significantly better understanding of the actions and properties of cytochrome b5, much work remains to be done to truly understand the molecular basis of its actions.
Acknowledgments
This work was supported by a National Institutes of Health Grant, GM035533, and a VA Merit Review grant to Lucy Waskell. The assistance of Launa Wakenhut in preparation of this manuscript is greatly appreciated.
Footnotes
Abbreviations: Cytochrome P450, P450 or cyt P450; meander-β bulge, .m-β
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ortiz de Montellano PR. Cytochrome P450: structure, mechanism, and biochemistry. Kluwer Academic/Plenum Publishers; New York, New York: 2005. [Google Scholar]
- 2.Ortiz de Montellano PR. Hydrocarbon Hydroxylation by Cytochrome P450 Enzymes. Chem Rev. 2010;110:932–948. doi: 10.1021/cr9002193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shaik S, Cohen Y, Wang HC, Kumar D, Thiel W. P450 Enzymes: Their Structure, Reactivity and Selectivity, Modeled by QM/MM Calculations. Chem Rev. 2010;110:949. doi: 10.1021/cr900121s. [DOI] [PubMed] [Google Scholar]
- 4.Paine MJ, Scrutton NS, Munro AW, Gutierrez A, Roberts GCK, Wolf CR. In: Cytochrome P450. 3rd. Ortiz de Montellano PR, editor. Kluwer Academic/Plenum Publishers; New York, Boston, Dordrecht, London, Moscow: 2005. pp. 115–148. [Google Scholar]
- 5.Schenkman JB, Jansson I. The many roles of cytochrome b5. Pharmacol Ther. 2003;97:139–152. doi: 10.1016/s0163-7258(02)00327-3. [DOI] [PubMed] [Google Scholar]
- 6.Vergeres G, Waskell L. Cytochrome b5: its function, structure, and membrane topology. Biochimie. 1995;77:604–620. doi: 10.1016/0300-9084(96)88176-4. [DOI] [PubMed] [Google Scholar]
- 7.Noble MA, Girvan HM, Smith SJ, Smith WE, Murataliev M, Guzov VM, Feyereisen R, Munro AW. Analysis of the interactions of cytochrome b5 with flavocytochrome P450 BM3 and its domains. Drug Metabolism Reviews. 2007;39:599–617. doi: 10.1080/03602530701468458. [DOI] [PubMed] [Google Scholar]
- 8.Peterson JA, Prough RA. In: Cytochrome P450: Structure, Mechanism, and Biochemistry. Ortiz de Montellano PR, editor. Plenum Press; New York: 1986. pp. 89–188. [Google Scholar]
- 9.Canova-Davis E, Waskell L. The identification of the heat-stable microsomal protein required for methoxyflurane metabolism as cytochrome b5. J Biol Chem. 1984;259:2541–2546. [PubMed] [Google Scholar]
- 10.Gorsky LD, Coon MJ. Effects of conditions for reconstitution with cytochrome b5 on the formation of products in cytochrome P-450-catalyzed reactions. Drug Metab Dispos. 1986;14:89–96. [PubMed] [Google Scholar]
- 11.Gruenke LD, Konopka K, Cadieu M, Waskell L. The stoichiometry of the cytochrome P-450-catalyzed metabolism of methoxyflurane and benzphetamine in the presence and absence of cytochrome b5. J Biol Chem. 1995;270:24707–24718. doi: 10.1074/jbc.270.42.24707. [DOI] [PubMed] [Google Scholar]
- 12.Finn RD, McLaughlin LA, Ronseaux S, Rosewell I, Houston JB, Henderson CJ, Wolf CR. Defining the in Vivo Role for Cytochrome b5 in Cytochrome P450 Function through the Conditional Hepatic Deletion of Microsomal Cytochrome 5. J Biol Chem. 2008;283:31385–31393. doi: 10.1074/jbc.M803496200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McLaughlin LA, Ronseaux S, Finn RD, Henderson CJ, Wolf CR. Deletion of microsomal cytochrome b5 profoundly affects hepatic and extrahepatic drug metabolism. Mol Pharmacol. 2010;78:269–278. doi: 10.1124/mol.110.064246. [DOI] [PubMed] [Google Scholar]
- 14.Aoyama T, Nagata K, Yamazoe Y, Kato R, Matsunaga E, Gelboin HV, Gonzalez FJ. Cytochrome b5 potentiation of cytochrome P450 catalytic activity demonstrated by a vaccinia virus-mediated in situ reconstitution system. Proc Natl Acad Sci U S A. 1990;87:5425–5429. doi: 10.1073/pnas.87.14.5425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Urban P, Truan G, Gautier JC, Pompon D. Xenobiotic metabolism in humanized yeast: engineered yeast cells producing human NADPH-cytochrome P-450 reductase, cytochrome b5, epoxide hydrolase and P450s. Biochemical Society Transactions. 1993;21:1028–1034. doi: 10.1042/bst0211028. [DOI] [PubMed] [Google Scholar]
- 16.Duarte MP, Palma BB, Gilep AA, Laires A, Oliveira JS, Usanov SA, Rueff J, Kranendonk M. The stimulatory role of human cytochrome b5 in the bioactivation activities of human CYP1A2, 2A6, and 2E1: a new cell expression system to study cytochrome P450 mediated biotransformation. Mutagenesis. 2005;20:93–100. doi: 10.1093/mutage/gei012. [DOI] [PubMed] [Google Scholar]
- 17.Yamazaki H, Nakano M, Imai Y, Ueng YF, Guengerich FP, Shimada T. Roles of Cytochrome b5 in the Oxidation of Testosterone and Nifedipine by Recombinant Cytochrome P450 3A4 and by Human Liver Microsomes. Arch Biochem Biophys. 1996;325:174–182. doi: 10.1006/abbi.1996.0022. [DOI] [PubMed] [Google Scholar]
- 18.Canova-Davis E, Chiang JY, Waskell L. Obligatory role of cytochrome b5 in the microsomal metabolism of methoxyflurane. Biochem Pharmacol. 1985;34:1907–1912. doi: 10.1016/0006-2952(85)90307-7. [DOI] [PubMed] [Google Scholar]
- 19.Katagiri M, Kagawa N, Waterman MR. The role of cytochrome b5 in the biosynthesis of androgens by human P450 C17. Arch Biochem Biophys. 1995;317:343–347. doi: 10.1006/abbi.1995.1173. [DOI] [PubMed] [Google Scholar]
- 20.Hegesh E, Hegesh J, Kaftory A. Congenital Methemoglobinemia with a deficiency of cytochrome b5. N England J Medicine. 1986;314:757–761. doi: 10.1056/NEJM198603203141206. [DOI] [PubMed] [Google Scholar]
- 21.Giordano SJ, Kaftory A, Steggles AW. A splicing mutation in the cytochrome b5 gene from a patient with congenital methemoglobinemia and pseudohermaphrodism. Hum Genet. 1994;93:568–570. doi: 10.1007/BF00202825. [DOI] [PubMed] [Google Scholar]
- 22.Zhang H, Gruenke L, Arscott D, Shen A, Kasper CB, Harris DL, Glavanovich M, Johnson R, Waskell L. Determination of the rate of reduction of oxyferrous cytochrome P450 2B4 by 5-deazaFAD T491V cytochrome P450 reductase. Biochemistry. 2003b;42:11594–11603. doi: 10.1021/bi034968u. [DOI] [PubMed] [Google Scholar]
- 23.Bridges A, Gruenke L, Chang YT, Vakser IA, Loew G, Waskell L. Identification of the binding site on cytochrome P450 2B4 for cytochrome b5 and cytochrome P450 reductase. J Biol Chem. 1998;273:17036–17049. doi: 10.1074/jbc.273.27.17036. [DOI] [PubMed] [Google Scholar]
- 24.Page CC, Moser CC, Chen XX, Dutton PL. Natural Engineering Principles of Electron Tunnelling in Biological Oxidation-reduction. Nature. 1999;402:47–52. doi: 10.1038/46972. [DOI] [PubMed] [Google Scholar]
- 25.Page CC, Moser CC, Dutton PL. Mechanism for electron transfer within and between proteins. Curr Opin Chem Biol. 2003;7:551–556. doi: 10.1016/j.cbpa.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 26.Hasemann CA, Kurumbail RG, Boddupalli SS, Peterson JA, Deisenhofer J. Structure and function of cytochromes P450: a comparative analysis of three crystal structures. Structure. 1995;3:41–62. doi: 10.1016/s0969-2126(01)00134-4. [DOI] [PubMed] [Google Scholar]
- 27.Tamburini PP, Schenkman JB. Purification to homogeneity and enzymological characterization of a functional covalent complex composed of cytochromes P-450 isozyme 2 and b5 from rabbit liver. Proc Natl Acad Sci U S A. 1987;84:11–15. doi: 10.1073/pnas.84.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang H, Im SC, Waskell L. Cytochrome b5 increases the rate of product formation by cytochrome P450 2B4 and competes with cytochrome P450 reductase for a binding site on cytochrome P450 2B4. J Biol Chem. 2007;282:29766–29776. doi: 10.1074/jbc.M703845200. [DOI] [PubMed] [Google Scholar]
- 29.Zhang H, Hamdane D, Im SC, Waskell L. Cytochrome b5 inhibits electron transfer from NADPH-cytochrome P450 reductase to ferric cytochrome P450 2B4. J Biol Chem. 2008;283:5217–5225. doi: 10.1074/jbc.M709094200. [DOI] [PubMed] [Google Scholar]
- 30.French JS, Guengerich FP, Coon MJ. Interactions of cytochrome P-450, NADPH-cytochrome P-450 reductase, phospholipid, and substrate in the reconstituted liver microsomal enzyme system. J Biol Chem. 1980;255:4112–4119. [PubMed] [Google Scholar]
- 31.Haines DC, Tomchick DR, Machius M, Peterson A. Pivotal role of water in the mechanism of P450BM-3. Biochemistry. 2001;40:13456–13465. doi: 10.1021/bi011197q. [DOI] [PubMed] [Google Scholar]
- 32.Harris DL, Park JY, Gruenke L, Waskell L. Theoretical study of the ligand-CYP2B4 complexes: effect of structure on binding free energies and heme spin state. Proteins. 2004;55:895–914. doi: 10.1002/prot.20062. [DOI] [PubMed] [Google Scholar]
- 33.Mak PJ, Im SC, Zhang H, Waskell L, Kincaid JA. Resonance Raman studies of cytochrome P450 2B4 in its interactions with substrates and redox partners. Biochemistry. 2008;47:3950–3963. doi: 10.1021/bi800034b. [DOI] [PubMed] [Google Scholar]
- 34.Gruenke LD, Sun J, Loehr TM, Waskell L. Resonance Raman spectral properties and stability of manganese protoporphyrin IX cytochrome b5. Biochemistry. 1997;36:7114–7125. doi: 10.1021/bi970407p. [DOI] [PubMed] [Google Scholar]
- 35.Deng TJ, Macdonald IDG, Simianu MD, Sykora M, Kincaid JR, Sligar SG. Hydrogen-bonding interactions in the active sites of cytochrome P450cam and its site-directed mutants. J Am Chem Soc. 2001;123:269–278. doi: 10.1021/ja001517d. [DOI] [PubMed] [Google Scholar]
- 36.Clarke TA, Im SC, Bidwai A, Waskell L. The role of the length and sequence of the linker domain of cytochrome b5 in stimulating cytochrome P450 2B4 catalysis. J Biol Chem. 2004;279:36809–36818. doi: 10.1074/jbc.M406055200. [DOI] [PubMed] [Google Scholar]
- 37.Williams PA, Cosme J, Sridhar V, Johnson EF, McRee DE. Mammalian microsomal cytochrome P450 monooxygenase: structural adaptations for membrane binding and functional diversity. Mol Cell. 2000;5:121–131. doi: 10.1016/s1097-2765(00)80408-6. [DOI] [PubMed] [Google Scholar]
- 38.Vergeres G, Waskell L. Expression of cytochrome b5 in yeast and characterization of mutants of the membrane-anchoring domain. J Biol Chem. 1992;267:12583–12591. [PubMed] [Google Scholar]
- 39.Pompon D, Coon MJ. On the mechanism of action of cytochrome P-450 oxidation and reduction of the ferrous dioxygen complex of liver microsomal cytochrome P-450 by cytochrome b5. J Biol Chem. 1984;259:15377–15385. [PubMed] [Google Scholar]
- 40.Mitoma JY, Ito A. The carboxyl-terminal 10 amino acid residues of cytochrome b5 are necessary for its targeting to the endoplasmic reticulum. The EMBO Journal. 1992;II doi: 10.1002/j.1460-2075.1992.tb05513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Honsho M, Mitoma JY, Ito A. Retention of cytochrome b5 in the endoplasmic reticulum is transmembrane and luminal domain-dependent. J Biol Chem. 1998;273:20860–20866. doi: 10.1074/jbc.273.33.20860. [DOI] [PubMed] [Google Scholar]
- 42.Mulrooney SB, Meinhardt DR, Waskell L. The alpha-helical membrane spanning domain of cytochrome b5 interacts with cytochrome P450 via nonspecific interactions. Biochim Biophys Acta. 2004;1674:319–326. doi: 10.1016/j.bbagen.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 43.Reichmann D, Phillip Y, Carmi A, Schreiber G. On the contribution of water-mediated interactions to protein-complex stability. Biochemistry. 2008;47:1051–1060. doi: 10.1021/bi7019639. [DOI] [PubMed] [Google Scholar]
- 44.Wang M, Roberts DL, Paschke R, Shea TM, Siler-Masters BS, Kim JJ. Three-dimensional structure of NADPH-cytochrome P450 reductase: Prototype for FMN- and FAD-containing enzymes. Proc Natl Acad Sci U S A. 1997;94:8411–8416. doi: 10.1073/pnas.94.16.8411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hamdane D, Xia C, Im SC, Zhang H, Kim JJ, Waskell L. Structure and function of an NADPH-cytochrome P450 oxidoreductase in an open conformation capable of reducing cytochrome P450. J Biol Chem. 2009;284:11374–11384. doi: 10.1074/jbc.M807868200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krebs WG, Gerstein M. The morph server: a standardized system for analyzing and visualizing macromolecular motions in a database framework. Nucleic Acids Res. 2000;28:1665–1675. doi: 10.1093/nar/28.8.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ingelman-Sundberg M, Johansson I. Cytochrome b5 as electron donor to rabbit liver cytochrome P450LM2 in reconstituted phospholipid vesicles. Biochem Biophys Res Commun. 1980;97:582–586. doi: 10.1016/0006-291x(80)90303-4. [DOI] [PubMed] [Google Scholar]
- 48.Zhang H, Gruenke L, Saribas AS, Im SC, Shen AL, Kasper CB, Waskell L. Preparation and characterization of a 5′-deazaFAD T491V NADPH-cytochrome P450 reductase. Biochemistry. 2003a;42:6804–6813. doi: 10.1021/bi030081m. [DOI] [PubMed] [Google Scholar]
- 49.Davydov R, Makris TM, Kofman V, Werst DE, Sligar SG, Hoffman BM. Hydroxylation of camphor by reduced oxy-cytochrome P450cam: mechanistic implications of EPR and ENDOR studies of catalytic intermediates in native and mutant enzymes. J Am Chem Soc. 2001;123:1403–1415. doi: 10.1021/ja003583l. [DOI] [PubMed] [Google Scholar]
- 50.Bosterling B, Trudell JR, Trevor AJ, Bendix M. Lipid-protein interactions as determinants of activation or inhibition by cytochrome b5 of cytochrome P-450-mediated oxidations. J Biol Chem. 1982;257:4375–4380. [PubMed] [Google Scholar]
- 51.Gorsky LD, Koop DR, Coon MJ. On the stoichiometry of the oxidase and monooxygenase reactions catalyzed by liver microsomal cytochrome P-450. Products of oxygen reduction. J Biol Chem. 1984;259:6812–6817. [PubMed] [Google Scholar]
- 52.Loida PJ, Sligar SG. Molecular recognition in cytochrome P450: mechanism for the control of uncoupling reactions. Biochemistry. 1993;32:11530–11538. doi: 10.1021/bi00094a009. [DOI] [PubMed] [Google Scholar]
- 53.Zhang H, Myshkin E, Waskell L. Role of cytochrome b5 in catalysis by cytochrome P450 2B4. Biochem Biophys Res Commun. 2005;338:499–506. doi: 10.1016/j.bbrc.2005.09.022. [DOI] [PubMed] [Google Scholar]


