Abstract
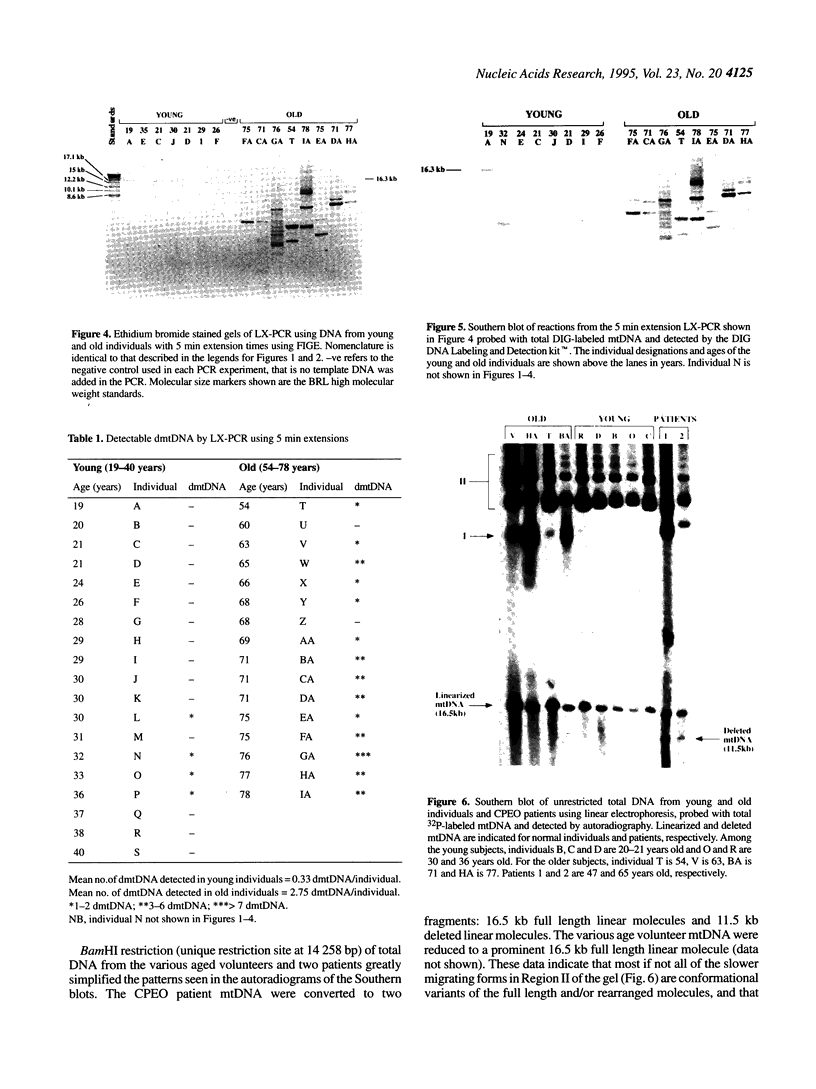
Several reports have shown that individual mitochondrial DNA (mtDNA) deletions accumulate with age. However, the overall extent of somatic mtDNA damage with age remains unclear. We have utilized full-length PCR to concurrently screen for multiple mtDNA rearrangements in total DNA extracted from skeletal muscle derived from physiologically normal individuals (n = 35). This revealed that both the number and variety of mtDNA rearrangements increases dramatically between young and old individuals (P < 0.0001). We further examined the mtDNA from both the younger and older subjects by Southern blot analysis and observed an age-related increase in mtDNA(s) comparable in size to mtDNA products unique to patients with known mtDNA deletions. These data imply that a wide spectrum of mtDNA rearrangements accumulate in old individuals, which correlates with the marked age related decrease in OXPHOS capacity observed in post-mitotic tissues.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Bandy B., Davison A. J. Mitochondrial mutations may increase oxidative stress: implications for carcinogenesis and aging? Free Radic Biol Med. 1990;8(6):523–539. doi: 10.1016/0891-5849(90)90152-9. [DOI] [PubMed] [Google Scholar]
- Boffoli D., Scacco S. C., Vergari R., Solarino G., Santacroce G., Papa S. Decline with age of the respiratory chain activity in human skeletal muscle. Biochim Biophys Acta. 1994 Apr 12;1226(1):73–82. doi: 10.1016/0925-4439(94)90061-2. [DOI] [PubMed] [Google Scholar]
- Bowling A. C., Mutisya E. M., Walker L. C., Price D. L., Cork L. C., Beal M. F. Age-dependent impairment of mitochondrial function in primate brain. J Neurochem. 1993 May;60(5):1964–1967. doi: 10.1111/j.1471-4159.1993.tb13430.x. [DOI] [PubMed] [Google Scholar]
- Cheng S., Higuchi R., Stoneking M. Complete mitochondrial genome amplification. Nat Genet. 1994 Jul;7(3):350–351. doi: 10.1038/ng0794-350. [DOI] [PubMed] [Google Scholar]
- Chung S. S., Weindruch R., Schwarze S. R., McKenzie D. I., Aiken J. M. Multiple age-associated mitochondrial DNA deletions in skeletal muscle of mice. Aging (Milano) 1994 Jun;6(3):193–200. doi: 10.1007/BF03324239. [DOI] [PubMed] [Google Scholar]
- Corral-Debrinski M., Horton T., Lott M. T., Shoffner J. M., Beal M. F., Wallace D. C. Mitochondrial DNA deletions in human brain: regional variability and increase with advanced age. Nat Genet. 1992 Dec;2(4):324–329. doi: 10.1038/ng1292-324. [DOI] [PubMed] [Google Scholar]
- Corral-Debrinski M., Shoffner J. M., Lott M. T., Wallace D. C. Association of mitochondrial DNA damage with aging and coronary atherosclerotic heart disease. Mutat Res. 1992 Sep;275(3-6):169–180. doi: 10.1016/0921-8734(92)90021-g. [DOI] [PubMed] [Google Scholar]
- Cortopassi G. A., Arnheim N. Detection of a specific mitochondrial DNA deletion in tissues of older humans. Nucleic Acids Res. 1990 Dec 11;18(23):6927–6933. doi: 10.1093/nar/18.23.6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortopassi G. A., Shibata D., Soong N. W., Arnheim N. A pattern of accumulation of a somatic deletion of mitochondrial DNA in aging human tissues. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7370–7374. doi: 10.1073/pnas.89.16.7370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadaleta M. N., Rainaldi G., Lezza A. M., Milella F., Fracasso F., Cantatore P. Mitochondrial DNA copy number and mitochondrial DNA deletion in adult and senescent rats. Mutat Res. 1992 Sep;275(3-6):181–193. doi: 10.1016/0921-8734(92)90022-h. [DOI] [PubMed] [Google Scholar]
- Hayakawa M., Hattori K., Sugiyama S., Ozawa T. Age-associated oxygen damage and mutations in mitochondrial DNA in human hearts. Biochem Biophys Res Commun. 1992 Dec 15;189(2):979–985. doi: 10.1016/0006-291x(92)92300-m. [DOI] [PubMed] [Google Scholar]
- Lee C. M., Chung S. S., Kaczkowski J. M., Weindruch R., Aiken J. M. Multiple mitochondrial DNA deletions associated with age in skeletal muscle of rhesus monkeys. J Gerontol. 1993 Nov;48(6):B201–B205. doi: 10.1093/geronj/48.6.b201. [DOI] [PubMed] [Google Scholar]
- Linnane A. W., Marzuki S., Ozawa T., Tanaka M. Mitochondrial DNA mutations as an important contributor to ageing and degenerative diseases. Lancet. 1989 Mar 25;1(8639):642–645. doi: 10.1016/s0140-6736(89)92145-4. [DOI] [PubMed] [Google Scholar]
- Melov S., Lithgow G. J., Fischer D. R., Tedesco P. M., Johnson T. E. Increased frequency of deletions in the mitochondrial genome with age of Caenorhabditis elegans. Nucleic Acids Res. 1995 Apr 25;23(8):1419–1425. doi: 10.1093/nar/23.8.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miquel J., Economos A. C., Fleming J., Johnson J. E., Jr Mitochondrial role in cell aging. Exp Gerontol. 1980;15(6):575–591. doi: 10.1016/0531-5565(80)90010-8. [DOI] [PubMed] [Google Scholar]
- Müller-Höcker J., Seibel P., Schneiderbanger K., Kadenbach B. Different in situ hybridization patterns of mitochondrial DNA in cytochrome c oxidase-deficient extraocular muscle fibres in the elderly. Virchows Arch A Pathol Anat Histopathol. 1993;422(1):7–15. doi: 10.1007/BF01605127. [DOI] [PubMed] [Google Scholar]
- Münscher C., Müller-Höcker J., Kadenbach B. Human aging is associated with various point mutations in tRNA genes of mitochondrial DNA. Biol Chem Hoppe Seyler. 1993 Dec;374(12):1099–1104. doi: 10.1515/bchm3.1993.374.7-12.1099. [DOI] [PubMed] [Google Scholar]
- Münscher C., Rieger T., Müller-Höcker J., Kadenbach B. The point mutation of mitochondrial DNA characteristic for MERRF disease is found also in healthy people of different ages. FEBS Lett. 1993 Feb 8;317(1-2):27–30. doi: 10.1016/0014-5793(93)81484-h. [DOI] [PubMed] [Google Scholar]
- Richter C. Do mitochondrial DNA fragments promote cancer and aging? FEBS Lett. 1988 Dec 5;241(1-2):1–5. doi: 10.1016/0014-5793(88)81018-4. [DOI] [PubMed] [Google Scholar]
- Shoffner J. M., Fernhoff P. M., Krawiecki N. S., Caplan D. B., Holt P. J., Koontz D. A., Takei Y., Newman N. J., Ortiz R. G., Polak M. Subacute necrotizing encephalopathy: oxidative phosphorylation defects and the ATPase 6 point mutation. Neurology. 1992 Nov;42(11):2168–2174. doi: 10.1212/wnl.42.11.2168. [DOI] [PubMed] [Google Scholar]
- Shoffner J. M., Lott M. T., Lezza A. M., Seibel P., Ballinger S. W., Wallace D. C. Myoclonic epilepsy and ragged-red fiber disease (MERRF) is associated with a mitochondrial DNA tRNA(Lys) mutation. Cell. 1990 Jun 15;61(6):931–937. doi: 10.1016/0092-8674(90)90059-n. [DOI] [PubMed] [Google Scholar]
- Shoubridge E. A., Karpati G., Hastings K. E. Deletion mutants are functionally dominant over wild-type mitochondrial genomes in skeletal muscle fiber segments in mitochondrial disease. Cell. 1990 Jul 13;62(1):43–49. doi: 10.1016/0092-8674(90)90238-a. [DOI] [PubMed] [Google Scholar]
- Soong N. W., Hinton D. R., Cortopassi G., Arnheim N. Mosaicism for a specific somatic mitochondrial DNA mutation in adult human brain. Nat Genet. 1992 Dec;2(4):318–323. doi: 10.1038/ng1292-318. [DOI] [PubMed] [Google Scholar]
- Takasawa M., Hayakawa M., Sugiyama S., Hattori K., Ito T., Ozawa T. Age-associated damage in mitochondrial function in rat hearts. Exp Gerontol. 1993 May-Jun;28(3):269–280. doi: 10.1016/0531-5565(93)90034-b. [DOI] [PubMed] [Google Scholar]
- Tokunaga M., Mita S., Sakuta R., Nonaka I., Araki S. Increased mitochondrial DNA in blood vessels and ragged-red fibers in mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes (MELAS). Ann Neurol. 1993 Mar;33(3):275–280. doi: 10.1002/ana.410330308. [DOI] [PubMed] [Google Scholar]
- Trounce I., Byrne E., Marzuki S. Decline in skeletal muscle mitochondrial respiratory chain function: possible factor in ageing. Lancet. 1989 Mar 25;1(8639):637–639. doi: 10.1016/s0140-6736(89)92143-0. [DOI] [PubMed] [Google Scholar]
- Wallace D. C. Mitochondrial DNA mutations in diseases of energy metabolism. J Bioenerg Biomembr. 1994 Jun;26(3):241–250. doi: 10.1007/BF00763096. [DOI] [PubMed] [Google Scholar]
- Wallace D. C. Mitochondrial genetics: a paradigm for aging and degenerative diseases? Science. 1992 May 1;256(5057):628–632. doi: 10.1126/science.1533953. [DOI] [PubMed] [Google Scholar]
- Yen T. C., Chen Y. S., King K. L., Yeh S. H., Wei Y. H. Liver mitochondrial respiratory functions decline with age. Biochem Biophys Res Commun. 1989 Dec 29;165(3):944–1003. doi: 10.1016/0006-291x(89)92701-0. [DOI] [PubMed] [Google Scholar]
- Zhang C., Linnane A. W., Nagley P. Occurrence of a particular base substitution (3243 A to G) in mitochondrial DNA of tissues of ageing humans. Biochem Biophys Res Commun. 1993 Sep 15;195(2):1104–1110. doi: 10.1006/bbrc.1993.2158. [DOI] [PubMed] [Google Scholar]









