Abstract
Background
Previously, we have demonstrated that extending a continuous femoral nerve block from overnight to four days following total knee arthroplasty provides clear benefits during the infusion, but not subsequent to catheter removal. However, there were major limitations in generalizing the results of that investigation, and we subsequently performed a very similar study using a multicenter format, with many healthcare providers, in patients on general orthopedic wards; thus, greatly improving inference of the results to the general population. Not surprisingly, the perioperative/short-term outcomes differed greatly from the first, more-limited, study. We now present a prospective follow-up study of the previously published, multicenter, randomized, controlled clinical trial to investigate the possibility that an extended ambulatory continuous femoral nerve block decreases long-term pain, stiffness, and functional disability following total knee arthroplasty; which greatly improves inference of the results to the general population.
Methods
Subjects undergoing total knee arthroplasty received a continuous femoral nerve block with ropivacaine 0.2% from surgery until the following morning, at which time patients were randomized to either continue perineural ropivacaine (n=28) or normal saline (n=26). Patients were discharged with their catheter and a portable infusion pump, and catheters were removed on postoperative day 4. Health-related quality-of-life was measured using the Western Ontario and McMaster Universities Osteoarthritis (WOMAC) Index preoperatively and then at 7 days, as well as 1, 2, 3, 6, and 12 months following surgery. This index evaluates pain, stiffness, and physical functional disability. For inclusion in the analysis, we required a minimum of four of the six time points, including day 7 and at least two of months 3, 6, and 12.
Results
The two treatment groups had similar WOMAC scores for the mean area under the curve calculations (point estimate for the difference in mean area under the curve for the two groups [overnight infusion group – extended infusion group]=3.8, 95% confidence interval: −3.8 to +11.3; p=0.32) and at all individual time points (p>0.05).
Conclusions
This investigation found no evidence that extending an overnight continuous femoral nerve block to four days improves (or worsens) subsequent pain, stiffness, or physical function following TKA in patients of multiple centers convalescing on general orthopedic wards.
Introduction
Total knee arthroplasty (TKA) often results not only in severe perioperative pain and debilitation, but chronic pain, joint stiffness, and functional disability many months or even years following the procedure.1 Persistent postoperative pain is strongly associated with pre-operative pain, poorly controlled post-operative pain, and the type of surgery.2 Providing potent perioperative analgesia accelerates passive knee flexion in the first two months following TKA;3 but whether persistent pain and functional disability is decreased remains undetermined.
We previously reported, for example, that extending a cFNB from overnight to four days following TKA improves analgesia during the infusion.4 However, a study of health-related quality-of-life during the first year for subjects of our original study failed to detect any long-term benefits from extending the cFNB to four days.5 Unfortunately, there were limitations in generalizing the results of that investigation, and we therefore subsequently performed a very-similar study in a multicenter format, with many healthcare providers, in patients on general orthopedic wards.6
We now present a prospective follow-up study of the previously-published, multicenter, randomized, controlled clinical trial to investigate the possibility that an extended ambulatory cFNB decreases long-term pain, stiffness, and functional disability following TKA;6 which greatly improves inference of the results to the general population. We hypothesized that an improvement in pain, stiffness, and functional ability would be greater not only at one week, but also at 1, 2, 3, 6, and 12 months following TKA in patients who received a four-day cFNB compared with an overnight cFNB in the immediate postoperative period.
Materials and Methods
The Institutional Review Board at each participating clinical center approved all study procedures and the trial was prospectively registered at clinicaltrials.gov (NCT00419276). All subjects provided written, informed consent; and because this was a multi-center trial, a Data Safety Monitoring Board (University of California San Diego, San Diego, California) reviewed data and adverse events every six months. Details of the study methods have been published previously.6 In brief, patients offered enrollment included adults (18–75 years) with osteoarthritis scheduled for primary, unilateral, tricompartment, cemented TKA via a 12–18 cm midline skin incision and parapatellar approach who desired a continuous femoral nerve block for postoperative analgesia.
Study intervention
Subjects received a femoral nerve block and perineural catheter (StimuCath, Arrow International, Reading, PA) followed by a perineural ropivacaine, 0.2%, infusion (6 mL/h basal; 4 mL patient-controlled bolus; 30-min lockout) from surgery until the following morning, at which time patients were randomized to either continue perineural ropivacaine (“extended infusion”, n=39) or switched to normal saline (“overnight infusion”, n=38). Randomization was performed in a triple-masked fashion (patients, investigators, statisticians) with stratification according to clinical site.
At 18:00 on postoperative day (POD) 2 (36 h following randomization), a portable infusion pump (Pain Pump 2 Blockaid, Stryker Instruments, Kalamazoo, MI) containing 400 mL of the same study solution (basal 5 mL/h; bolus 4 mL; lock-out 60 min) replaced the previous infusion pump. Patients were discharged with their pump and perineural catheter in situ as early as 10:00 on POD 3. In the evening of POD 4, patients’ caretakers removed the femoral catheters with physician instructions provided by telephone.
Outcome measurements
The current study was a planned secondary analysis of prospectively-collected data involving pain, joint stiffness, and physical functional disability. Various instruments are available that convert health status into quantifiable values. The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) is an instrument specifically designed to evaluate clinically important, patient-relevant changes in health-related quality-of-life following treatment interventions in patients with osteoarthritis of the knee.7 The WOMAC evaluates three dimensions of health-related quality-of-life: pain, stiffness, and physical functional disability with 5, 2, and 17 questions, respectively. An ordinal Likert scale from 0 to 4 is used for each question, with lower scores indicating lower levels of symptoms or physical disability.7 Each subscale is summated to a maximum score of 20, 8, and 68, respectively. The individual dimensions are always analyzed separately, and investigators have often added a “global” score, which is calculated by summating the scores for the 3 subscales.8 The questionnaire may be self-administered or administered via a telephone call, and takes 5–10 minutes to complete.9 Because it is a proprietary instrument, the questionnaire itself may not be published and is therefore not included in an appendix.
Since its inception two decades ago, the WOMAC has been translated into 60 languages and used in several hundred published clinical trials.10 It has been rigorously examined, demonstrating excellent construct validity, responsiveness, and test-retest reliability in patients following total knee replacement,7;11–13 and is therefore recommended in the Osteoarthritis International Research Society’s guidelines for clinical trials.10;14
Therefore, to investigate the relationship between postoperative analgesic technique and subsequent pain, stiffness, and functional disability, a baseline WOMAC was administered prior to surgery (POD 0), and again at 7 days as well as 1, 2, 3, 6, and 12 months following surgery. The baseline measurement was a self-administered written questionnaire, while subsequent measurements were administered via the telephone. Scores from self-administered and telephone-administered WOMAC instruments have a demonstrated error rate of 0.9–2.6%.9
Statistical analysis
The study was powered for the previously-published primary end point: time to attain three discharge criteria (adequate analgesia, independence from intravenous analgesics, and ambulation of at least 30 m).6 To analyze the WOMAC responses, the WOMAC scores were joined by straight lines between time-points from POD 7 (t=0.25 months) to t=12 months. The personal progress estimated mean area under the curve was defined as the integral of this curve from 0.25 to 12, divided by 11.75 months. The WOMAC hypotheses asked the question of whether overall personal means over a continuum for 12 months of the WOMAC scores (mean area under the curve) differ between treatment groups.
The mean area under the curve measurements were compared by a two-sided t-test with a pooled variance estimate, as the primary question of the null hypothesis was that the two groups have the same WOMAC profile over time. For inclusion in this specific analysis, we required a minimum of four of the six time points, including day 7 and at least two of months 3, 6, and 12. For missing time-points, we applied a slightly modified trapezoidal rule, commonly used in pharmacokinetic studies, which effectively imputes missing values by linear interpolation between the values on either side of the one missing; or in the case of month 12, linear extrapolation from the values of months three and six. The only modification occurs if the extrapolated value falls below zero, in which case a value of zero was used as month 12. These rules were set up before the study was conducted. Additional secondary analyses involved time-point by time-point comparison of the changes from baseline in AUC for total scores, plus the same for pain, stiffness, and functional subscales.
Results
Subjects were enrolled during a 29-month period between April 2007 and August 2009. Details of the study results for the immediate postoperative period have been published previously.6 Data acquisition for the current study involving WOMAC scores occurred 7 days to 12 months following enrollment of each subject. No patient was lost to follow-up. However, for the mean area under the curve calculations, follow-up WOMAC data meeting our stringent inclusion criteria (a minimum of four of the six time points, including day 7 and at least two of months 3, 6, and 12) were available from 28 subjects (72%) from the extended infusion and 26 (68%) subjects from the overnight infusion groups.
The two treatment groups had similar WOMAC scores for the mean area under the curve calculations (point estimate for the difference in mean area under the curve for the two groups [overnight infusion group – extended infusion group] = 3.8, 95% confidence interval: −3.8 to +11.3; p=0.32). In addition, the two treatment groups had similar total WOMAC scores at all individual time points in terms of both raw scores and changes from baseline (p>0.05; Fig. 1 and 2, and Table 1). Although there appeared to be a trend towards improved WOMAC scores in the extended infusion group at Month 2 (p=0.09), this difference disappeared after adjusting for baseline values (p=0.62).
Figure 1.
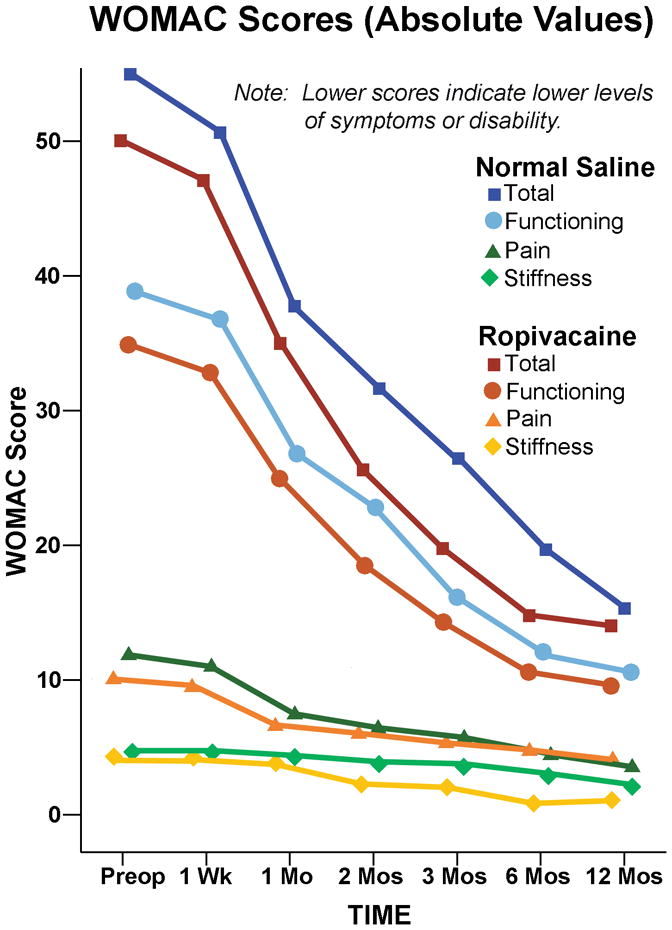
Effect of an extended femoral perineural ropivacaine infusion on health-related quality-of-life following tricompartment knee arthroplasty, as measured with the Western Ontario and McMaster Universities Osteoarthritis Index. Data are expressed means for patients randomly assigned to an extended continuous femoral nerve block (perineural ropivacaine from surgery through postoperative day 4) or overnight continuous femoral nerve block (perineural ropivacaine from surgery through 06:00 postoperative day 1 followed by perineural normal saline through postoperative day 4). The two treatment groups had similar WOMAC scores for the mean area under the curve calculations (point estimate for the difference in mean area under the curve for the two groups [overnight infusion group – extended infusion group]=3.8, 95% confidence interval: −3.8 to +11.3; p=0.32).
Figure 2.
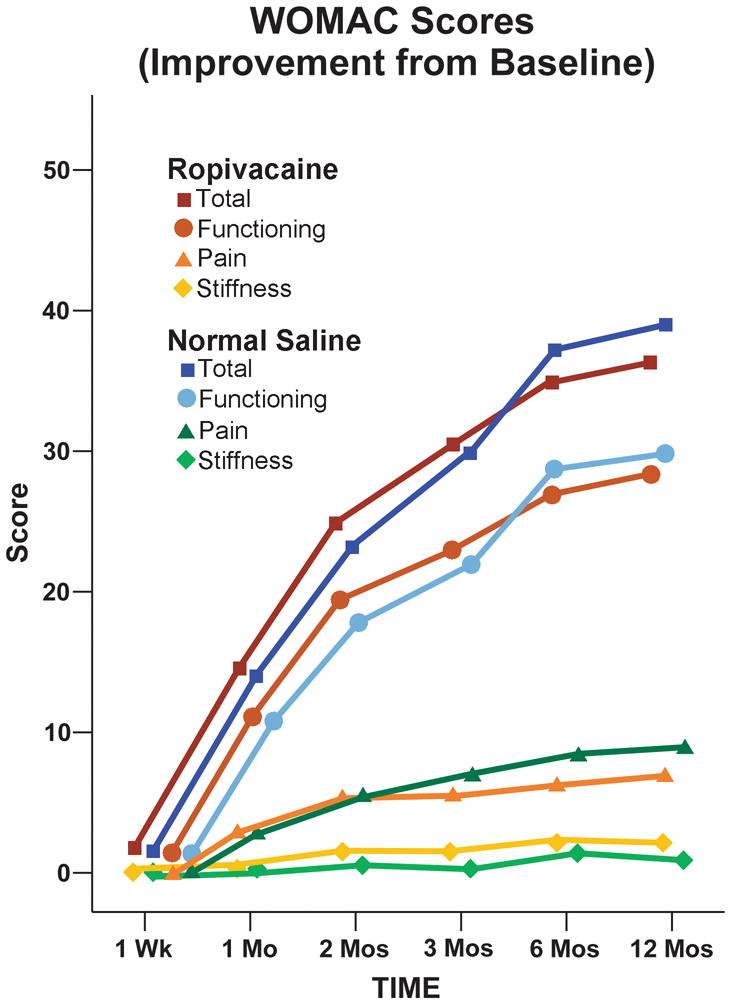
Effect of an extended femoral perineural ropivacaine infusion on improvement from preoperative baseline of health-related quality-of-life following tricompartment knee arthroplasty, as measured with the Western Ontario and McMaster Universities Osteoarthritis Index. Data are expressed as mean for patients randomly assigned to an extended continuous femoral nerve block (perineural ropivacaine from surgery through postoperative day 4) or overnight continuous femoral nerve block (perineural ropivacaine from surgery through 06:00 postoperative day 1 followed by perineural normal saline through postoperative day 4). The two treatment groups had similar scores at all individual time points (p>0.05).
Table 1.
Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) Scores.
| Absolute Values | Total minus Baseline (Improvement) | |||||
|---|---|---|---|---|---|---|
| Infusion: | Extended | Overnight | P-Value | Extended | Overnight | P-Value |
| Time | Mean (SD) [N] | Mean (SD) [N] | Mean (SD) [N] | Mean (SD) [N] | ||
| 0 (at surgery) | 50.0 (17.9) [28] | 55.5 (17.2) [26] | N/A | N/A | N/A | N/A |
| 1 Week | 47.3 (16.1) [28] | 53.3 (17.6) [26] | 0.19 | 2.8 (19.4) [28] | 2.3 (21.1) [26] | 0.93 |
| 1 Month | 35.2(19.8) [25] | 38.6 (14.4) [25] | 0.49 | 15.8 (16.0) [25] | 16.0 (15.0) [25] | 0.96 |
| 2 Months | 26.0 (14.0) [23] | 33.9 (15.2) [18] | 0.09 | 26.8 (19.4) [23] | 23.7 (21.5) [18] | 0.62 |
| 3 Months | 19.6 (12.2) [27] | 24.8 (18.3) [23] | 0.24 | 30.4 (21.4) [27] | 30.2 (22.3) [23] | 0.98 |
| 6 Months | 15.2 (13.7) [21] | 18.3 (17.6) [22] | 0.53 | 34.0 (22.7) [21] | 37.2 (22.7) [22] | 0.45 |
| 12 Months | 14.1 (14.6) [19] | 16.5 (19.4) [15] | 0.68 | 36.4 (20.3) [19] | 39.0 (21.5) [15] | 0.49 |
| AUC | 19.6 (11.6) [28] | 23.4 (15.8) [26] | 0.32 | 2.8 (19.4) [28] | 2.3 (21.1) [26] | 0.93 |
N/A: Not applicable
AUC: Area Under the Curve
A retrospective power calculation indicates that a study of 54 subjects (26 in one group and 28 in the other) has 80% power at P=0.05 (two-sided) to detect a difference of 0.78 standard deviations in mean AUC. This would translate to a sensitivity of about a 10-unit difference between the groups.
Discussion
This prospective, multicenter investigation found no evidence that extending an overnight cFNB to 4 days decreases subsequent post-infusion pain, stiffness, or functional disability between 7 days and 12 months following TKA. In contrast, multiple randomized, controlled trials have demonstrated clear benefits during the infusion.3;4;6;15;16
Pre-emptive analgesia
There are both theoretical reasons and clinical data suggesting that improving analgesia in the immediate postoperative period may decrease long-term pain, reduce joint stiffness, and improve functional status.17–21 For example, there are analogous data involving continuous epidural infusion effects on post-thoracotomy pain in which patients randomized to receive a thoracic bupivacaine/morphine epidural infusion from before surgery through 48 postoperative hours reported superior analgesia during the infusion when compared with patients randomized to receive intravenous patient-controlled morphine21 (similar to cFNB and TKA as reported in other studies3;15). After the epidural catheter was removed, patients who had received two days of epidural infusion had a lower risk of thoracotomy-associated pain at two (50% vs. 87%, p<0.05) and six months (45% vs. 78%, p<0.05) following surgery, as compared with patients receiving only intravenous opioids. Thus, the lack of long-term effect for an extended-duration cFNB of the current study is disappointing. While there may be short-term analgesic benefits following cFNB discontinuation two—randomized, controlled studies removed catheters on postoperative day 2 and still found a small improvement in pain scores 24–48 hours later16;22—there are currently little data to suggest that persistent chronic pain following catheter removal may be reduced with cFNB following TKA.
Study Limitations
A limitation of our study is that the control group received an initial femoral nerve block followed by an overnight cFNB, and not simply a single-injection femoral nerve block and/or opioids as is common in many practices in the United States. For TKA patients who do not receive any single-injection and/or continuous peripheral nerve block(s), the differences in postoperative health-related quality-of-life compared with a four-day cFNB as provided in this study may be much greater in the months following surgery. More importantly, WOMAC scores were secondary outcomes for the original study and thus do not have the statistical strength of primary outcomes. Nonetheless, our results do not suggest that there was any large effect of extending the cFNB from overnight to four postoperative days. In addition, the individual means, variances, and covariances at and between specific time points provided by this study may be used as planning parameters for future investigations. Lastly, the intervention protocol used in this investigation reflected our clinical practice during the study period. However, little data is available to define the optimal post-TKA infusion protocol. It is possible that an alternative infusion protocol would result in different findings than our study.
It is noteworthy that, as previously reported, there were a total of 4 falls during the cFNB in subjects receiving perineural ropivacaine, compared with none receiving perineural saline (p=0.24).6 Since that initial publication, a causal relationship has been demonstrated between continuous peripheral nerve blocks involving the femoral nerve and the risk of falling after knee and hip arthroplasty.23 Combined with the negative WOMAC results of the current and previous studies,5 the optimal duration of cFNB following knee arthroplasty must be questioned—the benefits of an extended duration infusion must be balanced against the potential risks.
Acknowledgments
The authors gratefully acknowledge the invaluable assistance of Thomas Peatman, MD, Attending Physician, Department of Orthopedics, Alta Bates Summit Medical Center (Oakland, California); Beverly Morris, RN, CNP, MBA, Educator, Department of Nursing, University of California San Diego (San Diego, California); Eliza Ferguson, BS, and Rosalita Maldonado, BS, both Research Coordinators, Department of Anesthesiology, University of California San Diego (San Diego, California); and Vickie Nolan, RN, CCRC, Clinical Research Manager, Jordan Research and Education Institute, Alta Bates Summit Medical Center (Oakland, California).
Funding Sources: Funding for this project was provided by the National Institutes of Health grant GM077026 from the National Institute of General Medical Sciences (Bethesda, Maryland); National Institutes of Health grants RR00082, RR000827, and RR025208 from the National Center for Research Resources (Bethesda, Maryland); the Department of Anesthesiology, University of California San Diego (San Diego, California); Stryker Instruments (Kalamazoo, Michigan); and Teleflex Medical (Research Triangle Park, North Carolina). The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of these entities.
Footnotes
This study was selected as a “Best of Meeting” presentation at the Spring 2010 ASRA meeting.
Conflicts of Interest: Teleflex Medical and Stryker Instruments provided funding for this investigation, these two companies had absolutely no input into any aspect of study conceptualization, design, and implementation; data collection, analysis and interpretation; or manuscript preparation. The funding from these companies was used by each institution to help defray research coordinator expenses for the original randomized clinical trial. Drs. Mariano and Loland previously conducted continuous peripheral nerve block workshops for Stryker Instruments. No other author has a personal financial interest in this research.
References
- 1.March LM, Cross MJ, Lapsley H, Brnabic AJ, Tribe KL, Bachmeier CJ, Courtenay BG, Brooks PM. Outcomes after hip or knee replacement surgery for osteoarthritis. A prospective cohort study comparing patients’ quality of life before and after surgery with age-related population norms. Med J Aust. 1999;171:235–8. [PubMed] [Google Scholar]
- 2.Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367:1618–25. doi: 10.1016/S0140-6736(06)68700-X. [DOI] [PubMed] [Google Scholar]
- 3.Singelyn FJ, Deyaert M, Joris D, Pendeville E, Gouverneur JM. Effects of intravenous patient-controlled analgesia with morphine, continuous epidural analgesia, and continuous three-in-one block on postoperative pain and knee rehabilitation after unilateral total knee arthroplasty. Anesth Analg. 1998;87:88–92. doi: 10.1097/00000539-199807000-00019. [DOI] [PubMed] [Google Scholar]
- 4.Ilfeld BM, Le LT, Meyer RS, Mariano ER, Vandenborne K, Duncan PW, Sessler DI, Enneking FK, Shuster JJ, Theriaque DW, Berry LF, Spadoni EH, Gearen PF. Ambulatory continuous femoral nerve blocks decrease time to discharge readiness after tricompartment total knee arthroplasty: a randomized, triple-masked, placebo-controlled study. Anesthesiology. 2008;108:703–13. doi: 10.1097/ALN.0b013e318167af46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ilfeld BM, Meyer RS, Le LT, Mariano ER, Williams BA, Vandenborne K, Duncan PW, Sessler DI, Enneking FK, Shuster JJ, Maldonado RC, Gearen PF. Health-related quality of life after tricompartment knee arthroplasty with and without an extended-duration continuous femoral nerve block: a prospective, 1-year follow-up of a randomized, triple-masked, placebo-controlled study. Anesth Analg. 2009;108:1320–5. doi: 10.1213/ane.0b013e3181964937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ilfeld BM, Mariano ER, Girard PJ, Loland VJ, Meyer RS, Donovan JF, Pugh GA, Le LT, Sessler DI, Shuster JJ, Theriaque DW, Ball ST. A multicenter, randomized, triple-masked, placebo-controlled trial of the effect of ambulatory continuous femoral nerve blocks on discharge-readiness following total knee arthroplasty in patients on general orthopaedic wards. Pain. 2010;150:477–84. doi: 10.1016/j.pain.2010.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–40. [PubMed] [Google Scholar]
- 8.Jones CA, Voaklander DC, Johnston DW, Suarez-Almazor ME. The effect of age on pain, function, and quality of life after total hip and knee arthroplasty. Arch Intern Med. 2001;161:454–60. doi: 10.1001/archinte.161.3.454. [DOI] [PubMed] [Google Scholar]
- 9.Bellamy N, Campbell J, Hill J, Band P. A comparative study of telephone versus onsite completion of the WOMAC 3.0 osteoarthritis index. J Rheumatol. 2002;29:783–6. [PubMed] [Google Scholar]
- 10.Bellamy N. WOMAC: a 20-year experiential review of a patient-centered self-reported health status questionnaire. J Rheumatol. 2002;29:2473–6. [PubMed] [Google Scholar]
- 11.Wright JG, Young NL. A comparison of different indices of responsiveness. J Clin Epidemiol. 1997;50:239–46. doi: 10.1016/s0895-4356(96)00373-3. [DOI] [PubMed] [Google Scholar]
- 12.Angst F, Aeschlimann A, Steiner W, Stucki G. Responsiveness of the WOMAC osteoarthritis index as compared with the SF-36 in patients with osteoarthritis of the legs undergoing a comprehensive rehabilitation intervention. Ann Rheum Dis. 2001;60:834–40. [PMC free article] [PubMed] [Google Scholar]
- 13.McConnell S, Kolopack P, Davis AM. The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC): a review of its utility and measurement properties. Arthritis Rheum. 2001;45:453–61. doi: 10.1002/1529-0131(200110)45:5<453::aid-art365>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 14.Brazier JE, Harper R, Munro J, Walters SJ, Snaith ML. Generic and condition-specific outcome measures for people with osteoarthritis of the knee. Rheumatology (Oxford) 1999;38:870–7. doi: 10.1093/rheumatology/38.9.870. [DOI] [PubMed] [Google Scholar]
- 15.Capdevila X, Barthelet Y, Biboulet P, Ryckwaert Y, Rubenovitch J, d’Athis F. Effects of perioperative analgesic technique on the surgical outcome and duration of rehabilitation after major knee surgery. Anesthesiology. 1999;91:8–15. doi: 10.1097/00000542-199907000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Salinas FV, Liu SS, Mulroy MF. The effect of single-injection femoral nerve block versus continuous femoral nerve block after total knee arthroplasty on hospital length of stay and long-term functional recovery within an established clinical pathway. Anesth Analg. 2006;102:1234–9. doi: 10.1213/01.ane.0000198675.20279.81. [DOI] [PubMed] [Google Scholar]
- 17.Ryu J, Saito S, Yamamoto K, Sano S. Factors influencing the postoperative range of motion in total knee arthroplasty. Bull Hosp Jt Dis. 1993;53:35–40. [PubMed] [Google Scholar]
- 18.Shoji H, Solomonow M, Yoshino S, D’Ambrosia R, Dabezies E. Factors affecting postoperative flexion in total knee arthroplasty. Orthopedics. 1990;13:643–9. doi: 10.3928/0147-7447-19900601-08. [DOI] [PubMed] [Google Scholar]
- 19.Perkins FM, Kehlet H. Chronic pain as an outcome of surgery. A review of predictive factors. Anesthesiology. 2000;93:1123–33. doi: 10.1097/00000542-200010000-00038. [DOI] [PubMed] [Google Scholar]
- 20.Akeson WH, Amiel D, Abel MF, Garfin SR, Woo SL. Effects of immobilization on joints. Clin Orthop. 1987:28–37. [PubMed] [Google Scholar]
- 21.Senturk M, Ozcan PE, Talu GK, Kiyan E, Camci E, Ozyalcin S, Dilege S, Pembeci K. The effects of three different analgesia techniques on long-term postthoracotomy pain. Anesth Analg. 2002;94:11–5. doi: 10.1213/00000539-200201000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Williams BA, Kentor ML, Vogt MT, Irrgang JJ, Bottegal MT, West RV, Harner CD, Fu FH, Williams JP. Reduction of verbal pain scores after anterior cruciate ligament reconstruction with 2-day continuous femoral nerve block: a randomized clinical trial. Anesthesiology. 2006;104:315–27. doi: 10.1097/00000542-200602000-00018. [DOI] [PubMed] [Google Scholar]
- 23.Ilfeld BM, Duke BK, Donohue MC. Association between lower extremity continuous peripheral nerve blocks and patient falls following knee and hip arthroplasty. Anesth Analg. doi: 10.1213/ANE.0b013e3181fb9507. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]


