Abstract
Initiated by the finding that platelets express functional CD40 ligand (CD40L, CD154), many new roles for platelets have been discovered in unanticipated areas, including the immune response. When current literature is considered as a whole, the picture that is emerging begins to show that platelets are able to significantly affect, for better or worse, the overall health and condition of the mammalian host. Animal models have made significant contributions to our expanding knowledge of platelet function, much of which is anticipated to be clinically relevant. While still mostly circumstantial, the evidence supports a critical role for CD40L in many normal and disease processes.
Keywords: T lymphocyte, B lymphocyte, platelet, CD40L, immunity, inflammation
Introduction
The discovery of functional CD40L expression by platelets [1] has led to interesting and novel investigations into areas well beyond traditional platelet coagulation pathways. While platelets have long been known to possess innate immune functions, CD40L expression extends potential modulatory capacity to inflammatory processes mediated by the CD40-CD40L axis, the most well-known being adaptive immune function. Specifically, CD40L is critical in the production of T-dependent isotype switched antibodies by B cells, as well as dendritic cell activation which significantly enhances the CD8 T cell response via antigen presentation. These topics have been well-described and firmly establish CD40L as a molecule central to adaptive immunity [2]. Since platelet numbers in circulation are second only to red blood cells, their overall CD40L bulk is high, providing the potential to deliver significant signaling capacity that could modulate early responses to alterations in homeostasis. Platelets have been described to play roles in wound repair and healing, innate control of bacteria, viruses, fungi, protozoans, leukocyte recruitment and extravasation, adaptive B and T cell responses involved in pathogen clearance, and autoinflammatory conditions such as systemic lupus erythematosus (SLE), arthritis, and atherosclerosis [3–5]. This broad range of activity is due to the large number of preformed mediators expressed/released by platelets upon activation, and the broad activity of those molecules. While most of these functions do not depend on CD40L, it can make a measurable contribution in the area of thrombosis, atherosclerosis, and adaptive immunity.
Platelets and adaptive immunity
CD40L is a pivotal molecule in adaptive immune function. In its absence, T-dependent humoral immunity is debilitated [6], and several important T cell functions are compromised, including antigen presenting cell (APC) activation, lytic activity, cross presentation and memory formation [7]. When platelets were reported to express functional CD40L, it became intriguing to hypothesize that platelets could somehow participate in or influence adaptive immune responses. The initial report that indicated platelets could promote B and T cell responses via CD40L first demonstrated that platelet CD40L could activate dendritic cells (DC)in vitro [8], an observation confirmed by several other groups [9–12]. DC are arguably the most important link between innate and adaptive immunity since they are thought to be the only cell capable of activating naïve T cells sufficiently for induction of a productive cellular immune response [13]. If platelet CD40L can activate DC in vivo, then a logical prediction is that T cell responses should also be enhanced by platelet CD40L. Using CD40L−/− mice it was demonstrated that 24 hours prior to intravenous immunization with adenovirus, adoptively transferred wild-type platelets significantly enhanced CD8 T cell antigen specific activity as measured by in vitro lytic function, IFNγ production and vivo lytic function [8, 14]. Further evidence of the importance of platelets to T cell responses is the observation that platelet depletion in normal mice results in a reduction of CD8 T cell lytic activity [14]. While platelet depletion does not directly measure the contribution of platelet CD40L, it does indicate that a fully CD40L-intact immune system does not function optimally in the absence of platelets, which can express CD40L. Additionally, LCMV infection in mice resulted in an 80% reduction of the number of reactive cytotoxic T lymphocytes (CTL) in the absence of platelets [15]. Interestingly, upon platelet reconstitution in the depleted mice, CTL cell numbers were increased indicating that platelets may also play a role in T cell expansion during an immune response. This observation was partially dependent on CD40L expression by platelets.
Platelets also have been reported to modulate T cell responses through non-CD40L dependent mechanisms. In a hepatitis model, platelet depletion was shown to prevent CTL entry into the liver, which protected acute organ damage [16]. The same observation was made by simply inhibiting platelet activation/function through administration of aspirin or clopidogrel. Alternatively, through the release of serotonin, platelets were reported to inhibit hepatic T cell infiltration into the intralobular region, thus protecting the organ from acute CTL damage [17]. However, this results in higher viral titers in the liver and could contribute to chronic hepatitis. It has also been shown that platelets and T cells reciprocate in an immune response amplification pathway. This is accomplished by T cell triggering of CD40 on platelets causing them to release RANTES, which binds to endothelial cells and mediates T cell recruitment [18].
Using the murine CD40L−/− model, platelet CD40L also was shown to mediate a dramatic effect on antibody production. Due to the absence of CD40L on CD4 T cells, these mice are unable to produce T-dependent isotype-switched antibody above background levels since they are unable to form germinal centers (GC) [19], the structures of intense B cell proliferation that leads to class switch recombination, somatic hypermutation, and differentiation into memory or plasma cells [6]. Therefore, after adoptive transfer of normal platelets into CD40L−/− mice, any IgG produced upon immunization can be directly attributed to platelet CD40L. It was reported that normal platelets in CD40L−/− mice enabled modest, but significant, production of anti-adenovirus IgG upon immunization [8]. This antibody was transient and occurred in the absence of GC formation, but demonstrated the principle that platelet CD40L could participate in B cell immune responses. One possible explanation was that this function only occurred in the artificial setting where CD40L+ CD4 T cells were absent. Subsequent studies demonstrated that minimal numbers of adoptively transferred normal CD4 T cells conferred the ability to form low numbers of GC in CD40L−/− mice upon intravenous adenovirus immunization [20]. When normal platelets were co-transferred with CD4 T cells, GC formation was significantly increased as was adenovirus-specific IgG. This confirmed that platelet CD40L contributed in a significant manner to IgG antibody production in the presence of normal T cells. To assess the role of platelet-derived CD40L in a CD40L intact host, depletion of platelets in normal mice dramatically decreased the amount of IgG produced against adenovirus, confirming the requirement of platelets for the normal B cell response, as also seen in the T cell compartment [14, 20]. A notable observation of these studies was that platelets demonstrated the most influence on antibody/GC formation when antigen dose was comparatively low, or in a limited presence of contributing CD4 T cells. This suggested that a role for platelets in adaptive host defense might be most apparent in studies approaching a more physiologic introduction of antigen, such as a wound model [21], where antigen dose is comparatively low as well as precursor T/B cell numbers. This setting was accomplished by determining a subcutaneous dose of adenovirus that is just below the threshold required to induce a detectable adaptive immune response. The appropriate dose was then delivered in a defined amount of porcine type I collagen to mimic a basement membrane exposure along with delivery of antigen (type 5 adenovirus recombinant for a membrane form of chicken ovalbumin (Ad5mOVA)) [14]. When normal mice were injected subcutaneously with low-dose Ad5mOVA in collagen, but not PBS, OVA-specific T cell responses were markedly enhanced. This observation was dependent on the ability of platelets to be able to respond to collagen as shown by blocking antibodies to gpVI and transgenic mice whose platelets cannot be activated by collagen (gpVI−/−), and to express CD40L, as T cell responses were not enhanced in CD40L−/− mice unless they were first given normal platelets. Mice immunized with Ad5mOVA in collagen were also shown to be significantly protected from a lethal challenge of virulent Listeria monocytogenes recombinant for OVA.
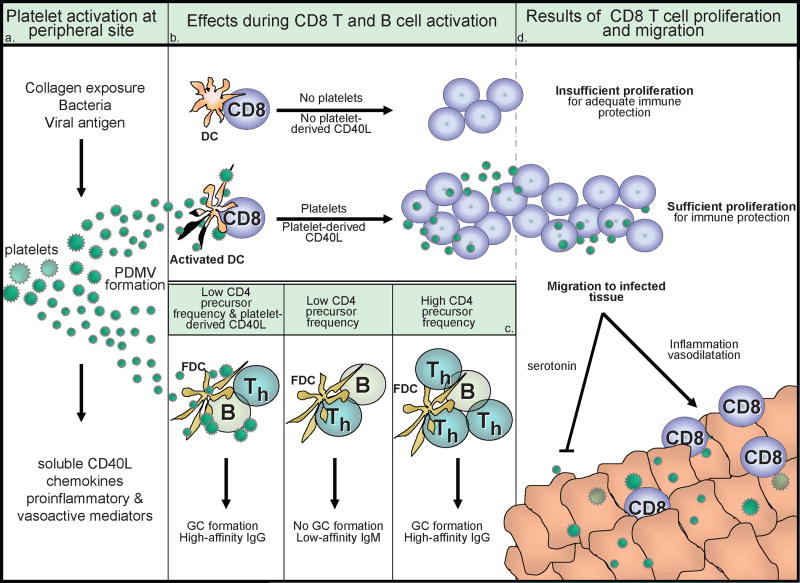
The mechanism by which platelets, thought to be largely restricted to circulation, are able to gain access to DC in draining lymph nodes to enhance T cell responses is uncertain. And while present in large numbers in the spleen, platelets are observed only in the red pulp, separated from the white pulp containing T and B cells. Although platelets can directly bind, communicate, and prompt specific functions of T and B cells and DC in vitro, the direct role this plays in vivo is only starting to be understood [22]. In a step towards defining the in vivo mechanism, it was shown that platelet-derived microvesicles (largely comprised of microparticles) released upon activation are capable of eliciting the same antibody/GC responses as intact platelets when adoptively transferred to CD40L−/− mice prior to immunization [23]. These data support the hypothesis that platelets, activated distally in the periphery, release microparticles that traffic to the area of B and T cell immunologic induction to enhance the adaptive response. To accomplish this, it is still uncertain whether platelets/microparticles directly contact lymphoctyes, or enhance the lymphocyte response through better activation of DC or follicular DC, the APC responsible for B cell activation. This is distinctly possible since microparticles as cellular messengers has previously been discussed [24]. Figure 1 provides a simplified summary diagram of what may occur in vivo.
Figure 1. The effect of platelets on adaptive immune responses during activation, proliferation, and migration.
(a) Platelets become activated at site of injury or after contact with bacteria and viral particles, breaking into platelet-derived microvescicles (PDMV) and secreting soluble inflammatory mediators, including sCD40L. (b) In the absence of platelets or when platelet-derived CD40L is not available, decreased numbers of antigen specific T cells are generated leading to inadequate immune protection. However, in the presence of platelets or when platelets are the sole source of CD40L in a CD40L-deficient environment, increased numbers of antigen specific T cells are generated leading to adequate immune protection. This could potentially be mediated by platelet-induced activation of dendritic cells (DC). Platelets have also been shown to contribute to CD8 T cell proliferation as in (d). (c) When platelets are immunodepleted from WT mice or when platelet-derived CD40L is not available in a CD40L-deficient environment, T dependent isotype switching is very limited. When platelets or PDMV and limiting numbers of CD4 T cells are available, germinal center (GC) formation and isotype switching is enabled. This could potentially be mediated by platelet-induced activation of follicular dendritic cells (FDC) or direct stimulation of B cells. Platelets are not necessary for GC formation when high frequencies of CD4 T helper cells are available. (d) Platelets have also been shown to affect T cell accumulation in infected tissues. Platelet-derived serotonin can inhibit CD8 T cell migration into infected liver, but platelets also have a long-standing role for the induction of vasodilation and allowing CD8 T cell tethering/rolling.
Other functions of platelet CD40L
While platelet CD40L may help in host defense, there is also indirect evidence that platelets may contribute to inflammatory diseases. Diabetes [25], inflammatory bowel disease [26], atherosclerosis [27], and immune thrombocytopenia [28]are among those conditions where platelets circulate in higher numbers and higher states of activation [29]. Of note is the enhanced amount of circulating soluble CD40L (sCD40L) which is attributed to platelets [30], present in many inflammatory diseases. Currently, it is unknown if this plays a role in disease initiation/progression or is simply a marker of disease and lacks significant biologic activity.
Platelet CD40L is able to interact with many other cells besides lymphocytes and DC, including other platelets [31, 32], endothelial cells [1], granulocytes [33], fibroblasts [34], and macrophages and monocytes [35]. The exact nature and results of these interactions are not always clear, but it is documented that endothelial cells can be activated by platelet CD40L to release mediators that are chemoattractants for leukocytes, potentially providing signals to facilitate inflammation at sites of injury [1]. Transfusion-related acute lung injury, an uncommon but often fatal blood transfusion reaction, may be due to CD40L released by platelets which then binds to sensitized neutrophils in the lungs causing them to degranulate and produce symptoms [36]. Macrophages are well known to participate and influence immune responses, but are also documented to contribute to fibrosis present in atherosclerosis, which may be directed partially by platelet CD40L [37]. Furthermore, in atherosclerosis-prone apoE−/− mice, platelet CD40L plays a crucial role in leukocyte activation leading to atherosclerosis [27]. Another interesting contribution to this observation is that platelet CD40L decreased regulatory T cell number, permitting accelerated atherosclerosis. And finally, platelet CD40L is able to activate other platelets by binding to platelet-expressed CD40 [31, 32]. In support of this observation, it is documented that platelets from CD40L−/− mice exhibit reduced aggregation in vitro and thrombus stability in vivo [38]. These observations may address the tragic clinical result of unexplained fatal thromboembolism in SLE patients undergoing experimental anti-CD40L monoclonal antibody therapy [39] as well as the report that anti-CD40L immune complexes can activate platelets through transgenically expressed human FcγRIIa and result in pulmonary thrombosis [40]. Either way, the role of platelet CD40L in thrombosis and immunity is becoming more prominent and may have clinical implications.
Conclusions
The field of platelet biology has been revolutionized since the discovery of functional CD40L on platelets. While their innate immune function has been documented but overlooked until recently, we now know that platelets can play a much larger role in homeostasis than just coagulation. However, platelets can play a role in the dark side of CD40L function as well. It will be important to determine if immunotherapy which frequently relies on T cell CD40L has any role to play in adverse advents such as thrombosis, and whether exploiting platelet CD40L for immunotherapy or specific silencing to control autoimmunity will have clinical application.
Abbreviations
- CD40L
CD40 Ligand (also CD154)
- SLE
systemic lupus erythematosus
- DC
dendritic cell
- IFNγ
interferon gamma
- CTL
cytotoxic T lymphocyte
- GC
germinal center
- RANTES
regulated upon activation normal T cell expressed and secreted
- Ad5mOVA
adenovirus serotype 5 recombinant for membrane-bound chicken ovalbumin
- sCD40L
soluble CD40L
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Henn V, Slupsky JR, Grafe M, Anagnostopoulos I, Forster R, Muller-Berghaus G, Kroczek RA. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature. 1998;391:591–594. doi: 10.1038/35393. [DOI] [PubMed] [Google Scholar]
- 2.Elgueta R, Benson MJ, de Vries VC, Wasiuk A, Guo Y, Noelle RJ. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol Rev. 2009;229:152–172. doi: 10.1111/j.1600-065X.2009.00782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yeaman MR. The role of platelets in antimicrobial host defense. Clin Infect Dis. 1997;25:951–968. doi: 10.1086/516120. quiz 969–970. [DOI] [PubMed] [Google Scholar]
- 4.Klinger MH. Platelets and inflammation. Anat Embryol (Berl) 1997;196:1–11. doi: 10.1007/s004290050075. [DOI] [PubMed] [Google Scholar]
- 5.Sowa JM, Crist SA, Ratliff TL, Elzey BD. Platelet influence on T- and B-cell responses. Arch Immunol Ther Exp (Warsz) 2009;57:235–241. doi: 10.1007/s00005-009-0032-y. [DOI] [PubMed] [Google Scholar]
- 6.Wolniak KL, Shinall SM, Waldschmidt TJ. The germinal center response. Crit Rev Immunol. 2004;24:39–65. doi: 10.1615/critrevimmunol.v24.i1.20. [DOI] [PubMed] [Google Scholar]
- 7.Bevan MJ. Helping the CD8(+) T-cell response. Nat Rev Immunol. 2004;4:595–602. doi: 10.1038/nri1413. [DOI] [PubMed] [Google Scholar]
- 8.Elzey BD, Tian J, Jensen RJ, Swanson AK, Lees JR, Lentz SR, Stein CS, Nieswandt B, Wang Y, Davidson BL, Ratliff TL. Platelet-mediated modulation of adaptive immunity. A communication link between innate and adaptive immune compartments. Immunity. 2003;19:9–19. doi: 10.1016/s1074-7613(03)00177-8. [DOI] [PubMed] [Google Scholar]
- 9.Kaneider NC, Kaser A, Tilg H, Ricevuti G, Wiedermann CJ. CD40 ligand-dependent maturation of human monocyte-derived dendritic cells by activated platelets. Int J Immunopathol Pharmacol. 2003;16:225–231. doi: 10.1177/039463200301600307. [DOI] [PubMed] [Google Scholar]
- 10.Czapiga M, Kirk AD, Lekstrom-Himes J. Platelets deliver costimulatory signals to antigen-presenting cells: a potential bridge between injury and immune activation. Exp Hematol. 2004;32:135–139. doi: 10.1016/j.exphem.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Martinson J, Bae J, Klingemann HG, Tam Y. Activated platelets rapidly up-regulate CD40L expression and can effectively mature and activate autologous ex vivo differentiated DC. Cytotherapy. 2004;6:487–497. doi: 10.1080/14653240410005249. [DOI] [PubMed] [Google Scholar]
- 12.Duffau P, Seneschal J, Nicco C, Richez C, Lazaro E, Douchet I, Bordes C, Viallard JF, Goulvestre C, Pellegrin JL, Weil B, Moreau JF, Batteux F, Blanco P. Platelet CD154 potentiates interferon-alpha secretion by plasmacytoid dendritic cells in systemic lupus erythematosus. Sci Transl Med. 2:47ra63. doi: 10.1126/scitranslmed.3001001. [DOI] [PubMed] [Google Scholar]
- 13.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 14.Elzey BD, Schmidt NW, Crist SA, Kresowik TP, Harty JT, Nieswandt B, Ratliff TL. Platelet-derived CD154 enables T-cell priming and protection against Listeria monocytogenes challenge. Blood. 2008;111:3684–3691. doi: 10.1182/blood-2007-05-091728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iannacone M, Sitia G, Isogawa M, Whitmire JK, Marchese P, Chisari FV, Ruggeri ZM, Guidotti LG. Platelets prevent IFN-alpha/beta-induced lethal hemorrhage promoting CTL-dependent clearance of lymphocytic choriomeningitis virus. Proc Natl Acad Sci U S A. 2008;105:629–634. doi: 10.1073/pnas.0711200105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iannacone M, Sitia G, Isogawa M, Marchese P, Castro MG, Lowenstein PR, Chisari FV, Ruggeri ZM, Guidotti LG. Platelets mediate cytotoxic T lymphocyte-induced liver damage. Nat Med. 2005;11:1167–1169. doi: 10.1038/nm1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lang PA, Contaldo C, Georgiev P, El-Badry AM, Recher M, Kurrer M, Cervantes-Barragan L, Ludewig B, Calzascia T, Bolinger B, Merkler D, Odermatt B, Bader M, Graf R, Clavien PA, Hegazy AN, Lohning M, Harris NL, Ohashi PS, Hengartner H, Zinkernagel RM, Lang KS. Aggravation of viral hepatitis by platelet-derived serotonin. Nat Med. 2008;14:756–761. doi: 10.1038/nm1780. [DOI] [PubMed] [Google Scholar]
- 18.Danese S, de la Motte C, Reyes BM, Sans M, Levine AD, Fiocchi C. Cutting edge: T cells trigger CD40-dependent platelet activation and granular RANTES release: a novel pathway for immune response amplification. J Immunol. 2004;172:2011–2015. doi: 10.4049/jimmunol.172.4.2011. [DOI] [PubMed] [Google Scholar]
- 19.Xu J, Foy TM, Laman JD, Elliott EA, Dunn JJ, Waldschmidt TJ, Elsemore J, Noelle RJ, Flavell RA. Mice deficient for the CD40 ligand. Immunity. 1994;1:423–431. doi: 10.1016/1074-7613(94)90073-6. [DOI] [PubMed] [Google Scholar]
- 20.Elzey BD, Grant JF, Sinn HW, Nieswandt B, Waldschmidt TJ, Ratliff TL. Cooperation between platelet-derived CD154 and CD4+ T cells for enhanced germinal center formation. J Leukoc Biol. 2005;78:80–84. doi: 10.1189/jlb.1104669. [DOI] [PubMed] [Google Scholar]
- 21.Viswanathan K, Dhabhar FS. Stress-induced enhancement of leukocyte trafficking into sites of surgery or immune activation. Proc Natl Acad Sci U S A. 2005;102:5808–5813. doi: 10.1073/pnas.0501650102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morrell CN, Sun H, Swaim AM, Baldwin WM. 3rd, Platelets an inflammatory force in transplantation. Am J Transplant. 2007;7:2447–2454. doi: 10.1111/j.1600-6143.2007.01958.x. [DOI] [PubMed] [Google Scholar]
- 23.Sprague DL, Elzey BD, Crist SA, Waldschmidt TJ, Jensen RJ, Ratliff TL. Platelet-mediated modulation of adaptive immunity: unique delivery of CD154 signal by platelet-derived membrane vesicles. Blood. 2008;111:5028–5036. doi: 10.1182/blood-2007-06-097410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shantsila E, Kamphuisen P, Wand Lip GY. Circulating microparticles in cardiovascular disease: implications for atherogenesis and atherothrombosis. J Thromb Haemost. 2010 doi: 10.1111/j.1538–7836.2010.04007.x. [DOI] [PubMed] [Google Scholar]
- 25.Ray DM, Spinelli SL, O’Brien JJ, Blumberg N, Phipps RP. Platelets as a novel target for PPARgamma ligands: implications for inflammation, diabetes, and cardiovascular disease. BioDrugs. 2006;20:231–241. doi: 10.2165/00063030-200620040-00004. [DOI] [PubMed] [Google Scholar]
- 26.Collins CE, Cahill MR, Newland AC, Rampton DS. Platelets circulate in an activated state in inflammatory bowel disease. Gastroenterology. 1994;106:840–845. doi: 10.1016/0016-5085(94)90741-2. [DOI] [PubMed] [Google Scholar]
- 27.Lievens D, Zernecke A, Seijkens T, Soehnlein O, Beckers L, Munnix IC, Wijnands E, Goossens P, van Kruchten R, Thevissen L, Boon L, Flavell RA, Noelle RJ, Gerdes N, Biessen EA, Daemen MJ, Heemskerk JW, Weber C, Lutgens E. Platelet CD40L mediates thrombotic and inflammatory processes in atherosclerosis. Blood. 2010 doi: 10.1182/blood-2010-01-261206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Solanilla A, Pasquet JM, Viallard JF, Contin C, Grosset C, Dechanet-Merville J, Dupouy M, Landry M, Belloc F, Nurden P, Blanco P, Moreau JF, Pellegrin JL, Nurden AT, Ripoche J. Platelet-associated CD154 in immune thrombocytopenic purpura. Blood. 2005;105:215–218. doi: 10.1182/blood-2003-07-2367. [DOI] [PubMed] [Google Scholar]
- 29.Danese S, Fiocchi C. Platelet activation and the CD40/CD40 ligand pathway: mechanisms and implications for human disease. Crit Rev Immunol. 2005;25:103–121. doi: 10.1615/critrevimmunol.v25.i2.20. [DOI] [PubMed] [Google Scholar]
- 30.Danese S, Katz JA, Saibeni S, Papa A, Gasbarrini A, Vecchi M, Fiocchi C. Activated platelets are the source of elevated levels of soluble CD40 ligand in the circulation of inflammatory bowel disease patients. Gut. 2003;52:1435–1441. doi: 10.1136/gut.52.10.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prasad KS, Andre P, He M, Bao M, Manganello J, Phillips DR. Soluble CD40 ligand induces beta3 integrin tyrosine phosphorylation and triggers platelet activation by outside-in signaling. Proc Natl Acad Sci U S A. 2003;100:12367–12371. doi: 10.1073/pnas.2032886100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Inwald DP, McDowall A, Peters MJ, Callard RE, Klein NJ. CD40 is constitutively expressed on platelets and provides a novel mechanism for platelet activation. Circ Res. 2003;92:1041–1048. doi: 10.1161/01.RES.0000070111.98158.6C. [DOI] [PubMed] [Google Scholar]
- 33.Manfredi AA, Rovere-Querini P, Maugeri N. Dangerous connections: neutrophils and the phagocytic clearance of activated platelets. Curr Opin Hematol. 2010;17:3–8. doi: 10.1097/MOH.0b013e3283324f97. [DOI] [PubMed] [Google Scholar]
- 34.Boilard E, Nigrovic PA, Larabee K, Watts GF, Coblyn JS, Weinblatt ME, Massarotti EM, Remold-O’Donnell E, Farndale RW, Ware J, Lee DM. Platelets amplify inflammation in arthritis via collagen-dependent microparticle production. Science. 2010;327:580–583. doi: 10.1126/science.1181928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McGregor L, Martin J, McGregor JL. Platelet-leukocyte aggregates and derived microparticles in inflammation, vascular remodelling and thrombosis. Front Biosci. 2006;11:830–837. doi: 10.2741/1840. [DOI] [PubMed] [Google Scholar]
- 36.Khan SY, Kelher MR, Heal JM, Blumberg N, Boshkov LK, Phipps R, Gettings KF, McLaughlin NJ, Silliman CC. Soluble CD40 ligand accumulates in stored blood components, primes neutrophils through CD40, and is a potential cofactor in the development of transfusion-related acute lung injury. Blood. 2006;108:2455–2462. doi: 10.1182/blood-2006-04-017251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lievens D, Eijgelaar WJ, Biessen EA, Daemen MJ, Lutgens E. The multi-functionality of CD40L and its receptor CD40 in atherosclerosis. Thromb Haemost. 2009;102:206–214. doi: 10.1160/TH09-01-0029. [DOI] [PubMed] [Google Scholar]
- 38.Andre P, Prasad KS, Denis CV, He M, Papalia JM, Hynes RO, Phillips DR, Wagner DD. CD40L stabilizes arterial thrombi by a beta3 integrin--dependent mechanism. Nat Med. 2002;8:247–252. doi: 10.1038/nm0302-247. [DOI] [PubMed] [Google Scholar]
- 39.Yazdany J, Davis J. The role of CD40 ligand in systemic lupus erythematosus. Lupus. 2004;13:377–380. doi: 10.1191/0961203304lu1030oa. [DOI] [PubMed] [Google Scholar]
- 40.Robles-Carrillo L, Meyer T, Hatfield M, Desai H, Davila M, Langer F, Amaya M, Garber E, Francis JL, Hsu YM, Amirkhosravi A. Anti-CD40L immune complexes potently activate platelets in vitro and cause thrombosis in FCGR2A transgenic mice. J Immunol. 2010;185:1577–1583. doi: 10.4049/jimmunol.0903888. [DOI] [PubMed] [Google Scholar]