Abstract
Background
Multiple Sclerosis (MS) is an inflammatory demyelinating disease of the human central nervous system. While the clinical impact of gray matter pathology in MS brains is unknown, 30–40% of MS patients demonstrate memory impairment. The molecular basis of this memory dysfunction has not yet been investigated in MS patients.
Method
To investigate possible mechanisms of memory impairment in MS patients, we compared morphological and molecular changes in myelinated and demyelinated hippocampi from postmortem MS brains.
Findings
Demyelinated hippocampi had minimal neuronal loss but significant decreases in synaptic density. Neuronal proteins essential for axonal transport, synaptic plasticity, glutamate neurotransmission, glutamate homeostasis and memory/learning were significantly decreased in demyelinated hippocampi, but not in demyelinated motor cortices from MS brains.
Interpretation
Collectively, these data support hippocampal demyelination as a cause of synaptic alterations in MS patients and establish that the neuronal genes regulated by myelination reflect specific functions of neuronal subpopulations.
Keywords: Multiple Sclerosis, hippocampus, demyelination, memory
INTRODUCTION
Multiple Sclerosis (MS), an inflammatory-mediated demyelinating disease of the central nervous system (CNS), affects more than 2.5 million people worldwide 1,2. Although traditionally considered a white matter disease, grey matter demyelination and associated neuronal pathology play significant roles in the pathogenesis of permanent neurological disability in MS patients 1 and may cause the cognitive dysfunction found in over 50% of MS patients 3. Brain imaging studies have correlated white matter lesion load and brain atrophy with cognitive dysfunction in MS patients 4,5. A subgroup of cognitively impaired MS patients, however, have low white matter lesion load and minimal physical disability, raising the possibility that hippocampal demyelination may cause memory dysfunction in MS patients 5. Recent imaging studies have correlated altered hippocampal magnetic resonance imaging (MRI) measures6 and increased hippocampal atrophy 7 with memory dysfunction in MS patients. Hippocampal demyelination has been detected in 53% to 79% of postmortem MS hippocampi 8,9. These studies also support the concept that hippocampal demyelination causes a greater decrease in synaptic density compared to neuronal loss, thereby leading to a “dying back” axonopathy.
The hippocampus orchestrates the formation, maintenance and retrieval of memory through an equilibrated cascade of molecular events 10,11. These include axonal transport, synaptic plasticity, glutamate homeostasis, activation of calcium/calmodulin kinase II (CaMKII), phosphorylation of the transcription factor cyclic AMP binding protein (CREB), and transcription of glutamate receptors 11–14. While the role of myelin in regulating neuronal molecules involved in hippocampal function has not been investigated, myelin-axonal interactions can modulate neuronal gene expression 15. While the signaling mechanisms responsible for this gene regulation remain to be determined, the neuronal genes regulated by myelination are likely to reflect the specialized functions of different neuronal subpopulations. This hypothesis predicts that demyelination of motor and hippocampal neurons would alter expression of different neuronal genes.
This report describes molecular and cellular changes that result from demyelination of the hippocampus in brains from individuals with MS. Hippocampal demyelination negatively impacts molecules involved in axonal transport, synaptic integrity, glutamate homeostasis, synaptic plasticity and memory/learning. The molecular changes observed in demyelinated hippocampi were not detected in demyelinated MS motor cortex or in hippocampi from Alzheimer’s disease (AD) brains. These data support the concept that myelin is essential for the maintenance of normal memory function by the hippocampus. These results also demonstrate that neuronal genes regulated by myelination reflect the specialized functions of different neuronal subpopulations.
METHODS
Tissue collection and characterization
All brains were collected as part of the tissue procurement program approved by the Cleveland Clinic Institutional Review Board. Brains were removed according to a rapid autopsy protocol, sliced (1 cm thick), and slices either fixed in 4% paraformaldehyde for morphological studies or rapidly frozen for biochemical analysis. Patient demographics are listed in Supplemental Table I. Sections (30 µm thick) from fixed blocks of the hippocampus were cut on a sliding microtome, microwaved in 10mM citric acid buffer (pH 6.0) for 5 minutes, incubated in 3% hydrogen peroxide and 1% Triton X-100 in phosphate buffered saline for 30 minutes and immunostained by the avidin-biotin complex procedure with diaminobenzidine (DAB) for myelin proteolipid protein (PLP) & MHC Class II or HuR as described previously 16. Extent of demyelination and neuronal status were determined from PLP and HuR staining. Sections used for double-labeling experiments were pretreated as above and incubated with two primary antibodies for 3–5 days followed by fluorescently-conjugated and biotinylated secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA) for 1–2 hours.
Microarray assay, statistical analysis and functional categories
Four control, four myelinated MS and four demyelinated MS hippocampi were selected from the frozen control and MS brain slices (see supplementary Table I. Frozen blocks of hippocampus were characterized for demyelination and other frank pathology using 14 µm sections. Myelinated and demyelinated hippocampi were selected based on immunohistochemical analysis of PLP. Sixty-um-thick sections from control, myelinated and demyelinated MS hippocampi were collected and used for RNA isolation. Details of RNA isolation, labeling and microarray analysis have been described previously 17,18. RNA was compared on Affymetrix U133A microarrays, intensity was determined using Microarray Analysis Suite software 5.0 (Affymetrix, Santa Clara, CA), imported to Genespring version 7.0 (Agilent Technologies, Redwood City, CA) and normalized to the median of all genes. Univariate and principle component analysis were used to determine intensity distribution and eliminate sample outliers. The number of false positives arising from multiple comparisons was controlled using the false discovery rate (FDR) procedure of Benjamini and Hochberg 19 at the conventional FDR threshold of 5%. Significantly altered genes were queried through the DAVID Functional annotation tool (http://david.abcc.ncifcrf.gov). To identify significantly enriched biological groups, we applied the Benjamini and Hochberg 19 FDR correction over the standard t-test.
Quantitative RT-PCR
Hippocampal tissue isolated from five control, five myelinated and five demyelinated hippocampi was reverse transcribed as detailed previously 17,18. Gene-specific PCR was performed using SYBR Green I kit and a Roche Lightcycler (Roche Diagnostics, Indianapolis, IN) and standardized to 18S RNA (Ambion Inc, Austin, TX). Primer sequences are appended (supplemental Table SII). Each sample was run in triplicate with melting curve analysis to detect primer dimer artifacts. Normal distribution of the data was confirmed using a Shapiro-Wilk test (Analyse-It Software, Leeds, UK). Threshold cycle of each gene was normalized against GAPDH to calculate delta Ct values and relative expression changes.
Measurement of Synaptic density
Thirty micron thick sections of myelinated (n=3) and demyelinated MS hippocampus (n=3) were fluorescently labeled with synaptophysin (green) and MAP2 (red). Synapses surrounding the area around neuronal cell body were acquired at × 63 magnification, transferred to a work station and split into the three (red. blue and green) color channels using ImageJ. The images were coded and the experimenter was blinded to sample identity throughout the analyses. Total neuronal area (cell body and perikarya in red channel) and number of synapses (in contact with the neuronal circumference in green channel) was measured using Photoshop 7.0 software (Adobe Systems, San Jose, CA). Synaptic density as a percentage of total neuronal area was determined. Data were analyzed with Student’s t-tests.
Western blots
For protein analysis, 60 µm-thick frozen hippocampal sections from control (n=3) MS myelinated (n=3) and MS demyelinated (n=3) brains were homogenized in protein extraction buffer. Protein was extracted as described previously 17,18. 20 µg protein was separated on 4–12% NuPage Bis-Tris gels (Invitrogen, Carlsbad, CA) and transferred to PVDF membranes (Invitrogen Inc. Carlsbad CA). After blocking (5% non-fat milk), membranes were placed in primary antibodies overnight, incubated in horseradish peroxidase-tagged secondary antibodies, treated with Supersignal Pico detection reagent (Pierce Inc. Rockford IL) and then exposed to Kodak X-Omat film (Kodak Inc, Rochester, NY). Membranes were stripped and subsequently re-probed with glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The average intensity for each band was quantified with ImageJ software (NIH, Bethesda, MD). The antibodies used in the study are commercially available, well characterized and are detailed in supplemental Table SIII.
Statistical Analysis
All quantitative data are expressed as mean ± S.E.M. The statistical significance of differences between groups in RT-PCR, western blots, synaptic density was analyzed by Student's t-test. Differences were considered significant at a P-value < 0.05.
RESULTS
Demyelination and neuronal gene expression
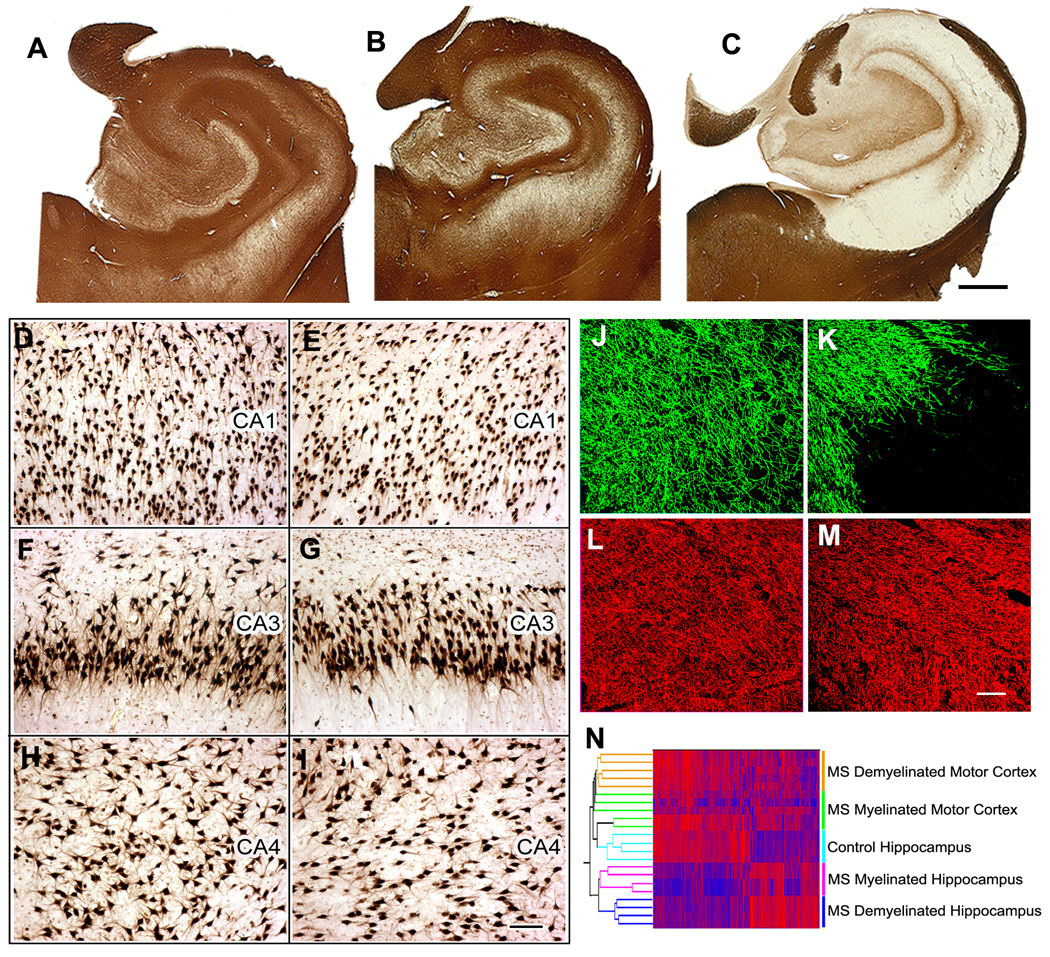
We studied hippocampi from the brains of 22 individuals with MS and 9 control patients without neurological disease. (Patient details are listed in Supplementary Table S1). Compared to control brains (Fig. 1A), ten MS hippocampi had no or minimal demyelination (Fig. 1B), while twelve were extensively demyelinated (Fig. 1C). Pathological changes characteristic of AD or ischemic injury were not detected in these hippocampi or adjoining entorhinal cortices. Compared to control hippocampi, demyelinated MS hippocampi contained modest microglial activation (Supplementary Fig. S1) and astrocytosis. Although significant neuronal loss has been reported in the CA1 and CA2/3 regions of demyelinated MS hippocampi 9, we did not detect obvious neuronal loss in demyelinated CA1, CA3, or CA4 regions (Fig. 1D, G and I) when compared to control hippocampi (Fig. 1D, F and H). Quantification of Hu-positive neuronal nuclei revealed a 10–20% decrease in neuronal density in some, but not all areas of demyelinated hippocampi. Axonal densities were similar in myelinated (Fig. 1J and L) and demyelinated (Fig. 1K and M) hippocampi and demyelinated axons appeared healthy with little evidence of dystrophy, swelling or transection (Fig. 1M). We next compared microarray gene expression profiles from age-matched control (n=4), MS myelinated (n=4) and MS demyelinated hippocampi (n=4). For comparison, we included microarray gene profiles from myelinated and demyelinated MS motor cortex. Based on condition-based clustering, gene expression profiles in demyelinated MS hippocampus were significantly different from those in demyelinated MS motor cortex (Fig. 1N). Only 32 transcripts were significantly different between control and myelinated MS hippocampi. In contrast, 799 gene transcripts were significantly different in demyelinated MS hippocampi compared to myelinated hippocampi. Of the 120 genes altered in demyelinated MS motor cortex, only 5 were also changed in demyelinated hippocampus and four of these encoded for myelin proteins (Supplementary Fig. S2). Classification of the significantly altered genes identified “intracellular transport” (p=0.008), “ubiquitin-dependent processes” (p=0.001), “synaptic transmission” (p=0.001), “behavior” (p=0.032), and “learning/memory” (p=0.028) as major biological categories affected by hippocampal demyelination. Of equal interest are the mRNAs not altered in demyelinated MS hippocampi, including the neuron-specific neurofilament heavy (NF-H), medium (NF-M) and light (NF-L) subunits, TAU and microtubule associated protein 2 (MAP2) (Supplementary Fig. S3a). Protein levels of Hu-R and NF-L were also unchanged in demyelinated MS hippocampi (Supplementary Fig. S3b). Most of these neuronal housekeeping genes were significantly decreased in AD hippocampi 20, reflecting the neuronal degeneration that is the pathological hallmark of AD brains (Supplemental Fig. S3c). These results establish that 1) loss of myelin leads to gene changes which reflect specific neuronal functions (hippocampal vs. motor neurons) and disease pathogenesis (MS vs. AD) and 2) hippocampal demyelination alters mRNAs that encode neuronal proteins involved in synaptic integrity and memory function.
Figure 1.
Hippocampal demyelination is common in multiple sclerosis (MS) brains and is associated with relative preservation of neurons and a distinct gene expression profile. (Panel A–C) Immunostaining for proteolipid protein (PLP) detected preservation of myelin in all control (A) and 10 of 22 MS hippocampi (B). Twelve of 22 MS hippocampi had significant or complete loss of myelin (C). (Panel D–I) Neuronal status in demyelinated MS hippocampus was determined by HuR immunohistochemistry. When compared to control hippocampi (Panels D, F, H), significant neuronal loss was not detected in CA1, CA3 or CA4 regions of demyelinated MS hippocampi (Panels E, G, I). (Panel J–M) Demyelinated hippocampal axons appear healthy. Double labeling immunofluorescence for myelin (myelin basic protein (MBP), green) and axons (SMI32, red) showed loss of myelin (K) with relative preservation of axons (M) in MS demyelinated hippocampus compared to control hippocampus (MBP, J; SMI32, I). (N) Demyelination alters hippocampal mRNA transcripts. Dual clustering of mRNA expression levels arranged samples into discrete clusters based upon myelin status (myelinated and demyelinated) and location (hippocampus vs motor cortex). High mRNA levels are indicated by red and blue denotes low expression levels. Scale bars: A–C: 2mm, D–I: 100µm, J–M:50µm
Hippocampal demyelination decreases the expression of neuronal genes that mediate fast axonal transport
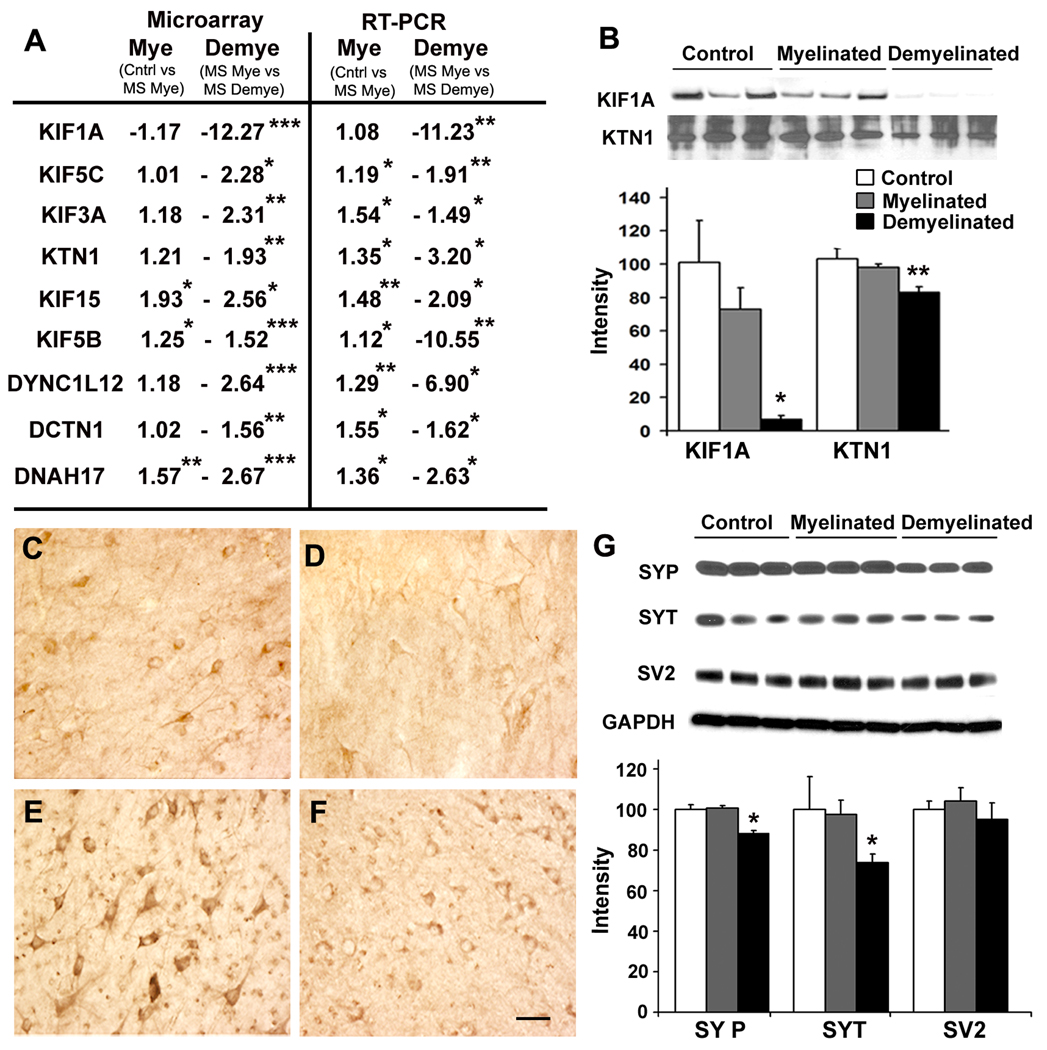
Increased kinesin-mediated anterograde axonal transport leads to enhanced memory function in rodents 21. KIF1A, the major microtubule motor responsible for fast anterograde axonal transport, was significantly decreased at the mRNA level (Fig. 2A) and protein level (Fig. 2B) in demyelinated MS hippocampi. KIF1A was enriched in hippocampal neurons in myelinated (Fig. 2C) and demyelinated MS hippocampi (Fig. 2D), excluding loss of oligodendrocytes as a source of decreased KIF1A. In addition to KIF1A, mRNAs encoding KIF3A, KIF15, KIF5B, KIF5C and kinectin1 (KTN1) were significantly decreased in demyelinated MS hippocampi (Fig. 2A). KTN1, a membrane anchor protein for kinesin-driven vesicle transport 22, was significantly decreased at the protein level (Fig. 2B) and enriched in neurons in demyelinated (Fig. 2E) and myelinated MS hippocampi (Fig. 2F). Hippocampal demyelination also alters retrograde axonal transport genes, as mRNAs encoding 3 key dynein molecules were also significantly decreased (Fig. 2A). These microtubule motor changes were specific to demyelinated hippocampi, as none of these transcripts were significantly changed in demyelinated motor cortex from MS patients (Supplementary Fig. S4).
Figure 2.
Hippocampal demyelination decreases proteins involved in axonal transport and enriched in synaptic vesicles. (Panels A–F) Both mRNA and protein transcripts of fast axonal transport genes were decreased in demyelinated MS hippocampal neurons. (A) mRNA encoding kinesin and dynein proteins were significantly decreased in microarray analysis (left), and verified by quantitative RT-PCR (right). mRNA levels of Kinesin 1A (KIF1A), Kinesin 5C (KIF5C), Kinesin 3A (KIF3A), Kinectin 1 (KTN1), Kinesin 15 (KIF15) Kinesin 5B (KIF5B), Dynein cytoplasmic light chain 12 (DYNC1L12), Dynactin 1 (DCTN1) and Dynein axonemal heavy polypeptide 17 (DNAH17) were significantly decreased in demyelinated compared to myelinated hippocampi. KIF1A and KTN1 were also reduced at the protein level (B). Immunohistochemistry shows presence of KIF1A and KTN1 in neurons in myelinated (Panels C, E) and demyelinated hippocampi (Panels D, F). (G) Synaptic vesicle proteins were decreased in demyelinated MS hippocampus. Protein levels of synaptophysin (SYP) and synaptotagmin (SYT) were significantly decreased in demyelinated MS hippocampus compared to myelinated MS hippocampus. Levels of synaptic vesicle glycoprotein-2, (SV2) were similar between control, myelinated MS and demyelinated MS hippocampi. GAPDH was used to verify equal loading of total protein. Scale Bars: C–F: 50µm, Error bars indicate S.E.M, * p<0.05, **p<0.005, ***p<0.0005
Reductions in KIF1A have been associated with decreases in the synaptic vesicle proteins synaptophysin and synaptotagmin, but not in synaptic vesicle glycoprotein-2 (SV2) 23. Levels of synaptophysin and synaptotagmin, but not SV2, were decreased in demyelinated MS hippocampi when compared to control and myelinated hippocampi (Fig. 2G). Hippocampal demyelination therefore decreases levels of proteins essential for anterograde and retrograde fast axonal transport and reduces the synaptic vesicle-enriched proteins synaptophysin and synaptogamin.
Reduced synaptic density in demyelinated hippocampus
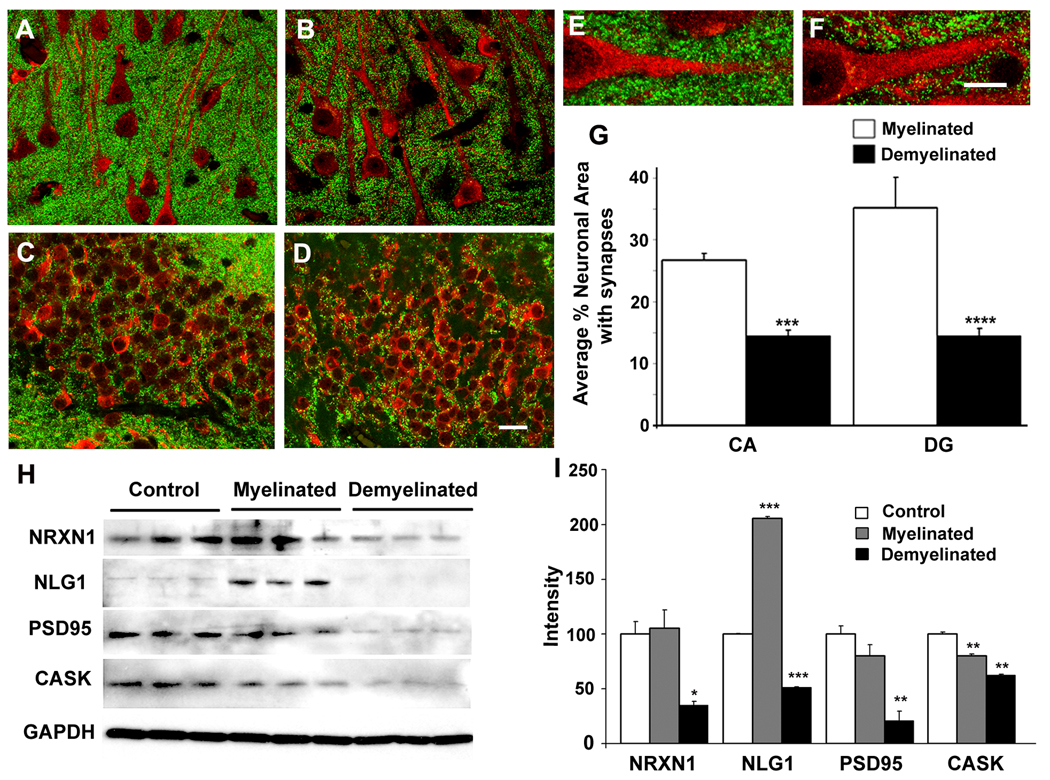
Synaptic pruning can reduce long term potentiation (LTP) and contribute to cognitive dysfunction 24. Therefore, we used confocal microscopy to quantify the density of synaptophysin-positive pre-synaptic terminals in CA1 and dentate gyrus (DG) of myelinated (Figs. 3A, 3C, 3E) and demyelinated (Figs. 3B, 3D and 3F) MS hippocampi. Compared to myelinated hippocampi, synaptophysin-positive punctae were decreased 1.85 fold (p= 1× 10−9) in demyelinated CA1 and 2.4-fold (p = 0.0049) in demyelinated DG (Fig. 3G). Loss of myelin therefore causes a significant decrease in the number of synapses associated with hippocampal neurons in MS brains.
Figure 3.
Hippocampal demyelination deceases pre-synaptic terminals and levels of proteins that help maintain synapses. (Panels A–F) Confocal images of MS hippocampus immunostained with antibodies specific to MAP2 (red) and synaptophysin (green) show punctuate pre-synaptic terminals (green) surrounding neurons in CA1 (Panels A, B) and dentate gyrus (Panels C, D) regions in MS myelinated (Panels A, C) and MS demyelinated (Panels B, D) hippocampus. Compared to myelinated MS hippocampi (E), pre-synaptic terminals were significantly decreased (G) in demyelinated MS hippocampi (F). (H) Levels of proteins essential for maintenance of synapses are decreased in demyelinated MS hippocampus. Neurexin 1 (NRXN1), neuroligin 1 (NLG1), post-synaptic density protein 95 (PSD95) and calmodulin-associated serine/threonin kinase (CASK) were significantly decreased in demyelinated MS hippocampi compared to myelinated MS hippocampi (I). GAPDH was used a loading control. Scale Bars: A–D: 20µm, E–F: 10µm, Error bars indicate S.E.M, * p<0.05, **p<0.005, ***p<0.0005, ****p<0.00005
We next inquired whether hippocampal demyelination alters the expression of molecules enriched in synaptic terminals. Neurexins and neuroligins are neuron-specific synaptic cell adhesion molecules that help maintain contact between pre- and post-synaptic specializations 25. mRNA (Supplementary Fig. S5) and protein (Figs. 3H–I) levels of both neurexin and its ligand neuroligin were significantly decreased in MS demyelinated hippocampi. The neurexin/neuroligin junction is bound by calmodulin-associated serin/threonin kinase (CASK) on the neurexin side and post-synaptic density protein (PSD95) on the neuroligin side 25. Both PSD95 and CASK were significantly decreased at the protein (Fig. 3H–I) and mRNA (Supplementary Fig. S5) levels in demyelinated MS hippocampi. Taken together, these results show that loss of myelin disrupts the maintenance and number of hippocampal synapses.
Reduced glutamate receptors and transporters in demyelinated MS hippocampus
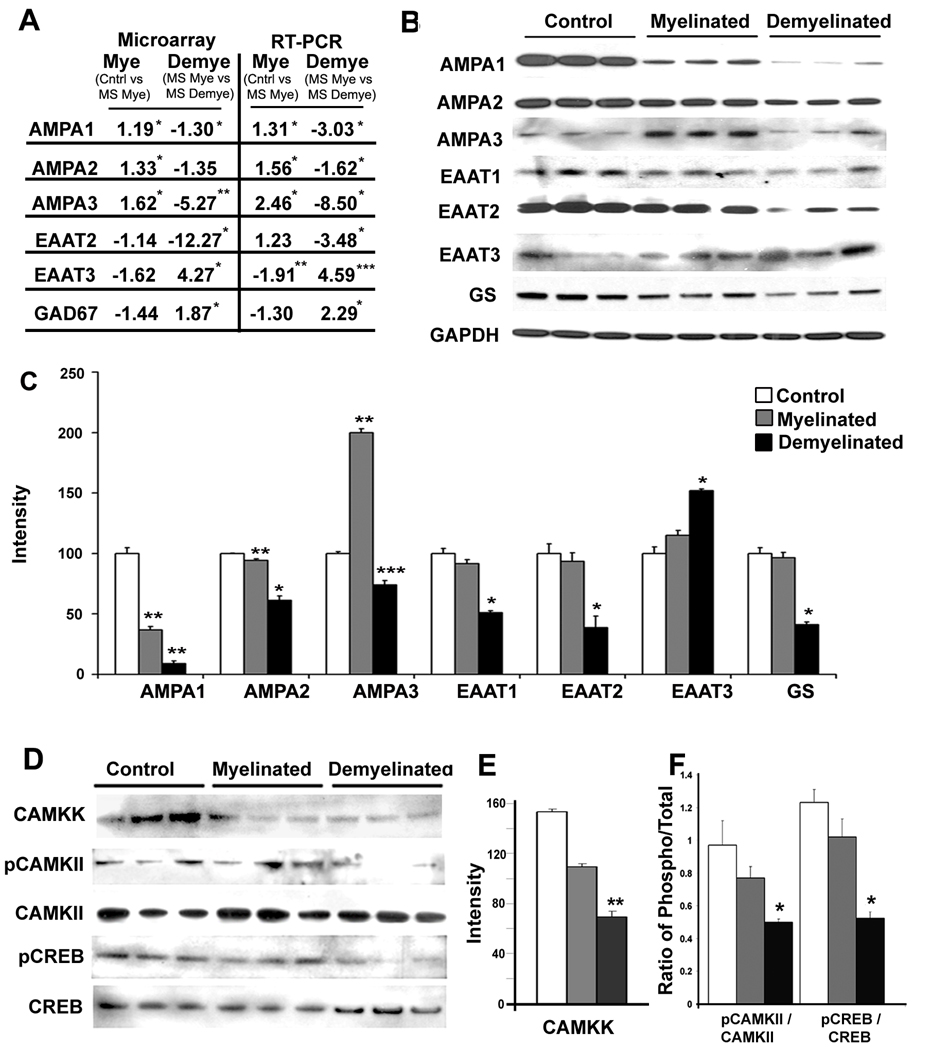
Glutamate neurotransmission plays a significant role in synaptic plasticity, memory, and cell survival 26. Increased levels of AMPA receptors favor enhanced LTP, increased synaptic plasticity 27, and improved performance in hippocampal-dependent memory tasks 28. mRNAs encoding AMPA 1 and AMPA 3 receptors were significantly decreased in demyelinated MS hippocampi compared to myelinated hippocampi in microarray analysis (Fig. 4A). AMPA 2 transcripts were also reduced in the microarray analysis, but did not reach statistical significance (Fig. 4A). However, mRNA levels measured by RT-PCR (Fig. 4A) and protein levels of all three AMPA receptors (Fig. 4B) were significantly decreased in demyelinated MS hippocampi. AMPA receptors present at the post-synaptic sites impact firing and signaling through NMDA receptors 29. mRNAs encoding NMDAR2A, 2B, 2C, 2D and NMDAR3B subunits were significantly reduced in demyelinated MS hippocampi (Supplemental Fig. S5a). In addition to its effects on ionotropic glutamate receptors, hippocampal demyelination also significantly reduced mRNA levels of the metabotropic glutamate receptors MGLUR1, MGLUR2, MGLUR3 and MGLUR4 (Supplementary Fig. S6a). These results support a reduction in both fast and slow glutamate neurotransmission in demyelinated MS hippocampus.
Figure 4.
Demyelination leads to decreased expression of glutamate receptors, glutamate transporters and key intermediates of memory/learning in MS hippocampus. (A–C) mRNA and protein levels of glutamate receptors and transporters were decreased in demyelinated MS hippocampus. (A) mRNAs encoding AMPA1, AMPA2, AMPA3, and EAAT2 were significantly decreased while mRNAs encoding EAAT3 and GAD67 were significantly increased in MS demyelinated hippocampi as measured by microarray analysis (left) and validated by quantitative RT-PCR (right). (B) Protein levels of AMPA receptors (AMPA1, AMPA2, AMPA3), glutamate transporters (EAAT1, EAAT2) and glutamine synthase (GS) were decreased in demyelinated MS hippocampi, while the neuronal glutamate transporter EAAT3 was significantly increased (C). (D–F) Key intermediates of memory/learning are decreased in demyelinated MS hippocampi. Phosphorylated CAMKII (E) and phosphorylated CREB (F), but not total CAMKII and total CREB, were significantly decreased in demyelinated MS hippocampi. Error bars indicate S.E.M., *p<0.05, ** P<0.005, ***p<0.0005
We next asked if hippocampal demyelination leads to dysregulation of extracellular glutamate. Astrocyte-enriched glutamate transporters (EAAT1 and 2) remove excess synaptic glutamate from the synapse and recycle glutamate by metabolism through glutamine synthase (GS) 30. Astrocytic EAAT2 is responsible for the clearance of 95% of extracellular glutamate 30. Hippocampal demyelination significantly decreased mRNA (Fig. 4A) and protein (Figs. 4B–C) levels of EAAT1, EAAT2 and GS, while levels of the neuronal transporter EAAT3 were increased (Fig. 4A–C). EAAT2-positive astrocytes were abundant in control and MS hippocampi and EAAT2 immunoreactivity was decreased in demyelinated hippocampi (Supplementary Fig. S6b–d). In contrast, the neuronal glutamate transporter EAAT3 immunoreactivity was increased in CA neurons of MS demyelinated hippocampi (Fig. S6g) compared to MS myelinated (Fig. S6f) and control hippocampi (Fig. S6e). These results support reduced glutamate clearance from the synaptic cleft both by astrocytic glutamate transporters and GS. Glutamate can also be converted to GABA by the enzyme glutamate decarboxylase (GAD). GAD67 mRNA was significantly increased in MS demyelinated hippocampi (Fig. 4A), suggesting increased synthesis of GABA. mRNA encoding the GABA receptor subunits GABAβ3, GABAγ2 and GABAα5 as well as protein levels of GABAα5 were significantly increased in MS demyelinated hippocampi compared to MS myelinated hippocampi (Supplementary Fig. S7a–b). Taken together, these data show that demyelination of the hippocampus leads to decreased levels of glutamate receptors, downregulation of glial glutamate transporters and GS, increased levels of GABA receptors, and possible extracellular accumulation of glutamate.
Hippocampal demyelination decreases key intermediates of memory function and neuronal survival
Memory and learning involve an intricate signal transduction cascade, including activation of glutamate receptors, entry of Ca(2+), and subsequent activation of Ca(2+)/calmodulin-dependent protein kinases (CaM kinases) 10,11. Significant decreases in AMPA, NMDA and mGLUR receptors in MS demyelinated hippocampus should result in impaired activation of CaM kinases in MS hippocampus. Indeed, the level of activated CAMKII was significantly decreased in demyelinated hippocampi (Fig. 4D). We next investigated whether this decrease is due to an inability of the upstream CAM dependent kinase kinase (CAMKK) to phosphorylate CAMKII. CAMKK protein levels were significantly decreased in MS demyelinated hippocampi (Fig. 4E). One of the consequences of decreased activation of CAMKII is downregulation of CREB, which is a ubiquitous transcription factor and a key molecule for learning and memory 10. While total CREB levels were similar (Fig. 4D), phosphorylated or activated CREB was significantly decreased in demyelinated MS hippocampi compared to myelinated hippocampi (Fig. 4F). Hippocampal demyelination therefore leads to decreased levels of activated CAMKII and CREB, two molecules essential for memory and learning.
DISCUSSION
Despite recent interest in hippocampal pathology in MS patients, the molecular basis of memory dysfunction in MS has not been investigated. Our data establish that hippocampal demyelination leads to decreased expression of neuronal proteins involved in axonal transport, synaptic plasticity, glutamate homeostasis, memory/learning and neuronal survival. Surprisingly, very few of the neuronal gene products altered in demyelinated MS hippocampus were altered in demyelinated MS motor cortex. Memory dysfunction and hippocampal demyelination are common in MS patients. Our studies therefore provide a molecular basis for memory decline found in individuals with MS and establish that myelination modulates neuronal gene expression in a manner that reflects the specialized functions of different neuronal populations.
Our studies are consistent with previous pathological studies that have documented hippocampal demyelination in postmortem MS brain 8,9. This includes the overall incidence of hippocampal demyelination and decreases in synaptic terminals. Our approach differs from previous studies as it includes a molecular comparison of myelinated and completely demyelinated hippocampi. Hippocampi were obtained from chronic patients with an average disease duration of 26.6 years. The retention of 80 – 90% of neuronal perikarya in these demyelinated hippocampi is encouraging, as it identifies the demyelinated hippocampal neuron as a viable and abundant therapeutic target. In contrast to AD patients, who are diagnosed at late and possibly irreversible stages of cognitive decline, more than 50% of MS patients are diagnosed in the 3rd and 4th decade of life. MS, therefore, may be the disease of choice for testing therapies that prevent memory decline. Hence, it was important to identify the molecular and cellular changes that accompany hippocampal demyelination. We have identified therapeutic targets that could enhance memory function in MS patients.
Neurogenesis in the adult hippocampus and its possible role in synaptic plasticity has been proposed in various neurological disorders including AD, epilepsy, depression and Parkinson’s disease 31,32. While we recently provided evidence for neurogenesis in chronic white matter lesions in MS brains 33, neurogenesis in MS hippocampus has not been investigated. Correlation of cognitive dysfunction demyelination and neurogenesis in MS hippocampus would require development of non-invasive imaging techniques that detect new neurons and demyelinated hippocampi. Conventional brain imaging techniques cannot distinguish myelinated and demyelinated hippocampi, so there is no data on when hippocampi demyelinate during the clinical course of MS. Most of the MS patients who donated brains to the present studies were cognitively impaired, but not tested for memory deficits. Since most of our data were derived from individuals with long disease duration, it is impossible to distinguish primary from secondary changes by analysis of postmortem brains. Data from acute demyelination of the mouse hippocampi will therefore, provided valuable insight into the primary effects of hippocampal demyelination on neuronal protein expression. The neuronal gene with the greatest decrease in demyelinated MS hippocampus was KIF1A, the major molecular motor for anterograde transport of synaptic vesicles to the pre-synaptic terminal. Reduced synaptic vesicle transport and subsequent decreased synaptic firing could account for the majority of gene changes detected in demyelinated post-synaptic hippocampal neurons. In support of this hypothesis, KIF1A mutants show motor and sensory disturbances with significant decreases in synaptic vesicle density, neuronal afferent stimulation and glutamate neurotransmission 23. The major regulators of synaptic glutamate, astrocytic EAAT 1 and 2, were significantly reduced in demyelinated hippocampi despite astrocytosis. Since increased extracellular glutamate is detrimental to neuronal survival and function, future therapeutic approaches to normalize extracellular glutamate levels in demyelinated hippocampi may partially delay or reverse cognitive decline in MS patients.
In summary, we describe molecular alterations in demyelinated hippocampal neurons that are known to cause memory dysfunction in experimental animal models. The incidence of memory impairment in MS patients is reported to be over 30%. Advances in imaging hippocampal changes in living MS patients will increase our understanding of the incidence and dynamics of hippocampal pathology and will set the stage for the development of reliable surrogate markers for demonstrating the efficacy of future therapies to reduce memory decline in MS patients. The data presented here identify several molecular targets for such therapies.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank Dr. Christopher Nelson for his assistance in editing the manuscript. We would also like to thank Cynthia Schwanger, RN and LifeBanc for the MS tissue donation program. The authors would like to thank Dr. J. D Rothstein from JHU, for the EAAT2 and EAAT3 antibodies.
FUNDING: The work is in part supported by NMSS RG-4280 (RD), NIH NS38667 and NIH NS35058 (BDT).
Footnotes
COMPETING INTERESTS: None
References
- 1.Trapp BD, Nave KA. Multiple sclerosis: an immune or neurodegenerative disorder? Annu Rev Neurosci. 2008;31:247–269. doi: 10.1146/annurev.neuro.30.051606.094313. [DOI] [PubMed] [Google Scholar]
- 2.Noseworthy JH. Progress in determining the causes and treatment of multiple sclerosis. Nature. 1999;399:A40–A47. doi: 10.1038/399a040. [DOI] [PubMed] [Google Scholar]
- 3.Chiaravalloti ND, DeLuca J. Cognitive impairment in multiple sclerosis. Lancet Neurol. 2008;7:1139–1151. doi: 10.1016/S1474-4422(08)70259-X. [DOI] [PubMed] [Google Scholar]
- 4.Bobholz JA, Rao SM. Cognitive dysfunction in multiple sclerosis: a review of recent developments. Curr Opin Neurol. 2003;16:283–288. doi: 10.1097/01.wco.0000073928.19076.84. [DOI] [PubMed] [Google Scholar]
- 5.Rao SM, Leo GJ, Aubin-Faubert P. On the nature of memory disturbance in multiple sclerosis. J Clin Exp Neuropsychol. 1989;11:699–712. doi: 10.1080/01688638908400926. [DOI] [PubMed] [Google Scholar]
- 6.Roosendaal SD, Moraal B, Vrenken H, et al. In vivo MR imaging of hippocampal lesions in multiple sclerosis. J Magn Reson Imaging. 2008;27:726–731. doi: 10.1002/jmri.21294. [DOI] [PubMed] [Google Scholar]
- 7.Sicotte NL, Kern KC, Giesser BS, et al. Regional hippocampal atrophy in multiple sclerosis. Brain. 2008;131:1134–1141. doi: 10.1093/brain/awn030. [DOI] [PubMed] [Google Scholar]
- 8.Geurts JJ, Bo L, Roosendaal SD, et al. Extensive hippocampal demyelination in multiple sclerosis. J Neuropathol Exp Neurol. 2007;66:819–827. doi: 10.1097/nen.0b013e3181461f54. [DOI] [PubMed] [Google Scholar]
- 9.Papadopoulos D, Dukes S, Patel R, et al. Substantial archaeocortical atrophy and neuronal loss in multiple sclerosis. Brain Pathol. 2009;19:238–253. doi: 10.1111/j.1750-3639.2008.00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barco A, Bailey CH, Kandel ER. Common molecular mechanisms in explicit and implicit memory. J Neurochem. 2006;97:1520–1533. doi: 10.1111/j.1471-4159.2006.03870.x. [DOI] [PubMed] [Google Scholar]
- 11.Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294:1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- 12.Carlezon WA, Jr, Duman RS, Nestler EJ. The many faces of CREB. Trends Neurosci. 2005;28:436–445. doi: 10.1016/j.tins.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 13.Kelleher RJ, III, Govindarajan A, Tonegawa S. Translational regulatory mechanisms in persistent forms of synaptic plasticity. Neuron. 2004;44:59–73. doi: 10.1016/j.neuron.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 14.Wayman GA, Lee YS, Tokumitsu H, et al. Calmodulin-kinases: modulators of neuronal development and plasticity. Neuron. 2008;59:914–931. doi: 10.1016/j.neuron.2008.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nave KA, Trapp BD. Axon-glial signaling and the glial support of axon function. Annu Rev Neurosci. 2008;31:535–561. doi: 10.1146/annurev.neuro.30.051606.094309. [DOI] [PubMed] [Google Scholar]
- 16.Trapp BD, Peterson J, Ransohoff RM, et al. Axonal transection in the lesions of multiple sclerosis. N Engl J Med. 1998;338:278–285. doi: 10.1056/NEJM199801293380502. [DOI] [PubMed] [Google Scholar]
- 17.Dutta R, McDonough J, Yin X, et al. Mitochondrial dysfunction as a cause of axonal degeneration in multiple sclerosis patients. Ann Neurol. 2006;59:478–489. doi: 10.1002/ana.20736. [DOI] [PubMed] [Google Scholar]
- 18.Dutta R, McDonough J, Chang A, et al. Activation of the ciliary neurotrophic factor (CNTF) signalling pathway in cortical neurons of multiple sclerosis patients. Brain. 2007;130:2566–2576. doi: 10.1093/brain/awm206. [DOI] [PubMed] [Google Scholar]
- 19.Hochberg Y, Benjamini Y. More powerful procedures for multiple significance testing. Stat Med. 1990;9:811–818. doi: 10.1002/sim.4780090710. [DOI] [PubMed] [Google Scholar]
- 20.Blalock EM, Geddes JW, Chen KC, et al. Incipient Alzheimer's disease: microarray correlation analyses reveal major transcriptional and tumor suppressor responses. Proc Natl Acad Sci U S A. 2004;101:2173–2178. doi: 10.1073/pnas.0308512100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Puthanveettil SV, Monje FJ, Miniaci MC, et al. A new component in synaptic plasticity: upregulation of kinesin in the neurons of the gill-withdrawal reflex. Cell. 2008;135:960–973. doi: 10.1016/j.cell.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar J, Yu H, Sheetz MP. Kinectin, an essential anchor for kinesin-driven vesicle motility. Science. 1995;267:1834–1837. doi: 10.1126/science.7892610. [DOI] [PubMed] [Google Scholar]
- 23.Yonekawa Y, Harada A, Okada Y, et al. Defect in synaptic vesicle precursor transport and neuronal cell death in KIF1A motor protein-deficient mice. J Cell Biol. 1998;141:431–441. doi: 10.1083/jcb.141.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neves G, Cooke SF, Bliss TV. Synaptic plasticity, memory and the hippocampus: a neural network approach to causality. Nat Rev Neurosci. 2008;9:65–75. doi: 10.1038/nrn2303. [DOI] [PubMed] [Google Scholar]
- 25.Sudhof TC. Neuroligins and neurexins link synaptic function to cognitive disease. Nature. 2008;455:903–911. doi: 10.1038/nature07456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sheng M, Kim MJ. Postsynaptic signaling and plasticity mechanisms. Science. 2002;298:776–780. doi: 10.1126/science.1075333. [DOI] [PubMed] [Google Scholar]
- 27.Lu W, Man H, Ju W, et al. Activation of synaptic NMDA receptors induces membrane insertion of new AMPA receptors and LTP in cultured hippocampal neurons. Neuron. 2001;29:243–254. doi: 10.1016/s0896-6273(01)00194-5. [DOI] [PubMed] [Google Scholar]
- 28.Mack V, Burnashev N, Kaiser KM, et al. Conditional restoration of hippocampal synaptic potentiation in Glur-A-deficient mice. Science. 2001;292:2501–2504. doi: 10.1126/science.1059365. [DOI] [PubMed] [Google Scholar]
- 29.Sudhof TC, Malenka RC. Understanding synapses: past, present, and future. Neuron. 2008;60:469–476. doi: 10.1016/j.neuron.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rothstein JD, Dykes-Hoberg M, Pardo CA, et al. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16:675–686. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- 31.Deng W, Aimone JB, Gage FH. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010;11:339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 33.Chang A, Smith MC, Yin X, et al. Neurogenesis in the chronic lesions of multiple sclerosis. Brain. 2008;131:2366–2375. doi: 10.1093/brain/awn157. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.