Abstract
Endocannabinoids are lipid mediators of the same cannabinoid (CB) receptors that mediate the effects of marijuana. The endocannabinoid system (ECS) consists of CB receptors, endocannabinoids, and the enzymes involved in their biosynthesis and degradation, and is present both in brain and peripheral tissues, including the liver. The hepatic ECS is activated in various liver diseases, which contributes to the underlying pathologies. In cirrhosis of various etiologies, activation of vascular and cardiac CB1 receptors by macrophage- and platelet-derived endocannabinoids contribute to the vasodilated state and cardiomyopathy, which can be reversed by CB1 blockade. In mouse models of liver fibrosis, activation of CB1 receptors on hepatic stellate cells is fibrogenic, and CB1 blockade slows the progression of fibrosis. Fatty liver induced by high-fat diets or chronic alcohol feeding depend on activation of peripheral, including hepatic CB1 receptors, which also contribute to insulin resistance and dyslipidemias. Although the documented therapeutic potential of CB1 blockade is limited by neuropsychiatric side effects, these may be mitigated by using novel, peripherally restricted CB1 antagonists.
The Endocannabinoid System
Marijuana has been used for its psychoactive and medicinal properties for millennia. As other plant-derived substances, marijuana has been slow to yield its secrets, with insights into its mechanism of action beginning to emerge only during the last decades. The existence of specific CB receptors in mammalian tissues was first revealed by radioligand binding, followed by the molecular cloning of two G protein-coupled cannabinoid receptors (1). CB1 receptors are the most abundant receptors in the mammalian brain, but are also expressed in peripheral tissues, including various cell types of the liver, at much lower yet functionally relevant concentrations (2–8). CB2 receptors are expressed primarily in immune and hematopoietic cells, and have also been detected in the liver in certain pathological states (9, 10). Additional CB receptors may exist (11), but their potential role in liver biology is unknown.
The discovery of CB receptors triggered a search for endogenous ligands. Arachidonoyl ethanolamide (AEA, anandamide) was the first such ligand discovered (12), with 2-arachidonoyl glycerol (2-AG) identified 3 years later (13, 14). Additional endogenous ligands have since been identified (1) but have received less attention. AEA and 2-AG are generated on demand in response to a rise in intracellular calcium or metabotropic receptor activation (1). Their biosynthesis from membrane phospholipid precursors may proceed along multiple, parallel pathways (15, 16). Once released, they remain largely membrane associated due to their hydrophobic nature, and can be taken up by cells via a high affinity uptake mechanism (17), which is followed by their enzymatic degradation. AEA is metabolized primarily by the membrane associated fatty-acid amide hydrolase (FAAH)(18), whereas 2-AG is preferentially degraded by monoglyceride lipase (MAGL)(19).
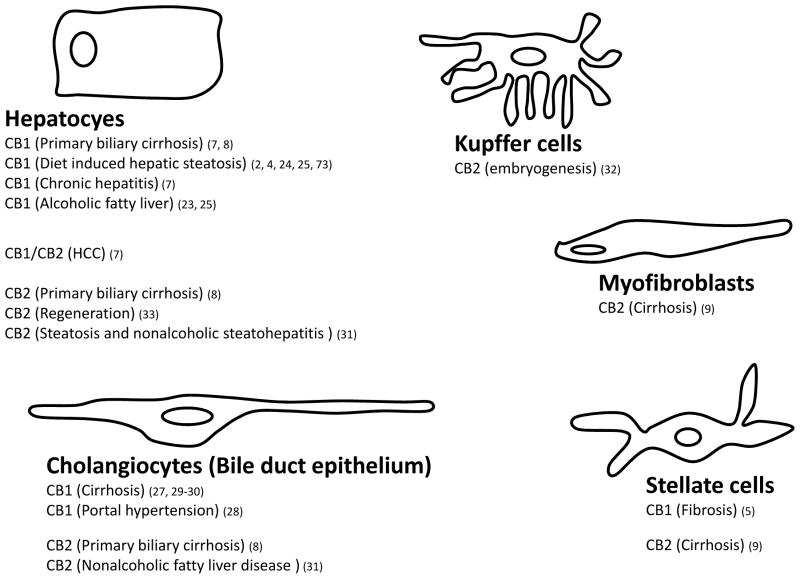
The psychoactive properties of cannabinoids and the abundance of CB1 receptors in the brain have suggested that the ECS is primarily a neuronal signaling system, therefore evidence for the presence and functional importance of the ECS in the liver (2) was unexpected. Indeed, early studies of brain CB1 receptors have used the liver as negative control (20). However, recent reports have documented low level CB1 expression in whole liver (2–4, 21–23), hepatocytes (6, 23–25), stellate cells (5, 26), and hepatic vascular endothelial cells (27–30)(see Fig. 1). CB1 receptors are present in human hepatocytes (25) and in whole human liver, with increased expression noted in hepatocellular carcinoma (7) and primary biliary cirrhosis (8). CB2 receptors are undetectable in normal liver, but are induced in pathological conditions such as NAFLD (31), embryonic state (32), liver fibrosis (9), regenerating liver (33), and in hepatocellular carcinoma (7). Hepatic endocannabinoids levels are similar to those in brain (2, 26), whereas FAAH expression is higher in liver than in brain. Evidence implicating the ECS in the regulation of hepatic hemodynamics, fibrogenesis as well as lipid metabolism, and in the dysregulation of these functions in pathological states such as cirrhosis, NAFLD, alcoholic fatty liver and ischemia-reperfusion injury is discussed below.
Figure 1. Cellular distribution of hepatic CB1 and CB2 receptors and their involvement in liver diseases.
Numbers in parentheses refer to original reports as listed in References.
Endocannabinoids and Altered Hemodynamics in Cirrhosis
The potent hypotensive action of THC has long been recognized and it even prompted attempts to exploit it for the treatment of hypertension (34). This and the similar effect of synthetic cannabinoid analogs and endocannabinoids are mediated by CB1 receptors located, in part, in the peripheral cardiovascular system (35), and play a pathogenic role in various forms of shock (36, 37), including endotoxic shock (38–40).
Advanced liver cirrhosis is associated with endotoxemia and hypotension, suggesting endocannabinoid involvement. Indeed, cirrhosis in rats is accompanied by progressive hypotension reversible by CB1 blockade (27), which also reduces the elevated portal venous pressure and mesenteric blood flow. The likely source of endocannabinoids is activated macrophages, in which LPS induces CD14-dependent synthesis of anandamide (38, 41). AEA levels are elevated in circulating macrophages of cirrhotic rats or patients, and such macrophages injected into normal rats elicit CB1-mediated hypotension (27, 42). Cirrhosis increases CB1 expression in vascular endothelial cells (27), or in mesenteric arteries (29, 43), and increases the vasodilator potency of AEA (29, 43, 44). In cirrhotic patients, circulating AEA, but not 2-AG, levels are increased in peripheral blood but not in hepatic veins or liver tissue, suggesting that the liver is not its source (45).
These findings implicate AEA as mediator of the vasodilated state in cirrhosis. Although in one study of cirrhotic patients, the increase in circulating AEA did not correlate with the degree of hepatic and renal dysfunction (46), in another study of patients with primary biliary cirrhosis, the CB1 expression in hepatocytes and biliary epithelial cells and CB2 expression in hepatocytes and cholangiocytes were positively correlated with the severity of the histological stage (8). Cirrhosis is associated with renal sodium retention, which has been attributed, in part, to portal hypertension secondary to liver parenchymal damage and fibrosis (47). In cirrhotic rats, rimonabant dose-dependently reduced ascites by ensuring a less positive sodium balance (48).
AEA induces CB1-mediated mesenteric vasodilation independent of NO (49). However, the effect of higher doses of AEA is resistant to CB1 blockade (49), and may be mediated via putative ‘AEA receptors’, implicated in the mesenteric vasorelaxant effect of AEA observed in CB1/CB2 double knockout mice(11, 50), which may also contribute to mesenteric vasodilation in cirrhosis.
The hyperdynamic circulation of advanced cirrhosis is associated with increased cardiac output and tachycardia. However, the cirrhotic heart has an underlying decrease in contractility and β-adrenergic hyposensitivity referred to as ‘cirrhotic cardiomyopathy’ (51), which has been attributed to endocannabinoid activation of cardiac CB1 receptors, based on pharmacological studies using isolated myocardial preparations from bile duct-ligated rats (52). In vivo studies, using an intraventricular pressure-volume microcatheter system, revealed a profound decrease in baseline cardiac contractility, which was acutely normalized by CB1 blockade (53). The suppression of cardiac contractility by CB1 receptor activation may involve inhibition of L-type calcium channels (54) and/or reductions in myocardial cAMP content (55). Of the 2 major endocannabinoids, AEA is more likely to be involved, as suggested by a cirrhosis-related increase in myocardial AEA, but not 2-AG, levels (53).
These findings raise the therapeutic potential of CB1 blockade in treating the hemodynamic abnormalities in advanced liver cirrhosis. Because the increase in mesenteric blood flow may precipitate the rupture of varicosities and also contributes to ascites formation, CB1 blockade may avert these potentially fatal complications, thus keeping patients alive until a liver transplant becomes available.
Endocannabinoids and Liver Fibrosis
CB2 receptors, which are normally undetectable in the liver, are prominently expressed in the cirrhotic human liver, and are also detectable in non-parenchymal liver cells in fibrotic mouse liver (9). THC suppresses the proliferation and induces apoptosis of human hepatic myofibroblasts and stellate cells via CB2 receptors (9), and thus may be antifibrotic and hepatoprotective (56). Accordingly, CB2−/− mice had an enhanced response to fibrogenic stimuli (9). CB2 receptor activation by AEA also inhibits the hyperplastic proliferation of cholangiocytes, a frequent consequence of extrahepatic biliary obstruction, cholestatis and toxic liver injury. This was associated with increased production of reactive oxygen species and cell death, via the induction of the AP-1 complex and thioredoxin-1 (3). In cirrhotic rats, chronic treatment with the CB2-selective agonist JWH-133 attenuated cellular markers of fibrosis (57), and enhanced the regenerative response to acute liver injury. Accordingly, CB2−/− mice had delayed liver regeneration in response to CCl4-induced injury, whereas JWH-133 treatment reduced the injury and accelerated liver regeneration (33). These findings signal the therapeutic potential of non-psychoactive CB2 agonists in the treatment of liver fibrosis.
Paradoxically, in patients with hepatitis C virus infection, daily cannabis use increased rather than protected from fibrosis progression (58). Thus, endocannabinoids also exert a profibrotic effect, possibly mediated by CB1 receptors. This is compatible with the finding of increased CB1 expression in stellate cells and hepatic myofibroblasts in cirrhotic human liver, and in the livers of mice with three different models of fibrosis (5). Genetic or pharmacological ablation of CB1 receptors protected mice against liver injury, as reflected in the reduced expression of smooth muscle α-actin and transforming growth factor-β(5). 2-AG is the likely fibrogenic mediator, as its hepatic level is preferentially increased by CCl4-treatment of mice (26) and rats (53). Higher doses of 2-AG induce apoptosis in activated HSC in vitro through a receptor-independent, membrane cholesterol-dependent mechanism, which means that 2-AG also has antifibrotic activity (26). AEA had a similar effect, although the eventual cell death was by necrosis rather than apoptosis (59). For both endocannabinoids, these effects occur at the 2-50 μM range. The hepatic concentration of AEA is orders of magnitude below such levels, whereas 2-AG may reach low micromolar concentrations (26). As the pro-apoptotic effect of 2-AG is independent of cannabinoid receptors, it could contribute to the reduction in fibrotic activity observed following CB1 blockade (48). The profibrotic and adverse hemodynamic effects of CB1 activation could provide a rationale for the use of CB1 antagonists in the medical management of advanced liver cirrhosis.
Endocannabinoids and the Metabolic Syndrome
The CB1-mediated appetite promoting effect of endocannabinoids (60) was the primary impetus for the development of brain-penetrant CB1 receptor antagonists for the treatment of obesity. The first-in-class compound, rimonabant, caused weight reduction and improved the associated cardiometabolic risk factors, but neuropsychiatric side effects, including depression and anxiety, have prevented its approval in the United States and led to its withdrawal from the market in other countries (reviewed in(61)). Accumulating evidence indicates, however, that the metabolic effects of endocannabinoids are mediated, at least in part, by peripheral CB1 receptors, as discussed in some detail below. Indeed, a non brain-penetrant CB1 antagonist was recently reported to retain the beneficial metabolic effects of rimonabant without producing the behavioral effects that predict neuro-psychiatric side effects in humans, which may revive interest in the therapeutic potential of CB1 antagonism (62).
Reduced food intake is not the primary mechanism of weight reduction by CB1 blockade in obesity. In mice with diet-induced obesity (DIO), chronic rimonabant caused a transient reduction in food intake and sustained weight loss, indicating food intake-independent effects on energy balance (2, 63). Increased de novo hepatic lipogenesis has been documented in DIO mice (2, 64, 65) as well as in people with NAFLD (66, 67), and may be mediated by endocannabinoids. Indeed, lipogenic gene expression and the rate of de novo hepatic lipogenesis were increased by CB1 agonists and decreased by CB1 antagonists in rodents (2, 25, 68–70). High-fat diet increases hepatic CB1 expression (2, 21, 25, 69) and the hepatic levels of AEA (2). Thus, endogenous AEA acting via hepatic CB1 receptors contributes to increased de novo lipogenesis in mouse models of obesity. CB1-mediated lipogenesis may explain the finding that in patients with chronic hepatitis C infection, daily cannabis smoking was an independent risk factor for steatosis severity, but not for obesity (71).
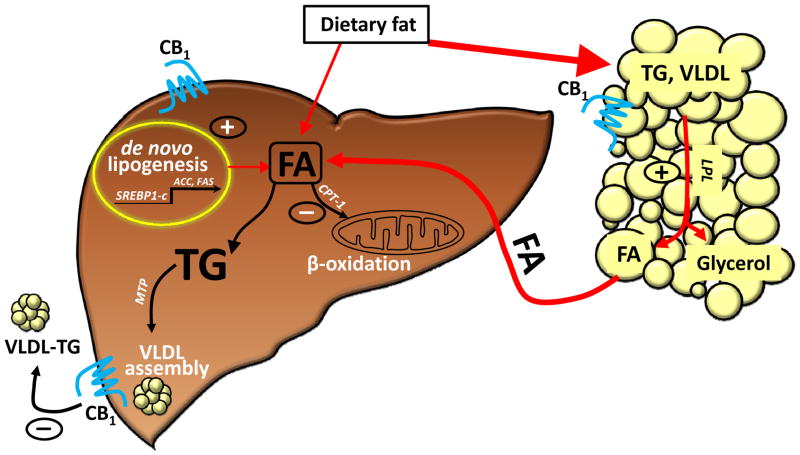
Although lipogenesis via hepatic CB1 receptors may contribute to lipid accumulation, extrahepatic CB1 must play a major role, because liver-specific CB1−/− mice are only partially protected from steatosis (24), whereas CB1−/− mice with selective transgenic re-expression of CB1 in hepatocytes remain largely resistant to the steatotic effect of high-fat diet, similar to their global CB1 knockout littermates (62). The source of liver fat may be adipose tissue, as activation of CB1 receptors in adipocytes promotes lipogenesis (72), and the released fatty acids may be taken up and converted to triglycerides by the liver (4). On the other hand, the rapid depletion of excess hepatic triglycerides following CB1 blockade may involve hepatic CB1 receptors, as indicated by the increased rate of secretion of triglyceride-rich VLDL from the liver of both DIO and ob/ob mice following treatment with a peripherally restricted CB1 antagonist (62)(see Fig. 2).
Figure 2. CB1 mechanisms involved in liver fat accumulation.
The major pathway is CB1 activation of lipoprotein lipase (LPL) in adipose tissue, resulting in increased fatty-acid (FA) release and transfer to liver (72). Additional mechanisms mediated via hepatic CB1 receptors include increased de novo hepatic lipogenesis, decreased FA oxidation (2) and decreased secretion of TG-rich VLDL (62).
Endocannabinoids are also involved in the diet-induced decrease in fatty-acid oxidation. The activity of hepatic carnitine palmitoyltransferase-1 (CPT-1), the rate limiting enzyme in mitochondrial fatty acid β-oxidation, is suppressed by either high-fat diet or treatment with a CB1 agonist, and both effects are prevented by rimonabant (24). Conversely, hepatic CPT-1 activity is increased in CB1−/− mice (24), and in DIO mice following chronic CB1 blockade (24, 62, 73). Adiponectin is a key stimulator of fatty acid β-oxidation, and CB1 blockade increases plasma adiponectin (74). The improved insulin sensitivity following CB1 blockade was found to have both adiponectin-dependent (75, 76) and adiponectin-independent components (76), although the role of adiponectin in the effects of CB1 blockade on hepatic mitochondrial function and fatty acid oxidation has not been explored. Increased energy expenditure, due to increased fat oxidation, following CB1 blockade has been documented using indirect calorimetry in rats (77–79) and mice (62). These effects likely contribute to the food-intake-independent sustained weight loss (62, 80) as well as reversal of hepatic steatosis (62, 81, 82) following chronic CB1 blockade.
The DIO-related hypertrygliceridemia was modestly attenuated, whereas the accompanying increase in plasma LDL cholesterol and decrease in HDL cholesterol were absent in both CB1−/− and LCB1−/− mice on the high-fat diet. This suggests that hepatic CB1 mediate diet-induced changes in hepatic lipoprotein metabolism and/or secretion. In a recent study, treatment of mice with an inhibitor of monoglyceride lipase resulted in elevated hepatic levels of 2-AG, increased hepatic expression of SREBP1c and FAS, hypertriglyceridemia, and accumulation in plasma of apoE-depleted, triglyceride-rich apolipoproteins (68). These changes were absent in CB1−/− or ApoE−/− mice and could be prevented by CB1 blockade. Furthermore, despite the elevated hepatic lipogenic gene expression, triglyceride secretion rates were unchanged, but triglyceride clearance from plasma was inhibited (68). In contrast, in DIO mice with long-term upregulation of the ECS, peripheral CB1 blockade does increase triglyceride secretion (62), as discussed above.
High fat diets also result in elevated plasma insulin and leptin levels accompanied by hyperglycemia, indicating insulin resistance (24, 83), as well as leptin resistance (84, 85). Interestingly, both CB1−/− and LCB1−/− mice remained glucose-tolerant and insulin sensitive and did not display the hyperleptinemia associated with high-fat diets (24). Moreover, the insulin and leptin resistance of DIO mice was normalized by the peripheral CB1 antagonist, AM6545 (62). There is also evidence that THC induces glucose intolerance in humans (86) and rodents, via activation of CB1 receptors (87). Thus, endocannabinoids and hepatic CB1 play an important role in diet-induced insulin and leptin resistance.
Diet-induced insulin resistance involves adipose tissue, skeletal muscle and liver, and interactions among the three tissues through neurogenic (88) and/or humoral factors (89). In mice, high-fat diet induces CB1 expression in skeletal muscle (90), and CB1 blockade increases insulin-induced glucose uptake and phosphorylation in skeletal muscle of genetically obese mice (79). The possibility that activation of hepatic CB1 may influence insulin sensitivity of extrahepatic tissues via the release of soluble mediators, remains to be explored.
CB2 receptors may also be involved in diet-induced hormonal and metabolic changes. In rats, the selective CB2 agonist JWH-133 improved glucose tolerance, whereas the CB2-antagonist AM630 had the opposite effect and it also prevented the effect of JWH-133 (91). These are opposite to the glucose intolerance induced by CB1 receptor activation (see above), and could minimize the effects of mixed CB1/CB2 agonists on glucose homeostasis. The well documented insulin sensitization by chronic CB1 blockade (92, 93) may be due to reversal of the action of AEA, which has low CB2 efficacy (94). This is also consistent with findings that high-fat diet-induced glucose intolerance and insulin resistance are associated with increased hepatic AEA, but not 2-AG, levels (2).
In a recent study, CB2 expression was strongly induced by both steatosis and non-alcoholic steatohepatitis (31), which suggests CB2 involvement in hepatic fat metabolism. Indeed, a modest increase in CB2 expression was reported in hepatocytes from both ob/ob and DIO mice. On the other hand, CB2−/− mice were resistant to diet-induced steatohepatitis, and were less insulin resistant than wild-type littermates on the same diet. Furthermore, JWH-133 increased the hepatic accumulation of triglycerides in DIO mice (95). The CB2-induced insulin resistance suggested by these findings in mice is opposite to the insulin sensitizing effect of CB2 agonists in rats (91). Further studies are needed to resolve this discrepancy.
Alcoholic Fatty Liver
Chronic alcoholism may lead to steatosis that can further progress into steatohepatitis, liver cirrhosis and hepatocellular carcinoma. Ethanol enhances hepatic lipogenesis (96, 97) and decreases fatty acid oxidation (98). The similar mechanisms of diet- and alcohol-induced steatosis, together with ethanol’s ability to increase endocannabinoid levels, at least in brain (99), suggest ECS involvement in alcoholic fatty liver. Indeed, exposure of male mice to a low fat, liquid ethanol diet for 4 weeks increased hepatic CB1 expression and 2-AG but not AEA levels. 2-AG was increased in HSC, but not in hepatocytes. The expression of diacylglycerol lipase-β(DAGLβ) was also increased in HSC (23), suggesting increased biosynthesis of 2-AG. Rimonabant treatment attenuated the ethanol-induced steatosis without affecting alcohol intake and blood ethanol levels, suggesting CB1 involvement. This was further supported by the resistance to ethanol-induced steatosis of both CB1−/− and LCB1−/− mice (23).
The hepatic nuclear expression of SREBP1c and its target FAS were increased, whereas CPT-1 expression and activity decreased in ethanol-fed mice, in agreement with earlier findings (97). In both CB1−/− and LCB1−/− mice, effects of ethanol on SREB1c, FAS and CPT-1 were blunted or absent. Furthermore, CPT-1 activity was increased and resistant to suppression by ethanol in both CB1 knockout strains (23). This supports the notion that in AFLD, hepatic lipogenesis is increased and fatty acid oxidation is decreased via CB1 activation.
CB1−/− hepatocytes are resistant to ethanol-induced steatosis, whereas the ethanol increases 2-AG exclusively in HSC. This suggests a paracrine mechanism whereby HSC-derived 2-AG activates CB1 receptors on adjacent hepatocytes to stimulate lipogenesis and inhibit fatty acid oxidation in the latter. Indeed, co-culturing HSC from alcohol-fed mice with hepatocytes from control mice resulted in increased lipogenic gene expression in the latter. The paracrine effect of ethanol-primed HSC was blunted when the hepatocytes in the co-culture were from LCB1−/− mice, confirming the role of CB1 receptors (23). This paracrine interaction, together with high levels of retinoic acid in HSC and its well known role in the control of gene expression, prompted a study of the possible role of retinoic acid and its receptors in regulating hepatic CB1 expression. CB1 expression in mouse or human isolated hepatocytes was upregulated by RARγ or panRAR agonists, and the effect could be attenuated by siRNA knockdown of RARγ, but not other RAR subtypes (25). Both CB1 and RARγ were upregulated in hepatocytes from mice fed either high-fat diet or liquid alcohol diet. Furthermore, 2-AG upregulated CB1 in normal but not in retinaldehyde dehydrogenase-1−/− hepatocytes, which are deficient in retinoic acid. Thus, CB1 ‘autoinduction’ may also involve retinoic acid (25). Interestingly, autoinduction of hepatic CB1 receptors is also suggested by the finding that chronic rimonabant treatment of DIO mice reversed the diet-induced upregulation of hepatic CB1 (4). The findings reviewed above suggest that CB1 antagonists could be effective in the treatment of both AFLD and NAFLD.
Endocannabinoids and Ischemia-Reperfusion Injury
Ischemia/reperfusion (I/R) injury may develop in conditions where blood and oxygen supply to a tissue is transiently disrupted and then restored. Hepatic I/R injury is a potentially fatal complication of liver surgery, including liver transplantation. Substances that improve hypoxia tolerance may also protect against I/R injury. Cannabinoids induce hypomotility and hypothermia, both of which result in reduced oxygen demand. The metabolic effects of cannabinoids that promote energy storage and reduce energy expenditure (see above) may also reduce oxygen demand. There is evidence that endocannabinoids acting via CB2 protect against hepatic I/R injury (100, 101).
In mice, segmental ischemia followed by reperfusion (but not ischemia alone) markedly increased the hepatic levels of AEA and 2-AG, which correlated with the severity of tissue damage (100). I/R-induced tissue damage, including neutrophil infiltration and lipid peroxidation, was attenuated by pretreatment with JWH-133 in wild-type but not in CB2−/− mice, in which the damage was more severe than in wild-type littermates (100). Another potent and selective CB2 agonist, HU-308, caused similar effects and also attenuated I/R-induced hepatocyte apoptosis and mitigated the TNFα-induced expression of the cell adhesion molecules ICAM-1 and VCAM-1 in hepatic sinusoidal endothelial cells (101). Thus, CB2 agonists may afford protection at multiple levels against I/R injury, highlighting their therapeutic potential.
CB1 receptor blockade also protects the liver from I/R injury and superimposed endotoxaemia (102). In this study, rats subjected to LPS plus I/R had an immediate increase in CB1 expression in perisinusoidal hepatocytes. Rimonabant treatment reduced both tissue necrosis and serum ALT in the late phase of reperfusion, and attenuated the oxidative injury (102). Further studies with peripherally restricted CB1 receptor antagonists could reinforce the therapeutic potential of this approach.
Hepatic Encephalopathy, Autoimmune Hepatitis
Hepatic encephalopathy is a neuropsychiatric syndrome that may accompany acute liver failure. The underlying mechanisms are not completely understood, although there is evidence for the pathogenic role of ammonia, alterations in various central neurotransmitter systems as well as altered cerebrovascular function. Mice with thioacetamide-induced fulminant liver failure have elevated brain 2-AG content. Treatment of such mice with 2-AG or the CB2 agonist HU-308 improved the neurological score and cognitive function, and these effects were blocked by a CB2 antagonist. The beneficial effects of CB2 agonists could be mimicked by treatment with the CB1 antagonist rimonabant (103). In another study by the same group, thioacetamide teatment or BDL induced CB2 expression in the brain, and also resulted in activation of AMP-activated protein kinase (AMPK). The absence of both effects in CB2−/− mice indicated the role of CB2 receptors (104), although there is also evidence for the additional involvement of TRPV1 receptors (105).
Cannabidiol (CBD) is a non-psychoactive constituent of marijuana with no significant CB1 or CB2 activity. CBD was found to improve cognitive and motor function as well as the neuroinflammation found in hepatic encephalopathy (106). The cerebral inflammatory response of mice to BDL was reduced by CBD treatment, and the effect was attributed to indirect activation of hippocampal A2A adenosine receptors. It is possible that combined treatment with a CB2 agonists and CBD offers additive therapeutic benefits in hepatic encephalopathy.
In a murine model of concanavalin A (ConA)-induced autoimmune hepatitis, THC attenuated the hepatitis, as judged by decreased plasma levels of liver enzymes and inflammatory cytokines and reduced tissue injury (107). Interestingly, FAAH−/− mice responded with reduced hepatic damage to ConA treatment, suggesting hepatoprotection by endogenous AEA (107). In contrast, the results of another study suggest that hepatoprotection may be achieved by blocking CB1 receptors (108).
Concluding Remarks
The ECS is present in the liver and is involved in the control of various hepatic functions with important therapeutic implications. Increased CB1 activity contributes to the hemodynamic abnormalities and promotes fibrosis in liver cirrhosis, whereas CB1 blockade attenuates and delays these changes. Endocannabinoids acting via hepatic CB1 receptors have emerged as mediators of both diet-induced and alcoholic fatty liver which, together, account for the majority of cirrhosis in Western societies. Additionally, hepatic CB1 activation contributes to obesity-related insulin- and leptin-resistance and dyslipidemias. This provides strong rationale for the therapeutic use of CB1 antagonists in these conditions. Although neuropsychiatric side effects limit the therapeutic potential of brain-penetrant CB1 antagonists, the recent emergence of second generation, peripherally-restricted CB1 antagonists may mitigate this problem. Additionally, non-psychoactive CB2 agonists may offer therapeutic benefit in attenuating liver injury and promoting tissue repair in the fibrotic liver.
References
- 1.Pacher P, Batkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev. 2006;58:389–462. doi: 10.1124/pr.58.3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Osei-Hyiaman D, DePetrillo M, Pacher P, Liu J, Radaeva S, Batkai S, Harvey-White J, et al. Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. J Clin Invest. 2005;115:1298–1305. doi: 10.1172/JCI23057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeMorrow S, Francis H, Gaudio E, Ueno Y, Venter J, Onori P, Franchitto A, et al. Anandamide inhibits cholangiocyte hyperplastic proliferation via activation of thioredoxin 1/redox factor 1 and AP-1 activation. Am J Physiol Gastrointest Liver Physiol. 2008;294:G506–519. doi: 10.1152/ajpgi.00304.2007. [DOI] [PubMed] [Google Scholar]
- 4.Jourdan T, Djaouti L, Demizieux L, Gresti J, Verges B, Degrace P. CB1 antagonism exerts specific molecular effects on visceral and subcutaneous fat and reverses liver steatosis in diet-induced obese mice. Diabetes. 59:926–934. doi: 10.2337/db09-1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teixeira-Clerc F, Julien B, Grenard P, Tran Van Nhieu J, Deveaux V, Li L, Serriere-Lanneau V, et al. CB1 cannabinoid receptor antagonism: a new strategy for the treatment of liver fibrosis. Nat Med. 2006;12:671–676. doi: 10.1038/nm1421. [DOI] [PubMed] [Google Scholar]
- 6.Michalopoulos GK, Bowen WC, Mule K, Luo J. HGF-, EGF-, and dexamethasone-induced gene expression patterns during formation of tissue in hepatic organoid cultures. Gene Expr. 2003;11:55–75. doi: 10.3727/000000003108748964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu X, Liu Y, Huang S, Liu G, Xie C, Zhou J, Fan W, et al. Overexpression of cannabinoid receptors CB1 and CB2 correlates with improved prognosis of patients with hepatocellular carcinoma. Cancer Genet Cytogenet. 2006;171:31–38. doi: 10.1016/j.cancergencyto.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 8.Floreani A, Lazzari R, Macchi V, Porzionato A, Variola A, Colavito D, Leon A, et al. Hepatic expression of endocannabinoid receptors and their novel polymorphisms in primary biliary cirrhosis. J Gastroenterol. 2009 doi: 10.1007/s00535-009-0122-y. [DOI] [PubMed] [Google Scholar]
- 9.Julien B, Grenard P, Teixeira-Clerc F, Van Nhieu JT, Li L, Karsak M, Zimmer A, et al. Antifibrogenic role of the cannabinoid receptor CB2 in the liver. Gastroenterology. 2005;128:742–755. doi: 10.1053/j.gastro.2004.12.050. [DOI] [PubMed] [Google Scholar]
- 10.Ashton JC, Rahman RM, Nair SM, Sutherland BA, Glass M, Appleton I. Cerebral hypoxia-ischemia and middle cerebral artery occlusion induce expression of the cannabinoid CB2 receptor in the brain. Neurosci Lett. 2007;412:114–117. doi: 10.1016/j.neulet.2006.10.053. [DOI] [PubMed] [Google Scholar]
- 11.Begg M, Pacher P, Batkai S, Osei-Hyiaman D, Offertaler L, Mo FM, Liu J, et al. Evidence for novel cannabinoid receptors. Pharmacol Ther. 2005;106:133–145. doi: 10.1016/j.pharmthera.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 12.Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- 13.Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, Yamashita A, et al. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun. 1995;215:89–97. doi: 10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]
- 14.Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, Gopher A, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- 15.Liu J, Wang L, Harvey-White J, Osei-Hyiaman D, Razdan R, Gong Q, Chan AC, et al. A biosynthetic pathway for anandamide. Proc Natl Acad Sci U S A. 2006;103:13345–13350. doi: 10.1073/pnas.0601832103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simon GM, Cravatt BF. Anandamide Biosynthesis Catalyzed by the Phosphodiesterase GDE1 and Detection of Glycerophospho-N-acyl Ethanolamine Precursors in Mouse Brain. J Biol Chem. 2008;283:9341–9349. doi: 10.1074/jbc.M707807200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fowler CJ. The pharmacology of the cannabinoid system--a question of efficacy and selectivity. Mol Neurobiol. 2007;36:15–25. doi: 10.1007/s12035-007-0001-6. [DOI] [PubMed] [Google Scholar]
- 18.McKinney MK, Cravatt BF. Structure and function of fatty acid amide hydrolase. Annu Rev Biochem. 2005;74:411–432. doi: 10.1146/annurev.biochem.74.082803.133450. [DOI] [PubMed] [Google Scholar]
- 19.Dinh TP, Carpenter D, Leslie FM, Freund TF, Katona I, Sensi SL, Kathuria S, et al. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc Natl Acad Sci U S A. 2002;99:10819–10824. doi: 10.1073/pnas.152334899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galiegue S, Mary S, Marchand J, Dussossoy D, Carriere D, Carayon P, Bouaboula M, et al. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur J Biochem. 1995;232:54–61. doi: 10.1111/j.1432-1033.1995.tb20780.x. [DOI] [PubMed] [Google Scholar]
- 21.Quarta C, Bellocchio L, Mancini G, Mazza R, Cervino C, Braulke LJ, Fekete C, et al. CB(1) signaling in forebrain and sympathetic neurons is a key determinant of endocannabinoid actions on energy balance. Cell Metab. 2010;11:273–285. doi: 10.1016/j.cmet.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 22.Lee TY, Lee KC, Chang HH. Modulation of the cannabinoid receptors by andrographolide attenuates hepatic apoptosis following bile duct ligation in rats with fibrosis. Apoptosis. 2010;15:904–914. doi: 10.1007/s10495-010-0502-z. [DOI] [PubMed] [Google Scholar]
- 23.Jeong WI, Osei-Hyiaman D, Park O, Liu J, Batkai S, Mukhopadhyay P, Horiguchi N, et al. Paracrine activation of hepatic CB1 receptors by stellate cell-derived endocannabinoids mediates alcoholic fatty liver. Cell Metab. 2008;7:227–235. doi: 10.1016/j.cmet.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 24.Osei-Hyiaman D, Liu J, Zhou L, Godlewski G, Harvey-White J, Jeong WI, Batkai S, et al. Hepatic CB(1) receptor is required for development of diet-induced steatosis, dyslipidemia, and insulin and leptin resistance in mice. J Clin Invest. 2008;118:3160–3169. doi: 10.1172/JCI34827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mukhopadhyay B, Liu J, Osei-Hyiaman D, Godlewski G, Mukhopadhyay P, Wang L, Jeong WI, et al. Transcriptional regulation of cannabinoid receptor-1 expression in the liver by retinoic acid acting via retinoic acid receptor-gamma. J Biol Chem. 2010;285:19002–19011. doi: 10.1074/jbc.M109.068460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siegmund SV, Qian T, de Minicis S, Harvey-White J, Kunos G, Vinod KY, Hungund B, et al. The endocannabinoid 2-arachidonoyl glycerol induces death of hepatic stellate cells via mitochondrial reactive oxygen species. Faseb J. 2007;21:2798–2806. doi: 10.1096/fj.06-7717com. [DOI] [PubMed] [Google Scholar]
- 27.Batkai S, Jarai Z, Wagner JA, Goparaju SK, Varga K, Liu J, Wang L, et al. Endocannabinoids acting at vascular CB1 receptors mediate the vasodilated state in advanced liver cirrhosis. Nat Med. 2001;7:827–832. doi: 10.1038/89953. [DOI] [PubMed] [Google Scholar]
- 28.Biecker E, Sagesser H, Reichen J. Vasodilator mRNA levels are increased in the livers of portal hypertensive NO-synthase 3-deficient mice. Eur J Clin Invest. 2004;34:283–289. doi: 10.1111/j.1365-2362.2004.01331.x. [DOI] [PubMed] [Google Scholar]
- 29.Domenicali M, Ros J, Fernandez-Varo G, Cejudo-Martin P, Crespo M, Morales-Ruiz M, Briones AM, et al. Increased anandamide induced relaxation in mesenteric arteries of cirrhotic rats: role of cannabinoid and vanilloid receptors. Gut. 2005;54:522–527. doi: 10.1136/gut.2004.051599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moezi L, Gaskari SA, Liu H, Baik SK, Dehpour AR, Lee SS. Anandamide mediates hyperdynamic circulation in cirrhotic rats via CB(1) and VR(1) receptors. Br J Pharmacol. 2006;149:898–908. doi: 10.1038/sj.bjp.0706928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mendez-Sanchez N, Zamora-Valdes D, Pichardo-Bahena R, Barredo-Prieto B, Ponciano-Rodriguez G, Bermejo-Martinez L, Chavez-Tapia NC, et al. Endocannabinoid receptor CB2 in nonalcoholic fatty liver disease. Liver Int. 2007;27:215–219. doi: 10.1111/j.1478-3231.2006.01401.x. [DOI] [PubMed] [Google Scholar]
- 32.Buckley NE, Hansson S, Harta G, Mezey E. Expression of the CB1 and CB2 receptor messenger RNAs during embryonic development in the rat. Neuroscience. 1998;82:1131–1149. doi: 10.1016/s0306-4522(97)00348-5. [DOI] [PubMed] [Google Scholar]
- 33.Teixeira-Clerc F, Belot MP, Manin S, Deveaux V, Cadoudal T, Chobert MN, Louvet A, et al. Beneficial paracrine effects of cannabinoid receptor 2 on liver injury and regeneration. Hepatology. 2010 doi: 10.1002/hep.23779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zaugg HE, Kyncl J. New antihypertensive cannabinoids. J Med Chem. 1983;26:214–217. doi: 10.1021/jm00356a017. [DOI] [PubMed] [Google Scholar]
- 35.Lake KD, Compton DR, Varga K, Martin BR, Kunos G. Cannabinoid-induced hypotension and bradycardia in rats mediated by CB1-like cannabinoid receptors. J Pharmacol Exp Ther. 1997;281:1030–1037. [PubMed] [Google Scholar]
- 36.Wagner JA, Varga K, Ellis EF, Rzigalinski BA, Martin BR, Kunos G. Activation of peripheral CB1 cannabinoid receptors in haemorrhagic shock. Nature. 1997;390:518–521. doi: 10.1038/37371. [DOI] [PubMed] [Google Scholar]
- 37.Wagner JA, Hu K, Bauersachs J, Karcher J, Wiesler M, Goparaju SK, Kunos G, et al. Endogenous cannabinoids mediate hypotension after experimental myocardial infarction. J Am Coll Cardiol. 2001;38:2048–2054. doi: 10.1016/s0735-1097(01)01671-0. [DOI] [PubMed] [Google Scholar]
- 38.Varga K, Wagner JA, Bridgen DT, Kunos G. Platelet- and macrophage-derived endogenous cannabinoids are involved in endotoxin-induced hypotension. Faseb J. 1998;12:1035–1044. doi: 10.1096/fasebj.12.11.1035. [DOI] [PubMed] [Google Scholar]
- 39.Wang Y, Liu Y, Sarker KP, Nakashima M, Serizawa T, Kishida A, Akashi M, et al. Polymyxin B binds to anandamide and inhibits its cytotoxic effect. FEBS Lett. 2000;470:151–155. doi: 10.1016/s0014-5793(00)01313-2. [DOI] [PubMed] [Google Scholar]
- 40.Kohro S, Imaizumi H, Yamakage M, Masuda Y, Namiki A, Asai Y, Maruyama I. Anandamide absorption by direct hemoperfusion with polymixin B-immobilized fiber improves the prognosis and organ failure assessment score in patients with sepsis. J Anesth. 2006;20:11–16. doi: 10.1007/s00540-005-0366-5. [DOI] [PubMed] [Google Scholar]
- 41.Liu J, Batkai S, Pacher P, Harvey-White J, Wagner JA, Cravatt BF, Gao B, et al. Lipopolysaccharide induces anandamide synthesis in macrophages via CD14/MAPK/phosphoinositide 3-kinase/NF-kappaB independently of platelet-activating factor. J Biol Chem. 2003;278:45034–45039. doi: 10.1074/jbc.M306062200. [DOI] [PubMed] [Google Scholar]
- 42.Ros J, Claria J, To-Figueras J, Planaguma A, Cejudo-Martin P, Fernandez-Varo G, Martin-Ruiz R, et al. Endogenous cannabinoids: a new system involved in the homeostasis of arterial pressure in experimental cirrhosis in the rat. Gastroenterology. 2002;122:85–93. doi: 10.1053/gast.2002.30305. [DOI] [PubMed] [Google Scholar]
- 43.Yang YY, Lin HC, Huang YT, Lee TY, Hou MC, Wang YW, Lee FY, et al. Role of Ca2+-dependent potassium channels in in vitro anandamide-mediated mesenteric vasorelaxation in rats with biliary cirrhosis. Liver Int. 2007;27:1045–1055. doi: 10.1111/j.1478-3231.2007.01551.x. [DOI] [PubMed] [Google Scholar]
- 44.Orliac ML, Peroni R, Celuch SM, Adler-Graschinsky E. Potentiation of anandamide effects in mesenteric beds isolated from endotoxemic rats. J Pharmacol Exp Ther. 2003;304:179–184. doi: 10.1124/jpet.102.041095. [DOI] [PubMed] [Google Scholar]
- 45.Caraceni P, Viola A, Piscitelli F, Giannone F, Berzigotti A, Cescon M, Domenicali M, et al. Circulating and hepatic endocannabinoids and endocannabinoid-related molecules in patients with cirrhosis. Liver Int. 2010;30:816–825. doi: 10.1111/j.1478-3231.2009.02137.x. [DOI] [PubMed] [Google Scholar]
- 46.Fernandez-Rodriguez CM, Romero J, Petros TJ, Bradshaw H, Gasalla JM, Gutierrez ML, Lledo JL, et al. Circulating endogenous cannabinoid anandamide and portal, systemic and renal hemodynamics in cirrhosis. Liver Int. 2004;24:477–483. doi: 10.1111/j.1478-3231.2004.0945.x. [DOI] [PubMed] [Google Scholar]
- 47.Bernardi M. Renal sodium retention in preascitic cirrhosis: expanding knowledge, enduring uncertainties. Hepatology. 2002;35:1544–1547. doi: 10.1053/jhep.2002.33714. [DOI] [PubMed] [Google Scholar]
- 48.Domenicali M, Caraceni P, Giannone F, Pertosa AM, Principe A, Zambruni A, Trevisani F, et al. Cannabinoid type 1 receptor antagonism delays ascites formation in rats with cirrhosis. Gastroenterology. 2009;137:341–349. doi: 10.1053/j.gastro.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 49.Garcia N, Jr, Jarai Z, Mirshahi F, Kunos G, Sanyal AJ. Systemic and portal hemodynamic effects of anandamide. Am J Physiol Gastrointest Liver Physiol. 2001;280:G14–20. doi: 10.1152/ajpgi.2001.280.1.G14. [DOI] [PubMed] [Google Scholar]
- 50.Jarai Z, Wagner JA, Varga K, Lake KD, Compton DR, Martin BR, Zimmer AM, et al. Cannabinoid-induced mesenteric vasodilation through an endothelial site distinct from CB1 or CB2 receptors. Proc Natl Acad Sci U S A. 1999;96:14136–14141. doi: 10.1073/pnas.96.24.14136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gaskari SA, Honar H, Lee SS. Therapy insight: Cirrhotic cardiomyopathy. Nat Clin Pract Gastroenterol Hepatol. 2006;3:329–337. doi: 10.1038/ncpgasthep0498. [DOI] [PubMed] [Google Scholar]
- 52.Gaskari SA, Liu H, Moezi L, Li Y, Baik SK, Lee SS. Role of endocannabinoids in the pathogenesis of cirrhotic cardiomyopathy in bile duct-ligated rats. Br J Pharmacol. 2005;146:315–323. doi: 10.1038/sj.bjp.0706331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Batkai S, Mukhopadhyay P, Harvey-White J, Kechrid R, Pacher P, Kunos G. Endocannabinoids acting at CB1 receptors mediate the cardiac contractile dysfunction in vivo in cirrhotic rats. Am J Physiol Heart Circ Physiol. 2007;293:H1689–1695. doi: 10.1152/ajpheart.00538.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gebremedhin D, Lange AR, Campbell WB, Hillard CJ, Harder DR. Cannabinoid CB1 receptor of cat cerebral arterial muscle functions to inhibit L-type Ca2+ channel current. Am J Physiol. 1999;276:H2085–2093. doi: 10.1152/ajpheart.1999.276.6.H2085. [DOI] [PubMed] [Google Scholar]
- 55.Howlett AC. Cannabinoid receptor signaling. Handb Exp Pharmacol. 2005:53–79. doi: 10.1007/3-540-26573-2_2. [DOI] [PubMed] [Google Scholar]
- 56.Lotersztajn S, Teixeira-Clerc F, Julien B, Deveaux V, Ichigotani Y, Manin S, Tran-Van-Nhieu J, et al. CB2 receptors as new therapeutic targets for liver diseases. Br J Pharmacol. 2008;153:286–289. doi: 10.1038/sj.bjp.0707511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Munoz-Luque J, Ros J, Fernandez-Varo G, Tugues S, Morales-Ruiz M, Alvarez CE, Friedman SL, et al. Regression of fibrosis after chronic stimulation of cannabinoid CB2 receptor in cirrhotic rats. J Pharmacol Exp Ther. 2008;324:475–483. doi: 10.1124/jpet.107.131896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hezode C, Roudot-Thoraval F, Nguyen S, Grenard P, Julien B, Zafrani ES, Pawlotsky JM, et al. Daily cannabis smoking as a risk factor for progression of fibrosis in chronic hepatitis C. Hepatology. 2005;42:63–71. doi: 10.1002/hep.20733. [DOI] [PubMed] [Google Scholar]
- 59.Yang Q, Liu HY, Zhang YW, Wu WJ, Tang WX. Anandamide induces cell death through lipid rafts in hepatic stellate cells. J Gastroenterol Hepatol. 2010;25:991–1001. doi: 10.1111/j.1440-1746.2009.06122.x. [DOI] [PubMed] [Google Scholar]
- 60.Di Marzo V, Goparaju SK, Wang L, Liu J, Batkai S, Jarai Z, Fezza F, et al. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature. 2001;410:822–825. doi: 10.1038/35071088. [DOI] [PubMed] [Google Scholar]
- 61.Rosenson RS. Role of the endocannabinoid system in abdominal obesity and the implications for cardiovascular risk. Cardiology. 2009;114:212–225. doi: 10.1159/000230691. [DOI] [PubMed] [Google Scholar]
- 62.Tam J, Vemuri VK, Liu J, Batkai S, Mukhopadhyay B, Godlewski G, Osei-Hyiaman D, et al. Peripheral CB1 cannabinoid receptor blockade improves cardiometabolic risk in mouse models of obesity. J Clin Invest. 2010;120:2953–2966. doi: 10.1172/JCI42551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ravinet Trillou C, Delgorge C, Menet C, Arnone M, Soubrie P. CB1 cannabinoid receptor knockout in mice leads to leanness, resistance to diet-induced obesity and enhanced leptin sensitivity. Int J Obes Relat Metab Disord. 2004;28:640–648. doi: 10.1038/sj.ijo.0802583. [DOI] [PubMed] [Google Scholar]
- 64.Lin J, Yang R, Tarr PT, Wu PH, Handschin C, Li S, Yang W, et al. Hyperlipidemic effects of dietary saturated fats mediated through PGC-1beta coactivation of SREBP. Cell. 2005;120:261–273. doi: 10.1016/j.cell.2004.11.043. [DOI] [PubMed] [Google Scholar]
- 65.Biddinger SB, Almind K, Miyazaki M, Kokkotou E, Ntambi JM, Kahn CR. Effects of diet and genetic background on sterol regulatory element-binding protein-1c, stearoyl-CoA desaturase 1, and the development of the metabolic syndrome. Diabetes. 2005;54:1314–1323. doi: 10.2337/diabetes.54.5.1314. [DOI] [PubMed] [Google Scholar]
- 66.Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115:1343–1351. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Westerbacka J, Kotronen A, Fielding BA, Wahren J, Hodson L, Perttila J, Seppanen-Laakso T, et al. Splanchnic Balance of Free Fatty Acids, Endocannabinoids, and Lipids in Subjects With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2010 doi: 10.1053/j.gastro.2010.06.064. [DOI] [PubMed] [Google Scholar]
- 68.Ruby MA, Nomura DK, Hudak CS, Mangravite LM, Chiu S, Casida JE, Krauss RM. Overactive endocannabinoid signaling impairs apolipoprotein E-mediated clearance of triglyceride-rich lipoproteins. Proc Natl Acad Sci U S A. 2008;105:14561–14566. doi: 10.1073/pnas.0807232105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jourdan T, Djaouti L, Demizieux L, Gresti J, Verges B, Degrace P. CB1 antagonism exerts specific molecular effects on visceral and subcutaneous fat and reverses liver steatosis in diet-induced obese mice. Diabetes. 2010;59:926–934. doi: 10.2337/db09-1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Son MH, Kim HD, Chae YN, Kim MK, Shin CY, Ahn GJ, Choi SH, et al. Peripherally acting CB1-receptor antagonist: the relative importance of central and peripheral CB1 receptors in adiposity control. Int J Obes (Lond) 2010;34:547–556. doi: 10.1038/ijo.2009.253. [DOI] [PubMed] [Google Scholar]
- 71.Hezode C, Zafrani ES, Roudot-Thoraval F, Costentin C, Hessami A, Bouvier-Alias M, Medkour F, et al. Daily cannabis use: a novel risk factor of steatosis severity in patients with chronic hepatitis C. Gastroenterology. 2008;134:432–439. doi: 10.1053/j.gastro.2007.11.039. [DOI] [PubMed] [Google Scholar]
- 72.Cota D, Marsicano G, Tschop M, Grubler Y, Flachskamm C, Schubert M, Auer D, et al. The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J Clin Invest. 2003;112:423–431. doi: 10.1172/JCI17725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Flamment M, Gueguen N, Wetterwald C, Simard G, Malthiery Y, Ducluzeau PH. Effects of the cannabinoid CB1 antagonist, rimonabant, on hepatic mitochondrial function in rats fed a high fat diet. Am J Physiol Endocrinol Metab. 2009 doi: 10.1152/ajpendo.00169.2009. [DOI] [PubMed] [Google Scholar]
- 74.Despres JP, Golay A, Sjostrom L. Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N Engl J Med. 2005;353:2121–2134. doi: 10.1056/NEJMoa044537. [DOI] [PubMed] [Google Scholar]
- 75.Migrenne S, Lacombe A, Lefevre AL, Pruniaux MP, Guillot E, Galzin AM, Magnan C. Adiponectin is required to mediate rimonabant-induced improvement of insulin sensitivity but not body weight loss in diet-induced obese mice. Am J Physiol Regul Integr Comp Physiol. 2009;296:R929–935. doi: 10.1152/ajpregu.90824.2008. [DOI] [PubMed] [Google Scholar]
- 76.Watanabe T, Kubota N, Ohsugi M, Kubota T, Takamoto I, Iwabu M, Awazawa M, et al. Rimonabant ameliorates insulin resistance via both adiponectin-dependent and adiponectin-independent pathways. J Biol Chem. 2009;284:1803–1812. doi: 10.1074/jbc.M807120200. [DOI] [PubMed] [Google Scholar]
- 77.Herling AW, Gossel M, Haschke G, Stengelin S, Kuhlmann J, Muller G, Schmoll D, et al. CB1 receptor antagonist AVE1625 affects primarily metabolic parameters independently of reduced food intake in Wistar rats. Am J Physiol Endocrinol Metab. 2007;293:E826–832. doi: 10.1152/ajpendo.00264.2007. [DOI] [PubMed] [Google Scholar]
- 78.Kunz I, Meier MK, Bourson A, Fisseha M, Schilling W. Effects of rimonabant, a cannabinoid CB1 receptor ligand, on energy expenditure in lean rats. Int J Obes (Lond) 2008;32:863–870. doi: 10.1038/ijo.2008.3. [DOI] [PubMed] [Google Scholar]
- 79.Liu YL, Connoley IP, Wilson CA, Stock MJ. Effects of the cannabinoid CB1 receptor antagonist SR141716 on oxygen consumption and soleus muscle glucose uptake in Lep(ob)/Lep(ob) mice. Int J Obes (Lond) 2005;29:183–187. doi: 10.1038/sj.ijo.0802847. [DOI] [PubMed] [Google Scholar]
- 80.Ravinet Trillou C, Arnone M, Delgorge C, Gonalons N, Keane P, Maffrand JP, Soubrie P. Anti-obesity effect of SR141716, a CB1 receptor antagonist, in diet-induced obese mice. Am J Physiol Regul Integr Comp Physiol. 2003;284:R345–353. doi: 10.1152/ajpregu.00545.2002. [DOI] [PubMed] [Google Scholar]
- 81.Gary-Bobo M, Elachouri G, Gallas JF, Janiak P, Marini P, Ravinet-Trillou C, Chabbert M, et al. Rimonabant reduces obesity-associated hepatic steatosis and features of metabolic syndrome in obese Zucker fa/fa rats. Hepatology. 2007;46:122–129. doi: 10.1002/hep.21641. [DOI] [PubMed] [Google Scholar]
- 82.DeLeve LD, Wang X, Kanel GC, Atkinson RD, McCuskey RS. Prevention of hepatic fibrosis in a murine model of metabolic syndrome with nonalcoholic steatohepatitis. Am J Pathol. 2008;173:993–1001. doi: 10.2353/ajpath.2008.070720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Poirier B, Bidouard JP, Cadrouvele C, Marniquet X, Staels B, O’Connor SE, Janiak P, et al. The anti-obesity effect of rimonabant is associated with an improved serum lipid profile. Diabetes Obes Metab. 2005;7:65–72. doi: 10.1111/j.1463-1326.2004.00374.x. [DOI] [PubMed] [Google Scholar]
- 84.Wang J, Obici S, Morgan K, Barzilai N, Feng Z, Rossetti L. Overfeeding rapidly induces leptin and insulin resistance. Diabetes. 2001;50:2786–2791. doi: 10.2337/diabetes.50.12.2786. [DOI] [PubMed] [Google Scholar]
- 85.El-Haschimi K, Pierroz DD, Hileman SM, Bjorbaek C, Flier JS. Two defects contribute to hypothalamic leptin resistance in mice with diet-induced obesity. J Clin Invest. 2000;105:1827–1832. doi: 10.1172/JCI9842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hollister LE, Reaven GM. Delta-9-tetrahydrocannabinol and glucose tolerance. Clin Pharmacol Ther. 1974;16:297–302. doi: 10.1002/cpt1974162297. [DOI] [PubMed] [Google Scholar]
- 87.Bermudez-Siva FJ, Serrano A, Diaz-Molina FJ, Sanchez Vera I, Juan-Pico P, Nadal A, Fuentes E, et al. Activation of cannabinoid CB1 receptors induces glucose intolerance in rats. Eur J Pharmacol. 2006;531:282–284. doi: 10.1016/j.ejphar.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 88.Uno K, Katagiri H, Yamada T, Ishigaki Y, Ogihara T, Imai J, Hasegawa Y, et al. Neuronal pathway from the liver modulates energy expenditure and systemic insulin sensitivity. Science. 2006;312:1656–1659. doi: 10.1126/science.1126010. [DOI] [PubMed] [Google Scholar]
- 89.Yang Q, Graham TE, Mody N, Preitner F, Peroni OD, Zabolotny JM, Kotani K, et al. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 2005;436:356–362. doi: 10.1038/nature03711. [DOI] [PubMed] [Google Scholar]
- 90.Pagotto U, Marsicano G, Cota D, Lutz B, Pasquali R. The emerging role of the endocannabinoid system in endocrine regulation and energy balance. Endocr Rev. 2006;27:73–100. doi: 10.1210/er.2005-0009. [DOI] [PubMed] [Google Scholar]
- 91.Bermudez-Silva FJ, Sanchez-Vera I, Suarez J, Serrano A, Fuentes E, Juan-Pico P, Nadal A, et al. Role of cannabinoid CB2 receptors in glucose homeostasis in rats. Eur J Pharmacol. 2007;565:207–211. doi: 10.1016/j.ejphar.2007.02.066. [DOI] [PubMed] [Google Scholar]
- 92.Doyon C, Denis RG, Baraboi ED, Samson P, Lalonde J, Deshaies Y, Richard D. Effects of rimonabant (SR141716) on fasting-induced hypothalamic-pituitary-adrenal axis and neuronal activation in lean and obese Zucker rats. Diabetes. 2006;55:3403–3410. doi: 10.2337/db06-0504. [DOI] [PubMed] [Google Scholar]
- 93.Scheen AJ, Finer N, Hollander P, Jensen MD, Van Gaal LF. Efficacy and tolerability of rimonabant in overweight or obese patients with type 2 diabetes: a randomised controlled study. Lancet. 2006;368:1660–1672. doi: 10.1016/S0140-6736(06)69571-8. [DOI] [PubMed] [Google Scholar]
- 94.Gonsiorek W, Lunn C, Fan X, Narula S, Lundell D, Hipkin RW. Endocannabinoid 2-arachidonyl glycerol is a full agonist through human type 2 cannabinoid receptor: antagonism by anandamide. Mol Pharmacol. 2000;57:1045–1050. [PubMed] [Google Scholar]
- 95.Deveaux V, Cadoudal T, Ichigotani Y, Teixeira-Clerc F, Louvet A, Manin S, Nhieu JT, et al. Cannabinoid CB2 receptor potentiates obesity-associated inflammation, insulin resistance and hepatic steatosis. PLoS One. 2009;4:e5844. doi: 10.1371/journal.pone.0005844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lieber CS, Schmid R. The effect of ethanol on fatty acid metabolism; stimulation of hepatic fatty acid synthesis in vitro. J Clin Invest. 1961;40:394–399. doi: 10.1172/JCI104266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.You M, Fischer M, Deeg MA, Crabb DW. Ethanol induces fatty acid synthesis pathways by activation of sterol regulatory element-binding protein (SREBP) J Biol Chem. 2002;277:29342–29347. doi: 10.1074/jbc.M202411200. [DOI] [PubMed] [Google Scholar]
- 98.You M, Matsumoto M, Pacold CM, Cho WK, Crabb DW. The role of AMP-activated protein kinase in the action of ethanol in the liver. Gastroenterology. 2004;127:1798–1808. doi: 10.1053/j.gastro.2004.09.049. [DOI] [PubMed] [Google Scholar]
- 99.Basavarajappa BS, Saito M, Cooper TB, Hungund BL. Stimulation of cannabinoid receptor agonist 2-arachidonylglycerol by chronic ethanol and its modulation by specific neuromodulators in cerebellar granule neurons. Biochim Biophys Acta. 2000;1535:78–86. doi: 10.1016/s0925-4439(00)00085-5. [DOI] [PubMed] [Google Scholar]
- 100.Batkai S, Osei-Hyiaman D, Pan H, El-Assal O, Rajesh M, Mukhopadhyay P, Hong F, et al. Cannabinoid-2 receptor mediates protection against hepatic ischemia/reperfusion injury. Faseb J. 2007;21:1788–1800. doi: 10.1096/fj.06-7451com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rajesh M, Pan H, Mukhopadhyay P, Batkai S, Osei-Hyiaman D, Hasko G, Liaudet L, et al. Cannabinoid-2 receptor agonist HU-308 protects against hepatic ischemia/reperfusion injury by attenuating oxidative stress, inflammatory response, and apoptosis. J Leukoc Biol. 2007;82:1382–1389. doi: 10.1189/jlb.0307180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Caraceni P, Pertosa AM, Giannone F, Domenicali M, Grattagliano I, Principe A, Mastroleo C, et al. Antagonism of the cannabinoid CB-1 receptor protects rat liver against ischaemia-reperfusion injury complicated by endotoxaemia. Gut. 2009;58:1135–1143. doi: 10.1136/gut.2007.147652. [DOI] [PubMed] [Google Scholar]
- 103.Avraham Y, Israeli E, Gabbay E, Okun A, Zolotarev O, Silberman I, Ganzburg V, et al. Endocannabinoids affect neurological and cognitive function in thioacetamide-induced hepatic encephalopathy in mice. Neurobiol Dis. 2006;21:237–245. doi: 10.1016/j.nbd.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 104.Dagon Y, Avraham Y, Ilan Y, Mechoulam R, Berry EM. Cannabinoids ameliorate cerebral dysfunction following liver failure via AMP-activated protein kinase. Faseb J. 2007;21:2431–2441. doi: 10.1096/fj.06-7705com. [DOI] [PubMed] [Google Scholar]
- 105.Avraham Y, Zolotarev O, Grigoriadis NC, Poutahidis T, Magen I, Vorobiav L, Zimmer A, et al. Cannabinoids and capsaicin improve liver function following thioacetamide-induced acute injury in mice. Am J Gastroenterol. 2008;103:3047–3056. doi: 10.1111/j.1572-0241.2008.02155.x. [DOI] [PubMed] [Google Scholar]
- 106.Magen I, Avraham Y, Ackerman Z, Vorobiev L, Mechoulam R, Berry EM. Cannabidiol ameliorates cognitive and motor impairments in mice with bile duct ligation. J Hepatol. 2009;51:528–534. doi: 10.1016/j.jhep.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 107.Hegde VLHS, Cravatt BF, Hofseth LJ, Nagarkatti M, Nagarkatti PS. Attenuation of experimental autoimmune hepatitis by exogenous and endogenous cannabinoids: involvement of regulatory T cells. Molecular Pharmacology. 2008 doi: 10.1124/mol.108.047035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kojima M, Kato N, Hirano D, Ochi T, Nii A, Shinjo K, Eda H. Selective CB1 cannabinoid receptor antagonist, SR141716A, attenuates liver injury induced by Concanavalin A. Hepatol Res. 2009;39:408–414. doi: 10.1111/j.1872-034X.2008.00473.x. [DOI] [PubMed] [Google Scholar]