Abstract
Background
Heart failure in patients with preserved left ventricular systolic function (HFpEF) is a prevalent disease characterized by exercise intolerance, with poorly understood pathophysiology. We hypothesized that recruitable contractility is impaired in HFpEF, accounting for the appearance of symptoms with exertion.
Methods and Results
Echocardiographic analysis of myocardial performance was performed at baseline and after a modified dobutamine protocol (max dose 16 mcg/kg/min) in participants with known HFpEF and age and sex matched controls. The primary outcome variable was change in contractile reserve, measured as a change in ejection fraction (EF). Recruitable contractility was decreased in HFpEF participants compared to controls (Δ EF HFpEF = 0.4±1.9% vs. control = 19.0±1.4%; p<0.001). During dobutamine infusion velocities increased in control participants but remained unchanged in the HFpEF group, yielding a significant difference between groups (p<0.05) for both longitudinal displacement and velocity.
Conclusion
Patients with HFpEF have an impaired contractile response to adrenergic stimulation. The blunted response to adrenergic stimulation in the HFpEF group suggests that these patients may be unable to respond to periods of increased cardiac demand. This inability to increase contractility appropriately suggests abnormalities of systolic function in this disease, and may contribute to exertional intolerance in HFpEF.
Keywords: diastolic heart failure, contractile reserve, stress echocardiography, dobutamine
Introduction
There are more than 5 million people with heart failure (HF) in the United States, approximately 50% of whom have preserved ejection fraction (HFpEF), with the number of cases increasing as the population ages.1–3 No therapeutic interventions have been demonstrated to reduce mortality in HFpEF. In contrast, mortality in patients with HF and impaired systolic function has decreased over the last 20 years.2, 4, 5 Arguably, progress in treating HFpEF has been hampered by a lack of understanding of the underlying pathophysiology, leading to inadequate diagnosis and treatment.6
Multiple clinical variables have been shown to be associated with HFpEF; however, none is sufficiently sensitive or specific to serve as a definitive diagnostic test for HFpEF.7–9 It is known that HFpEF is associated with aging and affects women more often than men. There is a clear association with hypertension as well.10, 11 Since aging and hypertension are almost universally associated with impaired left ventricular (LV) relaxation and an abnormal LV filling pattern, this is a common finding in patients with HFpEF.3, 12 Many people with evidence of abnormal LV relaxation and filling, however, do not have symptoms or signs of heart failure, leading to speculation that other pathophysiologic mechanisms contribute to the clinical syndrome of HFpEF.
Because impaired exertional tolerance is a universal finding in patients with HFpEF,7, 13, 14 we hypothesized that impaired contractile reserve may contribute to this syndrome. Most non-invasive imaging of these patients occurs at rest, and is at least superficially normal. Recognizing that increases in heart rate and adrenergic stimulation normally augment myocardial contractility during periods of elevated physiological stress, we tested the hypothesis that HFpEF patients would have an abnormal response to pharmacological stress with dobutamine infusion. Patients and age-matched controls underwent a low dose dobutamine protocol with comprehensive echocardiographic imaging.
Methods
Study Participants
We enrolled 9 control participants and 10 patients with HFpEF. All HF patients had an ejection fraction (EF) > 45%, had been diagnosed with HFpEF by a cardiologist, had exhibited symptoms or signs of fluid overload, and were NYHA Class II or III at the time of enrollment. Half of the HFpEF participants had been previously hospitalized with decompensated heart failure. All HFpEF participants were followed in the Heart Failure Clinic at the University of Wisconsin, had been treated aggressively for their heart failure, and none had other significant comorbidities that might explain their exertional symptoms. HFpEF subjects with clinically significant coronary artery disease, symptoms of active ischemia, severe renal dysfunction, ventricular arrhythmias or aortic aneurysms were excluded. Control participants were age and sex matched, without any evidence of heart failure on careful history, physical examination and review of medical records. Written informed consent was provided by all participants included in the present study. The protocol was approved by the University of Wisconsin Human Subjects Committee.
Experimental Protocol
All participants were studied in a fasting state in the Clinical and Translational Research Center at the University of Wisconsin. All vasoactive medications, including β-blockers, were held for 24 hours prior to study commencement. Briefly, participants arrived, had baseline vital signs measured, and then had an intravenous line placed. After 20 minutes of quiet rest, vital signs were repeated. Baseline echocardiographic images were then obtained. After the rest images were obtained, dobutamine was infused at incremental doses of 2, 4, 8, and 16 mcg/kg/min IV at 25-minute intervals. During the last 15 minutes of each dose increment, echocardiographic data acquisition was repeated. Criteria for stopping the dobutamine infusion included hypotension, angina, significant dyspnea, clinically significant arrhythmia, attainment of 85% maximal predicted heart rate, 90% cavity obliteration or completion of full protocol.
Echocardiographic Studies
Standard 2-dimensional echocardiographic imaging, pulsed Doppler interrogation of mitral inflow, and tissue Doppler imaging of the annular region of the left ventricle were performed under resting conditions and at each dobutamine dose. 2-D Doppler tissue imaging (DTI) of the lateral, septal, anterior and posterior walls was performed in apical views using a high frame rate acquisition for VVI analysis. In addition, 2D imaging was obtained at high frame rates (>220 frames/s, 3.5 MHz) for offline strain-rate analysis. All data were digitized and stored for later offline analysis (Syngo Analysis Systems, Siemens Medical).
Echocardiographic Analysis
Quantification of left ventricular and atrial volumes, and left ventricular mass were performed according to the recommendations of the American Society of Echocardiography.15 Ejection fraction was assessed using Simpson’s Rule. Detailed analysis of myocardial deformation, including velocity, strain rate and displacement was conducted using Velocity Vector Imaging (VVI, Siemens Medical). Measurements of velocity, strain rate and displacement were analyzed in the longitudinal dimension from apical 2- and 4-chamber views, and analyzed in the radial dimension from the short axis view. The basal segments of the lateral and septal walls were analyzed. Values for velocity and strain rate were assessed at peak systole, during early diastole, and during atrial systole. Ventricular dimensions and function were evaluated offline by two experienced readers blinded to study group assignment. Inter-reader variability was less than 5% for all echocardiographic measures.
Statistical Analyses
The primary outcome measure was change in LV ejection fraction with dobutamine infusion compared to baseline within and between groups. Comparisons were performed with both repeated measures one-way and two-way ANOVA, followed by either a post-hoc Holm-Sidak or Student-Newman-Keuls -test for significance. Comparisons were performed between groups at each dose using two-way repeated measures ANOVA. One-way repeated measures ANOVA was used to assess differences within groups across different dobutamine doses. A p-value ≤0.05 was used to define a significant result.
Secondary analyses included assessment of myocardial velocities and strain rate, to better detail the dobutamine response in the 2 groups. Similar to the primary endpoint, comparisons of these echocardiographic measurements to baseline values within each group, and also between groups, were made with a both repeated measures one-way and two-way ANOVAs, followed by a post-hoc Bonferroni t-test for significance.
Results
Clinical characteristics and cardiac function
Clinical characteristics of the study population are shown in Table 1. The HFpEF participants were predominantly female, and ranged in age from 42–88 (median = 63). The HFpEF patients had a higher prevalence of smoking than the control group. Both groups were overweight, with similar resting heart rates and blood pressures. No control participants were being treated with antihypertensive medications. The majority of HFpEF patients were on treatment for hypertension with multidrug regimens. While exercise tolerance was not evaluated, all HFpEF patients reported decreased exercise tolerance, as determined by medical history and study questionnaire, while no control participants reported this symptom. No HFpEF subject had a significant secondary explanation for their dyspnea, as participants were excluded if they had any evidence of significant non-cardiac disease causing dyspnea. Most patients completed the dobutamine protocol, however one HFpEF participant developed severe hypertension at dobutamine doses ≥8 mcg/kg/min. Data from this participant obtained at lower dobutamine doses were included in the analysis.
Table 1.
Clinical Data
| Control (n=9) | HFpEF (n=10) | |
|---|---|---|
| n (females, males) | 7, 2 | 9, 1 |
| Age [median (range)] | 65 (36–80) | 60 (42–88) |
| Concommitant Diseases: | ||
| Diabetes Mellitus | 1/9 (11%) | 2/10 (20%) |
| Hypertension | 0/9 (0%) | 6/10 (60%) |
| Hyperlipidemia | 2/9 (22%) | 6/10 (60%) |
| Smoker | 0/9 (0%) | 2/10 (20%) |
| Medications: | ||
| Beta-blocker | 0/9 (0%) | 7/10 (70%) |
| ACE Inhibitor | 0/9 (0%) | 4/10 (40%) |
| Calcium channel Blocker | 0/9 (0%) | 2/10 (20%) |
| Diuretics | 0/9 (0%) | 7/10 (70%) |
| Vitals: | ||
| Height (cm) | 162.3 ± 3.1 | 170.9 ± 8.3 |
| Weight (kg) | 70.0 ± 5.8 | 85.7 ± 9.1 |
| BMI (kg/m2) | 26.7 ± 2.3 | 30.3 ± 3.7 |
| Resting HR (bpm) | 62.0 ± 3.0 | 63.4 ± 3.2 |
| Baseline SBP (mmHg) | 125.1 ± 4.5 | 132.6 ± 7.6 |
| Baseline DBP (mmHg) | 71.4 ± 1.1 | 68.0 ± 2.9 |
BMI = body mass index, HR = heart rate, bpm = beats per minute, SBP = systolic blood pressure, DBP = diastolic blood pressure, mmHg = millimeters of mercury. All values are mean ± SEM.
Standard echocardiographic data obtained from 2D and Doppler measurements at baseline are shown in Table 2. The ventricles of the participants with HFpEF were of normal size, although left ventricular end diastolic dimension was increased compared to controls, as was the left ventricular mass index. Relative wall thickness was normal, despite the presence of hypertension in this population. LA volume, however, was 80% larger in HFpEF than in control participants, consistent with the presence of HF. Despite the presence of clinical heart failure in all subjects, echocardiographic evidence of diastolic dysfunction was not present in most subjects. (Table 2),
Table 2.
Echocardiographic Data
| Control | HFpEF | |
|---|---|---|
| Baseline Ejection Fraction (%) | 68.4 ± 1.6 | 63.2 ± 3.2 |
| Max (16mcg/kg/min) Ejection Fraction (%) | 85.6 ± 1.7 | 63.6 ± 2.9* |
| Chamber Dimensions: | ||
| LVEDD (mm) | 42.6 ± 1.1 | 47.1 ± 1.5* |
| Septum (mm) | 8.3 ± 0.4 | 9.6 ± 0.5 |
| Posterior Wall (mm) | 9.9 ± 0.3 | 10.1 ± 0.3 |
| LA Volume (cm3) | 38.9 ± 3.3 | 61.2 ± 5.7* |
| LVMI (g/m2) | 82.8 ± 7.2 | 104.0 ± 5.7* |
| Relative Wall Thickness (RWT) | 0.47 ± 0.02 | 0.44 ± 0.02 |
| Mitral Valve Flow: | ||
| Deceleration Time (ms) | 217.2 ± 17.1 | 216.4 ± 20.2 |
| IVRT (ms) | 100.1 ± 8.4 | 93.0 ± 9.0 |
| E (m/s) | 0.87 ± 0.07 | 0.84 ± 0.06 |
| A (m/s) | 0.73 ± 0.06 | 0.79 ± 0.07 |
| E/A | 1.32 ± 0.21 | 1.15 ± 0.14 |
| E′ (cm/s) | 13.0 ± 1.0 | 15.0 ± 2.0 |
| E/E′ | 6.68 ± 0.72 | 6.26 ± 0.59 |
p<0.05 between groups.
LVEDD = left ventricular end diastolic diameter, LA volume = left atrial volume, LVMI = left ventricular mass index, Ea = mitral annular velocity, lateral wall, IVRT = isovolumic relaxation time, E/A = ratio of peak early and late diastolic mitral flow. All values are mean ± SEM.
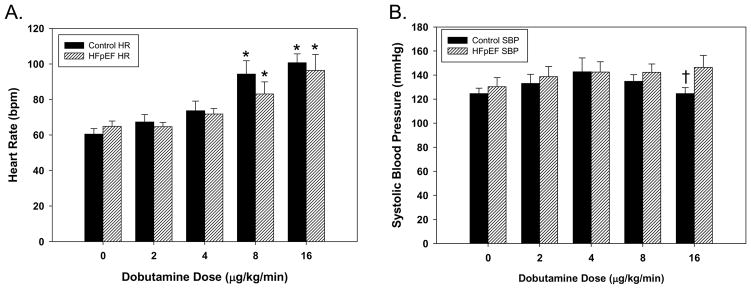
In both control and HFpEF participants, dobutamine had a similar dose-dependent effect to increase HR with no significant HR differences between groups at any dose (Figure 1). However, the effect of dobutamine on systolic blood pressure (SBP) differed in the two groups at the maximal level of dobutamine infusion (16mcg/kg/min). At lower doses of dobutamine infusion, there was no difference in SBP between groups. In control participants, SBP rose and fell at the higher doses of dobutamine infusion, whereas HFpEF participants hadconsistently increased SBP with increasing doses of dobutamine, such that the SBP was significantly higher at 16 mcg/kg/min in HFpEF than control participants (Figure 1). End systolic volume was similar for both groups at baseline (HFpEF = 26.9 ± 3.6ml vs. control = 26.3 ± 2.2ml) but differed significantly at 16 mcg/kg/min (HFpEF = 25.0 ± 3.8ml vs. control = 14.7 ± 2.5ml; p=0.04), whereas no significant differences were detected for end diastolic volume at either baseline (HFpEF = 75.7 ± 7.8ml vs. control = 70.2 ± 6.3ml) or at the maximal dobutamine dosage (HFpEF = 64.0 ± 8.5ml vs. control = 72.0 ± 7.7ml).
Figure 1. A dose-dependent effect of dobutamine on heart rate and systolic blood pressure in control and HFpEF participants.
The dose-dependent effect of dobutamine on (A) heart rate (HR; bars) and (B) systolic blood pressure (SBP; circles) in HFpEF participants (hatched bars, open circles) did not differ significantly from control participants (black bars, black circles). The data represents the mean ± SEM, *p<0.05 significant difference vs. baseline; † significant difference (p<0.05) between control and HFpEF groups.
Inter-reader variability for all measurements was low, e.g., measurements of tissue Doppler E/E′ in the control group was 6.64 ± 0.8mm vs. 6.78 ± 0.6mm (p = 0.96).
Contractile reserve
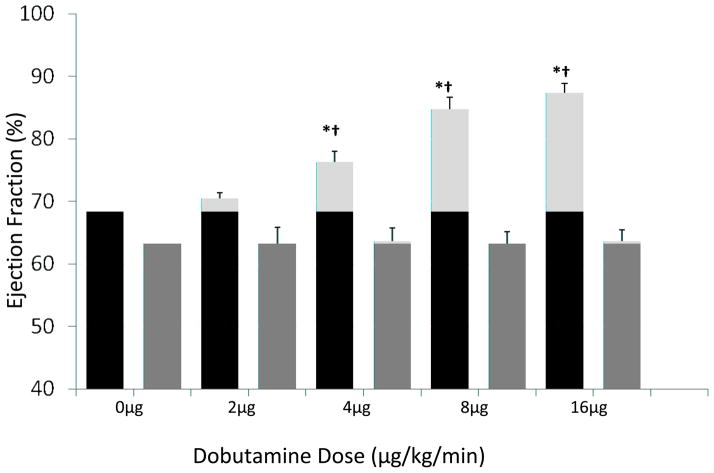
At baseline, EF in HFpEF (63.2±3.2%) and control (68.4±1.6%) participants was not significantly different. While EF increased with dobutamine in a dose-dependent manner in control participants, no increase in EF was evident in HFpEF participants (Figure 2). Thus, contractile reserve, defined as change in EF, was reduced in HFpEF participants compared to controls at low dose (4mcg/kg/min; ΔEF HFpEF = 0.4 ± 2.1 vs. control = 7.9 ± 1.7, p<0.02) and the maximal 16 mcg/kg/min dose (Δ EF HFpEF = 0.4±1.9% vs. control = 19.0±1.4%, p<0.001).
Figure 2. A dose-dependent effect of dobutamine on contractile reserve in control and HFpEF participants.
Ejection fraction was evaluated in control (black bars) and HFpEF participants (dark grey bars) at base line and following infusion of 2–16 mcg/kg/min of dobutamine (ΔEF% = light grey bars). The data are the mean ± SEM. * The values evaluated following dobutamine infusion are different (p<0.001–0.03) versus baseline. †The values evaluated following dobutamine infusion in HFpEF participants are significantly different (p<0.001–0.03) from controls at the same dosage.
Longitudinal displacement and velocity
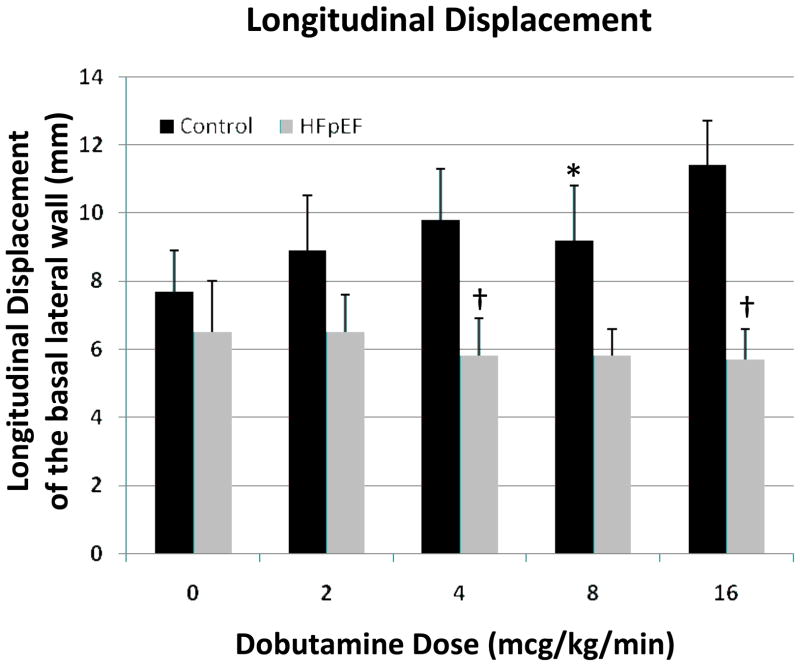
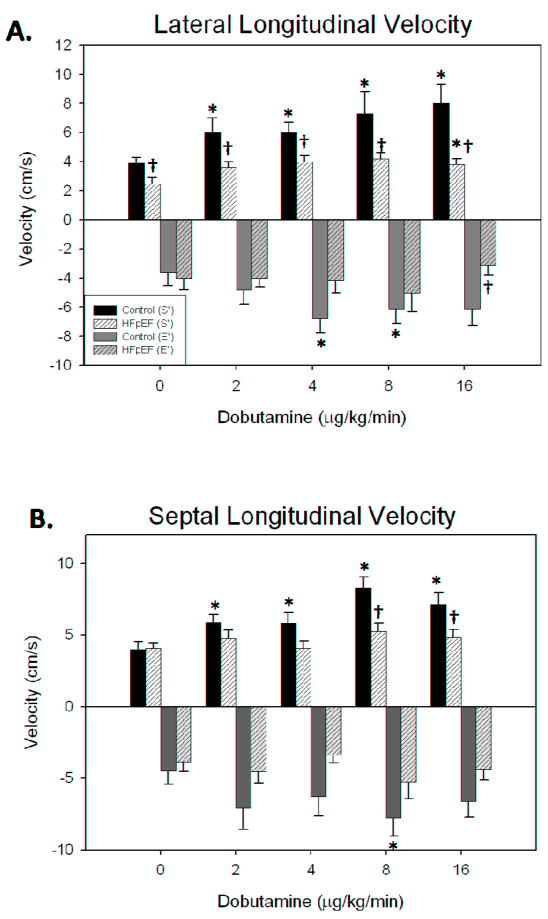
Myocardial displacement (Figure 3) and velocity tangential to the long axis of the ventricle (Figure 4) were analyzed using the basal LV segments. At baseline, there was no significant difference in mean peak longitudinal displacement between HFpEF (6.5±1.4 mm) and control (7.5±1.1 mm) participants. The dobutamine response in HFpEF participants was blunted relative to control participants (Figures 4 and 5). For example, 4 mcg/kg/min infusion of dobutamine increased the displacement of lateral segment from 7.5 ± 1.1 mm to 9.4 ± 1.4 mm in control participants with no significant change seen in HFpEF patients (6.5±1.4 mm to 5.0 ± 0.8 mm). A similar response was seen in the basal septal segment. Peak systolic velocity measured in the basal lateral segment was significantly different between the two groups at baseline, and failed to increase significantly in HFpEF, but increased significantly in control participants, such that velocity was significantly greater in controls than in HFpEF under all conditions (Figure 4). Significant differences were also detected for the septal basal segment between groups at higher dobutamine doses, although not at baseline, likely due to the lower velocities in the septal region. VVI-determined early diastolic velocities were only significantly different at maximum dobutamine doses, and only in the lateral segment (Figure 4).
Figure 3. Longitudinal displacement before and after infusion of dobutamine.
Longitudinal displacement of the basal segment of left ventricular free wall was evaluated by echocardiography in control (black bars) and HFpEF (gray bars) participants before and during dobutamine infusion (2–16 mcg/kg/min) for 10 min. * The values evaluated following dobutamine infusion are different (p<0.05) versus baseline. †The values evaluated following dobutamine infusion in HFpEF participants are significantly different (p<0.05) from controls at the same dosage.
Figure 4. A dose-dependent effect of dobutamine on systolic and diastolic longitudinal velocities in control and HFpEF participants.
A dose-dependent effect of dobutamine on both the systolic (dark, S′) and diastolic (light, E′) longitudinal velocities of the basal lateral segment in control (black/grey filled bars) and HFpEF (hatched bars) was evaluated echocardiographically. The values are the mean ± SEM. The values evaluated following dobutamine infusion in are significantly different (* p<0.05) from those evaluated at baseline. The values evaluated following dobutamine infusion are significantly different († p<0.01–0.05) between groups.
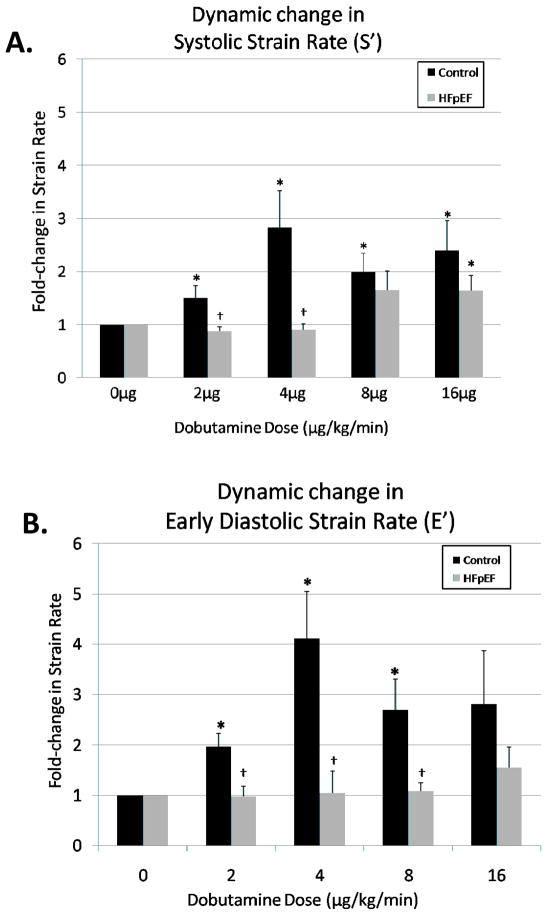
Figure 5. A dose-dependent effect of dobutamine on systolic and early diastolic strain rate in control and HFpEF participants.
Control (black) and HFpEF (grey) participants. The data are the mean ± SEM. * The values evaluated following dobutamine infusion in are significantly different (p<0.05) from those at baseline. † The values evaluated following dobutamine infusion are significantly different (p<0.05) between groups.
Radial velocities and displacement increased modestly with dobutamine in both groups, typically reaching maximal values at 4mcg/kg/min for velocity measurements and 8mcg/kg/min for displacement measurements, with no further increases at higher doses of dobutamine. No significant differences were found between groups in the radial dimension for either velocity or displacement.
Strain Rate
Systolic strain rate and early diastolic strain rate yielded robust statistical differences following dobutamine infusion both between groups and when compared to baseline values for the control group. The systolic component of strain rate rose significantly in the control group, while an increase in strain rate in the HFpEF participants was not evident at lower dobutamine doses. When comparing the increases in strain rate, differences between groups were found at the lower dobutamine doses (2 and 4mcg/kg/min, Figure 5).
Similarly early diastolic values of strain rate increased reproducibly in the control group, with a far less impressive increase in HFpEF participants. The magnitude of the difference between the groups in early diastolic strain rate was maximal at 4mcg/kg/min (Control 2.5 ± 0.4 s−1 vs. HFpEF 1.2 ± 0.2 s−1). Differences did not reach statistical significance between groups for the remaining dosages, most likely due to variability and small sample size.
When examining change in strain rate vs. baseline, significant changes were found between the control and HFpEF groups at 2mcg/kg/min, 4mcg/kg/min and 8mcg/kg/min for the early diastolic component (E′) of strain rate (Figure 5), and at 2mcg/kg/min and 4mcg/kg/min for the systolic (S′) component of strain rate (Figure 5) measured in the basal lateral free wall.
Discussion
We report here the use of a low-dose dobutamine protocol to assess myocardial recruitable contractility in participants with HFpEF compared to age-matched controls. We have demonstrated impaired contractile reserve with adrenergic stimulation, as manifested by the failure to increase ejection fraction during dobutamine stimulation, and, more subtly, failure of systolic and diastolic strain rate to increase with dobutamine in HFpEF participants. We believe a phenotype of impaired contractile reserve may contribute to the symptoms of exertional intolerance universally seen in this disease.
Baseline ventricular function
In the present study, there was no discernible difference between groups for all measured contractile parameters at baseline, excluding lateral segment velocity. All study participants had a baseline ejection fraction >50% and had similar baseline heart rates, myocardial velocities and strain rates. These data agree with previously published findings16, 17 and highlight the often indiscernible nature of this heart disease at rest. In addition, the HFpEF participants in this study did not have LVH, a finding commonly associated with HFpEF which some consider diagnostic in the right clinical setting.3 This reflects the aggressive nature of the antihypertensive and heart failure therapy the patients were receiving.
Recently, it has been suggested that subtle systolic deficiencies, measured through depressed myocardial contractile performance (altered mitral annular velocities), exist in HFpEF, and that the preserved systolic function or ejection fraction was the result of an increase in relative wall thickness.18 However, our patient and control cohorts exhibit similar relative wall thickness; thus, while a systolic component of HFpEF at rest may in fact be present in patients with more severe abnormalities of cardiac hypertrophy, we conclude that the preserved function and inability to differentiate the HFpEF from control participants at rest in patients with perhaps less obvious myocardial disease warrants a need for more robust diagnostics. Further, reports of altered systolic function in HFpEF at rest are inconclusive, reporting that some participants have a definitive systolic decrement, 19 while others do not,20 suggesting that these findings are not universally present and thus not sensitive diagnostically. These reports indicate that the clinical disease HFpEF may include a spectrum of systolic dysfunction, which by definition will not be useful diagnostically. Our finding of differences in longitudinal myocardial velocities indicates that a systolic component of HFpEF may exist at rest; however, the parameter is not sensitive enough for accurate diagnosis.
Despite the presence of clinical heart failure, echocardiographic assessment of diastolic function using tissue Doppler imaging did not show slowed myocardial relaxation in most HFpEF subjects. This is consistent with previous reports in which diastolic impairment was not closely associated with the presence of HFpEF 10, 21–23. A larger study population may be necessary to detect diastolic differences and more accurately reflect the heterogeneous population of HFpEF.
Contractile Reserve
The HFpEF participants exhibited minimal change in ejection fraction with low dose β-adrenergic stimulation in a ramped protocol, despite heart rate and blood pressure responses that did not differ from control participants at all but the highest dobutamine dose. Possible mechanisms for a decreased sensitivity to β-adrenergic stimulation in HFpEF are defects in β-adrenergic receptor density or signaling, defects in the ability to augment myocyte calcium cycling and/or a dysfunctional myofilament response to augmented calcium cycling. The inability of the ventricle to increase ejection fraction with stress, due in part to an inability to decrease end systolic volume, indicates that this diastolic disease in fact has a systolic component as well. Thus we suggest that the symptom of exercise intolerance may be a result of impaired contractile reserve, rather than the result of pure diastolic dysfunction, as was previously reported.24
Contractility in HFpEF
At baseline, indices of myocardial contractility such as longitudinal displacement (Figure 3) and velocity (Figure 4) were similar between control and HFpEF participants, an observation consistent with earlier studies.16, 25 Dobutamine, a β-adrenoceptor agonist, increased both the longitudinal displacement and velocities in a dose-dependent manner in control but not in HFpEF participants. The lack of dobutamine effects on longitudinal displacement and velocities in HFpEF reported here suggests that dynamic myocardial contractility is depressed in this condition, which has been proposed to be a result of diminished compliance of ventricular tissue.26, 27 Reduced compliance may directly interfere with the Frank-Starling mechanism, potentially by increasing the lattice spacing between thick and thin filaments and reducing cross-bridge formation, which would lead to reduced myocardial contractility. A similar lack of increase in longitudinal shortening velocity has previously been reported in HFpEF participants performing an exercise protocol.17, 18, 25
The robust difference found at the lower doses of dobutamine for longitudinal displacement, velocity and strain rate, indicate that discernible differences may be identified reliably at quite low doses of β-agonist, rather than at the typical levels used in stress testing which are significantly higher than the doses used in this protocol. In previous studies using higher doses of dobutamine the primary finding has been an altered diastolic component of HFpEF, e.g. impaired relaxation,24 thus our lower-dose dobutamine method may allow for more sensitive study of the systolic component of HFpEF. In addition, at lower dobutamine doses, the heart rate effects of the β-agonist are less profound, allowing more precise Doppler analysis of myocardial behavior in the absence of the confounding effects of high heart rate. Additionally, at lower doses of dobutamine infusion, effects on SBP were similar to control participants, eliminating differences in afterload as a potential explanation of the impaired contractile response.
Chronotropic Responsiveness
At the relatively low doses of dobutamine used here, heart rate increases were minimized, allowing us to focus on the contractile behavior of the myocardium. Interestingly, however, both groups had similar increases in heart rate during the dobutamine protocol. It has previously been suggested that decreased chronotropic responsiveness may contribute to the exercise intolerance seen in HFpEF.14, 28 In our study, chronotropic incompetence did not contribute to the abnormal inotropic response seen in the HFpEF group. Our data suggest, instead, that an impaired force-frequency relationship may contribute to exercise intolerance in HFpEF, since the heart rate increase in the HFpEF group was similar to that seen in controls, yet the ability to increase ejection fraction was diminished in the HFpEF group. Impaired force-frequency responses are thought to be due to impaired calcium handling in myocytes with an altered competition between sarcoplasmic Ca2+ uptake via the Ca2+ pump and diastolic Ca2+ extrusion via the sodium-calcium exchanger demonstrated in severely failing human hearts with systolic HF.29
Possible underlying mechanisms of pathophysiological function
To date, the suspected pathophysiology of HFpEF has been postulated to be excessive fibrosis30 and myocyte hypertrophy, leading to impaired left ventricular filling and reduced diastolic distensibility and/or stiffness.12, 31 Our results indicate that in addition to the above factors, there is also a decrease in the sensitivity of myocardial responsiveness to β-adrenergic stimulation. The mechanism of this decreased responsiveness is unclear. Although it may be explained by decreased β-adrenergic receptor density or more distal defects in cAMP/PKA-dependent signaling, recent studies indicate that reduced cytosolic calcium levels, related to post-receptor alterations of the sarcoplasmic reticulum, contribute to reduced responsiveness.32 Thus, both impaired left ventricular filling 27 and poor contractile reserve may contribute to deficient pump function and impaired exercise tolerance 28 in HFpEF.
Clinical Implications
Echocardiography for diagnosis of HFpEF can be inconclusive,8, 33 although necessary to rule out other forms of heart failure.34 Here we present a potential diagnostic technique, using a low dose dobutamine infusion in conjunction with standard echocardiographic imaging, which may provide more accurate diagnosis of HFpEF in difficult cases. Distinguishing between HFpEF and normal patients is rarely a challenge; however, distinguishing HFpEF from other etiologies of exertional shortness of breath such as chronic pulmonary disorders is of intense clinical interest. It remains to be shown whether the myocardial response to dobutamine differs between participants with HFpEF and those with normal EF and non-myocardial causes of dyspnea.
Limitations
While a larger validation cohort is needed to confirm the results presented here, the ability to detect differences in contractile responses in a limited number of patients with similar resting echocardiographic parameters supports the diagnostic potential of the low-dose dobutamine echocardiography for HFpEF in clinical settings. The fact that many patients with HFpEF have comorbidities and limited exercise tolerance increases the potential utility of a modality employing pharmacological stimulation, rather than exercise as a means of assessing contractile reserve. Confirmatory studies investigating more heterogenous populations are needed to confirm the results and demonstrate specificity to HFpEF. As this study investigated a small, white, intensively managed population, with non-hypertrophied ventricles and minimal diastolic dysfunction, extension to a more typical HFpEF population is important. Recent studies have indicated that the underlying pathophysiologic mechanisms responsible for the phenotype of HFpEF may be varied in different cohorts of patients 35; thus, while the present study offers a potentially valuable diagnostic tool, elucidating the precise pathophysiological mechanism in various cohorts of patients may ultimately be required before we understand the mechanism of HFpEF comprehensively. It is important, for example, that future studies demonstrate that the impaired dobutamine response is specific to HFpEF and not present in hypertensive patients without HF. There were pharmacological (β-blockade) and physiological (hypertension) differences between the HFpEF and control groups, which may have impacted the results of this study; these factors were minimized by chronic aggressive treatment of hypertension and the cessation of β-blocker therapy 24 hours prior to the study session. While symptoms to suggest ischemia, including wall motion abnormalities and ECG changes, were absent throughout the dobutamine protocol in all research subjects, it is impossible to exclude subclinical ischemia as a contributing factor to the failure to increase LVEF in HFpEF participants.
Summary
Echocardiographic analysis of myocardial responses during dobutamine infusion demonstrates impaired recruitable contractility in participants with HFpEF. The impaired response results in failure to increase ejection fraction and decrease end-systolic volume appropriately for a given level of β-adrenergic stimulation. These results may help explain the prominent exertional intolerance seen in this patient population, despite apparently normal myocardial function at rest. In addition, if verified, the absence of dobutamine responsiveness may be a useful diagnostic tool in suspected HFpEF. The precise underlying molecular mechanism of this behavior is yet to be determined.
Acknowledgments
We are grateful to Dr. Richard Moss for helpful assistance with data interpretation.
Funding Sources
Dr. Sweitzer was funded in part through NIH AG01022 K23. Dr. Norman was funded in part through NIH T32 T32-HL 07936. Dr. Margulies was funded by NIH AG17022. Supported by grant 1UL1RR025011 from the Clinical and Translational Science Award (CTSA) program of the National Center for Research Resources, NIH.
Footnotes
Disclosures: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Owan TE, Redfield MM. Epidemiology of diastolic heart failure. Prog Cardiovasc Dis. 2005;47(5):320–332. doi: 10.1016/j.pcad.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 2.Redfield MM, Jacobsen SJ, Burnett JC, Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. Jama. 2003;289(2):194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 3.Zile MR, Brutsaert DL. New concepts in diastolic dysfunction and diastolic heart failure: Part I: diagnosis, prognosis, and measurements of diastolic function. Circulation. 2002;105(11):1387–1393. doi: 10.1161/hc1102.105289. [DOI] [PubMed] [Google Scholar]
- 4.Fitzgibbons TP, Meyer TE, Aurigemma GP. Mortality in diastolic heart failure: an update. Cardiol Rev. 2009;17(2):51–55. doi: 10.1097/CRD.0b013e318194527d. [DOI] [PubMed] [Google Scholar]
- 5.Somaratne JB, Berry C, McMurray JJ, Poppe KK, Doughty RN, Whalley GA. The prognostic significance of heart failure with preserved left ventricular ejection fraction: a literature-based meta-analysis. Eur J Heart Fail. 2009;11(9):855–862. doi: 10.1093/eurjhf/hfp103. [DOI] [PubMed] [Google Scholar]
- 6.Najjar SS. Heart failure with preserved ejection fraction failure to preserve, failure of reserve, and failure on the compliance curve. Journal of the American College of Cardiology. 2009;54(5):419–421. doi: 10.1016/j.jacc.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lester SJ, Tajik AJ, Nishimura RA, Oh JK, Khandheria BK, Seward JB. Unlocking the mysteries of diastolic function: deciphering the Rosetta Stone 10 years later. J Am Coll Cardiol. 2008;51(7):679–689. doi: 10.1016/j.jacc.2007.09.061. [DOI] [PubMed] [Google Scholar]
- 8.Kasner M, Westermann D, Steendijk P, Gaub R, Wilkenshoff U, Weitmann K, Hoffmann W, Poller W, Schultheiss HP, Pauschinger M, Tschope C. Utility of Doppler echocardiography and tissue Doppler imaging in the estimation of diastolic function in heart failure with normal ejection fraction: a comparative Doppler-conductance catheterization study. Circulation. 2007;116(6):637–647. doi: 10.1161/CIRCULATIONAHA.106.661983. [DOI] [PubMed] [Google Scholar]
- 9.Vasan RS, Levy D. Defining diastolic heart failure: a call for standardized diagnostic criteria. Circulation. 2000;101(17):2118–2121. doi: 10.1161/01.cir.101.17.2118. [DOI] [PubMed] [Google Scholar]
- 10.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355(3):251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 11.Chen MA. Heart failure with preserved ejection fraction in older adults. Am J Med. 2009;122(8):713–723. doi: 10.1016/j.amjmed.2009.01.038. [DOI] [PubMed] [Google Scholar]
- 12.Westermann D, Kasner M, Steendijk P, Spillmann F, Riad A, Weitmann K, Hoffmann W, Poller W, Pauschinger M, Schultheiss HP, Tschope C. Role of left ventricular stiffness in heart failure with normal ejection fraction. Circulation. 2008;117(16):2051–2060. doi: 10.1161/CIRCULATIONAHA.107.716886. [DOI] [PubMed] [Google Scholar]
- 13.Ennezat PV, Lefetz Y, Marechaux S, Six-Carpentier M, Deklunder G, Montaigne D, Bauchart JJ, Mounier-Vehier C, Jude B, Neviere R, Bauters C, Asseman P, de Groote P, Lejemtel TH. Left ventricular abnormal response during dynamic exercise in patients with heart failure and preserved left ventricular ejection fraction at rest. J Card Fail. 2008;14(6):475–480. doi: 10.1016/j.cardfail.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 14.Kitzman DW, Groban L. Exercise intolerance. Heart Fail Clin. 2008;4(1):99–115. doi: 10.1016/j.hfc.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18(12):1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Baicu CF, Zile MR, Aurigemma GP, Gaasch WH. Left ventricular systolic performance, function, and contractility in patients with diastolic heart failure. Circulation. 2005;111(18):2306–2312. doi: 10.1161/01.CIR.0000164273.57823.26. [DOI] [PubMed] [Google Scholar]
- 17.Wachter R, Schmidt-Schweda S, Westermann D, Post H, Edelmann F, Kasner M, Luers C, Steendijk P, Hasenfuss G, Tschope C, Pieske B. Blunted frequency-dependent upregulation of cardiac output is related to impaired relaxation in diastolic heart failure. Eur Heart J. 2009 doi: 10.1093/eurheartj/ehp341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fukuta H, Little WC. Contribution of systolic and diastolic abnormalities to heart failure with a normal and a reduced ejection fraction. Prog Cardiovasc Dis. 2007;49(4):229–240. doi: 10.1016/j.pcad.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 19.Surucu H, Tatli E, Degirmenci A, Okudan S, Boz H. Subtle systolic dysfunction may be associated with the tendency to develop diastolic heart failure in patients with preserved left ventricular ejection fraction. Echocardiography. 2009;26(4):365–370. doi: 10.1111/j.1540-8175.2008.00824.x. [DOI] [PubMed] [Google Scholar]
- 20.Dokainish H, Sengupta R, Pillai M, Bobek J, Lakkis N. Assessment of left ventricular systolic function using echocardiography in patients with preserved ejection fraction and elevated diastolic pressures. Am J Cardiol. 2008;101(12):1766–1771. doi: 10.1016/j.amjcard.2008.02.070. [DOI] [PubMed] [Google Scholar]
- 21.Burkhoff D, Maurer MS, Packer M. Heart failure with a normal ejection fraction: is it really a disorder of diastolic function? Circulation. 2003;107(5):656–658. doi: 10.1161/01.cir.0000053947.82595.03. [DOI] [PubMed] [Google Scholar]
- 22.Melenovsky V, Borlaug BA, Rosen B, Hay I, Ferruci L, Morell CH, Lakatta EG, Najjar SS, Kass DA. Cardiovascular features of heart failure with preserved ejection fraction versus nonfailing hypertensive left ventricular hypertrophy in the urban Baltimore community: the role of atrial remodeling/dysfunction. J Am Coll Cardiol. 2007;49(2):198–207. doi: 10.1016/j.jacc.2006.08.050. [DOI] [PubMed] [Google Scholar]
- 23.Persson H, Lonn E, Edner M, Baruch L, Lang CC, Morton JJ, Ostergren J, McKelvie RS. Diastolic dysfunction in heart failure with preserved systolic function: need for objective evidence:results from the CHARM Echocardiographic Substudy-CHARMES. J Am Coll Cardiol. 2007;49(6):687–694. doi: 10.1016/j.jacc.2006.08.062. [DOI] [PubMed] [Google Scholar]
- 24.Chattopadhyay S, Alamgir MF, Nikitin NP, Rigby AS, Clark AL, Cleland JG. Lack of Diastolic Reserve in Patients with Heart Failure and Normal Ejection Fraction. Circ Heart Fail. 2009 doi: 10.1161/CIRCHEARTFAILURE.108.824888. [DOI] [PubMed] [Google Scholar]
- 25.Tan YT, Wenzelburger F, Lee E, Heatlie G, Leyva F, Patel K, Frenneaux M, Sanderson JE. The pathophysiology of heart failure with normal ejection fraction: exercise echocardiography reveals complex abnormalities of both systolic and diastolic ventricular function involving torsion, untwist, and longitudinal motion. J Am Coll Cardiol. 2009;54(1):36–46. doi: 10.1016/j.jacc.2009.03.037. [DOI] [PubMed] [Google Scholar]
- 26.Lam CS, Roger VL, Rodeheffer RJ, Bursi F, Borlaug BA, Ommen SR, Kass DA, Redfield MM. Cardiac structure and ventricular-vascular function in persons with heart failure and preserved ejection fraction from Olmsted County, Minnesota. Circulation. 2007;115(15):1982–1990. doi: 10.1161/CIRCULATIONAHA.106.659763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zile MR, Baicu CF, Gaasch WH. Diastolic heart failure--abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med. 2004;350(19):1953–1959. doi: 10.1056/NEJMoa032566. [DOI] [PubMed] [Google Scholar]
- 28.Borlaug BA, Melenovsky V, Russell SD, Kessler K, Pacak K, Becker LC, Kass DA. Impaired chronotropic and vasodilator reserves limit exercise capacity in patients with heart failure and a preserved ejection fraction. Circulation. 2006;114(20):2138–2147. doi: 10.1161/CIRCULATIONAHA.106.632745. [DOI] [PubMed] [Google Scholar]
- 29.Rossman EI, Petre RE, Chaudhary KW, Piacentino V, 3rd, Janssen PM, Gaughan JP, Houser SR, Margulies KB. Abnormal frequency-dependent responses represent the pathophysiologic signature of contractile failure in human myocardium. J Mol Cell Cardiol. 2004;36(1):33–42. doi: 10.1016/j.yjmcc.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 30.Martos R, Baugh J, Ledwidge M, O’Loughlin C, Conlon C, Patle A, Donnelly SC, McDonald K. Diastolic heart failure: evidence of increased myocardial collagen turnover linked to diastolic dysfunction. Circulation. 2007;115(7):888–895. doi: 10.1161/CIRCULATIONAHA.106.638569. [DOI] [PubMed] [Google Scholar]
- 31.Krum H, Abraham WT. Heart failure. Lancet. 2009;373(9667):941–955. doi: 10.1016/S0140-6736(09)60236-1. [DOI] [PubMed] [Google Scholar]
- 32.Quaile MP, Rossman EI, Berretta RM, Bratinov G, Kubo H, Houser SR, Margulies KB. Reduced sarcoplasmic reticulum Ca(2+) load mediates impaired contractile reserve in right ventricular pressure overload. J Mol Cell Cardiol. 2007;43(5):552–563. doi: 10.1016/j.yjmcc.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 33.Sanderson JE. Heart failure with a normal ejection fraction. Heart. 2007;93(2):155–158. doi: 10.1136/hrt.2005.074187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aurigemma GP, Gaasch WH. Clinical practice. Diastolic heart failure. N Engl J Med. 2004;351(11):1097–1105. doi: 10.1056/NEJMcp022709. [DOI] [PubMed] [Google Scholar]
- 35.Maurer MS, Kronzon I, Burkhoff D. Ventricular pump function in heart failure with normal ejection fraction: insights from pressure-volume measurements. Prog Cardiovasc Dis. 2006;49(3):182–195. doi: 10.1016/j.pcad.2006.08.007. [DOI] [PubMed] [Google Scholar]