Abstract
CD4 and CD8 co-receptors play critical roles in T cell development and activation by interacting both with T cell receptors and MHC molecules. Although homologs of these genes have been identified in many jawed vertebrates, there are still unresolved gaps concerning their evolution and specialization in MHC interaction and T cell function. Using experimental and computational procedures we identified CD4, CD8α and CD8β gene homologs both in Xenopus tropicalis, whose full genome has been sequenced, and its sister species X. laevis. Multiple alignments of deduced amino acid sequences reveal a poor conservation of the residues involved in binding of CD4 to MHC class II, and CD8α to class I in non-mammalian species, presumably related to the coevolutionary pressure of MHC I and II genes. Phylogenetic study suggests that Xenopodinae co-receptor genes are more closely related to their homologs in other tetrapods than those of bony fish. Furthermore, the developmental and cell-specific expression patterns of these genes in X. laevis are very similar to that of mammals. X. laevis CD4 is mainly expressed by peripheral non-CD8 T cells and detected in the thymus as early as four days post-fertilization (dpf) at the onset of thymic organogenesis. CD8β expression is specific to adult surface CD8+ T cells and thymocytes, and is first detected in the thymus at five dpf in parallel with productive TCRγ transrcipts, whereas productive TCRβ and α rearrangements are not detected before 7–9 dpf.
Keywords: genome mining, IgSF, evolution, T cell ontogeny
1. Introduction
Both CD4 and CD8 molecules are cell surface co-receptor glycoproteins of the immunoglobulin (Ig) superfamily that are involved in T cell development and antigen recognition by T cells [1]. Both CD4 and CD8 expressing T cells use the same repertoire of T cell receptors (TCRs), but respond to antigens associated with different types of Major Histocompatibility Complex (MHC) molecules [2]. CD4 and CD8 co-receptors interact with nonpolymorphic regions of MHC Class II and Class I molecules respectively that are conserved between mammalian species. These interactions lead to increased intercellular adhesion and enhanced stimulation of T cells. In addition, the polymorphic regions of the MHC molecules interact with the T cell receptor [3]. T cells develop in the thymus where they progress from CD4 and CD8 double negative to double positive stage, and then finally to a single positive stage in which either CD4 or CD8, but not both are expressed [4].
In mammals, CD4 is a 55kDa monomer with an extracellular region consisting of four Ig-like domains (D1–D4). D1 and D3 are V-like domains containing 9 β strands (ABCC′ C″DEFG) with characteristics of the Ig superfamily including a pair of cysteine residues and a conserved tryptophan residue [5, 6]. D2 and D4 are C-like domains that contain 7β strands (ABCC′EFG). On the other hand, CD8 is either a disulphide-linked homodimer of two α chains, or a heterodimer with one α and one β-chain. The extracellular regions of CD8α and β chains are similar; they consist of a single NH2 terminal Ig-like domain, an extended stalk hinge region of 50 (α) or 30 (β) amino acids that may be glycosylated, a transmembrane domain and a cytoplasmic tail [7, 8]. There is a conserved binding motif p56lck within the cytoplasmic tail of CD8α but not CD8β [9]. Although CD8αα and CD8αβ are similar in tertiary structure they differ in tissue distribution, ligand specificity and efficiency of antigen presentation (reviewed in [10, 11]). During TCR mediated MHC peptide recognition, CD8 acts as a TCR co-receptor and interacts with MHC class I and β2-microglobulin (β2m) [12]. CD8α associates with β2m and the α2 and α3 domains of MHC class Ia molecules using its A/B β strands and its complementarity determining (CDR)-like region. The CDR-like region of CD8αα and the MHC class I α3 domain are critical for forming the CD8αα.pMHC class I complex [13].
Although CD4 and CD8 gene homologs have been identified in all classes of jawed vertebrates, functional and differential expression studies are still limited outside of mammals and birds [14–16]. In addition, several species of teleost fish possess an additional CD4-like T cell co-receptor, CD4REL, that has only two Ig domains [17, 18]. CD4 and CD4REL receptors are presumably expressed by distinct T cell populations. A second CD4-like gene with four Ig domains has also been reported in fugu and trout [19, 20]. It is unclear whether these additional genes are the result of the particular evolutionary history of fish, since this groups has undergone a rapid expansion and diversification aside from the main branch that lead to mammals. A CD4-like surface molecule with two Ig domains has also been reported in the lamprey, a jawless fish with an adaptive immune system that is convergent but not homologous to the jawed vertebrate adaptive immune system [21]. Concerning CD8, besides the genetic linkage of CD8α and CD8β, the developmental expression and cell type-specific expression is also poorly known outside homeothermic vertebrates. Whether the functions of CD4 and CD8 co-receptors in T cell activation and T cell development has been fixed in multiple steps or whether these functions have diversified in some species during evolution is still unclear.
Amphibians occupy a key intermediate position between fishes and mammals/chicken in the evolutionary radiation of vertebrates, and species from the Anuran amphibian subfamily Xenopodinae provide a unique model for studying evolution of the immune system (reviewed in [22]). Multiple lines of evidence indicate that the Xenopodinae subfamily is composed of two divergent genera named Xenopus and Silurana [23], [24]. Xenopus laevis is a representative species of the Xenopus genus, whereas Xenopus tropicalis is a representative species of the Silurana genus. These two species, X. laevis and X. tropicalis, whose divergence from a common ancestor is estimated to be over 65 million years ago (MYA), are serving as valuable non-mammalian animal models in the study of early vertebrate development and comparative immunology [24]. Importantly, the immune system of the frog Xenopus laevis is extensively characterized [22]. In particular, thymocyte differentiation and peripheral CD8 T cells have been characterized to some extent phenotypically and functionally thanks to the availability of monoclonal antibodies (reviewed in [22]). Notably, MHC-class I restriction and antigen-specificity of cytotoxic CD8 T cells has been demonstrated [25]. In contrast, little is still known about CD4 T cells besides indirect evidence of class II-dependent T helper-like function in T-B collaboration and MHC-mismatched mixed lymphocyte reaction [26, 27]. Recently, X. tropicalis has been selected as a model organism for a whole genome sequencing project (www.jgi.doe.gov/xenopus) because compared to X. laevis it is diploid and develops faster, which is advantageous for genetic analysis [22, 28]. Analysis of the X. tropicalis genome revealed an extensive degree of conserved gene synteny with human, mouse and chicken genomes and also a high degree of conservation of genes associated with human disease [28].
We took advantage of the X. tropicalis annotated genome sequence to characterize and compare CD4 and CD8 gene homologs in X. tropicalis and X. laevis. We also determined the tissue specific expression profiles of these genes in X. laevis adult and during early development. Our study reveals that Xenopodinae co-receptor genes are more related to their respective homologs in other tetrapods than to those in bony fish. Furthermore, developmental, tissue and cell-specific expression patterns of these genes in X. laevis are very similar to that of mammals.
2. Materials and Methods
2.1. Animals
Adult and larval outbred X. laevis were obtained from the X. laevis Research Resource for Immunobiology at the University of Rochester Medical Center (http://www.urmc.rochester.edu/mbi/resources/Xenopus/). Larval development stages were determined according to [29]. Adults and larvae were euthanized with 0.5% and 0.1% Tricaine methanesulfonate (TMS), respectively. All animals were handled under strict laboratory and University Committee on Animal Resources (UCAR) regulations (Approval number 100577/2003-151 and 2004-199).
2.2. GenBank accessions of cDNA clones
Xenopus EST cDNA clones were obtained from the I.M.A.G.E. Consortium through ATCC (USA) or Research Genetics Inc (USA), sequenced, and submitted to GenBank. Accession numbers for these cDNAs are EB-728260 (CD4), EB-645787 (CD8β), EB-472568, and EB-646935 (CD8α).
2.3. RNA extraction, cDNA synthesis, RACE-PCR and RT-PCR
Total RNA was extracted with Trizol reagent (Invitrogen), and quantified with SmartSpec spectrophotometer (BioRad). 5′ RACE-Ready cDNAs were synthesized using SMART cDNA amplification kit (clonetech Laboratories, inc.), cloned into pGEM-T Easy (Promega Corp.) and sequenced. 500 ng of total RNA was used to synthesize cDNA with iScript first strand cDNA synthesis kit (BioRad) according to the manufacturer’s protocol. Negative reverse transcriptase (RT) controls were run for each sample at the same time. cDNAs were diluted two times and 1μl was used as a template in all PCR reactions (Genescript Corp.) The primers used for RT-PCR are listed in Table 1.
Table 1.
List of primers used for RT-PCR and RACE
| Gene | Primer name | Sequence (5′ – 3′) |
|---|---|---|
| β-Actin | b-actin-ex3-F1 | GGTGTCATGGTTGGAATGG |
| b-actin-ex3-R3 | TGTGGGTTACACCATCACCTG | |
| CD4 | CD4-SP-F1 | TCCATCTCTGACATCCCCTC |
| CD4-D4-R2 | TCACCAGACACACGTCCATT | |
| CD8α | CD8-a-49-F2 | AAGCCACCTACGACTACCACCAA |
| CD8-a-296-R2 | CCGTTCTTCTCAGTCTCAGGCAC | |
| CD8β | CD8-b-SP-F4 | TCATCATCTCTTTCTGGGGC |
| CD8-b-cyt-R3 | AATTCAGTGGGTGCTTCCTG | |
| Rag1 | Rag1-F-415 | GCGCCAAGAATCTGTGTCACT |
| Rag1-R-2 | GTTCTGTTTCATGGTTGTCTACCA | |
| TCRα | Alpha-TCR-C3-2 | TTCAGGGACCTCTGGTTGCTTC |
| Alpha-TCR-C5 | ACACATCAGCCTCAATCCATCC | |
| TCRβ | TCR-BV1-F | CACCCAGGAGCCAAGATC |
| TCR-BV3-F | TGACGGTGAATCCTGGAGAC | |
| TCR-BV8-F | GACCAAAAGTTCTTAGCG | |
| TCR-BC-R | CGATAGCCGTGACAATGAGC | |
| TCRγ | TCRg-V-96F | AAGTCAGCTGAAGAGGATCC |
| TCRg-C-424R | AGAAGTTCTCAAGCAGGCAG | |
| 5′ RACE CD4 | CD4-5′-RACE-322 | CACTGATGTGTCGTGAGAATGTGG |
| 5′ RACE CD8α | CD8a-5′-RACE-210 | GGCGAGTAGCAGAAGAGAACACA |
| 5′ RACE CD8β | CD8b-5′-RACE-207 | CCAGACCACAGCACTGCAGA |
2.4. Northern blot analysis
Total RNA samples (15 μg) were separated on 1% formaldehyde-agarose gel, transferred to Zeta-probe GT membrane (BioRad) by capillary transfer in 20X SSC, UV crosslinked and hybridized with 32p labeled probes under stringent conditions (62°C, 0.5%SSC and 0.2% SDS). Probes were fragments digested from EST clones of CD4 (650bp), CD8α (550bp), or CD8β (600bp). As a control for RNA integrity a probe encoding X. laevis EF-1α was used. The sizes of mRNAs were determined using a 0.24–9.5-Kb RNA ladder (Invitrogen). Currently, due to unavailability of CD4 monoclonal antibody (mAb) the splenocytes were sorted into CD8+ and CD8− (containing CD4+ cells), by making use of a Xenopus-specific anti-CD8 mAb (AM22, mouse IgM isotype [30]) and magnetic microbeads (Miltenyi Biotec, Auburn, CA) coupled to mouse-specific anti-μ, following the manufacturer’s instructions. Purity (>95%) was controlled by fluorescence microscopy using biotinylated anti-CD8 mAbs and FITC-conjugated streptavidin (data not shown).
2.5. Phylogenetic analysis
Available deduced amino acid sequences were retrieved from GenBank using ENTREZ at the NCBI. Multiple nucleotide and amino acid sequence alignments of CD4, CD8α and β amino acids were generated using Clustal X. Phylogenetic analysis was performed using Molecular Evolutionary Genetics Analysis (MEGA, version 4.1). Phylogenetic trees were generated for individual V domains and C domains or the full length CD4 molecule. The trees for CD8α/β were generated using either the Igsf V domains or the full length sequences. The neighbor joining method with pairwise deletion of gaps and p-distances (proportion of differences) was used to make the trees. Minimum evolution and maximum parsimony methods were also used for comparison. Numbers on nodes represent percentages of 1000 bootstrap replicates supporting each partition.
3. Results
3.1. Identification of CD4 and CD8 gene homologs in X. tropicalis and X. laevis
The genome of the only diploid species of the Xenopodinae subfamily, X. tropicalis, has recently been fully sequenced and assembled. We used in silico analysis and searched the X. tropicalis genomic database version 4.1 (http://genome.jgi-psf.org/Xentr4/Xentr4.home.htm) for CD4 and CD8 like sequences using the tblastn algorithm as well as gene synteny using Metazome (http://www.metazome.net/index.php). A X. tropicalis CD4 gene homolog (XtCD4) composed of 10 exons was identified on scaffold 827 adjacent to LAG3 that is also composed of 10 exons. The intron/exon organization of XtCD4 is very similar to that of other vertebrates. In particular, the distinctive split of the variable domain 1 (D1) into two exons that is observed in mammals, chicken and bony fish also occurs in XtCD4. The intervening intron separates the sequence encoding the C′ and C″ strand in XtCD4 as in other vertebrates. We did not find evidence for additional CD4-like or CD4REL homologs by searching the X. tropicalis genome sequence. The XtCD4 gene lies in a preserved syntenic region that contains 9 gene homologs present in chicken, mouse and human (Fig. 1). In contrast, the genomic locus containing putative CD4 genes in bony fish shares a limited close range synteny; very few of the CD4 neighboring genes in zebrafish and fugu are found in this region in tetrapods including X. tropicalis. Note that there are still some uncertainties concerning the assembly and annotations of putative CD4 gene models in zebrafish, fugu and pufferfish, especially concerning the status of the LAG3 gene. A putative LAG3 gene model was found in a previous genome assembly version (Z5) of the zebrafish, and a linkage of CD4, CD4-like and LAG3 was established in a BAC clone of trout [31]. However, LAG3 appears to be absent in the last assembly version of zebrafish (Zv8), fugu (Fv4) and pufferfish (Tetraodon v8.0). Whether one of the two CD4 gene models (CD4L-1, CD4L-2) represents in fact a LAG3 gene is under investigation [32]
Figure 1.
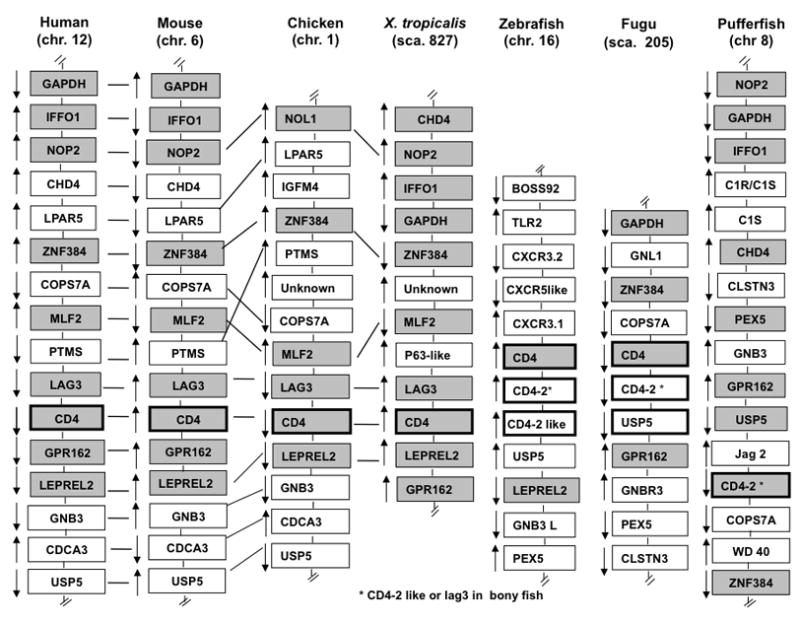
Synteny relationship for CD4 genes between human chromosome 12, mouse chromosome 6, chicken chromosome 1, Xenopus tropicalis scaffold 827, zebrafish chromosome 16, fugu scaffold 205, and pufferfish chromosome 8. These genes were identified in the genome database using ENSEMBL and the unannotated genes were checked by BLAST search using NCBI. Xenopus tropicalis genome assembly 4.1 was used. Arrows indicate gene orientation. Gray boxes indicate syntenic genes. Not drawn to scale.
On scaffold 280 of X. tropicalis, we identified a CD8α gene homolog (XtCD8α; 280:197001-216000) consisting of 5 exons followed by an ill annotated sequence that revealed to be a bona fide CD8β encoding gene (XtCD8β; 280:240001-246000) composed of 4 exons. Both genes are closely linked in the same orientation and are in a large genomic region of as many as 15 genes syntenic with their mammalian and chicken homologs (Fig. 2). A large portion of this syntenic region has an inversion in X. tropicalis. Although CD8 α and β are also tightly linked in bony fish, they are in a completely different genomic region that is not syntenic with tetrapods.
Figure 2.

Synteny relationship for CD8α/β genes between human chromosome 2, mouse chromosome 6, chicken chromosome 4, Xenopus tropicalis scaffold 280, zebrafish chromosome 21, fugu scaffold 116, and pufferfish chromosome 11. Arrows indicate gene orientation. Gray boxes indicate syntenic genes.
The high degree of close range gene syntenic conservation in X. tropicalis and other endothermic vertebrates for both the CD4 and CD8 loci suggests a stabilization of these genomic regions during evolution of tetrapods and highlights the distinctive evolutionary history of bony fish.
3.2. Structural features of Xenopodinae T cell co-receptors
To obtain support for the X. tropicalis CD4 and CD8 gene models, we searched the EST databases and found expressed transcripts for each gene model. We used these X. tropicalis EST sequences to search the X. laevis EST databases. We identified EST clones encoding putative X. laevis CD4 and CD8 gene homologs that were obtained from the IMAGE consortium, sequenced them and used the sequence to design primers to fully clone and sequence each X. laevis gene homolog (XlCD4, XlCD8α and XlCD8β) by RACE-PCR.
CD4
Multiple alignments of XtCD4 and XlCD4 with other available deduced amino acid sequences show an overall conservation of their molecular structure including the four extracellular Ig domains D1–D4 (D1 and D3 are V-like whereas D2 and D4 are C like), a transmembrane domain and a cytoplasmic tail (Supplementary Fig. S1). The cysteine residues involved in disulphide bonds in B and F strands of D1 and D4, as well as the F strand of D2 are conserved. The signature motif WXC in the F strand of D4 is also conserved. There are five N-linked glycosylation sites: one in D1, D2 and D3, and two in D4. The cytoplasmic domain contains a conserved recognition site for the p56lck with the consensus sequence motif ‘RXCQC’.
There are however several noticeable differences. First, the ‘FLXK’ motif formed by F43, L44, X45 and K46, that in human has been shown to bind MHC class II [33], is not conserved in Xenopodinae (Fig. 3). Although a phenylalanine is present further downstream (Phe63) in Xenopodinae CD4s as well as several other charged residues (lysine and arginine) that could participate in a tertiary interaction, the conformation of this region and whether it binds class II in Xenopodinae is unknown. It is noteworthy that this motif is also poorly conserved in bony fish, chicken and several mammalian species. To further investigate the potential impact of the amino acid sequence divergence on the conformation of this region in Xenopus, we used the SWISS-MODEL workspace to generate a predicted CD4 structure. As expected, protein structure homology modeling reveals that X. laevis CD4 adopts a different overall three-dimensional structure compared to human CD4 (Supplementary Fig. S2). Interestingly, despite the differences in residues, X. laevis CD4 residues that align with the MHC class II interacting motif of human CD4 adopt a similar conformation. In contrast, the similar putative class II interacting region of X. tropicalis CD4 does not overlay with human CD4 domain 1. Therefore, it appears that both primary and tertiary structure of the putative class II of CD4 interacting region is poorly conserved in vertebrates.
Figure 3.

Multiple alignment of the MHC class II binding motif in domain 1 of vertebrate CD4. Phenylalanine (F43), leucine (L44) and lysine (K46) residues of human CD4 known to bind to MHC class II are shown. The boxes indicate putative binding sites. Abbreviations in the alignment: Chimp (Chimpanzee); Dolp (Dolphin); Xtrop (X. tropicalis); Xlaevis (X.laevis).
A second difference is the number and position of the cysteine residues that are different when compared to those of mammals. Xenopodinae CD4 sequences have three cysteine residues in D3 (supplementary figure S1, positions 236, 241 and 287 marked with double line). This feature is also seen in chicken (one cysteine) and fish (two cysteines) whereas mammals lack cysteine residues in domain 3 altogether. Additionally, Cys130 present in D2 in human, primates, rabbit and mouse, is absent in Xenopodinae, ruminants, pig, dolphins, marsupials, chicken, turkey and bony fish. A third notable feature is the ‘WXC’ signature in the F strand that is conserved in D2 of all vertebrates, but not Xenopodinae where the tryptophan is replaced by a tyrosine (Supplementary Fig S1). The difference in this motif and the overall sequence is suggestive of a divergent folding of domain 2 in Xenopodinae, although it is unknown how this affects the overall structure of the CD4 molecule. Finally, the cytoplasmic dileucine motif involved in CD4 internalization in mammals is not conserved in Xenopodinae CD4, since one hydrophobic leucine is replaced by the hydrophilic neutral amino acid asparagine (N; Supplementary Fig S1). This cytoplasmic dileucine motif is also lacking in chicken and bony fish (Fig. S1), which suggests that this feature is limited to mammals.
CD8
Alignments of all available CD8α and β deduced amino acid sequences ranging from fish to human show that both XtCD8 and XlCD8 have an overall preserved structure including an extracellular Igsf-V-like domain, a proximal hinge region, a transmembrane and a cytoplasmic domain (Supplementary Fig. S3). The two cysteines involved in the canonical intrachain disulphide bonding to form the V domain are conserved in all species including those of the Xenopodinae subfamily. The hinge region is rich in serine, proline and threonine and contains several potential O-linked glycosylation sites. A notable element is that the cytoplasmic tail of both Xenopodinae CD8α chains possesses a p56lck binding motif (CXC) that is conserved in all mammals and chicken but absent in bony fish species including fugu (Supplementary Fig. S3), pufferfish and zebrafish (data not shown).
On the other hand, as in the case of CD4, some residues found to be important in mammals for interaction between CD8α chain, β2m and conserved residues of the MHC class I molecules are not conserved in the Xenopodinae sequences nor in other non-mammalian vertebrates such as chicken and several bony fish species (Supplementary Fig. S4). These residues include arginine (R4), lysine (K21) and leucine (L25) in the A and B strands. The wide variability found among the various vertebrate classes shows species-specific selection in the interaction between CD8 and MHC class I, as it has been previously suggested in X. laevis based on the presence of a motif different from mammals but conserved between multiple class I alleles of X. laevis [34].
Other differences are observed in the hinge region of Xenopodinae CD8α chain. In addition to the two cysteines, C143 and C160, involved in dimerization and found in all mammals and some fish, Xenopodinae CD8α has an additional cystein in position 141, which could be responsible for a unique intradomain disulphide bond. Variations in the number and position of cysteine residues is also observed in chicken and fugu [16, 35]. The structural and functional consequences of these differences are unknown. Also, the N-linked glycosylation motif varies considerably in different vertebrate CD8α sequences. Xenopodinae CD8α sequences have a single putative N-linked glycosylation. In contrast, human, wallaby, chicken, and many fish species don’t have any N-linked glycosylation, whereas mouse and opossum have three [36].
Xenopodinae CD8β also has specific similarities and differences when compared to other vertebrate species. Like other vertebrate species the two cysteines (C23 and C98) in the Igsf-V domain are conserved. In contrast, the two cysteines in the hinge region (C137 and C150) that are involved in the formation of interchain disulphide bonds in the CD8αβ heterodimer in mammals and conserved in many vertebrates, differ in X. tropicalis, but not X. laevis, where C150 is replaced by a serine. This is also the case in salmon and trout where there is a threonine or serine substitution. On the other hand, Xenopodinae and fish have an additional cysteine at position 139. Finally, Xenopodinae CD8β chains have a distinctive di-glycine bulge found in the G strand (not present in CD8α), as seen in most mammals and chicken. This pattern is not present in fish, and opossum.
In summary, the conservation of certain residues between Xenopodinae, mammals and chicken suggests structural conservation and implies that Xenopodinae CD4 and CD8α and β genes are more closely related to their homologs in other tetrapods than to those in bony fish. However, the species-specific variation of the putative sites interacting with MHC molecules suggests co-evolution of each T cell co-receptor with its cognate MHC molecules.
3.3. Phylogenetic analysis
To further analyze the evolutionary relationships of the CD4 and CD8 gene families, Neighbor joining consensus trees of amino acid alignments (i.e., nucleotide data gave similar results, data not shown) were constructed with individual Ig extracellular domains of available sequences in the databank, including Xenopoidinae sequences. The robustness of each topology was evaluated by 1000 bootstrap replicates (Fig 4 and 5). The CD4 trees were rooted using human and mouse CD2 that is also composed of a variable and constant domain, and has a distinct evolutionary history. The CD8 tree was rooted using human and mouse variable CD4 domains. Using the V-like domain of CD2 gave similar results (data not shown). We also generated trees using the Minimum evolution and Maximum Parsimony methods, which gave results comparable to the Neighbor joining method (data not shown).
Figure 4.


Phylogenetic analysis of the deduced amino acid sequences of the V-like domain 1 and 3 (A), and C-like domain 2 and 4 (B) of CD4. The trees were made using the neighbour-joining method with pairwise deletion of gaps and p-distance. Percentage values represent 1000 bootstrappings (only those > 30 % are displayed). We used human and mouse CD2 as an outgroup. The arrow indicates the node that separate bony fish from tetrapodes. Accession numbers are as follows: human CD4, NP_000607; mouse CD4, NP_038516; chimpanzee CD4, NP_001009043; rhesus monkey CD4, BAA09671; rat CD4, NP_036837; dolphin CD4, AAQ03208; pig CD4, NP_001001908; rabbit CD4, NP_001075782; goat CD4, ACG76115; sheep CD4, NP_001123374; cattle CD4, NP_001096695; opussum CD4, NP_001092760; wallaby CD4, ABR22561; turkey CD4, CAP04927; chicken CD4, NP_989980; duck CD4, ACL 68187; X. tropicalis CD4, (XP_002941730); X. laevis CD4 (Temporary accession # HQ116782); zebrafish CD4-4, NP_001128568; Salmon CD4-like, NP_001117083; trout CD4, NP_001118011; fugu CD4, NP_001072091; catfish CD4 like, ABD93355; carp CD4-like, BAF94326; pufferfish CD4-4a, EF601919.1; human CD2, NP_001758.2; mouse CD2, AAH53731.
Figure 5.

Phylogenetic analysis of the CD8 family. The phylogenetic tree was generated using ClustalX and Mega 4.1) with the deduced amino acid sequences of Igsf domain of CD8α and CD8β. The tree was made using the neighbour-joining method with pairwise deletion of gaps and p-distance. Percentage values represent 1000 bootstrappings. Human and mouse CD4 were used as outgroup. Accession numbers are as follows: Human CD8α, AAH25715; mouse CD8α, NP_001074579; chimpanze CD8α, XP_001138772; squirrel monkey CD8α, CAB41462; cattle CD8α, NP_776440; pig CD8α, ABK30934; dog CD8α, NP_001002935; opussum CD8α, ABX79403; duck CD8α, AAW63062; chicken CD8α, NP_990566; X. tropicalis CD8α, (protein ID 173532); X. laevis CD8α (Temporary accession # HQ116783); zebrafish CD8α, NP_001035138; fugu CD8α, NP_001072086; salmon CD8α, AAW23967; trout CD8α, NP_001117735. Human CD8β, NP_742097; mouse CD8β, NP_033988; chimpanze CD8β, XP_001139269; squirrel monkey CD8β, Q9XSM7; cattle CD8β, NP_001098814; pig CD8β, BAD06313; dog CD8β, XP_865047; opussum CD8β, NP_001139803; cat CD8β, NP_001009867; chicken CD8β, CAA72261; X. tropicalis CD8β (protein ID 173534); X. laevis CD8β (Temporary accession #HQ116784); fugu CD8β, NP_001103475; salmon CD8β, NP_001117056; trout CD8β, NP_001117480; human CD4, NP_000607; mouse CD4, NP_038516.
As expected, in all trees X. laevis clusters closely to its sister species X. tropicalis. The topology of V-like C1 and C3 CD4 sequences reveals a clear clustering of each Xenopodinae individual domain with the respective CD4 domain of mammals and chicken, whereas bony fish form a distinct clade with high bootstrap value (Fig 4A, arrow). In the case of C-like domains, bony fish also branch away from tetrapods including Xenopodinae for D4. However, the topology of D2 is less definite. Most basal roots have poor bootstrap support and the D2 of Xenopodinae sequences, while tightly related appear to be divergent to all other vertebrate D2 sequences. The divergence of Xenopodinae CD4 domain 2 from other vertebrates may suggest a specialization in these amphibian species that has been maintained for at least 65 million years (i.e., the time of divergence of X. laevis and X. tropicalis from a common ancestor).
Phylogenetic analysis of CD8α and CD8β genes indicates a closer relationship of Xenopodinae CD8 with homologs in other tetrapods than with homologs in bony fish, which group as a separate clade. The distinction is particularly pronounced for the V domain of CD8β (Fig. 5). The unequivocal separation between CD8α and β for all species clearly indicates that gene duplication occurred at an early stage of evolution before the split of bony fish from the common ancestor of jawed vertebrates. Interestingly, the CD8β V domain of bony fish clusters away from other CD8β V domains. This divergence is supported by a high bootstrap value and is not maintained when the whole molecule is used to generate the tree. This suggests that the extracellular domain of CD8β has specialized in fish or that CD8β in bony fish and tetrapods are not orthologous and have emerged twice independently.
3.4. Expression analysis of CD4 and CD8 molecules
CD8 T cells are characterized to some extent in X. laevis (reviewed in [37]). In addition, two Xenopus-specific anti-CD8 mAbs are available and have been used to characterize CD8 T cell populations, as well as the CD8 co-receptor at the protein level [30, 38, 39]. We, therefore, took advantage of the Xenopus-specific anti-CD8 mAb AM22 to purify splenic CD8 T cells and CD8-depleted splenocytes by magnetic microbeads (MACS). Purified CD8 T cells were more than 95% pure, and the CD8-depeleted cell population contained less than 10% CD8 T cells, as determined by flow cytometry (data not shown). Northern blot analysis reveals that XlCD8α and β genes are primarily expressed by CD8 T cells, whereas XlCD4 is preferentially expressed by CD8-depleted splenocytes (Fig. 6A). No or very weak signal was detected with purified IgM+ B cells (presumably due to a few contaminating T cells), and the A6 kidney cell line was completely negative for all these transcripts. Low levels of CD4 transcripts were also detected in liver, kidneys and intestine, whereas CD8α and CD8β signals were only detectable in the intestine.
Figure 6.
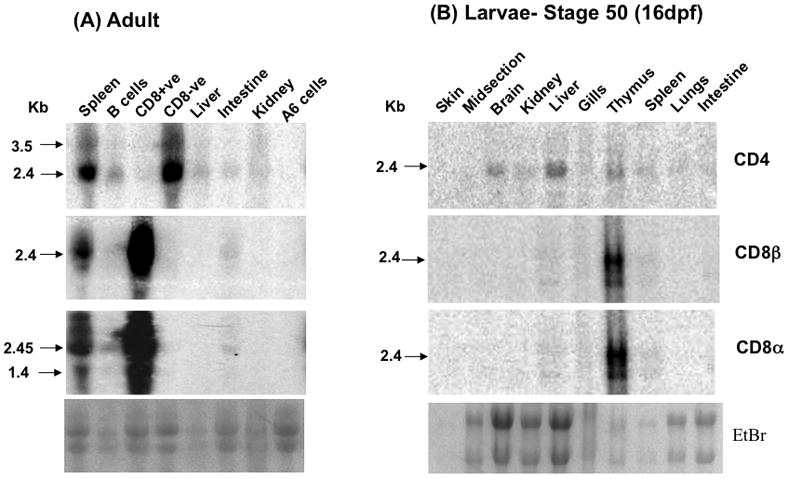
(A). CD4 and CD8α/β mRNA expression pattern in adult X. laevis was cell type specific by Northern blotting. Spleenocytes were MACS separated to sort out CD8+ve, CD8-ve cells and B cells. Other tissues used in the blot were liver, intestine, and kidney from the same animal. We also tested the expression of these genes in the A6 (Xenopus kidney) cell line used as a negative control. The gel picture shown at the bottom is our loading control. Marker is shown on left side of the blots. Probes used in adult and larval blot were made by fragments specific from CD4, CD8α and CD8β and they sizes were 550bp, 650bp and 600bp, respectively. (B) CD4 and CD8α/β mRNA expression pattern in X. laevis larvae (stage 50, 16dpf) in various tissues by Northern blotting. The tissues used were skin, midsection, brain, kidney, liver, gills, thymus, spleen, lungs and intestine. The gel pictures shown at the bottom of each panel are our loading controls. Sizes are determined by the marker shown on left side of the blots. Probes used in adult and larval blots were made by fragments specific to CD4, CD8α and CD8β and their sizes were 550bp, 650bp and 600bp, respectively.
Similar tissue-specific expression was found in pre-metamorphic larvae 15–16 days post-fertilization (dpi) or stage 50 (Fig. 6B). CD4 displayed a wide tissue expression including thymus and spleen, as well as brain, kidneys, liver, gills, lung and intestine. In contrast, CD8α and CD8β, were mainly detected in the thymus and weakly in spleen, liver and gills. In addition, the level of CD8α and CD8β expression in the thymus was considerably higher than CD4. Importantly, at the larval stage used (st 50), the spleen has not reached its full size and contains only a few T cells, which explains the weak signal detected for these transcripts.
In summary, the tissue- and cell-specific expression patterns of CD4 and CD8 genes in larvae and adults of the Xenopodinae subfamily are very similar to mammals.
3.5. Developmental expression of CD4 and CD8
To obtain further evidence of the conserved involvement of CD4 and CD8 co-receptors in T cell function, we determined their expression profile during early development from stage 40 to 48 (4–8 dpf), focusing mainly in the thymus, and intestine as secondary immune tissue since at this developmental stage the spleen has not yet differentiated. RT-PCR was performed on tissues pooled from 20 individual tadpoles using CD4, CD8α and β-specific primers. All minus reverse transcriptase controls were negative and each amplified band was cloned and sequenced to verify its authenticity. In several independent experiments (one is shown in Fig. 7, and summary in Table 2), CD4 transcripts were consistently detected prior to CD8 as early as 4 dpf (stage 41/42) both in the thymus and intestine, whereas both CD8α and β signal were not detectable until one day later at stage 43/44 and only in the thymus. No CD4 or CD8 expression was found at 3 dpf. Expression of all these genes was found in thymus and intestine at 6 dpf (stage 45/46). Interestingly, full length TCRγ transcripts were already detected in the thymus at this stage using a forward primer specific for one V sequence and a reverse primer specific for the C region. In contrast, no complete TCRβ transcripts were detected with consensus primers for the Vβ8 family (Fig. 7) as well as Vβ3 and Vβ1 families (data not shown). Full length TCRβ was found in the thymus only from day 8–9 (stage 47/48) onward (Supplementary Fig. 4; Table 2). Since sterile transcription of Cβ and other incomplete products (e.g., J-C) are generated at early stages of thymocyte differentiation in Xenopus as in mammals [40, 41], only full length VDJ TCR transcripts are indicative of a fully productive rearrangement. We chose as representative Vβ family Vβ1, Vβ3 and Vβ8 because they have been previously shown to be well expressed in larvae, and the Vβ8 family is composed by multiple gene segments [41]. Note that Rag 1 expression, indicative of TCR gene rearrangement activity was detected as early as 4 dpf in the thymus (st 41/42).
Figure 7.
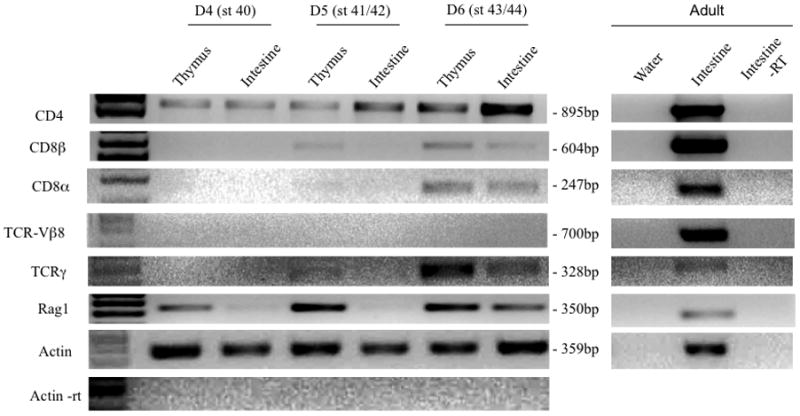
Expression patterns of CD4 and CD8 transcripts during early development in X. laevis. Total RNA was extracted from the thymocytes and intestine from 4 days post fertilization (dpf) to 6 dpf larvae and subjected to RT-PCR analysis at 40 cycles (except actin, 25 cycles) with primers specific for CD4, CD8α, CD8β, TCRγ TCRβ, and RAG1. β-Actin served as loading control, adult intestine as positive control, β-Actin -RT as control for genomic DNA contamination, and water as negative control. The corresponding stages of development are shown in bracket adjacent to dpf. CD4 is detected as early as 4 dpf in the thymus and intestine whereas CD8α and CD8β are detected at 5 dpf in the thymus. Full-length TCRγ transcripts are detected at 6 dpf.
Table 2.
Summary of CD4, CD8α, CD8β, and TCRγ and TCRβ onset of expression in thymus and intestine of X. laevis at early developmental stages by RT-PCR#
| Eggs | St 29/30 (1.5 dpf) | St 41/42 (4 dpf) | St 43/44 (5 dpf) | St 45/46 (6 dpf) | St 47 (7 dpf) | St 48 (8 dpf) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fr | Mp | Thy | Int | Thy | Int | Thy | Int | Thy | Int | Thy | Int | ||
| CD4 | ND (2/2) | ND (2/2) | ND (2/2) | + (2/3) | + (2/3) | + (2/3) | + (2/3) | + (2/3) | + (3/3) | + (3/3) | + (3/3) | + (2/2) | + (2/2) |
| CD8α | ND (2/2) | ND (2/2) | ND (2/2) | ND (3/3) | ND (3/3) | +/− (2/3) | ND (3/3) | + (2/3) | + (2/3) | + (3/3) | + (3/3) | + (2/2) | + (2/2) |
| CD8β | ND (2/2) | ND (2/2) | ND (2/2) | ND (3/3) | ND (2/3) | +/− (2/3) | ND (3/3) | + (1/3) | + (2/3) | + (3/3) | + (3/3) | + (2/2) | + (2/2) |
| ΔTCRγ | ND (2/2) | ND (2/2) | ND (2/2) | ND (3/3) | ND (2/3) | +/− (3/3) | ND (3/3) | + (3/3) | + (3/3) | + (3/3) | + (3/3) | + (2/2) | + (2/2) |
| ΔTCRβ | ND (2/2) | ND (2/2) | ND (2/2) | ND (3/3) | ND (3/3) | ND (3/3) | ND (3/3) | ND (3/3) | ND (3/3) | +/− (2/3) | ND (2/3) | + (2/2) | + (2/2) |
| ΔTCRα | ND (2/2) | ND (2/2) | ND (2/2) | ND (3/3) | ND (3/3) | ND (3/3) | ND (3/3) | ND (3/3) | ND (3/3) | ND (3/3) | ND (3/3) | +/− (2/2) | ND (2/2) |
Each RT-PCR (30 cycles on 1μg of reverse-transcribed RNA) was performed on tissues from 5–20 pooled outbred tadpoles (different progenies). The numbers of observed expression pattern over the total number of experiments is indicated in parenthesis. +: Consistent expression; +/−: weak, barely detectable; ND: not detected. Fr: front portion; Mp: mid portion; Thy: thymus; Int: intestine.
forward primer sits in the variable region and reverse primer in the constant region.
In summary, our data indicate that in X. laevis the CD4 gene is expressed before CD8α and β genes, at the onset of thymocyte differentiation (5 dpf), and that TCRγ is also expressed in the thymus at this developmental stage at least two days before any full length TCRβ can be detected.
4. Discussion
In this study we have identified and extensively characterized CD4, CD8α and CD8β gene homologs in two amphibian species belonging to two different genera of the Xenopodinae subfamily, X. laevis and X. tropicalis. Collectively, our analysis that includes close range gene synteny, genomic organization, molecular structure, phylogeny, and differential and developmental expression, strongly suggests that a strong selection, related to their critical T cell development and function, has been maintained on these three gene homologs in the whole tetrapod lineage. However, our data also reveal Xenopodinae-specific divergence of certain domains and/or motifs indicative of specialization and presumably co-evolution with their cognate MHC genes.
4.1. Genomic and molecular stabilization of the T cell co-receptors in the tetrapod lineage
Because of its key position in the evolution of tetrapods, the whole-genome sequence of X. tropicalis provides a powerful source of information for better understanding the evolutionary history of genes involved in immunity [28]. The extensive annotation and initial analysis of the X. tropicalis genome revealed a high degree of shared gene synteny with human, mouse and chicken, while still maintaining primordial features (review in [22]). The genetic stability of X. tropicalis and X. laevis, with preservation of ancestral features of immune related genes has been clearly demonstrated for the MHC genomic region [34]. The present study provides further evidence of the preserved short-range synteny of two genomic regions containing important T cell co-receptors, CD4 and CD8, between amphibian and mammals. This situation contrasts with that of teleost fish models (e.g., zebrafish, fugu, puffer fish) where the genomic loci containing the CD4 and CD8 genes share little (CD4) to no (CD8) short-range synteny with tetrapods. Concerning the CD4 genomic region, as mentioned in the results section, the draft genome sequences available for zebrafish, fugu and pufferfish are still not fully reliable. However, it is quite probable that this genomic region has not been as stable as in tetrapods. Few neighboring genes are shared between tetrapods and anyone of the three fish genomes, and the shared synteny between the three fish genome is only partial. In addition, the number and organization of CD4 and CD4-like genes appear to be variable within bony fish. Although, CD4 has clearly been shown to be closely linked to a CD4-like gene with only two Ig domains, and LAG3 in fish [31], the status of the LAG3 gene in the three draft genomes, with respect to putative additional CD4 and CD4-like genes still remains to be resolved. The divergence between bony fish and tetrapods is even more accentuated with the CD8 locus, none of the 10 genes neighboring the CD8α and β in tetrapods are found in the CD8 locus of zebrafish, pufferfish and fugu.
Phylogenetic analysis of both nucleotide and amino acid sequences indicates a closer relationship of Xenopodinae CD4 and CD8 co-receptors with their homologs in other tetrapods when compared with bony fish. This suggests some stabilization in the evolution of these receptors presumably due to the constraint of their function in T cell development and regulation. On the other hand, when the Ig V-like D1 and D3, as well as the C-like domain D4 are analyzed separately, all respective domains form a distinct cluster, and within each cluster Xenopodinae homologs are always more closely related to tetrapod homologs than to those of bony fish (Figure 4A and 4B). Further analysis of the sequence indicates that some residues are very distinct; in particular the tryptophan of the signature motif in the F strand of D2 is changed to tyrosine only in Xenopodinae.
Concerning CD8 sequences, the segregation into a CD8α and β cluster with deep rooting is consistent with an ancient origin of these gene paralogs by duplication coincident with the emergence of vertebrates. However, whereas CD8α sequences form a relatively homogeneous cluster within Xenopodinae in an intermediate position between bony fish and other tetrapods, phylogenetic analysis reveals a striking divergence of bony fish CD8β genes, especially when only the V domain is considered (Fig 5). We can propose two possible non-exclusive scenarios to explain such a divergence topology. First, CD8β may have undergone a rapid evolution and specialization driven by the postulated expansion of bony fish. The deep rooting that separates fugu from trout and salmon would be consistent with such a specie-specific specialization. Alternatively, two distinct duplication events may have taken place: one in a fish ancestor giving fish CD8β, and a second later on in a tetrapod ancestor giving a tetrapod CD8β. Because of its wider expression pattern and function, CD8α may have been less prone to specialization. In such a scenario, CD8β in bony fish and tetrapods would not be orthologous.
4.2. Co-evolution of MHC and T cell co-receptors
In humans, the F43, L44, and K46 residues in domain 1 of CD4 are involved in binding of CD4 to MHC class II molecules. These residues are not conserved in Xenopodinae but there is a phenylalanine available downstream in both the species. This feature is also seen in mouse and chicken where the position of the phenylalanine is changed. Furthermore, protein structure homology modeling (SWISS-MODEL) predicts that the three-dimensional structure of the putative MHC class II interacting region is also poorly conserved between X. laevis and X. tropicalis when compared with human CD4. Similarly, in the case of CD8α the residues arginine (R4), lysine (K21) and leucine (L25) involved in binding to class I are not conserved in other species (Supplementary Fig. 3). Because of the complexity of the homo or hetero-dimeric structure of CD8 molecules, we did not attempt computer modeling. It has been previously suggested that there is co-evolution of each T cell co-receptor with its cognate MHC molecules in X. laevis based on the presence of a motif different from mammals but conserved between the multiple class I alleles [42]. Consistent with this idea, the putative MHC binding motif of CD4 and CD8α are not conserved but rather species specific. Nevertheless, it is possible that selective pressure has acted more at the overall conformational level rather than at each specific residue.
4.3. Conservation of developmental and cell-specific expression of T cell co-receptors
Our study provides further molecular evidence that similar to mammals, X. laevis displays two main T cell subsets in the periphery. X. laevis CD8 T cells are recognized by a well characterized mAb (AM22) that stain 85–90% of total thymocytes and 20–30% of splenic lymphocytes that co-express pan-T cell marker (XT-1), high CD5 and CD45 levels [43–45]. These cells are not detectable in animals that had been thymectomized at early developmental stages before the migration of stem cells into the thymus [43]. In addition, cell-mediated cytotoxicity of AM22 positive cells is Ag-specific and MHC class I-restricted [25]. The AM22 mAb binds a polypeptide of 30–32 kDa as determined by Western blotting [30], and it immunoprecipitates a dimeric complex of 65 kDa under nonreducing conditions that resolves to a 35 kDa band and a 30 to 32kDa band under reducing conditions [38]. Here, we have now confirmed that CD8α and CD8β gene are almost exclusively co-expressed by cells expressing surface CD8 molecules recognized by the AM22 mAb. In mammals and birds, CD8α is also expressed as a homodimer by some NK cells, and in mouse but not human by dendritic cells [46]. Since the AM22 mAb is unlikely to distinguish CD8αα homodimers from CDαβ heterodimers at the cell surface it is possible that a fraction of purified CD8 splenocytes are also non-T cells (NK and/or DCs) in Xenopus. The higher signal intensity of the CD8α detected in Northern analysis would be consistent with the contribution from CD8β-negative non-T cells.
In contrast to CD8 T cells, the absence of reliable markers has so far hampered the characterization of CD4 T cell subsets in Xenopus. Our gene expression study constitutes a first step and should permit the generation of antibodies in a near future. The preferential expression in CD8-depleted splenocytes, and absence of signal in purified splenic B cell, is consistent with the presence of a bona fide CD4 T cell subset in adult spleen. Similarly the preferential expression in thymus, both in adults and larvae from early developmental stages, suggests the presence of a CD4 T cell subset. However, as in the case of non-T cells expressing CD8α, CD4 is also expressed in a variety of non-T cells in mammals. These include, DCs, monocytes, macrophages, and thymic stromal cells [47]. Therefore, more studies will be needed to determine which cell types express CD4 in Xenopus.
The detection of CD4 transcripts as early as 4 dpf in the thymus before CD8 and far before expression of TCR transcripts suggests that CD4 is expressed by some thymic stromal cells, although Rag 1 gene expression indicates that gene rearrangements are already taking place. Interestingly, the earliest detectable CD8α and β expression coincides with the first detection of a productive TCRγ mRNA, and the expression of these three genes markedly increases one day later, whereas productive TCRβ transripts are hardly detectable at 7 dpi. This strongly suggests that as in mammals and chicken, rearrangement of TCRγ genes, and therefore differentiation of γδT cells occurs before TCRβ rearrangements. The detection of a significant TCRγ signal in the intestine at 6 dpi further supports that γδT cells differentiate earlier than αβT cells, and emigrate out from the thymus. The detection of CD8α and β transcripts is in good agreement with the detection of the CD8 antigen by immunfluorescence microscopy [48].
So far, our study of developmental and cell-type specific expression T cell co-receptors has been limited to X. laevis, for the obvious reasons of reagents availability and the extensive characterization of the immune system of this species. However, we think that the results we have obtained in X. laevis can be largely extended to X. tropicalis, owing to the high degree of conservation of immunologically-relevant genes revealed by whole genome sequence data. In addition, embryogenesis and early developmental process are fundamentally similar between the two species. Nevertheless, it would be advantageous in the future to integrate X. tropicalis in immunological studies by taking advantage of the genetics and genomics resource to generate tools (e.g., transgenic animals) and reagent (e.g., antibodies).
Supplementary Material
Acknowledgments
The expert animal husbandry provided by David Albright and Tina Martin is gratefully appreciated. We also would like to thank Hristina Nedelkovska and Dr. Guangchun Chen for their critical reading of the manuscript.
Abbreviations and symbols used (in alphabetical order)
- BLAST
Basic Local Alignment Search Tool
- Cyt
cytoplasmic tail
- IgSF
Immunoglobulin Superfamily
- MHC
Major Histocompatibility Complex
- TM
transmembrane domain
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Leahy DJ. A structural view of CD4 and CD8. FASEB J. 1995;9(1):17–25. doi: 10.1096/fasebj.9.1.7821755. [DOI] [PubMed] [Google Scholar]
- 2.Wange RL, Samelson LE. Complex complexes: signaling at the TCR. Immunity. 1996;5(3):197–205. doi: 10.1016/s1074-7613(00)80315-5. [DOI] [PubMed] [Google Scholar]
- 3.Parnes JR. Molecular biology and function of CD4 and CD8. Adv Immunol. 1989:44265–311. doi: 10.1016/s0065-2776(08)60644-6. [DOI] [PubMed] [Google Scholar]
- 4.Germain RN. T-cell development and the CD4-CD8 lineage decision. Nat Rev Immunol. 2002;2(5):309–22. doi: 10.1038/nri798. [DOI] [PubMed] [Google Scholar]
- 5.Maddon PJ, Littman DR, Godfrey M, Maddon DE, Chess L, Axel R. The isolation and nucleotide sequence of a cDNA encoding the T cell surface protein T4: a new member of the immunoglobulin gene family. Cell. 1985;42(1):93–104. doi: 10.1016/s0092-8674(85)80105-7. [DOI] [PubMed] [Google Scholar]
- 6.Clark SJ, Jefferies WA, Barclay AN, Gagnon J, Williams AF. Peptide and nucleotide sequences of rat CD4 (W3/25) antigen: evidence for derivation from a structure with four immunoglobulin-related domains. Proc Natl Acad Sci U S A. 1987;84(6):1649–53. doi: 10.1073/pnas.84.6.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Littman DR, Thomas Y, Maddon PJ, Chess L, Axel R. The isolation and sequence of the gene encoding T8: a molecule defining functional classes of T lymphocytes. Cell. 1985;40(2):237–46. doi: 10.1016/0092-8674(85)90138-2. [DOI] [PubMed] [Google Scholar]
- 8.Norment AM, Lonberg N, Lacy E, Littman DR. Alternatively spliced mRNA encodes a secreted form of human CD8 alpha. Characterization of the human CD8 alpha gene. J Immunol. 1989;142(9):3312–9. [PubMed] [Google Scholar]
- 9.Chalupny NJ, Ledbetter JA, Kavathas P. Association of CD8 with p56lck is required for early T cell signalling events. EMBO J. 1991;10(5):1201–7. doi: 10.1002/j.1460-2075.1991.tb08061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ellmeier W, Sawada S, Littman DR. The regulation of CD4 and CD8 coreceptor gene expression during T cell development. Annu Rev Immunol. 1999:17523–54. doi: 10.1146/annurev.immunol.17.1.523. [DOI] [PubMed] [Google Scholar]
- 11.Wong JS, Wang X, Witte T, Nie L, Carvou N, Kern P, et al. Stalk region of beta-chain enhances the coreceptor function of CD8. J Immunol. 2003;171(2):867–74. doi: 10.4049/jimmunol.171.2.867. [DOI] [PubMed] [Google Scholar]
- 12.Salter RD, Benjamin RJ, Wesley PK, Buxton SE, Garrett TP, Clayberger C, et al. A binding site for the T-cell co-receptor CD8 on the alpha 3 domain of HLA-A2. Nature. 1990;345(6270):41–6. doi: 10.1038/345041a0. [DOI] [PubMed] [Google Scholar]
- 13.Sun J, Leahy DJ, Kavathas PB. Interaction between CD8 and major histocompatibility complex (MHC) class I mediated by multiple contact surfaces that include the alpha 2 and alpha 3 domains of MHC class I. J Exp Med. 1995;182(5):1275–80. doi: 10.1084/jem.182.5.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Powell F, Lawson M, Rothwell L, Kaiser P. Development of reagents to study the turkey’s immune response: Identification and molecular cloning of turkey CD4, CD8alpha and CD28. Dev Comp Immunol. 2009;33(4):540–6. doi: 10.1016/j.dci.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Duncan LG, Nair SV, Deane EM. Molecular characterisation and expression of CD4 in two distantly related marsupials: the gray short-tailed opossum (Monodelphis domestica) and tammar wallaby (Macropus eugenii) Mol Immunol. 2007;44(15):3641–52. doi: 10.1016/j.molimm.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 16.Suetake H, Araki K, Akatsu K, Somamoto T, Dijkstra JM, Yoshiura Y, et al. Genomic organization and expression of CD8alpha and CD8beta genes in fugu Takifugu rubripes. Fish Shellfish Immunol. 2007;23(5):1107–18. doi: 10.1016/j.fsi.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 17.Edholm ES, Stafford JL, Quiniou SM, Waldbieser G, Miller NW, Bengten E, et al. Channel catfish, Ictalurus punctatus, CD4-like molecules. Dev Comp Immunol. 2007;31(2):172–87. doi: 10.1016/j.dci.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 18.Moore LJ, Somamoto T, Lie KK, Dijkstra JM, Hordvik I. Characterisation of salmon and trout CD8alpha and CD8beta. Mol Immunol. 2005;42(10):1225–34. doi: 10.1016/j.molimm.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 19.Suetake H, Araki K, Suzuki Y. Cloning, expression, and characterization of fugu CD4, the first ectothermic animal CD4. Immunogenetics. 2004;56(5):368–74. doi: 10.1007/s00251-004-0694-x. [DOI] [PubMed] [Google Scholar]
- 20.Dijkstra JM, Somamoto T, Moore L, Hordvik I, Ototake M, Fischer U. Identification and characterization of a second CD4-like gene in teleost fish. Mol Immunol. 2006;43(5):410–9. doi: 10.1016/j.molimm.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 21.Pancer Z, Mayer WE, Klein J, Cooper MD. Prototypic T cell receptor and CD4-like coreceptor are expressed by lymphocytes in the agnathan sea lamprey. Proc Natl Acad Sci U S A. 2004;101(36):13273–8. doi: 10.1073/pnas.0405529101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robert J, Ohta Y. Comparative and developmental study of the immune system in Xenopus. Dev Dyn. 2009;238(6):1249–70. doi: 10.1002/dvdy.21891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Evans BJ, Kelley DB, Tinsley RC, Melnick DJ, Cannatella DC. A mitochondrial DNA phylogeny of African clawed frogs: phylogeography and implications for polyploid evolution. Mol Phylogenet Evol. 2004;33(1):197–213. doi: 10.1016/j.ympev.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 24.Evans BJ. Genome evolution and speciation genetics of clawed frogs (Xenopus and Silurana) Front Biosci. 2008:134687–706. doi: 10.2741/3033. [DOI] [PubMed] [Google Scholar]
- 25.Robert J, Gantress J, Rau L, Bell A, Cohen N. Minor histocompatibility antigen-specific MHC-restricted CD8 T cell responses elicited by heat shock proteins. J Immunol. 2002;168(4):1697–703. doi: 10.4049/jimmunol.168.4.1697. [DOI] [PubMed] [Google Scholar]
- 26.Blomberg B, Bernard CC, Du Pasquier L. In vitro evidence for T-B lymphocyte collaboration in the clawed toad, Xenopus. Eur J Immunol. 1980;10(11):869–76. doi: 10.1002/eji.1830101112. [DOI] [PubMed] [Google Scholar]
- 27.Watkins D, Harding F, Cohen N. In vitro proliferative and cytotoxic responses against Xenopus minor histocompatibility antigens. Transplantation. 1988;45(2):499–501. doi: 10.1097/00007890-198802000-00054. [DOI] [PubMed] [Google Scholar]
- 28.Hellsten U, Harland RM, Gilchrist MJ, Hendrix D, Jurka J, Kapitonov V, et al. The genome of the Western clawed frog Xenopus tropicalis. Science. 2010;328(5978):633–6. doi: 10.1126/science.1183670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nieuwkoop PD, Faber J. Normal tables of Xenopus laevis (Daudin) New York, London: Garland Publishing; 1994. [Google Scholar]
- 30.Flajnik MF, Ferrone S, Cohen N, Du Pasquier L. Evolution of the MHC: antigenicity and unusual tissue distribution of Xenopus (frog) class II molecules. Mol Immunol. 1990;27(5):451–62. doi: 10.1016/0161-5890(90)90170-5. [DOI] [PubMed] [Google Scholar]
- 31.Laing KJ, Zou JJ, Purcell MK, Phillips R, Secombes CJ, Hansen JD. Evolution of the CD4 family: teleost fish possess two divergent forms of CD4 in addition to lymphocyte activation gene-3. J Immunol. 2006;177(6):3939–51. doi: 10.4049/jimmunol.177.6.3939. [DOI] [PubMed] [Google Scholar]
- 32.Ohashi K, Takizawa F, Tokumaru N, Nakayasu C, Toda H, Fischer U, et al. A molecule in teleost fish, related with human MHC-encoded G6F, has a cytoplasmic tail with ITAM and marks the surface of thrombocytes and in some fishes also of erythrocytes. Immunogenetics. 2010;62(8):543–59. doi: 10.1007/s00251-010-0460-1. [DOI] [PubMed] [Google Scholar]
- 33.Bowers K, Pitcher C, Marsh M. CD4: a co-receptor in the immune response and HIV infection. Int J Biochem Cell Biol. 1997;29(6):871–5. doi: 10.1016/s1357-2725(96)00154-9. [DOI] [PubMed] [Google Scholar]
- 34.Ohta Y, Goetz W, Hossain MZ, Nonaka M, Flajnik MF. Ancestral organization of the MHC revealed in the amphibian Xenopus. J Immunol. 2006;176(6):3674–85. doi: 10.4049/jimmunol.176.6.3674. [DOI] [PubMed] [Google Scholar]
- 35.Luhtala M. Chicken CD4, CD8alphabeta, and CD8alphaalpha T cell co-receptor molecules. Poult Sci. 1998;77(12):1858–73. doi: 10.1093/ps/77.12.1858. [DOI] [PubMed] [Google Scholar]
- 36.Duncan LG, Nair SV, Deane EM. The marsupial CD8 gene locus: molecular cloning and expression analysis of the alpha and beta sequences in the gray short-tailed opossum (Monodelphis domestica) and the tammar wallaby (Macropus eugenii) Vet Immunol Immunopathol. 2009;129(1–2):14–27. doi: 10.1016/j.vetimm.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 37.Robert J, Goyos A, Nedelkovska H. Xenopus, a unique comparative model to explore the role of certain heat shock proteins and non-classical MHC class Ib gene products in immune surveillance. Immunol Res. 2009 doi: 10.1007/s12026-009-8094-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robert JLD. Xenopus lymphoid tumor cell lines. In: IL, editor. Manual of Immunological Methods. London: Academic; 1997. p. 2367. [Google Scholar]
- 39.Robert J, Du Pasquier L. Xenopus lymphoid tumor cell lines. In: Lefkovitz I, editor. Manual of Immunological Methods. London: Academic; 1997. p. 2367. [Google Scholar]
- 40.Abbey JL, O’Neill HC. Expression of T-cell receptor genes during early T-cell development. Immunol Cell Biol. 2008;86(2):166–74. doi: 10.1038/sj.icb.7100120. [DOI] [PubMed] [Google Scholar]
- 41.Meier E. T cell development and the diversification and selection of the Xenopus T cell recpetor beta chain repertoire. Alberta CA: University of Alberta; 2003. [Google Scholar]
- 42.Shum BP, Avila D, Du Pasquier L, Kasahara M, Flajnik MF. Isolation of a classical MHC class I cDNA from an amphibian. Evidence for only one class I locus in the Xenopus MHC. J Immunol. 1993;151(10):5376–86. [PubMed] [Google Scholar]
- 43.Gravenor I, Horton TL, Ritchie P, Flint E, Horton JD. Ontogeny and thymus-dependence of T cell surface antigens in Xenopus: flow cytometric studies on monoclonal antibody-stained thymus and spleen. Dev Comp Immunol. 1995;19(6):507–23. doi: 10.1016/0145-305x(95)00030-w. [DOI] [PubMed] [Google Scholar]
- 44.Robert J, Sung M, Cohen N. In vitro thymocyte differentiation in MHC class I-negative Xenopus larvae. Dev Comp Immunol. 2001;25(4):323–36. doi: 10.1016/s0145-305x(00)00066-5. [DOI] [PubMed] [Google Scholar]
- 45.Robert J, Cohen N. In vitro differentiation of a CD4/CD8 double-positive equivalent thymocyte subset in adult Xenopus. Int Immunol. 1999;11(4):499–508. doi: 10.1093/intimm/11.4.499. [DOI] [PubMed] [Google Scholar]
- 46.Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nat Rev Immunol. 2002;2(3):151–61. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- 47.Tucek CL, Boyd RL. Surface expression of CD4 and Thy-1 on mouse thymic stromal cells. Int Immunol. 1990;2(7):593–601. doi: 10.1093/intimm/2.7.593. [DOI] [PubMed] [Google Scholar]
- 48.Robert J, Cohen N. Ontogeny of CTX expression in xenopus. Dev Comp Immunol. 1998;22(5–6):605–12. doi: 10.1016/s0145-305x(98)00028-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


