Introduction
Clinical Perspective
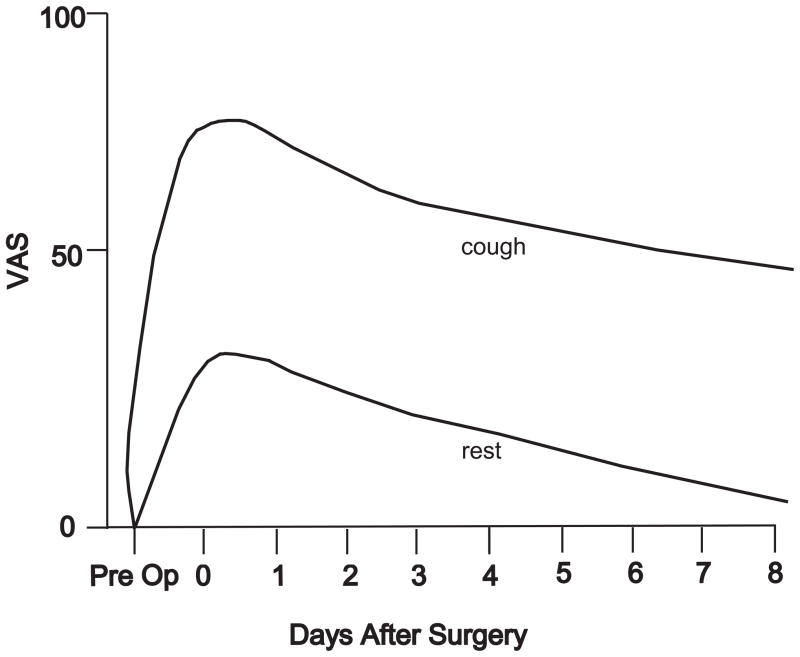
Acute postoperative pain remains a significant medical problem. Patients undergoing outpatient ambulatory surgery have clinically significant postoperative pain even when administration of oral opioids and nonopioid adjuncts are optimized [1]. Regional analgesic techniques improve pain control except their use is limited to a minority of all surgical patients [8; 31]. For those patients undergoing major surgical procedures, ongoing pain or pain at rest, and pain during activities are important clinical symptoms. Pain at rest is usually moderate; the average visual analogue pain scale (VAS) is 3 to 4 out of 10 during the first 2 to 3 days after surgery [24]. These pain scores occur even when parenteral treatments are administered. Usually, pain at rest resolves within the first week after surgery (see schematic, Fig. 1). Pain with activities, such as coughing or walking is severe during the first 2 to 3 days; the average visual analogue scale can be as great as 7 to 8. Pain with activities is moderate or severe for many days and even weeks later. Functional capability is limited during this period as well; thus, pain can be moderate and the activities like ability to cough or walking distance to evoke this pain are reduced [16; 36]. Greater opioid dosing to further reduce pain is limited by side effects like nausea, vomiting, ileus, respiratory depression and sedation [8].
Figure 1.
Schematic of postoperative pain after major surgery in patients with optimized parenteral opioid analgesia. Top line is pain with cough; bottom line is pain at rest. VAS=visual analogue scale.
Clinically, our goal is to advance simple, safe, effective therapies that will greatly reduce postoperative pain. In the last 10 to 15 years, gabapentinoids and cyclooxygenase-2 inhibitors have been trialed extensively and regional anesthesia is advancing in acceptance [9] In general, we have had difficulty making major improvements in the overall treatment of clinical postoperative pain for the majority of patients.
Scientific Perspective
Currently, we recognize that the etiology and treatment of pain produced by surgery is different than other clinical pain conditions like rheumatoid arthritis, fibromyalgia, or acute herpes zoster [6; 23]. Many pain models may be useful for mechanistic studies but less valuable for developing novel targets to treat particular clinical pain conditions. In order to advance our treatment for acute postoperative pain, we must recognize, that many preclinical pain models, such as antigen-specific inflammation or receptor-specific, chemical stimuli (i.e., formalin or capsaicin), do not necessarily translate well to incisional pain mechanisms [15; 44; 46; 47].
Theoretically, if the etiology and pathophysiology of the clinical condition are mimicked by the experimental model, analgesic target discovery may be improved. A second goal for preclinical rodent pain research is to make the endpoints for nociceptive testing have proximate translatability to clinical pain and analgesia [34] Others have suggested that plasma levels of drugs for antinociception in animals and plasma concentrations for analgesia in human subjects be compared to improve the translatability of the models and the behavior in rodents [34]. Limitations of pain models and behaviors may be one of several factors contributing to our limited success in developing new treatments for acute postoperative pain [20; 32]
Because there has been a considerable debate regarding the clinical utility of rodent pain models, ourselves and others undertook studies on the etiology of nociception caused by incisions [2; 12; 21; 26; 28; 39] Perhaps improving our understanding of the mechanisms for postoperative pain could increase the chances of developing new treatments for patients.
It is our hope that research on incisional pain mechanisms and relating these preclinical results to patients symptoms has advanced our understanding of animal pain models, incision-induced pain-related behaviors in animal models, and postoperative pain mechanisms in patients. The long-term goal of postoperative pain research and acute incisional pain studies is for patients to undergo painless or nearly painless surgery.
Preclincal studies
Rat Plantar Incision
Several years ago, it was noted that few studies utilized incisions to understand mechanisms of pain caused by surgery. At the time of our model development, many pain behaviors utilized hindpaw injections into the glabrous skin and nociception was measured using behaviors associated with the hindpaw withdrawal [14]. We undertook experiments using hindpaw incision to utilize similar behaviors and to compare these responses to other models [5] Other preclinical surgical models were developed.
For plantar incision, we use general anesthesia. The hindpaw is sterilized and the glabrous skin is incised usually through the skin and underlying plantar fascia, which are in close apposition in vivo (Fig. 1A–D). After incising to the fascia, an underlying flexor digitorum brevis muscle is encountered. This muscle contracts the lateral four digits of the hindpaw. This underlying flexor muscle is elevated, divided and retracted. Then the skin overlying the muscle is closed with sutures. The rat is allowed to recover from general anesthesia and pain behaviors can be tested as early as 1 hr after incision. In patients who undergo surgical procedures that access deep tissues, skin is incised and underlying muscles are divided and retracted so that inflamed organs or cancerous tumors can be removed. Therefore, the somatic injury after plantar incision has some similarities to the somatic injury of some patients undergoing surgery.
Evoked pain behaviors
A variety of pain-related, nociceptive behaviors can be measured after plantar incision [45] Reduced heat withdrawal latency is greatest the day of incision and sustained for approximately 5 days and is completely resolved by 7 to 10 days after the procedure. Withdrawal threshold to punctate monofilament application adjacent to the incision for mechanical testing is also markedly decreased and this response is sustained for 5 to 10 days. Both primary mechanical hyperalgesia and primary heat hyperalgesia are present in patients after surgery. Finally, other pain behaviors have been measured. These include Randall-Selitto mechanical withdrawal threshold [40], weight bearing [40] and secondary mechanical withdrawal threshold [45].
Guarding
Immediately after plantar incision, there is a small amount of spontaneous pain behavior, such as licking of the incised hindpaw. This usually resolves 30 min to 1 hour after emergence from anesthesia [5] We have observed that rats guard the incised hindpaw early in the postoperative period. We have measured nonevoked pain behavior using a cumulative pain score that examines the position in which both paws are placed on a plastic mesh floor. A cumulative pain score is obtained depending on the position in which the paw is observed during the majority of the 1 min scoring period over 1 hour. Guarding is increased after incision and gradually returns towards pre-incision values over the next 2 to 3 days. The rapid resolution of guarding pain after plantar incision, while a robust mechanical and heat hyperalgesia are still evident, suggests different mechanisms.
We were interested in the relationship of guarding behavior to spontaneous activity (SA) in nociceptive pathways and postoperative pain in patients. If SA in part, signals nonevoked, guarding pain, in nociceptors and dorsal horn neurons, both measures, guarding and SA, may be in part translatable to pain at rest in patients after surgery.
In vivo neurophysiology experiments
Spontaneous activity in nociceptive pathways after incision
A key to translational pain research is to identity sensitized nociceptors and dorsal horn neurons that encode enhanced nociception so that targets transmitting these amplified signals can be evaluated for therapeutic potential. Ongoing or SA, one aspect of sensitization, is a stimulus-independent measurement of sensitization.
Characteristics of nociceptor sensitization after injury may include [23]
spontaneous activity,
decreased response threshold,
increased response to a suprathreshold stimulus,
expansion of the receptive field,
an increase in the proportion of nociceptors responding to a particular stimulus [33; 35]
We have undertaken a series of electrophysiologic studies in rats that had undergone plantar incision one day earlier and examined primary afferent and dorsal horn neuron activity and compared SA to unincised, sham operated animals. Most studies in our laboratory include not only measurements of ongoing SA but also, heat, mechanical and in some cases chemical sensitivity. SA is perhaps the most robust form of sensitization produced in post-injury states because no stimulus is required [19] This review will focus on the generation of or an increase in the magnitude of SA of nociceptors and dorsal horn neurons produced by incisions.
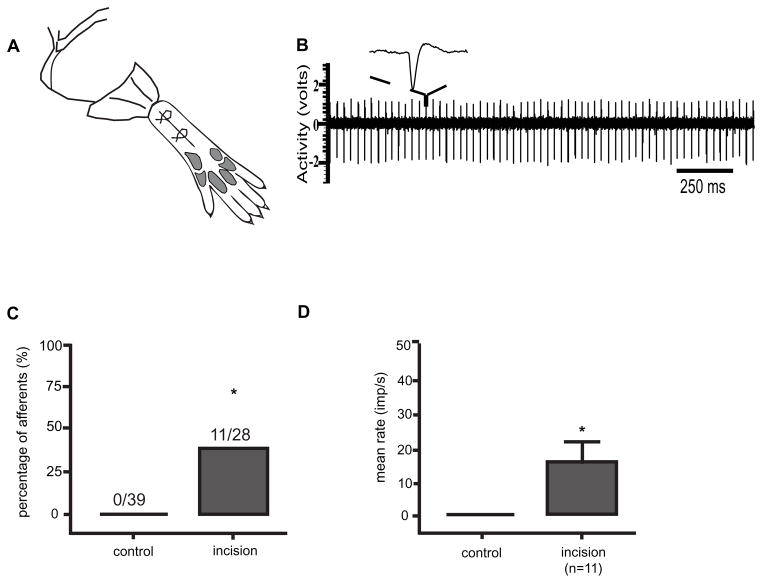
One day after plantar incision, guarding behavior is usually significantly elevated [5]. We compared the SA of primary afferent nociceptors from rats that had undergone plantar incision [29] which included incision in skin, fascia and muscle one day earlier to those that had undergone sham operation (Fig. 3). The nociceptors studied from the incision group innervated, at least in part, the area of the incision. Thirty-nine afferent fibers were recorded in the sham-operated group and 28 fibers were recorded in the plantar incision group on postoperative day 1, Eleven of 28 nociceptors had SA in the incision group; the average frequency was approximately 17 imp/sec. In this study, the majority of spontaneously active Aδ and C-fibers’ activity was greater than 15 imp/sec after incision. In the sham group, no afferents were spontaneously active. The origin of the SA was the incised tissue because local anesthetic infiltrated into the receptive field of the nociceptor in the hindpaw eliminated the activity [29] It was surprising that the rate of SA was quite high in some nociceptive fibers.
Figure 3.
Spontaneous activity in nociceptors one day after plantar incision [29]. A: Schematic of in vivo recording from nociceptors from rats that underwent hindpaw incision. B: Example of spontaneous action potentials recorded from nociceptor in rat that underwent plantar incision. C: Percentage of nociceptors with spontaneous activity in the control group that underwent a sham operation and group that underwent skin, fascia and muscle incision [29; 42]. D: Mean rate of spontaneous activity of nociceptors that underwent skin, fascia and muscle incision [29; 42].
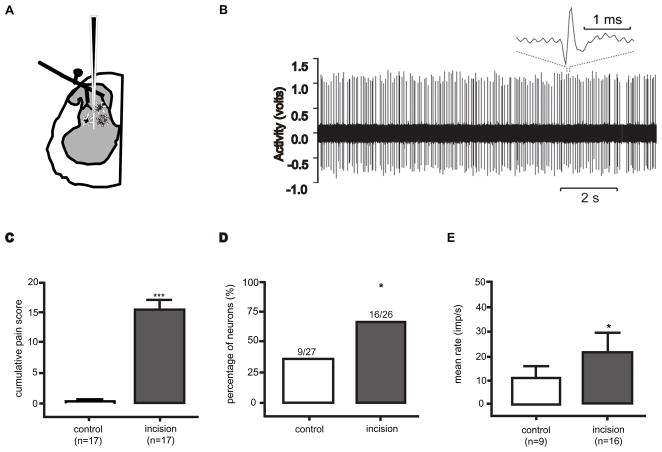
We performed the corresponding experiment but this time recording dorsal horn neurons [43] We measured behavior in rats that had undergone skin, fascia and muscle incision on postoperative day 1 and compared the behavior to rats that underwent a sham operation (Fig. 4). The mean guarding pain scores were 15 and 0 in the incised and sham operated groups, respectively. Rats then underwent electrophysiology experiments, recordings of single dorsal horn neuron activity under general anesthesia. These dorsal horn neurons received input from afferent fibers innervating the glabrous skin of the hindpaw and had a mechanoreceptive field that again included at least part of the area of the incision. SA was identified in 9 of 27 neurons in the control group and 16 of 26 neurons in the incision group. The average dorsal horn SA in the incised rats was 19 imp/sec and was 10 imp/sec in the sham operated group. In this series, both the proportion of neurons with SA and the magnitude of SA were greater in the neurons from the incised group. Bupivacaine injection into the skin and deep tissue of the hindpaw reduced the level of SA in the incised group to the same level to that in the sham operated group [43] Bupivacaine did not affect the magnitude of SA in the sham group. In summary, even though SA is intrinsic to some dorsal horn neurons, the proportion of neurons with SA and amount of SA were both increased one day after incision. Altogether, increased spinal dorsal horn neuron activity remained largely dependent on ongoing primary afferent input on postoperative day 1 when guarding behavior was evident. The SA was quite high in dorsal horn neurons and in agreement with the magnitude of activity in primary afferent nociceptors in similar groups of animals (Fig. 2).
Figure 4.
Spontaneous activity in dorsal horn neurons one day after plantar incision [43]. A: Schematic of dorsal horn neuron recording. B: Example of spontaneous action potentials recorded from dorsal horn neuron in a rat that underwent plantar incision and reproduced from Pain 144: 329–339 with permission of the International Association for the Study of Pain® (IASP®). The figure may not be reproduced for any other purpose without permission.. C: Guarding pain score in rats that underwent a sham (control) operation and group that underwent skin, fascia and muscle incision. D: Percentage of dorsal horn neurons with spontaneous activity in the control group and group that underwent skin, fascia and muscle incision. E: Mean rate of spontaneous activity of dorsal horn neurons in the control and incision groups. C–D adapted from [43] European Journal of Pain, Vol 13, Issue 8, Xu J, Richebe P, Brennan TJ, Separate groups of dorsal horn neurons transmit spontaneous activity and mechanosensitivity one day after plantar incision, p. 823 and 824, Copyright 2010, with permission from Elsevier.
Figure 2.
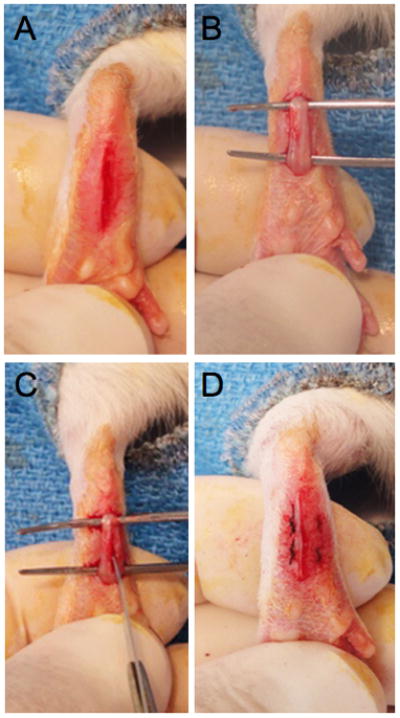
Photographs of the different stages of the rat plantar incision. A: A one cm longitudinal incision is made through the skin and fascia starting 0.5 cm from the proximal edge of the heel and extending toward the distal aspect of the paw. B–C: The underlying flexor muscle is elevated and also incised longitudinally. The muscle is split and dissected longitudinally. D: After hemostasis, the wound is apposed with two mattress sutures of 5-0 nylon.
Tissues contributing to guarding pain and SA of nociceptive pathways
Three previous studies identified cutaneous nociceptors and examined SA before and after skin injury using scalpel blades or needles. In two separate studies, investigators used needles to penetrate the receptive fields of both A-delta and C-fiber nociceptors recorded from cutaneous nerves in the cat [3; 7] Activation during needle penetrations occurred and occasionally there were after discharges following the needle injury; however, no sustained SA was generated after the injury. Finally, Hammaleinin et al. recorded afferent fibers from the tibial nerve innervating the hindpaw of the rat hindpaw [13] Activity was generated during incision and other forms of sensitization were evident; yet, no nociceptors developed ongoing, sustained SA after incision.
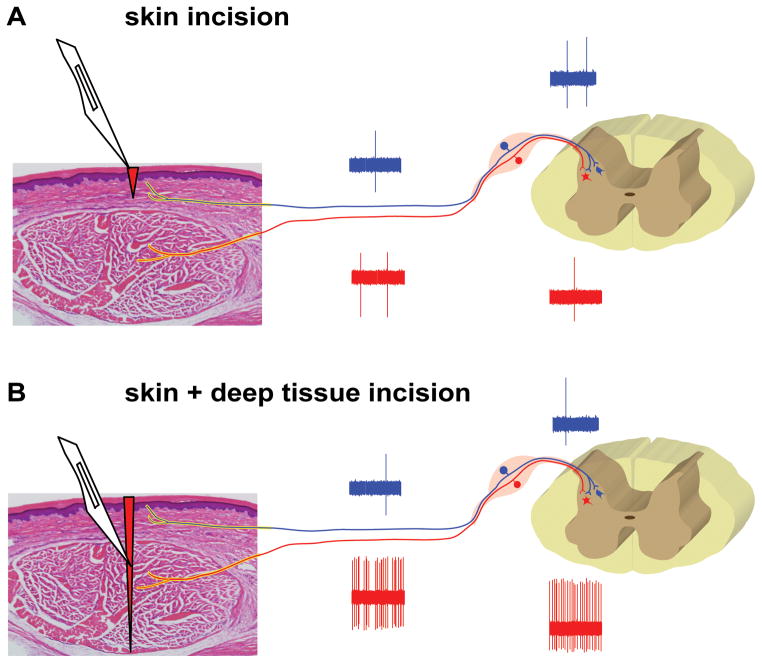
It was surprising that nociceptors could not be activated immediately after incision because dorsal horn neurons generate some sustained SA during and after the same incisions [30; 38; 46] Dorsal horn neurons receive convergent input from a variety of tissues whereas nociceptors innervate specific tissues. Perhaps by selecting cutaneous nociceptors and incising their receptive fields, these cutaneous nociceptors did not generate SA after incision. We hypothesized that deep tissue incision that included fascia and muscle would generate SA in nociceptive pathways and produce unprovoked guarding pain but a skin only incision would not.
We undertook a series of experiments incising skin only, skin, fascia and muscle (deep tissue) or sham (no operation) surgery. Guarding behavior was measured in separate groups of rats after sham, skin, or skin plus deep tissue incision 1 day later. A fourth group was studied 7 days after skin plus deep tissue incision when pain behaviors generally resolve. These same groups then underwent in vivo recording of single fiber nociceptors innervating the area of the incision (Fig. 5A–C).
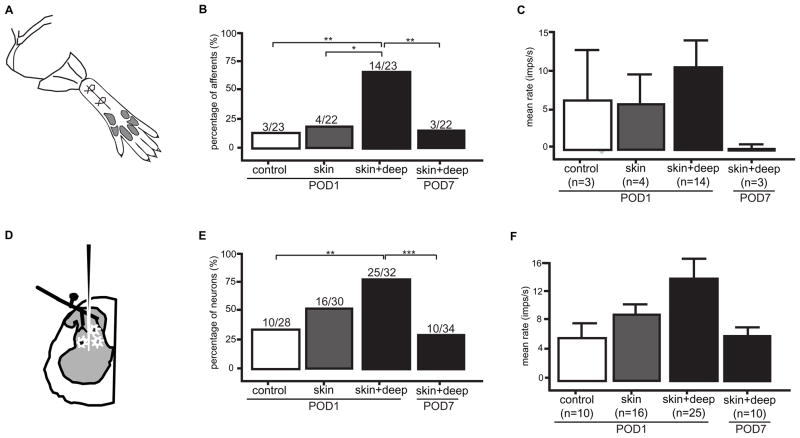
Figure 5.
Spontaneous activity in nociceptors and dorsal horn neurons. A–C. Nociceptor recordings in rats that underwent a sham (control) operation, a skin incision, a skin, fascia and muscle incision and a group 7 days after skin, fascia and muscle incision. Percentage of nociceptors with spontaneous activity (B) and average spontaneous activity (C). B–C from [42]. Figure B–C from Xu J, Brennan TJ. Guarding pain and spontaneous activity of nociceptors after skin versus skin plus deep tissue incision. Anesthesiology 2010; 112:153–64 with permission from Lippincott, Williams, & Wilkins. D–F. Dorsal horn neuron recordings in rats that underwent a sham (control) operation, a skin incision, a skin, fascia and muscle incision and a group 7 days after skin, fascia and muscle incision. Percentage of dorsal horn neurons with spontaneous activity (E) and average spontaneous activity (F). E–F adapted from [41]. Figure E–F has been reproduced with permission of the International Association for the Study of Pain® (IASP®). The figure may not be reproduced for any other purpose without permission.
Compared with the sham control group, skin incision induced a small amount of guarding on the day of incision only, whereas skin plus deep tissue incision caused guarding for 5 days after incision and this behavior completely resolved on postoperative day 7 [41; 42]. On postoperative day 1, skin incision (18%) produced a similar prevalence of SA in nociceptors as the sham operated group (13%), whereas skin plus deep tissue incision generated a greater prevalence of SA in nociceptors (61%); the rate of SA also tended to be greater after skin plus deep tissue incision (10 ± 3 imp/sec) versus the control group (6 ± 6) and skin incision group (6 ± 3 imp/sec). Seven days after skin plus deep tissue incision, when pain behaviors had resolved, the prevalence of SA (14%) and the amount of SA (0.3 ± 0.1) were similar as the sham group [42]. Incision of skin was not sufficient to produce guarding pain behavior but incision that included skin fascia and muscle was sufficient. In addition, skin incision was sufficient to produce the typical reduced heat withdrawal latency and reduced mechanical withdrawal threshold on postoperative day 1 [41; 42].
An comparable series of experiments in four similar groups of rats was undertaken except in these four groups (Fig. 5D–F), dorsal horn neurons were recorded on postoperative day 1 [41] Pain behaviors again demonstrated the importance of deep muscle incision on the generation of guarding but normal heat and mechanical responses after skin only incision. On postoperative day 1, skin incision (53%) produced a similar prevalence of SA in dorsal horn neurons as the sham operated group (36%), whereas skin plus deep tissue incision generated a greater prevalence of SA in dorsal horn neurons (78%); the rate of SA also tended to be greater after skin plus deep tissue incision (14 ± 3 imp/sec) versus the control group (6 ± 2) and skin incision group (9 ± 2 imp/sec). Seven days after skin plus deep tissue incision, when pain behaviors had resolved, the prevalence of SA (30%) and the amount of SA (6 ± 2) were similar as the sham group. Bupivacaine infiltration into the incision decreased SA in the skin plus deep tissue incision group to the same level as the sham operated rats on postoperative day 1 [41]
These data demonstrated incised deep tissue rather than skin had a central role in the genesis of guarding behavior and SA in nociceptors and nociceptive transmitting dorsal horn neurons (Fig. 6). Nociceptor and dorsal horn SA was associated with guarding behavior after incision. Decreased heat withdrawal latency and decreased mechanical withdrawal threshold occurred with skin incision only.
Figure 6.
Schematic for the development guarding pain and SA in the nociceptive pathways after plantar incision. (A) An incision in the skin only (epidermis and dermis layer) induces minimal SA in nociceptors and DHNs which receive predominately cutaneous input. (B) An incision including skin and deep tissue (fascia and muscle layer) results in sustained SA in muscle-innervating primary afferents and the DHNs receiving input from muscle. From Xu J, Brennan TJ. Guarding pain and spontaneous activity of nociceptors after skin versus skin plus deep tissue incision. Anesthesiology 2010; 112:153–64 with permission from Lippincott, Williams, & Wilkins.
Human studies
Forearm incision
Data from human studies support these preclinical incisional pain studies [17; 18]. First, when an incision was made in the forearm of human volunteers, pain diminished soon after the blade had been pulled out from the skin; spontaneous pain, rapidly decreased and resolved within 30 min after incision. However, mechanical hyperalgesia was evident. The hyperalgesia was similar in magnitude and duration to the mechanical withdrawal thresholds in rats after plantar incision. Thus, forearm incision in volunteers has similarities to skin incision of the rat hindpaw.
Clinical studies
In support of this concept, two surgical approaches for a unilateral total hip arthroplasty, a minimally invasive approach and a conventional approach, were recently compared [11]. Both approaches used the same length of skin incision, 20 cm. The minimally invasive approach preserved the underlying muscles whereas muscles were incised and divided in the conventional approach. The minimally invasive approach resulted in significantly less postoperative pain and opioid consumption than the conventional approach.
In a complimentary study [27], two groups of patients underwent total hip arthroplasty with the same amount of deep tissue dissection through different lengths of skin incision (10 vs 20 cm). There was no difference in postoperative pain between groups when the same degree of deep tissue injury occurred. Together, these postoperative pain studies indicate that reducing the amount of deep tissue injury decreases pain at rest and opioid consumption whereas varying the magnitude of the skin incision did not affect pain at rest or opioid use.
Implications for animal models, clinical disease states, and drug discovery
In preclinical behavioral studies, there has been a significant emphasis on exaggerated withdrawal responses in models of pathologic pain states. This began with testing changes in heat withdrawal latency and subsequently punctate mechanical withdrawal thresholds. Both heat hyperalgesia [22] and decreased mechanical pain threshold [37] occur in both surgical patients. Because there has been increasing awareness of the limitations of these evoked behavioral responses, the applicability of cutaneous hyperalgesia to patient’s postoperative pain symptoms may be limited.
The implications of our data are the following:
Clinical studies with local anesthetic infiltration and nerve blockade indicate pre-incision analgesia has little clinical benefit compared to administration of analgesic drugs later after surgery [25]. Basic science data indicate that early after surgery, primary afferent activation and peripheral sensitization are profound when patient’s postoperative pain is greatest [29; 41–43]. Central sensitization occurs early in the postoperative period but its precise role in clinical acute pain is not clear [10]. Central sensitization likely contributes to referred pain and secondary hyperalgesia; perhaps it is related to chronic post-traumatic pain [4].
There is profound primary afferent and dorsal horn neuron activation 1 day after plantar incision [29; 42]. Despite evidence that some nociceptors have ongoing activity as great or greater than 20 imp/sec, no obvious licking, biting or scratching of the hindpaw is evident one day after plantar incision. These data suggest that behaviors resulting from ongoing activity in nociceptive pathways may be modest and difficult to detect behaviorally but may represent very clinically significant acute pain. The demonstration of high ongoing activity in dorsal horn neurons and primary afferent fibers suggests that these neurophysiology experiments may detect activation of the nociceptive system when spontaneous nociceptive behaviors are limited.
Using evoked stimuli to test the effect of an analgesic drug could fail to capture drugs effective against ongoing activity in nociceptive pathways, the correlate to pain at rest. Thus, a useful analgesic drug may be overlooked when only evoked responses are tested.
Tests that utilize mechanical and heat stimuli may largely evaluate cutaneous sensitivity after incision. This may apply to other models as well. Skin injury and skin testing may have limited clinical relevance and may in part have contributed to the limited discovery of new analgesic drugs. The skin is certainly an important model system, but clinical pathophysiologic relevance may be limited for postoperative patients.
Conclusion
Clinical studies indicate that improvements in postoperative pain control will advance perioperative medicine. Models for pain and nociception continue to be refined and evaluated. For postoperative models, the behavioral responses after incision and neurophysiologic studies on activities of primary afferent and dorsal horn neurons indicate different tissues have unique responses to incision. These studies will hopefully improve our understanding of incisional pain mechanisms and the behavioral aspects of activation and sensitization of nociceptive pathways.
Footnotes
Conflict of interest statement
The author has no conflict of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Apfelbaum JL, Chen C, Mehta SS, Gan TJ. Postoperative pain experience: results from a national survey suggest postoperative pain continues to be undermanaged. Anesth Analg. 2003;97:534–540. doi: 10.1213/01.ANE.0000068822.10113.9E. table of contents. [DOI] [PubMed] [Google Scholar]
- 2.Beitz AJ, Newman A, Shepard M, Ruggles T, Eikmeier L. A new rodent model of hind limb penetrating wound injury characterized by continuous primary and secondary hyperalgesia. J Pain. 2004;5:26–37. doi: 10.1016/j.jpain.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Bessou P, Perl ER. Response of cutaneous sensory units with unmyelinated fibers to noxious stimuli. J Neurophysiol. 1969;32:1025–1043. doi: 10.1152/jn.1969.32.6.1025. [DOI] [PubMed] [Google Scholar]
- 4.Brennan TJ, Kehlet H. Preventive analgesia to reduce wound hyperalgesia and persistent postsurgical pain: not an easy path. Anesthesiology. 2005;103:681–683. doi: 10.1097/00000542-200510000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Brennan TJ, Vandermeulen EP, Gebhart GF. Characterization of a rat model of incisional pain. Pain. 1996;64:493–501. doi: 10.1016/0304-3959(95)01441-1. [DOI] [PubMed] [Google Scholar]
- 6.Brennan TJ, Zahn PK, Pogatzki-Zahn EM. Mechanisms of incisional pain. Anesthesiol Clin North America. 2005;23:1–20. doi: 10.1016/j.atc.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 7.Burgess PR, Perl ER. Myelinated afferent fibres responding specifically to noxious stimulation of the skin. J Physiol. 1967;190:541–562. doi: 10.1113/jphysiol.1967.sp008227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dahl JB, Kehlet H. Postoperative pain and its management. In: McMahon SB, Koltzenburg M, editors. Wall and Melzack’s Textbook of Pain. 5. Amsterdam, the Netherlands: Elsevier Churchill Living stone; 2006. pp. 635–652. [Google Scholar]
- 9.Dahl JB, Mathiesen O, Kehlet H. An expert opinion on postoperative pain management, with special reference to new developments. Expert Opin Pharmacother. 2010;11:2459–2470. doi: 10.1517/14656566.2010.499124. [DOI] [PubMed] [Google Scholar]
- 10.Dirks J, Moiniche S, Hilsted KL, Dahl JB. Mechanisms of postoperative pain: clinical indications for a contribution of central neuronal sensitization. Anesthesiology. 2002;97:1591–1596. doi: 10.1097/00000542-200212000-00035. [DOI] [PubMed] [Google Scholar]
- 11.Dorr LD, Maheshwari AV, Long WT, Wan Z, Sirianni LE. Early pain relief and function after posterior minimally invasive and conventional total hip arthroplasty. A prospective, randomized, blinded study. J Bone Joint Surg Am. 2007;89:1153–1160. doi: 10.2106/JBJS.F.00940. [DOI] [PubMed] [Google Scholar]
- 12.Duarte AM, Pospisilova E, Reilly E, Hamaya Y, Mujenda F, Strichartz GR. Reduction of post-incisional allodynia by subcutaneous bupivacaine: findings with a new model in the hairy skin of the rat. Anesthesiology. 2005;103:113–125. doi: 10.1097/00000542-200507000-00018. [DOI] [PubMed] [Google Scholar]
- 13.Hamalainen MM, Gebhart GF, Brennan TJ. Acute effect of an incision on mechanosensitive afferents in the plantar rat hindpaw. J Neurophysiol. 2002;87:712–720. doi: 10.1152/jn.00207.2001. [DOI] [PubMed] [Google Scholar]
- 14.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 15.Honore P, Wade CL, Zhong C, Harris RR, Wu C, Ghayur T, Iwakura Y, Decker MW, Faltynek C, Sullivan J, Jarvis MF. Interleukin-1alphabeta gene-deficient mice show reduced nociceptive sensitivity in models of inflammatory and neuropathic pain but not post-operative pain. Behav Brain Res. 2006;167:355–364. doi: 10.1016/j.bbr.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 16.Ilfeld BM, Mariano ER, Girard PJ, Loland VJ, Meyer RS, Donovan JF, Pugh GA, Le LT, Sessler DI, Shuster JJ, Theriaque DW, Ball ST. A multicenter, randomized, triple-masked, placebo-controlled trial of the effect of ambulatory continuous femoral nerve blocks on discharge-readiness following total knee arthroplasty in patients on general orthopaedic wards. Pain. 2010;150:477–484. doi: 10.1016/j.pain.2010.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawamata M, Takahashi T, Kozuka Y, Nawa Y, Nishikawa K, Narimatsu E, Watanabe H, Namiki A. Experimental incision-induced pain in human skin: effects of systemic lidocaine on flare formation and hyperalgesia. Pain. 2002;100:77–89. doi: 10.1016/s0304-3959(02)00233-6. [DOI] [PubMed] [Google Scholar]
- 18.Kawamata M, Watanabe H, Nishikawa K, Takahashi T, Kozuka Y, Kawamata T, Omote K, Namiki A. Different mechanisms of development and maintenance of experimental incision-induced hyperalgesia in human skin. Anesthesiology. 2002;97:550–559. doi: 10.1097/00000542-200209000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Liang YF, Haake B, Reeh PW. Sustained sensitization and recruitment of rat cutaneous nociceptors by bradykinin and a novel theory of its excitatory action. J Physiol. 2001;532:229–239. doi: 10.1111/j.1469-7793.2001.0229g.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mao J. Translational pain research: achievements and challenges. J Pain. 2009;10:1001–1011. doi: 10.1016/j.jpain.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin TJ, Buechler NL, Kahn W, Crews JC, Eisenach JC. Effects of laparotomy on spontaneous exploratory activity and conditioned of operant responding in the rat - A model for postoperative pain. Anesthesiology. 2004;101:191–203. doi: 10.1097/00000542-200407000-00030. [DOI] [PubMed] [Google Scholar]
- 22.Martinez V, Fletcher D, Bouhassira D, Sessler DI, Chauvin M. The evolution of primary hyperalgesia in orthopedic surgery: quantitative sensory testing and clinical evaluation before and after total knee arthroplasty. Anesth Analg. 2007;105:815–821. doi: 10.1213/01.ane.0000278091.29062.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meyer RA, Ringkamp M, Campbell JN, Raja SN. Peripheral mechanisms of cutaneous nociception. In: McMahon SB, Koltzenburg M, editors. Wall and Melzack’s Textbook of Pain. 5. Amsterdam, the Netherlands: Elsevier Churchill Living stone; 2006. pp. 3–34. [Google Scholar]
- 24.Moiniche S, Dahl JB, Erichsen CJ, Jensen LM, Kehlet H. Time course of subjective pain ratings, and wound and leg tenderness after hysterectomy. Acta Anaesthesiol Scand. 1997;41:785–789. doi: 10.1111/j.1399-6576.1997.tb04784.x. [DOI] [PubMed] [Google Scholar]
- 25.Moiniche S, Kehlet H, Dahl JB. A qualitative and quantitative systematic review of preemptive analgesia for postoperative pain relief: the role of timing of analgesia. Anesthesiology. 2002;96:725–741. doi: 10.1097/00000542-200203000-00032. [DOI] [PubMed] [Google Scholar]
- 26.Nara T, Saito S, Obata H, Goto F. A rat model of postthoracotomy pain: behavioural and spinal cord NK-1 receptor assessment. Can J Anaesth. 2001;48:665–676. doi: 10.1007/BF03016201. [DOI] [PubMed] [Google Scholar]
- 27.Ogonda L, Wilson R, Archbold P, Lawlor M, Humphreys P, O’Brien S, Beverland D. A minimal-incision technique in total hip arthroplasty does not improve early postoperative outcomes. A prospective, randomized, controlled trial. J Bone Joint Surg Am. 2005;87:701–710. doi: 10.2106/JBJS.D.02645. [DOI] [PubMed] [Google Scholar]
- 28.Pitcher GM, Ritchie J, Henry JL. Nerve constriction in the rat: model of neuropathic, surgical and central pain. Pain. 1999;83:37–46. doi: 10.1016/s0304-3959(99)00085-8. [DOI] [PubMed] [Google Scholar]
- 29.Pogatzki EM, Gebhart GF, Brennan TJ. Characterization of Adelta- and C-fibers innervating the plantar rat hindpaw one day after an incision. J Neurophysiol. 2002;87:721–731. doi: 10.1152/jn.00208.2001. [DOI] [PubMed] [Google Scholar]
- 30.Pogatzki EM, Vandermeulen EP, Brennan TJ. Effect of plantar local anesthetic injection on dorsal horn neuron activity and pain behaviors caused by incision. Pain. 2002;97:151–161. doi: 10.1016/s0304-3959(02)00014-3. [DOI] [PubMed] [Google Scholar]
- 31.Popping DM, Zahn PK, Van Aken HK, Dasch B, Boche R, Pogatzki-Zahn EM. Effectiveness and safety of postoperative pain management: a survey of 18 925 consecutive patients between 1998 and 2006: a database analysis of prospectively raised data. Br J Anaesth. 2008;101:832–840. doi: 10.1093/bja/aen300. [DOI] [PubMed] [Google Scholar]
- 32.Quessy SN. The challenges of translational research for analgesics: the state of knowledge needs upgrading and some uncomfortable deficiencies remain to be urgently addressed. J Pain. 2010;11:698–700. doi: 10.1016/j.jpain.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 33.Reeh PW, Bayer J, Kocher L, Handwerker HO. Sensitization of nociceptive cutaneous nerve fibers from the rat’s tail by noxious mechanical stimulation. Exp Brain Res. 1987;65:505–512. doi: 10.1007/BF00235973. [DOI] [PubMed] [Google Scholar]
- 34.Rice AS, Cimino-Brown D, Eisenach JC, Kontinen VK, Lacroix-Fralish ML, Machin I, Mogil JS, Stohr T. Animal models and the prediction of efficacy in clinical trials of analgesic drugs: a critical appraisal and call for uniform reporting standards. Pain. 2008;139:243–247. doi: 10.1016/j.pain.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 35.Schmidt R, Schmelz M, Forster C, Ringkamp M, Torebjork E, Handwerker H. Novel classes of responsive and unresponsive C nociceptors in human skin. J Neurosci. 1995;15:333–341. doi: 10.1523/JNEUROSCI.15-01-00333.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singelyn FJ, Deyaert M, Joris D, Pendeville E, Gouverneur JM. Effects of intravenous patient-controlled analgesia with morphine, continuous epidural analgesia, and continuous three-in-one block on postoperative pain and knee rehabilitation after unilateral total knee arthroplasty. Anesth Analg. 1998;87:88–92. doi: 10.1097/00000539-199807000-00019. [DOI] [PubMed] [Google Scholar]
- 37.Stubhaug A, Breivik H, Eide PK, Kreunen M, Foss A. Mapping of punctuate hyperalgesia around a surgical incision demonstrates that ketamine is a powerful suppressor of central sensitization to pain following surgery. Acta Anaesthesiol Scand. 1997;41:1124–1132. doi: 10.1111/j.1399-6576.1997.tb04854.x. [DOI] [PubMed] [Google Scholar]
- 38.Vandermeulen EP, Brennan TJ. Alterations in ascending dorsal horn neurons by a surgical incision in the rat foot. Anesthesiology. 2000;93:1294–1302. doi: 10.1097/00000542-200011000-00024. discussion 1296A. [DOI] [PubMed] [Google Scholar]
- 39.Weber J, Loram L, Mitchell B, Themistocleous A. A model of incisional pain: the effects of dermal tail incision on pain behaviours of Sprague Dawley rats. J Neurosci Methods. 2005;145:167–173. doi: 10.1016/j.jneumeth.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 40.Whiteside GT, Harrison J, Boulet J, Mark L, Pearson M, Gottshall S, Walker K. Pharmacological characterisation of a rat model of incisional pain. Br J Pharmacol. 2004;141:85–91. doi: 10.1038/sj.bjp.0705568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu J, Brennan TJ. Comparison of skin incision vs. skin plus deep tissue incision on ongoing pain and spontaneous activity in dorsal horn neurons. Pain. 2009;144:329–339. doi: 10.1016/j.pain.2009.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu J, Brennan TJ. Guarding pain and spontaneous activity of nociceptors after skin versus skin plus deep tissue incision. Anesthesiology. 2010;112:153–164. doi: 10.1097/ALN.0b013e3181c2952e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu J, Richebe P, Brennan TJ. Separate groups of dorsal horn neurons transmit spontaneous activity and mechanosensitivity one day after plantar incision. Eur J Pain. 2009;13:820–828. doi: 10.1016/j.ejpain.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 44.Zahn PK, Brennan TJ. Lack of effect of intrathecally administered N-methyl-D-aspartate receptor antagonists in a rat model for postoperative pain. Anesthesiology. 1998;88:143–156. doi: 10.1097/00000542-199801000-00022. [DOI] [PubMed] [Google Scholar]
- 45.Zahn PK, Brennan TJ. Primary and secondary hyperalgesia in a rat model for human postoperative pain. Anesthesiology. 1999;90:863–872. doi: 10.1097/00000542-199903000-00030. [DOI] [PubMed] [Google Scholar]
- 46.Zahn PK, Pogatzki-Zahn EM, Brennan TJ. Spinal administration of MK-801 and NBQX demonstrates NMDA-independent dorsal horn sensitization in incisional pain. Pain. 2005;114:499–510. doi: 10.1016/j.pain.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 47.Zahn PK, Subieta A, Park SS, Brennan TJ. Effect of blockade of nerve growth factor and tumor necrosis factor on pain behaviors after plantar incision. J Pain. 2004;5:157–163. doi: 10.1016/j.jpain.2004.02.538. [DOI] [PubMed] [Google Scholar]