Abstract
Iron cardiomyopathy in β-thalassemia major patients is associated with vitamin D deficiency. Stores of 25-OH-D3 are markedly reduced, while the active metabolite, 1-25-(OH)-D3, is normal or increased. Interestingly, the ratio of 25-OH-D3 to 1-25-(OH)-D3 (a surrogate for parathyroid hormone (PTH)) is the strongest predictor of cardiac iron. Increased PTH and 1-25-OH-D3 levels have been shown to up-regulate L-type voltage-gated calcium channels (LVGCC), the putative channel for cardiac iron uptake. Therefore, we postulate that vitamin D deficiency increases cardiac iron by altering LVGCC regulation. Hemojuvelin knockout mice were calcitriol treated, PTH treated, vitamin D-depleted, or untreated. Half of the animals in each group received the Ca2+-channel blocker verapamil. Mn2+ was infused to determine LVGCC activity. Hearts and livers were harvested for iron, calcium, and manganese measurements as well as histology. Cardiac iron did not differ amongst the treatment groups; however, liver iron was increased in vitamin D-depleted animals (p<0.0003). Cardiac iron levels did not correlate with manganese uptake, but were proportional to cardiac calcium levels (r2 = 0.6, p < 0.0001). Verapamil treatment reduced both cardiac (p <0.02) and hepatic (p < 0.003) iron levels significantly by 34% and 28%. The association between cardiac iron and calcium levels was maintained after verapamil treatment (r2 = 0.3, p < 0.008). Vitamin D-depletion is associated with an increase in liver, but not cardiac, iron accumulation. Cardiac iron uptake was strongly correlated with cardiac calcium stores and was significantly attenuated by verapamil, suggesting that cardiac calcium and iron are related.
Keywords: b-thalassemia, vitamin D, iron overload, hemojuvelin
Introduction
Vitamin D deficiency is epidemic in the United States and has been increasingly linked to a number of important cardiovascular complications, including exacerbation of hypertension, myocardial failure in end-stage renal disease, and ischemic and idiopathic cardiomyopathy [1–3]. Despite the statistical association, little is known about the mechanisms involved. Vitamin D deficiency is also highly prevalent in β-thalassemia patients [4]. β-thalassemia major (TM) is a severe congential anemia, requiring chronic blood transfusions, that results in iron overload. We have recently demonstrated a strong correlation between circulating vitamin D-25-OH, increased cardiac iron burden, and decreased myocardial function in β-thalassemia patients. Although deficient in vitamin D stores (25-OH-D3), these subjects have normal or increased levels of the biologically active metabolite of vitamin D (1-25-OH-D3), suggesting secondary hyperparathyroidism [5]. Reduced 25-OH-D3 levels normally lead to increased PTH levels to ensure adequate levels of 1-25-OH-D3. Previous work in myocyte culture suggests that PTH- and vitamin D (1-25-OH-D3) upregulate L-type voltage-gated Ca2+ channels (LVGCC) [6–8]. LVGCC are essential for calcium homeostasis and the dominant mechanism for transmembrane calcium influx into myocytes. Recent murine work also suggests that LVGCC play a role in iron transport into cardiomyocytes [9–10]. Therefore, we hypothesized that vitamin D deficiency increases cardiac iron uptake by increasing the number or activity of LVGCC.
Methods
Animals
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Institutional Animal Care and Use Committee of Children’s Hospital Los Angeles.
Many previous studies of iron overload in rodents have utilized injections of macromolecular iron, such as iron dextran. However, the rodent heart contains a rich network of interstitial phagocytes that take up macromolecular iron, dwarfing the iron stores observed in myocytes [11]. In iron dextran models, whole heart iron levels bear little relationship to myocyte iron stores, making it a poor model to study myocyte iron uptake. In contrast, the hemojuvelin knockout mouse (HJV KO) spontaneously accumulates hepatic, pancreatic, and cardiac iron, with parenchymal iron stores that mimic human histopathology [12–13].
Forty-eight eight-week-old hemojuvelin knockout mice were used for this study. Eight groups were established. Group 1 had no treatment. Group 2 received vitamin D (1-25-(OH)-D3). Group 3 received PTH. Group 4 was vitamin D depleted. Group 5 received verapamil only. Group 6 received vitamin D and verapamil. Group 7 received PTH and verapamil. Group 8 contained vitamin D depleted mice receiving verapamil. Mice were maintained on an iron supplemented chow (Fe 1400 ppm, base rodent diet 5001 (Ca 0.95%, Mn 70 ppm) (Newco Distributor, Ca)).Treatment duration was 6 weeks. Following the treatment period, animals were euthanized by cardiac puncture and organs harvested for quantitative iron, manganese, calcium, and tissue histology.
Vitamin D Deficiency
Vitamin D deficiency was induced by feeding breeding couples vitamin D-depleted chow in a UV-free environment [14]. Vitamin D-deficient offspring were raised on a vitamin D-free diet and used for the studies.
Drug Administration
Mice of group 2 and 6 received calcitriol. Calcitriol (Sigma Aldrich, Saint Louis, MO), a bioactive form of vitamin D, 0.4ug/kg was administered 3 times a week by IP injections for 6 weeks. Calcitriol at a dose of 1 ug/kg 3 times a week for 8 weeks has been shown to induce vascular calcification in rodents [15–16]. Since 1ug/kg seemed to be too aggressive (increased blood and pulse pressure), we opted for 0.4ug/kg. PTH (Bachem, Torrance, Ca) was administrated via Alzet pump continuously (subcutaneously) at a rate of 10 µg/kg/d. Mice of group 3 and 7 received PTH. In a PTH dose-response study, this dose has shown to maintain normal calcium and phosphorus levels in parathyroidectomized rats[17]. Mice of group 5–8 received verapamil treatment. In order to block L-type Ca2+-channels, verapamil (0.2mg/ml) was administered via drinking water ad libitum. A verapamil concentration of 0.1mg/ml effectively blocks LVGCC [9, 18].
Manganese Infusions
Short-term Mn2+ infusions are a surrogate for LVGCC activity. Previous studies using the Ca2+ channel blockers diltiazem and verapamil demonstrated effective blocking of Mn2+ influx at physiologic doses. Manganese activity was decreased after β-blocker administration and increased following inotrope treatment, consistent with known response of calcium transport through LVGCC [19–21].
All experimental mice were sedated with ketamine/xylazine (100mg/10mg) and infused via an IP line. MnCl2 was dissolved in PBS to deliver a dose of 0.4mg/kg/min for 30 minutes [20]. Thirty minutes post infusion, hearts were harvested and prepared for blood collection, inductively coupled plasma mass spectroscopy (ICP-MS), and histology.
Iron, Manganese, and Calcium Determination
Cardiac (30–50mg) and hepatic (70–100 mg) tissue samples were dissolved in equal aliquots of analysis-grade 70% nitric acid (Sigma Aldrich, Saint Louis, MO) and 30% hydrogen peroxide (Fisher Scientific, Pittsburgh, PA). Tissue digests were dried and then diluted to a final nitric acid concentration of 2%. Samples were processed using a Hewlett Packard 4500 Series ICP-MS (Hewlett Packard, Palo Alto, CA). Seven-element control standards were used. ICP standards (Sigma Aldrich, Saint Louis, MO) for each element were used to create desired dilutions with 2% nitric acid. The standard samples residual standard deviation averaged less than 5%, with a few exceptions for highly diluted samples. Isotope emissions of iron (Fe-56, Fe- 57, and Fe-58) were averaged together.
Histology
At the end of the study, sections of cardiac and hepatic tissues were fixed in 10% formalin, embedded in paraffin, and stained with Prussian blue and Hematoxylin and Eosin (H&E).
Statistical Analysis
All results are presented as means ± standard deviations. One-way analysis of variance was applied to determine statistical differences among groups. The mean of each treatment group was compared with the mean value from the control animals using Dunnett’s test, which corrects for multiple comparisons. P < 0.05 was considered significant. Where applicable, multivariate testing was used to determine the most influential variable.
Results
Animals in all groups appeared to tolerate treatments well, with the exception of the calcitriol treated mice. These animals showed signs of lethargy and stiffness. One animal with a PTH pump implant died unexpectedly two weeks post surgery.
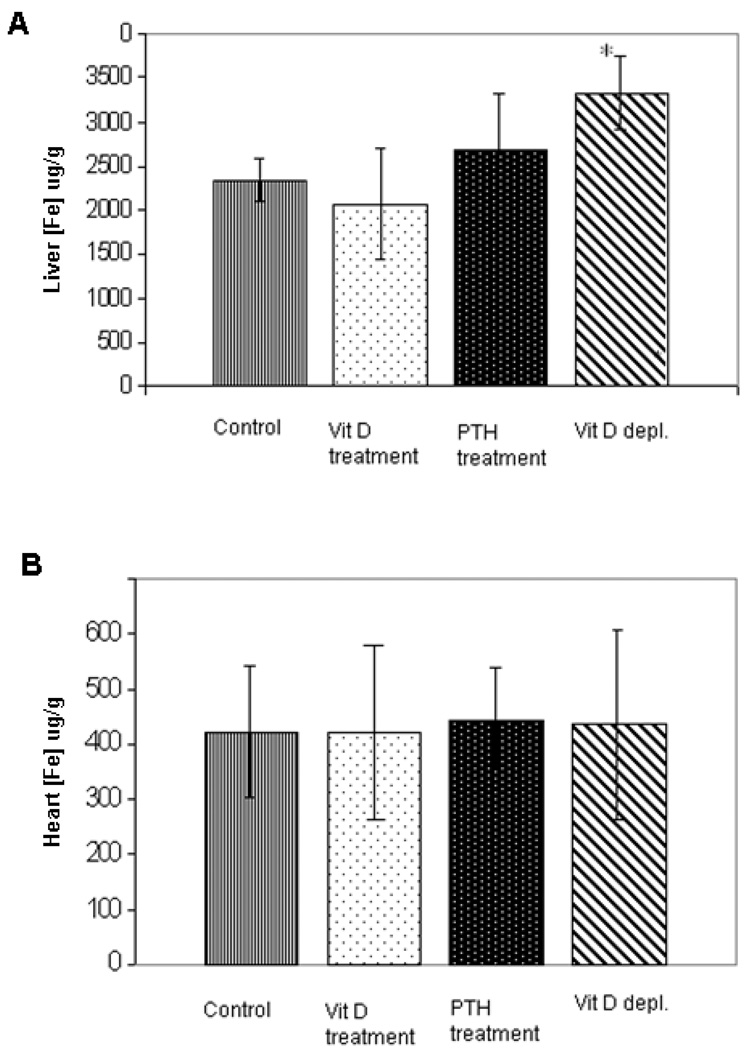
The effects of Vitamin D axis manipulations on cardiac and hepatic iron concentrations in non-verapamil treated animals are summarized in Figure 1. Liver iron levels of vitamin D-depleted animals were 43% higher compared to liver iron levels of control animals (p<0.003) (Fig. 1A). Despite increasing liver iron concentration, vitamin D depletion did not increase cardiac iron. In fact, cardiac iron levels were unchanged by any of the vitamin D axis perturbations (Fig. 1B).
Figure 1. Hepatic (top panel) and cardiac (lower panel) iron concentrations of following vitamin D axis manipulation in non-verapamil treated animals.
Iron levels are reported in units of mg/g wet weight. Liver iron levels of vitamin D-depleted animals were significantly higher than in other groups (p<0.003) (A). Cardiac iron levels were unchanged by any of the vitamin D axis perturbations (B). Data are presented as mean ± s.d.
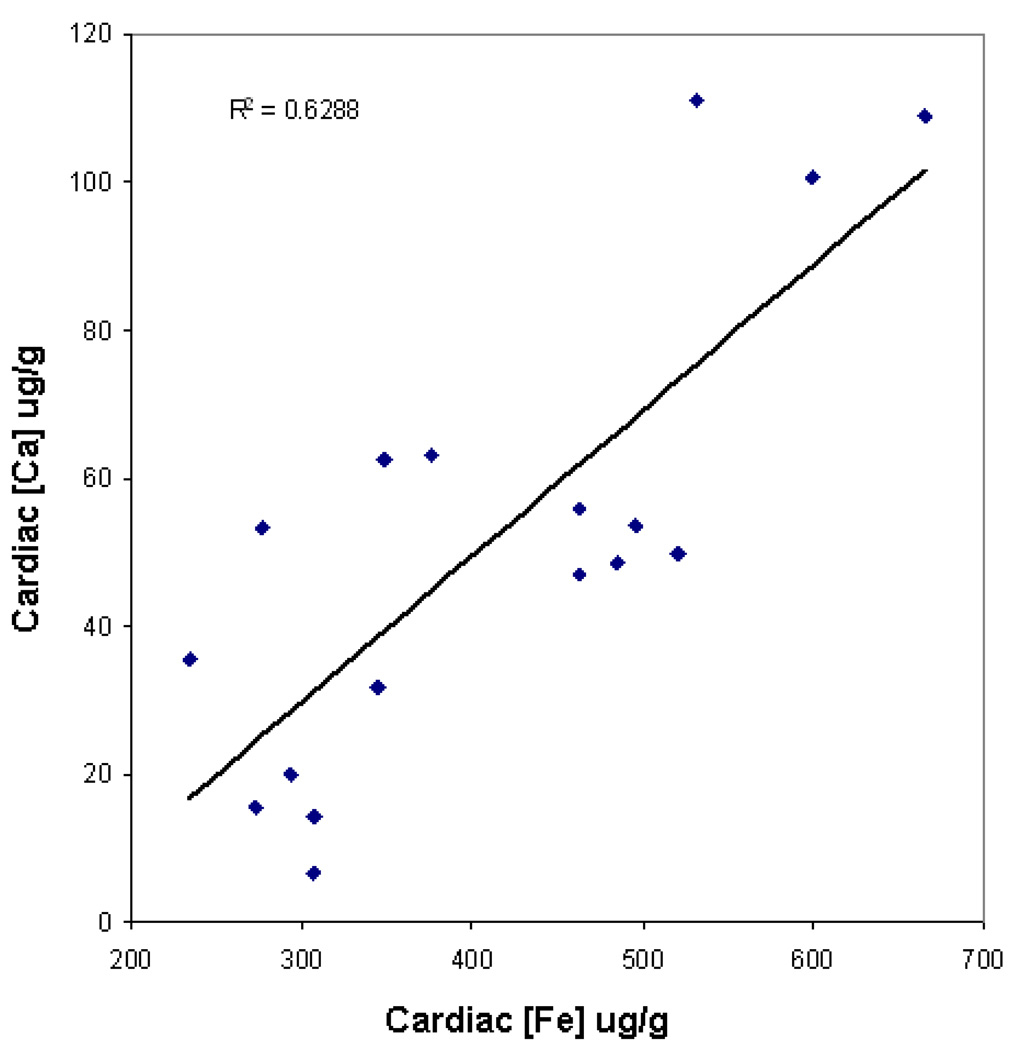
There was no correlation between cardiac iron levels and cardiac manganese levels. Despite this, cardiac iron was tightly correlated with cardiac calcium (R2= 0.63; p<0.0001, Figure 2), suggesting linked transport mechanisms.
Figure 2. Linear relationship between cardiac iron and cardiac calcium concentrations in non-verapamil treated animals.
Iron and calcium levels are reported in units of mg/g wet weight. Cardiac iron and cardiac calcium levels were tightly correlated (R2= 0.63; p<0.0001).
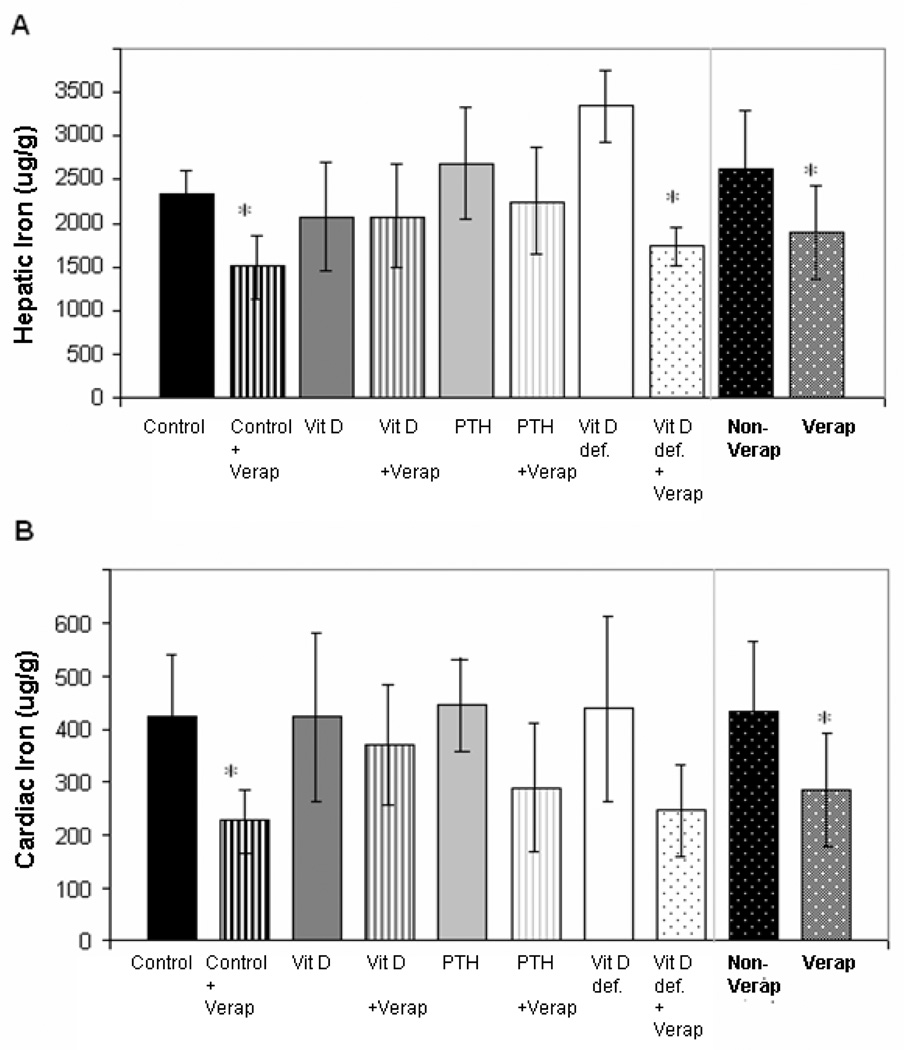
To explore this relationship further, we looked at the effects of verapamil in all four groups (Figure 3). Liver iron was reduced 28% in verapamil alone treated animals (p<0.001) (Figure 3 A). Effect size was largest in the control and vitamin D depleted animals, reaching statistical significance after Bonferroni correction. Cardiac iron levels trended lower after verapamil treatment (p = 0.018 – 0.047, non-significant after Bonferroni correction) in all groups except calcitriol-treated animals. Analysis of variance among the groups (p=0.10) did not reach statistical significance nor did post-hoc analysis for calcitriol supplementation (p=0.10 for Dunnett’s correction). Overall, cardiac iron was reduced 34% in verapamil treated animals. (p=0.011, right hand aspect of Figure 3 B).
Figure 3. Hepatic (top panel) and cardiac (lower panel) iron concentrations following vitamin D axis manipulation in verapamil treated animals.
Liver iron was reduced 28% in verapamil (verap) treated animals compared to non-verapamil (non-verap) treated ones (p<0.001)(right hand aspect of A). Effect size was largest in the control and vitamin D depleted animals, but not different in calcitriol-treated animals (B).Cardiac iron was reduced 34% in verapamil (verap) treated animals compared to non-verapamil (non-verap) treated ones (p=0.011)(right hand aspect of B). Following verapamil treatment, cardiac iron levels trended lower in all groups except calcitriol-treated animals (B). Data is presented as mean ± s.d.
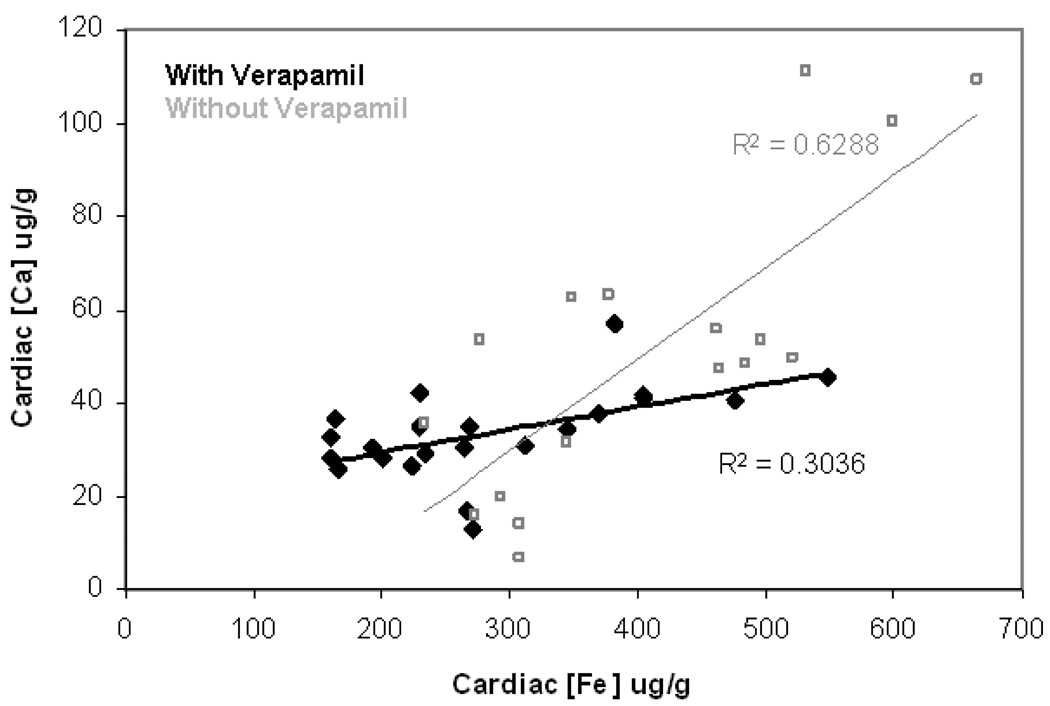
Correlation between cardiac iron and calcium was maintained during LVGCC blockade (Figure 4). Although the association remains statistically significant (R2=0.3, p<0.008), the slope is less steep in the presence of verapamil (filled versus open symbols). Verapamil lowered cardiac calcium levels an average of 35%. Similar to cardiac iron levels, reduction was similar in all treatment groups except calcitriol-treated animals (p = 0.15 by ANOVA).
Figure 4. Correlation between cardiac iron and calcium in verapamil treated animals.
The association between cardiac iron and cardiac calcium concentrations is statistically significant (R2=0.3, p<0.008). The slope is less steep in the presence of verapamil (filled symbols) than without verapamil (open symbols).
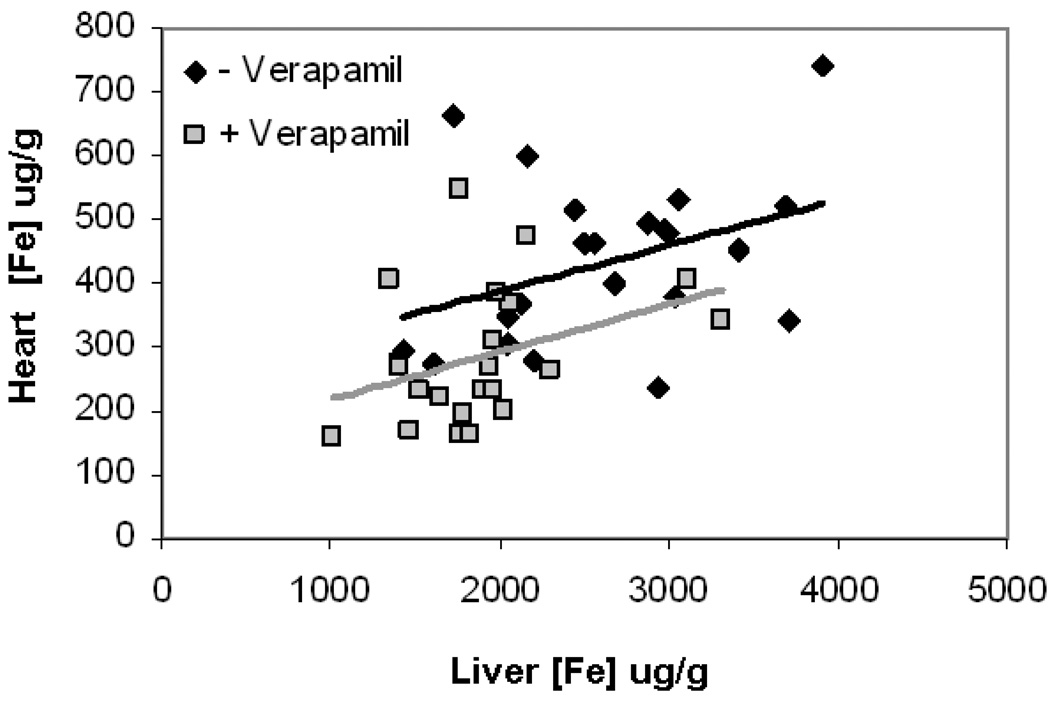
Since verapamil produced similar changes in cardiac and liver iron, one could postulate that verapamil is acting primarily by lowering total body iron stores. Figure 5 demonstrates a scatter gram of cardiac and liver iron in verapamil and non-verapamil treated animals. Linear regression analysis between cardiac and liver iron did not reach statistical significance, with or without verapamil (p=0.09). Slopes of the trends are parallel but the shift in cardiac iron is significantly larger than predicted by the change in liver iron alone. Multivariate testing showed that cardiac calcium is the main predictor for cardiac iron levels for both verapamil and non-verapamil treated animals. This suggests that more than one verapamil-mediated mechanism is involved. There was no strong correlation between liver iron and cardiac iron. The data suggest that in our animal model cardiac calcium and iron are linked.
Figure 5. Cardiac versus hepatic iron concentration with and without verapamil treatment.
Linear regression analysis between cardiac and liver iron did not reach statistical significance, with (gray squares) or without verapamil (black dimonds) (p=0.09). Slopes of the trends are parallel but the shift in cardiac iron is significantly larger than predicted by the change in liver iron alone.
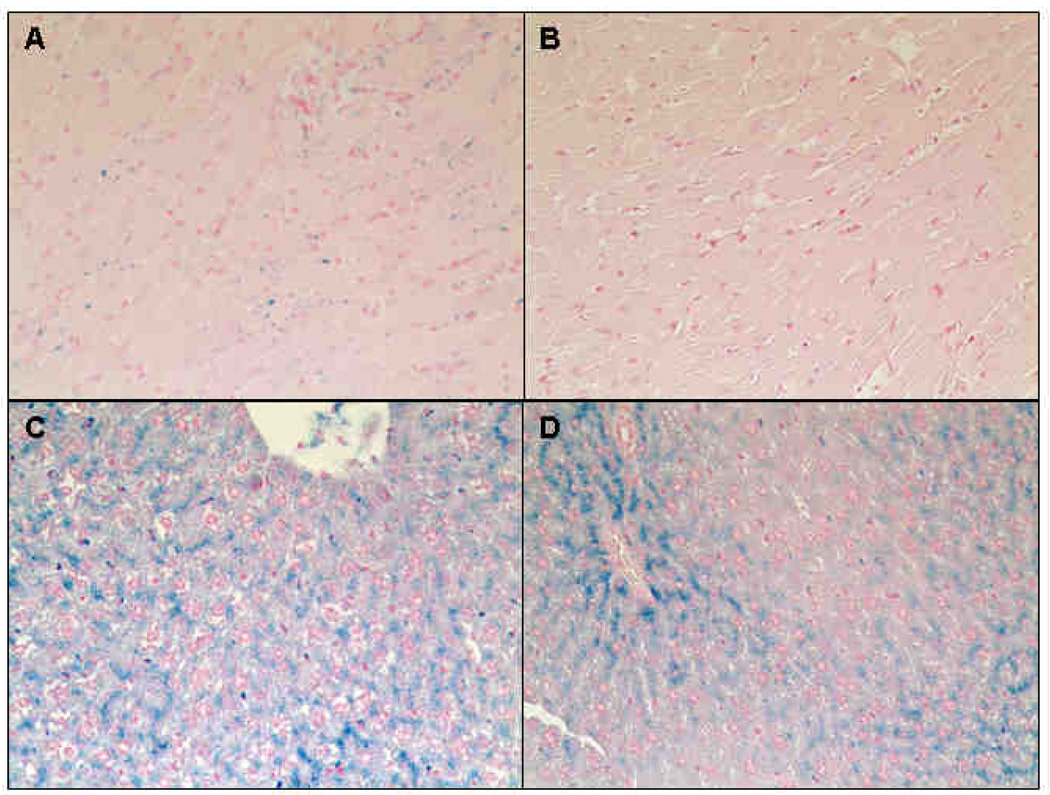
The effects of verapamil on tissue iron distribution are highlighted in Figure 6. A representative Prussian blue stained cardiac section from an untreated animal is shown in Figure 6A. Iron accumulation is exclusively observed in cardiomyocytes, with no evidence of peri-myocyte deposition commonly found in iron dextran animal models. Little stainable iron was evident in control animals treated with verapamil (Fig 6 B). Figure 6 C demonstrates representative liver iron staining for untreated control animals. Hepatic iron is mainly accumulated in the hepatocytes with a higher concentration surrounding the portal vein; no Kupffer cell loading is observed. Verapamil treatment preferentially cleared hepatocytes remote to the portal vein (Fig 6 D).
Figure 6. Representative histological examination of heart and liver following verapamil treatment.
Specimens were stained with Perl’s Prussian blue iron stain (A-D). Cardiac sections from an untreated animal is shown in Figure 6A. Iron was predominantly found in cardiomyocytes. Only minor iron staining was detectable in control animals treated with verapamil (B). In untreated animals, hepatic iron mainly accumulated in parenchyma cells, but not in Kuffper cells (C). Verapamil treatment cleared hepatocytes (D). Original magnifications is x200.
Discussion
The aim of this study was to examine the effects of vitamin D axis perturbation on iron uptake in the heart. Iron-induced cardiomyopathy remains the primary cause of mortality in β-thalassemia major patients [22–23]. Unfortunately, the kinetics of cardiac iron clearance are slow [24] making iron chelation therapy onerous and time-consuming. Patients who are unable to fully comply with therapy, which may take several years, have a high mortality [23]. Thus therapies that might prevent or reduce cardiac iron uptake are of considerable clinical interest. Given the statistical association between vitamin D deficiency and increased cardiac iron-overload in β-thalassemia patients, we wanted to determine whether vitamin D deficiency would prove to be an important and preventable risk factor for iron cardiomyopathy. To test this hypothesis, and explore possible mechanisms, we tried to replicate our observations in humans using an animal model of parenchymal iron overload.
Our data demonstrated that manipulation of the vitamin D axis did not alter cardiac iron levels across all groups. One possible explanation is that our observations in humans were an epiphenomenon, linked to iron cardiomyopathy through other physiologic correlates such as patient age, liver iron concentration, and inflammatory state. Alternatively, no animal model perfectly mimics the human condition. The hemojuvelin knockout condition could have unanticipated interactions with the vitamin D axis, either through the very low circulation hepcidin levels or by other signaling mechanisms. Normally, hemojuvelin is highly expressed in skeletal and cardiac muscles [12, 25], however, our mice have a complete null mutation of hemojuvelin. Therefore, it is possible that cardiac iron loading is regulated differently in our model compared to human patients. However, hemojuvelin’s role in tissue iron uptake and export has not been characterized. Tissue iron homeostasis is primarily modulated at the transcriptional level via iron-responsive elements/proteins (IRE/IRP), but there are many opportunities for modulation by hemojuvelin.). Also we can not exclude that a higher dose of PTH would have worked better. It is possible that the rat data was not a good starting point for our pilot and we perhaps should have given a significantly higher amount of PTH.
Not only was cardiac iron unaffected by vitamin D axis perturbations, but cardiac calcium levels were also unaffected. One possible explanation is that mice may not rely as strongly on vitamin D for calcium homeostasis since they are predominantly nocturnal. Alternatively, factors other than vitamin D status may have dominated in our experimental model. For example, the high iron chow used in these experiments may have interfered with calcium absorption. The global knockout of hemojuvelin may also have unrecognized effects on calcium absorption or regulation. Lastly, the heart may simply have robust calcium homeostasis mechanisms that compensated for differences in systemic calcium levels.
Vitamin D deficiency significantly increased hepatic iron levels. This may be due to increased iron absorption or decreased elimination. This question could be addressed using metabolic cages to quantify urinary and stool iron losses. Alternatively, one could study animals loaded by iron dextran injection because differences in liver iron would solely reflect differences in spontaneous iron elimination rates. Also, since verapamil reduced hepatic iron loading in vitamin D deficient mice, it is conceivable that a calcium dependent mechanism is responsible for increased liver iron accumulation during vitamin D deficiency.
If vitamin D deficiency does increase iron absorption, then vitamin D deficiency might be a treatable risk factor in patients with hyperabsorption syndromes such as thalassemia intermedia and hereditary hemochromatosis. The penetrance of severe iron overload in these disorders is quite variable and it is worth exploring whether vitamin D deficiency exacerbates hyperabsorption. However, further studies need to be done to elucidate the mechanistic link between vitamin D deficiency and hepatic iron overload in our model.
Even though previous studies have demonstrated that manganese is a surrogate for LVGCC activity [19–20], cardiac iron and cardiac manganese were uncorrelated. There are at least four possible explanations, (1) cardiac iron does not travel through LVGCC, (2) acute manganese infusions are not representative of long term LVGCC activity, (3) the specificity of manganese infusions is poorer than suggested by the literature and (4) manganese infusion may be also influenced by alteration in intracellular iron and calcium due to a feedback response on the LVGCC. LVGCC are modulated by the autonomic nervous system, which in turn may be have altered by the stress of animal handling, changes in environmental temperature, and the level of sedation. If these perturbations were large enough, the manganese flux may have borne little relationship to the “average” LVGCC activity over the 6 weeks of iron loading. In fact, since LVGCC are the dominant transmembrane transporter of calcium in the myocytes, one might argue that myocyte calcium levels are the best surrogate for chronic LVGCC activity.
In our study, the 34% reduction of cardiac iron levels with verapamil treatment was accompanied by a 28% reduction of liver iron. While it is tempting to postulate that cardiac iron changes simply reflected lower total body iron stores, the weak association between cardiac and liver iron represented in Figure 5 makes this hypothesis unlikely. As with vitamin D deficiency, verapamil-mediated iron loss could represent decreased iron absorption or increased iron elimination. The latter observation is not without precedent. Ludewiczek et al. reported that calcium channel blockade with nifedipine treatment decreases hepatic iron levels in iron-overloaded mice by prolonging DMT1 opening times [26]; this study prompted a clinical trial (NCT00712738).
In contrast, Oudit et al. demonstrated a 50% reduction in cardiac iron with verapamil treatment, without corresponding liver iron reduction [8]. The most likely explanation for this discrepancy is that Oudit et al used iron dextran injections to load their mice; iron dextran injections favor reticulo-endothelial cell loading while our present model exclusively loads hepatocytes and other parenchymal cells. Ludewiczek et al have shown that calcium channel blockers can affect DMT1 opening time. DMT1 is up regulated in the cell membranes of iron-overloaded hepatocytes, but Kupffer cells lacked this expression [27]. Therefore, verapamil treatment would only alter iron levels in hepatocytes.
Whether iron loads the myocardium through LVGCC remains an open question. In support, cardiac calcium and cardiac iron are closely correlated through a wide range of physiologic perturbations. Direct LVGCC blockage also lowers cardiac iron. However, correlation does not imply causality. Verapamil clearly has unanticipated systemic effects. Blockage of cardiac calcium influx may impair other important calcium-mediated processes in the myocyte such as receptor-mediated and nonspecific endocytosis; the role of these processes in cardiac iron transport has not been characterized. The relationship between cardiac iron and cardiac calcium was also maintained in the presence of LVGCC blockade (albeit a weaker association) suggesting contributions from other divalent transporters.
Since verapamil seems to influence iron transport at multiple levels, an alternative mechanism to probe the relationship between LVGCC activity and cardiac iron is desired. As cardiac calcium flux through LVGCC is modulated by the autonomic nervous system, we postulate that beta-blockers might decrease calcium and iron uptake through voltage gated mechanisms while having little influence on parallel transport mechanisms.
In summary, neither vitamin D deficiency, PTH suprasufficiency, nor calcitriol overdose modulated cardiac iron levels in a juvenile hemochromatosis model. Cardiac iron and cardiac calcium were strongly correlated and verapamil treatment decreased cardiac and liver iron proportionally. Vitamin D deficient animals exhibited more severe hepatic iron loading. Further work will be necessary to probe the mechanisms of these interactions and their translation to humans.
Acknowledgements
Support for this project was provided by the American Heart Association and the National Institute of Health (HL075592-01A1). The authors also gratefully acknowledge Dr. Nathan Dalleska for the assistance with and use of his mass spectrometry facilities and Dr. Nancy Andrews for kindly providing us with Hemojuvelin KO mice.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 2.Tangpricha V, Pearce EN, Chen TC, Holick MF. Vitamin D insufficiency among free-living healthy young adults. Am J Med. 2002;112:659–662. doi: 10.1016/s0002-9343(02)01091-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weber KT, Simpson RU, Carbone LD. Vitamin D and calcium dyshomoeostasis-associated heart failure. Heart. 2008;94:540–541. doi: 10.1136/hrt.2007.126359. [DOI] [PubMed] [Google Scholar]
- 4.Napoli N, Carmina E, Bucchieri S, Sferrazza C, Rini GB, Di Fede G. Low serum levels of 25-hydroxy vitamin D in adults affected by thalassemia major or intermedia. Bone. 2006;38:888–892. doi: 10.1016/j.bone.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 5.Wood JC, Claster S, Carson S, Menteer JD, Hofstra T, Khanna R, et al. Vitamin D deficiency, cardiac iron and cardiac function in thalassaemia major. Br J Haematol. 2008;141:891–894. doi: 10.1111/j.1365-2141.2008.07135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bogin E, Massry SG, Harary I. Effect of parathyroid hormone on rat heart cells. J Clin Invest. 1981;67:1215–1227. doi: 10.1172/JCI110137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rampe D, Lacerda AE, Dage RC, Brown AM. Parathyroid hormone: an endogenous modulator of cardiac calcium channels. Am J Physiol. 1991;261:H1945–H1950. doi: 10.1152/ajpheart.1991.261.6.H1945. [DOI] [PubMed] [Google Scholar]
- 8.De Boland AR, Boland RL. Non-genomic signal transduction pathway of vitamin D in muscle. Cell Signal. 1994;6:717–724. doi: 10.1016/0898-6568(94)00042-5. [DOI] [PubMed] [Google Scholar]
- 9.Oudit GY, Sun H, Trivieri MG, Koch SE, Dawood F, Ackerley C, et al. L-type Ca(2+) channels provide a major pathway for iron entry into cardiomyocytes in iron-overload cardiomyopathy. Nat Med. 2003;9:1187–1194. doi: 10.1038/nm920. [DOI] [PubMed] [Google Scholar]
- 10.Tsushima RG, Wickenden AD, Bouchard RA, Oudit GY, Liu PP, Backx PH. Modulation of iron uptake in heart by L-type Ca2+ channel modifiers: possible implications in iron overload. Circ Res. 1999;84:1302–1309. doi: 10.1161/01.res.84.11.1302. [DOI] [PubMed] [Google Scholar]
- 11.Otto-Duessel M, Aguilar M, Moats R, Wood J. Antioxidant Mediated Effects in a Gerbil Model of Iron Overload. Blood. 2006;108:28b. doi: 10.1159/000109879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang FW, Pinkus JL, Pinkus GS, Fleming MD, Andrews NC. A mouse model of juvenile hemochromatosis. J Clin Invest. 2005;115:2187–2191. doi: 10.1172/JCI25049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Niederkofler V, Salie R, Arber S. Hemojuvelin is essential for dietary iron sensing, and its mutation leads to severe iron overload. J Clin Invest. 2005;115:2180–2186. doi: 10.1172/JCI25683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giulietti A, Gysemans C, Stoffels K, van Etten E, Decallonne B, Overbergh L, et al. Vitamin D deficiency in early life accelerates Type 1 diabetes in non-obese diabetic mice. Diabetologia. 2004;47:451–462. doi: 10.1007/s00125-004-1329-3. [DOI] [PubMed] [Google Scholar]
- 15.Cardus A, Panizo S, Parisi E, Fernandez E, Valdivielso JM. Differential effects of vitamin D analogs on vascular calcification. J Bone Miner Res. 2007;22:860–866. doi: 10.1359/jbmr.070305. [DOI] [PubMed] [Google Scholar]
- 16.Bas A, Lopez I, Perez J, Rodriguez M, Aguilera-Tejero E. Reversibility of calcitriol-induced medial artery calcification in rats with intact renal function. J Bone Miner Res. 2006;21:484–490. doi: 10.1359/JBMR.051211. [DOI] [PubMed] [Google Scholar]
- 17.Berdud I, Martin-Malo A, Almaden Y, Aljama P, Rodriguez M, Felsenfeld AJ. The PTH-calcium relationship during a range of infused PTH doses in the parathyroidectomized rat. Calcif Tissue Int. 1998;62:457–461. doi: 10.1007/s002239900460. [DOI] [PubMed] [Google Scholar]
- 18.Chandra M, Shirani J, Shtutin V, Weiss LM, Factor SM, Petkova SB, et al. Cardioprotective effects of verapamil on myocardial structure and function in a murine model of chronic Trypanosoma cruzi infection (Brazil Strain): an echocardiographic study. International Journal for Parasitology. 2002;32:207–215. doi: 10.1016/s0020-7519(01)00320-4. [DOI] [PubMed] [Google Scholar]
- 19.Hu TC, Bao W, Lenhard SC, Schaeffer TR, Yue T, Willette RN, et al. Simultaneous assessment of left-ventricular infarction size, function and tissue viability in a murine model of myocardial infarction by cardiac manganese-enhanced magnectic resonance imaging (MEMRI) NMR Biomed. 2004;17:620–626. doi: 10.1002/nbm.933. [DOI] [PubMed] [Google Scholar]
- 20.Hu TC, Pautler RG, Macgowan GA, Koretsky AP. Manganese-enhanced mri of mouse heart during changes in inotropy. magnetic resonance in medicine. 2001;46:884–890. doi: 10.1002/mrm.1273. [DOI] [PubMed] [Google Scholar]
- 21.Wendland MF. Applications of manganese-enhanced magnetic resonance imaging (MEMRI) to imaging of the heart. NMR Biomed. 2004;17:581–594. doi: 10.1002/nbm.943. [DOI] [PubMed] [Google Scholar]
- 22.Borgna-Pignatti C, Rugolotto S, De Stefano P, Piga A, Di Gregorio F, Gamberini MR, et al. Survival and disease complications in thalassemia major. Ann N Y Acad Sci. 1998;850:227–231. doi: 10.1111/j.1749-6632.1998.tb10479.x. [DOI] [PubMed] [Google Scholar]
- 23.Davis BA, Porter JB. Results of long term iron chelation treatment with deferoxamine. Adv Exp Med Biol. 2002;509:91–125. doi: 10.1007/978-1-4615-0593-8_6. [DOI] [PubMed] [Google Scholar]
- 24.Anderson LJ, Westwood MA, Holden S, Davis B, Prescott E, Wonke B, et al. Myocardial iron clearance during reversal of siderotic cardiomyopathy with intravenous desferrioxamine: a prospective study using T2* cardiovascular magnetic resonance. Br J Haematol. 2004;127:348–355. doi: 10.1111/j.1365-2141.2004.05202.x. [DOI] [PubMed] [Google Scholar]
- 25.Papanikolaou G, Samuels M, Ludwig Eea. Mutations in HFE2 cause iron overload in chromosome 1q-linked juvenile hemochromatosis. Nature Genetics. 2004;36:77–82. doi: 10.1038/ng1274. [DOI] [PubMed] [Google Scholar]
- 26.Ludwiczek S, Theurl I, Muckenthaler MU, Jakab M, Mair SM, Theurl M, et al. Ca2+ channel blockers reverse iron overload by a new mechanism via divalent metal transporter-1. Nat Med. 2007;13:448–454. doi: 10.1038/nm1542. [DOI] [PubMed] [Google Scholar]
- 27.Trinder D, Oates PS, Thomas C, Sadleir J, Morgan EH. Localisation of divalent metal transporter 1 (DMT1) to the microvillus membrane of rat duodenal enterocytes in iron deficiency, but to hepatocytes in iron overload. Gut. 2000;46:270–276. doi: 10.1136/gut.46.2.270. [DOI] [PMC free article] [PubMed] [Google Scholar]