Abstract
A large proportion of oncology outpatients with bone metastasis report unrelieved pain that significantly interferes with daily functioning and quality of life. However, little is known about the longitudinal pattern of pain intensity and analgesic prescriptions or use. Moreover, despite considerable advantages, the use of sophisticated statistical techniques, such as hierarchical linear modeling (HLM) has not been applied to the study of pain and analgesic outcomes. In a prospective longitudinal study, HLM was used to explore predictors of pain intensity and analgesic prescription and intake at the time of enrollment into the study (intercept) and over the course of 6 weeks (trajectory) in a sample of oncology outpatients with bone metastasis who received standard care for pain. In addition to corroborating known predictors of pain intensity, previously unrecognized variables were found that appear to affect both pain and analgesic outcomes. Importantly, some of the predictors of the trajectories of pain intensity and analgesic use (i.e., pain-related distress and pain management index (PMI) scores) are particularly amenable to interventions. Findings from this study suggest that sophisticated statistical modeling can be used in pain research to identify individual risk factors and propose novel targets that can be used to improve pain management in oncology outpatients with bone metastasis.
Keywords: hierarchical linear modeling, cancer pain, analgesic prescription, analgesic intake, pain management, distress
Introduction
Pain is a significant problem for oncology patients. In fact, the majority of patients with metastatic cancer experience pain or require analgesic medications on a daily basis [5]. While pain can result from a number of causes, bone metastasis is the most common cause of cancer pain, occurring in 60% to 84% of patients [26]. More than 70% of patients with bone metastasis report moderate to severe pain [10; 5; 42] that has a significant impact on their functional status and quality of life [34].
Although metastasis to the bone signals disease progression and is associated with pain, substantial variability exists in the time course of progression and the pain experience, which is not predicted by the magnitude of bone involvement [26]. Therefore, effective pain management of these patients requires a multidisciplinary approach that incorporates both biological and psychosocial processes [10; 27]. The identification of factors that influence this variability and predict the experience of pain across time would provide valuable information allowing for early detection of high-risk individuals, and potentially leading to novel therapeutic approaches to pain prevention and management, as well as advocating for individually tailored treatment.
A consistent theme in the cancer pain literature is inadequate pain management. In fact, more than a third of patients with moderate to severe pain are prescribed weak or no analgesics at all [12; 42]. The Pain Management Index (PMI) provides a method of quantifying and evaluating pain treatment, by comparing a patient's worst pain rating with the most potent analgesic prescribed. Negative scores denote inadequate treatment, while positive scores conservatively indicate adequate pain treatment [4]. Using this measure, 14% to 45% of outpatients with metastatic cancer had inadequate pain management [30; 5]. This proportion has steadily increased [30] despite efforts to implement international pain management guidelines [41]. Although undertreatment of pain is widely reported in the oncology field, a paucity of research exists on how analgesic prescriptions and analgesic intake change over time. Studies by our group found that adherence rates to overall analgesic regimens was 41% to 55% [29; 39] identifying lack of adherence as an important factor that contributes to inadequate pain management in oncology patients. Additionally, pain levels tend to vary over time [26; 14]. However, the identification of individual characteristics that predict the trajectories of analgesic prescriptions and intake remains largely unexplored.
In this descriptive longitudinal study, oncology outpatients with bone metastasis who were receiving standard care for pain management were followed for 6 weeks in order to determine the trajectories of pain ratings (average and worst) and analgesic prescription and intake (i.e., Medication Quantification Scores (MQS) prescribed and MQS taken [25], respectively). The purposes of this study were to determine whether pain intensity and MQS scores changed over time, and to determine whether these outcome measures displayed specific trajectories that were predicted by an individual's demographic or clinical characteristics. Hierarchical linear modeling (HLM), which has considerable advantages over the standard repeated measures analysis of variance (ANOVA) in terms of its flexibility and specificity, was used to model individual change across time [3; 8].
Methods
Patient Population
This descriptive, longitudinal study was part of a randomized clinical trial (RCT) that evaluated the effects of a psychoeducational intervention on cancer pain management [28]. For the purposes of this study, the analyses were limited to participants who received standard care for pain management, in an effort to depict the “natural” trajectories of pain and analgesic prescriptions and intake over time. Participants were 88 oncology outpatients, who were experiencing pain from bone metastasis. Patients were recruited from seven outpatient settings in Northern California: a university-based cancer center, two community based oncology practices, one health maintenance organization, one outpatient radiation therapy center, one veteran's affairs facility, and one military hospital. All participants were adult oncology outpatients (>18) who were able to read, write, and understand English. All had Karnofsky Performance Status (KPS) scores [19] of ≥50, average pain intensity scores of ≥2.5 on a 0 to 10 numeric rating scale (NRS), and radiographic evidence of bone metastasis. The study was approved by the Human Subjects Committee at the University of California San Francisco, and at each of the study sites. All patients signed a written informed consent.
Procedure
Study procedures are described in detail elsewhere [40]. Briefly, patients were approached in the outpatient setting by a recruitment nurse who explained the study and obtained written informed consent. Patients were then randomly assigned to either the standard care or intervention group. At the time of enrollment, patients completed a demographic questionnaire, the KPS, and 1 week prior to the first study visit patients rated their level of pain intensity on a daily basis. At the beginning and end of the study, patients’ medical records were reviewed. Patients in the standard care arm received the patient version of the Cancer Pain Guideline published by the Agency for Health Care Policy and Research (AHCPR) [17], and were seen by a research nurse in their homes at weeks 1, 3, and 6. Telephone interviews were conducted at weeks 2, 4, and 5. The focus of the visits and phone calls was on monitoring patients’ level of adherence with completion of a pain management diary. Using the pain management diary, patients were asked to report on their pain experience and medication intake, on a nightly basis. A separate analysis of this dataset revealed a 98% adherence rate for completion of the pain management diary [29].
At the initiation of the study, patients completed the Pain Experience Scale (PES), which consists of 9 (10 mm) visual analog scales that evaluated knowledge about pain and its management and 4 scales that evaluated various aspects of pain perception. Items that assessed pain perception included current pain, satisfaction with pain relief, and how upsetting the pain was to the patient and his or her family caregiver (i.e., pain-related distress) from “none” to “a great deal”[9]. At enrollment, patients were asked to report the number of hours per day their cancer-pain currently lasted (range 0 to 24 hours). Measures of “distress” and “hours per day in pain” at the time of enrollment were used for the purposes of these analyses.
The nurses assigned to the patients in the standard care group were a different group of nurses than those who administered the intervention. These nurses received training in how to obtain informed consent and how to teach the patients to complete the study questionnaires. The patients in the standard care group did not receive any intervention. The fidelity of this portion of the study was monitored by having all of the sessions between the nurses and the patients in the standard care group tape recorded and evaluated by the principal investigator and project director on an ongoing basis.
Outcome Measures
The primary outcome measures for this study were pain intensity ratings (i.e., average and worst pain) and quantity of analgesic medications prescribed and taken. To obtain measures of pain intensity, patients reported average and worst pain intensity at enrollment and nightly throughout the 6 week study period using a descriptive NRS that ranged from 0 (“no pain”) to 10 (“worst pain imaginable”). To determine prescription and intake of analgesic medications, the research nurse recorded the name, dose, and administration schedule for all of the prescribed and over-the-counter pain medications. Then, on a daily basis, patients recorded the time and amount of opioid, nonopioid, and coanalgesic medications taken on an around-the-clock (ATC) and on an as-needed (i.e., PRN) basis. If a change in the analgesic prescription occurred, patients were instructed to make the change in their diary. The research nurse verified the patients’ current pain medication regimen at each study visit and checked diary entries for completeness.
From this diary information, Medication Quantification Scale (MQS) scores for prescribed and taken nonopioid, opioid, and adjuvant analgesics medications were calculated using the method described by Masters Steedman et al.[25]. The MQS provides a method of quantifying analgesic use by multiplying detriment weights applied to each medication by the medication dosage level. Individual scores for each medication were summed to create a quantitative index of total analgesic medications prescribed (MQS prescribed) and taken (MQS taken), that were amenable to statistical analyses.
Data Analysis
Descriptive statistics and frequency distributions were generated on the sample characteristics using SPSS™ Version 18.0. HLM, based on full maximum likelihood estimation, was done using the software developed by Raudenbush and colleagues [32]. The repeated measures of pain and MQS scores were conceptualized as being nested within individuals. Compared with other methods of analyzing change, HLM has two major advantages. First, HLM can accommodate unbalanced designs which allows for the analysis of data when the number and the spacing of the assessments vary across respondents. Although every patient was to be assessed on a pre-specified schedule, the actual number of assessments was not necessarily the same for all of the patients due to simple scheduling conflicts or the wellness of the participant. Second, HLM has the ability to model individual change, which helps to more accurately identify complex patterns of change that are often averaged and therefore overlooked by other methods [33; 32].
With HLM, the repeated measures of the outcome variables (i.e., pain and MQS) are nested within individuals and the analysis of change in pain and MQS scores has two levels: within persons (intra-individual; Level 1) and between persons (inter-individual; Level 2). At Level 1, the outcome is conceptualized as varying within individuals and is a function of person-specific change parameters plus error. At Level 2, these person-specific change parameters are multivariate outcomes that vary across individuals. These Level 2 outcomes can be modeled as a function of demographic or clinical characteristics that vary between individuals, plus an error associated with the individual. Combining Level 1 with Level 2 results in a mixed model with fixed and random effects [32; 24; 23].
Four separate HLM analyses were done to evaluate changes over time in average pain, worst pain, MQS-prescribed, and MQS-taken scores. Each HLM analysis proceeded in two stages. At Level 1, intra-individual variability in the outcome measure over time was examined. Time in days, refers to the length of time from the enrollment visit to completion of the study six weeks later (i.e., 42 days). Three Level 1 models, which represented that the patients’ pain or MQS levels (a) did not change over time (i.e., no time effect), (b) changed at a constant rate (i.e., linear time effect), and (c) changed at a rate that accelerates or decelerates over time (i.e., quadratic effect) were compared. At this point, the Level 2 model was constrained to be unconditional (i.e., no predictors) and likelihood ratio tests were used to determine the best model. These analyses answered the first research aim and identified the change parameters that best described individual changes in the outcome measures over time.
Level 2 of the HLM analysis, which addressed the second research aim, examined inter-individual differences in the trajectories of the outcome measures by modeling the individual change parameters (i.e., intercept and linear slopes) as a function of proposed predictors.. These exploratory analyses were conducted using the potential predictors listed in Tables 1 and 2. For this type of analysis, intercepts and slopes vary across individuals and are therefore random effects at Level 2. An unstructured covariance matrix was used for the random intercepts and slopes. To improve estimation efficiency and construct a model that was parsimonious, an exploratory Level 2 analysis was done in which each potential predictor was assessed to see if it would result in a better fitting model if it alone was added as a Level 2 predictor. Predictors with a t-value of < 2.0, which indicates a lack of a significant effect, were dropped from subsequent model testing [32]. All of the potentially significant predictors from the exploratory analyses were entered into the model to predict each individual change parameter. Only predictors that maintained a significant contribution in conjunction with other variables were retained in the final model. A p-value of < 0.05 indicates statistical significance. Readers are referred to a few basic references additional information on the use of HLM for longitudinal research[36; 16].
Table 1.
Variables identified from exploratory analyses as potential predictors of average and worst pain, based on t-values ≥ |2.00|, indicated by filled boxes (■) for standard care group.
| Variable | Average Pain | Worst Pain | ||
|---|---|---|---|---|
| | ||||
| Intercept | Trajectory | Intercept | Trajectory | |
| Demographic/Clinical | ||||
| Gender | ||||
| Ethnicity | ||||
| Age | ||||
| Living Alone | ||||
| Married/Partnered | ||||
| Education | ■ | |||
| Employment | ||||
| Karnofsky Performance | ||||
| Pain Characteristics | ||||
| Pain duration | ■ | |||
| Baseline average daily pain | ||||
| Baseline pain at its worst | ||||
| Baseline pain at its least | ||||
| Current average daily pain | ||||
| Current pain at its worst | ||||
| Current pain at its least | ||||
| Hours of day pain lasts | ■ | ■ | ||
| % Pain relief in last week | ■ | |||
| PMI Score | ■ | ■ | ||
| Pain upsetting to patient (Distress) | ■ | ■ | ■ | ■ |
| Pain upsetting to caregiver | ■ | ■ | ||
| Pain knowledge at baseline | ■ | ■ | ||
| Change in knowledge | ||||
| Confident to manage pain | ||||
| Motivated to take meds | ||||
| Satisfied with relief | ■ | |||
| Associated Symptoms | ||||
| Tension | ||||
| Depression | ||||
| Anger | ||||
| Vigor | ||||
| Fatigue | ||||
| Confusion | ||||
| Total mood disturbance | ||||
Table 2.
Variables identified from exploratory analyses as potential predictors of pain medications prescribed and taken, based on t-values ≥ |2.00|, indicated by filled boxes (■) for standard care group.
| Variable | Prescribed | Taken | ||
|---|---|---|---|---|
| | ||||
| Intercept | Linear | Intercept | Linear | |
| Demographic/Clinical | ||||
| Gender | ||||
| Ethnicity | ||||
| Age | ||||
| Living Alone | ||||
| Married/Partnered | ||||
| Education | ||||
| Employment | ||||
| Karnofsky Performance | ■ | |||
| Pain Characteristics | ||||
| Pain duration | ||||
| Baseline average daily pain | ||||
| Baseline pain at its worst | ||||
| Baseline pain at its least | ||||
| Current average daily pain | ||||
| Current pain at its worst | ||||
| Current pain at its least | ||||
| Hours of day pain lasts | ||||
| % Pain relief in last week | ||||
| PMI Score | ■ | |||
| Pain upsetting to patient (Distress) | ■ | |||
| Pain upsetting to caregiver | ||||
| Pain knowledge at baseline | ■ | ■ | ||
| Change in knowledge | ■ | |||
| Confident to manage pain | ||||
| Motivated to take meds | ||||
| Satisfied with relief | ||||
| Associated Symptoms | ||||
| Tension | ■ | |||
| Depression | ■ | |||
| Anger | ■ | |||
| Vigor | ||||
| Fatigue | ||||
| Confusion | ||||
| Total mood disturbance | ■ | |||
| SF-36 Mental Component | ■ | |||
Results
Patient Characteristics
The demographic, disease, and treatment characteristics of the 88 patients are presented in Table 3. Patients were approximately 59 years old, primarily female (74%), white (89%), well-educated (14.7 years), and had a mean KPS score of 71.5 (±12.06).
Table 3.
Demographic and disease characteristics of the patients (N = 88)
| Characteristic | Mean (SD) |
|---|---|
| Age (years) | 59.1 (13.1) |
| Education (years) | 14.7 (3.3) |
| KPS | 71.5 (12.1) |
| Sex | % |
| Male | 26.1 |
| Female | 73.9 |
| Lives Alone | 20.5 |
| Marital Status | |
| Married/partnered | 65.9 |
| Other | 34.1 |
| Ethnicity | |
| White | 88.6 |
| Other | 11.4 |
| Employment | |
| Employed | 25.0 |
| Other | 75.0 |
| Diagnosis | |
| Breast | 51.7 |
| Prostate | 12.6 |
| Lung | 14.9 |
| Other | 20.6 |
| Current Therapy | |
| Chemotherapy | 44.8 |
| Hormonal therapy | 31.0 |
| Radiation therapy | 14.9 |
| Biotherapy | 1.1 |
| No treatment | 15.3 |
Individual and Mean Change in Outcome Measures
The Level 1 HLM analyses examined how each of the outcome measures (i.e., average and worst pain, and MQS-prescribed and taken) changed over the course of the 6 week study period. Two models were estimated in which the function of time was linear and quadratic. Because the final estimate of fixed effects revealed that quadratic terms had no significant effect, all outcome measures were fit to a linear model. It should be noted that the mean scores for the various groups depicted in all of the figures are estimated or predicted means based on the HLM analyses.
Average Pain
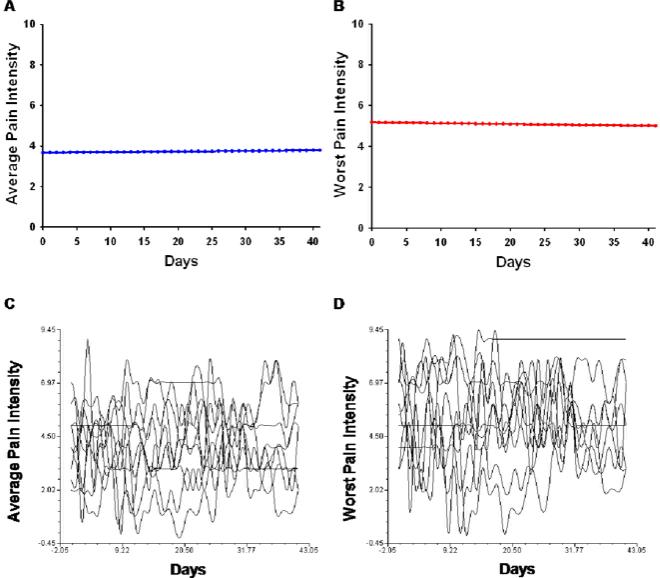
The estimates of the linear change model are presented in Table 4 (unconditional model). Because the model has no covariates (i.e., unconditional), the intercept represents the estimated amount of average pain (i.e., 3.67 on a 0 to 10 scale) at the time of the initial visit. The estimated linear rate of change in average pain was 0.003 (ns). Figure 1A displays the trajectory for average pain over the course of 6 weeks. According to the unconditional model, although pain persisted throughout the study, ratings of average pain did not change significantly across the study period.
Table 4.
Hierarchical linear models of average and worst pain scores.
| Average Pain | Coefficient (SE) | |
|---|---|---|
| Variable | Unconditional Model | Final Model |
| Fixed Effects | ||
| Intercept | 3.674 (0.176)b | 3.675 (0.128)b |
| Timea (linear rate of change) | 0.003 (0.005) | 0.003 (0.005) |
| Time invariant covariates | ||
| Intercept | ||
| Education | -0.109 (0.037)b | |
| Distress | 0.214 (0.043)b | |
| Hours per day in pain | 0.059 (0.017)b | |
| Satisfaction with pain relief | -0.128 (0.050)c | |
| PMI | -0.536 (0.135)b | |
| Linear | ||
| Distress × time | -0.004 (0.002)c | |
| Hours per day in pain × time | -0.002 (0.001)c | |
| Baseline average pain × time | 0.005 (0.003) | |
| Variance Components | ||
| In intercept | 2.603b | 1.318b |
| In linear rate | 0.002b | 0.002b |
| Goodness-of-fit deviance (parameters estimated) | 11569.628 (6) | 11507.978 (14) |
| Model Comparison (χ2 [df]) | 61.650 (8)c | |
| Worst Pain | Coefficient (SE) | |
|---|---|---|
| Variable | Unconditional Model | Final Model |
| Fixed Effects | ||
| Intercept | 5.175 (0.220)b | 5.177 (0.166)b |
| Timea (linear rate of change) | -0.004 (0.006) | -0.004 (0.006) |
| Time invariant covariates | ||
| Intercept | ||
| Distress | 0.313 (0.055)b | |
| Pain knowledge | -0.030 (0.010)c | |
| Total time in pain | 0.356 (0.119)c | |
| PMI | -0.579 (0.165)b | |
| Linear | ||
| Distress × time | -0.006 (0.002)c | |
| Baseline worst pain × time | 0.004 (0.003) | |
| Variance Components | ||
| In intercept | 4.097b | 2.249b |
| In linear rate | 0.003b | 0.003b |
| Goodness-of-fit deviance (parameters estimated) | 12554.329 (6) | 12497.442 (12) |
| Model Comparison (χ2 [df]) | 56.887 (6)c | |
Time was coded 0 at the time of the initial visit.
p < 0.001
p < 0.05
Abbreviation: PMI = Pain Medication Index
Figure 1.
Trajectories of average (A) and worst (B) pain scores using an unconditional model. A random selection (N = 10) of individual trajectories of average (C) and worst (D) pain scores across the 6 week study period.
Worst Pain
As shown in Table 4, in the unconditional model, the intercept represents the estimated amount of worst pain (i.e., 5.18) at the time of the initial visit. The estimated linear rate of change in worst pain was -0.004 (ns). Figure 1B displays the trajectory for worst pain over the course of 6 weeks. According to the unconditional model, although pain persisted throughout the study, worst pain ratings did not change significantly over time.
MQS Prescribed
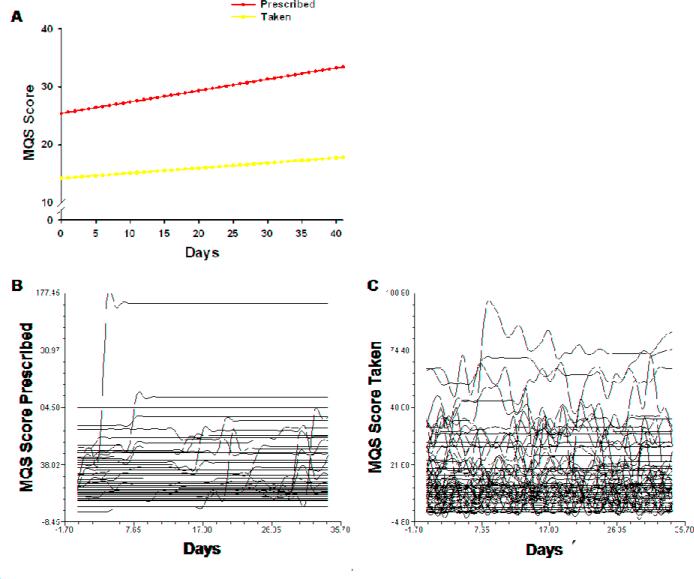
The estimates of the linear change model are presented in Table 5 (unconditional model). Because the model has no covariates (i.e., unconditional), the intercept represents the estimated MQS score for prescribed analgesics (i.e., 25.537) at the time of the initial study visit. The estimated linear rate of change in MQS-prescribed was 0.196 (p < 0.001). Figure 1C displays the trajectory for MQS-prescribed over the course of 6 weeks. According to the unconditional model, MQS scores for analgesic prescriptions increased steadily over time.
Table 5.
Hierarchical linear models of the quantity of analgesic medications prescribed and taken.
| Medications Prescribed | Coefficient (SE) | |
|---|---|---|
| Variable | Unconditional Model | Final Model |
| Fixed Effects | ||
| Intercept | 25.537 (2.448)b | 25.419 (2.107)b |
| Timea (linear rate of change) | 0.196 (0.047)b | 0.196 (0.046)b |
| Time invariant covariates | ||
| Intercept | ||
| Age | -0.447 (0.160)c | |
| Baseline Karnofsky score | -0.672 (0.175)b | |
| Distress | 1.96 (0.695)c | |
| Linear | ||
| Baseline worst pain × time | 0.042 (0.019)c | |
| Variance Components | ||
| In intercept | 511.799b | 378.308b |
| In linear rate | 0179b | 0.169b |
| Goodness-of-fit deviance (parameters estimated) | 18696.642 (6) | 18666.397 (10) |
| Model Comparison (χ2 [df]) | 30.245 (4)b | |
| Medications Taken | Coefficient (SE) | |
|---|---|---|
| Variable | Unconditional Model | Final Model |
| Fixed Effects | ||
| Intercept | 14.221 (1.581)b | 14.088 (1.464)b |
| Timea (linear rate of change) | 0.088 (0.036)b | 0.216 (0.067)b |
| Time invariant covariates | ||
| Intercept | ||
| Distress | 1.337 (0.480)b | |
| Hours per day in pain | 0.530 (0.223)b | |
| Linear | ||
| Gender × time | -0.174 (0.078)b | |
| Variance Components | ||
| In intercept | 212.936b | 182.048b |
| In linear rate | 0.101b | 0.095b |
| Goodness-of-fit deviance (parameters estimated) | 17259.659 (6) | 17242.047 (9) |
| Model Comparison (χ2 [df]) | 17.612 (3)b | |
Time was coded 0 at the time of the initial visit.
p < 0.001
p < 0.05
MQS Taken
As shown in Table 5, in the unconditional model, the intercept represents the estimated MQS score for consumed analgesic medication (i.e., 14.221) at the time of the initial visit. The estimated linear rate of change in MQS-taken was 0.088 (p < 0.05). Figure 2A displays the trajectory for MQS-taken the over the course of the 6 weeks. According to the unconditional model, MQS scores for analgesic intake increased steadily over time, but with a slope about half of that of analgesics prescribed.
Figure 2.
Trajectories of the quantity of medications prescribed and taken using an unconditional model. The entire sample (N = 88) of individual trajectories of analgesics prescribed (B) and taken (C) across the 6 week study period.
Although the results indicate a sample-wide lack of change in average and worst pain ratings, and a sample-wide increase in MQS prescribed and taken scores over time, they do not imply that all patients exhibited the same trajectories. The variance in individual change parameters estimated by the models (see “Variance Components”, Tables 4 and 5) suggested substantial inter-individual differences in the intercept and trajectories of the outcome measures. Spaghetti plots, that were used to visualize individual trajectories of random subsets of patients (Fig. 1C, 1D, 2B, and 2C), further demonstrated significant variability in pain and analgesic outcomes. Therefore, further examination of inter-individual differences in the individual change parameters was warranted.
Inter-individual Differences in the Trajectories of Outcome Measures
The Level 2 HLM analyses tested the hypothesis that the trajectories of the outcome measures varied based on specific person, disease, treatment, and/or symptom variables that were found to influence the various outcome measures (Tables 1 and 2). To improve estimation efficiency and construct models that were parsimonious, exploratory Level 2 analyses were done in which each potential predictor was assessed to see if it would result in a better fitting model if it alone was added as a Level 2 predictor. Predictors with a t-value of < 2.0, indicating lack of a significant effect, were dropped from subsequent model testing. All of the significant predictors from the exploratory analyses (marked in Tables 1 and 2 by filled squares) were entered into the models to predict each individual change parameter. Only predictors that maintained a significant contribution in conjunction with other variables were retained in the final models. For all outcome measures, differences were calculated based on 1 standard deviation (SD) above (e.g., high education) and below (e.g., low education) the mean score for that predictor variable.
Average Pain
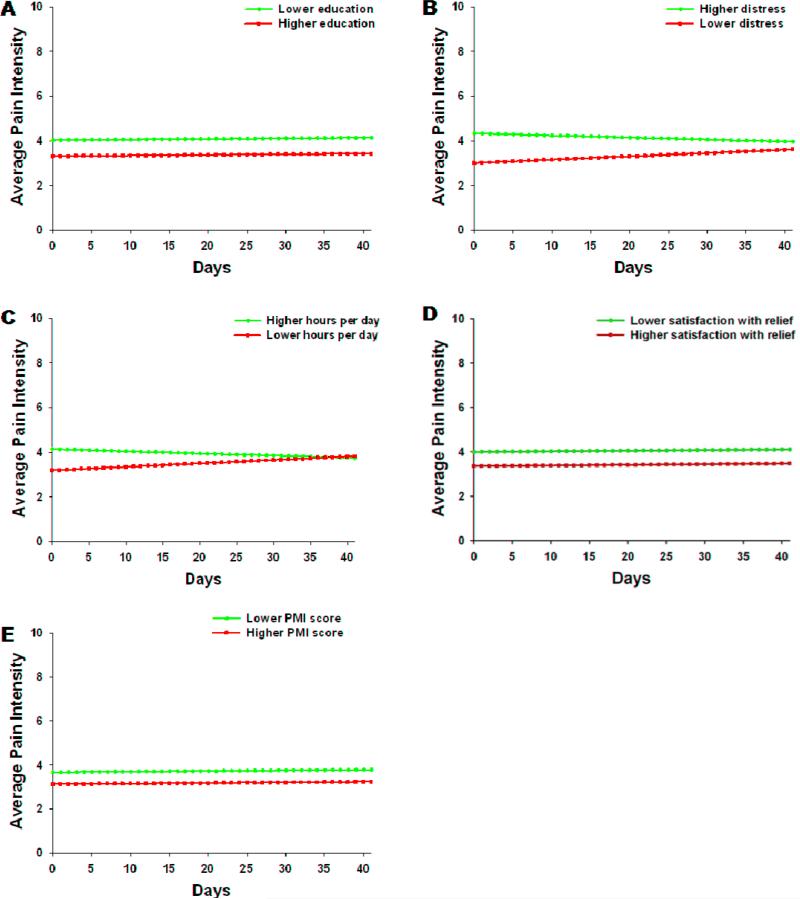
As shown in the final model in Table 4, five variables predicted inter-individual differences in the intercept for average pain: education, distress, hours per day in pain, satisfaction with pain relief, and PMI scores. The variables that predicted inter-individual differences in the slope parameters for average pain were distress and hours per day in pain. Although not a significant predictor, baseline average pain was entered into the final model in order to account for differences in average pain ratings at baseline, such that individual differences in trajectories would not be influenced by individual differences in baseline average pain ratings.
To illustrate the effects of each of these predictors on patients’ trajectories of average pain, Figure 3 displays the adjusted change curves for average pain that were estimated based on differences in the significant predictors reported above. In brief, patients with decreasing levels of education, higher levels of distress, higher number of hours per day in pain, lower satisfaction with relief, and lower PMI scores reported increased levels of average pain at baseline. Patients with higher distress and higher number of hours per day in pain reported decreases in average pain scores over the 6 weeks of the study.
Figure 3.
Trajectories of average pain scores by (A) education, (B) distress, (C) hours per day in pain, (D) satisfaction with pain relief, and (E) PMI score at the time of enrollment. Higher/lower differences were calculated based on 1 standard deviation above/below the mean.
Worst Pain
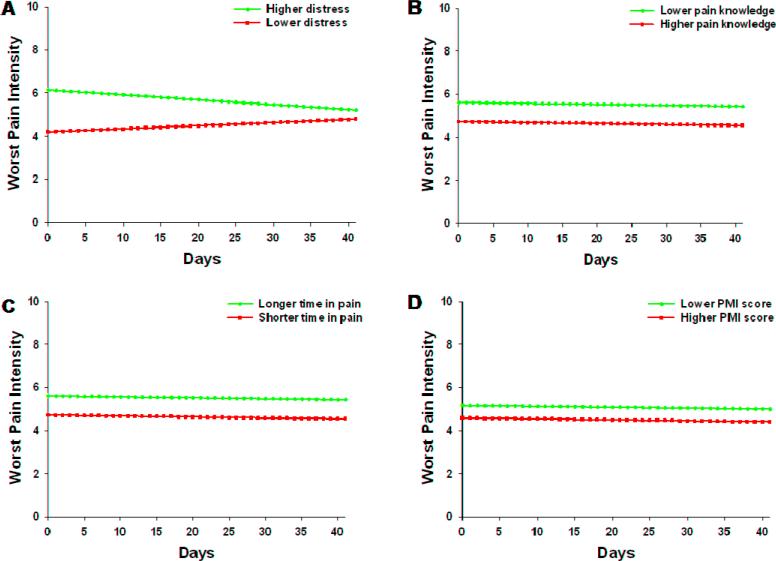
As shown in the final model in Table 4, the four variables that predicted inter-individual differences in the intercept for worst pain were distress, pain knowledge at baseline, total time in pain, and PMI scores. The variable that predicted inter-individual differences in the slope parameters for worst pain was distress at baseline. As was done for average pain, baseline worst pain was entered into the final model in order to account for differences in worst pain at baseline.
Figure 4 displays the adjusted change curves for worst pain that were estimated based on differences in the significant predictors reported above. Patients with greater distress, lower pain knowledge, longer total time in pain, and lower PMI scores reported relatively higher levels of worst pain at baseline. Those patients with higher levels of baseline distress reported decreases in worst pain over time.
Figure 4.
Trajectories of worst pain scores by distress (A), pain knowledge (B), total time in pain (C), and PMI score (D) at the time of enrollment. Higher/lower differences were calculated based on 1 standard deviation above/below the mean.
MQS Prescribed
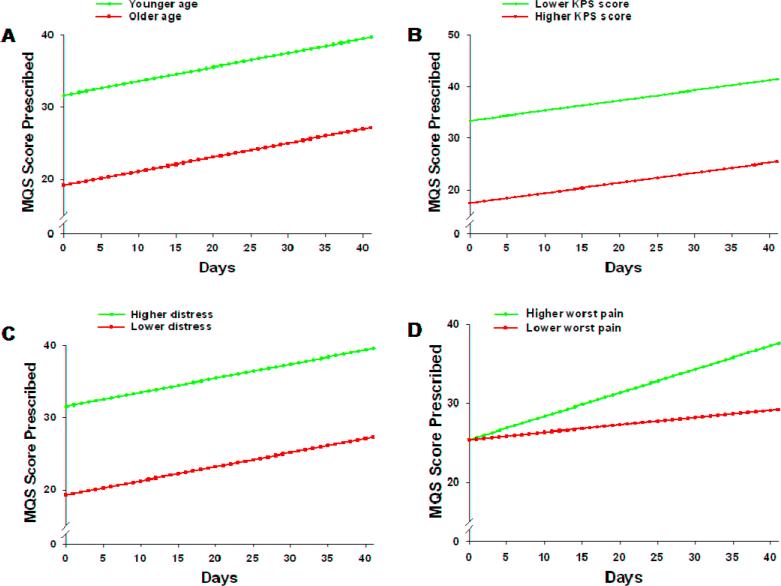
As shown in the final model in Table 5, the three variables that predicted inter-individual differences in the intercept for prescribed medication scores were age, KPS, and distress. The variable that predicted inter-individual differences in the slope parameter was worst pain at baseline. Figure 5 displays the adjusted change curves that were estimated based on differences in the significant predictors reported above. Participants who were younger, had lower KPS scores, and higher levels of distress had higher MQS scores at baseline, which increased steadily over time. Patients with higher ratings of worst pain at baseline displayed a steeper trajectory of increasing MQS scores for prescribed medications over time.
Figure 5.
Trajectories of MQS scores for analgesics prescribed by age (A), KPS score (B), distress (C), and worst pain (D) at the time of enrollment. Higher/lower differences were calculated based on 1 standard deviation above/below the mean.
MQS Taken
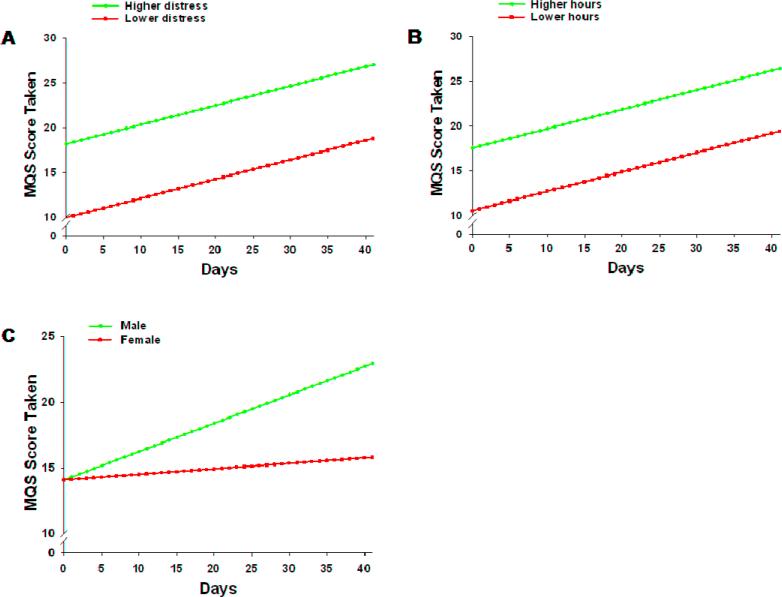
As shown in the final model in Table 5, the two variables that predicted inter-individual differences in the intercept for medication scores taken were distress and hours per day in pain. The variable that predicted inter-individual differences in the slope parameter was gender. Figure 6 displays the adjusted change curves that were estimated based on differences in these predictors. Participants with higher levels of distress and higher number of hours per day in pain consumed more analgesic medication at baseline, which increased steadily over time. Despite no initial baseline differences, male patients displayed an increase in analgesic consumption over time, while female patients did not change over time.
Figure 6.
Trajectories of MQS scores for consumed analgesics by distress (A), hours per day in pain (B), and gender (C). Higher/lower differences were calculated based on 1 standard deviation above/below the mean.
Discussion
This study is the first to use HLM to model individual trajectories in pain and analgesic outcomes. While initial analyses found that oncology outpatients with metastatic bone pain experienced no sample-wide changes in pain intensity, variance components suggested substantial inter-individual variability in average and worst pain, which allowed for testing of predictors. In contrast, quantity of analgesic medications prescribed and taken exhibited sample-wide increases over the course of the study, and testing of predictors improved both models.
Changes in Pain Intensity
While increases in average and worst pain are expected in the setting of bone metastasis [26; 14], both pain intensity ratings remained at approximately 3.7 and 5.2 across 6 weeks. The lack of increase in pain intensity scores may be related to the relatively short study duration. Alternatively, patients may have benefited from keeping a daily pain diary [35], which may have modified pain management strategies (as indicated by increasing analgesic prescriptions and intake), thus preventing pain intensity scores from increasing [28]. Finally, substantial individual variability in these patients’ experience of pain was revealed using the more complex HLM analyses.
It is not surprising that a somewhat distinct set of predictors were identified for average and worst pain, as they reflect different dimensions of the pain experience and have differential effects on function and quality of life [15]. Distinct predictors of average pain scores at enrollment included education, hours per day in pain, and satisfaction with pain relief. In a large community sample, education strongly predicted average pain in a similar direction, with lower levels of education associated with higher average pain scores [21]. Because average pain reflects pain experienced throughout the day, it is not surprising that patients who reported pain for longer periods on a daily basis reported higher scores. Nor is it surprising that patients who were more satisfied with pain relief reported lower scores at enrollment as it suggests their pain was more effectively managed. However, this finding contrasts with previous studies that reported high satisfaction scores in paitens with severe pain [6; 7; 18]. Hours per day in pain also predicted the trajectory of average pain ratings, such that patients who initially experienced more hours per day in pain reported a slight reduction in average pain scores, while patients who experienced fewer hours of pain per day reported a slight increase in average pain scores over 6 weeks. Although interesting, these trends were modest and likely not clinically meaningful.
Distinct predictors of higher worst pain scores at enrollment included lower pain-related knowledge scores and longer total time in pain. Knowledge about pain and analgesia is a factor ripe for intervention. In fact, participants in the psychoeducational intervention arm of this study showed a significant increase in pain knowledge [20] and a corresponding reduction in worst pain intensity over the course of the study [28]. In terms of total time in pain, over 55% of the patients in this study were in pain for over 7 months [28]. These patients’ higher worst pain scores may reflect more severe disease and/or tolerance to analgesic medications. This finding warrants further investigation.
Two variables significantly predicted both average and worst pain scores at enrollment: PMI scores (-0.55 ± 0.909) and pain-related distress (6.416 ± 3.131). Not surprisingly, lower PMI scores (indicative of inadequate pain management) were associated with increased pain intensity ratings at enrollment. This finding corroborates research detailing the undertreatment of cancer pain and further highlights the importance of prescribing appropriate analgesic medications based on pain severity ratings [1; 27]. Of note, this factor is amenable to intervention, as suggested by current cancer pain guidelines that emphasize the titration of analgesic medications based on pain severity [13].
Patients with greater levels of distress reported significantly higher average and worst pain scores than patients with lower levels of distress at enrollment. This finding stresses the important relationship between psychological processes and pain, and lends credence to a biopsychosocial framework of the study and treatment of pain. It also suggests that the affective component of the pain experience can predict the subjective sensation of pain, despite evidence that such components are differentiable [31]. Moreover, this finding proposes that interventions aimed at reducing pain-related distress, such as cognitive behavior therapy (CBT), hypnosis, or relaxation, may effectively reduce pain perception in these patients. Although such techniques are recommended for the treatment of cancer pain, substantial variability exists in patients’ responses to these interventions [22]. Using HLM, a group of patients was identified for whom pain-related distress was a significant problem. These patients comprise a subset of individuals who may be most responsive to such interventions.
Unexpectedly, patients with higher levels of distress at enrollment reported a reduction in pain intensity, while patients with lower levels of distress reported an increase in pain intensity over 6 weeks. It is plausible that patients with higher distress at enrollment displayed a response shift. They may have adapted to living with pain, accepted pain as a permanent part of their lives, and reported pain intensity accordingly [38]. Nonetheless, this rationale does not explain the increasing trajectory of pain intensity ratings in patients with low levels of distress initially. Perhaps this subset of patients was at an early stage in the disease process, and increasing pain intensity scores over the 6 weeks reflect disease progression. However, interestingly, there was no significant effect of disease characteristics on sample-wide pain outcomes, nor was there any correlation between degree of metastasis (i.e., number of metastatic sites) and pain-related distress at baseline (data not shown). This counterintuitive finding warrants further inquiry.
Changes in Analgesic Medications Prescribed and Taken
The quantity of medications prescribed was significantly and consistently higher than the quantity of medications taken, thus reinforcing the notion that a lack of adherence poses a significant barrier to adequate pain management [29]. Because these analyses included PRN medication, it is ppossible that the discrepancy between analgesics prescribed and taken may also reflect well controlled pain. However, a separate analysis suggests that the majority of these patients were experiencing moderate to severe pain and not adhering to their analgesic regimen [29]. Moreover, significant sample-wide increases in the amount of analgesic medications prescribed and taken were observed, which may be due to development of new pain management strategies, as discussed above.
Distinct predictors of analgesics prescribed at enrollment included age and KPS scores. Specifically, younger patients and patients with lower KPS scores were prescribed significantly more analgesic medication at the time of enrollment and throughout the study. Although worst pain did not predict the analgesic prescription at enrollment, worst pain scores significantly predicted the trajectory, with patients who reported higher worst pain displaying a sharper increase in prescriptions over the course of the study. This trend may reflect communication of patients’ worsening pain to their health care providers.
Hours per day in pain was a distinct predictor of analgesic medications taken at the time of enrollment, with longer time per day in pain predicting an increase in analgesic medications taken at enrollment and throughout the study. Gender was a significant predictor of the trajectory of medications taken, with males exhibiting a steeper increase in analgesic intake over 6 weeks. At least in the context of more potent analgesic medications, this finding may reflect higher efficacy of opioids in females than males [11].
As was true for pain intensity, pain-related distress was a significant predictor of both analgesic outcomes, with higher levels of baseline distress predicting greater quantities of analgesic medications prescribed and taken at baseline and over the course of the study. The fact that distress was identified as such a ubiquitous predictor of pain and analgesic outcomes strongly suggests that it is a prime target for therapeutic intervention in this population, and warrants further assessment.
Limitations
Some limitations of the study should be noted. The patient sample was fairly homogeneous, primarily comprising white, well-educated, and female patients, thus limiting the generalizability of our findings. Moreover, one of the major causes of pain in this sample was bone metastasis. It will be interesting to investigate trajectories of pain and analgesic outcomes in patients with treatment-related pain, such as chemotherapy-induced neuropathy. In addition, the use of bisphosphonates, which effectively reduces pain in patients with bone metastasis, was not evaluated [2]. Finally, although the predictors listed in Tables 1 and 2 are fairly comprehensive, they do not comprise an exhaustive list; therefore, other variables may have had an effect on the trajectories of pain and analgesia in this sample.
The Use of HLM in Pain Research
This paper highlights the important applicability of sophisticated statistical modeling techniques to longitudinal pain research. By employing HLM, we were able to identify and model the effects of predictors of individual change in pain and analgesic outcomes at the time of enrollment, and across the study period. HLM has rarely been applied to the study of pain, despite its powerful advantages. In addition to its flexibility and ability to model individual change, HLM is sensitive to clinically significant change [37], in that distinct trajectories are classified in a meaningful way (i.e., one standard deviation above or below the mean). Pain has been established as a highly subjective and idiosyncratic experience importantly influenced by a myriad of biopsychosocial factors. This individual variability in the perception of pain is therefore expected, but rarely accounted for in the field. This failure to appreciate inter-individual variability is unfortunate and can mask important patterns in the data that are specific to a particular subset of individuals. Finally, predictive variables identified by HLM analyses may represent important risk factors, and indicate important targets for intervention.
Acknowledgements
The authors would like to acknowledge the support and assistance of all the physicians, nurses, and other staff at our study sites as well as our project staff. We are especially grateful to all patients and family caregivers who participated in the PRO-SELF: Pain Control Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no potential conflicts of interest.
Reference List
- 1.Benedetti C, Brock C, Cleeland C, Coyle N, Dube JE, Ferrell B, Hassenbusch S, 3rd, Janjan NA, Lema MJ, Levy MH, Loscalzo MJ, Lynch M, Muir C, Oakes L, O'Neill A, Payne R, Syrjala KL, Urba S, Weinstein SM. NCCN Practice Guidelines for Cancer Pain. Oncology (Williston Park) 2000;14(11A):135–150. [PubMed] [Google Scholar]
- 2.Body JJ, Bartl R, Burckhardt P, Delmas PD, Diel IJ, Fleisch H, Kanis JA, Kyle RA, Mundy GR, Paterson AH, Rubens RD. Current use of bisphosphonates in oncology. International Bone and Cancer Study Group. J Clin Oncol. 1998;16(12):3890–3899. doi: 10.1200/JCO.1998.16.12.3890. [DOI] [PubMed] [Google Scholar]
- 3.Bryk AS, Raudenbush SW. Hierarchical linear models: Applications and data analysis methods. Advanced qualitative techniques in the social sciences, 1. Sage Publications, Inc.; Thousand Oaks, CA: 1992. [Google Scholar]
- 4.Cleeland C. Research in cancer pain. What we know and what we need to know. Cancer. 1991;67(3 Suppl):823–827. doi: 10.1002/1097-0142(19910201)67:3+<823::aid-cncr2820671412>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 5.Cleeland CS, Gonin R, Hatfield AK, Edmonson JH, Blum RH, Stewart JA, Pandya KJ. Pain and its treatment in outpatients with metastatic cancer. N Engl J Med. 1994;330(9):592–596. doi: 10.1056/NEJM199403033300902. [DOI] [PubMed] [Google Scholar]
- 6.Corizzo CC, Baker MC, Henkelmann GC. Assessment of patient satisfaction with pain management in small community inpatient and outpatient settings. Oncol Nurs Forum. 2000;27(8):1279–1286. [PubMed] [Google Scholar]
- 7.Dawson R, Spross JA, Jablonski ES, Hoyer DR, Sellers DE, Solomon MZ. Probing the paradox of patients’ satisfaction with inadequate pain management. J Pain Symptom Manage. 2002;23(3):211–220. doi: 10.1016/s0885-3924(01)00399-2. [DOI] [PubMed] [Google Scholar]
- 8.Dudley WN, McGuire DB, Peterson DE, Wong B. Application of multilevel growth-curve analysis in cancer treatment toxicities: the exemplar of oral mucositis and pain. Oncol Nurs Forum. 2009;36(1):E11–19. doi: 10.1188/09.ONF.E11-E19. [DOI] [PubMed] [Google Scholar]
- 9.Ferrell BR, Rhiner M, Rivera LM. Development and evaluation of the family pain questionnaire. J Psychosoc Oncol. 1993;10(4):21–35. [Google Scholar]
- 10.Foley KM. The treatment of pain in the patient with cancer. CA Cancer J Clin. 1986;36(4):194–215. doi: 10.3322/canjclin.36.4.194. [DOI] [PubMed] [Google Scholar]
- 11.Gear RW, Miaskowski C, Gordon NC, Paul SM, Heller PH, Levine JD. Kappa-opioids produce significantly greater analgesia in women than in men. Nat Med. 1996;2(11):1248–1250. doi: 10.1038/nm1196-1248. [DOI] [PubMed] [Google Scholar]
- 12.Ger LP, Ho ST, Wang JJ, Cherng CH. The prevalence and severity of cancer pain: a study of newly-diagnosed cancer patients in Taiwan. J Pain Symptom Manage. 1998;15(5):285–293. doi: 10.1016/s0885-3924(98)00017-7. [DOI] [PubMed] [Google Scholar]
- 13.Gordon DB, Dahl JL, Miaskowski C, McCarberg B, Todd KH, Paice JA, Lipman AG, Bookbinder M, Sanders SH, Turk DC, Carr DB. American pain society recommendations for improving the quality of acute and cancer pain management: American Pain Society Quality of Care Task Force. Arch Intern Med. 2005;165(14):1574–1580. doi: 10.1001/archinte.165.14.1574. [DOI] [PubMed] [Google Scholar]
- 14.Gralow J, Tripathy D. Managing metastatic bone pain: the role of bisphosphonates. J Pain Symptom Manage. 2007;33(4):462–472. doi: 10.1016/j.jpainsymman.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Harris K, Li K, Flynn C, Chow E. Worst, average or current pain in the Brief Pain Inventory: which should be used to calculate the response to palliative radiotherapy in patients with bone metastases? Clin Oncol (R Coll Radiol) 2007;19(7):523–527. doi: 10.1016/j.clon.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 16.Hedecker D, Gibbons RD. Longitudinal Data Analysis. John Wiley & Sons; Hoboken, NJ: 2006. [Google Scholar]
- 17.Jacox A, Carr DB, Payne R. New clinical-practice guidelines for the management of pain in patients with cancer. N Engl J Med. 1994;330(9):651–655. doi: 10.1056/NEJM199403033300926. [DOI] [PubMed] [Google Scholar]
- 18.Jenkinson C, Coulter A, Bruster S, Richards N, Chandola T. Patients’ experiences and satisfaction with health care: results of a questionnaire study of specific aspects of care. Qual Saf Health Care. 2002;11(4):335–339. doi: 10.1136/qhc.11.4.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karnofsky DA, Burchenal JH. The clinical evaluation fo chemotherapeutic agents in cancer. In: MacLeod CM, editor. Evaluation of Chemotherapeutic Agents. Columbia University Press; 1949. p. 196. [Google Scholar]
- 20.Kim JE, Dodd M, West C, Paul S, Facione N, Schumacher K, Tripathy D, Koo P, Miaskowski C. The PRO-SELF pain control program improves patients’ knowledge of cancer pain management. Oncol Nurs Forum. 2004;31(6):1137–1143. doi: 10.1188/04.ONF.1137-1143. [DOI] [PubMed] [Google Scholar]
- 21.Krueger AB, Stone AA. Assessment of pain: a community-based diary survey in the USA. Lancet. 2008;371(9623):1519–1525. doi: 10.1016/S0140-6736(08)60656-X. [DOI] [PubMed] [Google Scholar]
- 22.Kwekkeboom KL, Wanta B, Bumpus M. Individual difference variables and the effects of progressive muscle relaxation and analgesic imagery interventions on cancer pain. J Pain Symptom Manage. 2008;36(6):604–615. doi: 10.1016/j.jpainsymman.2007.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li LW. From caregiving to bereavement: trajectories of depressive symptoms among wife and daughter caregivers. J Gerontol B Psychol Sci Soc Sci. 2005;60(4):P190–198. doi: 10.1093/geronb/60.4.p190. [DOI] [PubMed] [Google Scholar]
- 24.Li LW. Longitudinal changes in the amount of informal care among publicly paid home care recipients. Gerontologist. 2005;45(4):465–473. doi: 10.1093/geront/45.4.465. [DOI] [PubMed] [Google Scholar]
- 25.Masters Steedman S, Middaugh SJ, Kee WG, Carson DS, Harden RN, Miller MC. Chronic-pain medications: equivalence levels and method of quantifying usage. Clin J Pain. 1992;8(3):204–214. [PubMed] [Google Scholar]
- 26.Mercadante S. Malignant bone pain: pathophysiology and treatment. Pain. 1997;69(1-2):1–18. doi: 10.1016/s0304-3959(96)03267-8. [DOI] [PubMed] [Google Scholar]
- 27.Miaskowski C, Cleary J, Burney R, Coyne P, Finley R, Foster R, Grossman S, Janjan N, Ray J, Syrjala K, Weisman S, Zahrbock C. Guideline for the Management of Cancer Pain in Adults and Children. Vol. 3. American Pain Society; Glenview, IL: 2005. [Google Scholar]
- 28.Miaskowski C, Dodd M, West C, Schumacher K, Paul SM, Tripathy D, Koo P. Randomized clinical trial of the effectiveness of a self-care intervention to improve cancer pain management. J Clin Oncol. 2004;22(9):1713–1720. doi: 10.1200/JCO.2004.06.140. [DOI] [PubMed] [Google Scholar]
- 29.Miaskowski C, Dodd MJ, West C, Paul SM, Tripathy D, Koo P, Schumacher K. Lack of adherence with the analgesic regimen: a significant barrier to effective cancer pain management. J Clin Oncol. 2001;19(23):4275–4279. doi: 10.1200/JCO.2001.19.23.4275. [DOI] [PubMed] [Google Scholar]
- 30.Mitera G, Zeiadin N, Kirou-Mauro A, Deangelis C, Wong J, Sanjeevan T, Sinclair E, Danjoux C, Barnes E, Tsao M, Sahgal A, Chow E. Retrospective Assessment of Cancer Pain Management in an Outpatient Palliative Radiotherapy Clinic Using the Pain Management Index. J Pain Symptom Manage. 39(2):259–267. doi: 10.1016/j.jpainsymman.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 31.Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science. 1997;277(5328):968–971. doi: 10.1126/science.277.5328.968. [DOI] [PubMed] [Google Scholar]
- 32.Raudenbush S, Bryk A. Hierarchical Linear Models: Applications and Data Analysis Methods. 2nd Ed. Sage Publications; Thousand Oaks, CA: 2002. [Google Scholar]
- 33.Raudenbush SW. Comparing personal trajectories and drawing causal inferences from longitudinal data. Annu Rev Psychol. 2001;52:501–525. doi: 10.1146/annurev.psych.52.1.501. [DOI] [PubMed] [Google Scholar]
- 34.Rustoen T, Moum T, Padilla G, Paul S, Miaskowski C. Predictors of quality of life in oncology outpatients with pain from bone metastasis. J Pain Symptom Manage. 2005;30(3):234–242. doi: 10.1016/j.jpainsymman.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 35.Schumacher KL, Koresawa S, West C, Dodd M, Paul SM, Tripathy D, Koo P, Miaskowski C. The usefulness of a daily pain management diary for outpatients with cancer-related pain. Oncol Nurs Forum. 2002;29(9):1304–1313. doi: 10.1188/02.ONF.1304-1313. [DOI] [PubMed] [Google Scholar]
- 36.Singer JD, Willett JB. Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. Oxford University Press; New York: 2003. [Google Scholar]
- 37.Speer DC, Greenbaum PE. Five methods for computing significant individual client change and improvement rates: support for an individual growth curve approach. J Consult Clin Psychol. 1995;63(6):1044–1048. doi: 10.1037//0022-006x.63.6.1044. [DOI] [PubMed] [Google Scholar]
- 38.Sprangers MA, Schwartz CE. Integrating response shift into health-related quality of life research: a theoretical model. Soc Sci Med. 1999;48(11):1507–1515. doi: 10.1016/s0277-9536(99)00045-3. [DOI] [PubMed] [Google Scholar]
- 39.Valeberg BT, Miaskowski C, Hanestad BR, Bjordal K, Moum T, Rustoen T. Prevalence rates for and predictors of self-reported adherence of oncology outpatients with analgesic medications. Clin J Pain. 2008;24(7):627–636. doi: 10.1097/AJP.0b013e31816fe020. [DOI] [PubMed] [Google Scholar]
- 40.West CM, Dodd MJ, Paul SM, Schumacher K, Tripathy D, Koo P, Miaskowski C. The PRO-SELF(c): Pain Control Program--an effective approach for cancer pain management. Oncol Nurs Forum. 2003;30(1):65–73. doi: 10.1188/03.ONF.65-73. [DOI] [PubMed] [Google Scholar]
- 41.WHO . Cancer pain relief. 2nd Edition. World Health Organization; City: Year. [Google Scholar]
- 42.Yau V, Chow E, Davis L, Holden L, Schueller T, Danjoux C. Pain management in cancer patients with bone metastases remains a challenge. J Pain Symptom Manage. 2004;27(1):1–3. doi: 10.1016/j.jpainsymman.2003.10.003. [DOI] [PubMed] [Google Scholar]