Abstract
Purpose
To understand the functional and preclinical efficacy of targeting urokinase plasminogen activator receptor (u-PAR) in ovarian cancer.
Experimental Design
Expression of u-PAR was studied in 162 epithelial ovarian cancers, including 77 pairs of corresponding primary and metastatic tumors. The effect of an antibody against u-PAR (ATN-658) on proliferation, adhesion, invasion, apoptosis, and migration was assessed in three (SKOV3ip1, HeyA8, and CaOV3) ovarian cancer cell lines. The impact of the u-PAR antibody on tumor weight, number, and survival was examined in corresponding ovarian cancer xenograft models and the mechanism by which ATN-658 blocks metastasis was explored.
Results
Only 8% of all ovarian tumors were negative for u-PAR expression. Treatment of SKOV3ip1, HeyA8, and CaOV3 ovarian cancer cells with the u-PAR antibody inhibited cell invasion, migration and adhesion. In vivo, anti-u-PAR treatment reduced the number of tumors and tumor weight in CaOV3 and SKOV3ip1 xenografts, and reduced tumor weight and increased survival in HeyA8 xenografts. Immunostaining of CaOV3 xenograft tumors and ovarian cancer cell lines showed an increase in active-caspase 3 and TUNEL staining. Treatment with u-PAR antibody inhibited α5-integrin and u-PAR colocalization on primary human omental ECM. Anti-u-PAR treatment also decreased the expression of urokinase, u-PAR, β3-integrin and fibroblast growth factor receptor-1 both in vitro and in vivo.
Conclusions
This study shows that an antibody against u-PAR reduces metastasis, induces apoptosis, and reduces the interaction between u-PAR and α5-integrin. This provides a rationale for targeting the u-PAR pathway in patients with ovarian cancer and for further testing of ATN-658 in this indication.
Keywords: urokinase, urokinase receptor, ovarian cancer, metastasis
Introduction
Ovarian cancer is the fifth leading cause of cancer death among US women. It has the highest mortality rate of all gynecologic tumors because most patients will experience recurrences and develop chemoresistant disease [1].The mechanism of ovarian cancer metastasis differs from that of hematogeneously metastasizing tumors, since ovarian cancer cells disseminate from the primary site and are carried by peritoneal fluid to peritoneal surfaces within the abdominal cavity, including the omentum. The first steps of metastasis to these sites involve a tightly regulated process of attachment, migration, and invasion to, and, proliferation on, mesothelium covered surfaces [2, 3].
A number of factors have been implicated as mediators of ovarian cancer metastasis, including integrins, growth factors, and proteases. The urokinase-type plasminogen activator (urokinase) is a serine protease that is first secreted as a proenzyme (pro-urokinase) and is then activated by proteolytic cleavage after binding to its specific cell-surface receptor, u-PAR [4, 5]. Urokinase catalyzes the activation of plasminogen to plasmin, which is critical for remodeling of the extracellular matrix (ECM). Besides regulating and focusing proteolysis at the invading edge of a tumor, u-PAR plays a critical role in cancer progression through its interaction with integrins and vitronectin, and as a regulator of angiogenesis.[6].
The expression of the uPA/u-PAR proteolytic system has been demonstrated in a number of different cancer types, and high endogenous intra-tumoral levels of both uPA and u-PAR are often present in advanced metastatic disease (ovarian cancer metastasis summarized in Supplementary Table S1) [7]. In patients with ovarian cancer, high levels of uPA, soluble u-PAR, and/or u-PAR have been detected in serum, ascites, and ovarian cancer tumors (primary and metastatic). However, it is not clear whether u-PAR is also a prognostic marker in patients with ovarian cancer, or what percentage of epithelial ovarian carcinomas actually express u-PAR. Moreover, the specific role of u-PAR in regulating the adhesion, migration, invasion, and metastasis of epithelial ovarian cancer, which are the key steps in ovarian cancer metastasis, remains to be determined.
Several approaches have been tested that target the u-PAR in pre-clinical models. Small molecules and peptides have been used to block the interaction of urokinase with the u-PAR, affecting downstream signaling. An antisense approach showed that the invasiveness of tumor cells was directly proportional to the density of surface u-PAR expression on cancer cells [8]. Gondi and colleagues used a small hairpin RNA to target u-PAR and showed a 65% regression of established gliomas in an intra-cranial tumor model [9]. These and other studies clearly show that the u-PAR is a potential target for cancer treatment. In view of the multifunctional properties of the u-PAR in the biology of epithelial tumors, and given that antibody based therapies have been established as clinically feasible and efficacious, we decided to test the anti-tumor effects of an antibody against u-PAR in several ovarian cancer models. Our goal was to determine if u-PAR inhibition is a viable strategy that should be further developed for ovarian cancer treatment.
We show here that the u-PAR is widely expressed in primary and metastatic ovarian carcinomas and that u-PAR inhibition, using a monoclonal antibody (ATN-658), reduces ovarian tumor metastasis in several pre-clinical models through the induction of apoptosis and the inhibition of u-PAR interactions with integrins and the ECM.
Translational Relevance
The urokinase plasminogen activator receptor (u-PAR) is an interesting and promising therapeutic target because of its important role in several tumor cell functions. We demonstrate that inhibiting the u-PAR with an antibody blocks the adhesion, migration, invasion, and metastasis of different ovarian cancer cells in vitro and in vivo, while affecting u-PAR’s regulation of adhesion receptors. We therefore believe that targeting the u-PAR system may enable us to block key steps of ovarian cancer progression and provide an approach appropriate for clinical evaluation.
Materials and Methods
Reagents and Cell Lines
Mouse monoclonal antibodies (IgG1) against u-PAR (ATN-658 for treatment and ATN-615 for immunohistochemistry) were developed and provided by Attenuon, LLC. (San Diego, CA). Both antibodies were raised against the D2D3 fragment of u-PAR immunized into Balb/C mice and bound to different epitopes as previously described [10]. The CaOV-3 human ovarian cancer cell line was from ATCC (Rockville, MD). SKOV3ip1 and HeyA8 cells were provided by Dr. Gordon Mills. Cell lines were validated by STR DNA fingerprinting using the AmpFϑSTR Identifiler PCR amplification kit (Applied Biosystems; Carlsbad, CA). The STR profiles were compared to known ATCC fingerprints, to the Cell Line Integrated Molecular Authentication database and to the MD Anderson fingerprint database. MONTY-1 was established by us [11]. The α5-integrin antibody used for immunofluorescence was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The αvβ3-integrin antibody (LM 609), β3-integrin antibody (B3A), and α5-integrin antibody (clone P1D6) were from Chemicon (Billerica, MA). Antibodies against FGFR1, caspase-3, and cleaved caspase-3 were from Cell Signaling (Beverly, MA). The rabbit urokinase antibody was from Abcam (Cambridge, MA). Quantitative real-time PCR primers for uPA, u-PAR, FGFR1, β3-integrin, and α5-integrin were purchased from Applied Biosystems (Foster City, CA).
Patient Tissue Samples
Tissue blocks from 162 patients with International Federation of Gynecology and Obstetrics (FIGO) stage I–IV ovarian cancer, who had undergone surgery performed by a Gynecologic Oncologist at the University of Chicago, were selected by a Gynecologic Pathologist (AM) after obtaining IRB approval. Clinical and histopathologic information was collected and updated as previously reported [12].
Tissue Microarray and Immunohistochemistry
Tissue microarray slides were deparaffinized and incubated with anti-u-PAR (mouse ATN-615) at a 1:200 dilution. The slides were stained using the Envision avidin–biotin-free detection system and counterstained with hematoxylin. Immunoscoring was performed with the Automated Cellular Imaging System (ACIS; Clarient, CA) [12]. Within the tissue core, the most representative tumor area of standardized size was selected at 100× magnification and the fraction of positively stained cells and the intensity of the staining read with the ACIS and confirmed by a Gynecologic Pathologist (AM). Readings were reported as a low (ACIS score < 120) or high (ACIS score ≥ 120) level of u-PAR expression in tumors. The median level of u-PAR was an ACIS score of 131. Overall survival estimates were computed using the Kaplan-Meier method, and comparisons between groups were analyzed using the log-rank test. Differences between primary tumors and corresponding metastases were evaluated using a two sample t-test with pooled variance.
Immunoblot Analysis
Cells were lysed in RIPA buffer and immunoblot analysis performed as previously described [12]. The following dilutions of antibodies were used: anti-u-PAR (1:2000), anti-uPA (1:1000), anti–β3-integrin (1:2000), anti-FGFR1 (1:2000).
Three-dimensional (3D) omental culture system
Specimens of human omentum were obtained from patients undergoing surgery for benign conditions under an IRB approved by the University of Chicago. Primary human mesothelial cells and fibroblasts were isolated from the omentum and purification was verified by vimentin, keratin 8, and prolyl-hydroxylase immunohistochemistry [13, 14]. The 3D omental culture system was assembled by plating 2,000 human primary fibroblasts/ well plus collagen; 18 hours later 10,000 human primary mesothelial cells were added to a 96-well culture plate.
Adhesion Assays
Matrigel (0.5µg) or the 3D omental culture assembled with 4,000 HPF and 20,000 HPMC were used to coat a 96-well dish, and a 4 hour adhesion assay was performed with fluorescently-labeled ovarian cancer cells [13]. Cells were treated with mouse IgG or anti-u-PAR (20µg/ml) at the time of cell plating or pretreated for 18 hours. Differences between treatments were evaluated using an unpaired, two-tailed Student’s t-test.
Migration and Invasion Assay
Fifty-thousand cells (unlabeled for collagen type I or Matrigel and fluorescently-labeled for 3D omentum culture) were plated on each well of a 24-well transwell plate that was either pre-coated with 20µg of collagen I, Matrigel, or contained the 3D omental culture system. The migration and invasion assays were performed as previously described [13]. Differences between treatments were evaluated using an unpaired, two-tailed Student’s t-test.
Omental ECM Isolation
ECM was isolated from primary human omentum, the most common site of ovarian cancer metastasis, in order to provide an environment that is more comparable to the endogenous situation. This omental ECM was used to determine the localization of u-PAR on ovarian cancer cells. . Fresh omentum was cut into small sections (1–2mm in thickness) and suspended in dispase solution (Sigma-Aldrich; St. Louis, MO) at 2mg/g of tissue and incubated for 2 hours at 4°C to remove all cells from the ECM. The tissue sections were rubbed over a cell sieve to separate the intact cells from the remaining ECM. Matrices were homogenized in high salt buffer (0.05M Tris pH 7.4, 3.4 M NaCl, 4uM N-ethylmaleimide) containing protease inhibitors (0.001mg/ml pepstatin, 0.01 mg/ml aprotonin, 2mM orthovanadate, and 1mM PMSF), and centrifuged three times at 7000g for 15 minutes. Supernatant was discarded. The pellet was incubated in 2M urea buffer (0.15M NaCl and 0.05 Tris pH7.4) at 1mL of buffer/g tissue rotating for 48 hours at 4°C to solubilize ECMs. The solution was centrifuged at 14000g for 20 minutes, and supernatant containing ECM collected. The concentration and sizes of ECMs isolated were analyzed using BCA protein concentration assay and SDS-PAGE. The protocol was adapted from Abberton KM et al. [15].
Immunofluorescence
Cells were plated on glass coverslips coated with omental ECM and 24-hours later FITC-labeled uPA was added for 30 minutes. For colocalization experiments, the cells were fixed with 4% paraformaldehyde in PBS for 30 min, and immunofluorescence performed. Primary antibodies against active caspase-3 (1:200) or α5-integrin (1:300), and secondary antibodies goat anti-mouse or anti-rabbit Alexafluor 645 (1:200; Invitrogen) were used. Nuclear counterstain was performed with Hoechst. Imaging was performed using a Zeiss LSM510 confocal microscope. For all 3D and colocalization experiments, Imaris 3D visualization and analysis software (Bitplane Inc.; Saint Paul, MN) was used. The ImarisColoc and Measurement Pro applications were used to measure mean percent colocalization of FITC-labeled uPA and α5-integrin in cells from the confocal images obtained with a 488 and 645 lasers on the Zeiss LSM510. Both automated (reported) and manually set thresholds were used in the analysis, but there was no difference in the two analyses. An independent sample t-test was used to determine significance of the mean percent of FITC-labeled uPA and α5-integrin colocalization in control and anti-u-PAR treated SKOV3ip1 cells.
Animal Experiments
CaOV3 (2×106), HeyA8 (1×106), or SKOV3ip1 (1×106) cells were injected intraperitoneally (i.p.). IgG or anti-u-PAR (ATN-658) (10 mg/kg) was administered twice a week starting on day 7 post tumor cell injection. Paclitaxel (0.03 mg/mouse) was injected once a week for 3 weeks (13 mice/group for CaOV3 cells; 10 mice/group for SKOV3ip1 or HeyA8 cells) starting on day 10. The 1.5 mg/kg dose was used to achieve an approximately 50% inhibition in metastasis to allow the evaluation of any additive or synergistic effect of the u-PAR antibody. The mice were sacrificed 63 (CaOV3), 22 (HeyA8), or 28 (SKOV3ip1) days after cancer cell injection, and the number of tumor colonies counted, dissected, collected, and weighed as described previously [12] and analyzed by unpaired, two-tailed Student’s t-test. For the survival study, HeyA8 cells (1 × 106), were injected i.p. into female athymic nude mice (10 mice/group), and animals were sacrificed at the first signs of distress per institutional guidelines. Kaplan-Meier survival estimates were calculated to determine significant changes in the survival study. Procedures involving animal care were approved by the Committee on Animal Care at the University of Chicago.
Quantitative Real-Time RT-PCR
Trizol reagent was used to isolate RNA according to manufacturer instructions (Invitrogen). Real-time quantitative RT-PCR was performed using the Prism7500 TaqMan PCR detector. Relative levels of mRNA gene expression were calculated using the 2−ΔΔCT method [16]. Differences between treatments were evaluated using an unpaired, two-tailed Student’s t-test.
Mouse Xenograft Tumor Immunohistochemistry and TUNEL Staining
Immunohistochemistry was performed on tumors as previously described [12]. TUNEL staining was performed according to the manufacturer’s protocol. The slides were stained with DAB reagent and counterstained with hematoxylin.
Apoptosis
SKOV3ip1 cells were treated for 48 hours with 20µg/ml u-PAR antibody or control IgG. The sub-G1 population was analyzed by FACS.
Microarray
RNA from each sample was labeled using the One-Cycle Eukaryotic Target Labeling assay from Affymetrix (Santa Clara, CA). The fragmented biotin-labeled cRNA was hybridized to Affymetrix GeneChip Human Genome U133 plus 2.0 arrays. Gene expression data were calculated from normalization of CEL files generated from the arrays. Our probe-level data were normalized using the invariant-set normalization procedure. The normalized probe-level data were converted to model-based gene expression indexes (MBEI, log with base 2) to be used as the values for gene expression. All microarray procedures were performed by the Functional Genomics Facility at the University of Chicago.
Results
u-PAR expression in human ovarian carcinoma
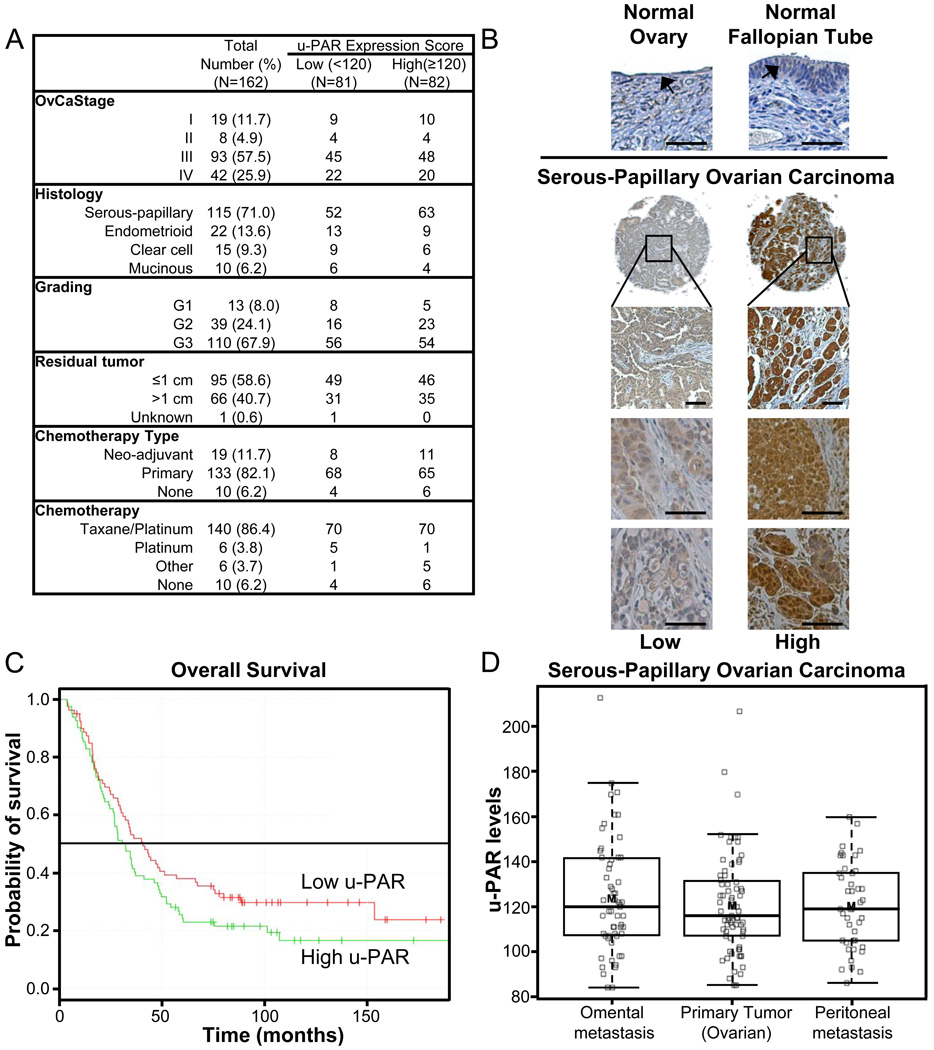
Previous studies have reported various expression levels of u-PAR in ovarian cancer (summarized in Supplementary Table S1) [17–24]. To clarify the percentage of patients with u-PAR positive ovarian cancers, a tissue microarray containing tumor tissue from 135 patients with advanced (FIGO stage III/IV) and 27 patients with early cancers (stage I/II) was stained for u-PAR. Only eight percent (13/162) of all patients had tumors that were negative for u-PAR expression. The u-PAR was expressed in all major histologic subtypes (serous-papillary, endometrioid, mucinous, and clear cell ovarian cancers; Figure 1A) in the epithelial tumor compartment (Fig. 1B). Weak expression was found in the stroma, but the expression in the tumor cells was consistently stronger. In univariate analysis, expression was independent of the classic clinico-pathologic parameters, including tumor grade, primary disease location (fallopian tube, peritoneum, or ovary), or ascites volume. This was expected, given the abundant expression of u-PAR in all tumors. u-PAR expression was also independent of the clinical response to platinum based chemotherapy (“platinum sensitivity”), and the amount of residual tumor at the end of surgery. Expression of u-PAR was low to absent in normal surface epithelium of the ovary and fallopian tube (Fig. 1B). To determine if expression of u-PAR is a prognostic factor in ovarian cancer, a Kaplan-Meier analysis was performed. The median overall survival in women with high (score ≥ 120) u-PAR expression was 33 months and 45 months in women with low (score < 120) u-PAR expression (p=0.15; Fig. 1C), a difference that did not reach statistical significance. Furthermore, there was no statistical difference in progression free survival (PFS) of women with low levels of u-PAR (median PFS 16.1 months) when compared to women with high levels (median PFS 13.1 months). There was also no difference in the level of u-PAR expression in early and late tumor stages, or between the primary ovarian tumor and the corresponding omental (n=61) or peritoneal metastases (n=50; Fig. 1D). Taken together, these data indicate that, while u-PAR expression was not shown to be a prognostic factor, u-PAR is expressed in the majority of ovarian tumors, both primary and metastatic, and, therefore, could prove to be a valuable target for the treatment of ovarian cancer.
Fig. 1. u-PAR expression in human ovarian cancer.
A, Clinical data of patients with stage I–IV ovarian cancer (n=162). Immunoscoring was performed with the Automated Cellular Imaging System, and the u-PAR expression scores are reported as high if greater than or equal to the median score (120) or low if less than median score. B, Representative pictures of immunohistochemical analysis of u-PAR expression in ovary, fallopian tube and ovarian carcinomas from 8 separate patients (4×, 100× or 200× magnification, scale bar=100 µm; arrow= surface epithelium). C, Overall survival of patients with low or high u-PAR expression in tumors (log rank test). D, u-PAR expression in primary and corresponding metastatic serous-papillary ovarian cancers. Immunohistochemical analysis of u-PAR expression in primary (n=80, ovarian), omental (n=61) and peritoneal (n=50) tumors. mean (thick line); 50% of population (box); 95% of cohort (dotted line).
u-PAR contributes to ovarian cancer cell adhesion, migration, and invasion
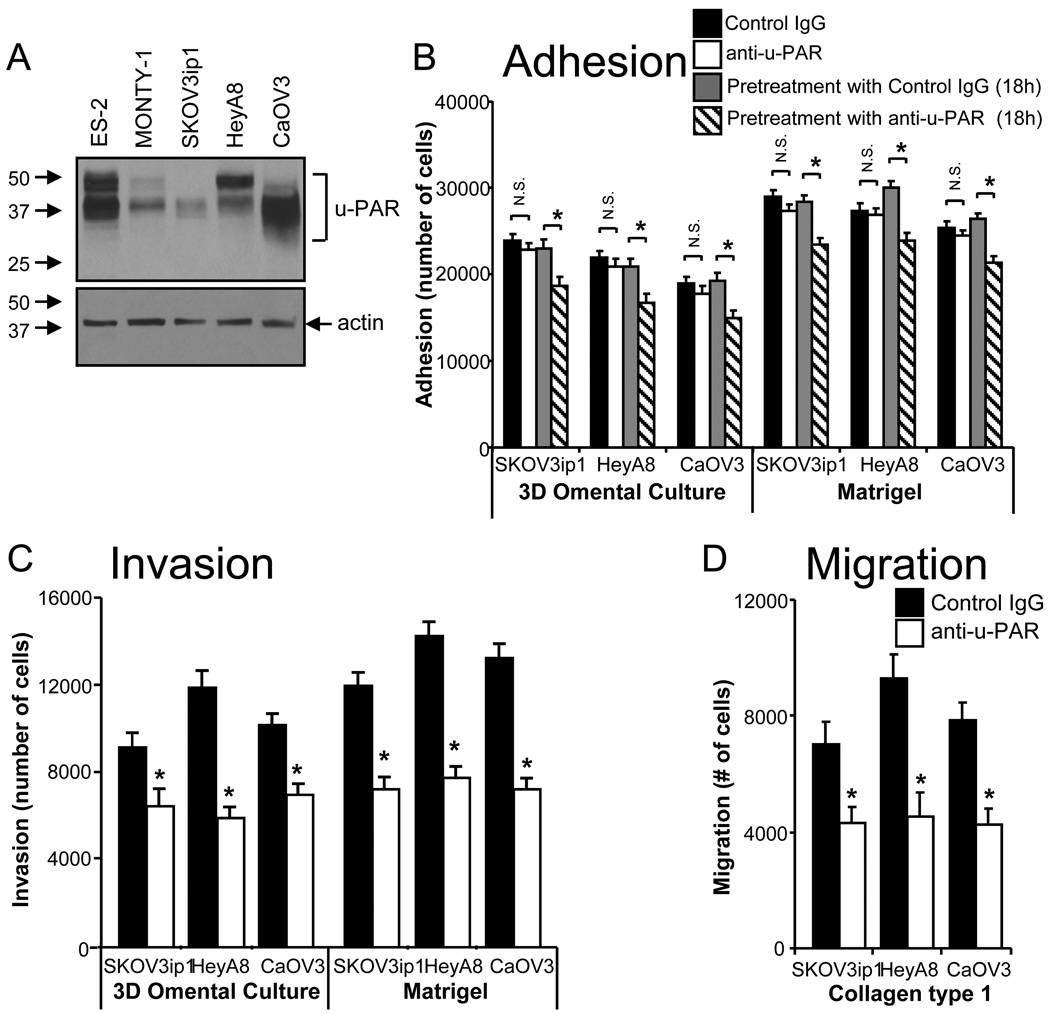
Given the expression of u-PAR in the majority of ovarian cancers, several ovarian cancer cell lines were characterized for their u-PAR expression. All cell lines, including the primary ovarian cancer cell line MONTY-1 [11], expressed at least a low level of u-PAR. CaOV3 (high-), HeyA8 (moderate-), or SKOV3ip1 (low u-PAR expression) were chosen for the subsequent experiments (Fig. 2A). Ovarian cancer metastasis involves the adhesion, migration, and invasion of tumor cells to the peritoneal cavity. To determine if u-PAR is involved in these processes, cells were treated with an u-PAR antibody (ATN-658) that inhibits the downstream signaling of u-PAR [10]. Simultaneous treatment of cells with the u-PAR antibody at the time of the assay had no effect on adhesion, whereas the pre-treatment of cells for 18 hours resulted in a modest inhibition of ovarian cancer cell adhesion (Fig. 2B). Dissociation of endogenous uPA and u-PAR with a mild acid wash had no effect on ovarian cancer cell adhesion (Supplementary Fig. S1). In contrast, the u-PAR antibody significantly inhibited ovarian cancer cell invasion through Matrigel, and through a 3D omental culture that mimics the microenvironment of ovarian cancer cells [13] (Fig. 2C), as well as migration through type I collagen (Fig. 2D). Anti-u-PAR treatment had no effect on ovarian cancer cell proliferation (Supplementary Fig. S2).
Fig. 2. Anti-u-PAR treatment of ovarian cancer cells reduces cell adhesion, migration, and invasion in vitro.
A, Immunoblot analysis of u-PAR levels in ovarian cancer cells, including the primary ovarian cancer cell line, MONTY-1. B, Effect of u-PAR antibody treatment on adhesion after 30 minutes to the 3D omental culture or Matrigel, with anti-u-PAR added at time of the assay or 18 hours prior to assay. C, Effect of u-PAR antibody treatment on ovarian cancer cell invasion into the 3D omental culture or Matrigel after 24 hours. D, Effect of u-PAR antibody treatment on ovarian cancer cell migration through collagen type 1 after 24 hours. Mouse IgG was added as the negative control. Each bar is the mean +/− standard deviation of n=5 (B–D), and is representative of three independent experiments. A student’s t-test compares the two treatments noted with brackets. * indicates p<0.01 between control IgG and experimental treatment and N.S. indicates not significant
Blocking u-PAR inhibits orthotopic ovarian cancer growth in mice
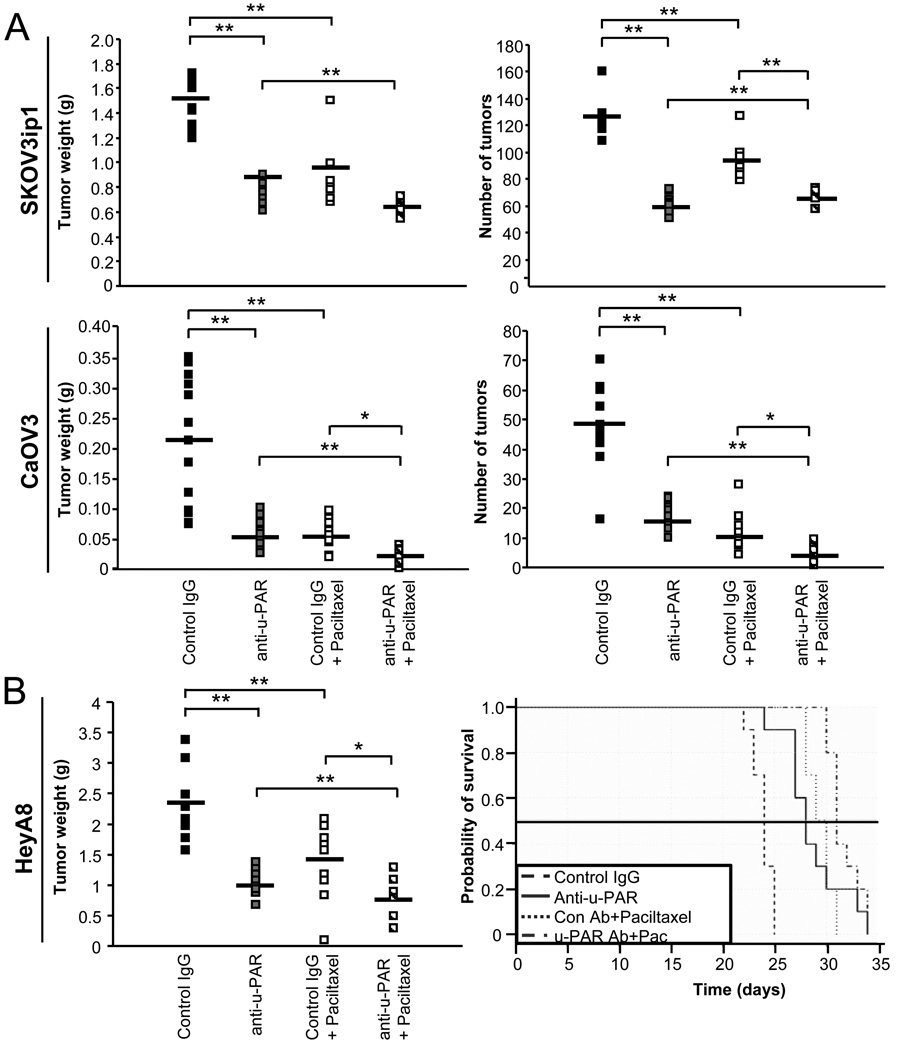
To evaluate the effect of the u-PAR antibody in vivo, we used the same ovarian cancer cell lines in an orthotopic ovarian cancer xenograft model. Cells were injected intraperitoneally, and 7 days later ATN-658 or mouse IgG (control) administered alone or in combination with paclitaxel, a drug currently used as a front-line treatment in patients with ovarian cancer. In the SKOV3ip1 and CaOV3-tumor bearing mice, anti-u-PAR treatment resulted in a statistically significant reduction in mean tumor weight (p<0.01) and number of metastasis (p<0.01) compared with the control IgG-treated mice (Fig. 3A). Paclitaxel treatment decreased mean tumor weight and the number of tumors (p<0.01). The combination of anti-u-PAR and paclitaxel treatment had a greater effect on the reduction of tumor weight (p<0.01) than either treatment alone in the SKOV3ip1 xenograft model, as well as on the reduction of tumor weight (p<0.05) and number (p<0.01) in the CaOV3 xenograft model. In the HeyA8-tumor bearing mice, which only produce a few solid tumors, anti-u-PAR treatment resulted in a decrease in tumor weight (p<0.01) compared with IgG control (Fig. 3B). Again, paclitaxel treatment resulted in a greater reduction of tumor weight, which also increased when combined with the u-PAR antibody. (p<0.05). To determine if anti-u-PAR treatment significantly changed survival in an OvCa xenograft model, Kaplan-Meier analysis of a survival study with the HeyA8 xenograft model was performed. Treatment with the u-PAR antibody led to increased survival in the HeyA8 xenograft model (Fig. 3B) compared with IgG-treated mice (p<0.0001). Paclitaxel increased overall survival rate regardless of antibody treatment (p<0.0001), and a further increase in survival rate was observed in mice treated with anti-u-PAR in combination with paclitaxel (p=0.002).
Fig. 3. Treatment with an u-PAR antibody significantly reduces tumor weight.
A, Tumor weight and number in SKOV3ip1 (10 mice/treatment) and CaOV3 (13 mice/treatment) ovarian cancer cell xenograft models treated with control IgG, u-PAR antibody (10 mg/kg) and/or paclitaxel (1.5 mg/kg). B, Tumor weight and survival of HeyA8 (10 mice/treatment) ovarian cancer cell xenograft models treated with control IgG, u-PAR antibody (10 mg/kg) and/or paclitaxel (1.5 mg/kg). For tumor weight, the mean (bar) and each animal (square) are presented (A–B). A student’s t-test compares the two treatments noted with brackets *indicates p<0.05, **indicates p<0.01 between control IgG and experimental treatment. For survival (B), the Kaplan-Meier survival curve is presented (p-values from log rank test results are presented in Results section).
u-PAR antibody treatment inhibits u-PAR and uPA expression
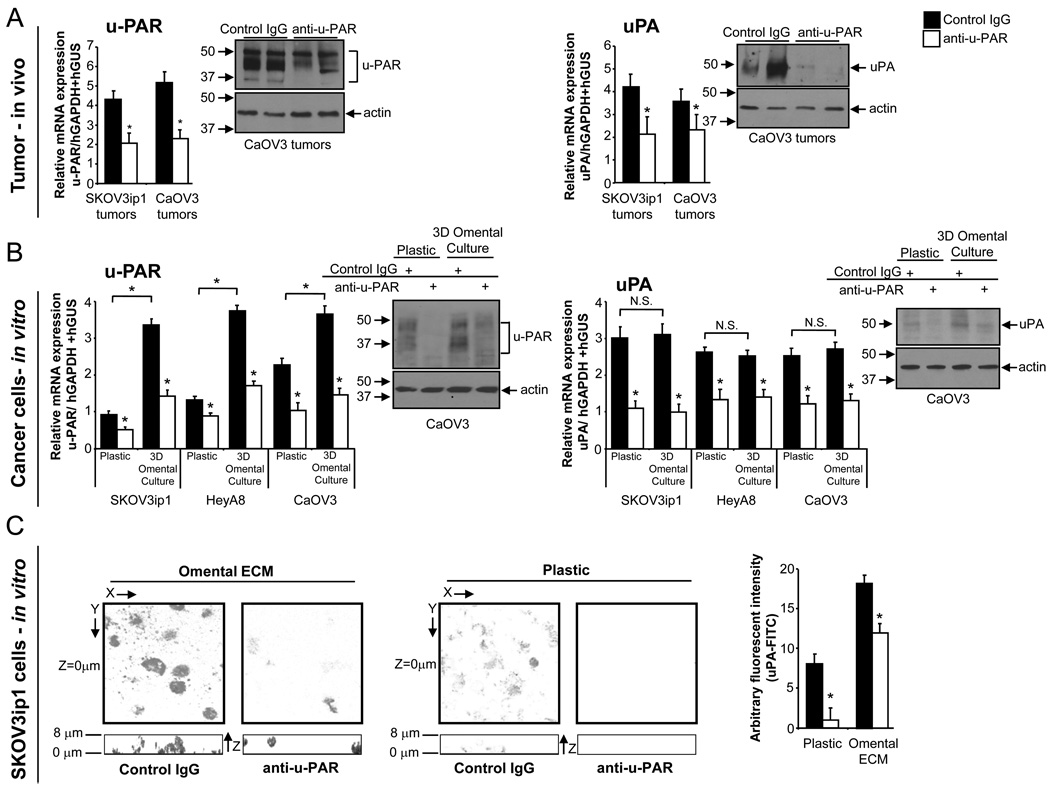
Next, we evaluated the effect of u-PAR antibody treatment on u-PAR and uPA expression in vivo and in vitro. Tumors from anti-u-PAR-treated mice had lower u-PAR and uPA mRNA and protein expression levels compared with tumors from mice treated with IgG (Fig. 4A). In vitro, fluorescently-labeled OvCa cells were cultured on plastic or added to the 3D omental culture, which was composed of a single layer of mesothelial cells overlaying fibroblasts plated in collagen ECM. Fluorescently-activated cell sorting was used to sort the OvCa cells from the 3D culture, and mRNA and protein expression levels were tested. The u-PAR mRNA and protein expression in all three tumor cell lines increased significantly upon co-culture with the 3D omental culture, suggesting that the interaction of cancer cells with mesothelial cells and fibroblasts induces u-PAR expression. These effects could be inhibited by at least 60% with u-PAR antibody treatment (Fig. 4B). While uPA mRNA and protein levels were not further induced by the 3D culture compared to plastic, its constitutive expression in the tumor cells was still blocked by the u-PAR antibody.
Fig. 4. Anti-u-PAR treatment decreases u-PAR and uPA expression in vivo and in vitro.
A–B, The effect of anti-u-PAR treatment on u-PAR and uPA was tested in vivo in the SKOV3ip1 and CaOV3 xenograft models and in vitro in SKOV3ip1, HeyA8, and CaOV3 cells grown on plastic or the 3D omental culture. Anti-u-PAR treatment significantly reduced u-PAR and uPA mRNA (qRT-PCR) and protein (immunoblot) levels in xenograft ovarian cancer tumors (A) and in ovarian cancer cells (B) cultured on plastic or the 3D culture. Bar graph show results representative of three independent experiments and shows results of (n=5, in vitro; n=3, in vivo) +/− standard deviation. C, 3D localization of FITC-labeled uPA (signal in gray) in SKOV3ip1 cells cultured on plastic or omental ECM (630× magnification). The XY images (square/top images) are taken at Z-plane (depth) of contact (Z=0 µm) between ovarian cancer cell and plastic/omental ECM, and the Z image (rectangle/bottom) is a cumulative image (Z-stack) of cells on plastic/omental ECM. The level of FITC-uPA in SKOV3ip1 cells on plastic or omental ECM with control IgG or anti-u-PAR treatment was measured by a fluorescent-plate reader, and reported as arbitrary fluorescent units. Each bar is the mean +/− standard deviation of n=5, and is representative of five independent experiments. A student’s t-test compares control to u-PAR antibody treatment in each graph (A–C) and the two treatments noted with brackets. * indicates p<0.05 and N.S. indicates not significant.
The effect of anti-u-PAR treatment on the localization of u-PAR in cancer cells cultured on omental ECM was investigated. We believed that ECM isolated from benign human omentum would provide a microenvironment that was more comparable to the human omentum, the most common site of OvCa metastasis, than Matrigel or collagen alone. u-PAR was localized on tumor cells using FITC-labeled uPA (Fig. 4C). Once again, anti-u-PAR treatment inhibited the constitutive u-PAR expression of cells cultured on plastic or omental ECM. Moreover, when cancer cells were treated with control IgG, u-PAR localized at the site of contact with primary human omental ECM (top square panels; bottom rectangular panels, 0 µm). However, after anti-u-PAR treatment, u-PAR did not cluster in this fashion, but was more uniformly distributed in the cells.
Blocking u-PAR affects integrin signaling and induces apoptosis
We then took an unbiased approach to investigate mRNA changes affected by u-PAR antibody treatment in vivo. Microarray analysis was performed on RNA isolated from mouse ovarian cancer cell xenograft tumors excised after four doses of u-PAR antibody or control IgG. Gene expression analysis revealed that the pathway most significantly regulated by ATN-658 treatment involved integrin signaling. The gene expression profile of integrin signaling in tumors from CaOV3 xenograft mice treated with anti-u-PAR compared to control IgG is shown in Fig. 5A. We confirmed and tested ten of the regulated genes, including ACTG2, the mRNA with greatest fold-difference between treated and untreated mice. u-PAR antibody treatment decreased ACTG2 protein and mRNA expressoin in vivo and in vitro (Supplementary Fig. S3).
Fig. 5. Anti-u-PAR treatment decreases integrin signaling.
A, Gene array analysis of expression changes of mRNAs involved in integrin signaling. Shown is relative fold-difference in the 19 genes which were significantly regulated at least 1.5-fold in anti-u-PAR compared to control IgG treatment. B, The effect of anti-u-PAR treatment on β3-integrin and FGFR1 was tested in vivo in the SKOV3ip1 and CaOV3 xenograft models and in vitro in SKOV3ip1 and CaOV3 cells grown on plastic or the 3D omental culture. Anti-u-PAR treatment significantly reduced β3-integrin and FGFR1 mRNA (qRT-PCR) and protein (immunoblot) levels in xenograft ovarian cancer tumors and in ovarian cancer cells cultured on plastic or the 3D culture. Bar graph shows results from three independent experiments (n=5, in vitro, B) and three animals (in vivo, B) +/− standard deviation. C, The effect of anti-u-PAR treatment on α5-integrin expression was determined in vivo. Anti-u-PAR treatment significantly reduced α5-integrin (q RT-PCR) and protein (immunoblot) levels in xenograft ovarian cancer tumors. Bar graph shows results from three animals (in vivo, C) +/− standard deviation. D, Colocalization of u-PAR and α5-integrin on omental ECM in ovarian cancer cells. SKOV3ip1 cells cultured on omental ECM were stained with FITC-labeled uPA and cofocal immunofluorescence for α5-integrin was performed. Bar graph shows mean percent of uPA-FITC colocalization with α5-integrin as calculated using the Imaris colocalization application (details in materials and methods). Bar graphs show mean +/− standard deviation (n=5, in vitro) and are representative of 3 independent experiments. A student’s t-test compares the two treatments noted with brackets. * indicates p<0.01.
In view of the inhibitory effect of u-PAR antibody treatment on ovarian cancer cell adhesion, migration, and invasion, we were particularly interested in confirming the down-regulation of two genes with known involvement in these processes, β3-integrin and FGFR1 [11, 25]. Treatment with the u-PAR antibody inhibited β3-integrin and FGFR1 mRNA and protein expression in vivo. This was confirmed in vitro in two cell lines (SKOV3ip1 and CaOV3). Treatment of cells on plastic, as well as on the 3D culture, resulted in an inhibition of β3-integrin and FGFR1 mRNA and protein expression (Fig. 5B).
Previous studies have shown that the association of u-PAR with the fibronectin receptor (α5β1-integrin) affects the expression and activation state of u-PAR, and that u-PAR is important for tumor cell invasion [26, 27]. Therefore, we tested to determine if the antibody would affect the expression of α5-integrin and the interaction of u-PAR and α5-integrin. Indeed, treatment with the u-PAR antibody in the CaOV3 xenograft model inhibited α5-integrin mRNA and protein expression (Fig. 5C). In vitro, the anti-u-PAR treatment inhibited colocalization of α5-integrin and u-PAR in SKOV3ip1 cells cultured on primary human omental ECM (Fig. 5D). For the colocalization experiments, the live cells were incubated with FITC-labeled uPA, just prior to fixation. Subsequently, immunofluorescence with an α5-integrin specific antibody, confocal microscopy, and image analysis were performed. Only mouse u-PAR antibodies were available for immunofluorescence, therefore, we localized u-PAR on tumor cells with FITC-labeled uPA to avoid a cross-reaction of secondary antibody with anti-u-PAR treatment. Pilot studies revealed that FITC-labeled uPA binds u-PAR in the presence or absence of anti-u-PAR treatment, which confirms previously published data demonstrating that ATN-658 does not inhibit uPA binding to uPAR [28].
The histologic appearance of u-PAR and IgG treated tumors was very similar and there was no difference between angiogenesis (micro vessel density) and proliferation (Ki-67) in tumors from u-PAR antibody or mouse IgG antibody treated mice (Fig. 6). However, anti-u-PAR treatment increased cleaved-caspase 3 expression and DNA fragmentation of ovarian tumor cells in CaOV3 and SKOV3ip1 xenografts (TUNEL staining, Fig. 6A). The effect of anti-u-PAR treatment on apoptosis was confirmed in vitro (Fig. 6B). Treatment with the u-PAR antibody increased expression of active caspase 3 in SKOV3ip1 and CaOV3 cells, DNA fragmentation of SKOV3ip1 cells, and the percent of apoptotic cells in SKOV3ip1 cells when compared to control antibody treated cells (Fig. 6B).
Fig. 6. Treatment with an u-PAR antibody increases apoptosis of ovarian cancer cells.
A, Effects of u-PAR antibody treatment in vivo. Representative images of tumors stained with hematoxylin and eosin are shown (100× and 200× magnification). Analysis of microvessel density (CD31), proliferation (Ki-67), and apoptosis (activated caspase 3 and TUNEL staining), by immunohistochemistry of CaOV3 xenograft tumor cross-sections. B, Effects of anti-u-PAR treatment for 48 hours in ovarian cancer cells cultured on plastic or 3D omental culture in vitro. Analysis of apoptosis by immunofluorescence and western blot for cleaved caspase 3, TUNEL staining, or propidium iodide staining with FACS (percent of sub-G1 fraction is indicated). Bar graph shows results of three animals (in vivo, A) +/− standard deviation and results (n=5, in vitro, B) representative of three independent experiments. A student’s t-test compares control to u-PAR antibody treatment in each graph. * indicates p<0.01. For immunoblot of caspase 3, CaOV3 cells on plastic were treated with staurosporine for 3 hours and protein lysates collected (positive control).
Discussion
Since the discovery of the u-PAR in 1985 [29, 30], 25 years of intensive research has elucidated its role as a multifunctional receptor involved in a myriad of tumor cell processes, including invasion, ECM remodeling, adhesion, migration, angiogenesis, and metastasis [4–6]. However, while u-PAR’s many roles in cancer makes it a very attractive therapeutic target, its potential has yet to be translated into a clinical benefit. As a first step towards defining u-PAR’s potential in ovarian cancer treatment, we characterized its expression level and found that over 90% of all epithelial ovarian cancers expressed u-PAR protein in the epithelial tumor compartment. u-PAR was expressed in both the primary tumor and the metastases and in early and as well as late tumor stages, suggesting that it is upregulated in early ovarian tumorigenesis. These results corroborate previously published studies on u-PAR expression in primary ovarian tumors. In a review of all published studies reporting u-PAR expression, we found seven studies that reported that greater than 80% of all ovarian cancer tumors express the u-PAR (summarized in Supplementary Table S1), and two studies that reported lower levels of u-PAR in ovarian cancer [22, 23]. These expression studies and the well-established importance of u-PAR in cancer biology suggest that all patients with the u-PAR expressing tumors could potentially benefit from u-PAR targeted therapy, and support our proposal to test u-PAR inhibition as an anti-cancer strategy.
The efficacy of the u-PAR antibody in the inhibition of various ovarian cancer cell functions was explored both in vitro and in vivo. We tested the effects of this antibody in three different ovarian cancer cell lines to increase the likelihood of generalizable results [31, 32]. Our results overwhelmingly show that the u-PAR antibody is able to inhibit the adhesion, migration, and invasion of all tested ovarian cancer cell lines in vitro and in a 3D model, as well as to inhibit metastasis in 3 different ovarian xenograft models. These results are in agreement with those of other investigators who have identified u-PAR as a therapeutic target in preclinical models of cancer. Two studies by the same group specifically investigated the targeting of u-PAR in ovarian cancer. First, Sato et al., using the OVMZ-6 ovarian cancer xenograft mouse model, identified two cyclic peptides which act as competitive antagonists of the uPA/u-PAR-interaction and were able to reduce tumor weight in vivo [33]. Second, Knor et al. successfully targeted u-PAR in OVMZ-6 ovarian cancer cells in culture, which inhibited colony formation [34]. The urokinase receptor has also been successfully inhibited using various techniques in other cancers, including DNAzymes (osteosarcoma) [35], siRNA (glioma) [9], monoclonal antibodies (pancreatic, colorectal and prostate) [10, 28, 36], and u-PAR antagonists (melanoma and colorectal cancer) [37, 38]. While most of these studies successfully targeted u-PAR in preclinical models of cancer and resulted in various degrees of disruption of known u-PAR functions, most of the reported methods used to inhibit u-PAR are not ready for further clinical development because of short half life of the agents, and concerns involving their purity, stable delivery, and safety. Monoclonal antibodies, however, have finally come of age as therapeutics, and several molecules have recently been approved as cancer therapies. Therefore, given the encouraging results in this and other pre-clinical studies [10, 36], we believe that an antibody against u-PAR has the potential to be advanced into clinical testing.
In view of previous observations that u-PAR directly binds to adhesion molecules, we investigated whether anti-u-PAR treatment affects u-PAR interaction with integrins. We found that the u-PAR antibody inhibited the association of u-PAR with α5β1-integrin. This finding is in agreement with results of the study by Wei et al., which determined that u-PAR directly binds and activates α5β1-integrin, causing a conformational change in α5β1-integrin that permits binding to a different fragment of fibronectin and increased invasion [27]. In addition, β3-integrin was consistently downregulated by anti-u-PAR treatment. In glioma cells, adenovirus-mediated down-regulation of u-PAR was also found to inhibit αvβ3-integrin expression [39]. Therefore, it is apparent that u-PAR inhibition modifies both the interaction and expression of integrins, suggesting that u-PAR can differentially affect integrin functions. In addition, anti-u-PAR treatment increased cleaved-caspase 3 expression, the percentage of apoptotic cells, and DNA fragmentation, explaining further why the treated tumors are smaller. Gondi and colleagues [40], also reported that u-PAR and uPA inhibition induces caspase-mediated apoptosis. Conversely, these results also suggest that u-PAR supports aberrant cell survival in tumor cells.
In summary, we show that u-PAR is widely expressed in ovarian carcinomas and that treatment with an u-PAR antibody targeted and inhibited u-PAR-dependent functions, including ovarian cancer cell invasion and metastasis. In view of the encouraging pre-clinical results shown in this and other studies targeting u-PAR, we propose that sufficient rationale exists to evaluate the effects of u-PAR inhibition in clinical cancer trials.
Supplementary Material
Acknowledgements
We thank Dr. Hal Chapman (UCSF, Pulmonary and Critical Care Division) for his kind advice, Dr. Karl Matlin (University of Chicago, Dept of Surgery) for use of the confocal microscope, and Gail Isenberg for editorial assistance. HAK is supported by the National Cancer Institute (K99CA134750). Ernst Lengyel holds a Clinical Scientist Award in Translational Research from the Burroughs Wellcome Fund, and is supported by grants from the Ovarian Cancer Research Fund (Liz Tilberis Scholars Program), and the National Cancer Institute (R01CA111882).
Footnotes
Author disclosures: The ATN-658 u-PAR antibody was supplied by Attenuon, which also provided funding for the animal experiments. Dr. Andrew Mazar was a former employee of Attenuon who developed the u-PAR antibody and has a patent application submitted on the antibody.
References
- 1.Cannistra SA. Cancer of the ovary. N Engl J Med. 2004;351:2519–2529. doi: 10.1056/NEJMra041842. [DOI] [PubMed] [Google Scholar]
- 2.Bast RC, Hennessy B, Mills GB. The biology of ovarian cancer: New opportunities for translation. Nat Rev Cancer. 2009;9:415–428. doi: 10.1038/nrc2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lengyel E. Ovarian cancer development and metastasis. Am J Pathol. 2010;177:1053–1064. doi: 10.2353/ajpath.2010.100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blasi F, Carmeliet P. uPAR: A versatile signalling orchestrator. Nat Rev Mol Cell Biol. 2002;3:932–943. doi: 10.1038/nrm977. [DOI] [PubMed] [Google Scholar]
- 5.Mazar AP. Urokinase plasminogen activator receptor choreographs multiple ligand interactions: Implications for tumor progression and therapy. Clin Cancer Res. 2008;14:5649–5655. doi: 10.1158/1078-0432.CCR-07-4863. [DOI] [PubMed] [Google Scholar]
- 6.Smith HW, Marshall CJ. Regulation of cell signalling by uPAR. Nat Rev Mol Cell Biol. 2010;11:23–36. doi: 10.1038/nrm2821. [DOI] [PubMed] [Google Scholar]
- 7.Dass K, Ahmad A, Azmi AS, Sarkar S, Sarkar FH. Evolving role of uPA/uPAR system in human cancers. Cancer Treatment Reviews. 2008;34:122–136. doi: 10.1016/j.ctrv.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Kook YH, Adamski J, Zelent A, Ossowski L. The effect of antisense inhibition of urokinase receptor in human squamous cell carcinoma on malignancy. EMBO J. 1994;13:3983–3991. doi: 10.1002/j.1460-2075.1994.tb06714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gondi CS, Lakka SS, Dinh DH, Olivero WC, Gujrati M, Rao JS. RNAi-mediated inhibition of cathepsin B and uPAR leads to decreased cell invasion, angiogenesis and tumor growth in gliomas. Oncogene. 2004;23:8486–8496. doi: 10.1038/sj.onc.1207879. [DOI] [PubMed] [Google Scholar]
- 10.Bauer TW, Liu W, Fan F, et al. Targeting of urokinase plasminogen activator receptor in human pancreatic carcinoma cells inhibits c-met and insulin-like growth factor-1 receptor-mediated migration and invasion and orthotopic tumor grwoth in mice. Cancer Res. 2005;17:7775–7781. doi: 10.1158/0008-5472.CAN-05-0946. [DOI] [PubMed] [Google Scholar]
- 11.Kaur S, Kenny HA, Jagadeeswaran S, et al. β3-integrin expression on tumor cells inhibits tumor progression, reduces metastasis, and is associated with a favorable prognosis in patients with ovarian cancer. Am J Pathol. 2009;175:2184–2196. doi: 10.2353/ajpath.2009.090028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sawada K, Radjabi AR, Shinomiya N, et al. C-Met overexpression is a prognostic factor in ovarian cancer and an effective target for inhibition of peritoneal dissemination and invasion. Cancer Res. 2007;67:1670–1680. doi: 10.1158/0008-5472.CAN-06-1147. [DOI] [PubMed] [Google Scholar]
- 13.Kenny HA, Krausz T, Yamada SD, Lengyel E. Use of a novel 3D culture model to elucidate the role of mesothelial cells, fibroblasts and extra-cellular matrices on adhesion and invasion of ovarian cancer cells. Int J Cancer. 2007;121:1463–1472. doi: 10.1002/ijc.22874. [DOI] [PubMed] [Google Scholar]
- 14.Kenny HA, Kaur S, Coussens L, Lengyel E. The initial steps of ovarian cancer cell metastasis are mediated by MMP-2 cleavage of vitronectin and fibronectin. J Clin Invest. 2008;118:1367–1379. doi: 10.1172/JCI33775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abberton KM, Bortolotto SK, Woods AA, et al. Myogel, a novel, basement membrane-rich, extracellular matrix derived from skeletal muscle, is highly adipogenic in vivo and in vitro. Cells Tissue Organs. 2008;188:347–358. doi: 10.1159/000121575. [DOI] [PubMed] [Google Scholar]
- 16.Pfaffl M. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:2002–2007. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Casslen B, Gustavsson B, Astedt B. Cell membrane receptors for urokinase plasminogen activator are increased in malignant ovarian tumors. Eur J Cancer. 1991;27:1445–1448. doi: 10.1016/0277-5379(91)90028-c. [DOI] [PubMed] [Google Scholar]
- 18.Borgfeldt C, Bendahl PO, Gustavsson B, et al. High tumor tissue concentration of urokinase plasminogen activator receptor is associated with good prognosis in patients with ovarian cancer. Int J Cancer. 2003;107:658–665. doi: 10.1002/ijc.11420. [DOI] [PubMed] [Google Scholar]
- 19.Borgfeldt C, Hansson S, Gustavsson B, Måsbäck A, Casslen B. Dedifferentiation of serous ovarian cancer from cystic to solid tumors is assocaited with increased expression of mRNA for urokinase plasminogen activator (uPA), its receptor (uPAR) and its inhibitor PAI-1. Int J Cancer. 2001;92:497–502. doi: 10.1002/ijc.1215. [DOI] [PubMed] [Google Scholar]
- 20.Tecimer C, Doering DL, Goldsmith LJ, Meyer JS, Abdulhay G, Wittliff JL. Clinical relevance of urokinase-type plasminogen activator, its receptor and inhibitor type 1 in ovarian cancer. Int J Gynecol Cancer. 2000;10:372–381. doi: 10.1046/j.1525-1438.2000.010005372.x. [DOI] [PubMed] [Google Scholar]
- 21.Schmalfeldt B, Kuhn W, Reuning U, et al. Primary tumor and metastasis in ovarian cancer differ in their content of urokinase-type plasminogen activator, its receptor, and inhibitors types 1 and 2. Cancer Res. 1995;55:3958–3963. [PubMed] [Google Scholar]
- 22.Mabrouk R, Ali-Labib R. Detection of urokinase plasminogen activator receptor and c-erbB-2 in sera of patients with breast and ovarian carcinoma. Clinical Biochemistry. 2003;36:537–543. doi: 10.1016/s0009-9120(03)00102-4. [DOI] [PubMed] [Google Scholar]
- 23.Chambers SK, Ivins CM, Carcangiu ML. Urokinase-type plasminogen activator in epithelial ovarian cancer: A poor prognostic factor, associated with advanced stage. Int J Gynecol Cancer. 1998;8:242–250. doi: 10.1002/(sici)1097-0215(19981023)79:5<449::aid-ijc1>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 24.Wang L, Madigan MC, Chen H, et al. Expression of urokinase plasminogen activator and its receptor in advanced epithelial ovarian cancer patients. Gynecol Oncol. 2009;114:265–272. doi: 10.1016/j.ygyno.2009.04.031. [DOI] [PubMed] [Google Scholar]
- 25.Suyama K, Shapiro I, Guttman M, Hazan RB. A signaling pathway leading to metastasis is controlled by N-cadherin and the FGF receptor. Cancer Cell. 2010;2:301–314. doi: 10.1016/s1535-6108(02)00150-2. [DOI] [PubMed] [Google Scholar]
- 26.Tarui T, Andronicos N, Czekay RP, et al. Critical role of integrin α5β1 in urokinase (uPA)/urokinase receptor (uPAR, CD87) signaling. J Biol Chem. 2003;278:29863–29872. doi: 10.1074/jbc.M304694200. [DOI] [PubMed] [Google Scholar]
- 27.Wei Y, Tang C, Kim Y, et al. Urokinase receptors are required for α5β1 integrin-mediated signaling in tumor cells. J Biol Chem. 2007;282:3929–3939. doi: 10.1074/jbc.M607989200. [DOI] [PubMed] [Google Scholar]
- 28.Rabbani SA, Ateeq B, Arakelian A, et al. An anti-urokinase plasminogen activator antibody (ATN-658) blocks prostate cancer invasion, migration, growth, and experimental skeletal metastasis in vitro and in vivo. Neoplasia. 2010;12:778–788. doi: 10.1593/neo.10296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stoppelli MP, Corti A, Soffientini A, Cassanni G, Blasi F, Assoian R. Differentiation-enhanced binding of the amino-terminal fragment of human urokinase plasminogen activator to a specific receptor on U937 monocytes. Proc Natl Acad Sci USA. 1985;82:4939–4943. doi: 10.1073/pnas.82.15.4939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vassalli J-D, Baccino D, Belin D. A cellular binding site for the Mr 55,000 form of the human plasminogen activator, urokinase. J Cell Biol. 1985;100:86–92. doi: 10.1083/jcb.100.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frederick PJ, Straughn JM, Alvarez RD, Buchsbaum HJ. Preclinical studies and clinical utilization of monoclonal antibodies in epithelial ovarian cancer. Gynecol Oncol. 2009;113:384–390. doi: 10.1016/j.ygyno.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 32.Voskoglou-Nomikos T, Pater JL, Seymour L. Clinical predicitive value of the in vitro cell line, human xenograft, and mouse allograft preclinical cancer models. Clin Cancer Res. 2003;9:4227–4239. [PubMed] [Google Scholar]
- 33.Sato S, Kopitz C, Schmalix WF, et al. High-affinity urokinase-derived cyclic peptides inhibiting urokinase/urokinase receptor-interaction: Effects on tumor growth and spread. FEBS Lett. 2002;528:212–216. doi: 10.1016/s0014-5793(02)03311-2. [DOI] [PubMed] [Google Scholar]
- 34.Knör S, Sato S, Huber T, et al. Development and evaluation of peptidic ligands, targeting tumour-associated urokinase plasminogen activator receptor (uPAR) for use in α-emitter therapy for disseminated ovarian cancer. Eur J Nucl Med Mol Imaging. 2008;35:53–64. doi: 10.1007/s00259-007-0582-3. [DOI] [PubMed] [Google Scholar]
- 35.de Bock CELZ, Itoh T, Morris D, Murrell G, Wang Y. Inhibition of urokinase receptor gene expression and cell invasion by anti-uPAR DNAzymes in osteosarcoma cells. FEBS Journal. 2005;272:3572–3582. doi: 10.1111/j.1742-4658.2005.04778.x. [DOI] [PubMed] [Google Scholar]
- 36.Van Buren G, Gray MJ, Dallas NA, et al. Targeting the urokinase plasminogen activator receptor with a monoclonal antibody impairs the growth of human colorectal cancer in the liver. Cancer. 2009;115:3360–3368. doi: 10.1002/cncr.24371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Min HY, Doyle LV, Vitt CR, et al. Urokinase receptor antagonists inhibit angiogenesis and primary tumor growth in syngeneic mice. Cancer Res. 1996;56:2428–2433. [PubMed] [Google Scholar]
- 38.Ignar DM, Andrews JL, Witherspoon SM, et al. Inhibition of establishment of primary and micrometastatic tumors by a urokinase plasminogen activator receptor antagonist. Clin Exp Metastasis. 1998;16:9–20. doi: 10.1023/a:1006503816792. [DOI] [PubMed] [Google Scholar]
- 39.Lutz V, Reuning U, Krüger A, et al. High level synthesis of recombinant soluble urokinase receptor (CD87) by ovarian cancer cells reduces intraperitoneal tumor growth and spread in nude mice. Biological Chemistry. 2001;382:789–798. doi: 10.1515/BC.2001.095. [DOI] [PubMed] [Google Scholar]
- 40.Gondi CS, Kandhukuri N, Dinh DH, Gujrati M, Rao JS. Downregulation of uPAR and uPA activates caspase mediated apoptosis, inhibits the PI3kinase/AKT pathway. Int J Oncol. 2007;31:19–27. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.