Abstract
Electrical remodeling of the heart occurs in response to both functional (i.e. altered electrical activation) and structural (i.e. heart failure, myocardial infarction, etc.) stressors. These electrophysiological changes produce a substrate that is vulnerable to malignant ventricular arrhythmias. Understanding the cellular and molecular mechanisms of electrical remodeling is important in elucidating potential therapeutic targets designed to alter maladaptive electrical remodeling. For example, primarily electrical remodeling occurs in response to altered patterns of electrical activation without significant structural remodeling. In contrast, secondary remodeling arises in response to a structural insult. In this article, we review cardiac electrical remodeling (primarily in the ventricle) with an emphasis on the mechanisms responsible for these adaptations. These mechanisms suggest novel therapeutic targets to manage or prevent the most devastating manifestation of heart diseases, sudden cardiac death (SCD).
Introduction
In recent years, cardiovascular death rates have fallen, and yet the proportion of cardiovascular death that is attributable to sudden cardiac death (SCD) is on the rise [1]. It is estimated that the incidence of sudden cardiac death is approximately 350,000 events per year in the United States. The most common etiology of SCD is the development of malignant ventricular arrhythmias resulting from complex structural and electrical remodeling that follows myocardial injury, most commonly secondary to coronary artery disease. Cardiac remodeling is often an adaptive response to a functional or structural stressor and plays an important role in both cardiovascular health and disease. Initially, these adaptations compensate and maintain cardiac performance, but over time, they can become maladaptive, causing progressive pump failure and/or malignant arrhythmias. Structural remodeling of the heart has been extensively reviewed and is beyond the scope of this paper [2, 3]. In addition to remodeling of mechanical and contractile properties of the heart, it has been more recently appreciated that various disease states can remodel key electrophysiological properties of the heart. Electrical remodeling occurs in both the atria and ventricle. Electrical remodeling in the atria has been linked to atrial arrhythmias such as atrial fibrillation and has been recently reviewed [4, 5]. In the ventricle, electrical remodeling produces an electrophysiological substrate for the development of potentially lethal ventricular arrhythmias. Therefore, in this article, we review cardiac electrical remodeling primarily in the ventricle, with an emphasis on the mechanisms responsible for these adaptations. We also discuss possible novel therapeutic targets to manage the consequence of ventricular electrical remodeling such as ventricular arrhythmias which lead to SCD.
Basic electrophysiological properties of the heart
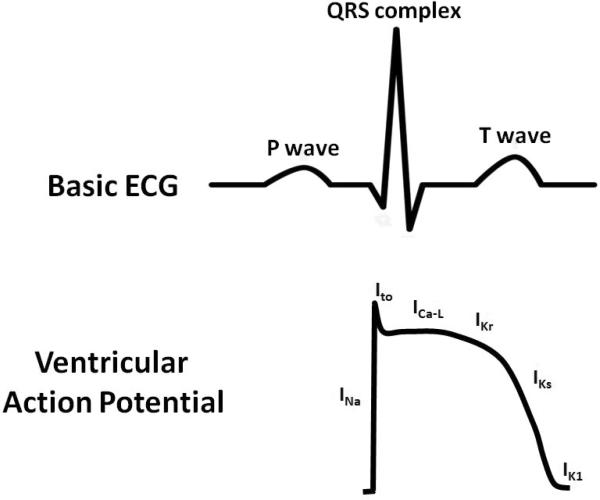
Normal electrical conduction in the heart allows for the coordinated propagation of electrical impulses that initiate atrial and ventricle contraction. The surface electrocardiogram (ECG) is a reflection of these cellular electrical events (Figure 1). For example, atrial depolarization is represented by the p-wave on the ECG. Ventricular depolarization and repolarization represented the QRS complex and T-wave, respectively. At the cellular level, the cardiac action potential is characterized by the interplay of depolarizing and repolarizing currents (Figure 1). In ventricular myocytes (i.e. QRS complex and T wave), activation of the Na+ current causes rapid depolarization (phase 0) followed by a brief period of repolarization (phase 1) secondary to activation of transient outward K+ current (Ito). Subsequently, depolarization is maintained (phase 2) by a balance of inward L-type Ca2+ current (ICa-L) and outward K+ currents (primarily Ikr but also IKs). Finally, repolarization (phases 3 and 4) occurs in response to inactivation of ICa-L and activation of multiple outward K+ currents (IKr, IKs and IK1). The subsequent of sections of this review will consider how these electrical properties of the heart remodel in health and disease.
Figure 1. Example of basic electrocardiogram (ECG) and ventricular action potential.
Top Panel: The ECG is a graphical representation of a coordinated sequence of electrical events in the heart during each heart beat. Atrial depolarization produces the P wave, while ventricular depolarization and repolarization produced the QRS complex and T wave, respectively. Bottom Panel: The ventricular action potential consists of an interplay of depolarizing and repolarizing currents. Abbreviations: INa = sodium current. ICa-L = L-type Ca2+ current. Ito = transient outward K+ current. IKr = rapid component of the delay rectifier K+ current. IKs = slow component of the delayed rectifier K+ current. IK1 = inward rectifier K+ current.
Electrical remodeling of the heart
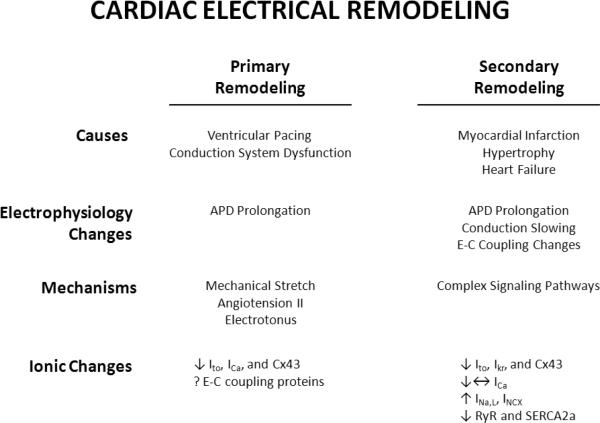
Electrical remodeling can be divided into primary and secondary remodeling (Figure 2). Primary electrical remodeling describes electrical remodeling that occurs primarily in response to a functional insult, such as an altered sequence of electrical activation. For example, during right ventricular pacing the normal sequence of electrical activation is altered because the initiating electrical impulse arises from ventricular myocytes in the right ventricle and not through the specialized purkinje system. In general, this type of electrical remodeling occurs in the absence of a primary structural insult to the myocardium [6, 7]. By contrast, secondary electrical remodeling develops as a result of a structural alteration such as heart failure (HF), hypertrophy, or myocardial infarction (54). The mechanisms responsible for primary and secondary electrical remodeling are complex and remain to be fully elucidated. However, recent research has shown that primary electrical remodeling also occurs in the absence of structural remodeling, and has provided important insights into the mechanisms underlying electrical remodeling of the heart in disease [8]. Furthermore, understanding the cellular and molecular mechanisms of electrical remodeling is important in elucidating potential therapeutic targets. Utilizing these insights to develop therapies designed to alter maladaptive electrical remodeling could transform the management of malignant arrhythmias.
Figure 2. Schematic of primary and secondary remodeling.
Abbreviations: INa-L = late sodium current. ICa-L = L-type Ca2+ current. Ito = transient outward K+ current. IKr = rapid component of the delay rectifier K+ current. INCX = sodium calcium exchanger current. Cx43 = Connexin 43. SERCA2a = sarcoplasmic reticulum Ca2+ ATPase. RyR = ryanodine receptor.
Primary electrical remodeling
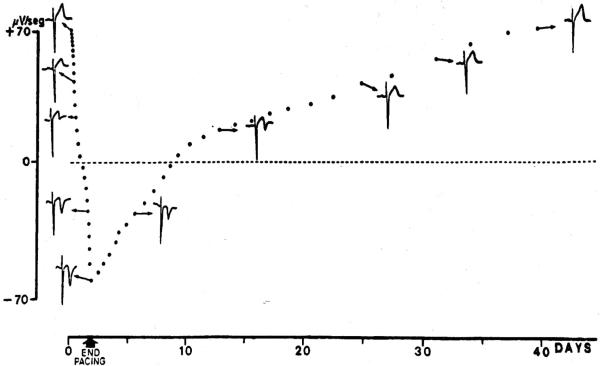
For the purpose of this review, we define electrical remodeling as a persistent change in the electrophysiological properties of the heart in response to a change in the sequence of electrical activation. As stated above, if these changes occur in the absence of significant structural changes, they are referred to as primary electrical remodeling. Also, changes in the rate of electrical activation can lead to primary electrical remodeling. This review will primarily focus on the role of altered sequence of electrical activation, and the reader is referred to recent reviews on the role of altered rate of activation in primary electrical remodeling [9,10]. Nearly 30 years ago, Rosenbaum, et al. described remodeling of cardiac repolarization in response to altered activation [9]. Specifically, following prolonged right ventricular (RV) pacing, the polarity of the T wave on the electrocardiogram (ECG) changes was persistently inverted even when normal activation was restored (Figure 3). This phenomenon was termed “cardiac memory” because the T wave polarity “remembered” the QRS polarity during the previous period of pacing [9]. The occurrence of cardiac memory represents significant remodeling of myocardial repolarization and occurs within minutes to hours of altered electrical activation (i.e. short-term memory)[10]. Furthermore, long periods of altered activation induce greater magnitude of T wave remodeling that persists for weeks to months (i.e. long-term memory)[10]. Understanding the mechanisms underlying cardiac memory, a clinical manifestation of primary electrical remodeling, might provide insights into the mechanisms underlying more complex electrical remodeling (i.e. secondary remodeling) as occurs following structural insults such as myocardial infarction.
Figure 3. ECG changes in T-wave memory.
Graph of T wave polarity and representative ECGs during right ventricular pacing and for 40 days post pacing. After progressive periods of pacing, progressive and persistent change in polarity of the T wave takes place. This change in T wave polarity persists for several days after the cessation of pacing. Adapted from The American Journal of Cardiology, Volume 50/Issue 2, Rosenbaum et al., Electrotonic modulation of the T wave and cardiac memory, pages 213–222, © (1982) with permission from Elsevier.
During normal activation of the ventricle through the His-Purkinje system, there is rapid electrical activation of both ventricles to produce synchronize mechanical contraction. In disease states that affect the cardiac conduction system or ventricular pacing, the propagation of impulse occurs through cell-to-cell conduction. The myocardial region from which activation initiates serves as the source, and the remainder of the myocardium becomes the sink. This generates a source-sink mismatch in which downstream depolarizing myocytes exert an electrotonic influence that prolongs repolarization in myocytes that are adjacent to the source [11–13]. Consequently, there is action potential prolongation in regions adjacent to the site of altered activation and shortening in distal regions [13]. The altered repolarization gradients that develop likely cause change in T-wave polarity. Electrotonus occurs within minutes after onset of altered activation and serves as a trigger for electrical remodeling (i.e. ion channel changes). The mechanisms responsible for specific ion channel remodeling in response to electrotonus is an active area of investigation. For example, attenuation of the epicardial phase 1 notch is evident within minutes after onset of altered activation [12, 14]. This is due to a decrease in the transient outward K+ current (Ito) in myocardial regions adjacent to site of altered activation [15]. The importance of Ito remodeling in short-term cardiac memory is supported by the observation that treatment with the Ito blocker 4-amino-pyridine prevents short-term memory, and that neonatal dogs which lack Ito are resistant to cardiac memory [15, 16].
Another important consequence of altered electrical activation is changes in the mechanical contractile properties of the heart, a process known as stretch-dependent remodeling [17]. Specifically, during altered activation, myocardial regions that are adjacent to the site of altered activation exhibit reduced mechanical strain, whereas distal regions are under significantly greater strain [8, 17]. Our laboratory recently investigated whether the changes in regional mechanical strain could induce electrical remodeling through a mechano-electrical feedback mechanism. Interestingly, we found that the most significant action potential remodeling occurred in myocardial regions that were under enhanced mechanical strain [8]. These findings were also evident in the pacing-induced model of dyssynchronous HF, in which the late-activated lateral LV region exhibits the most significant action potential remodeling [18]. Furthermore, recent studies have identified that short periods of mechanical stretch can also induce cardiac memory [19]. These observations suggest an important role for mechanical strain/stretch in inducing cardiac electrical remodeling.
Myocardial stretch is a potent stimulus for the local release of angiotensin II, and treatment with angiotensin receptor blockers attenuates the development of “short-term” memory [20, 21]. Importantly, angiotensin II was recently shown to decrease Ito in isolated epicardial myocytes. Following incubation with angiotensin II, Ito density was reduced with altered kinetics and attenuation of the phase1 notch was evident in epicardial myocytes [21]. Furthermore, the angiotensin II receptor (AT1) co-localizes to the α subunit (Kv4.3) of the channel carrying Ito, and exposure to angiotensin II caused internalization of the AT1 – Kv4.3 complex [22]. Angiotensin II also has a role in regulation of the β-subunit (KChIP2) through CREB mediated transcriptional regulation [23–25]. Therefore, angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers could have a beneficial role in prevention of cardiac memory.
In contrast to short-term memory, the ionic bases for chronic remodeling are complex and involve altered outward K+ currents, inward Ca2+ currents and changes in the quantity and distribution of cardiac gap junctions. The remodeling of the early-activated region following long-term pacing has been extensively studied; however, changes in the late-activated region remain to be fully elucidated. In long-term memory, remodeling of Ito results in a decreased current density, altered activation threshold (i.e. more positive) and delayed recovery from inactivation [15]. Furthermore, the rapid component of the delayed rectifier current (IKr) is remodeled in “long-term” memory [26]. Specifically, the normal transmural gradient of greater IKr in the epicardium compared to the endocardium is reversed in long-term memory [26]. In addition to the remodeling of outward K+ currents, long-term memory is associated with remodeling of the inward L-type Ca2+ current (Ica)[27]. In fact, treatment with L-type Ca2+ channel blockers has been shown to attenuate both short- and long-term cardiac memory [27]. Interestingly, only in long-term cardiac memory is the function of the L-type Ca2+ channel remodeled, and thus, the role of ICa,L in short-term memory is not clear. In long-term cardiac memory, ICa,L activates at a more positive membrane voltage, and recovery from inactivation is prolonged, both of which will prolong action potential duration (APD)[27]. It is postulated that the mechanisms responsible for these changes in ICa,L are secondary to the reduction of KChIP2, which has recently been shown to be an accessory subunit for the L-type Ca2+ channel [28].
Furthermore, Patel et al. demonstrated remodeling of the gap junction protein, connexin 43 (Cx43), following prolonged RV pacing [29]. They found reduced Cx43 expression primarily in the early activated myocardial segments. By contrast, Spragg et al. showed that Cx43 protein expression is not decreased in the late-activated myocardial segments, but its localization is lateralized with a reduction in conduction velocity in these regions[30]. The mechanisms responsible for Cx43 remodeling remain unclear, but angiotensin II has been postulated as a likely modulator of Cx43 in cardiac memory. Whether experimental agents that augment gap junction conduction will have a role in attenuating cardiac memory remains to be explored.
In summary, primary electrical remodeling occurs in response to alteration in the pattern of electrical activation (Figure 2). These changes alter electrotonic flow and myocardial strain throughout the heart, triggering distinct myocardial remodeling in the early-activated vs. late-activated regions. Electrotonic remodeling is apparent in early-activated regions, while mechanical strain-dependent remodeling occurs in the late-activated regions. These triggers underlie remodeling of repolarization via change in the expression and function of multiple ionic currents and gap junctions. Moreover, angiotensin II is important in the transduction altered myocardial stretch to remodeling of ionic currents and gap junctions. It is likely that other mechanisms are also involved in regulating primary electrical remodeling, and this is an active area of investigation. The molecular mechanisms driving remodeling of ionic currents and gap junctions in cardiac memory remain unclear. However, recently it was shown that the remodeling of KChIP2 (accessory subunit of Ito and ICa,L) in cardiac memory was mediated through the transcription factor CREB [31]. Furthermore, CREB expression is modulated by both angiotensin II binding to the AT1 receptor and by the intracellular actions of Ca2+ [31]. Finally, this likely explains why treatment with both angiotensin receptor and L-type Ca2+ channel antagonists attenuate the development of cardiac memory. Future studies that examine ionic remodeling and calcium handling remodeling in regions after primary electrical remodeling will provide important insights into common molecular mechanisms.
Secondary electrical modeling
One of the most devastating manifestations of cardiovascular disease (i.e. HF, myocardial infarction, etc.) is SCD secondary to fatal arrhythmias. The mechanisms responsible for these arrhythmias are complex and involve electrical remodeling of the myocardium in response to a structural insult, referred to as secondary electrical remodeling (Figure 2). In particular, secondary electrical remodeling involves alterations of numerous ion channels, excitation-contraction (EC)-coupling (i.e. altered sarcoplasmic reticulum [SR] Ca2+ cycling), and intercellular gap junctions. One of the hallmarks of secondary electrical remodeling is repolarization abnormalities, specifically a prolongation of action potential duration (APD). The ionic mechanisms responsible for remodeling of the cardiac action potential involve a complex interplay between altered outward K+ currents (Ik), inward Ca2+ currents (ICa) and the late component of the inward Na+ current (INa). In addition to altered current densities, the spatial distribution of IK, ICa and INa, are altered, most notably in HF. These changes markedly alter normal repolarization gradients in the heart and can contribute to the development of abnormal heart rhythms (arrhythmogenesis) [32]. It is important to note that secondary electrical remodeling is not only isolated to ventricular myocytes but is frequently seen in Purkinje cells and atrial myocardium. In fact, electrical remodeling of Purkinje cells is believed to produce a substrate that is particularly prone to the development of triggered ventricular arrhythmias [33]. Additionally, electrical remodeling of atrial myocytes increases susceptibility to atrial arrhythmias such as atrial fibrillation [34].
Similar to primary electrical remodeling, down-regulation of Ito is one of the most reproducible events during secondary electrical remodeling in the ventricle [35]. The primary effect of diminished Ito on the action potential (AP) is a slowing of early repolarization, primarily changing the shape of the AP with variable effect on APD. In contrast, the delayed rectifier K+ currents (i.e. IKr, and IKs) are largely responsible for phase 3 repolarization in cardiomyocytes. In secondary electrical remodeling, changes in IK1 IKr, and IKs are variable and seem to be related to the underlying cause of electrical remodeling (i.e. ischemic vs. non-ischemic cardiomyopathy) [36]. Recently, it was shown that altered expression of microRNAs might explain some of the IK changes seen in secondary remodeling [37, 38]. In particular, miR-133 expression was shown to be increased in the diabetic heart. Importantly, it was as shown that miR-133 acts to inhibit translation of HERG (subunit of IKr) [37]. In a separate study, miR-1 expression was increased in the infracted heart, causing inhibition of Kir2.1 (subunit of IK1) [38]. These microRNAs represent exciting new therapeutic targets in the management of secondary remodeling and its associated arrhythmic risk.
Pharmacological blockade of IK has shown limited promise in the management of arrhythmic risk in secondary electrical remodeling. In contrast, exciting new preclinical trials have shown promise for gene therapies targeting IK in secondary remodeling. In a porcine model, focal gene transfer in the border zone of myocardial infarction to silence IKr has been shown to abolish ventricular arrhythmias [39]. Moreover, overexpression of mir-1 and miR-133 has been shown to decrease the risk of arrhythmias in models of diabetes and myocardial infarction, respectively [37, 38].
Increasing evidence demonstrates that increased ICa,L density is an important mechanism for APD prolongation in mild to moderate hypertrophy [40]. However, in severe hypertrophy and HF, ICa-L is unchanged or decreased compared to control, highlighting the complexity of APD prolongation in HF. The most common alteration of ICa,L in HF is delayed ICa,L inactivation, secondary to diminished Ca2+-dependent inactivation. For example, the amount of Ca2+ released from the sarcoplasmic reticulum is diminished in HF, which results in diminished Ca2+-dependent ICa,L inactivation. Also, Wang et al. [41] recently demonstrated that the slowed ICa,L inactivation in HF requires Ca2+-calmodulin-dependent kinase (CAMKII). Interestingly, animal models of HF have enhanced/re-expression of fetal T-type Ca2+ current (ICa,T). It is postulated that ICa,T might enhance cardiac automaticity in HF [42, 43]. Unfortunately, clinical trials investigating the use of L-type Ca2+ channel antagonists in humans have failed to show a mortality benefit or reversal of secondary electrical remodeling [44].
Remodeling of INa is variable in HF, with reports of increased, unchanged and decreased peak INa [34]. These changes have very little impact on APD; rather, they primarily alter conduction velocity. In contrast, the late component of the INa,L, which accounts for only 1% of peak INa, is increased in HF. Importantly, increased INa,L (as occurs in patients with Long QT Syndrome Type III) can increase APD in HF, contributing to arrhythmogenesis in HF. Therapies targeted at blocking INa,L have shown promise in the management of secondary remodeling, and this is an active area of investigation [45, 46].
The sodium-calcium exchanger (NCX) is an electrogenic, bidirectional transporter (moving three Na+ ions across the membrane in exchange for one Ca2+ ion). In mild to moderate hypertrophy, NCX expression is increased, but INCX is decreased. Increased NCX expression in hypertrophy is hypothesized to be mediated by calcineurin [47] and altered targeting of NCX to the sarcolemma is postulated to cause decreased INCX. By contrast, NCX expression and function are increased in HF and have been linked to the development of delayed afterdepolarizations (DADs) and triggered ventricular arrhythmias [48]. The role of pharmacological blockade of NCX in the management of secondary electrical remodeling is unknown because there are no INCX blockers available for clinical use at this time. Preclinical investigation of the selective NCX blocker SEA-0400 has shown mixed results in the management of secondary electrical remodeling [49].
Abnormal Ca2+ handling is a hallmark of altered EC coupling in secondary electrical remodeling, resulting in decreased contractile force, impaired relaxation and increased susceptibility to ventricular arrhythmias. Impaired sarcoplasmic reticulum (SR) Ca2+ release is caused by impaired gating properties of the ryanodine receptor (RyR) complex, with variable alteration in RyR protein expression [50]. The exact mechanisms underlying abnormal RyR Ca2+ are complex and likely involve multiple pathways. For example, hyperphosphorylation of RyR causes a loss of FKBP12.6-RyR binding, which has been shown to increase RyR Ca2+ leak [51]. Both CAMKII and cAMP-dependent kinase (PKA) phosphorylate the RyR, yet, there remains debate over which is the predominant pathway mediating RyR leak in secondary electrical remodeling [51–55]. Additionally, other mechanisms, such as oxidative stress and altered S-nitrosylation of the RyR, have been implicated in enhanced RyR Ca2+ leak [56, 57]. For example, reduced S-nitrosylation of the RyR enhances diastolic calcium sparks in isolated myocytes. However, the mechanisms responsible for this observation are controversial. Whether enhanced RyR Ca2+ leak or diastolic calcium sparks in isolated myocytes translate to arrhythmogenesis in the intact heart is an active area of investigation. Spontaneous diastolic SR Ca2+ release in the intact heart can cause DAD's via INCX. Importantly, abnormal RyR release properties have been linked to DADs and triggered ventricular arrhythmias [58]. However, in isolation enhanced RyR Ca2+ leak is unlikely to be sufficient to produce arrhythmias in the intact heart. Increasing evidence indicates increased SR Ca2+loading is necessary for enhanced RyR Ca2+ leak to produce arrhythmias [59].
Impaired SR Ca2+ reuptake is a common finding in secondary electrical remodeling and is related to decreased sarcoplasmic reticulum Ca(2+) ATPase (SERCA2a) expression and function [60]. Also, decreased phosphorylation of phospholamban (PLB) secondary to reduced β-adrenergic sensitivity enhances PLB inhibition of SERCA2a. There is increasing evidence that decreased SERCA2a expression and/or function enhances arrhythmic risk [61–63]. In particular, our laboratory recently showed that SERCA2a expression is an important mechanism in the development of arrhythmogenic cardiac alternans. Moreover, increasing SERCA2a expression suppresses cardiac alternans and reduces arrhythmia susceptibility.
Therapies targeting abnormal SR Ca2+ handling are not currently available in clinical practice. However, preclinical trials have shown promise for therapies targeting RyR and SERCA2a function [64, 65]. Because abnormalities in EC-coupling increase both arrhythmic risk and cause contractile dysfunction targeting these abnormalities has great potential to ameliorate the effects of both structural and electrical remodeling.
Gap junctions provide low-resistance electrical coupling between adjacent cardiac myocytes and allow movement of ions and small molecules between cells. The intercellular channel of gap junctions is composed of two hemi-channel connexons that are each composed of six connexin protein subunits. Connexin 43, the major subtype of connexin in the ventricular, exhibits heterogeneous expression throughout the heart. These heterogeneities are important in defining differences regional electrophysiological properties within the heart [66]. Importantly, remodeling of gap junctions has been demonstrated in multiple models secondary electrical remodeling and has been linked to arrhythmic risk [67–70]. In HF, both lateralization and decreased expression of connexin43 (Cx43), the major subtype of connexin in the ventricle, have been described. Decreased expression and lateralization of Cx43 decreases conduction velocity and increases dispersion of repolarization, both of which provide the substrate for arrhythmias. Therapies targeting gap junctions are an active area of investigation but have not yet reached clinical testing. However, in preclinical testing rotigaptide, a peptide designed to enhance gap junction conduction, was shown to reduce susceptibility to arrhythmogenic cardiac alternans [71]. These observations highlight the potential for therapies targeting gap junction remodeling.
Concluding remarks
Electrical remodeling of the heart occurs in response to both functional (i.e. altered electrical activation) and structural (i.e. HF, myocardial infarction, etc.) stressors. These electrophysiological changes produce a substrate that is vulnerable to malignant ventricular arrhythmias. Understanding the cellular and molecular mechanisms of electrical remodeling is important in elucidating potential therapeutic targets designed to alter maladaptive electrical remodeling. The development of such therapies could revolutionize the management of malignant arrhythmias.
ACKNOWLEDGEMENTS
Support by RO1-HL54807
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Thom T, et al. Heart disease and stroke statistics--2006 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;113(6):e85–151. doi: 10.1161/CIRCULATIONAHA.105.171600. [DOI] [PubMed] [Google Scholar]
- 2.Hill JA, Olson EN. Cardiac plasticity. N Engl J Med. 2008;358(13):1370–80. doi: 10.1056/NEJMra072139. [DOI] [PubMed] [Google Scholar]
- 3.Olson EN. A decade of discoveries in cardiac biology. Nat Med. 2004;10(5):467–74. doi: 10.1038/nm0504-467. [DOI] [PubMed] [Google Scholar]
- 4.Carlsson L, Duker G, Jacobson I. New pharmacological targets and treatments for atrial fibrillation. Trends Pharmacol Sci. 2010;31(8):364–71. doi: 10.1016/j.tips.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Wijffels MC, et al. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation. 1995;92(7):1954–68. doi: 10.1161/01.cir.92.7.1954. [DOI] [PubMed] [Google Scholar]
- 6.Jeyaraj D, Ashwath M, Rosenbaum DS. Pathophysiology and clinical implications of cardiac memory. Pacing Clin Electrophysiol. 33(3):346–52. doi: 10.1111/j.1540-8159.2009.02630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Libbus I, Rosenbaum DS. Remodeling of cardiac repolarization: mechanisms and implications of memory. Card Electrophysiol Rev. 2002;6(3):302–10. doi: 10.1023/a:1016349613464. [DOI] [PubMed] [Google Scholar]
- 8.Jeyaraj D, et al. Mechanoelectrical feedback as novel mechanism of cardiac electrical remodeling. Circulation. 2007;115(25):3145–55. doi: 10.1161/CIRCULATIONAHA.107.688317. [DOI] [PubMed] [Google Scholar]
- 9.Oros A, Beekman JD, Vos MA. The canine model with chronic, complete atrioventricular block. Pharmacol Ther. 2008;119(2):168–78. doi: 10.1016/j.pharmthera.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 10.Stambler BS. Tachycardia-induced ventricular electrical remodeling: a perspective on unresolved experimental mechanisms and clinical implications. Heart Rhythm. 2006;3(11):1378–81. doi: 10.1016/j.hrthm.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 11.Rosenbaum MB, et al. Electrotonic modulation of the T wave and cardiac memory. Am J Cardiol. 1982;50(2):213–22. doi: 10.1016/0002-9149(82)90169-2. [DOI] [PubMed] [Google Scholar]
- 12.Ozgen N, Rosen MR. Cardiac memory: a work in progress. Heart Rhythm. 2009;6(4):564–70. doi: 10.1016/j.hrthm.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 13.Libbus I, Wan X, Rosenbaum DS. Electrotonic load triggers remodeling of repolarizing current Ito in ventricle. Am J Physiol Heart Circ Physiol. 2004;286(5):H1901–9. doi: 10.1152/ajpheart.00581.2003. [DOI] [PubMed] [Google Scholar]
- 14.Libbus I, Rosenbaum DS. Transmural action potential changes underlying ventricular electrical remodeling. J Cardiovasc Electrophysiol. 2003;14(4):394–402. doi: 10.1046/j.1540-8167.2003.02436.x. [DOI] [PubMed] [Google Scholar]
- 15.Costard-Jackle A, et al. Slow and long-lasting modulation of myocardial repolarization produced by ectopic activation in isolated rabbit hearts. Evidence for cardiac “memory”. Circulation. 1989;80(5):1412–20. doi: 10.1161/01.cir.80.5.1412. [DOI] [PubMed] [Google Scholar]
- 16.Geller JC, Rosen MR. Persistent T-wave changes after alteration of the ventricular activation sequence. New insights into cellular mechanisms of `cardiac memory'. Circulation. 1993;88(4 Pt 1):1811–9. doi: 10.1161/01.cir.88.4.1811. [DOI] [PubMed] [Google Scholar]
- 17.Yu H, et al. Transient outward current, Ito1, is altered in cardiac memory. Circulation. 1999;99(14):1898–905. doi: 10.1161/01.cir.99.14.1898. [DOI] [PubMed] [Google Scholar]
- 18.Coronel R, et al. Long-term cardiac memory in canine heart is associated with the evolution of a transmural repolarization gradient. Cardiovasc Res. 2007;74(3):416–25. doi: 10.1016/j.cardiores.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 19.Prinzen FW, et al. Mapping of regional myocardial strain and work during ventricular pacing: experimental study using magnetic resonance imaging tagging. J Am Coll Cardiol. 1999;33(6):1735–42. doi: 10.1016/s0735-1097(99)00068-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aiba T, et al. Electrophysiological consequences of dyssynchronous heart failure and its restoration by resynchronization therapy. Circulation. 2009;119(9):1220–30. doi: 10.1161/CIRCULATIONAHA.108.794834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sosunov EA, Anyukhovsky EP, Rosen MR. Altered ventricular stretch contributes to initiation of cardiac memory. Heart Rhythm. 2008;5(1):106–13. doi: 10.1016/j.hrthm.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 22.Sadoshima J, Izumo S. Molecular characterization of angiotensin II--induced hypertrophy of cardiac myocytes and hyperplasia of cardiac fibroblasts. Critical role of the AT1 receptor subtype. Circ Res. 1993;73(3):413–23. doi: 10.1161/01.res.73.3.413. [DOI] [PubMed] [Google Scholar]
- 23.Ricard P, et al. A role for the renin-angiotensin system in the evolution of cardiac memory. J Cardiovasc Electrophysiol. 1999;10(4):545–51. doi: 10.1111/j.1540-8167.1999.tb00711.x. [DOI] [PubMed] [Google Scholar]
- 24.Yu H, et al. Effects of the renin-angiotensin system on the current I(to) in epicardial and endocardial ventricular myocytes from the canine heart. Circ Res. 2000;86(10):1062–8. doi: 10.1161/01.res.86.10.1062. [DOI] [PubMed] [Google Scholar]
- 25.Ozgen N, et al. Determinants of CREB degradation and KChIP2 gene transcription in cardiac memory. Heart Rhythm. 7(7):964–70. doi: 10.1016/j.hrthm.2010.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patberg KW, et al. The cAMP response element binding protein modulates expression of the transient outward current: implications for cardiac memory. Cardiovasc Res. 2005;68(2):259–67. doi: 10.1016/j.cardiores.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 27.Patberg KW, et al. Cardiac memory is associated with decreased levels of the transcriptional factor CREB modulated by angiotensin II and calcium. Circ Res. 2003;93(5):472–8. doi: 10.1161/01.RES.0000088785.24381.2F. [DOI] [PubMed] [Google Scholar]
- 28.Obreztchikova MN, et al. I(Kr) contributes to the altered ventricular repolarization that determines long-term cardiac memory. Cardiovasc Res. 2006;71(1):88–96. doi: 10.1016/j.cardiores.2006.02.028. [DOI] [PubMed] [Google Scholar]
- 29.Plotnikov AN, et al. Role of L-type calcium channels in pacing-induced short-term and long-term cardiac memory in canine heart. Circulation. 2003;107(22):2844–9. doi: 10.1161/01.CIR.0000068376.88600.41. [DOI] [PubMed] [Google Scholar]
- 30.Thomsen MB, et al. Accessory subunit KChIP2 modulates the cardiac L-type calcium current. Circ Res. 2009;104(12):1382–9. doi: 10.1161/CIRCRESAHA.109.196972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel PM, et al. Altering ventricular activation remodels gap junction distribution in canine heart. J Cardiovasc Electrophysiol. 2001;12(5):570–7. doi: 10.1046/j.1540-8167.2001.00570.x. [DOI] [PubMed] [Google Scholar]
- 32.Spragg DD, et al. Abnormal conduction and repolarization in late-activated myocardium of dyssynchronously contracting hearts. Cardiovasc Res. 2005;67(1):77–86. doi: 10.1016/j.cardiores.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 33.Rosen MR, Cohen IS. Cardiac memory … new insights into molecular mechanisms. J Physiol. 2006;570(Pt 2):209–18. doi: 10.1113/jphysiol.2005.097873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akar FG, Rosenbaum DS. Transmural electrophysiological heterogeneities underlying arrhythmogenesis in heart failure. Circ Res. 2003;93(7):638–45. doi: 10.1161/01.RES.0000092248.59479.AE. [DOI] [PubMed] [Google Scholar]
- 35.Boyden PA, Hirose M, Dun W. Cardiac Purkinje cells. Heart Rhythm. 2010;7(1):127–35. doi: 10.1016/j.hrthm.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y, Hill JA. Electrophysiological remodeling in heart failure. J Mol Cell Cardiol. 2010;48(4):619–32. doi: 10.1016/j.yjmcc.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tomaselli GF, Marban E. Electrophysiological remodeling in hypertrophy and heart failure. Cardiovasc Res. 1999;42(2):270–83. doi: 10.1016/s0008-6363(99)00017-6. [DOI] [PubMed] [Google Scholar]
- 38.Akar FG, Tomaselli GF. Ion channels as novel therapeutic targets in heart failure. Ann Med. 2005;37(1):44–54. doi: 10.1080/07853890510007214. [DOI] [PubMed] [Google Scholar]
- 39.Xiao J, et al. MicroRNA miR-133 represses HERG K+ channel expression contributing to QT prolongation in diabetic hearts. J Biol Chem. 2007;282(17):12363–7. doi: 10.1074/jbc.C700015200. [DOI] [PubMed] [Google Scholar]
- 40.Yang B, et al. The muscle-specific microRNA miR-1 regulates cardiac arrhythmogenic potential by targeting GJA1 and KCNJ2. Nat Med. 2007;13(4):486–91. doi: 10.1038/nm1569. [DOI] [PubMed] [Google Scholar]
- 41.Sasano T, et al. Molecular ablation of ventricular tachycardia after myocardial infarction. Nat Med. 2006;12(11):1256–8. doi: 10.1038/nm1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hill JA. Electrical remodeling in cardiac hypertrophy. Trends Cardiovasc Med. 2003;13(8):316–22. doi: 10.1016/j.tcm.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 43.Wang Y, et al. Ca2+/calmodulin-dependent protein kinase II-dependent remodeling of Ca2+ current in pressure overload heart failure. J Biol Chem. 2008;283(37):25524–32. doi: 10.1074/jbc.M803043200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martinez ML, Heredia MP, Delgado C. Expression of T-type Ca(2+) channels in ventricular cells from hypertrophied rat hearts. J Mol Cell Cardiol. 1999;31(9):1617–25. doi: 10.1006/jmcc.1999.0998. [DOI] [PubMed] [Google Scholar]
- 45.Nuss HB, Houser SR. T-type Ca2+ current is expressed in hypertrophied adult feline left ventricular myocytes. Circ Res. 1993;73(4):777–82. doi: 10.1161/01.res.73.4.777. [DOI] [PubMed] [Google Scholar]
- 46.The effect of diltiazem on mortality and reinfarction after myocardial infarction. The Multicenter Diltiazem Postinfarction Trial Research Group. N Engl J Med. 1988;319(7):385–92. doi: 10.1056/NEJM198808183190701. [DOI] [PubMed] [Google Scholar]
- 47.Makielski JC, Valdivia CR. Ranolazine and late cardiac sodium current--a therapeutic target for angina, arrhythmia and more? Br J Pharmacol. 2006;148(1):4–6. doi: 10.1038/sj.bjp.0706713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scirica BM, et al. Effect of ranolazine, an antianginal agent with novel electrophysiological properties, on the incidence of arrhythmias in patients with non ST-segment elevation acute coronary syndrome: results from the Metabolic Efficiency With Ranolazine for Less Ischemia in Non ST-Elevation Acute Coronary Syndrome Thrombolysis in Myocardial Infarction 36 (MERLIN-TIMI 36) randomized controlled trial. Circulation. 2007;116(15):1647–52. doi: 10.1161/CIRCULATIONAHA.107.724880. [DOI] [PubMed] [Google Scholar]
- 49.Wang Z, et al. Na+-Ca2+ exchanger remodeling in pressure overload cardiac hypertrophy. J Biol Chem. 2001;276(21):17706–11. doi: 10.1074/jbc.M100544200. [DOI] [PubMed] [Google Scholar]
- 50.Bers DM, Pogwizd SM, Schlotthauer K. Upregulated Na/Ca exchange is involved in both contractile dysfunction and arrhythmogenesis in heart failure. Basic Res Cardiol. 2002;97(Suppl 1):I36–42. doi: 10.1007/s003950200027. [DOI] [PubMed] [Google Scholar]
- 51.Antoons G, Sipido KR. Targeting calcium handling in arrhythmias. Europace. 2008;10(12):1364–9. doi: 10.1093/europace/eun271. [DOI] [PubMed] [Google Scholar]
- 52.Hasenfuss G, Pieske B. Calcium cycling in congestive heart failure. J Mol Cell Cardiol. 2002;34(8):951–69. doi: 10.1006/jmcc.2002.2037. [DOI] [PubMed] [Google Scholar]
- 53.Marx SO, et al. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101(4):365–76. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- 54.Ai X, et al. Ca2+/calmodulin-dependent protein kinase modulates cardiac ryanodine receptor phosphorylation and sarcoplasmic reticulum Ca2+ leak in heart failure. Circ Res. 2005;97(12):1314–22. doi: 10.1161/01.RES.0000194329.41863.89. [DOI] [PubMed] [Google Scholar]
- 55.Curran J, et al. Beta-adrenergic enhancement of sarcoplasmic reticulum calcium leak in cardiac myocytes is mediated by calcium/calmodulin-dependent protein kinase. Circ Res. 2007;100(3):391–8. doi: 10.1161/01.RES.0000258172.74570.e6. [DOI] [PubMed] [Google Scholar]
- 56.Sipido KR. CaM or cAMP: linking beta-adrenergic stimulation to `leaky' RyRs. Circ Res. 2007;100(3):296–8. doi: 10.1161/01.RES.0000259326.68260.20. [DOI] [PubMed] [Google Scholar]
- 57.Yano M, et al. Mechanisms of Disease: ryanodine receptor defects in heart failure and fatal arrhythmia. Nat Clin Pract Cardiovasc Med. 2006;3(1):43–52. doi: 10.1038/ncpcardio0419. [DOI] [PubMed] [Google Scholar]
- 58.Gonzalez DR, et al. Impaired S-nitrosylation of the ryanodine receptor caused by xanthine oxidase activity contributes to calcium leak in heart failure. J Biol Chem. 2010;285(37):28938–45. doi: 10.1074/jbc.M110.154948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Terentyev D, et al. Redox modification of ryanodine receptors contributes to sarcoplasmic reticulum Ca2+ leak in chronic heart failure. Circ Res. 2008;103(12):1466–72. doi: 10.1161/CIRCRESAHA.108.184457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fozzard HA. Afterdepolarizations and triggered activity. Basic Res Cardiol. 1992;87(Suppl 2):105–13. doi: 10.1007/978-3-642-72477-0_10. [DOI] [PubMed] [Google Scholar]
- 61.Venetucci LA, et al. The sarcoplasmic reticulum and arrhythmogenic calcium release. Cardiovasc Res. 2008;77(2):285–92. doi: 10.1093/cvr/cvm009. [DOI] [PubMed] [Google Scholar]
- 62.Bers DM. Altered cardiac myocyte Ca regulation in heart failure. Physiology (Bethesda) 2006;21:380–7. doi: 10.1152/physiol.00019.2006. [DOI] [PubMed] [Google Scholar]
- 63.Cutler MJ, et al. Targeted SERCA2a gene expression identifies molecular mechanism and therapeutic target for arrhythmogenic cardiac alternans. Circ Arrhythm Electrophysiol. 2009;2(6):686–94. doi: 10.1161/CIRCEP.109.863118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.del Monte F, et al. Abrogation of ventricular arrhythmias in a model of ischemia and reperfusion by targeting myocardial calcium cycling. Proc Natl Acad Sci U S A. 2004;101(15):5622–7. doi: 10.1073/pnas.0305778101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wilson LD, et al. Heart failure enhances susceptibility to arrhythmogenic cardiac alternans. Heart Rhythm. 2009;6(2):251–9. doi: 10.1016/j.hrthm.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Prunier F, et al. Prevention of ventricular arrhythmias with sarcoplasmic reticulum Ca2+ ATPase pump overexpression in a porcine model of ischemia reperfusion. Circulation. 2008;118(6):614–24. doi: 10.1161/CIRCULATIONAHA.108.770883. [DOI] [PubMed] [Google Scholar]
- 67.Wehrens XH, et al. Protection from cardiac arrhythmia through ryanodine receptor-stabilizing protein calstabin2. Science. 2004;304(5668):292–6. doi: 10.1126/science.1094301. [DOI] [PubMed] [Google Scholar]
- 68.Strom M, et al. Gap junction heterogeneity as mechanism for electrophysiologically distinct properties across the ventricular wall. Am J Physiol Heart Circ Physiol. 2010;298(3):H787–94. doi: 10.1152/ajpheart.00887.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Akar FG, et al. Dynamic changes in conduction velocity and gap junction properties during development of pacing-induced heart failure. Am J Physiol Heart Circ Physiol. 2007;293(2):H1223–30. doi: 10.1152/ajpheart.00079.2007. [DOI] [PubMed] [Google Scholar]
- 70.Akar FG, et al. Mechanisms underlying conduction slowing and arrhythmogenesis in nonischemic dilated cardiomyopathy. Circ Res. 2004;95(7):717–25. doi: 10.1161/01.RES.0000144125.61927.1c. [DOI] [PubMed] [Google Scholar]
- 71.Dupont E, et al. Altered connexin expression in human congestive heart failure. J Mol Cell Cardiol. 2001;33(2):359–71. doi: 10.1006/jmcc.2000.1308. [DOI] [PubMed] [Google Scholar]
- 72.Poelzing S, Rosenbaum DS. Altered connexin43 expression produces arrhythmia substrate in heart failure. Am J Physiol Heart Circ Physiol. 2004;287(4):H1762–70. doi: 10.1152/ajpheart.00346.2004. [DOI] [PubMed] [Google Scholar]
- 73.Kjolbye AL, et al. Maintenance of intercellular coupling by the antiarrhythmic peptide rotigaptide suppresses arrhythmogenic discordant alternans. Am J Physiol Heart Circ Physiol. 2008;294(1):H41–9. doi: 10.1152/ajpheart.01089.2006. [DOI] [PubMed] [Google Scholar]