Abstract
Oxygen (O2) is essential for aerobic life; however, the level of O2, whether too low (hypoxia) or too high (hyperoxia), can induce oxidative injury and increase morbidity and mortality. Disruption of O2 homeostasis represents a major aspect of many disease etiologies and pathobiology. In the past, our laboratory has been using Drosophila melanogaster to investigate the cellular and molecular aspects of the response to hypoxia and oxidative stress. There are several advantages for using Drosophila as a model system, the most important one being an evolutionary conservation of genetic and signaling pathways from Drosophila to mammals. As a proof of this concept, we have shown that we can substantially improve the tolerance of human cells in culture by transfecting these cells with particular Drosophila genes. In this review, we summarize the recent findings from our laboratory using Drosophila as a model system to investigate the genetic basis of hypoxia/hyperoxia tolerance. We have done microarray studies and identified several oxidative stress resistance genes that play an important role in individual paradigms such as constant or intermittent hypoxia, short term (days) or long term (generations) hypoxia/hyperoxia. Our studies provide evidence that a pattern of oxidative stress is specific in inducing a gene expression profile which, in turn, plays an important role in modulating the phenotype. To improve our understanding of oxidative and hypoxic stress as well as its associated diseases, multi-disciplinary approaches are necessary and critical in the study of complicated issues in systems biology.
1. Introduction
Oxygen (O2) is essential for aerobic life; however, the level of O2, either too low or too high, can induce oxidative stress and increase morbidity and mortality. Disruption of O2 homeostasis represents a major aspect of disease etiology and pathobiology. Low oxygen (hypoxia) is often a major clinical problem and is associated with many diseases, for instance ischemic heart disease, cerebral ischemia (e.g., stroke, global ischemia, perinatal hypoxia-ischemia and trauma), complications of diabetes, pulmonary hypertension, obstetrical /perinatal complications (e.g., pre-eclampsia, retinopathy of prematurity and sudden infant death syndrome), High Altitude illnesses (such as high altitude pulmonary edema and high-altitude cerebral edema), and other consequences of cardio-respiratory disorders such as bronchopulmonary dysplasia and obstructive sleep apnea). Furthermore, conditions such as ischemia/perfusion of transplant and organ transplantation and cancer progression are important considerations in transplantation and cancer metastasis. High oxygen (hyperoxia) results in adverse effects on almost every organ including lung, retina, heart and brain, for instance, hyperoxia can lead to retinopathy of prematurity and lung injury.
The pattern of hypoxia is also important. For example, whether the organism experiences constant or intermittent hypoxia is important and these differences in pattern frequently occur in disease states. Intermittent hypoxia (IH) is associated with obstructive sleep apnea, central hypoventilation syndrome and intermittent vascular occlusion in sickle cell anemia. Constant hypoxia (CH) is associated with pulmonary disease such as asthma, and congenital heart disease with right to left shunt. Whether for IH or CH, various studies, using rodents as animal models have experimentally examined the effects of hypoxia on specific tissues such as heart, brain, and kidneys [1–5]. These studies have demonstrated that the response to low O2 is not only dependent on intensity and duration of the stimulus but also on the paradigm used.
For both short term and longer term hypoxia, the differences in the fundamental mechanisms underlying the responses to hypoxia are however not well understood. In spite of the fact that we know that IH and CH (short term or longer term) involve a differential expression of genes and pathways [1, 6, 7], we do not have a good appreciation as to whether these genes are important for the observed phenotype. While it is possible after obtaining results from microarray data to study the role of single or multiple genes in inducing the phenotype, it is rather difficult to perform such studies quickly in vivo in mice. Another way to approach this problem is by studying some of these questions in a model organism, as we have done in the past [8–10], to prove the role of certain genes in the phenotype and then investigate orthologs in mammals, such as rodents, and ultimately in humans. The advantage of using model systems such as Drosophila melanogaster is the relative speed with which one can perform such studies, especially because of 1) >65–70% of human disease genes are present in Drosophila, and 2) the availability of genetic markers and tools. For instance, many mutant/overexpression lines including P-element insertion and UAS lines are commercially available from a national Stock Center. A P-element is a transposon that is inserted within or around a gene and causes alterations in expression and function of specific genes. A UAS is a modified P-element containing a promoter (upstream activator sequences) that is activated by the Gal4 transcription factors. Therefore, overexpression of a gene of interest with a UAS construct can be obtained upon the presence of Gal4 drivers. By taking advantage of available P-element and UAS lines and testing their survival under hypoxic conditions, we are able to study the role of individual genes in hypoxia.
Our previous studies have shown that Drosophila is extremely resistant to hypoxia or even anoxia for a few hours [8, 9, 11]. The brain of these animals, for example, does not suffer any damage (as determined by light or electron-microscopy) after a period of anoxia that can induce irreversible injury and death in rodents [9, 11, 12]. In the past, we have used similar approaches including forward and reverse genetic approaches and identified several hypoxia-regulated genes [12]. We have also done microarray studies that have provided us with insight regarding tolerance of flies to long term (over many generations) hypoxia [13]. In this review, we will highlight three take-home messages: a) the pattern of hypoxia is important in inducing a differential gene expression profile which, in turn, plays an important role in modulating the phenotype; b) longer term hypoxia, such as after generations, may change the DNA sequence of organisms; and c) multi-disciplinary approaches for the study of hypoxia become critical especially in the study of a complicated issue such as hypoxia, including systems biology.
2. Short term hypoxia in Drosophila
We have applied short term CH or IH on Drosophila as a model system. For CH, the O2 level was maintained at 1% O2 continuously for 2.5 hours. For IH, the cycle consisted of a 4 min period of 1% O2 concentration alternating with 4 min of 21% O2 concentration. The ramp time was 1 min for 1%–21% O2 and around 10 minutes for 21%–1% O2. Hence, the total time of one complete IH cycle is about 20 min and flies were exposed to IH for 2.5 hours. Our current genome-wide study was designed to investigate gene expression changes and identify protective mechanism(s) in Drosophila melanogaster after exposure to severe (1% O2) hypoxia. We observed distinct responses to IH and CH in gene expression that varied in number and types of genes or gene families [14]. The role of candidate genes (up-or down-regulated) was then studied in hypoxia under severe CH or IH. Heat shock protein up-regulation (e.g., Hsp70 or Hsp23) led to a significant increase in adult survival during CH. In contrast, during IH treatment the up-regulation of specific genes such as Multi drug resistance 49 (Mdr49) and lethal (2) 08717 (l(2)08717) genes (P-element lines) provided survival advantage over controls. This demonstrated that the increased transcript levels following treatment with either paradigm played an important role in tolerance to severe hypoxia. Furthermore, by over-expressing Hsp70 in specific tissues, we found that up-regulation of Hsp70 in heart, brain and other singular specific organs was critical in the tolerance of flies to CH (Fig. 1). Of interest is that the up- or down- regulation of specific genes that were differentially expressed in one paradigm (say CH) were not beneficial in the other paradigm (say IH) and vice versa. These data provide further clues about the mechanisms by which IH or CH lead to cell injury and morbidity or adaptation and survival.
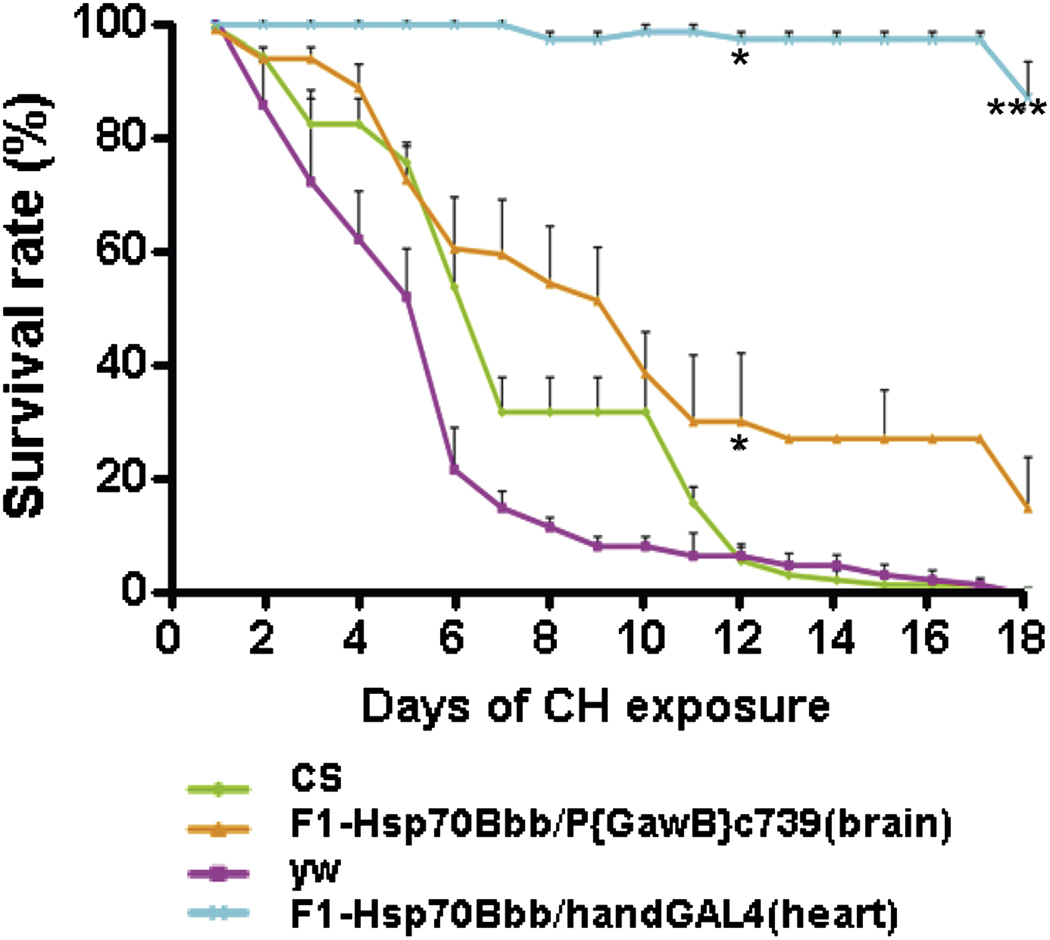
Fig. 1. Role of hsp70 in short term hypoxia (days).
Flies with tissue specific overexpression of Hsp70 were obtained by crossing UAS-hsp70 with various tissue specific Gal4 drivers. Progeny from these crosses along with Canton-S (CS) and yellow white (yw) serving as controls were exposed to 1.5 % O2 (constant hypoxia) and survival was scored every day. Flies with Hsp70 overexpression in the heart (Hsp70Bbb/handGal4) and brain (Hsp70Bbb/P{GawB}c739) have a remarkably increased survival in 1.5% O2 compared to control flies. * indicates p < 0.05 and *** indicates p < 0.001 (Azad et al., 2009).
3. Long term hypoxia in Drosophila
Some of the interesting advantages of Drosophila melanogaster are that a) life span and the time from a fertilized embryo to pupal eclosion is relatively short (e.g., days); b) they reproduce at a high rate (one female produces >300 eggs in its life time) and c) one can manipulate them genetically and tools are available to study at a molecular level a variety of questions such as those we are investigating here. When we think of short term and longer term hypoxia, while in the first paradigm (short term) changes occur over hours or days a longer term paradigm would span several generations since flies have a relatively limited life span. Hence, we not only embarked on the study of short term hypoxia but also used a long term protocol, i.e., across generations.
In order to perform such a long term study across generations, we used twenty-seven wild-type isogenic lines and these constituted the parental population that we used for the long-term experiment. At baseline, there was significant variability in hypoxia tolerance among these 27 lines, as determined by eclosion rate under 5% O2, an optimized O2 concentration to differentiate the genetic diversity within parental lines. The idea to use an O2 level that we decrease over time was to select for a more resistant strain of Drosophila melanogaster. In order to determine the level of O2 at which to initiate the long-term selection experiment, we performed a pilot study by culturing F1 embryos of the parental flies under different levels of hypoxia (e.g., 8%, 6% or 4% O2). We found that their survival rate was reduced at 6% O2, and no adult flies were actually obtained at 4% O2. At 8% O2 the majority of embryos (>80%) reached the adult stage. Therefore, hypoxia selection was initiated at 8% O2, an O2 level in which flies can develop throughout their life cycle. The O2 concentration was then gradually decreased by ~1% every 3 to 5 generations to maintain the selection pressure. By the 13th generation, flies could complete development and perpetually live in 5% O2; and by the 32nd generation, the adapted flies could even live perpetually under a severer level (4% O2), a lethal condition for the naïve ones [13]. We hypothesized that this is due to, at least partially, newly occurring mutations or recombination and selection of favorable alleles in the adapted population. To test this hypothesis, a subset of embryos from selected flies was cultured under normoxia for several consecutive generations. After 8 generations in normoxia, these adapted flies were re-introduced into the lethal hypoxic environment (4% O2), and again, the majority (>80%) of the flies completed their development and could be maintained in this extreme condition perpetually. This result strongly suggested that the hypoxia-tolerance in the selected flies had become a heritable trait. Of interest, these flies manifested a number of phenotypic changes. Some of these are a) the fact that these flies are much smaller at 4% O2 than the naïve ones and their reduced size is a result of a reduction in size and number of cells, and b) their O2 consumption/gram tissue, although reduced dramatically during 3% O2 hypoxia, this reduction was less than that in naïve flies.
Gene expression profiling showed striking differences between tolerant and naïve flies, in larvae and adults, both quantitatively and qualitatively [15]. Several genes from signal transduction pathways (e.g., Notch and Toll/Imd pathways) were significantly up-regulated in the selected flies, but metabolic genes were remarkably down-regulated in the larvae (Fig. 2A). The transcriptional suppressor, hairy, was up-regulated in the microarrays and its binding elements were present in the regulatory region of the specifically down-regulated metabolic genes but not others, and mutations in hairy significantly reduced hypoxia tolerance (Fig. 2B & 2C). We have concluded from these studies that, the hypoxia-selected flies: a) altered their gene expression and potentially their genetic code, and b) coordinated their metabolic suppression, especially during development, with hairy acting as a metabolic switch, thus playing a crucial role in hypoxia tolerance.
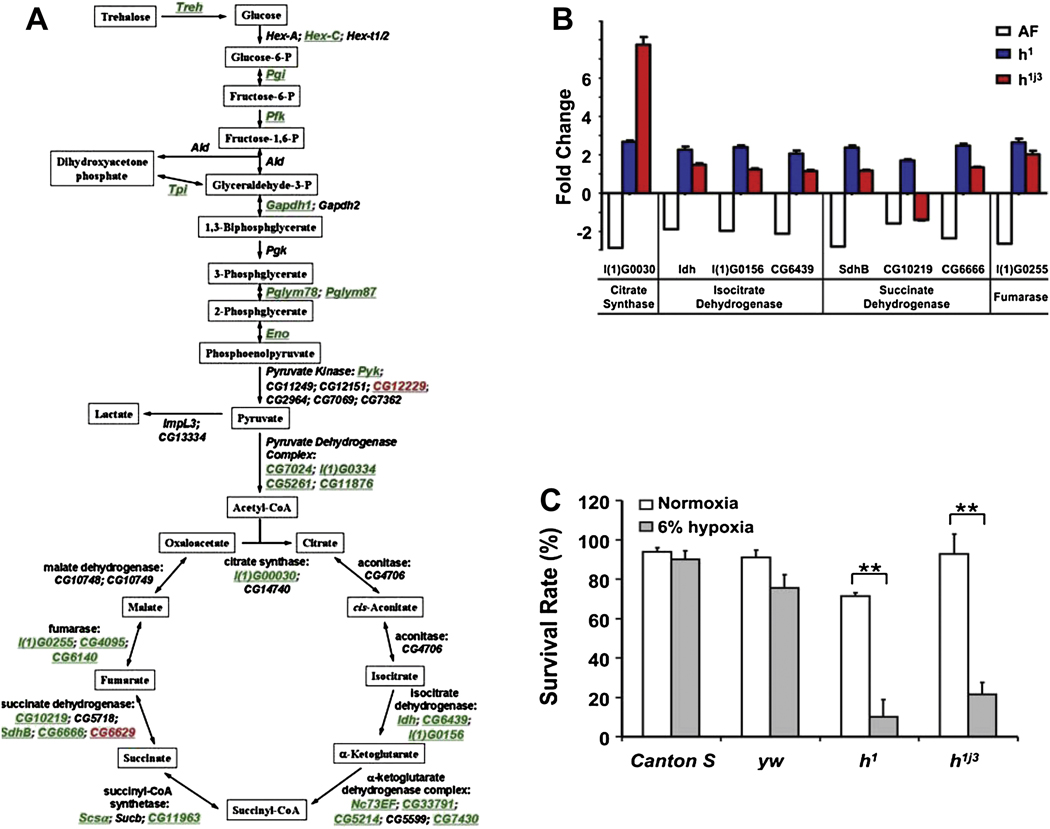
Fig. 2. Role of hairy in long term hypoxia (generations).
A) Schematic illustration of the alterations in genes encoding glycolytic and TCA cycle enzymes in hypoxia-selected flies as compared to control flies (green: down-regulation, black: not significantly changed, red: up-regulation). B) The expression of genes encoding TCA cycle enzymes, which are regulated by hairy at the transcriptional level, was determined in hypoxia-selected flies and in hairy loss-of-function mutants using real-time PCR. Individual genes encoding TCA cycle enzymes that were significantly down regulated in hypoxia-selected flies, had a significantly higher expression level in all hairy mutants than yw, control flies, except gene CG10219 (p<0.01). Gene description: l(1)G0030: citrate synthase; Idh: isocitrate dehydrogenase; l(1)G0156: isocitrate dehydrogenase; CG6439: isocitrate dehydrogenase; SdhB: succinate dehydrogenase; CG10219: succinate dehydrogenase; CG6666: succinate dehydrogenase; l(1)G0255: fumarase. C) Embryos from loss-of-function hairy mutants (h1 and h1j3) and controls (Canton-S and yw) were cultured at normoxic or 6% O2 hypoxic condition. The ratio of adult flies to pupae was determined as survival rate of each stock. The loss-of-function hairy mutants exhibited a significantly reduced rate of hypoxia survival (p < 0.01). ** indicates p<0.01 (Zhou et al., 2008).
We have, in addition to these microarray studies, performed re-sequencing studies across the genome of the adapted versus the naïve flies. We have also applied novel computing and analytical methods to identify a number of DNA regions under selection. Several of the hypoxia-selected regions containing genes encoding or regulating the Notch pathway appeared. We confirmed the contribution of Notch activation to hypoxia tolerance using a specific γ-secretase inhibitor, DAPT, which significantly reduced adult survival and lifespan in the hypoxia-selected flies. We also demonstrated that flies with loss-of-function Notch mutations or RNAi-mediated Notch knockdown had a significant reduction in hypoxia tolerance, but those with a gain-of-function had a dramatic, opposite effect. Using the UAS-Gal4 system, we also showed that specific over-expression of the Notch intracellular domain in glial cells was critical for conferring hypoxia tolerance. Novel analytical tools, genetic and bioinformatic strategies allowed us to discover that Notch activation plays a major and unsuspected role in this hypoxia tolerance in Drosophila melanogaster.
4. Long term hyperoxia in Drosophila
Prolonged exposure to hyperoxia generates excessive reactive oxygen species, induces cell death and oxidative stress responses, affects immune response and DNA integrity and modulates cell growth [16–20]. Disorders including neurodegenerative and chronic inflammatory diseases, as well as damage from ischemia and consequent reperfusion to the heart, lung, retina, brain, and other organs result, by and large, from oxidant injury. Mammalian aging can also be attributed, at least in part, to oxidant injury. Numerous studies have defined the phenotype of hyperoxia-induced injury and explored the underlying mechanisms, especially in lung and retina [17, 21–24]. In addition, the newborn mammal has been shown to be more resistant to hyperoxia challenge than the adult, possibly reflecting differing O2 defense mechanisms. However, conclusions drawn from many of these studies are at best correlative.
The importance of oxidative stress is not limited to mammalian tissues. Drosophila melanogaster has similar O2 response pathways to those of mammals and research on flies has enhanced our understanding of oxidant stress [8, 25, 26]. To study the mechanisms underlying hyperoxia tolerance, much like our studies in hypoxia, we have generated a Drosophila melanogaster strain that is extraordinarily resistant to high O2 levels through laboratory selection over many generations, mirror image selection experiments as detailed above. In brief, hyperoxia selection was initiated at 60% O2 and the O2 concentration was then gradually increased by ~10% every 3 to 5 generations to maintain the selection pressure. By the 13th generation, flies could complete development and perpetually live in 90% O2, a lethal condition for the naïve ones. Currently, these flies are living and reproducing at 90% – 95% O2 without any overt signs of injury [27].
We first demonstrated that tolerance to hyperoxia was also heritable in these flies and that these hyperoxia-selected flies exhibited phenotypic differences from naïve flies, such as a larger body size and increased weight by 20%. We have done microarray analysis using this novel hyperoxia-selected strain as a model to identify differentially expressed genes that are significantly altered by hyperoxia selection and to investigate the role of single genes that may functionally contribute to hyperoxia tolerance. Gene expression profiling revealed that many genes (i.e., 227 genes) were significantly altered in expression and two thirds of these genes were down-regulated. Using a mutant screen strategy, we studied the role of some altered genes (up- or down-regulated in the microarrays) by testing the survival of available corresponding P-element or UAS construct lines under hyperoxic conditions. A number of down-regulated genes including Tropomyosin 1, Glycerol 3 phosphate dehydrogenase, CG33129, and UGP (Fig. 3A) as well as the up-regulation of Diptericin and Attacin (anti-microbial peptides, AMP) (Fig. 3B) conferred tolerance to severe hyperoxia. We therefore identified several genes that were not only altered in hyperoxia-selected flies but we also proved that these play an important role in hyperoxia survival. Thus our study provides a molecular basis for understanding the mechanisms of hyperoxia tolerance.
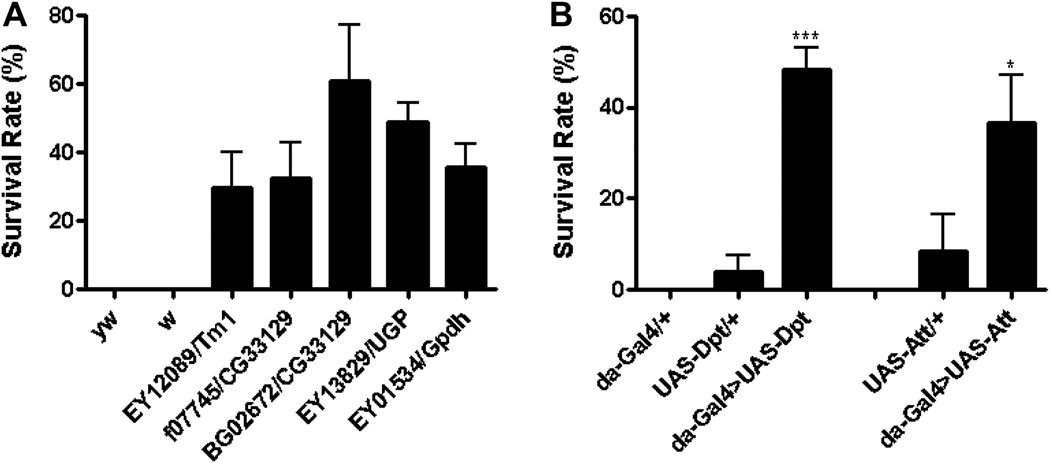
Fig. 3. Role of individual genes in long term hyperoxia (generations).
A) Embryos from 47 P-element insertion lines were collected and exposed to 90% O2 and the ratio of adult flies to pupae was determined. Embryos from five P-element insertion lines that were able to develop into adult stage as compared to no survival in control flies including yw and white (w). B) Flies with ubiquitous overexpression of antimicrobial peptide (AMP) genes were obtained by crossing UAS-AMP with da-Gal4. Embryos from these crosses were exposed to 90% O2 and survival rate was determined as above. Flies with overexpression of Diptericin (da-Gal4>UAS-Dpt, p < 0.001) and Attacin (da-Gal4>UAS-Att, p < 0.05) had a significantly increased survival rate in hyperoxia as compared to the controls. * indicates p < 0.05; *** indicates p < 0.001 (Zhao et al., 2010).
5. Conclusions
Studies of hypoxia and hyperoxia are of crucial importance for improving the understanding of many diseases that afflict humans across the age spectrum. These include diseases such as ischemic heart disease, stroke, perinatal and placental insufficiencies, cancer and a number of diseases that have at their basis oxidant stress and injury. O2 biology and O2 homeostasis is surfacing as one of the most important areas of research of human biology as it spans a whole range of conditions that lead to major morbidity and mortality world-wide.
Acknowledgements
Supported by NIH grant RO1NS037756 and PO1HD032573 to Gabriel G. Haddad.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fan C, Iacobas DA, Zhou D, Chen Q, Lai JK, Gavrialov O, Haddad GG. Gene expression and phenotypic characterization of mouse heart after chronic constant or intermittent hypoxia. Physiol Genomics. 2005;22(3):292–307. doi: 10.1152/physiolgenomics.00217.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Farahani R, Kanaan A, Gavrialov O, Brunnert S, Douglas RM, Morcillo P, Haddad GG. Differential effects of chronic intermittent and chronic constant hypoxia on postnatal growth and development. Pediatr Pulmonol. 2008;43(1):20–28. doi: 10.1002/ppul.20729. [DOI] [PubMed] [Google Scholar]
- 3.Iacobas DA, Fan C, Iacobas S, Haddad GG. Integrated transcriptomic response to cardiac chronic hypoxia: translation regulators and response to stress in cell survival. Funct Integr Genomics. 2008;8(3):265–275. doi: 10.1007/s10142-008-0082-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iacobas DA, Fan C, Iacobas S, Spray DC, Haddad GG. Transcriptomic changes in developing kidney exposed to chronic hypoxia. Biochem Biophys Res Commun. 2006;349(1):329–338. doi: 10.1016/j.bbrc.2006.08.056. [DOI] [PubMed] [Google Scholar]
- 5.Zhou D, Wang J, Zapala MA, Xue J, Schork NJ, Haddad GG. Gene expression in mouse brain following chronic hypoxia: role of sarcospan in glial cell death. Physiol Genomics. 2008;32(3):370–379. doi: 10.1152/physiolgenomics.00147.2007. [DOI] [PubMed] [Google Scholar]
- 6.Nanduri J, Nanduri RP. Cellular mechanisms associated with intermittent hypoxia. Essays Biochem. 2007;43:91–104. doi: 10.1042/BSE0430091. [DOI] [PubMed] [Google Scholar]
- 7.Wu W, Dave NB, Yu G, Strollo PJ, Kovkarova-Naumovski E, Ryter SW, Reeves SR, Dayyat E, Wang Y, Choi AM, Gozal D, Kaminski N. Network analysis of temporal effects of intermittent and sustained hypoxia on rat lungs. Physiol Genomics. 2008;36(1):24–34. doi: 10.1152/physiolgenomics.00258.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haddad GG, Sun Y, Wyman RJ, Xu T. Genetic basis of tolerance to O2 deprivation in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1997;94(20):10809–10812. doi: 10.1073/pnas.94.20.10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma E, Haddad GG. Anoxia regulates gene expression in the central nervous system of Drosophila melanogaster. Brain Res Mol Brain Res. 1997;46(1–2):325–328. doi: 10.1016/s0169-328x(97)00074-0. [DOI] [PubMed] [Google Scholar]
- 10.Chen Q, Behar KL, Xu T, Fan C, Haddad GG. Expression of Drosophila trehalose-phosphate synthase in HEK-293 cells increases hypoxia tolerance. J Biol Chem. 2003;278(49):49113–49118. doi: 10.1074/jbc.M308652200. [DOI] [PubMed] [Google Scholar]
- 11.Haddad GG, Wyman RJ, Mohsenin A, Sun Y, Krishnan SN. Behavioral and Electrophysiologic Responses of Drosophila melanogaster to Prolonged Periods of Anoxia. J Insect Physiol. 1997;43(3):203–210. doi: 10.1016/s0022-1910(96)00084-4. [DOI] [PubMed] [Google Scholar]
- 12.Ma E, Gu XQ, Wu X, Xu T, Haddad GG. Mutation in pre-mRNA adenosine deaminase markedly attenuates neuronal tolerance to O2 deprivation in Drosophila melanogaster. J Clin Invest. 2001;107(6):685–693. doi: 10.1172/JCI11625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou D, Xue J, Chen J, Morcillo P, Lambert JD, White KP, Haddad GG. Experimental selection for Drosophila survival in extremely low O2 environment. PLoS ONE. 2007;2(5):e490. doi: 10.1371/journal.pone.0000490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Azad P, Zhou D, Russo E, Haddad GG. Distinct mechanisms underlying tolerance to intermittent and constant hypoxia in Drosophila melanogaster. PLoS One. 2009;4(4):e5371. doi: 10.1371/journal.pone.0005371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou D, Xue J, Lai JC, Schork NJ, White KP, Haddad GG. Mechanisms underlying hypoxia tolerance in Drosophila melanogaster: hairy as a metabolic switch. PLoS Genet. 2008;4(10):e1000221. doi: 10.1371/journal.pgen.1000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barker GF, Manzo ND, Cotich KL, Shone RK, Waxman AB. DNA damage induced by hyperoxia: quantitation and correlation with lung injury. Am J Respir Cell Mol Biol. 2006;35(3):277–288. doi: 10.1165/rcmb.2005-0340OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhandari V, Choo-Wing R, Homer RJ, Elias JA. Increased hyperoxia-induced mortality and acute lung injury in IL-13 null mice. J Immunol. 2007;178(8):4993–5000. doi: 10.4049/jimmunol.178.8.4993. [DOI] [PubMed] [Google Scholar]
- 18.Lee PJ, Choi AM. Pathways of cell signaling in hyperoxia. Free Radic Biol Med. 2003;35(4):341–350. doi: 10.1016/s0891-5849(03)00279-x. [DOI] [PubMed] [Google Scholar]
- 19.Masalunga C, Rozycki HJ, Mainali ES. The impact of hyperoxia on the neonatal and adult developing dendritic cell. Pediatr Res. 2007;62(1):78–82. doi: 10.1203/PDR.0b013e3180674dc6. [DOI] [PubMed] [Google Scholar]
- 20.Ogawa Y, Tasaka S, Yamada W, Saito F, Hasegawa N, Miyasho T, Ishizaka A. Role of Toll-like receptor 4 in hyperoxia-induced lung inflammation in mice. Inflamm Res. 2007;56(8):334–338. doi: 10.1007/s00011-007-7052-z. [DOI] [PubMed] [Google Scholar]
- 21.Bhandari V, Elias JA. Cytokines in tolerance to hyperoxia-induced injury in the developing and adult lung. Free Radic Biol Med. 2006;41(1):4–18. doi: 10.1016/j.freeradbiomed.2006.01.027. [DOI] [PubMed] [Google Scholar]
- 22.Wright CJ, Zhuang T, La P, Yang G, Dennery PA. Hyperoxia-induced NF-kappaB activation occurs via a maturationally sensitive atypical pathway. Am J Physiol Lung Cell Mol Physiol. 2009;296(3):L296–L306. doi: 10.1152/ajplung.90499.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chetty A, Cao GJ, Manzo N, Nielsen HC, Waxman A. The role of IL-6 and IL-11 in hyperoxic injury in developing lung. Pediatr Pulmonol. 2008;43(3):297–304. doi: 10.1002/ppul.20777. [DOI] [PubMed] [Google Scholar]
- 24.Dasgupta C, Sakurai R, Wang Y, Guo P, Ambalavanan N, Torday JS, Rehan VK. Hyperoxia-induced neonatal rat lung injury involves activation of TGF-{beta} and Wnt signaling and is protected by rosiglitazone. Am J Physiol Lung Cell Mol Physiol. 2009;296(6):L1031–L1041. doi: 10.1152/ajplung.90392.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khurana V. Modeling Tauopathy in the fruit fly Drosophila melanogaster. J Alzheimers Dis. 2008;15(4):541–553. doi: 10.3233/jad-2008-15403. [DOI] [PubMed] [Google Scholar]
- 26.Morrow G, Tanguay RM. Mitochondria and ageing in Drosophila. Biotechnol J. 2008;3(6):728–739. doi: 10.1002/biot.200800015. [DOI] [PubMed] [Google Scholar]
- 27.Zhao HW, Zhou D, Nizet V, Haddad GG. Experimental selection for Drosophila survival in extremely high O2 environments. PLoS One. 5(7):e11701. doi: 10.1371/journal.pone.0011701. [DOI] [PMC free article] [PubMed] [Google Scholar]