Abstract
The endocrinology of the aging male is complex, with multiple hormones along the hypothalamic-pituitary-testicular (HPT) axis interacting with one another in feedback. As men age, there is a small and progressive (not precipitous, as in women) decline in several sex hormones, in particular testosterone and dehydroepiandrosterone, and related increases in luteinizing hormone, follicle-stimulating hormone, and sex hormone-binding globulin. The importance of these changes is wide-ranging because of the ubiquitous role of sex hormones in male physiology. This chapter discusses the endocrinology of the aging male. We provide an overview of the regulation of the HPT axis with an emphasis on the changes that occur with aging and the measurement of gonadal steroids, including hormone pulsatility, within-subject and circadian variations. The difficulties of assessing the symptoms of late-onset hypogonadism are highlighted. There is a comprehensive discussion of the epidemiology of sex hormone changes, including their age associations, prevalence of symptomatic hypogonadism, secular changes, risk factors, and the association of sex hormones with outcomes.
Keywords: Aging, androgens, hormones, hypogonadism, men, testosterone
II. Introduction
Aging is associated with degenerative changes in multiple organ systems. The rate and extent to which these occur depend on genetics, the presence of other disease processes, and the accumulated effects of socioeconomic, lifestyle, and environmental factors. Although there is no equivalence in men of the abrupt cessation of cyclical ovarian activity that occurs in women, a variable and inconsistent decrease in testosterone with increasing age is observed, albeit that even at very advanced age, sexual and reproductive function may be within normal limits. The age-related decrease in testosterone is primarily due to testicular dysfunction, at least in the absence of disorders that affect the hypothalamic-pituitary testicular (HPT) axis, for example obesity, although some reduction in central responsiveness of the HPT axis may also occur. The extent to which an age-related decrease in testosterone has direct consequences for physical or cognitive function as well as mood and overall quality of life, and the level of testosterone at which these occur remains incompletely resolved, as does the role of the testosterone supplementation.
This chapter discusses the endocrinology of the aging male, with particular focus on the biology and central regulation of the HPT axis, and the epidemiology of sex hormone changes, with implications for diagnosis and management of hypogonadism in aging men. Since testosterone is the most important androgen from a biological perspective and assays are widely available, this chapter focuses on testosterone, with attention paid to other androgens and other sex hormones as appropriate.
III. Biology and Central Regulation of the Hypothalamic-Pituitary-Testicular (HPT) Axis in Men
a. Gonadotrophin-releasing hormone
In a normal adult male, neurons in the preoptic area and the medial basal region of the hypothalamus secrete gonadotrophin-releasing hormone [GnRH] in a pulsatile manner. The periodicity and amplitude of GnRH secretion determine the pattern of secretion of the gonadotrophins, luteinizing hormone (LH) and follicle stimulating hormone (FSH), from the gonadotroph cells of the anterior pituitary.
It has been suggested, but not proven, that neuronal GnRH outflow in healthy men is reduced by 33–50% between the second and eighth decades of life. Feedback from testosterone induces a slowing of the hypothalamic pulse generator and consequently a decrease in the frequency of the LH pulsatile release, 1 but independent of this phenomenon testosterone has a role in maintaining physiological LH pulse frequency and incremental LH pulse size. In the face of eugonadal concentrations of testosterone, young and older men exhibit remarkably similar LH responses to a 300-fold dose range of exogenous GnRH, 2 an observation that implies an absence of a direct effect of aging on gonadotroph function. 2 GnRH dose shortens LH but not FSH secretory bursts and it has been reported that age elevates basal FSH but not LH secretion. 2 Nevertheless data from the European Male Aging Study (EMAS) demonstrates a clear elevation of basal LH with age. 3
Estrogen-dependent mechanisms also mediate testosterone negative feedback on GnRH expression, although there is no effect of targeted disruption of the estrogen receptor on the central regulation of reproductive function in males. Possible increases in circulating estrogen levels with increasing age, not be because of age per se, but as a result of increased body fat and aromatase activity, may lead to a decline in testosterone. In older men, aromatase inhibition increases testosterone levels. 4
Three peptides, kisspeptin, NKB, and dynorphin (DYN), colocalize in a single subpopulation of neurons in the hypothalamic arcuate nucleus (ARC). 5 The inputs from these neurons regulate pulsatile GnRH secretion and, importantly integrate signals relating to nutrition, photoperiod, stress, 6 and inflammation. 7 Accordingly, changes concomitant with aging that affect any of these homeostatic mechanisms may lead to changes in pulsatile GnRH release and testosterone production. The kisspeptins are a family of proteins encoded by the Kiss-1 gene. Kisspeptins and the kisspeptin receptor, GPR54, are pivotal to the central control of reproduction in rodents, primates and humans throughout the lifecycle, 8 and integrate gonadal steroid feedback, environmental and metabolic signals. There is evidence that leptin is a regulator of the hypothalamic KiSS-1 system. 9
Neurokinin B (NKB) and its receptor, NK3R, mediate a predominant effect on LH secretion. 10 NKB induces multiple-unit activity (MUA) bursts in the medial basal hypothalamus that relate directly to LH pulses. 11 One pulse per hour of GnRH sustains gonadotrophin synthesis and secretion. Slower pulse frequencies result in a decline in LH secretion but a rise in FSH secretion. 12 Impaired NKB signaling may specifically slow GnRH pulse frequencies. Dynorphin (DYN), like NKB, may be involved in generating changes in the rhythm of GnRH pulses, but in contrast to NKB inhibits bursts of multiple-unit activity in the medial basal hypothalamus. 11
b. Pituitary gonadotrophs and the gonadotrophins LH and FSH
GnRH is delivered to the anterior pituitary via the hypophyseal portal circulation where it binds to a 7-transmembrane domain, G-protein coupled, receptor (GnRHR) on the surface of gonadotrophs triggering the synthesis and secretion of the gonadotrophins FSH and LH. The gonadotrophins are composed of distinct hormone-specific β subunits paired with a common α subunit (αGSU). LH production is favored by fast pulse frequencies (> 1 pulse per h) and FSH favored by slow pulse frequencies (< 1 pulse per 2–3 h).
LH binds to the LH receptor on the plasma membrane of Leydig cells in the testis resulting in the synthesis of the enzymes of testosterone biosynthesis. LH is also required for Leydig cell differentiation and gonadal growth. A moderate decline in testosterone synthesis capacity may not necessarily be compensated completely by increased LH secretion even in young men, 13 suggesting that there is some tolerance within the system to fluctuating levels of gonadal steroids. FSH regulates spermatogenesis following its binding to the FSH receptor in the basal aspect of the plasma membrane of Sertoli cells in the testis. Deletion of the FSHβ gene does not cause infertility in male mice but does reduce testicular tubule size, sperm number and motility. FSH secretion is modulated by activin and inhibin. 14, 15
Activin is a homodimer produced by the Sertoli cells, peritubular and interstitial cells as well as the pituitary and hypothalamus, where it mediates effects on a purely paracrine basis. Activin binds to activin receptor type II in the gonadotroph cells in the pituitary and stimulates the secretion of FSH. Increase in FSH secretion is also thought to be via activin stimulation of GnRH in the hypothalamus. 16
Inhibin B is a heterodimeric glycoprotein that is predominantly produced in the Sertoli cells and it shows a diurnal rhythm parallel to that of testosterone. Inhibin’s production is stimulated by FSH and it in turn, inhibits the secretion of FSH via a negative feedback mechanism. Inhibin has also been shown to bind to activin receptor type II, reducing activin binding to the receptor and therefore activin’s stimulation of FSH secretion. 17, 18 FSH levels increase with the loss of germinal elements in the testis 19 and FSH has been used as a marker of spermatogenesis. Inhibin B used in combination with FSH is a more sensitive marker of spermatogenesis. 20
c. Gonadal- and tissue-derived sex steroids
Steroidogenic acute regulatory protein (StAR), rapidly synthesized in response to LH actively transports cholesterol from the outer to the inner mitochondrial membrane. The translocator protein (TSPO) mediates the StAR-induced cholesterol transport. Cytochrome P450 enzyme, CYP11A is located on the inner mitochondrial membrane and catalyses the rate limiting step of pregnenolone synthesis. The combined actions of the cytochrome P450 enzyme CYP17 and the HSD enzyme HSD3B2 convert pregnenalone into DHEA and then androstenedione. The enzyme 17β-HSD-3 converts androstenedione to testosterone, and DHEA into 5-androstene-3β,17β-diol which is in turn a substrate for HSD3B2 to form testosterone. 21 All steps beyond the formation of pregnenolone take place in the smooth endoplasmic reticulum.
Stress induced increases in glucocorticoids can suppress testosterone levels in adult males via a direct effect on the testis. Leydig cells express 11 β-HSD-1, an oxidoreductase, and 11 β-HSD-2, a unidirectional oxidase. Under normal physiological conditions, generation of NADPH, a byproduct of glucocorticoid metabolism by 11 β-HSD-1 potentiates testosterone biosynthesis, as NADPH is the cofactor used by steroidogenic enzymes such as 17β-HSD-3. The NAD+ generated drives 11 β-HSD-1 oxidase activity. Accordingly, it is only under stressful conditions when high input amounts of cortisol exceed the capacity of oxidative inactivation by 11 β–HSD that testicular cortisol levels increase to the extent that testosterone synthesis is inhibited. 21 With aging there is an increase in the cortisol production rate and free cortisol levels are increased 22 but whether this mediates age-related decreases in testicular function has not been established. At least in rats, there is an age related decline in 11 β-HSD-2 which therefore limits the degree of protection afforded from increasing glucocorticoid levels. 23
d. Testosterone and SHBG
Testosterone controls sexual differentiation (stabilization of the Wolfian ducts) and is active on skeletal muscle, libido and sexual function. Testosterone is present in plasma as free (unbound testosterone), albumin-bound and sex hormone-binding globulin [SHBG]-bound. SHBG is a plasma glycoprotein produced by hepatocytes and secreted into the blood.
The fraction of testosterone bound to SHBG in serum is proportional to the SHBG level. SHBG production in the liver is regulated by a number of hormones. Estrogen and related steroids, thyroid hormone and insulin increase SHBG levels. SHBG decreases in response to androgens, and in the presence of hypothyroidism, and insulin resistance. Hepatocyte nuclear factor-4 (HNF-4) recruits the transcription initiating complex to the human SHBG promoter. Lipogenesis induced by glucose or fructose inhibits hepatic SHBG expression by reducing cellular HNF-4 levels. 24
SHBG expression indirectly via thyroid hormone-mediated decreases de-novo synthesis of palmitate in the liver and consequently HNF-4 levels increase. 25 The human SHBG promoter also contains a PPAR-response element.
Plasma SHBG levels tend to increase with increasing age but remain inversely associated with plasma insulin and triglyceride levels irrespective of age. 26
The apparent metabolic clearance rate of testosterone is decreased in elderly as compared to younger men. Possible reasons for this include age-related changes in body composition, SHBG, hepatic blood flow, and presumably a range of other factors yet to be identified. 27
e. Dihydrotestosterone (DHT)
In certain target tissues testosterone is converted to 5α-dihydrotestosterone (DHT), which has a higher affinity than testosterone for both SHBG and the androgen receptor (AR). A 10-fold higher concentration of testosterone is required to achieve the AR mediated transcriptional effects of DHT. 28 Testosterone is converted to DHTby a membrane protein, steroid 5α-reductase (5αR). There are two types of 5αR each coded by a separate gene. The expression of 5αR1 is highest in hair follicles, sebaceous glands of the skin, and the liver, although its expression is widespread. 5αR2 is located primarily in the prostate, epididymis and seminal vesicles. DHT increases the expression of both 5αR1, 5αR2. Type 3 3α-hydroxysteroid dehydrogenase (HSD) (AKR1C2) catalyzes the reduction of 5α-DHT to yield the inactive androgen 3α-androstanediol (3α-diol), which can be oxidized back to DHT, at least in prostate stromal cells. 29 Type 3 3β-HSD reduces DHT to 3β-androstanediol, a potent ligand for ERβ. 29 DHT (and its precursor testosterone) can also be formed in bone, muscle, and the prostate from the circulating adrenal androgen DHEA, a pathway which may be of increasing importance with age.
f. Estrogen
Estrogen in the male can be synthesized locally from testosterone, by aromatase enzymes, in many tissues. 30, 31 This includes the brain, where estrogen may act via its classic nuclear receptors, or via rapid membrane actions. 32–34 The rate of whole body aromatization is higher in older men, 35 but the precise mechanisms are unclear.
In the presence of inactivating mutations in the CYP19 gene, several physiological disturbances have been identified in men, including skeletal, metabolic, and reproductive impairments. 30, 31 Studies in aromatase knockout mice generally recapitulate these sequelae. 36, 37
Estradiol suppresses LH and FSH to exogenously administered GnRH in GnRH-deficient men and aromatase inhibition increases LH and FSH indicating a role for estrogens in negative feedback. These effects are blocked by a GnRH agonist indicating the changes in hypothalamic GnRH secretion are responsible. In males, estrogen-dependent mechanisms also mediate testosterone negative feedback on GnRH expression and secretion. 38
IV. Measurement Issues
Proper assessment of HPT axis functioning relies critically on accurate assessment of analytes in human serum, and to a lesser extent and for specific syndromes (e.g., hypogonadism), on medical history or patient self-report.
Testosterone circulates predominantly bound to the plasma proteins SHBG and albumin, with high and low affinity respectively. A small and variable fraction is said to circulate as free testosterone. One or another of these circulating fractions of testosterone have been used as outcome measures in different studies, with various investigators attributing greater biological relevance to one measure as opposed to another. There is general consensus that the most relevant fraction to measure for both clinical and epidemiological purposes is total testosterone. The precision of many platform-based assays is suboptimal and there is limited standardization of processes both in terms of specimen collection and analysis. 39 Although liquid chromatography-tandem mass spectrometry (LC-MS/MS) is considered to be considerably more sensitive and precise, similar technical issues in relation to sample collection apply, and there are quite considerable variations in assays, which are technically difficult to undertake. While LC-MS/MS methodology represents a considerable advance, and in the case of some sex steroids, for example estrogen, permits reliable assessment of the very low plasma levels that are otherwise at the limits of detection of more conventional assays, harmonization of LC-MS/MS assays is required. 40 In the clinical setting, it generally accepted that a high-quality radioimmunoassay or chemiluminescence assay will provide sufficient information on testosterone levels in aging men. In obese men, free testosterone levels may also need to be measured.
Testosterone is secreted in a pulsatile fashion. 41 Since the pulse frequency is so rapid and the amplitude relatively low, a single blood sample is generally considered sufficient for most clinical or epidemiologic studies.42 Nonetheless, testosterone and other serum hormones exhibit considerable variability within subjects over time. 43 Current clinical guidelines suggest at least two measurements.
In adult men, there is a well-documented diurnal variation (particularly in younger subjects) in testosterone levels, which are highest in the early morning and progressively decline throughout the day to a nadir in the evening. 44 In older men, the diurnal variation is blunted. 45 Data on the clinical implications of keeping a consistent time window for the drawing of serum for testosterone assessment have been reported. 46 Thus, it is standard practice for samples to be obtained between 0800 and 1100 h.
The most substantial challenge to developing valid instruments to assess for symptoms attributable to hypogonadism, particularly with aging, is the non-specificity of these symptoms, 47 reflected in the low specificity of screening instruments for hypogonadism. 48–50 A recent publication showed that the presence of 3 sexual symptoms (decreased morning erections, erectile dysfunction, and decreased frequency of sexual thoughts) best predicted the presence of low testosterone, providing the first empirically-derived approach to defining hypogonadism. 51 The main limitation of this algorithm is the relatively high prevalence of these symptoms in the general population, making its use as a screening device especially challenging. We have previously shown that the relative percentages of low libido, ED, and two or more non-specific symptoms (e.g., fatigue, depressed mood) were elevated in men with low testosterone levels compared to men with testosterone levels in the normal range and that with increasing age, the specificity of symptoms for low testosterone appeared to increase 52 Zitzmann and colleagues have shown that some symptoms of hypogonadism might appear at higher concentrations of androgens than others,53 with variations between individuals,54 perhaps related to genetic differences that affect androgen sensitivity.
V. Epidemiologic and Clinical Research
a. Age Trends
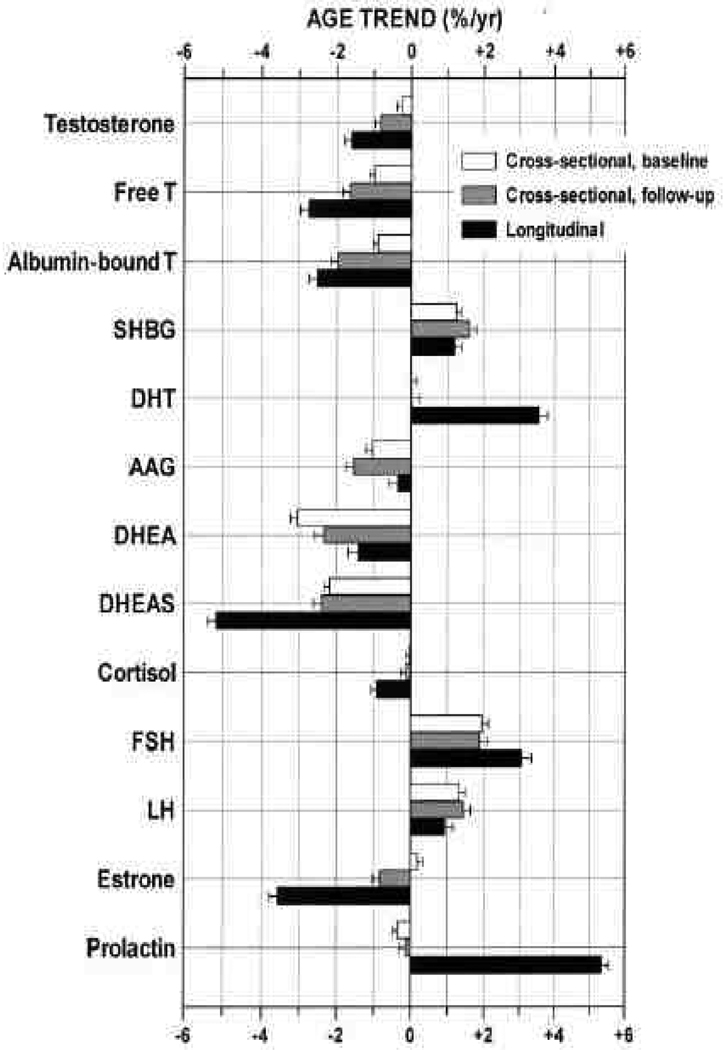
Because of the complex interrelations of the sex hormones with other hormone systems, common chronic diseases of aging (cancer, CVD, diabetes, depression, hyperlipidemia, arthritis), and associated risk factors for chronic disease (obesity, sedentariness, nutritional deficiency, smoking), there is still little consensus to what constitutes a normal sex hormone profile for an aging male. There is no male equivalent of the menopause, and even in very old men with healthy active lifestyles plasma testosterone levels and sexual function may be well within the range of normal. Nevertheless, it is well established that several of these sex hormone levels – though not all – undergo, on average, a gradual shift with age. Testosterone and DHEA decline, whereas LH, FSH, and SHBG rise.55 DHT remains constant despite the decline of its precursor testosterone (Figure 1). 55 It is by no means clear whether these shifts are universal, inevitable, or deleterious.
Figure 1.
Cross-sectional and 8-year longitudinal trends of testosterone, other androgens and metabolites, and related hormones in middle-aged men. Abbreviations: AAG, androstanediol glucuronide; DHEA, dehydroepiandrosterone; DHEAS, DHEA sulfate; DHT, dihydrotestosterone; FSH, follicle-stimulating hormone; LH, Luteinizing hormone; T, testosterone. Source: MMAS, Feldman et al, J Clin Endocrinol Metab 2002; 87(2):589–598. 55 Copyright 2002, The Endocrine Society. Used with permission.
i. Testosterone
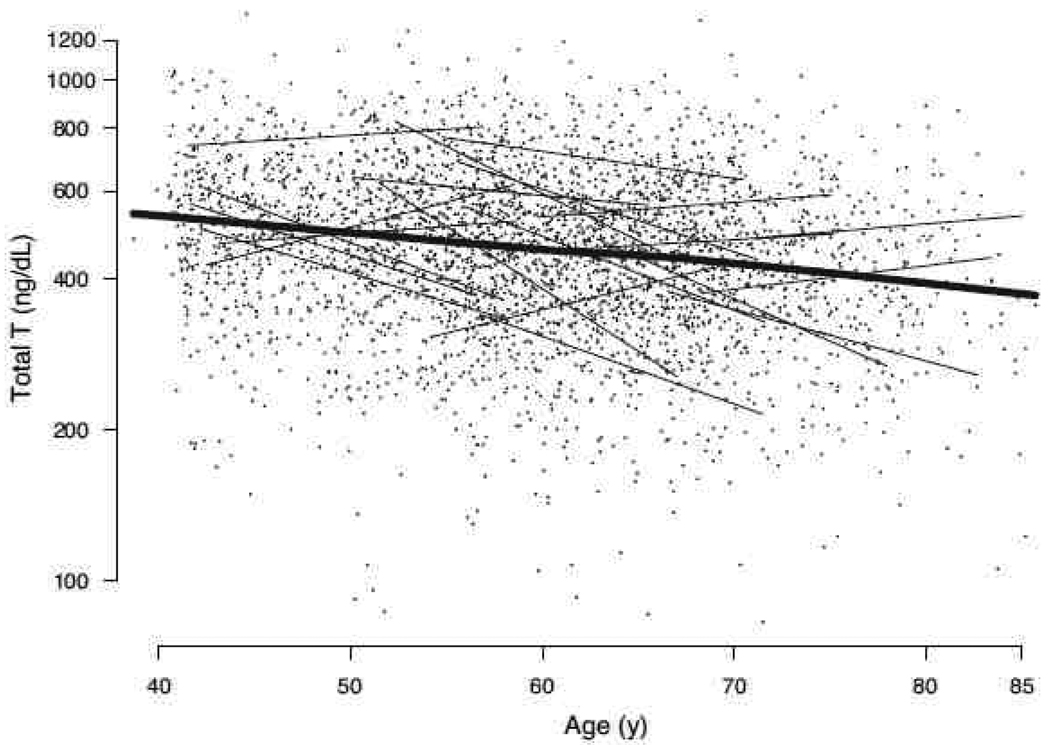
Longitudinal studies show an average annual decline of 1–2% total testosterone levels, with decline in free testosterone more rapid because of increases in SHBG with aging (Figure 1). 55–57 Nonetheless, the decline in testosterone observed in population studies is by no means universal and testosterone levels may be stable or even increase with age in some men (Figure 2). 58
Figure 2.
Total testosterone vs. age (natural log scale for all observations). Linear trajectories for 20 randomly chosen subjects are plotted (thin lines), demonstrating the substantial inter-subject variation in testosterone trends over time. A nonparametric, locally weighted regression smooth (thick line) depicts the linear decline in log testosterone values with age over all observations, which is generally outstripped by within-subject longitudinal decline. To convert total testosterone from nanograms per deciliter to nanomoles per liter, multiply by 0.0347. Source: MMAS, Travison et al, J Clin Endocrinol Metab 2007; 92(2):549– 555. 58 Copyright 2007, The Endocrine Society. Used with permission.
ii. DHEA
Studies agree that levels of the adrenal steroid DHEA and its sulfate (DHEAS), the most plentiful steroid in serum, decline with age more markedly than other hormones.59, 60 The adrenal steroid androstenedione (Ae) follows a similarly sharp decline.60 Massachusetts Male Aging Study (MMAS) data show DHEA, DHEAS, and Ae declining at 2–3% per year, both cross-sectionally 61 and longitudinally (Figure 1). 55
iii. DHT
DHT showed no cross-sectional age trend in MMAS 55 or other studies 60, 62–64 but increased within subjects between MMAS visits. 55. Androstanediol glucuronide (AAG) declined cross-sectionally with age in the MMAS sample, at 0.6% per year (but not longitudinally) and appeared to be related to prostate cancer 65 but the functional consequences of declines in AAG are not completely clear.
iv. Estrogen
The age trends of estrogen levels have been reported variously as declining 66 or remaining steady. 67 Estrogens were invariant with age in MMAS. 61
v. Gonadotrophins and regulation of the HPT axis with aging
There is general agreement that the pituitary gonadotrophins, LH and FSH (the function of which in men is to stimulate respectively testosterone secretion by Leydig cells and sperm production by Sertoli cells in the testes), increase in serum concentration with age in men. In MMAS, LH and FSH increased longitudinally at 1.1% and 3.5% per year, respectively. 55
The rise in FSH and LH with increasing age is consistent with the decline in testosterone, assuming normal operation of the feedback pathway by which low testosterone level signals the hypothalamic-pituitary axis to release FSH and LH.68 This has been further shown in a publication from the European Male Aging Study (EMAS), a cross-sectional survey on 3,200 community-dwelling men aged 40–79 yr in eight European countries. 3 The EMAS data show that, consistent with the longitudinal findings of MMAS (Figure 1), the core hormonal pattern with increasing age is suggestive of incipient primary testicular dysfunction with maintained total testosterone and progressively blunted free testosterone associated with higher LH. They also observed that obesity impairs hypothalamic/pituitary function, which is consistent with the observation that plasma INLS3 levels decrease with increasing age but are not related to obesity and are largely independent of LH levels. 69 The implication of this is that aging effects on testicular function may be compensated by increases in LH, but since obesity impairs hypothalamic/pituitary function independent of age, there should be no compensatory mechanism.
b. Prevalence
Most elderly men have testosterone levels within the normal range, with prevalence estimates of “low” (e.g., < 300 ng/dL (10.4 nmol/L)) serum testosterone generally between 10% and 25%. 66, 70 More appropriate prevalence estimates also account for presence of clinical symptoms. Data from the MMAS indicate that the prevalence of symptomatic hypogonadism is between 6%–12%, 71 which is similar to prevalence in the Boston Area Community Health (BACH) Survey (5.6%), 52 suggesting that there could be up to 4.7 million men American men 30–79 years with symptomatic hypogonadism.
Wu and colleagues 51 estimated the prevalence of hypogonadism in the EMAS, defined as the presence of at least 3 sexual symptoms (loss of morning erections, low sexual desire, and erectile dysfunction), total testosterone < 320 ng/dL (11 nmol/L), and free testosterone < 64 pg/mL (220 pmol/L). Using this definition, the overall prevalence of hypogonadism in the EMAS study population was 2.1% and increased with age from 0.1% for men 40 to 49 years of age to 5.1% for those 70 to 79 years.
c. Secular Trend
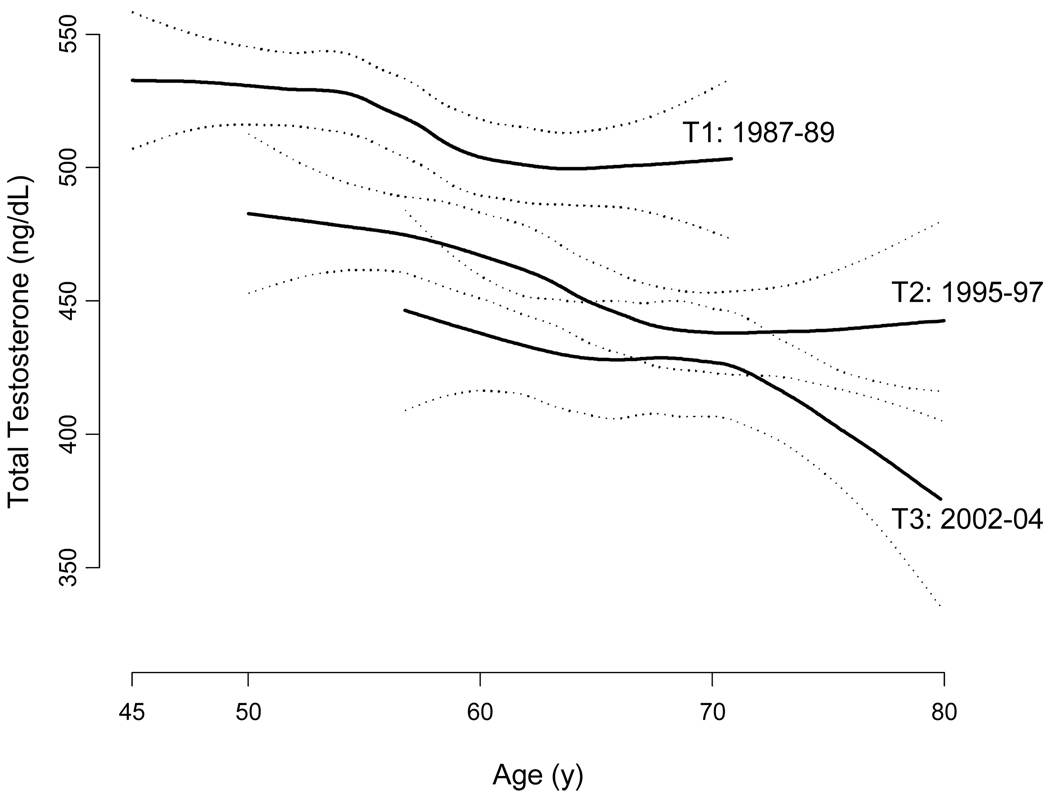
We published 72 results from the MMAS showing the first evidence of an age-independent secular decline in testosterone levels over the past three decades. The analyses indicated a 1% per year decrease in total testosterone. Because the secular effect is age independent (Figure 3), its existence is evidence that time-variant factors other than the aging of the population are having an effect on the distribution of testosterone in the general population. This finding was robust to control for secular changes in some of these factors, e.g., the increased prevalence of obesity and reduced prevalence of smoking. These observations are consistent with others showing population-level declines in sperm counts and increasing incidence rates of certain reproductive disorders in men. 73 The finding has been replicated in Denmark. 74
Figure 3.
Crude mean total testosterone concentrations, by MMAS study wave (T1, T2, T3), with confidence bands (dotted lines). Estimates are obtained from a generalized additive model with a lowess smoothing term. Source: MMAS, Travison et al., J Clin Endocrinol Metab 2007; 92(1):196–202. 72 Copyright 2007, The Endocrine Society. Used with permission.
d. Risk Factors
In addition to aging, modifiable lifestyle factors that are associated with testosterone levels, and therefore might have a role in modulating the decline with age, include tobacco and alcohol use, 75 caffeine intake, 76 social behavior mood, and severe psychosocial stress, 77 exercise, 56 obesity, 78 type 2 diabetes mellitus, 76 and the presence of obstructive sleep apnea and medication use. The MMAS has shown that comorbid conditions (e.g., diabetes, hypertension) and lifestyle influences may be as strongly associated with declining testosterone levels as is aging itself over the short- to mid-term. 58 This has implications for clinical practice. Diet and exercise have also shown influences on SHBG, thereby affecting the bioavailable pool of testosterone. 79
e. Association of Low Testosterone with Outcomes
The importance of a decline in testosterone is wide-ranging because of its ubiquitous role in male physiology, regulating sexual function and mood, muscle mass, secondary sex characteristics, liver function, lipid regulation, bone formation, erythropoiesis, and immune function. 68 In recent years, there has been an increasing interest in the role low testosterone levels or testosterone therapy may have with regard to important health outcomes, including not only the role of testosterone in sexual function or libido, but also its involvement in bone and muscle, metabolic disease, and survival. This has been highlighted by the Institute of Medicine report on testosterone in aging men. 80 The National Institutes of Health have initiated a coordinated series of 4 short-term randomized clinical trials examining the efficacy of testosterone administration for physical, sexual, and cognitive function, as well as vitality. Results from that series of trials will be available in the next 5 years.
Chapters 8, 9, 10 of this issue discuss the role of androgens in bone, metabolic/cardiovascular disease, and prostate health, respectively. Thus, this section only briefly touches on these issues, with more in-depth discussion of other targets.
i. Sexual Function
Low testosterone levels are associated with reduced libido. 81 There is debate regarding the effect of testosterone on erectile function in mildly hypogonadal men, 82 with studies showing no effect 83 or subgroup effects.84, 85 The confusion could stem from inadequate specification of androgen effects on sexual function. MMAS data show that the relationship between testosterone and ED is conditional on gonadotrophin level; testosterone is associated with ED only among men with high LH levels. 86 Some studies suggest that the testosterone concentration required for sexual activity is very low and that testosterone may affect sexual function only at markedly decreased testosterone levels 83 which is supported by a meta-analysis of 17 randomized placebo-controlled trials. 84 In summary, androgens exert weak (or even threshold) effects on erectile function, but probably play a more important role in libido. 87
ii. Body Composition, Muscle Strength, Physical Function, and Falls
Studies consistently show a significant relationship between low testosterone levels and body composition. 88, 89 The mechanisms linking sex hormones with body composition are not completely understood, but possible biological mechanisms include the effects of testosterone on regulation of mesenchymal stem cell differentiation 90 and muscle protein synthesis 91 through androgen receptor-mediated pathways, 90 activation of inflammatory pathways 92, 93 or increases in cortisol. 22
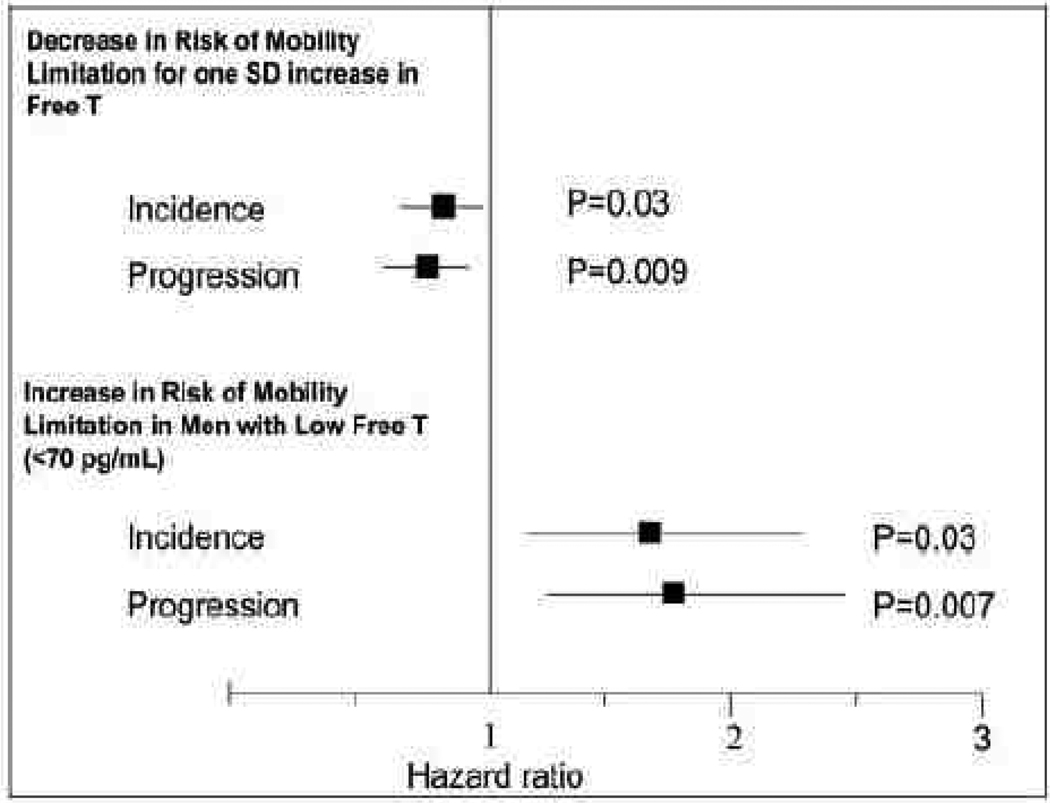
The relationship between aging, testosterone, muscle strength and overall physical function remains unclear. Low testosterone levels were not associated with 3-year declines in muscle strength or physical performance in two independent samples of older men. 94 In contrast, prospective data from the Framingham Offspring Study 95 showed an association between low free testosterone levels and a greater risk of incident or worsening mobility limitation in older men (Figure 4). In the Osteoporotic Fractures in Men (MrOS) cohort, 96 lower bioavailable testosterone levels were associated with increased fall risk, even with adjustment for physical performance, suggesting that the effect of testosterone on fall risk is mediated by other androgen actions (e.g., vision, coordination). At least one other study has related testosterone level to fall risk, 97 whereas another study did not. 98
Figure 4.
Longitudinal analyses of incident mobility limitation. Hazard ratios are for 1 SD increase in hormone levels, adjusting for age, BMI, smoking, and comorbidities (cardiovascular disease and cancer). As shown in the upper panel, each SD increase in free testosterone level was associated with 22% (OR 0.78; 95% CI 0.62–0.97) decrease in the risk of developing mobility limitation and 25% decrease n the risk of worsening mobility limitation (progression). The lower panel shows the association of low free testosterone (<2.5th percentile (<70.0 pg/ml)) at baseline examination 7 with the risk of developing (incident) mobility limitation at examination 8 or of reporting worsening mobility limitation (progression) at examination 8. The squares indicate point estimates for hormones, and the lines indicate 95% CI. Source: Framingham Offspring Study, Krasnoff et al., J Clin Endocrinol Metab 2010. 95 Copyright 2010, The Endocrine Society. Used with permission.
Interventional data show that testosterone administration improves body composition, including increasing lean mass and decreasing fat mass. 89 Despite its role in improving body composition, evidence in support of the concept that testosterone administration improves muscle strength and physical function is limited. In a meta-analysis that estimated the effect of testosterone therapy on men with hypogonadism, 99 the changes in muscle strength were inconsistent across trials. These findings have been replicated in recently-conducted dose-response studies 100, 101 and interventional trials. 102, 103 Bhasin has discussed limitations of physical function efficacy studies to date, which include the inclusion of men with low-normal testosterone levels or without functional limitations, failure of some studies to increase testosterone levels into the target range, the conceptual and practical difficulties in defining the indication for inclusion in a clinical trial, and the difficulty in measurement of functional endpoints. These limitations were addressed in a recently-published clinical trial of testosterone in elderly men with low testosterone levels (total testosterone 100–350 ng/dl or free testosterone <50 pg per ml) and limitations in mobility. 104 Despite a preliminary finding that testosterone improved leg-press and chest-press strength and ability to climb stairs while carrying a load, the trial was stopped because testosterone-treated men had 23 cardiovascular-related adverse events compared with only 5 such events in the placebo group.
iii. Bone metabolism, osteoporosis and fractures
In normal aging men, endogenous testosterone levels have been related to bone turnover markers, BMD, and hip structural geometry 105 although these findings are by no means universal. 67 Prevalence of osteoporosis is higher in men with low (12.2%) vs. normal (6.0%) testosterone levels. 106 The primary mechanism by which testosterone influences bone appears to be through aromatization to estradiol. 107 The limited data on endogenous testosterone levels and fracture are inconsistent, with low testosterone being associated with fractures in some studies 108 but not others. 109 The majority of studies of testosterone in association with bone outcomes have focused on BMD as assessed by dual x-ray absorptiometry (DXA). The limitations of DXA are known, with most fracture patients not meeting the WHO criterion for osteoporosis, 110 suggesting that other features of bone strength are relevant to fracture risk. Studies have shown that bone structural parameters (e.g., cortical and trabecular BMD, bone microarchitecture) are impaired in men with hypogonadism 111, 112 and that administration of exogenous testosterone may improve these parameters. 113
Two separate meta-analyses of testosterone trials in older men have shown significantly greater increases in lumbar spine BMD in testosterone-treated men than in those receiving placebo. 89, 114 The improvement in lumbar spine BMD was approximately 8%. 114 In general, trials that used intramuscular testosterone showed greater increment in vertebral BMD than those using transdermal testosterone delivery systems. 114
iv. Metabolic and Cardiovascular Disease Outcomes
Studies of induced hypogonadism have shown significant effects on glucose metabolism. Androgen deprivation in men with prostate cancer has been associated with increased insulin resistance, worse glycemic control, and a significant increase in risk of incident diabetes. 115 Low serum testosterone is associated with the development of metabolic syndrome 116, 117 and type 2 diabetes. 118 SHBG has been inversely correlated with type 2 diabetes. 119, 120
Improvement in insulin sensitivity with testosterone treatment has been reported in healthy 121 and diabetic 122 adult men. In studies conducted in men with central adiposity, testosterone has been shown to inhibit lipoprotein lipase activity in abdominal adipose tissue leading to decreased triglyceride uptake in central fat depots. 123
Testosterone may have direct effects on vascular reactivity and cardiac muscle. 124, 125 Although cross-sectional studies have shown an inverse correlation between sex steroids and CVD, most longitudinal studies have not. 126 There is evidence that the beneficial effects (if any) of testosterone, at least at the level of the endothelium, is mediated by conversion of testosterone to estradiol. 126, 127
Intervention studies of testosterone replacement on symptomatic coronary artery disease show some beneficial effects, 126, 128 but randomized controlled studies are relatively rare. A recent meta-analysis of incident cardiovascular events from 6 clinical trials of testosterone treatment showed a pooled odds ratio of 1.82 (95% CI 0.78, 4.23), indicating a non-significant increased risk among men on testosterone. The authors highlighted the heterogeneity of results across studies and the small number of events (14 events among men who received testosterone and 7 among placebo). 129
iv. Cognitive Function, Mood, and Quality of Life
Studies have also shown associations between androgens and quality of life outcomes such as cognitive function 130 and depressed mood or dysthmic disorder 62, 131. While the efficacy data are relatively weak, in some studies testosterone treatment significantly improved energy, mood, and subjective well-being. 132, 133
v. Mortality
Men with low testosterone levels are more likely to die prematurely, as reported in most 134–147 but not all 148–153 studies. It is not clear from these observational studies whether testosterone represents a causative factor or merely a risk marker, 154 although nearly every positive study reported that in sensitivity analyses excluding early deaths, results were fundamentally unchanged.
VI. Treatment
In men with classical hypogonadism, treatment is clearly indicated and men should be monitored appropriately. See Chapter X (Bhasin). There is considerable debate about the appropriateness of testosterone in aging men. The long-term safety or efficacy of testosterone replacement in aging men with late-onset hypogonadism has not been established, but as noted above, small-scale clinical studies suggest that testosterone may have beneficial effects.
VII. Practice Points
On average there is a small (1–2%) annual decrease in total testosterone levels with aging but the age-related change varies between individuals.
Factors related to disease, medication use, lifestyle behaviors, environmental exposures and psychosocial stress may be as important as age per se in leading to a testosterone declines, which implies that alternatives to testosterone therapy may be viable treatment options.
The diagnosis of hypogonadism must be based on at least two unequivocally low levels of testosterone on morning blood samples together with compatible symptoms.
Significant hypogonadism may occur at any age, and treatment with testosterone may be beneficial, but requires careful monitoring.
VIII. Research Needs
Assessment of the effects of aging on estrogen is required using well-validated assays with the requisite sensitivity.
Additional longitudinal studies to determine the interrelationships between sex steroid levels and physical and psychological function, disease, lifestyle factors, medication use, environmental factors, and psychosocial stress.
The factors underlying a possible secular decline in testosterone.
Appropriately constructed and validated questionnaires to empirically assess androgen action.
Understanding of the relationship between various sex steroids and specific symptoms attributable to hormonal deficiency in relation to age and health status.
Detailed study of the molecular physiology of the regulation of sex steroid production, action, and metabolism, with aging in relation to health outcomes.
The most appropriate circumstances for treatment with testosterone and quantifiable risks and benefits in each case.
Acknowledgements
Supported by Award Number R01AG020727 from the National Institute on Aging. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Aging or the National Institutes of Health. GAW is in receipt of project grant support from the National Health and Medical Research Council of Australia and the Australian Research Council.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: ABA is a consultant to Lilly USA, LLC (Indianapolis, IN). GAW is a consultant to Lawley Pharmaceuticals (Perth WA, Australia) and received speaking fees and research support from Bayer Schering Pharma AG and Organon.
Contributor Information
Andre B. Araujo, Director, Epidemiology, New England Research Institutes, Inc., 9 Galen Street, Watertown, MA 02472, Tel: 617.923.7747 x452, Fax: 617.673.9509, aaraujo@neriscience.com.
Gary A. Wittert, Head, Discipline of Medicine, The University of Adelaide, Principal Research Scientist, New England Research Institutes, Inc., Phone: +61 882225502, Fax: +61 882233870, gary.wittert@adelaide.edu.au.
Literature Cited
- 1.Veldhuis JD, Keenan DM, Pincus SM. Regulation of complex pulsatile and rhythmic neuroendocrine systems: the male gonadal axis as a prototype. Progress in Brain Research. 2010;181:79–110. doi: 10.1016/S0079-6123(08)81006-0. [DOI] [PubMed] [Google Scholar]
- 2.Iranmanesh A, Mulligan T, Veldhuis JD. Age in men does not determine gonadotropin-releasing hormone's dose-dependent stimulation of luteinizing hormone secretion under an exogenous testosterone clamp. Journal of Clinical Endocrinology and Metabolism. 2010 Jun;95(6):2877–2884. doi: 10.1210/jc.2009-2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu FC, Tajar A, Pye SR, Silman AJ, Finn JD, O'Neill TW, et al. Hypothalamic-pituitary-testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: the European Male Aging Study. Journal of Clinical Endocrinology and Metabolism. 2008 Jul;93(7):2737–2745. doi: 10.1210/jc.2007-1972. [DOI] [PubMed] [Google Scholar]
- 4.Leder BZ, Rohrer JL, Rubin SD, Gallo J, Longcope C. Effects of aromatase inhibition in elderly men with low or borderline-low serum testosterone levels. Journal of Clinical Endocrinology and Metabolism. 2004 Mar;89(3):1174–1180. doi: 10.1210/jc.2003-031467. [DOI] [PubMed] [Google Scholar]
- 5.Goodman RL, Lehman MN, Smith JT, Coolen LM, de Oliveira CV, Jafarzadehshirazi MR, et al. Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology. 2007 Dec;148(12):5752–5760. doi: 10.1210/en.2007-0961. [DOI] [PubMed] [Google Scholar]
- 6.Lehman MN, Coolen LM, Goodman RL. Minireview: kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: a central node in the control of gonadotropin-releasing hormone secretion. Endocrinology. 2010 Aug;151(8):3479–3489. doi: 10.1210/en.2010-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castellano JM, Bentsen AH, Romero M, Pineda R, Ruiz-Pino F, Garcia-Galiano D, et al. Acute inflammation reduces kisspeptin immunoreactivity at the arcuate nucleus and decreases responsiveness to kisspeptin independently of its anorectic effects. The American Journal of Physiology - Endocrinology and Metabolism. 2010 Jul;299(1):E54–E61. doi: 10.1152/ajpendo.00081.2010. [DOI] [PubMed] [Google Scholar]
- 8.Brioude F, Bouligand J, Trabado S, Francou B, Salenave S, Kamenicky P, et al. Non-syndromic congenital hypogonadotropic hypogonadism: clinical presentation and genotype-phenotype relationships. European Journal of Endocrinology / European Federation of Endocrine Societies. 2010 May;162(5):835–851. doi: 10.1530/EJE-10-0083. [DOI] [PubMed] [Google Scholar]
- 9.Castellano JM, Roa J, Luque RM, Dieguez C, Aguilar E, Pinilla L, et al. KiSS-1/kisspeptins and the metabolic control of reproduction: physiologic roles and putative physiopathological implications. Peptides. 2009 Jan;30(1):139–145. doi: 10.1016/j.peptides.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 10.Young J, Bouligand J, Francou B, Raffin-Sanson ML, Gaillez S, Jeanpierre M, et al. TAC3 and TACR3 defects cause hypothalamic congenital hypogonadotropic hypogonadism in humans. Journal of Clinical Endocrinology and Metabolism. 2010 May;95(5):2287–2295. doi: 10.1210/jc.2009-2600. [DOI] [PubMed] [Google Scholar]
- 11.Wakabayashi Y, Nakada T, Murata K, Ohkura S, Mogi K, Navarro VM, et al. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. Journal of Neuroscience. 2010 Feb 24;30(8):3124–3132. doi: 10.1523/JNEUROSCI.5848-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wildt L, Hausler A, Marshall G, Hutchison JS, Plant TM, Belchetz PE, et al. Frequency and amplitude of gonadotropin-releasing hormone stimulation and gonadotropin secretion in the rhesus monkey. Endocrinology. 1981 Aug;109(2):376–385. doi: 10.1210/endo-109-2-376. [DOI] [PubMed] [Google Scholar]
- 13.Woerdeman J, Kaufman JM, de Ronde W. In young men, a moderate inhibition of testosterone synthesis capacity is only partly compensated by increased activity of the pituitary and the hypothalamus. Clinical Endocrinology. 2010 Jan;72(1):76–80. doi: 10.1111/j.1365-2265.2009.03624.x. [DOI] [PubMed] [Google Scholar]
- 14.Kumar TR. FSHbeta knockout mouse model: a decade ago and into the future. Endocrine. 2009 Aug;36(1):1–5. doi: 10.1007/s12020-009-9199-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peltoketo H, Rivero-Muller A, Ahtiainen P, Poutanen M, Huhtaniemi I. Consequences of genetic manipulations of gonadotrophins and gonadotrophin receptors in mice. Annales d Endocrinologie. 2010 May;71(3):170–176. doi: 10.1016/j.ando.2010.02.022. [DOI] [PubMed] [Google Scholar]
- 16.Kumanov P, Nandipati KC, Tomova A, Robeva R, Agarwal A. Significance of inhibin in reproductive pathophysiology and current clinical applications. Reproductive Biomedicine Online. 2005 Jun;10(6):786–812. doi: 10.1016/s1472-6483(10)61124-8. [DOI] [PubMed] [Google Scholar]
- 17.de Kretser DM, Buzzard JJ, Okuma Y, O'Connor AE, Hayashi T, Lin SY, et al. The role of activin, follistatin and inhibin in testicular physiology. Molecular and Cellular Endocrinology. 2004 Oct 15;225(1–2):57–64. doi: 10.1016/j.mce.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 18.Meachem SJ, Nieschlag E, Simoni M. Inhibin B in male reproduction: pathophysiology and clinical relevance. European Journal of Endocrinology / European Federation of Endocrine Societies. 2001 Nov;145(5):561–571. doi: 10.1530/eje.0.1450561. [DOI] [PubMed] [Google Scholar]
- 19.Van Thiel DH, Sherins RJ, Myers GH, Jr, De Vita VT., Jr Evidence for a specific seminiferous tubular factor affecting follicle-stimulating hormone secretion in man. Journal of Clinical Investigation. 1972 Apr;51(4):1009–1019. doi: 10.1172/JCI106861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jorgensen N, Liu F, Andersson AM, Vierula M, Irvine DS, Auger J, et al. Serum inhibin-b in fertile men is strongly correlated with low but not high sperm counts: a coordinated study of 1,797 European and US men. Fertility and Sterility. 2010 Nov;94(6):2128–2134. doi: 10.1016/j.fertnstert.2009.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scott HM, Mason JI, Sharpe RM. Steroidogenesis in the fetal testis and its susceptibility to disruption by exogenous compounds. Endocrine Reviews. 2009 Dec;30(7):883–925. doi: 10.1210/er.2009-0016. [DOI] [PubMed] [Google Scholar]
- 22.Purnell JQ, Brandon DD, Isabelle LM, Loriaux DL, Samuels MH. Association of 24-hour cortisol production rates, cortisol-binding globulin, and plasma-free cortisol levels with body composition, leptin levels, and aging in adult men and women. Journal of Clinical Endocrinology and Metabolism. 2004 Jan;89(1):281–287. doi: 10.1210/jc.2003-030440. [DOI] [PubMed] [Google Scholar]
- 23.Koeva Y, Bakalska M, Atanassova N, Georgieva K, Davidoff M. Age-related changes in the expression of 11beta-hydroxysteroid dehydrogenase type 2 in rat Leydig cells. Folia Histochemica et Cytobiologica. 2009;47(2):281–287. doi: 10.2478/v10042-009-0021-3. [DOI] [PubMed] [Google Scholar]
- 24.Selva DM, Hogeveen KN, Innis SM, Hammond GL. Monosaccharide-induced lipogenesis regulates the human hepatic sex hormone-binding globulin gene. Journal of Clinical Investigation. 2007 Dec;117(12):3979–3987. doi: 10.1172/JCI32249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Selva DM, Hammond GL. Thyroid hormones act indirectly to increase sex hormone-binding globulin production by liver via hepatocyte nuclear factor-4alpha. Journal of Molecular Endocrinology. 2009 Jul;43(1):19–27. doi: 10.1677/JME-09-0025. [DOI] [PubMed] [Google Scholar]
- 26.Atlantis E, Martin SA, Haren MT, O'Loughlin PD, Taylor AW, Anand-Ivell R, et al. Demographic, physical and lifestyle factors associated with androgen status: the Florey Adelaide Male Ageing Study (FAMAS) Clinical Endocrinology. 2009 Aug;71(2):261–272. doi: 10.1111/j.1365-2265.2008.03463.x. [DOI] [PubMed] [Google Scholar]
- 27.Coviello AD, Lakshman K, Mazer NA, Bhasin S. Differences in the apparent metabolic clearance rate of testosterone in young and older men with gonadotropin suppression receiving graded doses of testosterone. Journal of Clinical Endocrinology and Metabolism. 2006 Nov;91(11):4669–4675. doi: 10.1210/jc.2006-0822. [DOI] [PubMed] [Google Scholar]
- 28.Grino PB, Griffin JE, Wilson JD. Testosterone at high concentrations interacts with the human androgen receptor similarly to dihydrotestosterone. Endocrinology. 1990 Feb;126(2):1165–1172. doi: 10.1210/endo-126-2-1165. [DOI] [PubMed] [Google Scholar]
- 29.Penning TM, Jin Y, Rizner TL, Bauman DR. Pre-receptor regulation of the androgen receptor. Molecular and Cellular Endocrinology. 2008 Jan 16;281(1–2):1–8. doi: 10.1016/j.mce.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharpe RM. The roles of oestrogen in the male. Trends in Endocrinology and Metabolism. 1998 Nov;9(9):371–377. doi: 10.1016/s1043-2760(98)00089-7. [DOI] [PubMed] [Google Scholar]
- 31.Jones ME, Boon WC, Proietto J, Simpson ER. Of mice and men: the evolving phenotype of aromatase deficiency. Trends in Endocrinology and Metabolism. 2006 Mar;17(2):55–64. doi: 10.1016/j.tem.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 32.Brann DW, Dhandapani K, Wakade C, Mahesh VB, Khan MM. Neurotrophic and neuroprotective actions of estrogen: basic mechanisms and clinical implications. Steroids. 2007 May;72(5):381–405. doi: 10.1016/j.steroids.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Micevych P, Dominguez R. Membrane estradiol signaling in the brain. Frontiers in Neuroendocrinology. 2009 Aug;30(3):315–327. doi: 10.1016/j.yfrne.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Toran-Allerand CD. Estrogen and the brain: beyond ER-alpha, ER-beta, and 17beta-estradiol. Annals of the New York Academy of Sciences. 2005 Jun;1052:136–144. doi: 10.1196/annals.1347.009. [DOI] [PubMed] [Google Scholar]
- 35.Lakshman KM, Kaplan B, Travison TG, Basaria S, Knapp PE, Singh AB, et al. The effects of injected testosterone dose and age on the conversion of testosterone to estradiol and dihydrotestosterone in young and older men. Journal of Clinical Endocrinology and Metabolism. 2010 Aug;95(8):3955–3964. doi: 10.1210/jc.2010-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roselli CE, Liu M, Hurn PD. Brain aromatization: classic roles and new perspectives. Seminars in Reproductive Medicine. 2009 May;27(3):207–217. doi: 10.1055/s-0029-1216274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simpson ER, Clyne C, Rubin G, Boon WC, Robertson K, Britt K, et al. Aromatase--a brief overview. Annual Review of Physiology. 2002;64:93–127. doi: 10.1146/annurev.physiol.64.081601.142703. [DOI] [PubMed] [Google Scholar]
- 38.Naftolin F, Garcia-Segura LM, Horvath TL, Zsarnovszky A, Demir N, Fadiel A, et al. Estrogen-induced hypothalamic synaptic plasticity and pituitary sensitization in the control of the estrogen-induced gonadotrophin surge. Reproductive Sciences. 2007 Feb;14(2):101–116. doi: 10.1177/1933719107301059. [DOI] [PubMed] [Google Scholar]
- 39.Rosner W, Auchus RJ, Azziz R, Sluss PM, Raff H. Position statement: Utility, limitations, and pitfalls in measuring testosterone: an Endocrine Society position statement. Journal of Clinical Endocrinology and Metabolism. 2007 Feb;92(2):405–413. doi: 10.1210/jc.2006-1864. [DOI] [PubMed] [Google Scholar]
- 40.Rosner W, Vesper H. Toward excellence in testosterone testing: a consensus statement. Journal of Clinical Endocrinology and Metabolism. 2010 Oct;95(10):4542–4548. doi: 10.1210/jc.2010-1314. [DOI] [PubMed] [Google Scholar]
- 41.Veldhuis JD, King JC, Urban RJ, Rogol AD, Evans WS, Kolp LA, et al. Operating characteristics of the male hypothalamo-pituitary-gonadal axis: pulsatile release of testosterone and follicle-stimulating hormone and their temporal coupling with luteinizing hormone. Journal of Clinical Endocrinology and Metabolism. 1987 Nov;65(5):929–941. doi: 10.1210/jcem-65-5-929. [DOI] [PubMed] [Google Scholar]
- 42.Vermeulen A, Verdonck G. Representativeness of a single point plasma testosterone level for the long term hormonal milieu in men. Journal of Clinical Endocrinology and Metabolism. 1992 Apr;74(4):939–942. doi: 10.1210/jcem.74.4.1548361. [DOI] [PubMed] [Google Scholar]
- 43.Brambilla DJ, O'Donnell AB, Matsumoto AM, McKinlay JB. Intraindividual variation in levels of serum testosterone and other reproductive and adrenal hormones in men. Clinical Endocrinology. 2007 Dec;67(6):853–862. doi: 10.1111/j.1365-2265.2007.02976.x. [DOI] [PubMed] [Google Scholar]
- 44.Diver MJ, Imtiaz KE, Ahmad AM, Vora JP, Fraser WD. Diurnal rhythms of serum total, free and bioavailable testosterone and of SHBG in middle-aged men compared with those in young men. Clinical Endocrinology. 2003 Jun;58(6):710–717. doi: 10.1046/j.1365-2265.2003.01772.x. [DOI] [PubMed] [Google Scholar]
- 45.Bremner WJ, Vitiello MV, Prinz PN. Loss of circadian rhythmicity in blood testosterone levels with aging in normal men. Journal of Clinical Endocrinology and Metabolism. 1983 Jun;56(6):1278–1281. doi: 10.1210/jcem-56-6-1278. [DOI] [PubMed] [Google Scholar]
- 46.Brambilla DJ, Matsumoto AM, Araujo AB, McKinlay JB. The effect of diurnal variation on clinical measurement of serum testosterone and other sex hormone levels in men. Journal of Clinical Endocrinology and Metabolism. 2009 Mar;94(3):907–913. doi: 10.1210/jc.2008-1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu FC. Commentary: Guideline for male testosterone therapy: a European perspective. Journal of Clinical Endocrinology and Metabolism. 2007 Feb;92(2):418–419. doi: 10.1210/jc.2006-2799. [DOI] [PubMed] [Google Scholar]
- 48.Heinemann LAJ, Zimmermann T, Vermeulen A, Thiel C. A new 'Aging Male's Symptoms' (AMS) rating scale Aging Male. 1999;2:105–114. [Google Scholar]
- 49.Morley JE, Charlton E, Patrick P, Kaiser FE, Cadeau P, McCready D, et al. Validation of a screening questionnaire for androgen deficiency in aging males. Metabolism. 2000;49(9):1239–1242. doi: 10.1053/meta.2000.8625. [DOI] [PubMed] [Google Scholar]
- 50.Smith KW, Feldman HA, McKinlay JB. Construction and field validation of a self-administered screener for testosterone deficiency (hypogonadism) in ageing men. Clinical Endocrinology. 2000 Dec;53(6):703–711. doi: 10.1046/j.1365-2265.2000.01152.x. [DOI] [PubMed] [Google Scholar]
- 51.Wu FC, Tajar A, Beynon JM, Pye SR, Silman AJ, Finn JD, et al. Identification of Late-Onset Hypogonadism in Middle-Aged and Elderly Men. New England Journal of Medicine. 2010 Jun 16; doi: 10.1056/NEJMoa0911101. [DOI] [PubMed] [Google Scholar]
- 52.Araujo AB, Esche GR, Kupelian V, O'Donnell AB, Travison TG, Williams RE, et al. Prevalence of symptomatic androgen deficiency in men. Journal of Clinical Endocrinology and Metabolism. 2007 Nov;92(11):4241–4247. doi: 10.1210/jc.2007-1245. [DOI] [PubMed] [Google Scholar]
- 53.Zitzmann M, Faber S, Nieschlag E. Association of specific symptoms and metabolic risks with serum testosterone in older men. Journal of Clinical Endocrinology and Metabolism. 2006 Nov;91(11):4335–4343. doi: 10.1210/jc.2006-0401. [DOI] [PubMed] [Google Scholar]
- 54.Kelleher S, Conway AJ, Handelsman DJ. Blood testosterone threshold for androgen deficiency symptoms. Journal of Clinical Endocrinology and Metabolism. 2004 Aug;89(8):3813–3817. doi: 10.1210/jc.2004-0143. [DOI] [PubMed] [Google Scholar]
- 55.Feldman HA, Longcope C, Derby CA, Johannes CB, Araujo AB, Coviello AD, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. Journal of Clinical Endocrinology and Metabolism. 2002 Feb;87(2):589–598. doi: 10.1210/jcem.87.2.8201. [DOI] [PubMed] [Google Scholar]
- 56.Zmuda JM, Cauley JA, Kriska A, Glynn NW, Gutai JP, Kuller LH. Longitudinal relation between endogenous testosterone and cardiovascular disease risk factors in middle-aged men. A 13-year follow-up of former Multiple Risk Factor Intervention Trial participants. American Journal of Epidemiology. 1997 Oct 15;146(8):609–617. doi: 10.1093/oxfordjournals.aje.a009326. [DOI] [PubMed] [Google Scholar]
- 57.Morley JE, Kaiser FE, Perry HM, 3rd, Patrick P, Morley PM, Stauber PM, et al. Longitudinal changes in testosterone, luteinizing hormone, and follicle-stimulating hormone in healthy older men. Metabolism. 1997 Apr;46(4):410–413. doi: 10.1016/s0026-0495(97)90057-3. [DOI] [PubMed] [Google Scholar]
- 58.Travison TG, Araujo AB, Kupelian V, O'Donnell AB, McKinlay JB. The relative contributions of aging, health, and lifestyle factors to serum testosterone decline in men. Journal of Clinical Endocrinology and Metabolism. 2007 Feb;92(2):549–555. doi: 10.1210/jc.2006-1859. [DOI] [PubMed] [Google Scholar]
- 59.Orentreich N, Brind JL, Vogelman JH, Andres R, Baldwin H. Long-term longitudinal measurements of plasma dehydroepiandrosterone sulfate in normal men. Journal of Clinical Endocrinology and Metabolism. 1992 Oct;75(4):1002–1004. doi: 10.1210/jcem.75.4.1400863. [DOI] [PubMed] [Google Scholar]
- 60.Belanger A, Candas B, Dupont A, Cusan L, Diamond P, Gomez JL, et al. Changes in serum concentrations of conjugated and unconjugated steroids in 40- to 80-year-old men. Journal of Clinical Endocrinology and Metabolism. 1994 Oct;79(4):1086–1090. doi: 10.1210/jcem.79.4.7962278. [DOI] [PubMed] [Google Scholar]
- 61.Gray A, Feldman HA, McKinlay JB, Longcope C. Age, disease, and changing sex hormone levels in middle-aged men: results of the Massachusetts Male Aging Study. Journal of Clinical Endocrinology and Metabolism. 1991;73(5):1016–1025. doi: 10.1210/jcem-73-5-1016. [DOI] [PubMed] [Google Scholar]
- 62.Barrett-Connor E, Von Muhlen DG, Kritz-Silverstein D. Bioavailable testosterone and depressed mood in older men: the Rancho Bernardo Study. Journal of Clinical Endocrinology and Metabolism. 1999 Feb;84(2):573–577. doi: 10.1210/jcem.84.2.5495. [DOI] [PubMed] [Google Scholar]
- 63.Deslypere JP, Vermeulen A. Leydig cell function in normal men: effect of age, life-style, residence, diet, and activity. Journal of Clinical Endocrinology and Metabolism. 1984 Nov;59(5):955–962. doi: 10.1210/jcem-59-5-955. [DOI] [PubMed] [Google Scholar]
- 64.Zumoff B, Strain GW, Kream J, O'Connor J, Rosenfeld RS, Levin J, et al. Age variation of the 24-hour mean plasma concentrations of androgens, estrogens, and gonadotropins in normal adult men. Journal of Clinical Endocrinology and Metabolism. 1982 Mar;54(3):534–538. doi: 10.1210/jcem-54-3-534. [DOI] [PubMed] [Google Scholar]
- 65.Mohr BA, Feldman HA, Kalish LA, Longcope C, McKinlay JB. Are serum hormones associated with the risk of prostate cancer? Prospective results from the Massachusetts Male Aging Study. Urology. 2001 May;57(5):930–935. doi: 10.1016/s0090-4295(00)01116-x. [DOI] [PubMed] [Google Scholar]
- 66.Orwoll E, Lambert LC, Marshall LM, Phipps K, Blank J, Barrett-Connor E, et al. Testosterone and estradiol among older men. Journal of Clinical Endocrinology and Metabolism. 2006 Apr;91(4):1336–1344. doi: 10.1210/jc.2005-1830. [DOI] [PubMed] [Google Scholar]
- 67.Araujo AB, Travison TG, Leder BZ, McKinlay JB. Correlations between serum testosterone, estradiol, and sex hormone-binding globulin and bone mineral density in a diverse sample of men. Journal of Clinical Endocrinology and Metabolism. 2008 Jun;93(6):2135–2141. doi: 10.1210/jc.2007-1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bagatell CJ, Bremner WJ. Androgens in men--uses and abuses. New England Journal of Medicine. 1996 Mar 14;334(11):707–714. doi: 10.1056/NEJM199603143341107. [DOI] [PubMed] [Google Scholar]
- 69.Anand-Ivell R, Wohlgemuth J, Haren MT, Hope PJ, Hatzinikolas G, Wittert G, et al. Peripheral INSL3 concentrations decline with age in a large population of Australian men. International Journal of Andrology. 2006 Dec;29(6):618–626. doi: 10.1111/j.1365-2605.2006.00714.x. [DOI] [PubMed] [Google Scholar]
- 70.Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. Journal of Clinical Endocrinology and Metabolism. 2001;86(2):724–731. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- 71.Araujo AB, O'Donnell AB, Brambilla DJ, Simpson WB, Longcope C, Matsumoto AM, et al. Prevalence and incidence of androgen deficiency in middle-aged and older men: estimates from the Massachusetts Male Aging Study. Journal of Clinical Endocrinology and Metabolism. 2004 Dec;89(12):5920–5926. doi: 10.1210/jc.2003-031719. [DOI] [PubMed] [Google Scholar]
- 72.Travison TG, Araujo AB, O'Donnell AB, Kupelian V, McKinlay JB. A population-level decline in serum testosterone levels in American men. Journal of Clinical Endocrinology and Metabolism. 2007 Jan;92(1):196–202. doi: 10.1210/jc.2006-1375. [DOI] [PubMed] [Google Scholar]
- 73.Carlsen E, Giwercman A, Keiding N, Skakkebaek NE. Evidence for decreasing quality of semen during past 50 years. British Medical Journal. 1992 Sep 12;305(6854):609–613. doi: 10.1136/bmj.305.6854.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Andersson AM, Jensen TK, Juul A, Petersen JH, Jorgensen T, Skakkebaek NE. Secular decline in male testosterone and sex hormone binding globulin serum levels in Danish population surveys. Journal of Clinical Endocrinology and Metabolism. 2007 Dec;92(12):4696–4705. doi: 10.1210/jc.2006-2633. [DOI] [PubMed] [Google Scholar]
- 75.Simon D, Preziosi P, Barrett-Connor E, Roger M, Saint-Paul M, Nahoul K, et al. Interrelation between plasma testosterone and plasma insulin in healthy adult men: the Telecom Study. Diabetologia. 1992 Feb;35(2):173–177. doi: 10.1007/BF00402551. [DOI] [PubMed] [Google Scholar]
- 76.Ferrini RL, Barrett-Connor E. Sex hormones and age: a cross-sectional study of testosterone and estradiol and their bioavailable fractions in community-dwelling men. American Journal of Epidemiology. 1998 Apr 15;147(8):750–754. doi: 10.1093/oxfordjournals.aje.a009519. [DOI] [PubMed] [Google Scholar]
- 77.Gray A, Jackson DN, McKinlay JB. The relation between dominance, anger, and hormones in normally aging men: results from the Massachusetts Male Aging Study. Psychosomatic Medicine. 1991;53(4):375–385. doi: 10.1097/00006842-199107000-00003. [DOI] [PubMed] [Google Scholar]
- 78.Derby CA, Zilber S, Brambilla D, Morales KH, McKinlay JB. Body mass index, waist circumference and waist to hip ratio and change in sex steroid hormones: the Massachusetts Male Ageing Study. Clinical Endocrinology. 2006 Jul;65(1):125–131. doi: 10.1111/j.1365-2265.2006.02560.x. [DOI] [PubMed] [Google Scholar]
- 79.Longcope C, Feldman HA, McKinlay JB, Araujo AB. Diet and sex hormone-binding globulin. Journal of Clinical Endocrinology and Metabolism. 2000;85(1):293–296. doi: 10.1210/jcem.85.1.6291. [DOI] [PubMed] [Google Scholar]
- 80.Liverman CT, Blazer DG, editors. Testosterone and aging: Clinical research directions. Washington D.C.: The National Academies Press; 2004. [PubMed] [Google Scholar]
- 81.Travison TG, Morley JE, Araujo AB, O'Donnell AB, McKinlay JB. The relationship between libido and testosterone levels in aging men. Journal of Clinical Endocrinology and Metabolism. 2006 Jul;91(7):2509–2513. doi: 10.1210/jc.2005-2508. [DOI] [PubMed] [Google Scholar]
- 82.Shabsigh R. Testosterone therapy in erectile dysfunction and hypogonadism. The Journal of Sexual Medicine. 2005 Nov;2(6):785–792. doi: 10.1111/j.1743-6109.2005.00139.x. [DOI] [PubMed] [Google Scholar]
- 83.Schiavi RC, White D, Mandeli J, Levine AC. Effect of testosterone administration on sexual behavior and mood in men with erectile dysfunction. Archives of Sexual Behavior. 1997 Jun;26(3):231–241. doi: 10.1023/a:1024518730222. [DOI] [PubMed] [Google Scholar]
- 84.Isidori AM, Giannetta E, Gianfrilli D, Greco EA, Bonifacio V, Aversa A, et al. Effects of testosterone on sexual function in men: results of a meta-analysis. Clinical Endocrinology. 2005 Oct;63(4):381–394. doi: 10.1111/j.1365-2265.2005.02350.x. [DOI] [PubMed] [Google Scholar]
- 85.Gray PB, Singh AB, Woodhouse LJ, Storer TW, Casaburi R, Dzekov J, et al. Dose-dependent effects of testosterone on sexual function, mood, and visuospatial cognition in older men. Journal of Clinical Endocrinology and Metabolism. 2005 Jul;90(7):3838–3846. doi: 10.1210/jc.2005-0247. [DOI] [PubMed] [Google Scholar]
- 86.Kupelian V, Shabsigh R, Travison TG, Page ST, Araujo AB, McKinlay JB. Is there a relationship between sex hormones and erectile dysfunction? Results from the Massachusetts Male Aging Study. Journal of Urology. 2006 Dec;176(6 Pt 1):2584–2588. doi: 10.1016/j.juro.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 87.Gooren LJ. Androgen levels and sex functions in testosterone-treated hypogonadal men. Archives of Sexual Behavior. 1987 Dec;16(6):463–473. doi: 10.1007/BF01541711. [DOI] [PubMed] [Google Scholar]
- 88.Araujo AB, Travison TG, Bhasin S, Esche GR, Williams RE, Clark RV, et al. Association between testosterone and estradiol and age-related decline in physical function in a diverse sample of men. Journal of the American Geriatrics Society. 2008 Nov;56(11):2000–2008. doi: 10.1111/j.1532-5415.2008.01965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Isidori AM, Giannetta E, Greco EA, Gianfrilli D, Bonifacio V, Isidori A, et al. Effects of testosterone on body composition, bone metabolism and serum lipid profile in middle-aged men: a meta-analysis. Clinical Endocrinology. 2005 Sep;63(3):280–293. doi: 10.1111/j.1365-2265.2005.02339.x. [DOI] [PubMed] [Google Scholar]
- 90.Singh R, Artaza JN, Taylor WE, Braga M, Yuan X, Gonzalez-Cadavid NF, et al. Testosterone inhibits adipogenic differentiation in 3T3-L1 cells: nuclear translocation of androgen receptor complex with beta-catenin and T-cell factor 4 may bypass canonical Wnt signaling to down-regulate adipogenic transcription factors. Endocrinology. 2006 Jan;147(1):141–154. doi: 10.1210/en.2004-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Brodsky IG, Balagopal P, Nair KS. Effects of testosterone replacement on muscle mass and muscle protein synthesis in hypogonadal men--a clinical research center study. Journal of Clinical Endocrinology and Metabolism. 1996 Oct;81(10):3469–3475. doi: 10.1210/jcem.81.10.8855787. [DOI] [PubMed] [Google Scholar]
- 92.Maggio M, Basaria S, Ble A, Lauretani F, Bandinelli S, Ceda GP, et al. Correlation between testosterone and the inflammatory marker soluble interleukin-6 receptor in older men. Journal of Clinical Endocrinology and Metabolism. 2006 Jan;91(1):345–347. doi: 10.1210/jc.2005-1097. [DOI] [PubMed] [Google Scholar]
- 93.Kupelian V, Chiu GR, Araujo AB, Williams RE, Clark RV, McKinlay JB. Association of sex hormones and C-reactive protein levels in men. Clinical Endocrinology. 2010 Apr;72(4):527–533. doi: 10.1111/j.1365-2265.2009.03713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schaap LA, Pluijm SM, Deeg DJ, Penninx BW, Nicklas BJ, Lips P, et al. Low testosterone levels and decline in physical performance and muscle strength in older men: findings from two prospective cohort studies. Clinical Endocrinology. 2008 Jan;68(1):42–50. doi: 10.1111/j.1365-2265.2007.02997.x. [DOI] [PubMed] [Google Scholar]
- 95.Krasnoff JB, Basaria S, Pencina MJ, Jasuja GK, Vasan RS, Ulloor J, et al. Free Testosterone Levels Are Associated with Mobility Limitation and Physical Performance in Community-Dwelling Men: The Framingham Offspring Study. Journal of Clinical Endocrinology and Metabolism. 2010 Apr 9; doi: 10.1210/jc.2009-2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Orwoll E, Lambert LC, Marshall LM, Blank J, Barrett-Connor E, Cauley J, et al. Endogenous testosterone levels, physical performance, and fall risk in older men. Archives of Internal Medicine. 2006 Oct 23;166(19):2124–2131. doi: 10.1001/archinte.166.19.2124. [DOI] [PubMed] [Google Scholar]
- 97.Szulc P, Claustrat B, Marchand F, Delmas PD. Increased risk of falls and increased bone resorption in elderly men with partial androgen deficiency: the MINOS study. Journal of Clinical Endocrinology and Metabolism. 2003 Nov;88(11):5240–5247. doi: 10.1210/jc.2003-030200. [DOI] [PubMed] [Google Scholar]
- 98.Schaap LA, Pluijm SM, Smit JH, van Schoor NM, Visser M, Gooren LJ, et al. The association of sex hormone levels with poor mobility, low muscle strength and incidence of falls among older men and women. Clinical Endocrinology. 2005 Aug;63(2):152–160. doi: 10.1111/j.1365-2265.2005.02315.x. [DOI] [PubMed] [Google Scholar]
- 99.Bhasin S, Calof OM, Storer TW, Lee ML, Mazer NA, Jasuja R, et al. Drug insight: Testosterone and selective androgen receptor modulators as anabolic therapies for chronic illness and aging. Nature Clinical Practice Endocrinology & Metabolism. 2006 Mar;2(3):146–159. doi: 10.1038/ncpendmet0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Storer TW, Magliano L, Woodhouse L, Lee ML, Dzekov C, Dzekov J, et al. Testosterone dose-dependently increases maximal voluntary strength and leg power, but does not affect fatigability or specific tension. Journal of Clinical Endocrinology and Metabolism. 2003 Apr;88(4):1478–1485. doi: 10.1210/jc.2002-021231. [DOI] [PubMed] [Google Scholar]
- 101.Storer TW, Woodhouse L, Magliano L, Singh AB, Dzekov C, Dzekov J, et al. Changes in muscle mass, muscle strength, and power but not physical function are related to testosterone dose in healthy older men. Journal of the American Geriatrics Society. 2008 Nov;56(11):1991–1999. doi: 10.1111/j.1532-5415.2008.01927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sattler FR, Castaneda-Sceppa C, Binder EF, Schroeder ET, Wang Y, Bhasin S, et al. Testosterone and growth hormone improve body composition and muscle performance in older men. Journal of Clinical Endocrinology and Metabolism. 2009 Jun;94(6):1991–2001. doi: 10.1210/jc.2008-2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Srinivas-Shankar U, Roberts SA, Connolly MJ, MD OC, Adams JE, Oldham JA, et al. Effects of Testosterone on Muscle Strength, Physical Function, Body Composition, and Quality of Life in Intermediate-Frail and Frail Elderly Men: A Randomized, Double-Blind, Placebo-Controlled Study. Journal of Clinical Endocrinology and Metabolism. 2010 Jan 8; doi: 10.1210/jc.2009-1251. [DOI] [PubMed] [Google Scholar]
- 104.Basaria S, Coviello AD, Travison TG, Storer TW, Farwell WR, Jette AM, et al. Adverse Events Associated with Testosterone Administration. New England Journal of Medicine. 2010 Jun 30; doi: 10.1056/NEJMoa1000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Khosla S, Melton LJ, 3rd, Atkinson EJ, O'Fallon WM, Klee GG, Riggs BL. Relationship of serum sex steroid levels and bone turnover markers with bone mineral density in men and women: a key role for bioavailable estrogen. Journal of Clinical Endocrinology and Metabolism. 1998 Jul;83(7):2266–2274. doi: 10.1210/jcem.83.7.4924. [DOI] [PubMed] [Google Scholar]
- 106.Fink HA, Ewing SK, Ensrud KE, Barrett-Connor E, Taylor BC, Cauley JA, et al. Association of testosterone and estradiol deficiency with osteoporosis and rapid bone loss in older men. Journal of Clinical Endocrinology and Metabolism. 2006 Oct;91(10):3908–3915. doi: 10.1210/jc.2006-0173. [DOI] [PubMed] [Google Scholar]
- 107.Falahati-Nini A, Riggs BL, Atkinson EJ, O'Fallon WM, Eastell R, Khosla S. Relative contributions of testosterone and estrogen in regulating bone resorption and formation in normal elderly men. Journal of Clinical Investigation. 2000 Dec;106(12):1553–1560. doi: 10.1172/JCI10942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Leblanc ES, Nielson CM, Marshall LM, Lapidus JA, Barrett-Connor E, Ensrud KE, et al. The effects of serum testosterone, estradiol, and sex hormone binding globulin levels on fracture risk in older men. Journal of Clinical Endocrinology and Metabolism. 2009 Jul 7; doi: 10.1210/jc.2009-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mellstrom D, Vandenput L, Mallmin H, Holmberg AH, Lorentzon M, Oden A, et al. Older men with low serum estradiol and high serum SHBG have an increased risk of fractures. Journal of Bone and Mineral Research. 2008 Oct;23(10):1552–1560. doi: 10.1359/jbmr.080518. [DOI] [PubMed] [Google Scholar]
- 110.Stone KL, Seeley DG, Lui LY, Cauley JA, Ensrud K, Browner WS, et al. BMD at multiple sites and risk of fracture of multiple types: long-term results from the Study of Osteoporotic Fractures. Journal of Bone and Mineral Research. 2003 Nov;18(11):1947–1954. doi: 10.1359/jbmr.2003.18.11.1947. [DOI] [PubMed] [Google Scholar]
- 111.Benito M, Gomberg B, Wehrli FW, Weening RH, Zemel B, Wright AC, et al. Deterioration of trabecular architecture in hypogonadal men. Journal of Clinical Endocrinology and Metabolism. 2003 Apr;88(4):1497–1502. doi: 10.1210/jc.2002-021429. [DOI] [PubMed] [Google Scholar]
- 112.Khosla S, Melton LJ, 3rd, Achenbach SJ, Oberg AL, Riggs BL. Hormonal and biochemical determinants of trabecular microstructure at the ultradistal radius in women and men. Journal of Clinical Endocrinology and Metabolism. 2006 Mar;91(3):885–891. doi: 10.1210/jc.2005-2065. [DOI] [PubMed] [Google Scholar]
- 113.Benito M, Vasilic B, Wehrli FW, Bunker B, Wald M, Gomberg B, et al. Effect of testosterone replacement on trabecular architecture in hypogonadal men. Journal of Bone and Mineral Research. 2005 Oct;20(10):1785–1791. doi: 10.1359/JBMR.050606. [DOI] [PubMed] [Google Scholar]
- 114.Tracz MJ, Sideras K, Bolona ER, Haddad RM, Kennedy CC, Uraga MV, et al. Testosterone use in men and its effects on bone health. A systematic review and meta-analysis of randomized placebo-controlled trials. Journal of Clinical Endocrinology and Metabolism. 2006 Jun;91(6):2011–2016. doi: 10.1210/jc.2006-0036. [DOI] [PubMed] [Google Scholar]
- 115.Basaria S, Muller DC, Carducci MA, Egan J, Dobs AS. Hyperglycemia and insulin resistance in men with prostate carcinoma who receive androgen-deprivation therapy. Cancer. 2006 Feb 1;106(3):581–588. doi: 10.1002/cncr.21642. [DOI] [PubMed] [Google Scholar]
- 116.Laaksonen DE, Niskanen L, Punnonen K, Nyyssonen K, Tuomainen TP, Valkonen VP, et al. Testosterone and sex hormone-binding globulin predict the metabolic syndrome and diabetes in middle-aged men. Diabetes Care. 2004 May;27(5):1036–1041. doi: 10.2337/diacare.27.5.1036. [DOI] [PubMed] [Google Scholar]
- 117.Kupelian V, Page ST, Araujo AB, Travison TG, Bremner WJ, McKinlay JB. Low sex hormone-binding globulin, total testosterone, and symptomatic androgen deficiency are associated with development of the metabolic syndrome in nonobese men. Journal of Clinical Endocrinology and Metabolism. 2006 Mar;91(3):843–850. doi: 10.1210/jc.2005-1326. [DOI] [PubMed] [Google Scholar]
- 118.Ding EL, Song Y, Malik VS, Liu S. Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. Journal of the American Medical Association. 2006 Mar 15;295(11):1288–1299. doi: 10.1001/jama.295.11.1288. [DOI] [PubMed] [Google Scholar]
- 119.Barrett-Connor E, Khaw KT, Yen SS. Endogenous sex hormone levels in older adult men with diabetes mellitus. American Journal of Epidemiology. 1990 Nov;132(5):895–901. doi: 10.1093/oxfordjournals.aje.a115732. [DOI] [PubMed] [Google Scholar]
- 120.Lakshman KM, Bhasin S, Araujo AB. Sex hormone-binding globulin as an independent predictor of incident type 2 diabetes mellitus in men. Journals of Gerontology Series A, Biological Sciences and Medical Sciences. 2010 May;65(5):503–509. doi: 10.1093/gerona/glq002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Simon D, Charles MA, Lahlou N, Nahoul K, Oppert JM, Gouault-Heilmann M, et al. Androgen therapy improves insulin sensitivity and decreases leptin level in healthy adult men with low plasma total testosterone: a 3-month randomized placebo-controlled trial. Diabetes Care. 2001 Dec;24(12):2149–2151. doi: 10.2337/diacare.24.12.2149. [DOI] [PubMed] [Google Scholar]
- 122.Kapoor D, Aldred H, Clark S, Channer KS, Jones TH. Clinical and biochemical assessment of hypogonadism in men with type 2 diabetes: correlations with bioavailable testosterone and visceral adiposity. Diabetes Care. 2007 Apr;30(4):911–917. doi: 10.2337/dc06-1426. [DOI] [PubMed] [Google Scholar]
- 123.Marin P. Testosterone and regional fat distribution. Obesity Research. 1995 Nov;3 Suppl 4:609S–612S. doi: 10.1002/j.1550-8528.1995.tb00233.x. [DOI] [PubMed] [Google Scholar]
- 124.Sader MA, Griffiths KA, Skilton MR, Wishart SM, Handelsman DJ, Celermajer DS. Physiological testosterone replacement and arterial endothelial function in men. Clinical Endocrinology. 2003 Jul;59(1):62–67. doi: 10.1046/j.1365-2265.2003.01796.x. [DOI] [PubMed] [Google Scholar]
- 125.Ong PJ, Patrizi G, Chong WC, Webb CM, Hayward CS, Collins P. Testosterone enhances flow-mediated brachial artery reactivity in men with coronary artery disease. American Journal of Cardiology. 2000 Jan 15;85(2):269–272. doi: 10.1016/s0002-9149(99)00630-x. [DOI] [PubMed] [Google Scholar]
- 126.Wu FC, von Eckardstein A. Androgens and coronary artery disease. Endocrine Reviews. 2003 Apr;24(2):183–217. doi: 10.1210/er.2001-0025. [DOI] [PubMed] [Google Scholar]
- 127.Arnlov J, Pencina MJ, Amin S, Nam BH, Benjamin EJ, Murabito JM, et al. Endogenous sex hormones and cardiovascular disease incidence in men. Annals of Internal Medicine. 2006 Aug 1;145(3):176–184. doi: 10.7326/0003-4819-145-3-200608010-00005. [DOI] [PubMed] [Google Scholar]
- 128.English KM, Steeds RP, Jones TH, Diver MJ, Channer KS. Low-dose transdermal testosterone therapy improves angina threshold in men with chronic stable angina: A randomized, double-blind, placebo-controlled study. Circulation. 2000 Oct 17;102(16):1906–1911. doi: 10.1161/01.cir.102.16.1906. [DOI] [PubMed] [Google Scholar]
- 129.Haddad RM, Kennedy CC, Caples SM, Tracz MJ, Bolona ER, Sideras K, et al. Testosterone and cardiovascular risk in men: a systematic review and meta-analysis of randomized placebo-controlled trials. Mayo Clinic Proceedings. 2007 Jan;82(1):29–39. doi: 10.4065/82.1.29. [DOI] [PubMed] [Google Scholar]
- 130.Janowsky JS. The role of androgens in cognition and brain aging in men. Neuroscience. 2006;138(3):1015–1020. doi: 10.1016/j.neuroscience.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 131.Seidman SN, Araujo AB, Roose SP, Devanand DP, Xie S, Cooper TB, et al. Low testosterone levels in elderly men with dysthymic disorder. American Journal of Psychiatry. 2002 Mar;159(3):456–459. doi: 10.1176/appi.ajp.159.3.456. [DOI] [PubMed] [Google Scholar]
- 132.Wang C, Alexander G, Berman N, Salehian B, Davidson T, McDonald V, et al. Testosterone replacement therapy improves mood in hypogonadal men--a clinical research center study. Journal of Clinical Endocrinology and Metabolism. 1996 Oct;81(10):3578–3583. doi: 10.1210/jcem.81.10.8855804. [DOI] [PubMed] [Google Scholar]
- 133.Snyder PJ, Peachey H, Berlin JA, Hannoush P, Haddad G, Dlewati A, et al. Effects of testosterone replacement in hypogonadal men. Journal of Clinical Endocrinology and Metabolism. 2000 Aug;85(8):2670–2677. doi: 10.1210/jcem.85.8.6731. [DOI] [PubMed] [Google Scholar]
- 134.Carrero JJ, Qureshi AR, Parini P, Arver S, Lindholm B, Barany P, et al. Low serum testosterone increases mortality risk among male dialysis patients. Journal of the American Society of Nephrology. 2009 Mar;20(3):613–620. doi: 10.1681/ASN.2008060664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Laughlin GA, Barrett-Connor E, Bergstrom J. Low serum testosterone and mortality in older men. Journal of Clinical Endocrinology and Metabolism. 2008 Jan;93(1):68–75. doi: 10.1210/jc.2007-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Shores MM, Moceri VM, Gruenewald DA, Brodkin KI, Matsumoto AM, Kivlahan DR. Low testosterone is associated with decreased function and increased mortality risk: a preliminary study of men in a geriatric rehabilitation unit. Journal of the American Geriatrics Society. 2004 Dec;52(12):2077–2081. doi: 10.1111/j.1532-5415.2004.52562.x. [DOI] [PubMed] [Google Scholar]
- 137.Shores MM, Matsumoto AM, Sloan Sloan KL, Kivlahan DR. Low serum testosterone and mortality in male veterans. Archives of Internal Medicine. 2006 Aug 14–28;166(15):1660–1665. doi: 10.1001/archinte.166.15.1660. [DOI] [PubMed] [Google Scholar]
- 138.Vikan T, Schirmer H, Njolstad I, Svartberg J. Low testosterone levels and SHBG levels and high estradiol levels are independent predictors of type 2 diabetes in men. European Journal of Endocrinology / European Federation of Endocrine Societies. 2010 Jan 8; doi: 10.1530/EJE-09-0943. [DOI] [PubMed] [Google Scholar]
- 139.Menke A, Guallar E, Rohrmann S, Nelson WG, Rifai N, Kanarek N, et al. Sex Steroid Hormone Concentrations and Risk of Death in US Men. American Journal of Epidemiology. 2010 Mar 1;171(5):583–592. doi: 10.1093/aje/kwp415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Haring R, Volzke H, Steveling A, Krebs A, Felix SB, Schofl C, et al. Low serum testosterone levels are associated with increased risk of mortality in a population-based cohort of men aged 20–79. European Heart Journal. 2010 Feb 17; doi: 10.1093/eurheartj/ehq009. [DOI] [PubMed] [Google Scholar]
- 141.Ponikowska B, Jankowska EA, Maj J, Wegrzynowska-Teodorczyk K, Biel B, Reczuch K, et al. Gonadal and adrenal androgen deficiencies as independent predictors of increased cardiovascular mortality in men with type II diabetes mellitus and stable coronary artery disease. International Journal of Cardiology. 2009 Apr 21; doi: 10.1016/j.ijcard.2009.03.072. [DOI] [PubMed] [Google Scholar]
- 142.Khaw KT, Dowsett M, Folkerd E, Bingham S, Wareham N, Luben R, et al. Endogenous testosterone and mortality due to all causes, cardiovascular disease, and cancer in men: European prospective investigation into cancer in Norfolk (EPIC-Norfolk) Prospective Population Study. Circulation. 2007 Dec 4;116(23):2694–2701. doi: 10.1161/CIRCULATIONAHA.107.719005. [DOI] [PubMed] [Google Scholar]
- 143.Lehtonen A, Huupponen R, Tuomilehto J, Lavonius S, Arve S, Isoaho H, et al. Serum testosterone but not leptin predicts mortality in elderly men. Age and Ageing. 2008 doi: 10.1093/ageing/afn048. [DOI] [PubMed] [Google Scholar]
- 144.Tivesten A, Vandenput L, Labrie F, Karlsson MK, Ljunggren O, Mellstrom D, et al. Low serum testosterone and estradiol predict mortality in elderly men. Journal of Clinical Endocrinology and Metabolism. 2009 Jul;94(7):2482–2488. doi: 10.1210/jc.2008-2650. [DOI] [PubMed] [Google Scholar]
- 145.Guder G, Frantz S, Bauersachs J, Allolio B, Ertl G, Angermann CE, et al. Low Circulating Androgens and Mortality Risk in Heart Failure. Heart. 2009 Oct 28; doi: 10.1136/hrt.2009.181065. [DOI] [PubMed] [Google Scholar]
- 146.Jankowska EA, Rozentryt P, Ponikowska B, Hartmann O, Kustrzycka-Kratochwil D, Reczuch K, et al. Circulating estradiol and mortality in men with systolic chronic heart failure. Journal of the American Medical Association. 2009 May 13;301(18):1892–1901. doi: 10.1001/jama.2009.639. [DOI] [PubMed] [Google Scholar]
- 147.Malkin CJ, Pugh PJ, Morris PD, Asif S, Jones TH, Channer KS. Low serum testosterone and increased mortality in men with coronary heart disease. Heart. 2010 Nov;96(22):1821–1825. doi: 10.1136/hrt.2010.195412. [DOI] [PubMed] [Google Scholar]
- 148.Araujo AB, Kupelian V, Page ST, Handelsman DJ, Bremner WJ, McKinlay JB. Sex steroids and all-cause and cause-specific mortality in men. Archives of Internal Medicine. 2007 Jun 25;167(12):1252–1260. doi: 10.1001/archinte.167.12.1252. [DOI] [PubMed] [Google Scholar]
- 149.Barrett-Connor E, Khaw KT. Endogenous sex hormones and cardiovascular disease in men. A prospective population-based study. Circulation. 1988 Sep;78(3):539–545. doi: 10.1161/01.cir.78.3.539. [DOI] [PubMed] [Google Scholar]
- 150.Haffner SM, Shaten J, Stern MP, Smith GD, Kuller L. Low levels of sex hormone-binding globulin and testosterone predict the development of non-insulin-dependent diabetes mellitus in men. MRFIT Research Group. Multiple Risk Factor Intervention Trial. American Journal of Epidemiology. 1996 May 1;143(9):889–897. doi: 10.1093/oxfordjournals.aje.a008832. [DOI] [PubMed] [Google Scholar]
- 151.Maggio M, Lauretani F, Ceda GP, Bandinelli S, Ling SM, Metter EJ, et al. Relationship between low levels of anabolic hormones and 6-year mortality in older men: the aging in the Chianti Area (InCHIANTI) study. Archives of Internal Medicine. 2007 Nov 12;167(20):2249–2254. doi: 10.1001/archinte.167.20.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Smith GD, Ben-Shlomo Y, Beswick A, Yarnell J, Lightman S, Elwood P. Cortisol, testosterone, and coronary heart disease: prospective evidence from the Caerphilly study. Circulation. 2005 Jul 19;112(3):332–340. doi: 10.1161/CIRCULATIONAHA.104.489088. [DOI] [PubMed] [Google Scholar]
- 153.Szulc P, Claustrat B, Delmas PD. Serum concentrations of 17beta-E2 and 25-hydroxycholecalciferol (25OHD) in relation to all-cause mortality in older men--the MINOS study. Clinical Endocrinology. 2009 Oct;71(4):594–602. doi: 10.1111/j.1365-2265.2009.03530.x. [DOI] [PubMed] [Google Scholar]
- 154.Platz EA. Low testosterone and risk of premature death in older men: analytical and preanalytical issues in measuring circulating testosterone. Clinical Chemistry. 2008 Jul;54(7):1110–1112. doi: 10.1373/clinchem.2008.104901. [DOI] [PubMed] [Google Scholar]