Abstract
The roles of cells within the nervous system are based on their properties of excitability, which are in part governed by voltage-gated ion channels. HCN channels underlie the hyperpolarization-activated current, Ih, an important regulator of excitability and rhythmicity through control of basic membrane properties. Ih is present in multiple neuronal types and regions of the central nervous system, and changes in Ih alter cellular input-output properties and neuronal circuitry important for behavior such as learning and memory. Furthermore, the pathophysiology of neurological diseases of both the central and peripheral nervous system involves defects in excitability, rhythmicity, and signaling, and animal models of many of these disorders have implicated changes in HCN channels and Ih as critical for pathogenesis. In this review, we focus on recent research elucidating the role of HCN channels and Ih in behavior and disease. These studies have utilized knockout mice as well as animal models of disease to examine how Ih may be important in regulating learning and memory, sleep, and consciousness, as well as how misregulation of Ih may contribute to epilepsy, chronic pain, and other neurological disorders. This review will help guide future studies aimed at further understanding the function of this unique conductance in both health and disease of the mammalian brain.
Keywords: Hyperpolarization-activated current, HCN channel, epilepsy, learning and memory, pain, Ih
1.1 Introduction
The major role of neurons within the central nervous system (CNS) and peripheral nervous system (PNS) is signaling, a process that relies on a vast repertoire of ion channels that mediate both the transmission and reception of signals, and influence the ultimate output of a neuron within a network. These ion channels are further classified as ligand- or voltage-gated, depending on how they are activated. Voltage-gated ion channels may be located anywhere along the axodendritic axis of a neuron, and their localization, combined with their specific functional properties, help determine their role within a given neuron (Lai and Jan, 2006).
Hyperpolarization-activated cyclic nucleotide-gated (HCN) channels underlie the hyperpolarization-activated current, Ih, a mixed Na+/K+ current that is activated by hyperpolarization rather than depolarization, despite the membership of HCN channels in the potassium channel superfamily (Robinson and Siegelbaum, 2003). Ih is conducted by four HCN channel subunits (HCN1–4) that assemble into homo- or heterotetrameric channels and are found in numerous excitable and non-excitable cell types, including many neurons of the CNS (Notomi and Shigemoto, 2004) and PNS (Dunlop et al., 2009), cardiomyocytes (Baruscotti et al., 2005), and taste cells (Stevens et al., 2001). HCN channel subunits differ significantly in their biophysical properties and sensitivity to cAMP (reviewed by (Biel et al., 2009; Lewis et al., 2010; Robinson and Siegelbaum, 2003; Wahl-Schott and Biel, 2008)). Voltage activation of HCN1 occurs at more depolarized potentials than HCN2–4, with HCN4 activated at the most hyperpolarized potentials. In similar order, the speed of channel activation is fastest for HCN1 and slowest for HCN4. Voltage activation of HCN4 is most dramatically depolarized by the presence of cAMP, whereas HCN1 is minimally cAMP-reponsive. Thus, the identity of HCN channels expressed within a particular cell serves to influence the overall properties of Ih.
The function of Ih in neurons stems from its unique ability to influence membrane properties such as resting membrane potential (RMP) and input resistance (RN), which in turn are important for determining a given neuron's role within a circuit. In a general sense, Ih plays two broad roles at the cellular level: 1) rhythmogenesis, and 2) control of dendritic integration and neuronal firing properties. Rhythmic oscillations of neuronal networks are paradigmatic of some physiological processes such as sleep, yet may also be observed in abnormal or pathological brain states, such as anesthesia or absence epilepsy (Luthi and McCormick, 1998; Pape, 1996). The unique hyperpolarization-activation of Ih permits it to act in a regenerative fashion, depolarizing neuronal membrane potential toward the firing threshold. Additionally, Ih plays an important role in non-rhythmically firing cells, such as excitatory principal neurons or inhibitory basket cells of the CNS, by modulating the amplitude and timing of both input and outputs (Aponte et al., 2006; Magee, 1999; Southan et al., 2000). These two cellular roles, rhythmogenesis and refinement of input-output properties, in turn are important on a neuronal network level.
In this review, we examine the function of Ih and HCN channels in behavior and disease. We review studies aimed at understanding how Ih may influence neuronal circuity designed for critical features of behavior such as learning and memory and sleep, as well as how misregulation of Ih and HCN channels may contribute to disorders of neuronal networks and excitability, such as epilepsy and chronic pain.
2.1 Ih in oscillatory thalamocortical neuronal networks
The thalamus consists of multiple nuclei containing a variety of types of neurons that differ in not only their cell-specific properties, such as type of neurotransmitter produced, but also in their connectivity, which may be either intrathalamic (i.e. connected to other thalamic nuclei), or extrathalamic. Two thalamic regions that express high levels of Ih are the dorsolateral geniculate nucleus (Ludwig et al., 2003; McCormick and Pape, 1990) and the ventrobasal (VB) complex (Santoro et al., 2000; Williams et al., 1997). In the absence of external innervation, thalamocortical (TC) neurons generate rhythmic bursts of action potentials at a frequency of 0.5–4 Hz due to Ih-mediated activation of the low-threshold calcium current IT leading to sodium spikes, followed by inactivation of IT, activation of Ih, and regeneration of the cycle (Luthi and McCormick, 1998; Pape, 1996). Normally, TC neurons are inhibited by GABAergic inhibitory post-synaptic potentials (IPSPs) from the neighboring reticular thalamic nucleus (RTN), which, like TC neurons, exhibit Ih mainly via expression of HCN2, (Abbas et al., 2006; Rateau and Ropert, 2006). TC and RTN neurons are both innervated by excitatory cortical inputs, and TC neurons also reciprocally project to the RTN, establishing a relatively simple circuit capable of generating oscillatory activity important for brain states discussed below (Steriade, 2005). Neocortical layer V pyramidal neurons also express high levels of Ih, with HCN channels comprised mostly of HCN1 (Notomi and Shigemoto, 2004). Thus, TC and corticothalamic (CT) connections are critical for gating information to the neocortex, and thus thought to be highly involved in defining states of consciousness, including sleep, arousal, and anesthesia (Franks, 2008). The relationship of these states to Ih and HCN channels are reviewed below.
2.1.1 Sleep and arousal
A major finding on electroencephalography (EEG) during non-rapid eye movement (non-REM) sleep in humans is coordinated rhythmic firing characterized by delta and spindle waves generated in the thalamus (Luthi and McCormick, 1998; McCormick and Bal, 1997). Delta waves, which have a frequency of 0.5–4 Hz, stem from the rhythmic interplay of Ih, IT, and Na+ spikes described above. Spindle waves, which are characterized by one to three second regularly spaced periods of 6–14 Hz activity superimposed on delta waves, are generated by the interaction of inhibitory neurons of the RTN and excitatory TC neurons (McCormick and Bal, 1997; McCormick and Pape, 1990). Rebound low-threshold Ca2+ spikes are generated in TC neurons following a subset of IPSPs mediated by GABAA receptors, which serve to remove inactivation of IT. Via a reciprocal excitatory connection, TC neurons subsequently fire a barrage of excitatory post-synaptic potentials (EPSPs) onto RTN neurons, restarting the cycle, which occurs at the observed frequency of spindle waves (McCormick and Bal, 1997). The slow kinetics of Ih are in part responsible for the periodicity of spindle waves (Bal and McCormick, 1996; McCormick and Pape, 1990).
Recently, Kanyshkova et al. examined the expression of HCN channels and Ih during development in rat TC neurons and correlated them with TC sleep patterns (Kanyshkova et al., 2009). During postnatal development between the third postnatal day (P3) and roughly four months of age, Ih current density in TC neurons was increased by 5.5-fold, the half-maximal voltage of activation (V50) was either unchanged or slightly hyperpolarized, and activation was significantly slowed. These changes were accompanied by decreased cAMP sensitivity. Molecularly, HCN1 and HCN2 protein was significantly increased with no significant change in HCN4, shifting the ratio of HCN1/HCN4 and HCN2/HCN4 subunits to higher values with age, although high levels of HCN4 expression persisted. Using EEG analysis, rats were found to develop differences in sleep and awake rhythms, such as slow-wave oscillations in non-REM sleep, beginning at P12, while at P7, there was little variation between the two states. Interestingly, computational modeling of a simple reciprocally connected TC/RTN neuron circuit demonstrated that oscillatory activity was highly dependent on Ih current conductance, and that when current was decreased to ~50% of adult levels, oscillations disappear. With these decreased current densities however, depolarizing the V50 by 15 mV led to the resumption of oscillatory activities. The authors correlated these findings with in vivo EEG results, whereby near P12, but not younger, the Ih current density and cAMP sensitivity permitted the commencement of slow wave oscillations during non-REM sleep (Kanyshkova et al., 2009).
These results further the understanding of the molecular basis of HCN channels in generating sleep-related oscillatory activity in the thalamus. Research has also focused on understanding the role of Ih in the transition between firing modes of TC neurons, specifically via monoaminergic nerve fibers innervating the thalamus from the brainstem, hypothalamus, and basal forebrain. The fibers, which signal using serotonin, noradrenaline, and histamine, enhance Ih in TC neurons (McCormick, 1992; Sun et al., 2003). In addition to monaminergic neurotransmitters, more recent research has found that peptides via multiple peptidergic connections to the thalamus may also influence Ih and TC firing modes. For example, vasoactive intestinal polypeptide (VIP) and pituitary adenylate cyclase-activating polypeptide (PACAP) are either expressed within the thalamus or by fibers innervating the thalamus (Sun et al., 2003). Application of these peptides in vitro to rat brain slices depolarized TC neuron RMP and decreased RN by activation of Ih, ultimately shifting the firing mode of these neurons from bursting to tonic (Sun et al., 2003). The activation resulted from a depolarizing shift in Ih V50, most likely resulting from increased cAMP levels following activation of PAC1 receptors. As both VIP and PACAP have been experimentally shown to play a role in the determination of REM sleep duration (Bourgin et al., 1997; Fang et al., 1995), endogenous neuropeptide control of Ih may be an important mechanism for regulation of sleep. Oppositely, orexin A, a peptide implicated in arousal, has recently been shown to inhibit Ih in layer V pyramidal neurons of the mouse prelimbic cortex (Li et al., 2009). The authors showed that orexin A, presumably through inhibition of Ih, enhanced the excitability of these neurons and may promote wakefulness, although further experimentation is required to definitively link orexin A's actions to Ih and HCN channels.
2.1.2 Anesthesia
As we have briefly reviewed, HCN channels and Ih in TC neurons impart critical membrane properties that facilitate synchronous oscillations required for sleep. It thus follows that Ih could be involved in the neural circuitry underlying anesthesia, a state with significant parallels to sleep that involves TC and CT neuronal circuits (Franks, 2008). Because inhibition of cortical or thalamic Ih may be sufficient for initiating the switch from tonic to burst firing of thalamic relay neurons, multiple studies have examined whether commonly used anesthetics may inhibit Ih in either cortical or TC neurons. Indeed, the anesthetics halothane (Chen et al., 2005b), isoflurane (Chen et al., 2009b), ketamine (see below), and propofol (see below) all are capable of inhibiting Ih current amplitude and/or hyperpolarizing Ih V50. Here we review evidence for the action of the anesthetics ketamine and propofol on Ih and HCN channels.
Ketamine is a commonly used drug for anesthesia and analgesia in medical settings, and is used illicitly for recreational purposes. Previous work has commonly ascribed ketamine's hypnotic function to its ability to non-competitively antagonize NMDA receptors at clinically relevant concentrations, yet experiments to prove this mechanism of action have not done so conclusively (Kelland et al., 1993; Petrenko et al., 2004). Chen et al. explored a potential effect of ketamine on HCN channels using both in vitro and in vivo experiments (Chen et al., 2009a). In HEK293 cells expressing HCN1 or HCN2 channels, as well as layer V cortical pyramidal cells (which predominantly express HCN1 and HCN2 subunits), treatment with a clinically-relevant level of ketamine caused a significant inhibition of Ih selectively through blockade of the HCN1 subunit, without inhibiting HCN2 (Chen et al., 2009a) Mice lacking HCN1 were also significantly more resistant to ketamine-induced loss of righting reflex (LORR) compared to wildtype mice. The authors hypothesized that ketamine induces its hypnotic effect by inhibition of cortical Ih, reduction of Ih-dependent dendritic shunting, and enhancing synaptic integration, leading to increased cortically-driven slow oscillatory activity (< 1 Hz) and hypnosis (Chen et al., 2009a).
The ability of propofol, another anesthetic that inhibits HCN1 (Chen et al., 2005a), to cause LORR was also significantly reduced in HCN1 knockout mice (Chen et al., 2009a). Multiple studies have implicated propofol in modulating Ih in both cortical pyramidal neurons as well as TC neurons. Cacheaux et al. found that clinically relevant concentrations of propofol inhibited Ih or slowed activation kinetics of HCN1, HCN2, and HCN4 in heterologous cells, with the greatest effect on HCN1 (Cacheaux et al., 2005). Propofol's anesthetic action likely involves TC neurons of the VB complex (Ying et al., 2006). In these neurons, propofol significantly hyperpolarized Ih V50 in a cAMP-independent manner, decreased Ih conductance, and slowed activation kinetics, with some of these changes in Ih lasting at least three hours following injection of an anesthetic dose (Cacheaux et al., 2005; Ying et al., 2006). Propofol also decreased the frequency and regularity of thalamic oscillations induced by tetanic stimulation of CT fibers in a manner identical to the HCN channel blocker ZD7288 (Ying et al., 2006). These results suggest that propofol may induce anesthesia by Ih-mediated changes in TC activity.
Studies have also shown that propofol acts on Ih in neocortical neurons. Chen et al. found application of 5 μM propofol substantially slowed Ih activation and inhibited Ih via hyperpolarization of V50 as well as caused a decrease in maximal available current (Chen et al., 2005a). Propofol hyperpolarized RMP and also decreased excitability, requiring more current input to achieve action potentials. This study, as well as other reports, found that in heterologous cells expressing recombinant HCN channel subunits, propofol was significantly selective for modifying Ih via HCN1 (Cacheaux et al., 2005; Chen et al., 2005a; Lyashchenko et al., 2007).
Although the mechanism underlying the inhibition of HCN1 channels by ketamine remains unknown, propofol's specificity for HCN1 has been studied. Propofol interacts with the conserved core domain of HCN1, as it was able to impart similar effects to channel opening, closing, and voltage gating on both wildtype HCN1 channels as well as a mutant channel lacking both the N-terminus and the C-terminus including the CNBD and C-linker (Lyashchenko et al., 2007). Propofol acts similarly whether applied intra- or extracellularly, and preferentially binds to closed-resting and closed-activated states (Lyashchenko et al., 2007). Propofol is structurally distinct from ketamine, so whether ketamine might share similar mechanisms with propofol remains to be determined by future studies.
2.2 Ih in learning and memory
One of the most important roles of neurons is integrating multiple synaptic inputs into a distinct output signal. This process is dependent upon the processing abilities of dendrites, which relies on not only dendritic morphology, but also the expression of voltage-gated ion channels (Magee and Johnston, 2005). Voltage-gated ion channels thus play a critical role in the induction and maintenance of synaptic and non-synaptic plasticity, which are thought to be important cellular correlates of learning and memory (Magee and Johnston, 2005).
Ih and HCN channels are highly expressed in the dendrites of multiple neuronal types, and figure prominently in the regulation of spatiotemporal inputs. In pyramidal neurons of hippocampal CA1 and neocortical layer V, Ih serves to dampen temporal summation of multiple synaptic inputs at the soma and sharpen the waveform of postsynaptic potential (Berger et al., 2001; Magee, 1999; Williams and Stuart, 2000). Furthermore, these effects are non-uniform throughout the dendrite, and thus Ih serves to normalize the effects of inputs regardless of their site on the dendritic tree. These neurons exhibit a striking distal dendritic enrichment of Ih and HCN channel proteins (Lorincz et al., 2002; Magee, 1998; Notomi and Shigemoto, 2004; Williams and Stuart, 2000), which was thought to underlie the ability of Ih to normalize inputs at differing dendritic locations. Studies of Purkinje cells, which express high levels of HCN1, have shown they do not express a distal gradient of Ih, yet also display a dampening of temporal summation that is location independent (Angelo et al., 2007). Thus, temporal summation properties appear to be controlled by the total number of HCN channels, while local dendritic processing is sensitive to HCN channel distribution (Angelo et al., 2007). These properties of Ih are important for understanding its contribution to certain elements of learning and memory.
2.2.1 Motor learning and memory
The first study of Ih in motor learning was performed using mice with genetic deletion of HCN1 (Nolan et al., 2003). These mice had either a global HCN1 knockout, or knockout restricted to the forebrain, and thus permitted an analysis of the importance of HCN1 in cerebellar function. Global HCN1 knockout mice had normal basic motor function, including ability to remain on a slow, constant speed rotarod. Interestingly, HCN1 knockout mice demonstrated deficits in their ability to learn to swim to a visible platform, despite having comparable abilities to wildtype mice on the initial trial, suggesting a defect in learned motor coordination. Similarly, on an accelerating rotarod test, HCN1 knockouts performed equally as well as controls on initial testing (Fig. 1A, B). However, after training, HCN1 knockout mice did not learn to balance as well on the rotating rod as controls, and repeat testing using forebrain-restricted HCN1 knockout mice demonstrated that this learning defect was forebrain-HCN1 independent, as the region-specific knockout mice learned the rotarod task similarly to controls. The defects in motor learning in global HCN1 knockout mice were most prominent at faster rotarod speeds. Tests of eyeblink conditioning showed that HCN1 knockout mice did not have differences in acquisition or extinction of the conditioned response, but did show shortened latency to the peak of the conditioned response. Taken together, HCN1 appeared to be required for a specific subset of motor learning and memory, involving fast and repeated coordinated movements.
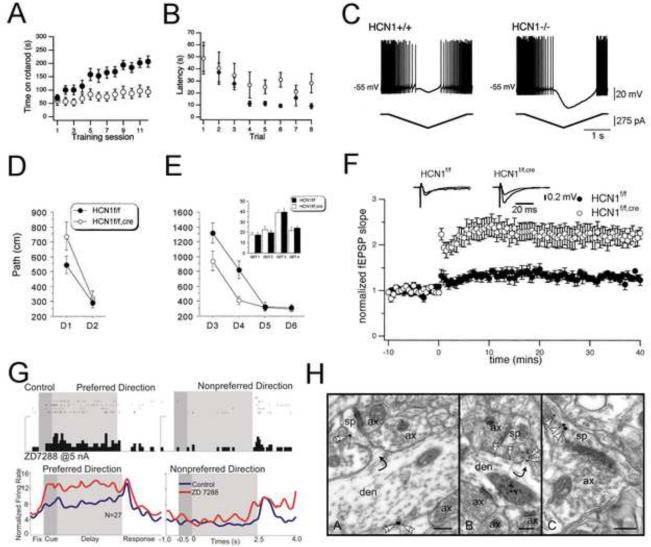
Figure 1. Ih and HCN channels contribute to physiology underlying learning and memory behaviors.
(A, B) Mice with full body deletion of HCN1 (◯) demonstrate impaired motor learning and memory versus controls (●) as tested on an accelerating rotarod (A) or in a visible platform water maze (B).
(C) Current ramps demonstrate that integration of inputs to cerebellar Purkinje cells is not history-independent in HCN1 knockout mice, which likely contributes to defects in fast repetitive coordinated movements.
(D, E) Forebrain-specific HCN1 knockout mice (HCN1f/f,cre) demonstrate enhanced spatial learning and memory in specific forms of a hidden platform water maze. D1, D2 (D) and D3–D6 (E) represent training days.
(F) Long-term potentiation at perforant path synapses to CA1 pyramidal neurons is enhanced by deletion of HCN1 from forebrain neurons. In contrast, LTP at Schaffer collateral synapses to CA1 neurons is unchanged by forebrain HCN1 deletion.
(G, top and bottom) Inhibition of Ih by ZD7288 in monkey PFC enhances delay-related firing in monkeys performing an oculomotor spatial delayed response task.
(H) Immunoelectron microscopy for HCN1 (left, middle panels) and α2A-adrenoreceptors (right panel) demonstrate that both are colocalized in dendritic spines in primate prefrontal cortex. Scale bars, 200 nm.
Figure adapted with permission from (Nolan et al., 2003) (A–C), (Nolan et al., 2004) (D–F), and (Wang et al., 2007) (G, H).
Nolan and colleagues used cellular physiology within the cerebellum to dissect how HCN1 may be required for this specific set of tasks by examining Purkinje cells, which express high levels of HCN1 (Notomi and Shigemoto, 2004; Santoro et al., 2000). Purkinje cell membranes from HCN1 knockout mice showed decreased slope conductance at potentials of −50 mV to −70 mV, which led to altered responses to inputs below threshold for spontaneous spiking (Nolan et al., 2003). Bidirectional current ramps beginning with hyperpolarization (and abolition of spontaneous spiking) followed by depolarization revealed remarkable differences between cells with and without HCN1. Beginning with a silent, non-spiking state, HCN1 knockout Purkinje neurons required more time to begin spiking, displayed a greater spiking frequency, and had a greater current threshold for the first spike. Furthermore, the current threshold and instantaneous spiking of wildtype Purkinje cells was similar regardless of the prior state, either spiking or silent, while these properties in HCN1 knockout cells were dramatically dependent upon the previous state (Fig. 1C). Taken together, Nolan et al. were able to correlate a specific deficit in a set of behavioral tasks to a deficit in the integrative properties of Purkinje cells, and hypothesized that proper motor learning was blocked by a decrease in LTD at parallel fiber synapses due to prolonged hyperpolarization (Nolan et al., 2003). These findings are of great importance in not only demonstrating a specific role for the HCN1 channel, but also in showing that in addition to synaptic plasticity, non-synaptic properties of neurons are important for learning and memory processes. Future experiments in which HCN1 is selectively deleted from Purkinje neurons could allow experimenters to pinpoint whether all of the observed motor learning phenotype stems from loss of HCN1 in Purkinje neurons, or from other regions of the brain outside of the forebrain.
2.2.2 Spatial learning and memory
We have previously discussed the importance of Ih for motor learning and focused on cerebellar Purkinje neurons. Now we focus on spatial learning and memory, a more long-term form of learning and memory that requires an intact hippocampus (Morris et al., 1982). Area CA1 of the hippocampus expresses significant levels of Ih, mediated primarily by HCN1 and HCN2, with current densities and protein levels increasing as a function of distance from the cell body in the apical dendrite (Lorincz et al., 2002; Magee, 1998; Notomi and Shigemoto, 2004). Although this distal dendritic enrichment does not appear to be required for Ih's ability to decrease temporal summation of distal inputs, it is nonetheless important for processes occurring locally to the site of inputs (Angelo et al., 2007), and the origin of inputs to distal dendrites of CA1 pyramidal neurons differs from the origin of more proximal inputs. Nolan et al. sought to understand how Ih might be important in learning and memory in the hippocampus (Nolan et al., 2004). These studies assessed the importance of forebrain HCN1 on tasks of spatial learning and memory as well as how Ih through HCN1 contributes to network activity known to be important for learning and memory. Mice lacking forebrain HCN1 (HCN1f/f,cre) were tested for spatial memory using multiple water maze paradigms. With a four day, four trials per day test to find a submerged platform beginning with a 15 s priming procedure prior to the first spatial learning trial, HCN1f/f,cre surprisingly learned the task faster than the HCN1f/f control group, although both groups demonstrated similar final levels of performance (Fig. 1D, E) (Nolan et al., 2004). This difference however was dependent upon the priming procedure. When the same test was performed with only one trial per day, HCN1f/f,cre mice also showed reduced path length to the submerged platform, which was not dependent upon a priming procedure. Thus, deletion of forebrain HCN1 enhanced both short-term and long-term forms of spatial memory.
Mechanistically, how might forebrain HCN1 subunits contribute to these behavioral observations? Hippocampal theta rhythm results from the concerted firing of neuronal populations at a frequency of 4–10 Hz, and is believed to be important for learning and memory processing in both rodents and humans (reviewed by (Buzsaki, 2002)). In the hippocampus, emergence of theta rhythm is likely due to the relationship between synaptic inputs and neuronal intrinsic electrical properties, such as resonance (Hu et al., 2002; Hutcheon and Yarom, 2000). Resonance refers to the ability of neurons to respond to inputs at specific, preferred frequencies, and CA1 pyramidal neurons in rodents show resonance at 2–7 Hz, roughly corresponding to the theta band (Leung and Yu, 1998; Pike et al., 2000). Hu et al. determined that two forms of theta-resonance exist in CA1 neurons, with one observed at potentials between −70 mV and −50 mV, and the other at more hyperpolarized potentials between −75 mV and −95 mV (Hu et al., 2002). The depolarized resonance form is due to the M-current, IM, and the persistent sodium current, INaP, while the hyperpolarized resonance form is due to Ih (Hu et al., 2002). Narayanan and Johnston demonstrated that resonance due to Ih was not absolutely uniform but rather depended upon a number of factors, including membrane potential, somatodendritic location, and activity, with increased resonance frequency observed at more distal sites on the dendrite corresponding with increased Ih density (Narayanan and Johnston, 2007). Resonance frequency was also increased by induction of activity-dependent, long-term plasticity (Narayanan and Johnston, 2007). It is thus likely that deletion of HCN1 would significantly alter the intrinsic resonant properties of CA1 pyramidal neurons and alter the properties of hippocampal theta rhythms in HCN1f/f,cre mice. Along these lines, non-genetic disruption of Ih has been shown to subtly yet specifically alter the theta rhythm (Kocsis and Li, 2004; Nolan et al., 2004). Injection of ZD7288 to block Ih in the medial septum of rats led to a decrease in theta frequency during waking motor activity and REM sleep, as well as in urethane-anesthetized rats either spontaneously or following pontine reticular formation stimulation (Kocsis and Li, 2004).
In CA1 of mice with HCN1 deletion, theta power was enhanced during wheel running and REM sleep, a change likely resulting from loss of HCN1 attenuation of low frequency inputs (Nolan et al., 2004). In HCN1f/f,cre mice, somatic recordings of EPSPs generated by stimulation of Schaffer collaterals changed the EPSP area but not amplitude. In contrast, EPSPs generated by stimulation of the perforant path exhibited both increased EPSP area and amplitude in HCN1f/f,cre mice. Remarkably, in HCN1f/f,cre mice LTP amplitude was also enhanced at perforant path synapses but not at Schaffer collateral synapses (Fig. 1F) (Nolan et al., 2004). Because Schaffer collateral synapses occur more proximally than perforant path synapses, the distal dendritic gradient of Ih and HCN1 in CA1 pyramidal neurons could explain the significant differences in effects of HCN1 deletion on LTP from these two pathways. Specifically, deletion of Ih leads to membrane hyperpolarization that enhances distal Ca2+ transients by removing resting inactivation of T- and N-type Ca2+ channels, facilitating LTP only at distal inputs with high levels of Ih (Tsay et al., 2007).
2.2.3 Working memory
Neurons of the prefrontal cortex (PFC) have primarily been implicated in underlying WM (Goldman-Rakic, 1999). These neurons demonstrate sustained tonic firing in response to “memory fields”, which are similar to the receptive fields of sensory neurons, such that they specifically fire in response to preferred content (Goldman-Rakic, 1999). Because of its time course, WM is thought to be supported by network activity within the PFC from recurrent excitatory connections rather than by synaptic plasticity, as well as potentially by intrinsic mechanisms (Day et al., 2005; McCormick et al., 2003; Shu et al., 2003). Thus, a major theme underlying studies of the cellular mechanisms of WM involves examining how signaling of PFC neurons may be modified. Because Ih participates substantially in the regulation of synaptic integration, it is a natural candidate for playing an important role in WM. Indeed, pyramidal neurons in the rodent homolog of the PFC, the prelimbic and infralimbic pyramidal neurons (PPNs), express a robust Ih that appears to mainly be carried via HCN1 and HCN1/HCN2 heteromers, as suggested by Ih kinetics as well as molecular studies (Day et al., 2005; Wang et al., 2007). In these neurons, Ih is complemented by expression of Kir2 channels (Kir2.2 and Kir2.3) and Kleak channels (KCNK3 and KCNK9), and the addition of these K+ conductances sets membrane potential in a range that allows Ih to regulate synaptic integration and inhibit temporal summation of EPSPs (Day et al., 2005). Thus, modulation of any of these channels within these neurons may lead to changes in synaptic integration important for WM. The α2A-adrenoreceptor (α2A-AR) appears to be one mechanism by which Ih is modified in PFC pyramidal neurons. Activation of α2A-ARs by norepinephrine can strengthen WM (Arnsten and Goldman-Rakic, 1985; Franowicz et al., 2002; Wang et al., 2007), while blockade dramatically impairs WM (Li and Mei, 1994; Wang et al., 2007), and increased levels of cAMP have also been found to impair WM (Wang et al., 2007). Wang et al. found that inhibiting Ih with ZD7288 in PFC of monkeys performing an oculomotor spatial delayed response task increased delay-related firing in both weak-spatially tuned neurons as well as neurons well-tuned for preferred direction (Fig. 1G) (Wang et al., 2007). The effect of yohimbine, an α2A-AR-antagonist that serves to inhibit delay-related firing, as well as the PDE4 inhibitor etazolate, a compound that serves to increase cAMP levels and also inhibit delay-related firing, was reversed by the selective Ih inhibitor ZD7288, suggesting that modulation of Ih may be a common endpoint for the mechanism of α2A-ARs and cAMP on WM. At the network level, recordings from ferret PFC demonstrated that blockade of Ih with ZD7288 dramatically increased the recurrent network activity of the up state, most likely through an enhancement of the ability of synaptic connections to generate action potentials. Inhibition of Ih in rat PFC via either ZD7288 or virally-delivered siRNA knockdown of HCN1 significantly enhanced ability in behavioral tests of spatial WM. Remarkably, immuno-electron microscopy for HCN1 and α2A-ARs in primate PFC revealed significant colocalization of the two proteins on distal dendritic spines of pyramidal neurons (Fig. 1H). Taken together, the findings of Wang et al. coalesce into a straightforward model for the role of Ih in WM. Specifically, α2A-AR activity modulates the levels of cAMP locally in regions containing HCN channels, and this control of Ih regulates network activity, with high levels of Ih decreasing network activity, and low levels of Ih increasing network activity and strengthening WM (Wang et al., 2007). Additional studies have surprisingly revealed that activation of α2-noradrenergic receptors does not inhibit Ih through the classical mechanism of adenylate cyclase inhibition by Gαi but rather via the phospholipase C-protein kinase C (PLC, PKC) activated by Gβγ, yet it remains unknown wheather this activation is through actions of PKC on the channel itself or through an intermediary (Carr et al., 2007). Finally, a recent report has shown that inhibition of Ih in the region, a manipulation that impairs the hippocampal theta rhythm, significantly impaired performance on a test of working memory (spontaneous alternations) but not a test of emotional long-term memory (continuous multiple trial inhibitory avoidance) (Cisse et al., 2008). Ih thus appears to play an important role in WM within multiple brain regions.
3.1 Misregulation of Ih in neurological disorders
So far we have reviewed physiological processes that depend on HCN channels and Ih to regulate membrane properties leading to specific oscillatory activity in groups of neurons as well as fine-tuning of synaptic integration. The study of Ih and basic membrane properties reinforces that concept that modest changes in neuronal circuits may have a considerable impact. For example, deletion of HCN2 in mice hyperpolarizes the RMP in TC neurons by ~12 mV (Ludwig et al., 2003). This change dramatically alters the firing profile of TC neurons from tonic to bursting, and mice lacking HCN2 demonstrate absence epilepsy (Chung et al., 2009; Ludwig et al., 2003). Additionally, cases of human cardiac disease have been linked to a specific mutation in hHCN4 leading to sinus bradycardia (Milanesi et al., 2006; Schulze-Bahr et al., 2003). Recently, multiple studies have suggested that mutations in hHCN1 and hHCN2 channel genes may be important in patients with epilepsy syndromes. A cohort of unrelated patients with idiopathic generalized epilepsy identified numerous variants of hHCN1 and hHCN2 that may result in altered channel assembly or voltage-conductance relationship (Tang et al., 2008). Furthermore, a screen of patients with febrile seizures (FS) or genetic epilepsy with febrile seizures plus (GEFS+) found a significantly increased frequency of a triple proline deletion in hHCN2, and functional analysis in oocytes demonstrated this mutation enhances Ih current by ~35% (Dibbens et al., 2010). Thus, mutations in human HCN channel genes may enhance neuronal excitability, contributing to the pathogenesis of these epilepsy syndromes. In some cases, genetic mutations might compliment “h-channelopathies”, which can be defined as transcriptional, translational, regulatory, or trafficking deficits leading to an altered expression of Ih in neurons (Poolos, 2005), to result in either the onset or maintenance of an epilepsy syndrome. Here we review some of the studies suggesting h-channelopathy may play a role in epilepsy and pain, although additional neurological diseases may also result from abnormal Ih (Table 1).
Table 1.
Ih and HCN channels in neurological disorders.
| Neurological disorder | Role for Ih or HCN channels | Selected references |
|---|---|---|
| Temporal lobe epilepsy and febrile seizures | Altered Ih current density or voltage gating in hippocampal neurons | (Bender et al., 2003; Brewster et al., 2002; Chen et al., 2001a; Dyhrfjeld-Johnsen et al., 2008; Jung et al., 2010; Jung et al., 2007; Shah et al., 2004; Shin et al., 2008) |
| HCN channel mislocalization in hippocampus | (Shin et al., 2008) | |
| Increased seizure susceptibility in mice lacking HCN1 | (Huang et al., 2009; Santoro et al., 2010) | |
| Absence epilepsy | Altered Ih current density or voltage gating in thalamic or cortical neurons | (Chung et al., 2009; Kole et al., 2007; Ludwig et al., 2003; Strauss et al., 2004) |
| Chronic Pain | Enhanced Ih leads to spontaneous firing in SNL and chronic compression models | (Chaplan et al., 2003; Yao et al., 2003) |
| Acute Pain | Blockade of Ih using ZD7288 injection reduces pain behaviors in acute pain models | (Dalle and Eisenach, 2005; Luo et al., 2007) |
| Alcohol addiction | EtOH enhances Ih amplitude in DA- midbrain neurons; Repeated administration of EtOH induces downregulation of Ih amplitude in DA-midbrain neurons | (Okamoto et al., 2006) |
| Parkinson's disease | Upregulation of “juvenile” pacemaking form by Ih is neuroprotective for SNc neurons | (Chan et al., 2007) |
Abbreviations: SNL, spinal nerve ligation; DA, dopaminergic; EtOH, ethanol; SNc, substantia nigra pars compacta
3.1.1 Epilepsy
Although epilepsy is a heterogeneous disease, at its root it is a disorder of neuronal excitability. Thus, defects in channels (such as HCN channels) that serve to regulate neuronal excitability and influence other ionic conductances are well situated to either cause or contribute to epileptogenesis. Here we briefly review evidence for h-channelopathy in FS, temporal lobe epilepsy (TLE), and absence epilepsy, three common forms of epilepsy that affect both children and adults.
Initial evidence suggesting that Ih may be altered in epilepsy and seizure disorders came from animal models of FS. These experiments demonstrated that a neurological insult such as FS induced long-lasting changes in Ih promote enhanced excitability and seizure susceptibility later in life. Chen et al. examined rat pups that had experienced hyperthermic seizures after raising their core temperature to 40–41.5 °C, simulating human children with significant fever (Chen et al., 1999). In rats that had undergone hyperthermic seizures one week prior to experiments, field recordings in area CA1 from Schaffer collateral stimulation demonstrated a surprising decrease in amplitude that was abolished by bicuculline treatment, suggesting a GABAA-mediated mechanism (Chen et al., 1999). Further experiments showed that this increased inhibition in field recordings was due to presynaptic upregulation of IPSPs, which persisted 10 weeks following seizures (Chen et al., 1999). Exploring the unconventional concept that enhanced inhibition might underlie epilepsy in FS, Chen et al. found that Ih was also significantly altered in this rodent FS model (Chen et al., 2001a). The V50 of Ih in CA1 pyramidal neurons was depolarized by ~3 mV both one week and nine weeks following experimental seizures, leading to both depolarized RMP and decreased RN. The enhanced Ih interacted with the augmented inhibitory input so that bursts of inhibitory input led to increased post-inhibitory rebound depolarization and cell firing (Chen et al., 2001a). Because rats treated with this paradigm are indeed more susceptible to chemoconvulsants such as kainic acid (Dube et al., 2000) and exhibit spontaneous seizures later in life (Dube et al., 2006), these results suggest that Ih modification may contribute to spontaneous seizures later in life of humans who experienced FS as children.
Because the changes in Ih following experimental FS persisted for weeks to months and were cAMP-independent (Chen et al., 2001a), experiments have examined whether transcriptional changes could account for such long-lasting modifications. Since multiple HCN channel subunits contribute to Ih, relative changes in any one or combination of subunits could dramatically alter the properties of the overall Ih by changing the stoichiometry of heteromeric HCN channels (Chen et al., 2001b). Rats that underwent FS showed regional changes in hippocampal HCN channel subunit transcripts compared to controls, whereas Hcn1 mRNA was decreased only in CA1 with no change in area CA3, while Hcn2 mRNA was increased in both CA1 and CA3, and Hcn4 was unchanged in either region (Brewster et al., 2002). The changes in Hcn1 and Hcn2 were also dependent upon rats actually having seizures, as no changes in mRNA levels were observed in rats that were made hyperthermic but also treated with pentobarbital to prevent seizures (Brewster et al., 2002). The mRNA changes in Hcn1 but not Hcn2 were long lasting, remaining apparent three months after FS. In addition to regulating transcription of HCN channel subunits, FS appears to influence heteromerization of HCN1 and HCN2 protein (Chen et al., 2001b). Induction of FS in young rats led to significantly increased interaction between HCN1 and HCN2 proteins as measured by reciprocal co-immunoprecipitation experiments, a long-lasting (at least 8 weeks) finding again requiring seizure activity specifically rather than just hyperthermia (Brewster et al., 2005). In agreement with mRNA levels, western blots for HCN1 showed decreased protein levels in experimental rats. However, HCN2 protein was unaffected despite a significant increase in Hcn2 mRNA, a finding the authors proposed could result from increased HCN2 protein turnover. The findings thus suggested an enhanced HCN2/HCN1 ratio and a potential mechanism for the increased heteromerization between HCN2 and the typically more abundant HCN1 (Brewster et al., 2005). Taken together, these studies demonstrated that changes in mRNA and protein levels of HCN channel subunits occur after experimental FS in young rats, although these biochemical results do not suffice to comprehensively explain the observed enhancement in Ih. Other regulators of Ih distinct from altered HCN channel subunit levels are also likely to be involved.
While models of FS generated initial interest in the relationship between HCN channels and epilepsy, chemoconvulsant models of the most common cause of intractable epilepsy in adults, TLE, have provided a wealth of reports that demonstrate both changes in HCN channels and Ih following experimental seizures, as well as changes in seizure susceptibility following manipulations of HCN channel subunits. Chemoconvulsant models of TLE involve administration of kainic acid or pilocarpine to an animal resulting in a prolonged seizure episode (status epilepticus, SE), followed by a seizure-free latent period and ultimately the onset of spontaneous seizures (Williams et al., 2007). These studies in general (but not always, see (Dyhrfjeld-Johnsen et al., 2008)) point to a role for Ih as an anti-excitatory influence on neuronal function, whereby its loss increases cellular excitability and may contribute to development of spontaneous seizures. In entorhinal cortex (EC) layer III pyramidal neurons, which provide excitatory input to the hippocampus, kainic acid seizures significantly enhanced excitability and reduced Ih within 24 hours and lasted for at least one week (Fig. 2A) (Shah et al., 2004). These changes were accompanied by an early decrease of HCN1 and HCN2 protein but normal protein levels after one week (Fig. 2B). Thus, although reduction of HCN subunits was implicated early in epileptogenesis, a functional deficit in these channels in EC layer III neurons remained one week following SE, likely resulting from post-translational mechanisms (Shah et al., 2004). Studies from human patients with chronic epilepsy have also shown changes in hippocampal HCN1 and HCN2 mRNA and protein. In patients with marked hippocampal sclerosis and dentate gyrus granule cell loss, HCN1 mRNA and protein was markedly upregulated compared to autopsy controls or epilepsy patients without hippocampal sclerosis, while HCN2 mRNA did not change significantly in any of the epileptic patients (Bender et al., 2003). Within the same study, many of the findings from human tissue were recapitulated by pilocarpine-treated rats (Bender et al., 2003).
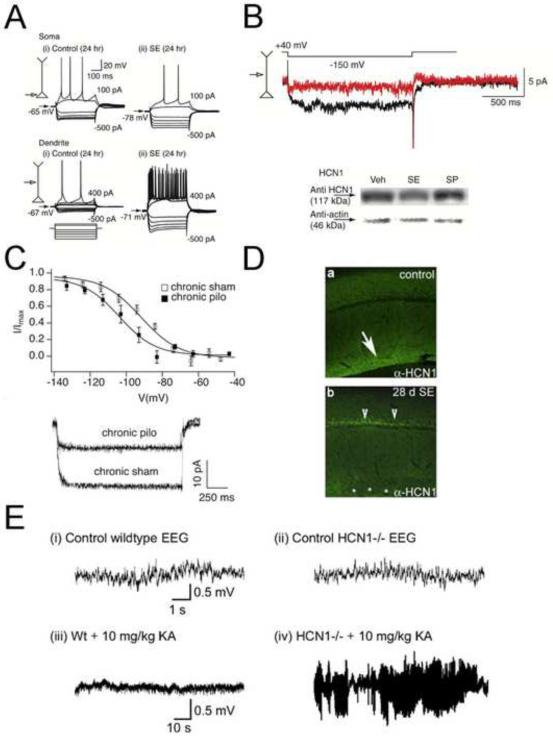
Figure 2. Animal models of temporal lobe epilepsy demonstrate modification of Ih and HCN channels leading to increased seizure susceptibility.
(A, B) Rats were treated with the chemoconvulsant drug kainic acid and underwent status epilepticus (SE) or were treated with vehicle. Twenty-four hours later, entorhinal cortex layer III somata and dendrites from rats undergoing SE demonstrated hyperpolarized RMP, and dendrites showed enhanced excitability to depolarizing current injection (A). Enhanced excitability in SE neurons was due to a decrease in Ih amplitude (B, upper panel, red trace) resulting from decreased HCN1 (B, lower panel) and HCN2 (data not shown) protein.
(C) Rats were treated with the chemoconvulsant drug pilocarpine or sham-injected. Three to five weeks later, Ih recorded from CA1 pyramidal neuron dendrites demonstrated a hyperpolarized V50 (upper panel) and decreased Ih current density (lower panel) in pilocarpine-treated rats but not sham-injected rats.
(D) Immunohistochemical staining demonstrated that as compared to control animals (a, upper panel), HCN1 immunoreactivity is decreased from distal dendrites and mislocalized to somata in hippocampal area CA1 28 days following kainic acid-induced SE (b, lower panel).
(E) EEG recordings demonstrate that sham-treated wildtype (i) and HCN1 knockout mice (ii) are not spontaneously epileptic, but when treated with 10 mg/kg kainic acid (KA), mice lacking HCN1 (iv) are significantly more susceptible to seizures than widtype mice (iii), which do not show any effect from this dose of kainic acid.
Figure adapted with permission from (Shah et al., 2004) (A, B), (Shin et al., 2008) (C), (Jung et al., 2007) (D), and (Huang et al., 2009) (E).
Decreased Ih through loss of HCN channel subunits has been found with the pilocarpine model of TLE. Jung et al. examined the relationship between seizure onset after pilocarpine administration and Ih and HCN channel protein in area CA1 of the hippocampus (Jung et al., 2007). Interestingly, about half of animals undergoing pilocarpine-induced SE evidenced spontaneous seizure activity within one week, and almost all (91%) of animals had a spontaneous seizure by 5 weeks (Jung et al., 2007). In CA1 neurons, dendritic Ih was downregulated within one week as well as after 5 weeks, and HCN1 and HCN2 channel protein levels were both decreased during the acute one-week period, although only HCN1 remained reduced after 5 weeks (Fig. 2C). To determine whether the downregulation of Ih and HCN channel proteins during the acute period was due to ongoing seizure activity after SE, rats were treated with pentobarbital after SE induction, which reduced spontaneous seizures. These rats also demonstrated downregulation of Ih current, HCN1, and HCN2 proteins, but surprisingly no change in V50, suggesting that ongoing seizure activity is required to modify Ih voltage gating but not current density or HCN channel protein expression (Jung et al., 2007). Importantly, in this model downregulation of Ih translated functionally into increased cellular excitability. A mechanism to explain altered Ih voltage gating after seizures has recently been published that identifies enhanced calcineurin (CaN) activity as a mediator of Ih V50 hyperpolarization in chronically epileptic animals following pilocarpine administration (Jung et al., 2010). In control slices, activation of CaN hyperpolarized Ih V50 in CA1 pyramidal neuron dendrites by ~14 mV with no change in maximal current amplitude. Blockade of CaN activity, as well as activation of p38 MAPK, in slices prepared from chronically epileptic animals partially reversed the hyperpolarized V50(Jung et al., 2010).
Other studies have found not a downregulation of HCN channel protein after chemically-induced SE but rather a mislocalization, suggesting that trafficking of HCN channels may be involved in epileptogenesis. Shin et al. utilized a model of TLE in which SE induced by kainic acid was terminated after one hour by pentobarbital to limit neuronal death, and found bidirectional changes in HCN channel surface expression and physiological function, without significant changes in total HCN channel expression (Shin et al., 2008). One to two days following SE, somatic Ih was upregulated in CA1 pyramidal neurons, a change likely mediated by increased neuronal surface expression of HCN1 protein. However, similar to findings in the pilocarpine model, one month following SE, somatic Ih was unchanged, but dendritic Ih was dramatically reduced, in this case due to decreased neuronal surface expression of both HCN1 and HCN2 subunits. These changes in surface expression were accompanied by a surprising redistribution of HCN1 and HCN2 subunits in CA1 dendritic fields, with both proteins being mislocalized to the stratum pyramidale, and HCN1 significantly removed from distal dendrites (Fig. 2D). These changes one month after SE were accompanied by a decreased association of HCN1 (but not HCN2) with the HCN channel auxiliary subunit TRIP8b, an alternatively spliced protein that controls both biophysical and trafficking properties of HCN channels (Lewis et al., 2009; Santoro et al., 2009; Santoro et al., 2004; Shin et al., 2008; Zolles et al., 2009).
Recent experiments have also examined whether animals lacking HCN channels are more susceptible to seizures or development of epilepsy (Huang et al., 2009; Santoro et al., 2010). While HCN1 knockout mice do not have baseline epileptic activity (Huang et al., 2009), they had a significantly reduced time to reach SE after administration of 20 mg/kg kainic acid, and the dose was lethal in all HCN1 knockouts but in no wild type mice (Huang et al., 2009). Using 10 mg/kg (a dose that does not cause SE in wildtype mice but sufficient to incite SE in knockouts without causing lethality) HCN1 knockout mice also demonstrated spontaneous seizures much more quickly after SE than wildtypes given twice this dose (Fig. 2E) (Huang et al., 2009). Similar findings of increased seizure susceptibility and death were also found with HCN1 knockout mice using the kindling and pilocarpine models (Santoro et al., 2010). These animal studies have recently been complimented by studies of human cortical tissue from patients with either TLE or frontal lobe epilepsy undergoing epilepsy surgery, which demonstrate a reduction of Ih in epileptic neocortical neurons correlating to the clinical severity of epilepsy (Wierschke et al., 2010).
Results from animal models of FS and TLE are not the only evidence for h-channelopathy in epilepsy. Absence epilepsy, a primary generalized epilepsy commonly seen in human children, may also involve changes in Ih and HCN channels. As discussed previously in this review, Ih in neurons involved in TC and CT communication is important in consciousness processes such as sleep and anesthesia. Absence seizures may also be thought of as brief departures from consciousness, and as such were hypothesized to involve dysfunctional Ih. Mice lacking HCN2 either from targeted or spontaneous knockout suffer from profound absence epilepsy characterized by both behavioral arrests and spike-wave discharges thought to arise due to a change in firing mode from tonic to burst in TC neurons (Chung et al., 2009; Ludwig et al., 2003). Two-photon calcium imaging in thalamic slices demonstrated significant oscillatory activity in HCN2 knockout mice, but not in wildtype mice, and the oscillatory activity was dependent upon T-type Ca2+-channel activation (Ludwig et al., 2003). Absence epilepsy in HCN2 knockout mice is suppressed by ethosuximide treatment, similar to the human condition (Chung et al., 2009; Ludwig et al., 2003). Additional evidence for Ih dysfunction in TC neurons is provided by analysis of the WAG/Rij rat genetic model of absence epilepsy. In these animals, the V50 of TC neuron Ih is hyperpolarized due to a change in cAMP responsivity, which is due to an upregulation of HCN1 mRNA and protein (Budde et al., 2005). Thus, absence epilepsy in these rats likely involves a mechanism whereby the firing mode of TC neurons can no longer effectively be regulated by cAMP, impairing the termination of burst firing (Budde et al., 2005).
Dysfunction of neocortical Ih has also been found in the WAG/Rij rat, suggesting that both thalamic and cortical Ih may be misregulated to facilitate synchronization and absence epilepsy. Layer 2/3 somatosensory cortical pyramidal neurons from WAG/Rij rats demonstrated decreased Ih current amplitude, slowed Ih activation kinetics, and a depolarized V50 compared to non-epileptic rat controls (Strauss et al., 2004). These changes affected EPSP properties as well as firing mode, leading to increased burst firing in these neurons. Molecularly, HCN1 protein, but not mRNA, was decreased in the neocortex, suggesting a post-transcriptional or translational defect (Strauss et al., 2004). Loss of neocortical HCN1 preceded development of spike-wave discharges, and thus may be epileptogenic (Kole et al., 2007).
Finally, correlative evidence for a role of Ih in generalized epilepsy syndromes comes from research detailing the specific effects of anti-epileptic medication on Ih properties. Lamotrigine, an anticonvulsant that is also particularly effective for neuropsychiatric disease such as bipolar disorder, reduced the excitability of hippocampal pyramidal neuron dendrites by upregulation of dendritic Ih, a result not found with the anticonvulsants carbamzepine and phenytoin (Poolos et al., 2002). Acetazolimide, an inhibitor of carbonic anhydrase, is an anticonvulsant used for absence epilepsy as well as some other forms of epilepsy. In TC neurons, acetazolamide depolarized the V50 of Ih due to an intracellular alkalinization, thus potentially terminating oscillatory activity in the thalamus (Munsch and Pape, 1999). Finally, gabapentin, another antiepileptic also in use for a wide variety of other neurological disorders, also enhanced Ih by increasing Ih conductance without change in V50 or kinetics (Surges et al., 2003).
3.1.2 Pain
Although the vast majority of interest in Ih for neurological disease has been within the epilepsy field, other lines of work have identified Ih as playing a potentially important role in pain (Dunlop et al., 2009). Ih and/or HCN channel subunits have been identified in dorsal root ganglion (DRG) neurons, Meissner's corpuscles, Merkel cells, as well as in axons of afferents to the superficial spinal dorsal horn and in motor neurons of the ventral spinal cord (Dunlop et al., 2009). Numerous physiological studies have been performed characterizing the role of Ih in these types of cells (reviewed in (Dunlop et al., 2009)). However, relatively few reports have linked Ih to the pathophysiology of pain. In general, most of this research has relied on using ZD7288 to block Ih. Since ZD7288 blocks Ih through all HCN channel subunit, it is unclear exactly which HCN channel subunit types might be involved in these mechanisms.
Because rhythmic discharges in the DRG are a hallmark finding following nerve transection injury, Chaplan et al. examined whether Ih and HCN channels are important for generating this rhythmicity as well as whether Ih blockade might provide therapeutic benefit for allodynia following such injuries (Chaplan et al., 2003). At the behavioral level, when the spinal nerve ligation (SNL) model for allodynia was performed on rats, systemic ZD7288 administration reduced neuropathic pain behavior, whereas lumbar intrathecal administration had no such effect. Physiologically, ZD7288 significantly reduced spontaneous firing following axotomy in both Aβ and Aδ neurons without conduction block. Analysis of single dissociated large DRG neurons from SNL or control rats indicated a significant increase in Ih in the SNL ganglia, accompanied by a depolarizing shift in V50 as well as depolarized RMP. Thus, Ih may contribute to neuropathic pain via its upregulation and initiation of spontaneous activity in large myelinated fibers (Chaplan et al., 2003). Similar physiological results were obtained by recordings from DRG neurons after chronic compression, which induced upregulation of Ih current density and rate of activation (Yao et al., 2003). In contrast, findings from Doan et al. suggest that enhanced peripheral nerve firing may result from inhibition of Ih (Doan et al., 2004). These studies examined HCN channels and Ih in sensory neurons isolated from rat nodose ganglion, and found that although HCN2 and HCN4 subunits were expressed in all nodose neurons, HCN1 was mainly expressed only in A-type neurons. CsCl was used to selectively inhibit Ih in nodose neurons, and the resulting increase in input resistance led to increased neuronal excitability. Similarly, inhibition of Ih in the aortic baroreceptor by CsCl promoted neuronal firing at pressures much lower than in control baroreceptors, which was reversed following CsCl removal (Doan et al., 2004). It is likely that nerve injury models change the intracellular milieu in which Ih is regulated, and thus make comparison of studies examining uninjured sensory nerve with animal models for chronic pain difficult. Targeted genetic deletion of HCN channel subunits may permit a more thorough understanding of the role of Ih and HCN channels in chronic pain.
Luo et al. examined whether Ih contributes to pain behaviors stemming from acute pain (Luo et al., 2007). Mild thermal injury (MTI) and SNL models were performed, and systemic administration of ZD7288 inhibited spontaneous pain and tactile allodynia. Local rather than systemic delivery of ZD7288 also repressed these pain behaviors, suggesting specific effects on HCN channels expressed in mechanosensory structures (Luo et al., 2007). Another study however using partial sciatic nerve injury or hind-paw incision and subsequent assessment of mechanical allodynia found that perineural administration of ZD7288, but not intraplantar administration, reduced pain behavior (Dalle and Eisenach, 2005). These findings thus also demonstrate a role for Ih in acute pain, although the locus of action may differ depending on injury model (Dunlop et al., 2009).
4.1 Conclusions and future perspectives
Here we have attempted to review the role of Ih and HCN channels in a specific set of physiological and pathological roles. Through control of basic membrane properties, Ih influences cellular excitability and synaptic integration, which in turn is required for basic behavior properties. Furthermore, derangement of Ih and HCN channels appears to contribute to a number of neurological disorders. What then are the most intriguing questions that remain in this field? Although tremendous strides have been made in the decade following the discovery of the molecular basis of Ih, future innovation will likely be spurred by a more detailed understanding of the real-time regulation of Ih and HCN channel biophysics and trafficking, since this conductance is endowed with the capability to enhance or depress activity in groups of neurons. Additionally, as has been reviewed here, exactly when and how Ih and HCN channel dysfunction contributes to human disease will undoubtedly remain an exciting area of future discovery. Human genetic studies have already identified significant variations in HCN channel genes potentially underlying epilepsy syndromes, and how these variations interface with acquired “h-channelopathy” is a wide-open area of research.
To conclude, we return to the question posed in the title of this review, which, albeit simplistic, summarizes much of the research aimed at understanding Ih and HCN channels in neurological disorders. In animal models of various epilepsy syndromes, as well as in human tissue from epilepsy patients, we have seen that in some cases Ih is too active, while in others it is not active enough. The relationship between Ih and cellular excitability is one of the most fundamental questions in this area of biology. In time, further understanding of current animal models of neurological disease, development of new models even more closely mimicking human neurological conditions, and direct study of human tissue from patients, will help clarify this simple yet intriguing question.
Acknowledgements
This work was supported by National Institutes of Health grants NS064757 (A.S.L.), NS05595, and NS059934 (D.M.C.).
Abbreviations
- α2A-AR
α2A-adrenoreceptor
- ADP
afterdepolarization
- CaN
calcineurin
- CNS
central nervous system
- CT
corticothalamic
- DRG
dorsal root ganglion
- EC
entorhinal cortex
- EEG
electroencephalography
- EPSP
excitatory post-synaptic potential
- FS
febrile seizures
- GEFS+
genetic epilepsy with febrile seizures plus
- HCN
hyperpolarization-activated cyclic-nucleotide gated
- Ih
hyperpolarization-activated current
- IPSP
inhibitory post-synaptic potential
- LORR
loss of righting reflex
- MTI
mild thermal injury
- PACAP
pituitary adenylate cyclase-activating polypeptide
- PNS
peripheral nervous system
- PFC
prefrontal cortex
- PKC
protein kinase C
- PLC
phospholipase C
- PNS
peripheral nervous system
- PPNs
prelimbic and infralimbic pyramidal neurons
- REM
rapid eye movement
- RMP
resting membrane potential
- RN
input resistance
- RTN
reticular thalamic nucleus
- SE
status epilepticus
- SNL
spinal nerve ligation
- TC
thalamocortical
- TLE
temporal lobe epilepsy
- V50
half-maximal voltage of activation
- VB
ventrobasal
- VIP
vasoactive intestinal polypeptide
- WM
working memory
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbas SY, Ying SW, Goldstein PA. Compartmental distribution of hyperpolarization-activated cyclic-nucleotide-gated channel 2 and hyperpolarization-activated cyclic-nucleotide-gated channel 4 in thalamic reticular and thalamocortical relay neurons. Neuroscience. 2006;141:1811–1825. doi: 10.1016/j.neuroscience.2006.05.034. [DOI] [PubMed] [Google Scholar]
- Angelo K, London M, Christensen SR, Hausser M. Local and global effects of I(h) distribution in dendrites of mammalian neurons. J Neurosci. 2007;27:8643–8653. doi: 10.1523/JNEUROSCI.5284-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aponte Y, Lien CC, Reisinger E, Jonas P. Hyperpolarization-activated cation channels in fast-spiking interneurons of rat hippocampus. J Physiol. 2006;574:229–243. doi: 10.1113/jphysiol.2005.104042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF, Goldman-Rakic PS. Alpha 2-adrenergic mechanisms in prefrontal cortex associated with cognitive decline in aged nonhuman primates. Science. 1985;230:1273–1276. doi: 10.1126/science.2999977. [DOI] [PubMed] [Google Scholar]
- Bal T, McCormick DA. What stops synchronized thalamocortical oscillations? Neuron. 1996;17:297–308. doi: 10.1016/s0896-6273(00)80161-0. [DOI] [PubMed] [Google Scholar]
- Baruscotti M, Bucchi A, Difrancesco D. Physiology and pharmacology of the cardiac pacemaker (“funny”) current. Pharmacol Ther. 2005;107:59–79. doi: 10.1016/j.pharmthera.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Bender RA, Soleymani SV, Brewster AL, Nguyen ST, Beck H, Mathern GW, Baram TZ. Enhanced expression of a specific hyperpolarization-activated cyclic nucleotide-gated cation channel (HCN) in surviving dentate gyrus granule cells of human and experimental epileptic hippocampus. J Neurosci. 2003;23:6826–6836. doi: 10.1523/JNEUROSCI.23-17-06826.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger T, Larkum ME, Luscher HR. High I(h) channel density in the distal apical dendrite of layer V pyramidal cells increases bidirectional attenuation of EPSPs. J Neurophysiol. 2001;85:855–868. doi: 10.1152/jn.2001.85.2.855. [DOI] [PubMed] [Google Scholar]
- Biel M, Wahl-Schott C, Michalakis S, Zong X. Hyperpolarization-activated cation channels: from genes to function. Physiol Rev. 2009;89:847–885. doi: 10.1152/physrev.00029.2008. [DOI] [PubMed] [Google Scholar]
- Bourgin P, Lebrand C, Escourrou P, Gaultier C, Franc B, Hamon M, Adrien J. Vasoactive intestinal polypeptide microinjections into the oral pontine tegmentum enhance rapid eye movement sleep in the rat. Neuroscience. 1997;77:351–360. doi: 10.1016/s0306-4522(96)00455-1. [DOI] [PubMed] [Google Scholar]
- Brewster A, Bender RA, Chen Y, Dube C, Eghbal-Ahmadi M, Baram TZ. Developmental febrile seizures modulate hippocampal gene expression of hyperpolarization-activated channels in an isoform- and cell-specific manner. J Neurosci. 2002;22:4591–4599. doi: 10.1523/JNEUROSCI.22-11-04591.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewster AL, Bernard JA, Gall CM, Baram TZ. Formation of heteromeric hyperpolarization-activated cyclic nucleotide-gated (HCN) channels in the hippocampus is regulated by developmental seizures. Neurobiol Dis. 2005;19:200–207. doi: 10.1016/j.nbd.2004.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budde T, Caputi L, Kanyshkova T, Staak R, Abrahamczik C, Munsch T, Pape HC. Impaired regulation of thalamic pacemaker channels through an imbalance of subunit expression in absence epilepsy. J Neurosci. 2005;25:9871–9882. doi: 10.1523/JNEUROSCI.2590-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G. Theta oscillations in the hippocampus. Neuron. 2002;33:325–340. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- Cacheaux LP, Topf N, Tibbs GR, Schaefer UR, Levi R, Harrison NL, Abbott GW, Goldstein PA. Impairment of hyperpolarization-activated, cyclic nucleotide-gated channel function by the intravenous general anesthetic propofol. J Pharmacol Exp Ther. 2005;315:517–525. doi: 10.1124/jpet.105.091801. [DOI] [PubMed] [Google Scholar]
- Carr DB, Andrews GD, Glen WB, Lavin A. alpha2-Noradrenergic receptors activation enhances excitability and synaptic integration in rat prefrontal cortex pyramidal neurons via inhibition of HCN currents. J Physiol. 2007;584:437–450. doi: 10.1113/jphysiol.2007.141671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CS, Guzman JN, Ilijic E, Mercer JN, Rick C, Tkatch T, Meredith GE, Surmeier DJ. `Rejuvenation' protects neurons in mouse models of Parkinson's disease. Nature. 2007;447:1081–1086. doi: 10.1038/nature05865. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Guo HQ, Lee DH, Luo L, Liu C, Kuei C, Velumian AA, Butler MP, Brown SM, Dubin AE. Neuronal hyperpolarization-activated pacemaker channels drive neuropathic pain. J Neurosci. 2003;23:1169–1178. doi: 10.1523/JNEUROSCI.23-04-01169.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Aradi I, Thon N, Eghbal-Ahmadi M, Baram TZ, Soltesz I. Persistently modified h-channels after complex febrile seizures convert the seizure-induced enhancement of inhibition to hyperexcitability. Nat Med. 2001a;7:331–337. doi: 10.1038/85480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Baram TZ, Soltesz I. Febrile seizures in the developing brain result in persistent modification of neuronal excitability in limbic circuits. Nat Med. 1999;5:888–894. doi: 10.1038/11330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Wang J, Siegelbaum SA. Properties of hyperpolarization-activated pacemaker current defined by coassembly of HCN1 and HCN2 subunits and basal modulation by cyclic nucleotide. J Gen Physiol. 2001b;117:491–504. doi: 10.1085/jgp.117.5.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Shu S, Bayliss DA. Suppression of ih contributes to propofol-induced inhibition of mouse cortical pyramidal neurons. J Neurophysiol. 2005a;94:3872–3883. doi: 10.1152/jn.00389.2005. [DOI] [PubMed] [Google Scholar]
- Chen X, Shu S, Bayliss DA. HCN1 channel subunits are a molecular substrate for hypnotic actions of ketamine. J Neurosci. 2009a;29:600–609. doi: 10.1523/JNEUROSCI.3481-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Shu S, Kennedy DP, Willcox SC, Bayliss DA. Subunit-Specific Effects of Isoflurane on Neuronal Ih in HCN1 Knockout Mice. J Neurophysiol. 2009b;101:129–140. doi: 10.1152/jn.01352.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Sirois JE, Lei Q, Talley EM, Lynch C, 3rd, Bayliss DA. HCN subunit-specific and cAMP-modulated effects of anesthetics on neuronal pacemaker currents. J Neurosci. 2005b;25:5803–5814. doi: 10.1523/JNEUROSCI.1153-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung WK, Shin M, Jaramillo TC, Leibel RL, LeDuc CA, Fischer SG, Tzilianos E, Gheith AA, Lewis AS, Chetkovich DM. Absence epilepsy in apathetic, a spontaneous mutant mouse lacking the h channel subunit, HCN2. Neurobiol Dis. 2009;33:499–508. doi: 10.1016/j.nbd.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisse RS, Krebs-Kraft DL, Parent MB. Septal infusions of the hyperpolarization-activated cyclic nucleotide-gated channel (HCN-channel) blocker ZD7288 impair spontaneous alternation but not inhibitory avoidance. Behav Neurosci. 2008;122:549–556. doi: 10.1037/0735-7044.122.3.549. [DOI] [PubMed] [Google Scholar]
- Dalle C, Eisenach JC. Peripheral block of the hyperpolarization-activated cation current (Ih) reduces mechanical allodynia in animal models of postoperative and neuropathic pain. Reg Anesth Pain Med. 2005;30:243–248. doi: 10.1016/j.rapm.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Day M, Carr DB, Ulrich S, Ilijic E, Tkatch T, Surmeier DJ. Dendritic excitability of mouse frontal cortex pyramidal neurons is shaped by the interaction among HCN, Kir2, and Kleak channels. J Neurosci. 2005;25:8776–8787. doi: 10.1523/JNEUROSCI.2650-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibbens LM, Reid CA, Hodgson B, Thomas EA, Phillips AM, Gazina E, Cromer BA, Clarke AL, Baram TZ, Scheffer IE, Berkovic SF, Petrou S. Augmented currents of an HCN2 variant in patients with febrile seizure syndromes. Ann Neurol. 2010;67:542–546. doi: 10.1002/ana.21909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doan TN, Stephans K, Ramirez AN, Glazebrook PA, Andresen MC, Kunze DL. Differential distribution and function of hyperpolarization-activated channels in sensory neurons and mechanosensitive fibers. J Neurosci. 2004;24:3335–3343. doi: 10.1523/JNEUROSCI.5156-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube C, Chen K, Eghbal-Ahmadi M, Brunson K, Soltesz I, Baram TZ. Prolonged febrile seizures in the immature rat model enhance hippocampal excitability long term. Ann Neurol. 2000;47:336–344. [PMC free article] [PubMed] [Google Scholar]
- Dube C, Richichi C, Bender RA, Chung G, Litt B, Baram TZ. Temporal lobe epilepsy after experimental prolonged febrile seizures: prospective analysis. Brain. 2006;129:911–922. doi: 10.1093/brain/awl018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop J, Vasilyev D, Lu P, Cummons T, Bowlby MR. Hyperpolarization-activated cyclic nucleotide-gated (HCN) channels and pain. Curr Pharm Des. 2009;15:1767–1772. doi: 10.2174/138161209788186281. [DOI] [PubMed] [Google Scholar]
- Dyhrfjeld-Johnsen J, Morgan RJ, Foldy C, Soltesz I. Upregulated H-current in hyperexcitable CA1 dendrites after febrile seizures. Front Cell Neurosci. 2008;2:2. doi: 10.3389/neuro.03.002.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang J, Payne L, Krueger JM. Pituitary adenylate cyclase activating polypeptide enhances rapid eye movement sleep in rats. Brain Res. 1995;686:23–28. doi: 10.1016/0006-8993(95)00443-t. [DOI] [PubMed] [Google Scholar]
- Franks NP. General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nat Rev Neurosci. 2008;9:370–386. doi: 10.1038/nrn2372. [DOI] [PubMed] [Google Scholar]
- Franowicz JS, Kessler LE, Borja CM, Kobilka BK, Limbird LE, Arnsten AF. Mutation of the alpha2A-adrenoceptor impairs working memory performance and annuls cognitive enhancement by guanfacine. J Neurosci. 2002;22:8771–8777. doi: 10.1523/JNEUROSCI.22-19-08771.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS. The “psychic” neuron of the cerebral cortex. Ann N Y AcadSci. 1999;868:13–26. doi: 10.1111/j.1749-6632.1999.tb11270.x. [DOI] [PubMed] [Google Scholar]
- Hu H, Vervaeke K, Storm JF. Two forms of electrical resonance at theta frequencies, generated by M-current, h-current and persistent Na+ current in rat hippocampal pyramidal cells. J Physiol. 2002;545:783–805. doi: 10.1113/jphysiol.2002.029249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, Walker MC, Shah MM. Loss of dendritic HCN1 subunits enhances cortical excitability and epileptogenesis. J Neurosci. 2009;29:10979–10988. doi: 10.1523/JNEUROSCI.1531-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutcheon B, Yarom Y. Resonance, oscillation and the intrinsic frequency preferences of neurons. Trends Neurosci. 2000;23:216–222. doi: 10.1016/s0166-2236(00)01547-2. [DOI] [PubMed] [Google Scholar]
- Jung S, Bullis JB, Lau IH, Jones TD, Warner LN, Poolos NP. Downregulation of dendritic HCN channel gating in epilepsy is mediated by altered phosphorylation signaling. J Neurosci. 2010;30:6678–6688. doi: 10.1523/JNEUROSCI.1290-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S, Jones TD, Lugo JN, Jr., Sheerin AH, Miller JW, D'Ambrosio R, Anderson AE, Poolos NP. Progressive dendritic HCN channelopathy during epileptogenesis in the rat pilocarpine model of epilepsy. J Neurosci. 2007;27:13012–13021. doi: 10.1523/JNEUROSCI.3605-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanyshkova T, Pawlowski M, Meuth P, Dube C, Bender RA, Brewster AL, Baumann A, Baram TZ, Pape HC, Budde T. Postnatal expression pattern of HCN channel isoforms in thalamic neurons: relationship to maturation of thalamocortical oscillations. J Neurosci. 2009;29:8847–8857. doi: 10.1523/JNEUROSCI.0689-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelland MD, Soltis RP, Boldry RC, Walters JR. Behavioral and electrophysiological comparison of ketamine with dizocilpine in the rat. Physiol Behav. 1993;54:547–554. doi: 10.1016/0031-9384(93)90248-e. [DOI] [PubMed] [Google Scholar]
- Kocsis B, Li S. In vivo contribution of h-channels in the septal pacemaker to theta rhythm generation. Eur J Neurosci. 2004;20:2149–2158. doi: 10.1111/j.1460-9568.2004.03678.x. [DOI] [PubMed] [Google Scholar]
- Kole MH, Brauer AU, Stuart GJ. Inherited cortical HCN1 channel loss amplifies dendritic calcium electrogenesis and burst firing in a rat absence epilepsy model. J Physiol. 2007;578:507–525. doi: 10.1113/jphysiol.2006.122028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai HC, Jan LY. The distribution and targeting of neuronal voltage-gated ion channels. Nat Rev Neurosci. 2006;7:548–562. doi: 10.1038/nrn1938. [DOI] [PubMed] [Google Scholar]
- Leung LS, Yu HW. Theta-frequency resonance in hippocampal CA1 neurons in vitro demonstrated by sinusoidal current injection. J Neurophysiol. 1998;79:1592–1596. doi: 10.1152/jn.1998.79.3.1592. [DOI] [PubMed] [Google Scholar]
- Lewis AS, Estep CM, Chetkovich DM. The fast and slow ups and downs of HCN channel regulation. Channels (Austin) 2010;4 doi: 10.4161/chan.4.3.11630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis AS, Schwartz E, Chan CS, Noam Y, Shin M, Wadman WJ, Surmeier DJ, Baram TZ, Macdonald RL, Chetkovich DM. Alternatively spliced isoforms of TRIP8b differentially control h channel trafficking and function. J Neurosci. 2009;29:6250–6265. doi: 10.1523/JNEUROSCI.0856-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Chen F, Ye J, Chen X, Yan J, Li Y, Xiong Y, Zhou Z, Xia J, Hu Z. The Modulation of Orexin A on HCN Currents of Pyramidal Neurons in Mouse Prelimbic Cortex. Cereb Cortex. 2009 doi: 10.1093/cercor/bhp241. [DOI] [PubMed] [Google Scholar]
- Li BM, Mei ZT. Delayed-response deficit induced by local injection of the alpha 2-adrenergic antagonist yohimbine into the dorsolateral prefrontal cortex in young adult monkeys. Behav Neural Biol. 1994;62:134–139. doi: 10.1016/s0163-1047(05)80034-2. [DOI] [PubMed] [Google Scholar]
- Lorincz A, Notomi T, Tamas G, Shigemoto R, Nusser Z. Polarized and compartment-dependent distribution of HCN1 in pyramidal cell dendrites. Nat Neurosci. 2002;5:1185–1193. doi: 10.1038/nn962. [DOI] [PubMed] [Google Scholar]
- Ludwig A, Budde T, Stieber J, Moosmang S, Wahl C, Holthoff K, Langebartels A, Wotjak C, Munsch T, Zong X, Feil S, Feil R, Lancel M, Chien KR, Konnerth A, Pape HC, Biel M, Hofmann F. Absence epilepsy and sinus dysrhythmia in mice lacking the pacemaker channel HCN2. Embo J. 2003;22:216–224. doi: 10.1093/emboj/cdg032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L, Chang L, Brown SM, Ao H, Lee DH, Higuera ES, Dubin AE, Chaplan SR. Role of peripheral hyperpolarization-activated cyclic nucleotide-modulated channel pacemaker channels in acute and chronic pain models in the rat. Neuroscience. 2007;144:1477–1485. doi: 10.1016/j.neuroscience.2006.10.048. [DOI] [PubMed] [Google Scholar]
- Luthi A, McCormick DA. H-current: properties of a neuronal and network pacemaker. Neuron. 1998;21:9–12. doi: 10.1016/s0896-6273(00)80509-7. [DOI] [PubMed] [Google Scholar]
- Lyashchenko AK, Redd KJ, Yang J, Tibbs GR. Propofol inhibits HCN1 pacemaker channels by selective association with the closed states of the membrane embedded channel core. J Physiol. 2007;583:37–56. doi: 10.1113/jphysiol.2007.136465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee JC. Dendritic hyperpolarization-activated currents modify the integrative properties of hippocampal CA1 pyramidal neurons. J Neurosci. 1998;18:7613–7624. doi: 10.1523/JNEUROSCI.18-19-07613.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee JC. Dendritic lh normalizes temporal summation in hippocampal CA1 neurons. Nat Neurosci. 1999;2:508–514. doi: 10.1038/9158. [DOI] [PubMed] [Google Scholar]
- Magee JC, Johnston D. Plasticity of dendritic function. Curr Opin Neurobiol. 2005;15:334–342. doi: 10.1016/j.conb.2005.05.013. [DOI] [PubMed] [Google Scholar]
- McCormick DA. Neurotransmitter actions in the thalamus and cerebral cortex and their role in neuromodulation of thalamocortical activity. Prog Neurobiol. 1992;39:337–388. doi: 10.1016/0301-0082(92)90012-4. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Bal T. Sleep and arousal: thalamocortical mechanisms. Annu Rev Neurosci. 1997;20:185–215. doi: 10.1146/annurev.neuro.20.1.185. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Pape HC. Properties of a hyperpolarization-activated cation current and its role in rhythmic oscillation in thalamic relay neurones. J Physiol. 1990;431:291–318. doi: 10.1113/jphysiol.1990.sp018331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Shu Y, Hasenstaub A, Sanchez-Vives M, Badoual M, Bal T. Persistent cortical activity: mechanisms of generation and effects on neuronal excitability. Cereb Cortex. 2003;13:1219–1231. doi: 10.1093/cercor/bhg104. [DOI] [PubMed] [Google Scholar]
- Milanesi R, Baruscotti M, Gnecchi-Ruscone T, DiFrancesco D. Familial sinus bradycardia associated with a mutation in the cardiac pacemaker channel. N Engl J Med. 2006;354:151–157. doi: 10.1056/NEJMoa052475. [DOI] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Munsch T, Pape HC. Upregulation of the hyperpolarization-activated cation current in rat thalamic relay neurones by acetazolamide. J Physiol. 1999;519(Pt 2):505–514. doi: 10.1111/j.1469-7793.1999.0505m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan R, Johnston D. Long-term potentiation in rat hippocampal neurons is accompanied by spatially widespread changes in intrinsic oscillatory dynamics and excitability. Neuron. 2007;56:1061–1075. doi: 10.1016/j.neuron.2007.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan MF, Malleret G, Dudman JT, Buhl DL, Santoro B, Gibbs E, Vronskaya S, Buzsaki G, Siegelbaum SA, Kandel ER, Morozov A. A behavioral role for dendritic integration: HCN1 channels constrain spatial memory and plasticity at inputs to distal dendrites of CA1 pyramidal neurons. Cell. 2004;119:719–732. doi: 10.1016/j.cell.2004.11.020. [DOI] [PubMed] [Google Scholar]
- Nolan MF, Malleret G, Lee KH, Gibbs E, Dudman JT, Santoro B, Yin D, Thompson RF, Siegelbaum SA, Kandel ER, Morozov A. The hyperpolarization-activated HCN1 channel is important for motor learning and neuronal integration by cerebellar Purkinje cells. Cell. 2003;115:551–564. doi: 10.1016/s0092-8674(03)00884-5. [DOI] [PubMed] [Google Scholar]
- Notomi T, Shigemoto R. Immunohistochemical localization of Ih channel subunits, HCN1-4, in the rat brain. J Comp Neurol. 2004;471:241–276. doi: 10.1002/cne.11039. [DOI] [PubMed] [Google Scholar]
- Okamoto T, Harnett MT, Morikawa H. Hyperpolarization-activated cation current (Ih) is an ethanol target in midbrain dopamine neurons of mice. J Neurophysiol. 2006;95:619–626. doi: 10.1152/jn.00682.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape HC. Queer current and pacemaker: the hyperpolarization-activated cation current in neurons. Annu Rev Physiol. 1996;58:299–327. doi: 10.1146/annurev.ph.58.030196.001503. [DOI] [PubMed] [Google Scholar]
- Petrenko AB, Yamakura T, Fujiwara N, Askalany AR, Baba H, Sakimura K. Reduced sensitivity to ketamine and pentobarbital in mice lacking the N-methyl-D-aspartate receptor GluRepsilonl subunit. Anesth Analg. 2004;99:1136–1140. doi: 10.1213/01.ANE.0000131729.54986.30. table of contents. [DOI] [PubMed] [Google Scholar]
- Pike FG, Goddard RS, Suckling JM, Ganter P, Kasthuri N, Paulsen O. Distinct frequency preferences of different types of rat hippocampal neurones in response to oscillatory input currents. J Physiol. 2000;529(Pt 1):205–213. doi: 10.1111/j.1469-7793.2000.00205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poolos NP. The h-channel: a potential channelopathy in epilepsy? Epilepsy Behav. 2005;7:51–56. doi: 10.1016/j.yebeh.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Poolos NP, Migliore M, Johnston D. Pharmacological upregulation of h-channels reduces the excitability of pyramidal neuron dendrites. Nat Neurosci. 2002;5:767–774. doi: 10.1038/nn891. [DOI] [PubMed] [Google Scholar]
- Rateau Y, Ropert N. Expression of a functional hyperpolarization-activated current (Ih) in the mouse nucleus reticularis thalami. J Neurophysiol. 2006;95:3073–3085. doi: 10.1152/jn.00922.2005. [DOI] [PubMed] [Google Scholar]
- Robinson RB, Siegelbaum SA. Hyperpolarization-activated cation currents: from molecules to physiological function. Annu Rev Physiol. 2003;65:453–480. doi: 10.1146/annurev.physiol.65.092101.142734. [DOI] [PubMed] [Google Scholar]
- Santoro B, Chen S, Luthi A, Pavlidis P, Shumyatsky GP, Tibbs GR, Siegelbaum SA. Molecular and functional heterogeneity of hyperpolarization-activated pacemaker channels in the mouse CNS. J Neurosci. 2000;20:5264–5275. doi: 10.1523/JNEUROSCI.20-14-05264.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro B, Lee JY, Englot DJ, Gildersleeve S, Piskorowski RA, Siegelbaum SA, Winawer MR, Blumenfeld H. Increased seizure severity and seizure-related death in mice lacking HCN1 channels. Epilepsia. 2010 doi: 10.1111/j.1528-1167.2010.02554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro B, Piskorowski RA, Pian P, Hu L, Liu H, Siegelbaum SA. TRIP8b splice variants form a family of auxiliary subunits that regulate gating and trafficking of HCN channels in the brain. Neuron. 2009;62:802–813. doi: 10.1016/j.neuron.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro B, Wainger BJ, Siegelbaum SA. Regulation of HCN channel surface expression by a novel C-terminal protein-protein interaction. J Neurosci. 2004;24:10750–10762. doi: 10.1523/JNEUROSCI.3300-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze-Bahr E, Neu A, Friederich P, Kaupp UB, Breithardt G, Pongs O, Isbrandt D. Pacemaker channel dysfunction in a patient with sinus node disease. J Clin Invest. 2003;111:1537–1545. doi: 10.1172/JCI16387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah MM, Anderson AE, Leung V, Lin X, Johnston D. Seizure-induced plasticity of h channels in entorhinal cortical layer III pyramidal neurons. Neuron. 2004;44:495–508. doi: 10.1016/j.neuron.2004.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin M, Brager D, Jaramillo TC, Johnston D, Chetkovich DM. Mislocalization of h channel subunits underlies h channelopathy in temporal lobe epilepsy. Neurobiol Dis. 2008;32:26–36. doi: 10.1016/j.nbd.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu Y, Hasenstaub A, McCormick DA. Turning on and off recurrent balanced cortical activity. Nature. 2003;423:288–293. doi: 10.1038/nature01616. [DOI] [PubMed] [Google Scholar]
- Southan AP, Morris NP, Stephens GJ, Robertson B. Hyperpolarization-activated currents in presynaptic terminals of mouse cerebellar basket cells. J Physiol. 2000;526(Pt 1):91–97. doi: 10.1111/j.1469-7793.2000.t01-1-00091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M. Sleep, epilepsy and thalamic reticular inhibitory neurons. Trends Neurosci. 2005;28:317–324. doi: 10.1016/j.tins.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Stevens DR, Seifert R, Bufe B, Muller F, Kremmer E, Gauss R, Meyerhof W, Kaupp UB, Lindemann B. Hyperpolarization-activated channels HCN1 and HCN4 mediate responses to sour stimuli. Nature. 2001;413:631–635. doi: 10.1038/35098087. [DOI] [PubMed] [Google Scholar]
- Strauss U, Kole MH, Brauer AU, Pahnke J, Bajorat R, Rolfs A, Nitsch R, Deisz RA. An impaired neocortical Ih is associated with enhanced excitability and absence epilepsy. Eur J Neurosci. 2004;19:3048–3058. doi: 10.1111/j.0953-816X.2004.03392.x. [DOI] [PubMed] [Google Scholar]
- Sun QQ, Prince DA, Huguenard JR. Vasoactive intestinal polypeptide and pituitary adenylate cyclase-activating polypeptide activate hyperpolarization-activated cationic current and depolarize thalamocortical neurons in vitro. J Neurosci. 2003;23:2751–2758. doi: 10.1523/JNEUROSCI.23-07-02751.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surges R, Freiman TM, Feuerstein TJ. Gabapentin increases the hyperpolarization-activated cation current Ih in rat CA1 pyramidal cells. Epilepsia. 2003;44:150–156. doi: 10.1046/j.1528-1157.2003.36802.x. [DOI] [PubMed] [Google Scholar]
- Tang B, Sander T, Craven KB, Hempelmann A, Escayg A. Mutation analysis of the hyperpolarization-activated cyclic nucleotide-gated channels HCN1 and HCN2 in idiopathic generalized epilepsy. Neurobiol Dis. 2008;29:59–70. doi: 10.1016/j.nbd.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsay D, Dudman JT, Siegelbaum SA. HCN1 channels constrain synaptically evoked Ca2+ spikes in distal dendrites of CA1 pyramidal neurons. Neuron. 2007;56:1076–1089. doi: 10.1016/j.neuron.2007.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl-Schott C, Biel M. HCN channels: Structure, cellular regulation and physiological function. Cell Mol Life Sci. 2008 doi: 10.1007/s00018-008-8525-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Ramos BP, Paspalas CD, Shu Y, Simen A, Duque A, Vijayraghavan S, Brennan A, Dudley A, Nou E, Mazer JA, McCormick DA, Arnsten AF. Alpha2A-adrenoceptors strengthen working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell. 2007;129:397–410. doi: 10.1016/j.cell.2007.03.015. [DOI] [PubMed] [Google Scholar]
- Wierschke S, Lehmann TN, Dehnicke C, Horn P, Nitsch R, Deisz RA. Hyperpolarization-activated cation currents in human epileptogenic neocortex. Epilepsia. 2010;51:404–414. doi: 10.1111/j.1528-1167.2009.02275.x. [DOI] [PubMed] [Google Scholar]
- Williams PA, Hellier JL, White AM, Staley KJ, Dudek FE. Development of spontaneous seizures after experimental status epilepticus: implications for understanding epileptogenesis. Epilepsia. 2007;48(Suppl 5):157–163. doi: 10.1111/j.1528-1167.2007.01304.x. [DOI] [PubMed] [Google Scholar]
- Williams SR, Stuart GJ. Site independence of EPSP time course is mediated by dendritic I(h) in neocortical pyramidal neurons. J Neurophysiol. 2000;83:3177–3182. doi: 10.1152/jn.2000.83.5.3177. [DOI] [PubMed] [Google Scholar]
- Williams SR, Turner JP, Hughes SW, Crunelli V. On the nature of anomalous rectification in thalamocortical neurones of the cat ventrobasal thalamus in vitro. J Physiol. 1997;505(Pt 3):727–747. doi: 10.1111/j.1469-7793.1997.727ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H, Donnelly DF, Ma C, LaMotte RH. Upregulation of the hyperpolarization-activated cation current after chronic compression of the dorsal root ganglion. J Neurosci. 2003;23:2069–2074. doi: 10.1523/JNEUROSCI.23-06-02069.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying SW, Abbas SY, Harrison NL, Goldstein PA. Propofol block of I(h) contributes to the suppression of neuronal excitability and rhythmic burst firing in thalamocortical neurons. Eur J Neurosci. 2006;23:465–480. doi: 10.1111/j.1460-9568.2005.04587.x. [DOI] [PubMed] [Google Scholar]
- Zolles G, Wenzel D, Bildl W, Schulte U, Hofmann A, Muller CS, Thumfart JO, Vlachos A, Deller T, Pfeifer A, Fleischmann BK, Roeper J, Fakler B, Klocker N. Association with the auxiliary subunit PEX5R/Trip8b controls responsiveness of HCN channels to cAMP and adrenergic stimulation. Neuron. 2009;62:814–825. doi: 10.1016/j.neuron.2009.05.008. [DOI] [PubMed] [Google Scholar]