Abstract
Dynorphin neuropeptides are believed to act as endogenous anticonvulsants, though direct evidence for such a role in humans is sparse. We now report pronounced increases of prodynorphin mRNA expression in the dentate gyrus of patients with temporal lobe epilepsy in comparison to controls. We detected a conspicuously right skewed, bimodal distribution of mRNA levels among patients, suggestive of a dynamic up-regulation of prodynorphin expression in epilepsy. Highest transcript levels were seen postictally. Our data argue for an essential role of dynorphin in the termination of seizures.
Keywords: dynorphin, neuropeptides, temporal lobe epilepsy, seizures, seizure protection
Growing evidence indicates that dynorphins and several other endogenous neuropeptides are involved in the modulation of the threshold for seizures. Dynorphins, products of the prodynorphin gene, are highly expressed in the dentate gyrus and were proposed to act as endogenous anticonvulsants. Evidence in support of this mainly comes from animal experiments and a few human studies. Knocking out the dynorphin gene in mice or depletion of hippocampal dynorphins by Borna disease virus infection in rats increases the susceptibility to seizures. κ-Opioid receptor agonists, mimicking effects of endogenous dynorphins reverse these clinical symptoms (Solbrig et al., 2006; Loacker et al., 2007). In patients with intractable mesial TLE dynorphin-immunoreactivity is markedly increased in dentate mossy fibers suggestive of an epilepsy-related role of dynorphins (Houser et al., 1990; Pirker et al., 2001). Finally, imaging studies using opioid receptor radioligands indicate ictal activation of opioid receptors in epilepsy patients, presumably because of the release of dynorphins to terminate seizures (Koepp et al., 1998).
Transcription of neuropeptides is generally characterized by a dynamic up- or down-regulation depending on the changing requirements of the respective networks. However, little is known about the transcriptional regulation of the prodynorphin gene in the context of human epilepsies. As this information is essential for a better understanding of the role of endogenous dynorphins in seizures, we quantified the degree of mRNA expression in hippocampal specimens of patients with TLE by in situ hybridization. We further correlated these results with two parameters which we hypothesized might influence the expression of dynorphins, i.e., recent preoperative seizures and a functional genetic variability in the prodynorphin gene promoter.
The study was approved by the local Institutional Board and informed consent was obtained from all patients. Specimens from 91 patients with pharmaco-resistant TLE and a mean age of 34 (4–65) yrs were included. Forty-nine patients were female, 42 were male. Ammon’s horn sclerosis was detected in 83 patients by magnetic resonance imaging and was verified by histopathology. Cell counts in Nissl stained sections revealed a loss of 56.2% ± 2.5% (mean ± SEM) granule cells in these specimens. Twelve hippocampi were obtained at routine autopsy from patients with no history of neurological or psychiatric disorders with a mean post mortem time of 14.5 (8–36) hr and a mean age at death of 56 (44–95) yrs. Causes of death were pulmonary embolism, carcinoma, melanoma, lymphoma, liver cirrhosis or myocardial infarction. The distribution of genotypes was similar as in epilepsy patients (two patients with LL-, six with LH- and four with HH-alleles; see Fig. 3 for TLE patients). The middle segments of hippocampi from 63 surgical and 12 autopsy specimens were fixed in 4% paraformaldehyde. Adjacent parts from all autopsy specimens and from 28 surgical hippocampi were also snap frozen in isopentane (−70°C, 3 min). Prodynorphin mRNA expression was determined in the dentate granule cell layer by in situ hybridization on 20-μm thick perpendicular sections. A synthetic oligonucleotide probe (CGGATCTTCCTGAGACCGAGTCACCACCTTGAACTGGCGCCGGAG), corresponding to bases 913–957 of the human prodynorphin mRNA (Gen bank No. NM_024411.2) was labeled with [35S]α-thio-dATP (1,300 Ci/mmol) by reaction with terminal deoxynucleotidyltransferase (both Roche, Vienna, Austria) and in situ hybridization was performed as described previously (Furtinger et al., 2001). Sections were exposed together with [14C]micro-scales three times (2, 7, and 21 d) to BioMax MR films (both Amersham Pharmacia Biothech, Buckinghamshire, UK).
FIGURE 3.
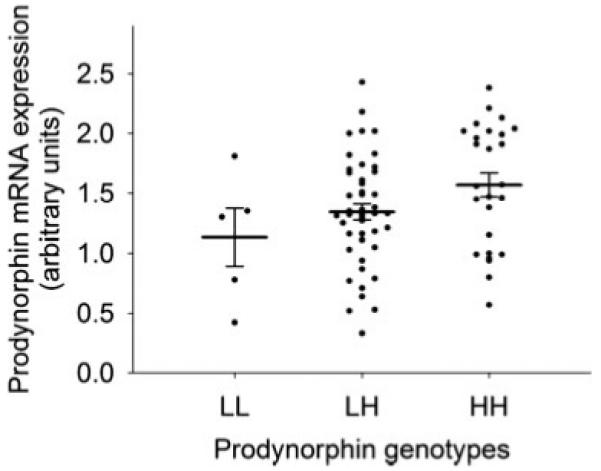
Association of prodynorphin promoter polymorphism genotypes (LL-, LH-, or HH-alleles) with prodynorphin mRNA expression over the dentate gyrus. Fourteen samples with normalized hybridization signal intensities of above 2.5 outside the basal expression curve were excluded from this analysis under the assumption that the transcription in these samples was up-regulated by other factors such as recent seizures. Circles represent individual samples and horizontal lines mark the mean and standard error of the mean for each genotype (mean ± SEM for LL-alleles: 1.13 ± 0.24, n = 5; LH-alleles: 1.34 ± 0.07, n = 46; HH-alleles: 1.57 ± 0.10, n = 26); in this subgroup there was a significant linear association between genotypes and hybridization signal (linear regression analysis, n = 77, P = 0.022).
Three independent experiments on overlapping paraformaldehyde fixed and snap frozen samples were performed. Hybridization signals from the three experiments were quantified by densitometry and merged by normalizing them to the same three post mortem controls used in each experiment. Densitometry was done on autoradiography films over the granule cell layer using ImageJ 1.34 (NIH, USA). Gray values were obtained at three different locations of the granule cell layer and the area adjacent to the granule cell layer (background) and converted to optical densities (RODs). Standard curves based on ROD values of the autoradiogryphy microscales were used for converting RODs of samples into nCi/g tissue (Furtinger et al., 2001). Correlation between the different experiments was achieved by calculating ratios of nCi/g values of each sample and mean nCi/g values obtained in the same experiment for the three post mortem standards. Mean ratios obtained in the PFA-fixed sections (1.7 ± 0.13 SEM, n = 63) were not significantly different from those obtained in snap frozen samples (1.9 ± 0.15 SEM, n = 28).
The prodynorphin promoter polymorphism was genotyped as described previously (Stogmann et al., 2002). Alleles with 1, 2, 3, or 4 repeats of a 68-bp element were identified. For simplification, alleles 1 and 2 were grouped together and are referred as L-alleles. The H-alleles group includes alleles 3 and 4. For investigating whether hybridization signals follow a normal distribution we employed the Kolmogorov-Smirnov-Test. To assess differences in hybridization signal intensities between patients with and without preoperative seizures and other clinical parameters (age at epilepsy onset, sex, side of seizure focus, seizure frequency, medication with specific antiepileptic drugs and hippocampal sclerosis) we used the Student’s t-test on logarithmically transformed hybridization data. For assessing an association between prodynorphin genotypes and hybridization signals we used a linear regression analysis. All statistical tests were calculated using the software SPSS v 14.0.1. As we considered this an explorative study, no correction for multiple testing was done.
In all patients and post mortem controls, prodynorphin mRNA was expressed by granule cells of the dentate gyrus, but as shown in Figure 1 there was a conspicuous up-regulation of prodynorphin transcripts in all TLE samples as compared to nonepilepsy controls. Quantifying signal intensities by densitometry revealed on average 6.7-fold increased transcript levels in epilepsy patients. (TLE samples, % of controls ±SEM: 669.8 ± 48.91%; P < 0.0001; n = 23 vs. 12 controls). We detected no correlation between age and prodynorphin mRNA levels in TLE specimens (r = 0.079, P = 0.805) nor in post mortem controls (r = −0.010, P = 0.963). On the other hand, there was a significant (P < 0.0086) negative correlation (r = −0.317) between duration of epilepsy (mean ± SEM: 21.4 ± 1.64; 3–51 years) and dynorphin mRNA expression (n = 56 patients for which data were available; mean duration, ±SEM: 21.4 ± 1.64; 3–51 years) indicating that the effect may be more pronounced in early epilepsy.
FIGURE 1.
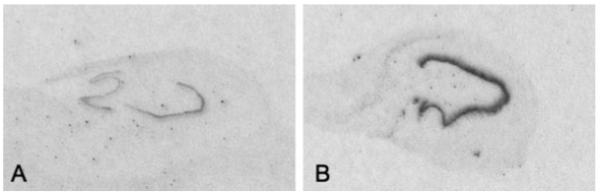
Film auto-radiographs depicting prodynorphin mRNA expression in the dentate gyrus of a representative control (A) and a TLE specimen (B).
To learn more about factors inducing up-regulation of prodynorphin mRNA we analyzed the distribution of hybridization signal intensities (averaged for each sample) within the group of epilepsy patients. The histogram data revealed a wide spread of prodynorphin mRNA levels among different patients with a highly right skewed, possibly bimodal distribution significantly departing from the normal distribution (Kolmogorov test, P = 0.01, Fig. 2A). The bimodal distribution suggests that factors causing the up-regulation of prodynorphin expression must have been operative to a varying degree at the time of tissue resection. In our subsequent analyses we considered two potentially responsible factors: (i) recent seizure(s) before surgery, as seizures are known to be potent inducers of gene expression, and (ii) differences in genotypes at a functional prodynorphin gene promoter.
FIGURE 2.
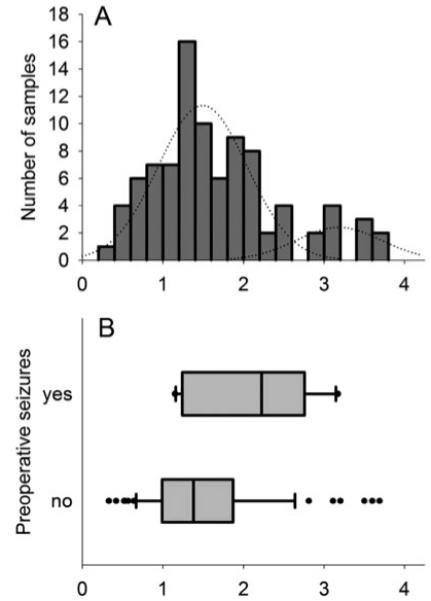
A. Histogramm of prodynorphin mRNA expression in the dentate gyrus (in situ hybridization signals intensities from 91 epileptic samples as normalized values, standardized to three nonepileptic reference control tissues). Bin width is 0.2 units. Data are clearly right skewed significantly departing from the normal distribution. Presumptive Gauss’ normal distribution curves for the two proposed populations are shown as thin lines (the cut-off point between the two curves was visually assessed at 2.5). B. Box-plot of normalized hybridization signal intensities (x-axis as in A) from 12 patients with confirmed seizures in the 48 hr before surgery (top box-plot) vs. 64 patients without evidence of preoperative seizures (bottom box-plot). Box-boundaries mark the 25th and the 75th percentiles and the line in the middle the median. Whiskers mark the 10th and the 90th percentile. Outliers are marked as separate points.
We identified 12 patients with documented seizures within a 48-h period before the operation. The interval between last seizure and surgery was between 3 and 43 h (24.38 ± 5.58 h, mean ± SEM) in these patients. In 64 patients, no evidence for recent preoperative seizures were found (though nocturnal, subtle or postictal amnesia-associated seizures might have been missed). For 15 patients investigated no reliable observations for the pre-surgical period were available. Conforming to our hypothesis the average hybridization signals were indeed significantly higher in the group of patients with recent preoperative seizures (t-test on logarithmically transformed normalized hybridization signals, P = 0.028, uncorrected for multiple testing, Fig. 2B). A rapid increase in dynorphin synthesis and its release is indicated by reduced binding of the opioid receptor ligand 11C-diprenorphine in positron emission tomography in the left parieto-temporo-occipital cortex of patients with reading-induced epilepsy due to increased occupancy or fast internalization of the receptors by the endogenous ligand (Koepp, 1998). This is followed by a rapid but reversible receptor up-regulation as shown by increased 11C-diprenorphine labeling about 8 hr after spontaneous partial seizures in TLE patients (Hammers et al., 2007).
To confirm that only recent seizures were associated with prodynorphin up-regulation and not just the severity of the epilepsy as such, we assessed the average monthly seizure frequencies and found no correlation with transcript levels. Neither did a range of other clinical parameters (age at epilepsy onset, sex, side of seizure focus, medication with specific antiepileptic drugs, and hippocampal sclerosis) correlate with the extent of prodynorphin mRNA expression (Supporting Information Table 1).
We also tested for an association of prodynorphin mRNA levels with a functional tandem repeat polymorphism in the prodynorphin promoter region. High-expression H-alleles of this polymorphism were previously shown to increase the transcriptional inducibility of the gene in vitro and in vivo (Zimprich et al., 2000; Rockman et al., 2005; Nikoshkov et al., 2008). There was no significant correlation between genotypes and mRNA levels in a linear regression analysis in the whole sample, which was due to the high spread of expression signals in patients homozygous for the low-expression L-alleles (n = 91, P = 0.2). As L-homozygous individuals were previously suggested to experience more seizures (Stogmann et al., 2002), which might confound the association between genotypes and transcription levels, we repeated the analysis in the subgroup of patients visually forming the first peak of the prodynorphin mRNA distribution, under the assumption that these were “basal” expression values not up-regulated by recent seizures. In this subgroup, we detected a significant linear association between genotypes and hybridization signal (linear regression analysis, n = 77, P = 0.022, Fig. 3).
The hippocampal formation with its excitatory circuits is prone to over-excitation. Under physiological conditions, dense networks of inhibitory neurons modulate and control these excitatory pathways. Dynorphin peptides are stored as cotransmitters in the excitatory (glutamate-ergic) mossy fibers and are released upon high frequency stimulation to provide a negative feedback on concomitant glutamate release from mossy fibers by presynaptic κ opioid receptors (Wagner et al., 1993; Weisskopf et al., 1993; Terman et al., 2000; Hammers et al., 2007).
Animal studies show that seizures induce marked release of dynorphin, accompanied by subsequent increases in its mRNA expression (Simonato and Romualdi, 1996). Our findings of (i) a large up-regulation of the prodynorphin gene in patients with TLE, (ii) the existence of a second high intensity peak in some patients, and (iii) the correlation of higher transcript levels with recent seizures fit well with the above sketched model of dynorphins’ inhibitory action in epilepsy. Moreover, the fact that we observed such large differences in gene expression in patients’ samples suggests that dynorphins indeed play a particularly relevant role in the context of TLE. This information (on the relevance) is important as the relative inhibitory contribution of functionally overlapping neuropeptide systems can change between species. Animal experiments can therefore not automatically be extrapolated to humans. For example, in some rat models the seizure induced up-regulation in dentate granule cells is much higher for neuropeptide Y than for dynorphins (Gall, 1988; Marksteiner et al., 1990; Douglass et al., 1991; Vezzani et al., 1999), whereas in humans neuropeptide Y transcripts are not increased in granule cells after seizures (Furtinger et al., 2001), which would suggest that dynorphins’ inhibitory function in the hippocampus is more pertinent in humans than in rats.
The prodynorphin promoter, particularly a 68-bp VNTR polymorphism has undergone a strong positive selection during primate and human evolution (Rockman et al., 2005). This polymorphism, which has been repeatedly shown to modulate the transcriptional activity of the gene in vivo and in vitro was correlated with prodynorphin transcript levels but only when confounding samples with very high expression levels were excluded. Although these findings would be compatible with an expression modifying role of this promoter variant, a statistically firm conclusion can not be drawn from the present data.
In conclusion, our findings lend weight to the hypothesis that dynorphins play a relevant role in the termination or inhibition of seizures in patients with temporal lobe epilepsy. Given the failure of current medical therapies to control seizures in a considerable proportion of patients, it would appear attractive to explore pharmacological alternatives that sustain dynorphin’s inhibitory activity.
Supplementary Material
Acknowledgment
We thank Elke Kirchmair for technical assistance.
Grant sponsor: European Union Grant FP6 EPICURE; Grant number: LSH-CT-2006-037315; Grant sponsor: Austrian Science Foundation; Grant numbers: P 19.464, SFB 35-12.
Footnotes
Additional Supporting Information may be found in the online version of this article.
REFERENCES
- Douglass J, Grimes L, Shook J, Lee PH, Hong JS. Systemic administration of kainic acid differentially regulates the levels of prodynorphin and proenkephalin mRNA and peptides in the rat hippocampus. Brain Res Mol Brain Res. 1991;9:79–86. doi: 10.1016/0169-328x(91)90132-h. [DOI] [PubMed] [Google Scholar]
- Furtinger S, Pirker S, Czech T, Baumgartner C, Ransmayr G, Sperk G. Plasticity of Y1 and Y2 receptors and neuropeptide Y fibers in patients with temporal lobe epilepsy. J Neurosci. 2001;21:5804–5812. doi: 10.1523/JNEUROSCI.21-15-05804.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall C. Seizures induce dramatic and distinctly different changes in enkephalin, dynorphin, and CCK immunoreactivities in mouse hippocampal mossy fibers. J Neurosci. 1988;8:1852–1862. doi: 10.1523/JNEUROSCI.08-06-01852.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammers A, Asselin MC, Hinz R, Kitchen I, Brooks DJ, Duncan JS, Koepp MJ. Upregulation of opioid receptor binding following spontaneous epileptic seizures. Brain. 2007;130:1009–1016. doi: 10.1093/brain/awm012. [DOI] [PubMed] [Google Scholar]
- Houser CR, Miyashiro JE, Swartz BE, Walsh GO, Rich JR, Delgado-Escueta AV. Altered patterns of dynorphin immunoreactivity suggest mossy fiber reorganization in human hippocampal epilepsy. J Neurosci. 1990;10:267–282. doi: 10.1523/JNEUROSCI.10-01-00267.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koepp MJ, Richardson MP, Brooks DJ, Duncan JS. Focal cortical release of endogenous opioids during reading-induced seizures. Lancet. 1998;352:952–955. doi: 10.1016/s0140-6736(97)09077-6. [DOI] [PubMed] [Google Scholar]
- Loacker S, Sayyah M, Wittmann W, Herzog H, Schwarzer C. Endogenous dynorphin in epileptogenesis and epilepsy: Anticonvulsant net effect via kappa opioid receptors. Brain. 2007;130:1017–1028. doi: 10.1093/brain/awl384. [DOI] [PubMed] [Google Scholar]
- Marksteiner J, Ortler M, Bellmann R, Sperk G. Neuropeptide Y biosynthesis is markedly induced in mossy fibers during temporal lobe epilepsy of the rat. Neurosci Lett. 1990;112:143–148. doi: 10.1016/0304-3940(90)90193-d. [DOI] [PubMed] [Google Scholar]
- Nikoshkov A, Drakenberg K, Wang X, Horvath MC, Keller E, Hurd YL. Opioid neuropeptide genotypes in relation to heroin abuse: Dopamine tone contributes to reversed mesolimbic proenkephalin expression. Proc Natl Acad Sci USA. 2008;105:786–791. doi: 10.1073/pnas.0710902105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirker S, Czech T, Baumgartner C, Maier H, Novak K, Furtinger S, Fischer-Colbrie R, Sperk G. Chromogranins as markers of altered hippocampal circuitry in temporal lobe epilepsy. Ann Neurol. 2001;50:216–226. doi: 10.1002/ana.1079. [DOI] [PubMed] [Google Scholar]
- Rockman MV, Hahn MW, Soranzo N, Zimprich F, Goldstein DB, Wray GA. Ancient and recent positive selection transformed opioid cis-regulation in humans. PLoS Biol. 2005;3:e387. doi: 10.1371/journal.pbio.0030387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonato M, Romualdi P. Dynorphin and epilepsy. Prog Neurobiol. 1996;50:557–583. doi: 10.1016/s0301-0082(96)00045-7. [DOI] [PubMed] [Google Scholar]
- Solbrig MV, Adrian R, Baratta J, Lauterborn JC, Koob GF. Kappa opioid control of seizures produced by a virus in an animal model. Brain. 2006;129:642–654. doi: 10.1093/brain/awl008. [DOI] [PubMed] [Google Scholar]
- Stogmann E, Zimprich A, Baumgartner C, Aull-Watschinger S, Hollt V, Zimprich F. A functional polymorphism in the prodynorphin gene promotor is associated with temporal lobe epilepsy. Ann Neurol. 2002;51:260–263. doi: 10.1002/ana.10108. [DOI] [PubMed] [Google Scholar]
- Terman GW, Drake CT, Simmons ML, Milner TA, Chavkin C. Opioid modulation of recurrent excitation in the hippocampal dentate gyrus. J Neurosci. 2000;20:4379–4388. doi: 10.1523/JNEUROSCI.20-12-04379.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezzani A, Sperk G, Colmers WF. Neuropeptide Y: Emerging evidence for a functional role in seizure modulation. Trends Neurosci. 1999;22:25–30. doi: 10.1016/s0166-2236(98)01284-3. [DOI] [PubMed] [Google Scholar]
- Wagner JJ, Terman GW, Chavkin C. Endogenous dynorphins inhibit excitatory neurotransmission and block LTP induction in the hippocampus. Nature. 1993;363:451–454. doi: 10.1038/363451a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisskopf MG, Zalutsky RA, Nicoll RA. The opioid peptide dynorphin mediates heterosynaptic depression of hippocampal mossy fibre synapses and modulates long-term potentiation. Nature. 1993;362:423–427. doi: 10.1038/362423a0. [DOI] [PubMed] [Google Scholar]
- Zimprich A, Kraus J, Woltje M, Mayer P, Rauch E, Hollt V. An allelic variation in the human prodynorphin gene promoter alters stimulus-induced expression. J Neurochem. 2000;74:472–477. doi: 10.1046/j.1471-4159.2000.740472.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


