Abstract
Background
Metastases from men with castration resistant prostate cancer (CRPC) harbor increased tumoral androgens vs. untreated prostate cancers (PCa). This may reflect steroid uptake by OATP/SLCO transporters. We evaluated SLCO gene expression in CRPC metastases and determined whether PCa outcomes are associated with single nucleotide polymorphisms (SNPs) in SLCO2B1 and SLCO1B3, transporters previously demonstrated to mediate androgen uptake.
Methods
Transcripts encoding 11 SLCO genes were analyzed in untreated PCa, and in metastatic CRPC tumors obtained by rapid autopsy. SNPs in SLCO2B1 and SLCO1B3 were genotyped in a population-based cohort of 1,309 Caucasian PCa patients. Median survival follow-up was 7.0 years (0.77–16.4). The risk of PCa recurrence/progression and PCa-specific mortality (PCSM) was estimated with Cox proportional hazards analysis.
Results
Six SLCO genes were highly expressed in CRPC metastases vs. untreated PCa, including SLCO1B3 (3.6 fold, p=0.0517) and SLCO2B1 (5.5 fold, p=0.0034). Carriers of the variant alleles SLCO2B1 SNP rs12422149 (HR 1.99, 95% CI 1.11 – 3.55) or SLCO1B3 SNP rs4149117 (HR 1.76, 95% CI 1.00 – 3.08) had an increased risk of PCSM.
Conclusions
CRPC metastases demonstrate increased expression of SLCO genes vs. primary PCa. Genetic variants of SLCO1B3 and SLCO2B1 are associated with PCSM. Expression and genetic variation of SLCO genes which alter androgen uptake may be important in PCa outcomes.
Impact
OATP/SLCO genes may be potential biomarkers for assessing risk of prostate cancer-specific mortality. Expression and genetic variation in these genes may allow stratification of patients to more aggressive hormonal therapy or earlier incorporation of non-hormonal based treatment strategies.
Keywords: prostate cancer, castration resistant, SLCO, steroid transport, genetic variation
Introduction
Androgens play a critical role in the development and progression of prostate cancer (PCa). (1, 2) While androgen deprivation therapy (ADT) remains the most effective treatment for men with advanced disease, the clinical course following ADT is uniformly marked by progression to castration resistant prostate cancer (CRPC). Many mechanisms proposed to confer a castration-resistant phenotype (e.g., androgen receptor (AR) over-expression, AR mutations with promiscuous ligand interactions, and enhanced AR signaling via co-regulator alterations) still require, or are enhanced by, the presence of AR ligands. (3-5) In this respect, we and others have demonstrated that the tissue response to castration is characterized by the presence of residual prostatic androgens at levels capable of activating the AR and maintaining androgen-regulated gene expression. (6-9) Moreover, tumor metastases from men with CRPC have been found to contain detectable testosterone levels that exceed androgen levels in the prostate tissue of eugonadal men. (10)
The source of residual tissue androgens present despite ADT has not been elucidated, but may reflect the uptake of adrenal androgens and intracellular conversion to testosterone, or de novo androgen synthesis from cholesterol or progesterone precursors. Accordingly, we and others have demonstrated that soft tissue and bone CRPC metastases express genes mediating steroid biosynthesis and adrenal androgen utilization. (10, 11)
Emerging data also suggest a potential role for steroid transport proteins encoded by the SLCO gene family in mediating the uptake of androgen into PCa cells and thereby influencing the clinical response to androgen suppression. (12, 13) The organic anion transporting polypeptides (OATP) are a superfamily of SLCO-encoded membrane transporters involved in the transport of bile acids, steroid conjugates, xenobiotics and a variety of clinically important drugs. (14) Several family members are known to mediate the uptake of steroids and steroidogenic precursors, including sulfated forms of pregnenolone, estrone and DHEA. Among these are SLCO1B1 and SLCO1B3, primarily expressed in the liver, and SLCO1A2 and SLCO2B1, more broadly distributed in liver, kidney, intestine and brain, as well as in steroidogenic tissues such as testis, ovary, mammary epithelium, placenta and adipose tissue. (14-19)
Consistent with a physiologic role for SLCO transporters in providing a pool of intracellular precursors for steroidogenic tissues, several studies have demonstrated SLCO transporter expression in human breast carcinomas, suggesting a role for SLCO mediated activity in the estrogen-dependent pathophysiology of this tumor (15, 20) Notably, Al Sarakbi et al observed a statistically significant association between the expression of SLCO2B1 and breast cancer grade, while Nozawa et al found that inhibition of estrone-sulfate transporter activity led to suppression of MCF-7 breast cancer cell proliferation. (21, 22)
Numerous studies have demonstrated that single nucleotide polymorphisms (SNPs) in SLCO genes can markedly alter substrate-specific transport efficiency, although few studies have specifically evaluated androgen transport. (23) Hamada et al recently demonstrated that a nonsynonymous SNP (rs4149117) in SLCO1B3 influenced testosterone uptake in transfected cells and was correlated with PCa outcomes. (13) Cells transfected with two copies of the G allele in rs4149117 showed impaired testosterone transport. Among 180 men with CRPC, those homozygous for the GG haplotype had a longer median survival (8.5 vs. 6.4 yrs; p=0.02) and significantly improved 10-year survival (42% vs. 23%, p=0.023). The authors also demonstrated increased OATP1B3 staining in primary prostate cancers compared to faint staining in samples of normal tissue or benign prostatic hyperplasia. Sharifi et al demonstrated that in 68 men treated with hormone suppression for biochemical relapse or metastatic disease, those with two copies of the G allele in this SLCO1B3 SNP had a longer time to androgen independent progression (1.2 years vs. 1.6 years, P<0.05). (12)
The identification of SLCO genes in breast cancer cells and the association of an SLCO genotype with response to ADT in men with advanced PCa suggest that transport genes mediating cellular steroid uptake may influence the growth and/or progression of hormone dependent tumors. The extent to which SLCO family gene members are expressed in untreated primary tumors or in CRPC metastases has not been previously reported. Moreover, whether genetic variation in these genes is associated with PCa survival in men with less advanced disease is unknown. We sought to determine expression of SLCO gene family members in untreated primary PCa and in CPRC metastases, and to determine the association of allelic variation in SLCO2B1 and SLCO1B3 with PCa outcomes in a large population-based cohort of men with PCa.
Methods
Tissue Analysis
Tissue Acquisition
All procedures involving human subjects were approved by the Institutional Review Board (IRB) of the University of Washington Medical Center. Matched samples of benign and tumor prostate tissue were obtained from 8 untreated patients undergoing radical prostatectomy for localized prostate cancer. Castration resistant tumors from 14 men with CRPC were obtained by rapid autopsy under the aegis of the University of Washington Prostate Cancer Donor Autopsy Program as previously described. (24) Autopsies were performed within 4-10 hours of death. Fresh tissue was embedded in freezing media (Tissue-Tek OCT Compound, Sakura Finetek) immediately after harvesting, snap frozen in liquid nitrogen and maintained at −80°C. Tissues utilized in this study included distant or local soft tissue metastases acquired from surgically or medically castrated patients (the latter with anorchid serum testosterone levels documented at <50 ng/dL), and included 2-4 metastatic lymph node, liver, bladder or lung tumor deposits per patient. Bone metastases were not included due to inconsistent RNA quality.
RNA Isolation
Areas consisting of >85% tumor tissue were grossly macro-dissected from 8 prostatectomy specimens and 22 metastases from 5 CRPC patients. Benign prostate samples were macro-dissected after being examined by H&E to ensure absence of tumor tissue, and homogenized in Trizol for isolation of total RNA and conversion to cDNA as previously described. (10) Laser capture micro-dissection was performed on an additional 69 metastases from 14 CRPC patients. All RNA samples were DNAse treated and amplified using oligo dT priming for first strand cDNA synthesis (to minimize priming off residual genomic DNA). Yield, purity and integrity of total and amplified RNA were determined by optical density measurements at wavelengths of 260 and 280nm (OD 260/280) and gel electrophoresis. The quality of cDNA conversion was assessed in studies demonstrating the amplification of genes encoding abundant (PSA, AR) vs. rare transcripts (SRD5A2, steroidogenic enzymes) as previously described. (10)
Quantitative RT-PCR
Reactions were performed in triplicate using an Applied Biosystems 7700 sequence detector with approximately 5 ng of cDNA, 1 μM of each primer pair and SYBR Green PCR master mix (Applied Biosystems, Foster City, CA). Primers specific for genes of interest were designed using the Web-based primer design service Primer3 provided by the Whitehead Institute for Biomedical Research. (25) Sequences are provided in Supplementary Table 1. The specificity of amplification in each reaction was assessed based on the melting point of the dissociation curve and reactions with Ct’s>35 were considered undetectable for that transcript. The mean cycle threshold (Ct) obtained for each gene was normalized to the expression of the housekeeping gene RPL13A in the same sample (the delta Ct). Samples with Ct above 35 were incorporated into the data analysis using a Ct value of 36 for purposes of calculation. The percent of samples that were undetectable varied for each gene (from 0% in SLCO2A1, SLCO2B1, SLCO3A1, SLCO4A1 to 8% in SLCO1B3; 13% in SLCO4C1 and SLCO5A1; 28% in SLCO1A2 and 39% in SLCO1B1) and did not represent a unique population. The Wilcoxan rank sum test was used to compare the mean delta Ct’s for each gene between the primary prostate cancers (n=8) and metastatic autopsy samples (n=16-22). P values < 0.05 were considered significant. The fold change was calculated from the difference in mean delta Ct’s between the sample groups (delta-delta CT method; fold = 2ΔΔCt).
Genotype Analysis
Study Population
The analyses of SLCO2B1 and SLCO1B3 genetic variation and PCa outcomes utilized data and DNA from patients (residents of King County, Washington) in one of two population-based studies of PCa risk factors. Incident cases (histologically confirmed PCa ascertained from the Seattle-Puget Sound SEER cancer registry) were diagnosed between January 1, 1993 – December 31, 1996 (Study I) or between January 1, 2002 – December 31, 2005 (Study II). A total of 2,244 eligible cases were identified and 1,754 (78%) participated in the study interview. Only Caucasian patients with DNA available (n=1,309) were included in these analyses. All study procedures and protocols were approved by the IRB of the Fred Hutchinson Cancer Research Center. In addition, genotyping was approved by the IRB of the National Human Genome Research Institute.
Genotyping
DNA was isolated from peripheral blood samples and stored at −80°C. SNPs in SLCO1B3 and SLCO2B1 were selected using publicly available data from the Genome Variation Server. (26) Haplotype tagging SNPs (tagSNPs) with a minor allele frequency > 5.0% were selected to maximize coverage of the transcript of interest (+ 5 kb upstream and downstream). SNP genotype was determined using the Applied Biosystems (ABI) SNPlex™ Genotyping System and allele calling was done with GeneMapper® software. (27) A total of seven (SLCO2B1) and five (SLCO1B3) SNPs were selected. Genotyping was successful in 98.0 – 99.4% of SNPs. Genotyping of blind duplicate samples (n = 141) was used for quality control with 100% agreement. The SNPs rs2306168 (SLCO2B1) and rs10743397 (SLCO1B3) had no genetic variability in our population and were excluded. For SLCO2B1, the SNPs analyzed included four tagSNPs, one SNP from the 5-prime untranslated region (rs2851069), and a coding non-synonymous SNP (rs12422149). For SLCO1B3, all SNPs were tagSNPs, including two coding non-synonymous SNPs (rs7311358 and rs4149117) in perfect linkage disequilibrium (for which only the rs4149117 data are reported).
Data Collection
Study patients completed standardized in-person interviews providing information on demographic and lifestyle factors, medical and family history, and number of prostate specific antigen (PSA) tests within the five year prior to diagnosis. Clinical information on PCa cases was obtained from the SEER cancer registry, including Gleason score, tumor stage, diagnostic PSA and primary therapy.
PCa Outcomes
All living cases who consented to future contact from Study I (n = 631) were sent a follow-up survey in January 2004. Information on physician diagnoses of PCa recurrence/progression, secondary therapy use, follow-up PSA values, and procedures such as subsequent biopsies and scans was collected along with dates and results. Outcomes were confirmed with review of medical records. Five hundred-twenty men completed the survey (83%). Those not completing the survey were younger at diagnosis (< 50 years), African-American, and had less education compared to responders (p-values < 0.05). No significant differences existed between tumor stage, Gleason score or primary treatment.
Disease recurrence/progression events were determined by multiple criteria. Date of recurrence was imputed for 13 patients who died from PCa but had unknown recurrence date with a multiple imputations methodology as previously described. (28, 29) PSA progression definitions varied by primary treatment: a follow-up PSA value of ≥ 0.2 in men who underwent radical prostatectomy (RP); nadir PSA + 2 ng/mL (Phoenix criteria) (30) for men treated with radiation (XRT); or any PSA rise in men treated with primary ADT. The date of last follow-up for recurrence/progression events was December 31, 2005. PCa-specific mortality (PCSM) was determined from the SEER registry, which links quarterly with the Washington State mortality database. Underlying cause of death was verified by a review of death certificates, with 99% agreement for PCa-specific deaths. The date of last follow-up for mortality was December 1, 2009.
Statistical Analysis
SNP alleles were in Hardy-Weinberg Equilibrium (HWE) by the Fisher’s exact test except for rs4944993 (SLCO2B1), which was then excluded from the analyses. Multivariate Cox proportional hazards models were created to estimate the hazard ratios (HR) and 95% CI for PCa recurrence/progression and PCSM adjusting for age, PSA level at diagnosis, Gleason score, stage, primary treatment, body mass index (BMI) and smoking status. All statistical analyses were conducted using STATA software, Version 11 (Stata, Inc., College Station, TX).
Results
Increased expression of SLCO genes in CRPC metastases vs. primary prostate cancers
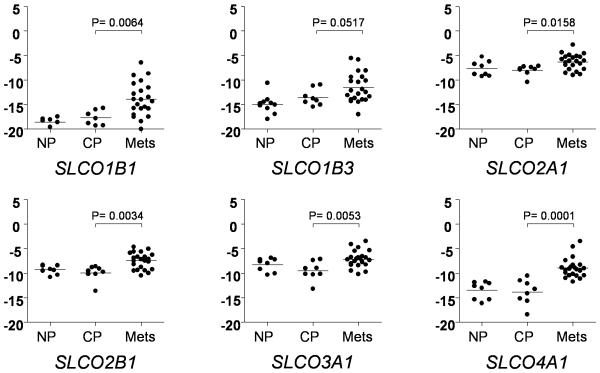
To determine whether SLCO gene expression and therefore OATP transport activity might be present in prostate tumors, we used qRT-PCR to evaluate the expression of these eleven genes in untreated prostate tumors and in advanced CRPC metastases. Transcripts encoding 9 of the 11 genes were reproducibly detected in prostate tumor samples and the CRPC metastases (Table 1). We did not find a significant difference in expression between the matched benign and cancer prostate samples for any of the SLCO genes (data not shown; of note, despite careful macro-dissection, contamination of cancer samples with benign epithelium cannot be ruled out). However, compared to the untreated primary prostate cancers, CRPC metastases demonstrated significantly increased expression of genes encoding six SLCO family members (Table 1). Dot plots depicting the expression level of these six transcripts in the CRPC metastases and in the untreated prostate tissues are shown in Figure 1. Interestingly, SLCO1B1 was detected in only 3 of the benign prostate samples (at a cycle threshold less than 35), but was detectable in a majority of the CRPC metastases.
Table 1.
Expression of SLCO genes in castration resistant prostate cancer
| Family | Prior name | Gene | Protein | Fold* | P value** | |
|---|---|---|---|---|---|---|
| OATP1 | OATP-A, OATP1 | SLCO1A2 | OATP1A2 | 1.1 | ns | |
| OATP-C, OATP2 | SLCO1B1 | OATP1B1 | 13.8 | 0.0064 | ||
| OATP8 | SLCO1B3 | OATP1B3 | 3.6 | 0.0517 | ||
| OATP-F | SLCO1C1 | OATP1C1 | nd | nd | ||
| OATP2 | SLCO2A1 | OATP2A1 | 3.2 | 0.0158 | ||
| OATP-B | SLCO2B1 | OATP2B1 | 5.5 | 0.0034 | ||
| OATP3 | OATP-D | SLCO3A1 | OATP3A1 | 5.4 | 0.0053 | |
| OATP4 | OATP-E | SLCO4A1 | OATP4A1 | 30 | 0.0001 | |
| OATP-H | SLCO4C1 | OATP4C1 | 1.9 | ns | ||
| OATP5 | OATP-J | SLCO5A1 | OATP5A1 | 1.1 | ns | |
| OATP6 | OATP-I | SLCO6A1 | OATP6A1 | nd | nd |
CRPC metastases vs. untreated prostate cancer
Wilcoxan rank sum test; nd - not detected; ns - not significant
Figure 1.
Transcript levels for the indicated SLCO genes in matched normal prostate tissue (NP) and prostate cancer tissue (PCa) from untreated men, and from CRPC metastases (Mets). Cycle thresholds (Ct) for each gene were normalized to the housekeeping gene RPL13A in the same sample. The y-axis is the RPL13A-normalized Ct; more positive numbers reflect higher transcript abundance. The fold change was calculated by the delta-delta CT method (fold = 2ΔΔCt). The Wilcoxan rank sum test was used to compare the mean Ct’s for each gene between the cancer prostate (PCa) and metastatic tumor (Mets) samples. P values < 0.05 were considered significant. Significant differences in expression between the CRPC Mets and PCa samples were not observed for SLCO1A2, SLCO4C1 and SLCO5A1 (data not shown).
Prevalent expression of multiple SLCO gene members in primary and metastatic CRPC tumors
To more carefully examine the range and distribution of SLCO gene expression in advanced prostate tumors, we expanded the initial cohort of CRPC tumors (isolated by gross macro-dissection) to include 69 tumor samples (isolated by laser capture micro-dissection) from 14 men with CRPC. For each subject we evaluated 2-10 soft tissue metastases as well as 1-2 primary samples (prostatic tumor simultaneously resected with the metastases at time of autopsy) to assess whether expression differed in the primary vs. metastatic environment. We found widespread but variable expression of multiple SLCO gene members across the CRPC samples (Figure 2), with certain patients consistently expressing multiple SLCO transporters in their tumor samples (e.g. patients 1-4), and others demonstrating low levels of SLCO gene expression (e.g. patients 11-14). Grouping of samples by patient of origin showed that gene expression was generally consistent across the metastatic samples of an individual patient, although not necessarily concordant between the primary tumor and metastases (paired analyses did not demonstrate consistent differences in primary vs. metastatic tumor deposits; data not shown). The primary and metastatic samples from individual patients occasionally demonstrated strikingly different patterns of SLCO gene expression, consistent with the known heterogeneity that characterizes prostate cancer metastases. (24, 31) For example, patients 6 and 7 had relatively low SLCO expression in the prostatic tumor samples harvested at autopsy, but abundant expression of multiple SLCO genes in their metastases. In contrast, patients 10 and 11 expressed multiple SLCO gene members in their prostatic samples without marked expression of these genes in their metastases. These data are consistent with the hypothesis that in patients whose tumors have strong SLCO gene expression, steroid uptake proteins may play a role CRPC progression
Figure 2.
Expression of SLCO genes by qRT-PCR in primary prostatic samples (P) and in metastatic (M) tumors obtained from 14 men with CRPC. Primary and metastatic samples were simultaneously harvested at autopsy and are grouped by patient of origin. The heatmap depicts the mean-centered expression of each gene across all samples. The scale is from bright green (lowest expression) to black (equivalent expression) to bright red (highest expression). Gray squares denote samples for which no transcript was detectable. Patients with prevalent vs. rare expression of SLCO genes are as denoted.
Association of SLCO2B1 and SLCO1B3 polymorphisms with PCa risk and PCSM
A cohort of 1,309 Caucasian PCa cases from the population-based studies described above had DNA available for genotyping, with selected characteristics shown in Table 2. The majority were diagnosed with localized disease (78%), had Gleason score ≤ 3+4 (85%) and a PSA value at diagnosis of < 10.0 ng/ml (69%). For the recurrence/progression analysis, 440 Caucasian men had genotyping results available for analysis. Of these, 30% had a recurrence/progression event (n = 143). The median follow-up time for recurrence/progression was 8.9 years (range 0.1 – 12.8 years). For PCSM, the median follow-up time was 7.0 years (range 0.8 – 16.4 years).
Table 2.
Distribution of selected characteristics of Caucasian population-based prostate cancer cases used for SLCO2B1 and SLCO1B3 SNP genotype analyses
| Total cases | 1,309 |
| Age | |
| 40-49 | 102 (7.8) |
| 50-54 | 189 (14.4) |
| 55-59 | 325 (24.8) |
| 60-64 | 395 (30.2) |
| 65-69 | 153 (11.7) |
| 70-74 | 145 (11.1) |
| Family history of prostate cancer * | |
| Negative | 1026 (78.4) |
| Positive | 283 (21.6) |
| PSA tests within 5 years prior to diagnosis | |
| None | 288 (22.1) |
| 1 – 2 | 320 (24.5) |
| 3+ | 638 (48.9) |
| Unknown | 58 (4.5) |
| BMI (weight/height2) | |
| < 25.0 | 429 (32.8) |
| 25.0 – 29.9 | 638 (48.7) |
| >= 30.0 | 242 (18.5) |
| Smoking status | |
| Never | 523 (40.0) |
| Former | 631 (48.2) |
| Current | 155 (11.8) |
| Diagnostic PSA (ng/mL) | |
| 0.0 – 3.9 | 178 (13.6) |
| 4.0 – 9.9 | 722 (55.2) |
| 10.0 – 19.9 | 191 (14.6) |
| >= 20 | 118 (9.0) |
| Missing | 100 (7.6) |
| Gleason score | |
| 2 – 6, 3+4 | 1103 (84.5) |
| 4+3, 8 – 10 | 202 (15.5) |
| Tumor stage | |
| Local | 1023 (78.2) |
| Regional | 254 (19.4) |
| Distant | 32 (2.4) |
| Treatment | |
| RP | 770 (58.8) |
| XRT +/− Hormones | 359 (27.4) |
| AD | 61 (4.7) |
| Other | 4 (0.3) |
| Watchful Waiting | 115 (8.8) |
First-degree family history of prostate cancer
Table 3 and Table 4 show the results for the multivariate Cox analysis for recurrence/progression and PCSM, respectively. None of the SNPs were associated with the risk of recurrence/progression. Death due to PCa was observed in 66 men (5% of cases). A total of 115 men who died of other causes were censored at time of death. Excluding these 115 from the analysis did not alter the results, and thus they were included in the final models. Having one or two copies of the variant A allele in SLCO2B1 SNP rs12422149 was associated with a 2-fold increased risk of PCSM (HR 1.99, 95% CI 1.11 – 3.55). Similarly, carriers of the variant T allele in SLCO1B3 SNP rs4149117 had a 76% increased risk of PCSM (HR 1.76, 95% CI 1.00 – 3.08). None of the other SNPs was associated with PCSM. There was no evidence for effect modification in those who received primary ADT.
Table 3.
Risk of prostate cancer recurrence/progression by SLCO2B1 and SLCO1B3 SNP genotypes in a population-based prostate cancer cohort*
| SNP | Genotype | Recurrence/Progression | HR (95% CI) ^ | ||
|---|---|---|---|---|---|
| No (n = 326) |
Yes (n = 143) |
||||
| SLCO2B1 | rs12422149 | GG | 254 (69.2) | 113 (30.8) | 1.00 (referent) |
| GA + AA | 47 (66.2) | 24 (33.8) | 1.09 (0.68 – 1.75) | ||
| SLCO2B1 | rs949069 | GG | 216 (69.5) | 95 (30.6) | 1.00 (referent) |
| GA + AA | 87 (66.4) | 44 (33.6) | 1.19 (0.82 – 1.73) | ||
| SLCO2B1 | rs2712819 | AA | 194 (70.6) | 81 (29.5) | 1.00 (referent) |
| AG + GG | 109 (65.7) | 57 (34.3) | 1.35 (0.95 – 1.91) | ||
| SLCO2B1 | rs2851069 | CC | 116 (70.3) | 49 (29.7) | 1.00 (referent) |
| CT + TT | 184 (66.9) | 91 (33.1) | 1.31 (0.92 – 1.89) | ||
| SLCO2B1 | rs7947726 | GG | 259 (69.1) | 116 (30.9) | 1.00 (referent) |
| AG + AA | 44 (67.7) | 21 (32.3) | 1.21 (0.75 – 1.95) | ||
| SLCO1B3 | rs4149117 | GG | 221 (68.0) | 104 (32.0) | 1.00 (referent) |
| GT + TT | 84 (70.0) | 36 (30.0) | 0.85 (0.57 – 1.26) | ||
| SLCO1B3 | rs3829311 | GG | 206 (68.0) | 97 (32.0) | 1.00 (referent) |
| GA + AA | 98 (69.5) | 43 (30.5) | 0.83 (0.57 – 1.21) | ||
| SLCO1B3 | rs4762803 | CC | 239 (68.3) | 111 (31.7) | 1.00 (referent) |
| CG + GG | 66 (70.2) | 28 (29.8) | 0.86 (0.56 – 1.32) | ||
Recurrence/progression data available for 469 cases, Variable number of cases due to failed genotyping
Adjusted for age, PSA at diagnosis, Gleason score, tumor stage, primary treatment, BMI and smoking status
Table 4.
Risk of prostate cancer-specific mortality by SLCO2B1 and SLCO1B3 SNP genotypes in a population-based prostate cancer cohort*
| SNP | Genotype | Prostate Cancer Death | HR (95% CI) ^ | ||
|---|---|---|---|---|---|
| No @ | Yes | ||||
| SLCO2B1 | rs12422149 | GG | 993 (95.6) | 46 (4.4) | 1.00 (referent) |
| GA + AA | 210 (92.5) | 17 (7.5) | 1.99 (1.11 – 3.55) | ||
| SLCO2B1 | rs949069 | GG | 811 (95.5) | 38 (4.5) | 1.00 (referent) |
| GA + AA | 397 (93.9) | 26 (6.2) | 1.39 (0.82 – 2.34) | ||
| SLCO2B1 | rs2712819 | AA | 777 (95.0) | 41 (5.0) | 1.00 (referent) |
| AG + GG | 431 (95.1) | 22 (4.9) | 1.02 (0.60 – 1.77) | ||
| SLCO2B1 | rs2851069 | CC | 452 (95.2) | 23 (4.8) | 1.00 (referent) |
| CT + TT | 752 (94.7) | 42 (5.3) | 1.21 (0.72 – 2.05) | ||
| SLCO2B1 | rs7947726 | GG | 999 (95.1) | 51 (4.9) | 1.00 (referent) |
| AG + AA | 204 (94.0) | 13 (6.0) | 0.75 (0.37 – 1.53) | ||
| SLCO1B3 | rs4149117 | GG | 885 (95.4) | 43 (4.6) | 1.00 (referent) |
| GT + TT | 326 (93.7) | 22 (6.3) | 1.76 (1.00 – 3.08) | ||
| SLCO1B3 | rs3829311 | GG | 812 (95.0) | 43 (5.0) | 1.00 (referent) |
| GA + AA | 396 (94.7) | 22 (5.3) | 1.28 (0.74 – 2.23) | ||
| SLCO1B3 | rs4762803 | CC | 958 (94.8) | 53 (5.2) | 1.00 (referent) |
| CG + GG | 251 (95.4) | 12 (4.6) | 1.16 (0.59 – 2.30) | ||
Variable number of cases due to failed genotyping
Includes 115 men who died of non-prostate cancer causes and who were censored at time of death
Adjusted for age, PSA at diagnosis, Gleason score, stage, primary treatment, BMI and smoking status
Discussion
The source of residual androgens present in prostate cancer tissues despite ADT has not been elucidated, but may reflect the uptake of adrenal androgens and intracellular conversion to testosterone, or de novo androgen synthesis from the uptake of cholesterol or progesterone precursors. While the uptake of steroid hormones is generally attributed to free diffusion across the lipid membrane, emerging data suggest a role for steroid transport proteins in actively mediating androgen uptake into prostate cells and thereby potentially influencing PCa outcomes.
In this study, we evaluated expression of SLCO-encoded membrane transporters in untreated primary PCa and in CPRC metastases, and evaluated genetic variation of SLCO2B1 and SLCO1B3 in association with PCa outcomes. We found significantly increased expression of many SLCO family members in CRPC metastases compared to untreated primary PCa. Moreover, individuals could be identified whose tumors were characterized by widespread or minimal transporter gene expression. An important limitation of gene expression data is that transcript level does not necessarily equate with protein expression; however, our findings are consistent with a hypothesis that, in certain individuals, steroid transport proteins may play a role in the tumor androgen levels observed in CRPC metastases. In our analysis of data from a population-based cohort of PCa patients, we found that two SNPs (SLCO2B1 SNP rs12422149 and SLCO1B3 SNP rs4149117) were associated with an increased risk of PCSM.
The increased risk of PCSM in carriers of the T vs. G allele in SLCO1B3 SNP rs4149117 (334T>G; adjusted HR 1.76, 95% CI 1.00 – 3.08), although of borderline significance, is consistent with the study of Hamada et al. (13) In their study of 180 men with CRPC, the HR for overall mortality was 1.57 (95% CI 1.11 – 2.24) for men carrying the T allele in rs4149117. Notably, they demonstrated that SLCO1B3 transfected cells actively transported testosterone, that testosterone uptake was higher for carriers of the T vs. G allele, and that men homozygous for the 334GG genotype (associated with impaired testosterone uptake) had a longer median survival (8.5 vs. 6.4 yrs; p=0.02) and improved 10-year survival (42% vs. 23%, p=0.023). In a separate study of 68 men treated with ADT for advanced PCa, those with two copies of the G allele in rs4149117 had a longer time to androgen independence (1.6 years vs. 1.2 years, P<0.05). (12) Of potential importance is that in the Caucasian population comprising these reports (as well as ours), SNP rs4149117 (334T>G) is in perfect linkage disequilibrium with SNP rs7311358 (699G>A). The study of Hamada et al found that only the double mutant haplotype demonstrated impaired testosterone transport in vivo. Thus, different outcomes might be observed in non-Caucasian populations where the SNPs may vary independently.
We also found an increased risk of PCSM in carriers of the A vs. G allele in SLCO2B1 SNP rs12422149 (935A>G; adjusted HR 1.99, 95% CI 1.11 – 3.55). This coding, non-synonymous SNP has been reported from in vitro studies to have altered cellular uptake of certain medications (i.e., montelukast) as well as DHEA-S. (32, 33) In a study of 538 castrate men with advanced PCa receiving ADT (a different setting than the population-based cohort of incident PCa cases in our study), the A allele in SLCO2B1 SNP rs12422149 was associated with a longer time to progression. (HR for progression 1.40; 95% CI 1.06-1.84 for the G vs. A allele).7 Interestingly, allelic variation in DHEA-S uptake by SLCO2B1 (and accordingly, any impact of cellular DHEA-S uptake on PCa outcome) might be anticipated to differ in the eugonadal and castrate settings, as testosterone has been demonstrated to inhibit SLCO2B1 mediated transport of DHEA-S. (23) Thus, the association of SLCO2B1 with PCa outcome may reflect the transport of DHEA-S in advanced disease, but transport of an alternative substrate in incident PCa cases.
We did not find any associations between SLCO1B3 or SLCO2B1 genotypes and PCa recurrence/progression. This may be due to several reasons. First, recurrence/progression data were only available for one-third of the cohort, which limited our power to detect a difference. Second, there can be significant variation (up to 35%) in reported PSA progression rates depending on how it is defined. (34) Further, considering the natural history of PCa, many men who experience recurrence/progression events will not die of their disease. (35) Finally, consistent with our findings of increased expression of SLCO gene members in metastatic tumors compared to primary tumors, it may be that alteration in SLCO activity is important in men with advanced, end stage tumors but not in men with early recurrence, which may be due to other mechanisms.
Our data from a population-based cohort of PCa patients suggest that genetic variation in the SLCO transport gene family is associated with PCa mortality. These findings are consistent with results from studies of men with advanced PCa, suggesting that genetic variation in SLCO genes may allow stratification of patients at higher risk for PCSM or poor response to ADT for consideration of either more aggressive hormonal therapies or incorporation of non-hormonal based treatment strategies. In addition, we observe significantly increased expression of SLCO gene members in CRPC metastases, suggesting steroid transport proteins may contribute to the elevated tumoral androgen levels observed during CRPC progression. Future studies include immunohistochemical assessment of OATP expression in primary and metastatic prostate cancer tissues, and using in vivo models to evaluate the ability of SLCO transporters to measurably alter intratumoral androgen levels. The family of OATP/SLCO steroid transport proteins may serve as novel biomarkers of response to ADT and common genetic variants in these genes may be associated with enhanced risk of PCa mortality. Thus, their role in PCa warrants further investigation.
Supplementary Material
Acknowledgements
We thank Peter Nelson and Philip Kantoff for thoughtful discussion; Ruth Dumpit, Tom Kim, Ilsa Coleman, Roger Coleman, Jared Lucas and Andrew Morgan for expert technical assistance; and Alex Moreno for administrative support.
Support: Prostate Cancer Foundation (Career Development Award to EAM and Synergy Award to RBM); Damon Runyon Cancer Research Foundation (Damon Runyon-Genentech Clinical Investigator Award CI-40-08 to EAM); National Institutes of Health (Pacific Northwest Prostate Cancer SPORE P50 CA97186 (JLS, Career Development Award to JLW, and Pilot Project Award to EAM); R01 CA056678 (JLS); R01 CA082664 (JLS); R01 CA092579 (JLS); T32 CA009168-30 (JLW)); with additional support from the Fred Hutchinson Cancer Research Center and the Intramural Program of the National Human Genome Research Institute (EMK and EAO).
Footnotes
Disclosure Statement: None
Phil Kantoff, personal communication
References
- 1.Nelson PS, Clegg N, Arnold H, et al. The program of androgen-responsive genes in neoplastic prostate epithelium. Proc Natl Acad Sci U S A. 2002;99:11890–5. doi: 10.1073/pnas.182376299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pritchard CC, Nelson PS. Gene expression profiling in the developing prostate. Differentiation. 2008;76:624–40. doi: 10.1111/j.1432-0436.2008.00274.x. [DOI] [PubMed] [Google Scholar]
- 3.Visakorpi T, Hyytinen E, Koivisto P, et al. In vivo amplification of the androgen receptor gene and progression of human prostate cancer. Nat Genet. 1995;9:401–6. doi: 10.1038/ng0495-401. [DOI] [PubMed] [Google Scholar]
- 4.Chen CD, Welsbie DS, Tran C, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33–9. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- 5.Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nat Rev Cancer. 2001;1:34–45. doi: 10.1038/35094009. [DOI] [PubMed] [Google Scholar]
- 6.Geller J, Albert J, Nachtsheim D, Loza D, Lippman S. Steroid levels in cancer of the prostate--markers of tumor differentiation and adequacy of anti-androgen therapy. Prog Clin Biol Res. 1979;33:103–11. [PubMed] [Google Scholar]
- 7.Mohler JL, Gregory CW, Ford OH, 3rd, et al. The androgen axis in recurrent prostate cancer. Clin Cancer Res. 2004;10:440–8. doi: 10.1158/1078-0432.ccr-1146-03. [DOI] [PubMed] [Google Scholar]
- 8.Page ST, Lin DW, Mostaghel EA, et al. Persistent intraprostatic androgen concentrations after medical castration in healthy men. J Clin Endocrinol Metab. 2006;91:3850–6. doi: 10.1210/jc.2006-0968. [DOI] [PubMed] [Google Scholar]
- 9.Mostaghel EA, Page ST, Lin DW, et al. Intraprostatic Androgens and Androgen-Regulated Gene Expression Persist after Testosterone Suppression: Therapeutic Implications for Castration-Resistant Prostate Cancer. Cancer Res. 2007;67:5033–41. doi: 10.1158/0008-5472.CAN-06-3332. [DOI] [PubMed] [Google Scholar]
- 10.Montgomery RB, Mostaghel EA, Vessella R, et al. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res. 2008;68:4447–54. doi: 10.1158/0008-5472.CAN-08-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stanbrough M, Bubley GJ, Ross K, et al. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res. 2006;66:2815–25. doi: 10.1158/0008-5472.CAN-05-4000. [DOI] [PubMed] [Google Scholar]
- 12.Sharifi N, Hamada A, Sissung T, et al. A polymorphism in a transporter of testosterone is a determinant of androgen independence in prostate cancer. BJU Int. 2008;102:617–21. doi: 10.1111/j.1464-410X.2008.07629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamada A, Sissung T, Price DK, et al. Effect of SLCO1B3 haplotype on testosterone transport and clinical outcome in caucasian patients with androgen-independent prostatic cancer. Clin Cancer Res. 2008;14:3312–8. doi: 10.1158/1078-0432.CCR-07-4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hagenbuch B, Meier PJ. The superfamily of organic anion transporting polypeptides. Biochim Biophys Acta. 2003;1609:1–18. doi: 10.1016/s0005-2736(02)00633-8. [DOI] [PubMed] [Google Scholar]
- 15.Pizzagalli F, Varga Z, Huber RD, Folkers G, Meier PJ, St-Pierre MV. Identification of steroid sulfate transport processes in the human mammary gland. J Clin Endocrinol Metab. 2003;88:3902–12. doi: 10.1210/jc.2003-030174. [DOI] [PubMed] [Google Scholar]
- 16.Tamai I, Nezu J, Uchino H, et al. Molecular identification and characterization of novel members of the human organic anion transporter (OATP) family. Biochem Biophys Res Commun. 2000;273:251–60. doi: 10.1006/bbrc.2000.2922. [DOI] [PubMed] [Google Scholar]
- 17.Alcorn J, Lu X, Moscow JA, McNamara PJ. Transporter gene expression in lactating and nonlactating human mammary epithelial cells using real-time reverse transcription-polymerase chain reaction. J Pharmacol Exp Ther. 2002;303:487–96. doi: 10.1124/jpet.102.038315. [DOI] [PubMed] [Google Scholar]
- 18.Ugele B, St-Pierre MV, Pihusch M, Bahn A, Hantschmann P. Characterization and identification of steroid sulfate transporters of human placenta. Am J Physiol Endocrinol Metab. 2003;284:E390–8. doi: 10.1152/ajpendo.00257.2002. [DOI] [PubMed] [Google Scholar]
- 19.Valle LD, Toffolo V, Nardi A, et al. Tissue-specific transcriptional initiation and activity of steroid sulfatase complementing dehydroepiandrosterone sulfate uptake and intracrine steroid activations in human adipose tissue. J Endocrinol. 2006;190:129–39. doi: 10.1677/joe.1.06811. [DOI] [PubMed] [Google Scholar]
- 20.Miki Y, Suzuki T, Kitada K, et al. Expression of the steroid and xenobiotic receptor and its possible target gene, organic anion transporting polypeptide-A, in human breast carcinoma. Cancer Res. 2006;66:535–42. doi: 10.1158/0008-5472.CAN-05-1070. [DOI] [PubMed] [Google Scholar]
- 21.Al Sarakbi W, Mokbel R, Salhab M, Jiang WG, Reed MJ, Mokbel K. The role of STS and OATP-B mRNA expression in predicting the clinical outcome in human breast cancer. Anticancer Res. 2006;26:4985–90. [PubMed] [Google Scholar]
- 22.Nozawa T, Suzuki M, Yabuuchi H, Irokawa M, Tsuji A, Tamai I. Suppression of cell proliferation by inhibition of estrone-3-sulfate transporter in estrogen-dependent breast cancer cells. Pharm Res. 2005;22:1634–41. doi: 10.1007/s11095-005-7096-0. [DOI] [PubMed] [Google Scholar]
- 23.Grube M, Kock K, Karner S, et al. Modification of OATP2B1-mediated transport by steroid hormones. Mol Pharmacol. 2006;70:1735–41. doi: 10.1124/mol.106.026450. [DOI] [PubMed] [Google Scholar]
- 24.Roudier MP, True LD, Higano CS, et al. Phenotypic heterogeneity of end-stage prostate carcinoma metastatic to bone. Hum Pathol. 2003;34:646–53. doi: 10.1016/s0046-8177(03)00190-4. [DOI] [PubMed] [Google Scholar]
- 25.Steve Rozen, Helen J. Skaletsky. Primer3 on the WWW for general users and for biologist programmers. In: Krawetz S, Misener S, editors. Bioinformatics Methods and Protocols: Methods in Molecular Biology. Humana Press; Totowa, NJ: 2000. pp. pp365–386. Source code available at http://fokker.wi.mit.edu/primer3/ [DOI] [PubMed] [Google Scholar]
- 26.Genome Variation Server [Internet] Seattle (WA): SeattleSNPs Program for Genomic Applications (Version 5.11) cited 2009, Sep 10. Available from: http://gvs.gs.washington.edu/GVS/
- 27.Applied Biosystems [Internet] Carlsbad (CA): Life Technologies Corporation. [cited 2009, Sep 10]. Available from: www.appliedbiosystems.com.
- 28.Wright JL, Kwon EM, Lin DW, et al. CYP17 polymorphisms and prostate cancer outcomes. Prostate. 70:1094–101. doi: 10.1002/pros.21143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harrell F. Regression Modeling Strategies. Springer; New York: 2001. [Google Scholar]
- 30.Roach M, 3rd, Hanks G, Thames H, Jr., et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65:965–74. doi: 10.1016/j.ijrobp.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 31.Shah RB, Mehra R, Chinnaiyan AM, et al. Androgen-independent prostate cancer is a heterogeneous group of diseases: lessons from a rapid autopsy program. Cancer Res. 2004;64:9209–16. doi: 10.1158/0008-5472.CAN-04-2442. [DOI] [PubMed] [Google Scholar]
- 32.Mougey EB, Feng H, Castro M, Irvin CG, Lima JJ. Absorption of montelukast is transporter mediated: a common variant of OATP2B1 is associated with reduced plasma concentrations and poor response. Pharmacogenet Genomics. 2009;19:129–38. doi: 10.1097/FPC.0b013e32831bd98c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang M, Oh WK, Xie W, et al. Genetic variations in SLCO2B1 and SLCO1B3 and the efficacy of androgen-deprivation therapy in prostate cancer patients; ASCO Genitourinary Cancers Symposium; 2010. [Google Scholar]
- 34.Stephenson AJ, Kattan MW, Eastham JA, et al. Defining biochemical recurrence of prostate cancer after radical prostatectomy: a proposal for a standardized definition. J Clin Oncol. 2006;24:3973–8. doi: 10.1200/JCO.2005.04.0756. [DOI] [PubMed] [Google Scholar]
- 35.Collette L. Prostate-specific antigen (PSA) as a surrogate end point for survival in prostate cancer clinical trials. Eur Urol. 2008;53:6–9. doi: 10.1016/j.eururo.2007.08.041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.