Abstract
Background
Studies in developed countries have shown highly active antiretroviral therapy (HAART) decreases incidence of severe opportunistic diseases (ODs) in HIV-infected patients beyond what is expected from CD4 changes.
Objective
To estimate the independent impact of HAART on reducing ODs and mortality in Côte d’Ivoire.
Methods
Within 2 longitudinal studies of HIV-infected adults (1996–2003), we identified time on cotrimoxazole alone and HAART plus cotrimoxazole. WHO stage 3–4 defining events and severe malaria were divided into those preventable and not preventable with cotrimoxazole. Incidence of ODs by CD4 stratum was estimated using incidence density analysis. CD4 at time of OD was estimated using linear interpolation. Using Poisson regression, we estimated the effect of HAART on OD incidence and mortality by CD4 stratum.
Results
Four hundred forty-six and 135 adults were followed during 6,216 and 3,412 person-months in the cotrimoxazole alone and HAART plus cotrimoxazole periods. There was a CD4-independent risk reduction for ODs and mortality during the HAART plus cotrimoxazole period compared to cotrimoxazole alone, which varied by time on HAART, CD4 stratum, and OD type. It was mainly seen after 6 months on HAART, and for ODs not preventable by cotrimoxazole. The HAART effect differed significantly by CD4 stratum (p=0.02), but was significant in all strata after 6 months on HAART.
Conclusions
In these sub-Saharan African adults, HAART initiation reduced ODs and mortality beyond what was expected through the HAART-induced CD4 increase. Further studies should examine practical implications of this independent “HAART effect” on clinical outcomes in patients on HAART.
Keywords: HAART, opportunistic disease, opportunistic infection, HIV, mortality
Introduction
In sub-Saharan Africa, HIV disease continues to be a leading cause of morbidity and mortality and opportunistic diseases (ODs) remain the most frequent cause of complications among HIV-infected patients [1, 2]. Rates of ODs increase substantially with worsening immune status, as determined by CD4 counts [3, 4]. Highly active antiretroviral therapy (HAART), introduced around 1996 in the US and Europe and more recently in resource-limited settings, dramatically reduces HIV-related morbidity and mortality, mainly by increasing CD4 counts [5–9].
Several studies in the US, Canada, and Europe suggest that HAART provides an independent, protective effect in reducing HIV complications beyond that referable to the increase in CD4 counts [10–14]. However, these data have not been seen from sub-Saharan Africa, where the spectrum of HIV morbidity differs from that in North America and Europe [3, 4, 15–17]. As HAART availability is rapidly increasing in sub-Saharan Africa through internationally-supported roll-out programs [1], it is important to examine whether HAART has an effect on OD incidence independent of CD4 count in that region. If it does, further questions include whether this “HAART effect” begins immediately after treatment initiation, whether it is similar across different CD4 count strata and whether it is the same for ODs that are preventable or not preventable with cotrimoxazole. The answers will help inform recommendations on HAART use in resource-limited countries, where multiple sequential HAART regimens are often not available. Our objectives were: 1) to estimate the impact of HAART within different CD4 count strata on the incidence of severe ODs and/or death, 2) to examine the impact of time since HAART initiation on these estimates and 3) to examine whether the effect of HAART is homogenous across different CD4 count strata in a therapeutic cohort of HIV-infected adults in Côte d’Ivoire, West Africa.
Methods
Population
The study population consisted of HIV-infected adults who participated in two studies in Côte d’Ivoire between 1996 and 2003: the Cotrimo-CI ANRS 059 randomized, controlled trial of cotrimoxazole prophylaxis (1996–1998) [3] and the Cotrame ANRS 1203 cohort study (1998–2003) [4]. Participants in both studies were followed through the same standardized procedures, which have been described elsewhere [3, 4, 16, 18]. For patients who participated in at least one of these two studies, we identified two periods of follow-up. In the first period, patients received cotrimoxazole prophylaxis but not HAART. For this period, all patients who started cotrimoxazole within the framework of at least one of the two studies were included. The entry date for the period was the individual date of cotrimoxazole initiation for each patient. The termination date for the period was the date when HAART became available for all patients in Abidjan, Côte d’Ivoire (December 31, 1998). We chose this stopping date because any later endpoint would have introduced the need to censor follow-up at the time of HAART initiation, introducing the risk of informative censoring due to individuals initiating HAART at differing disease states. In the second period, patients received both cotrimoxazole prophylaxis and HAART. For this period, all patients who started HAART within the framework of the Cotrame cohort were included. The entry date for the period was the date of HAART initiation. The termination date for the period was the Cotrame cohort closing date (July 31, 2003). The criteria used for HAART initiation were: CD4 count < 200 irrespective of WHO stage, CD4 count < 350 and WHO stage 3 disease, or WHO stage 4 disease irrespective of CD4 count.
Follow-up
For all patients in both periods, the standardized follow-up procedures included complete blood counts and CD4 counts every six months, standardized diagnosis of specific HIV-related morbidity, management of morbid events at the study clinic, and systematic investigation of vital status for subjects who did not keep scheduled appointments. CD4 counts were performed in two different laboratories: the CeDReS laboratory of the Treichville Hospital, and the Project Retro-CI laboratory. Patients from the Cotrame cohort who started HAART received antiretroviral drugs through the Côte d’Ivoire/UNAIDS Drug Access Initiative. In these pharmacies, blood samples for CD4 counts were systematically collected every three months. At the end of the Cotrame study, the results of these additional CD4 count measurements were obtained from the Côte d’Ivoire national database and merged with the cohort database. We performed sensitivity analyses to examine whether the frequency of CD4 count measurements influenced the results and found minimal effect [16].
Opportunistic diseases
The following specific diagnoses were included in the definition of a severe OD: (i) any WHO stage 3 or 4 defining diseases, including severe bacterial infections (pneumonia, bacterial enteritis, isolated bacteremia, invasive urogenital infection), severe fungal infections (cryptococcal infection and esophageal candidiasis), tuberculosis, isosporosis, toxoplasmic encephalitis, non-tuberculous mycobacteriosis, and other WHO clinical stage 3 and 4 defining illnesses (Table 1); and (ii) severe malaria, defined as all episodes of plasmodium parasitemia resulting in at least one day in the hospital. These severe ODs were further divided into two groups: those that are preventable and those that are not preventable with cotrimoxazole prophylaxis, as shown in several studies [3, 19–22] (Table 1). The diagnostic criteria used by the ANRS 059 and ANRS 1203 event documentation committees have been previously reported [3, 16].
Table 1.
Opportunistic diseases considered for the current analysis
| Opportunistic disease |
|---|
| Preventable with cotrimoxazole |
| Severe Bacterial Infections |
| Pneumonia |
| Bacterial enteritis |
| Isolated bacteremia |
| Invasive urogenital infection |
| Malaria |
| Toxoplasmic encephalitis |
| Isosporosis |
| Pneumocystis jirovecii pneumonia |
| Not preventable with cotrimoxazole |
| Severe Fungal Infections |
| Extra-pulmonary cryptococcal infection |
| Esophageal candidiasis |
| Tuberculosis |
| Non-tuberculous mycobacteriosis |
| Mycobacterium avium complex bacteremia |
| Other Severe Illnesses |
| Other WHO clinical stage 3 defining events |
| Other WHO clinical stage 4 defining events |
The first occurrence of any of these diagnoses for each individual in each treatment period was defined as the first severe OD event. Though each individual may have contributed follow-up time for multiple CD4 strata, only one first severe event was counted for each treatment period. For a given disease, an episode was defined as a new event if no past episode was known to have occurred, or if a past episode was known to have occurred but had been considered cured for more than one month. The following WHO clinical stage 4-defining diseases were considered not curable: cryptococcal meningitis, toxoplasmosis, non-tuberculous mycobacteriosis, invasive herpes simplex virus or cytomegalovirus diseases, isosporiasis, cryptosporidiosis, lymphoma, Kaposi’s sarcoma, chronic or recurrent genital herpes and unexplained weight loss. For these diseases, recurrences were not defined as new episodes (and were not taken into account for the present study). For tuberculosis, recurrence was considered as a new episode when the previous episode was considered cured according to international criteria [23].
Statistical analysis
During each follow-up period, we considered three endpoints successively: first severe OD, death, and a composite outcome of first severe OD or death. The primary analyses were performed within three clinically relevant CD4 count strata: ≤ 50/μl (major risk of death [16, 24]), 51–200/μl (initiation of HAART recommended irrespective of clinical stage [25]), and >200/μl (initiation of HAART should be considered [25]). CD4 count stratum was considered as a time-dependent covariate in the analysis. All available CD4 count measurements for each individual were used throughout the analysis, and were interpolated linearly between measurements. CD4 counts at the time of diagnosis of an OD were estimated by linear interpolation between two consecutive CD4 count measurements. We performed two sets of sensitivity analyses to examine whether the results were sensitive to the assumption underlying the modeling of CD4 counts at the time of diagnosis of an OD. In the first sensitivity analysis, we assumed that CD4 counts remain constant until the next measurement, at which time they change to the next measured value; in the second analysis, we assumed that CD4 counts change immediately to the level seen at the next measurement. We also performed additional sensitivity analyses to examine the sensitivity of the results to the CD4 count strata definitions. Dividing the data into more narrowly defined CD4 strata introduced more noise into the model, but similar trends were found in the results.
The analysis included four parts. First, we performed an incidence density analysis to estimate the incidence of a first severe OD during follow-up within each clinically important CD4 stratum for both groups (cotrimoxazole alone versus HAART plus cotrimoxazole). In patients on HAART, time on HAART was divided into two groups: months 0 through 6, and after month 6. This division of time was intended first to examine the effect of immune reconstitution, and second to distinguish morbidity occurring in patients with a low CD4 count due to HAART failure from morbidity occurring in patients with a low CD4 count because HAART had been recently introduced. Second, we estimated CD4 count stratum-specific OD incidence rates for both treatment groups stratified by likely response to cotrimoxazole (preventable or not preventable). Third, we built a multivariate Poisson regression model to estimate the HAART effect on severe OD incidence, death, and the composite outcome of severe OD or death, after adjusting for current CD4 count, WHO stage at the time of treatment initiation, age, and gender. WHO stage at the time of treatment initiation was defined as WHO stage at the time of cotrimoxazole initiation for the cotrimoxazole alone group and WHO stage at the time of HAART initiation for the HAART plus cotrimoxazole group. Fourth, we built a second Poisson regression model to perform a test for interaction between the CD4 and HAART effects to determine if CD4 count modified the impact of HAART on ODs and mortality. The Poisson regression approach was chosen for the latter analyses because it was the best fit for the data, allowing variance adjustment for non-independence among multiple observations contributed by the same individual with the use of generalized estimating equations [26, 27]. All analyses were performed using proc genmod in SAS 9.0. Robust standard errors were used for all analyses.
Results
Patients
The cotrimoxazole alone period consisted of 446 HIV-infected subjects followed between 1996 and 1998, with a total of 6,216 person-months of follow-up time (median 10.1 months per person). The HAART plus cotrimoxazole period consisted of 135 subjects followed between 1999 and 2003, contributing a total follow-up time of 3,412 person-months (median 25.5 months per person). At baseline, patients followed during the cotrimoxazole alone period were similar with respect to gender and age to those followed during the HAART plus cotrimoxazole period (Table 2A). Baseline CD4 counts were higher for the cotrimoxazole alone period. During follow-up in the cotrimoxazole alone period, patients had a median of two CD4 count measurements, while the median number of measurements during follow-up in the HAART plus cotrimoxazole period was eight (Table 2B).
Table 2.
| Table 2A: Baseline characteristics of patients included within the two periods | ||
|---|---|---|
| Characteristics | Cotrimoxazole alone period* | HAART plus cotrimoxazole period* |
| Number of patients | 446 | 135 |
| Baseline age, median (IQR) | 32 (27–38) | 35 (29–41) |
| Sex, female (%) | 265 (59%) | 85 (63%) |
| Baseline CD4 count/μl, median (IQR) | 248 (138–446) | 127 (58–226) |
| Baseline WHO stage, n (%) | ||
| Stage 3 | 244 (55%) | 61 (45%) |
| Stage 4 | 69 (15%) | 56 (41%) |
| Table 2B: Follow-up characteristics of patients included within the two periods | ||
|---|---|---|
| Characteristics | Cotrimoxazole alone period* | HAART plus cotrimoxazole period* |
| Follow-up time on treatment | ||
| Cumulative, person-months | ||
| Overall | 6,216 | 3,412 |
| By CD4 stratum | ||
| Within CD4 ≤ 50/μl | 615 | 102 |
| Within CD4 51–200/μl | 1,883 | 1,129 |
| Within CD4 >200/μl | 3,714 | 2,184 |
| Median (IQR), months | 10.1 (8.8–20.0) | 25.5 (15.2–37.0) |
| Number of events during follow-up | ||
| Death, number of events | ||
| Overall | 97 | 15 |
| By CD4 stratum | ||
| Within CD4 ≤ 50/μl | 33 | 6 |
| Within CD4 51–200/μl | 42 | 8 |
| Within CD4 >200/μl | 22 | 1 |
| By time since HAART initiation | ||
| 0–6 months | 8 | |
| > 6 months | 7 | |
| Severe morbidity, number of events | ||
| Overall | 217 | 73 |
| By CD4 stratum | ||
| Within CD4 ≤ 50/μl | 41 | 8 |
| Within CD4 51–200/μl | 84 | 25 |
| Within CD4 >200/μl | 92 | 40 |
| By time since HAART initiation | ||
| 0–6 months | 37 | |
| > 6 months | 36 | |
| CD4 measurements during follow-up | ||
| Number of measurements per patient, median (IQR), range | 2 (2–3), range: 1–7 | 8 (4–14), range: 1–23 |
| Number of measurements ≤ 50/μl; median (IQR) | 115; 17 (5–33) | 71; 15 (7–29) |
| Number of measurements 51–200/μl; median (IQR) | 355; 133 (90–168) | 423; 138 (98–171) |
| Number of measurements >200/μl; median (IQR) | 679; 390 (272–603) | 768; 336 (262–477) |
HAART: Highly active antiretroviral therapy; IQR: Interquartile range.
97 patients contributed successively to both periods.
Opportunistic disease incidence
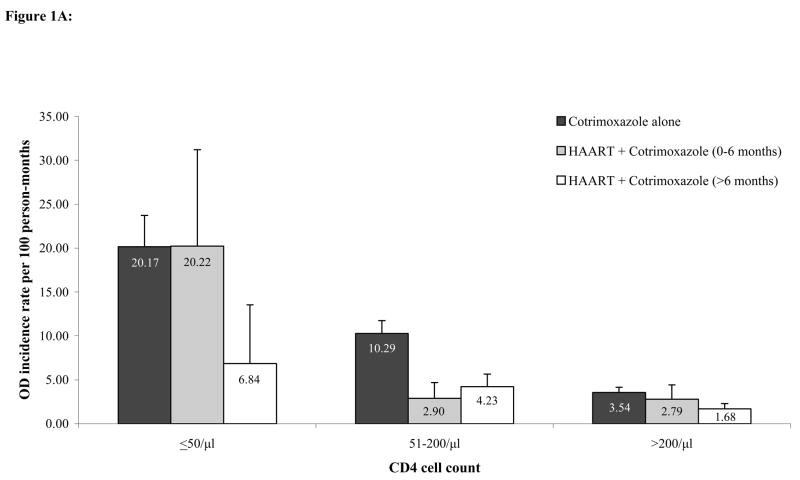
Figure 1A shows the estimated incidence of first severe OD, by current CD4 stratum and by treatment status. A consistently higher incidence of first severe OD across all CD4 strata was estimated during the cotrimoxazole alone period compared to the HAART plus cotrimoxazole period. Incidence rates of severe morbidity ranged from 20.2/100 person-months (PM) (95% CI 16.6–23.7) in the lowest CD4 stratum to 3.5/100PM (95% CI 2.9–4.1) in the highest CD4 stratum for the cotrimoxazole alone period, from 20.2/100PM (95% CI 9.2–31.2) to 2.8/100PM (95% CI 1.1–4.4) for the HAART plus cotrimoxazole 0–6 months period, and from 6.8/100PM (95% CI 0.1–13.5) to 1.7/100PM (95% CI 1.0–2.3) for the HAART plus cotrimoxazole >6 months period.
Figure 1.
Figure 1A: Crude incidence rates of any first severe opportunistic disease stratified by CD4 count and HAART use. Error bars represent the upper bound of the 95% confidence interval.
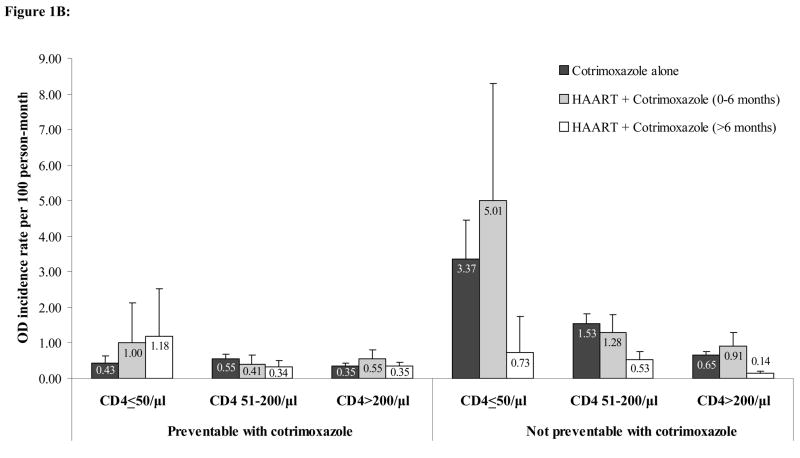
Figure 1B: Crude incidence rates of first severe opportunistic disease stratified by CD4 count, HAART use, and response to cotrimoxazole. Error bars represent the upper bound of the 95% confidence interval.
Figure 1B shows the estimated incidence of first severe OD, by current CD4 strata, treatment status, and type of OD (preventable or not preventable by cotrimoxazole). For severe ODs that are not preventable with cotrimoxazole prophylaxis, there was a consistent reduction in risk observed between the cotrimoxazole alone period and the HAART plus cotrimoxazole >6 months period across all three CD4 strata. Incidence rates of non-preventable severe ODs ranged from 3.4/100PM (95% CI 2.3–4.5) to 0.7/100PM (95% CI 0.5–0.8) for the cotrimoxazole alone period, from 5.0/100PM (95% CI 1.7–8.3) to 0.9/100PM (95% CI 0.5–1.3) for the HAART plus cotrimoxazole 0–6 months period, and from 0.7/100PM (95% CI −0.3–1.7) to 0.1/100PM (95% CI 0.07–0.2) for the HAART plus cotrimoxazole >6 months period. For severe ODs that are preventable with cotrimoxazole, incidences were generally much lower, ranging from 0.43/100PM (95% CI 0.2–0.6) to 0.35/100PM (95% CI 0.3–0.4) for the cotrimoxazole alone period, from 1.0/100PM (95% CI −0.13–2.13) to 0.5/100PM (95% CI 0.3–0.8) for the HAART plus cotrimoxazole 0–6 months period, and from 1.2/100PM (95% CI −0.2–2.5) to 0.3/100PM (95% CI 0.2–0.5) for the HAART plus cotrimoxazole >6 months period.
The independent effect of HAART
There was a CD4-independent reduction in risk of severe OD, death, or both, for patients on HAART compared to those on cotrimoxazole alone, adjusted for current CD4 count, WHO clinical stage at the time of treatment initiation, age, and gender (Table 3). This independent “HAART effect” was greater when patients were on HAART >6 months, and was greater for death than for severe ODs. Patients who had been on HAART for >6 months and for 0–6 months were 91% (RR 0.09, 95% CI 0.04–0.19) and 78% (RR 0.22, 95% CI 0.12–0.43) less likely to die than patients followed on cotrimoxazole alone. Patients who had been on HAART for >6 months and for 0–6 months were 28% (RR 0.72, 95% CI 0.49–1.26) and 21% (RR 0.79, 95% CI 0.53–0.98) less likely to experience severe ODs than patients followed on cotrimoxazole alone (Table 3).
Table 3.
Association between severe OD occurrence and/or mortality and treatment status: multivariate analysis
| Factor | Severe OD Relative Risk (95% CI) |
Mortality Relative Risk (95% CI) |
Severe OD or Mortality Relative Risk (95% CI) |
|---|---|---|---|
| CD4 stratum | |||
| ≤ 50/μl | 5.37 (3.87–7.43) | 5.33 (3.82–7.44) | 5.34 (4.10–6.96) |
| 51–200/μl | 2.42 (1.81–3.24) | 3.11 (2.34–4.14) | 2.68 (2.12–3.37) |
| >200/μl | 1.00 | 1.00 | 1.00 |
| WHO stage | |||
| 1,2 | 0.86 (0.61–1.21) | 0.64 (0.45–0.90) | 0.77 (0.58–1.02) |
| 3 | 0.96 (0.72–1.28) | 0.83 (0.62–1.10) | 0.91 (0.72–1.15) |
| 4 | 1.00 | 1.00 | 1.00 |
| Period of treatment | |||
| HAART + Cotrimoxazole | |||
| HAART >6 months | 0.72 (0.49–1.26) | 0.09 (0.04–0.19) | 0.40 (0.30–0.55) |
| HAART 0–6 months | 0.79 (0.53–0.98) | 0.22 (0.12–0.43) | 0.50 (0.35–0.73) |
| Cotrimoxazole alone | 1.00 | 1.00 | 1.00 |
OD: Opportunistic disease; HAART: Highly active antiretroviral therapy
CD4 counts in this analysis are current CD4 counts estimated as described in the Methods section. For each factor (CD4 stratum, WHO stage, Period of treatment), the risk ratios are adjusted for the other two factors as well as age and gender.
Interaction between CD4 and HAART effects
In the analysis of severe OD incidence, we did not find a significant interaction between the CD4 and HAART effects (p=0.11). In the analysis of the composite outcome of severe OD or death, there was a significant difference in the effect of HAART by the three different CD4 strata (p=0.02). While the effect of HAART >6 months after initiation was consistent across all CD4 strata, there was substantial variation in the reduction of ODs or death within the different CD4 count strata compared to cotrimoxazole during the first 6 months on therapy (Table 4). We were unable to perform the analysis for death alone due to a small number of cases.
Table 4.
Heterogeneity of the “HAART effect” by CD4 stratum
| Period of treatment | Model with interaction between CD4 and HAART effects | Model without interaction between CD4 and HAART effects | ||
|---|---|---|---|---|
| CD4 count stratum | Across all CD4 strata | |||
| ≤ 50/μl | 51–200/μl | > 200/μl | ||
| HAART + Cotrimoxazole | ||||
| HAART > 6 months | 0.36 (0.14–0.92) | 0.38 (0.25–0.58) | 0.43 (0.26–0.71) | 0.40 (0.30–0.55) |
| HAART 0–6 months | 0.94 (0.62–1.42) | 0.26 (0.13–0.51) | 0.71 (0.37–1.34) | 0.50 (0.35–0.73) |
| Cotrimoxazole | 1.00 | 1.00 | 1.00 | 1.00 |
HAART: Highly active antiretroviral therapy
Discussion
Using a well-described cohort with cumulative follow-up time of over 9,600 person-months, we found a CD4-independent additional beneficial effect of HAART compared to cotrimoxazole alone for HIV-infected adults in Côte d’Ivoire. After adjusting for current CD4 count, we estimated that HAART reduces the incidence of severe ODs by 21% during the first 6 months on HAART and 28% during subsequent follow-up on HAART. When mortality was added to the analysis, we estimated HAART reduces the incidence of a severe OD or death by 50% during the first 6 months on HAART and 60% during subsequent follow-up on HAART. The results also reveal that the risk reduction by HAART is not homogeneous across different CD4 count strata within the first 6 months on HAART.
These findings are consistent with previous studies from the United States and Europe. A similar effect was seen by Cole et al. in the Multicenter AIDS Cohort Study, McNaghten et al. in the Adult and Adolescent Spectrum of Disease Project, and Murphy et al. in the Viral Activation Transfusion Study, all in the US, as well as in Europe by Ledergerber et al. in the Swiss HIV Cohort Study, Mocroft et al. in the EuroSIDA Study, and Sabin in the Antiretroviral Therapy Cohort Collaboration [8, 12, 28–32]. Cole et al. examined the effect of HAART on the transition to AIDS or death, finding a significant reduction in this composite outcome, but they did not analyze OD events separately and did not stratify by time on HAART. McNaghten et al. and Mocroft et al. both found a significant reduction in mortality, but like Cole et al., they did not analyze OD events or stratify results by time on HAART. In contrast, the Swiss HIV Cohort Study showed a decrease in OD rates as a function of time on HAART, but did not include mortality, did not compare with a pre-HAART group, and did not provide an explicit stratification by CD4. The current study provided all three, most importantly the comparison to a pre-HAART period, allowing us to derive relative risk reductions from HAART use.
Sabin and the Antiretroviral Therapy Cohort Collaboration divided patient-time into two periods: 0–6 months and 7–12 months. They demonstrated that the greatest effect of HAART on the reduction of ODs occurred in the lowest CD4 stratum. We also observed the greatest effect of HAART in the lowest CD4 stratum. The Antiretroviral Therapy Cohort Collaboration studies did not provide a pre-HAART comparator group, as the cotrimoxazole alone group in our analysis did, and they were therefore unable to show the incremental effect of HAART over cotrimoxazole prophylaxis. Additionally, as the ODs were grouped by etiology, their event rates are not directly comparable to our overall event rates. However, the incidence of events in months 0–6 was significantly higher than the incidence of events in months 7–12. Similarly, the current study found a greater incremental benefit from HAART in the period >6 months after HAART initiation. We hypothesize that this trend is due to the occurrence of immune reconstitution inflammatory syndrome (IRIS) within the period of time directly following HAART initiation, leading to a higher incidence of OD events in the 0–6 months following HAART initiation.
In the current analysis, we found a generally greater risk reduction for death than for severe ODs, which is consistent with the results of Murphy et al., the only other group to examine the effect of HAART on both death and OD incidence [29]. However, they did not examine the composite outcome of OD or death, did not stratify results by current CD4 count, and did not stratify results by time. The greater effect on mortality seen in both studies may be due to the fact that many AIDS-related deaths occur from chronic causes, such as wasting and non-OD causes, and HAART may be more protective for those types of deaths than it is for ODs. This hypothesis is supported for wasting syndrome deaths, as pre-HAART body mass index has been repeatedly found to be associated with on-HAART mortality in sub-Saharan Africa [33–36].
To examine the effects of HAART and cotrimoxazole in patients receiving both treatments, we stratified ODs based on whether they were preventable with cotrimoxazole. Our data suggest that, in patients receiving both treatments, the effect of HAART was greater on the reduction of incidence of ODs that are not preventable with cotrimoxazole. However, since the study cohort used in this analysis provided follow-up time for both patients on cotrimoxazole alone and patients on HAART plus cotrimoxazole, and not follow-up time on HAART alone, we were unable to distinguish the benefit of cotrimoxazole from the benefit of HAART on reducing the incidence of ODs. This issue should be addressed by further studies.
This study has several limitations. First, due to limited statistical power, we were not able to establish a statistically significant interaction effect between the CD4 count and the HAART effect for severe ODs when they were not considered in combination with death. We were not able to establish any interaction effect for death events alone. Additionally, the numbers of each type of OD in our dataset were not sufficient to analyze the effect of HAART on individual ODs. Second, patients from the HAART plus cotrimoxazole group may have been more likely to have a history of prior OD at baseline than those from the cotrimoxazole alone group. Given that the incidence of severe OD recurrence may differ from the incidence of first occurrence, this may have biased the analysis for the association between OD incidence and treatment period.
However, rates of OD recurrence are likely to be higher than rates of first occurrence for most ODs [37, 38]. Therefore, this potential bias would have acted to mask an independent, protective effect of HAART, not to enhance it. Third, in the analyses on severe OD alone, follow-up was censored at the time of death for those patients who died without a severe morbid event, introducing the risk of informative censoring. However, this risk of selection bias does not exist for the analyses considering death alone and the composite outcome (severe OD or death). Since the analyses using each of the three outcomes gave similar results, we believe that the results showing the independent HAART effect on severe morbidity and/or mortality are robust. Fourth, CD4 count measurements were available every six months during the cotrimoxazole alone period and every three months during the HAART plus cotrimoxazole period. Results of a sensitivity analysis revealed that our findings were robust to the difference in CD4 count measurement frequency between the two groups. Due to the nature of data available at the time this analysis was conducted, there is almost twice as much follow-up time for the cotrimoxazole alone group, which is composed of many patients with short follow-up, while the HAART plus cotrimoxazole group is composed of fewer patients with longer follow-up. Finally, data on plasma HIV-1 RNA were not available from the cohort studies, so we were not able to adjust these analyses for viral load.
In conclusion, in this first study from sub-Saharan Africa to examine the association between HAART and severe morbidity or death, adjusted for current CD4 count, we found that HAART had an independent protective effect against both death and severe morbidity. This effect was greater after 6 months on HAART. If the effect observed in this analysis is also applicable in patients experiencing immunological or virological failure, there may be benefit gained from continuing an initial HAART regimen in the presence of observed failure in settings where additional HAART options are not available. Further studies are warranted to examine the HAART effect in patients experiencing failure. When considering only ODs preventable by cotrimoxazole, the HAART effect additional to cotrimoxazole was negligible. This highlights the specific importance of continued efforts to expand cotrimoxazole prophylaxis in sub-Saharan Africa.
Acknowledgments
Supported by the French Agence Nationale de Recherches sur le SIDA (ANRS 059, ANRS 1203), the French Ministry of Cooperation, and the US National Institute of Allergy and Infectious Diseases (R01 AI058736, K24 AI062476, K25 AI50436, CFAR P30 AI42851).
Footnotes
Author Contributions
EL, YY, KAF, and XA participated in the conception and design of the current study. EM, CS, and XA participated in the acquisition of data. All authors contributed to the analysis and interpretation of data. EL drafted the manuscript. All authors critically revised the manuscript for important intellectual content. EL, SDB, BW, and DG participated in the statistical analysis. EL, YY, KAF, and XA obtained funding. LLW provided administrative, technical, and material support. EL, YY, KAF, and XA supervised this work.
Ethics Approval
This work was approved by the Partners Human Research Committee of the Partners Healthcare System of Boston, MA, USA (Protocol #2003-P-01019/5), French Agence Nationale de Recherches sur le SIDA et les Hépatites (ANRS 1203), and the Comité National d’Ethique de Côte d’Ivoire.
Literature Cited
- 1.UNAIDS. Report on the Global AIDS Epidemic. [Accessed July 14, 2006]; Available at: http://www.unaids.org/en/HIV_data/2006GlobalReport/
- 2.Corbett EL, Churchyard GJ, Charalambos S, Samb B, Moloi V, Clayton TC, et al. Morbidity and mortality in South African gold miners: impact of untreated disease due to human immunodeficiency virus. Clin Infect Dis. 2002;34:1251–1258. doi: 10.1086/339540. [DOI] [PubMed] [Google Scholar]
- 3.Anglaret X, Chêne G, Attia A, Toure S, Lafont S, Combe P, et al. Early chemoprophylaxis with trimethoprim-sulphamethoxazole for HIV-1-infected adults in Abidjan, Cote d’Ivoire: a randomised trial. Cotrimo-CI Study Group. Lancet. 1999;353:1463–1468. doi: 10.1016/s0140-6736(98)07399-1. [DOI] [PubMed] [Google Scholar]
- 4.Anglaret X, Messou E, Ouassa T, Toure S, Dakoury-Dogbo N, Combe P, et al. Pattern of bacterial diseases in a cohort of HIV-1 infected adults receiving cotrimoxazole prophylaxis in Abidjan, Cote d’Ivoire. AIDS. 2003;17:575–584. doi: 10.1097/00002030-200303070-00013. [DOI] [PubMed] [Google Scholar]
- 5.Hammer SM, Squires KE, Hughes MD, Grimes JM, Demeter LM, Currier JS, et al. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. AIDS Clinical Trials Group 320 Study Team. N Engl J Med. 1997;337:725–733. doi: 10.1056/NEJM199709113371101. [DOI] [PubMed] [Google Scholar]
- 6.Palella FJ, Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 7.Hogg RS, Heath KV, Yip B, Craib KJ, O’Shaughnessy MV, Schechter MT, et al. Improved survival among HIV-infected individuals following initiation of antiretroviral therapy. JAMA. 1998;279:450–454. doi: 10.1001/jama.279.6.450. [DOI] [PubMed] [Google Scholar]
- 8.Mocroft A, Ledergerber B, Katlama C, Kirk O, Reiss P, d’Arminio Monforte A, et al. Decline in the AIDS and death rates in the EuroSIDA study: an observational study. Lancet. 2003;362:22–29. doi: 10.1016/s0140-6736(03)13802-0. [DOI] [PubMed] [Google Scholar]
- 9.Girardi E, Palmieri F, Cingolani A, Ammassari A, Petrosillo N, Gillini L, et al. Changing clinical presentation and survival in HIV-associated tuberculosis after highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2001;26:326–331. doi: 10.1097/00126334-200104010-00006. [DOI] [PubMed] [Google Scholar]
- 10.Miller V, Sabin CA, Phillips AN, Rottmann C, Rabenau H, Weidmann E, et al. The impact of protease inhibitor-containing highly active antiretroviral therapy on progression of HIV disease and its relationship to CD4 and viral load. AIDS. 2000;14:2129–2136. doi: 10.1097/00002030-200009290-00009. [DOI] [PubMed] [Google Scholar]
- 11.Jones JL, Hanson DL, Dworkin MS, DeCock KM. HIV-associated tuberculosis in the era of highly active antiretroviral therapy. The Adult/Adolescent Spectrum of HIV Disease Group. Int J Tuberc Lung Dis. 2000;4:1026–1031. [PubMed] [Google Scholar]
- 12.Cole SR, Hernan MA, Robins JM, Anastos K, Chmiel J, Detels R, et al. Effect of highly active antiretroviral therapy on time to acquired immunodeficiency syndrome or death using marginal structural models. Am J Epidemiol. 2003;158:687–694. doi: 10.1093/aje/kwg206. [DOI] [PubMed] [Google Scholar]
- 13.Barron Y, Cole SR, Greenblatt RM, Cohen MH, Anastos K, DeHovitz JA, et al. Effect of discontinuing antiretroviral therapy on survival of women initiated on highly active antiretroviral therapy. AIDS. 2004;18:1579–1584. doi: 10.1097/01.aids.0000131359.37210.1f. [DOI] [PubMed] [Google Scholar]
- 14.Gandhi T, Wei W, Amin K, Kazanjian P. Effect of maintaining highly active antiretroviral therapy on AIDS events among patients with late-stage HIV infection and inadequate response to therapy. Clin Infect Dis. 2006;42:878–884. doi: 10.1086/500210. [DOI] [PubMed] [Google Scholar]
- 15.Kaplan JE, Hu DJ, Holmes KK, Jaffe HW, Masur H, De Cock KM. Preventing opportunistic infections in human immunodeficiency virus-infected persons: implications for the developing world. Am J Trop Med Hyg. 1996;55:1–11. [PubMed] [Google Scholar]
- 16.Seyler C, Anglaret X, Dakoury-Dogbo N, Messou E, Toure S, Danel C, et al. Medium-term survival, morbidity and immunovirological evolution in HIV-infected adults receiving antiretroviral therapy, Abidjan, Cote d’Ivoire. Antivir Ther. 2003;8:385–393. [PubMed] [Google Scholar]
- 17.Holmes CB, Losina E, Walensky RP, Yazdanpanah Y, Freedberg KA. Review of human immunodeficiency virus type 1-related opportunistic infections in sub-Saharan Africa. Clin Infect Dis. 2003;36:652–662. doi: 10.1086/367655. [DOI] [PubMed] [Google Scholar]
- 18.Anglaret X, Toure S, Gourvellec G, Tchehy A, Zio L, Zaho M, et al. Impact of vital status investigation procedures on estimates of survival in cohorts of HIV-infected patients from Sub-Saharan Africa. J Acquir Immune Defic Syndr. 2004;35:320–323. doi: 10.1097/00126334-200403010-00015. [DOI] [PubMed] [Google Scholar]
- 19.Wiktor SZ, Sassan-Morokro M, Grant AD, Abouya L, Karon JM, Maurice C, et al. Efficacy of trimethoprim-sulphamethoxazole prophylaxis to decrease morbidity and mortality in HIV-1-infected patients with tuberculosis in Abidjan, Cote d’Ivoire: a randomised controlled trial. Lancet. 1999;353:1469–1475. doi: 10.1016/s0140-6736(99)03465-0. [DOI] [PubMed] [Google Scholar]
- 20.Currier JS, Williams P, Feinberg J, Becker S, Owens S, Fichtenbaum C, et al. Impact of prophylaxis for Mycobacterium avium complex on bacterial infections in patients with advanced human immunodeficiency virus disease. Clin Infect Dis. 2001;32:1615–1622. doi: 10.1086/320515. [DOI] [PubMed] [Google Scholar]
- 21.DiRienzo AG, van Der Horst C, Finkelstein DM, Frame P, Bozzette SA, Tashima KT. Efficacy of trimethoprim-sulfamethoxazole for the prevention of bacterial infections in a randomized prophylaxis trial of patients with advanced HIV infection. AIDS Res Hum Retroviruses. 2002;18:89–94. doi: 10.1089/08892220252779629. [DOI] [PubMed] [Google Scholar]
- 22.Dworkin MS, Ward JW, Hanson DL, Jones JL, Kaplan JE. Pneumococcal disease among human immunodeficiency virus-infected persons: incidence, risk factors, and impact of vaccination. Clin Infect Dis. 2001;32:794–800. doi: 10.1086/319218. [DOI] [PubMed] [Google Scholar]
- 23.WHO/Global Tuberculosis Program. Treatment of tuberculosis: guidelines for national programs. [Accessed January 22, 2007]; Available at: http://whqlibdoc.who.int/hq/2003/WHO_CDS_TB_2003.313_eng.pdf.
- 24.Egger M, May M, Chene G, Phillips AN, Ledergerber B, Dabis F, et al. Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. Lancet. 2002;360:119–129. doi: 10.1016/s0140-6736(02)09411-4. [DOI] [PubMed] [Google Scholar]
- 25.World Health Organization. World Health Organization. Antiretroviral Therapy for HIV Infection in Adults and Adolescents in Resource-limited Settings: Torwards Universal Access. Recommendations for a public health approach. [Accessed November 15, 2006]; Available at: http://www.who.int/hiv/pub/guidelines/arvguidelines2006.pdf.
- 26.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 27.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]
- 28.McNaghten AD, Hanson DL, Jones JL, Dworkin MS, Ward JW. Effects of antiretroviral therapy and opportunistic illness primary chemoprophylaxis on survival after AIDS diagnosis.Adult/Adolescent Spectrum of Disease Group. AIDS. 1999;13:1687–1695. doi: 10.1097/00002030-199909100-00012. [DOI] [PubMed] [Google Scholar]
- 29.Murphy EL, Collier AC, Kalish LA, Assmann SF, Para MF, Flanigan TP, et al. Highly active antiretroviral therapy decreases mortality and morbidity in patients with advanced HIV disease. Ann Intern Med. 2001;135:17–26. doi: 10.7326/0003-4819-135-1-200107030-00005. [DOI] [PubMed] [Google Scholar]
- 30.Ledergerber B, Egger M, Erard V, Weber R, Hirschel B, Furrer H, et al. AIDS-related opportunistic illnesses occurring after initiation of potent antiretroviral therapy: the Swiss HIV Cohort Study. JAMA. 1999;282:2220–2226. doi: 10.1001/jama.282.23.2220. [DOI] [PubMed] [Google Scholar]
- 31.Sabine C. AIDS events among individuals initiating HAART: do some patients experience a greater benefit from HAART than others? AIDS. 2005;19:1995–2000. doi: 10.1097/01.aids.0000189858.59559.d2. [DOI] [PubMed] [Google Scholar]
- 32.d’Arminio Monforte A, Sabin CA, Phillips A, Sterne J, May M, Justice A, et al. The changing incidence of AIDS events in patients receiving highly active antiretroviral therapy. Arch Intern Med. 2005;165:416–423. doi: 10.1001/archinte.165.4.416. [DOI] [PubMed] [Google Scholar]
- 33.Stringer JS, Zulu I, Levy J, Stringer EM, Mwango A, Chi BH, et al. Rapid scale-up of antiretroviral therapy at primary care sites in Zambia: feasibility and early outcomes. JAMA. 2006;296:782–793. doi: 10.1001/jama.296.7.782. [DOI] [PubMed] [Google Scholar]
- 34.Ferradini L, Jeannin A, Pinoges L, Izopet J, Odhiambo D, Mankhambo L, et al. Scaling up of highly active antiretroviral therapy in a rural district of Malawi: an effectiveness assessment. Lancet. 2006;367:1335–1342. doi: 10.1016/S0140-6736(06)68580-2. [DOI] [PubMed] [Google Scholar]
- 35.Etard JF, Ndiaye I, Thierry-Mieg M, Gueye NF, Gueye PM, Laniece I, et al. Mortality and causes of death in adults receiving highly active antiretroviral therapy in Senegal: a 7-year cohort study. AIDS. 2006;20:1181–1189. doi: 10.1097/01.aids.0000226959.87471.01. [DOI] [PubMed] [Google Scholar]
- 36.Zachariah R, Fitzgerald M, Massaquoi M, Pasulani O, Arnould L, Makombe S, et al. Risk factors for high early mortality in patients on antiretroviral treatment in a rural district of Malawi. AIDS. 2006;20:2355–2360. doi: 10.1097/QAD.0b013e32801086b0. [DOI] [PubMed] [Google Scholar]
- 37.Gilks CF, Ojoo SA, Ojoo JC, Brindle RJ, Paul J, Batchelor BI, et al. Invasive pneumococcal disease in a cohort of predominantly HIV-1 infected female sex-workers in Nairobi, Kenya. Lancet. 1996;347:718–723. doi: 10.1016/s0140-6736(96)90076-8. [DOI] [PubMed] [Google Scholar]
- 38.Gilks CF, Godfrey-Faussett P, Batchelor BI, Ojoo JC, Ojoo SJ, Brindle RJ, et al. Recent transmission of tuberculosis in a cohort of HIV-1-infected female sex workers in Nairobi, Kenya. AIDS. 1997;11:911–918. doi: 10.1097/00002030-199707000-00011. [DOI] [PubMed] [Google Scholar]