Abstract
Objectives
Resistance testing in HIV disease may provide long-term benefits that are not evident from short-term data. Our objectives were to estimate the long-term effectiveness, cost and cost-effectiveness of genotype testing in patients with extensive antiretroviral exposure.
Methods
We used an HIV simulation model to estimate the long-term effectiveness and cost-effectiveness of genotype testing. Clinical data incorporated into the model were from NARVAL, a randomized trial of resistance testing in patients with extensive antiretroviral exposure, and other randomized trials. Each simulated patient was eligible for up to three sequential regimens of antiretroviral therapy (i.e. two additional regimens beyond the trial-based regimen) using drugs not available at the time of the study, such as lopinavir/ritonavir, darunavir/ritonavir and enfuvirtide.
Results
In the long term, projected undiscounted life expectancy increased from 132.2 months with clinical judgement alone to 147.9 months with genotype testing. Median survival was estimated at 11.9 years in the resistance testing arm vs 10.4 years in the clinical judgement alone arm. Because of increased survival, the projected lifetime discounted cost of genotype testing was greater than for clinical judgement alone (€313 900 vs €263100; US$399 000 vs US$334 400). Genotype testing cost €69 600 (US$88 500) per quality-adjusted life year gained compared with clinical judgement alone.
Conclusions
In patients with extensive prior antiretroviral exposure, genotype testing is likely to increase life expectancy in the long term as a result of the increased likelihood of receiving two active new drugs. Genotype testing is associated with cost-effectiveness comparable to that of strategies accepted in patients with advanced HIV disease, such as enfuvirtide use.
Keywords: cost-effectiveness, costs, genotype testing, HIV infection, long-term effectiveness
Introduction
For many patients infected with HIV, antiretroviral therapy (ART) fails to result in complete viral suppression [1,2]. In HIV-infected patients failing therapy, several studies have compared virological response in patients given genotype resistance tests with virological response in patients whose therapy was guided by clinical judgement alone [3–6]. These studies indicate that short-term virological response to a new antiretroviral regimen can be improved when the results of resistance tests are available to guide drug choices [3–6]. As a result, current guidelines recommend the use of resistance testing in patients who are failing therapy [7,8].
However, a recent meta-analysis of randomized controlled trials (RCTs) comparing resistance testing and clinical judgement alone in guiding physicians’ choice of salvage regimens for treatment-experienced HIV-infected patients showed only a small virological benefit for genotype resistance testing over clinical judgement alone [9]. Furthermore, an RCT that evaluated resistance testing compared with clinical judgement alone in patients with extensive prior antiretroviral exposure did not demonstrate any clinical benefit of resistance testing over clinical judgement alone in the short term [10]. Consequently, it has been suggested that genotype resistance testing may be most useful for patients with limited antiretroviral exposure and few resistance mutations, and that further studies are needed to define the utility of these tests in patients who are highly drug-experienced [11].
Traditionally, the efficacy of resistance testing has been evaluated using short-term surrogate endpoints. However, use of these short-term surrogate markers fails to capture the HIV resistance ‘cost’ associated with antiretroviral therapy. Past RCTs evaluating resistance testing have shown that the number of both individual drugs and drug classes used by patients was greater when the regimens were chosen by clinical judgement alone [10,12]. Thus, subsequent therapy choices in these patients may be more difficult than in those for whom regimens were chosen using results of resistance testing. By preserving future drug options, resistance testing may provide a long-term benefit that is not evident from short-term data, especially in patients with extensive prior antiretroviral exposure.
Combining results from an RCT of resistance testing in treatment-experienced patients [10] with data from other trials assessing the efficacy of new antiretroviral drugs available since the end of the resistance testing trial, we projected the long-term clinical impact and cost-effectiveness of genotype testing in HIV-infected patients with extensive prior antiretroviral exposure.
Methods
Study design
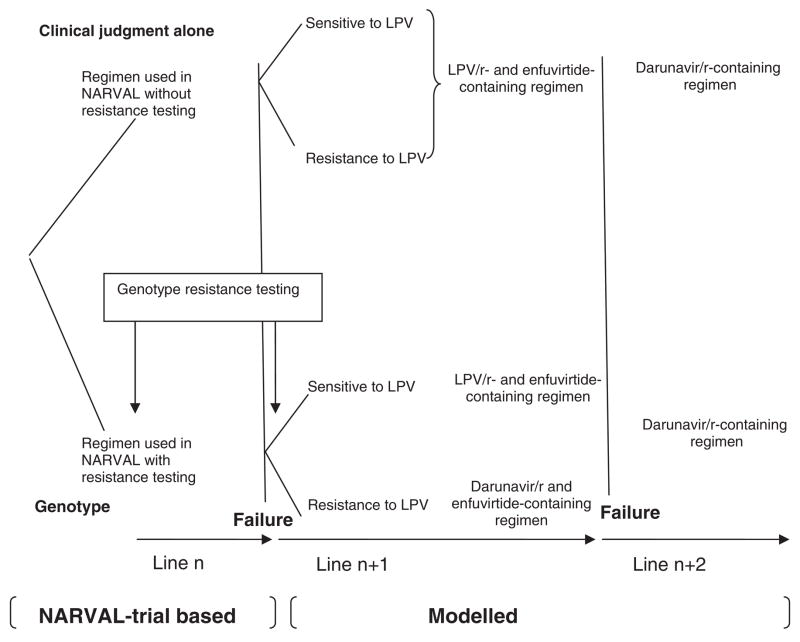
We used a previously described state-transition model of HIV disease [13–16] and a first-order Monte Carlo simulation to project patient outcomes beyond the endpoints of an RCT of resistance testing in patients with extensive prior antiretroviral exposure [10] to evaluate the long-term effectiveness and cost-effectiveness of genotype testing compared with clinical judgement alone. Each simulated patient was eligible for up to three sequential regimens of ART (i.e. two additional regimens beyond the regimen used in the trial) (Fig. 1). Model outcomes included life expectancy and median survival time. In addition, to estimate the cost-effectiveness of genotype testing, quality-adjusted life expectancy (QALE), lifetime costs and incremental cost-effectiveness ratios were also estimated. The incremental cost-effectiveness ratio is defined as the additional cost of a specific strategy compared with the next least expensive strategy, divided by its additional clinical benefit [17]. For the cost-effectiveness analysis, we adopted a modified societal perspective and discounted both costs and clinical benefits at 3% per year [17]. All costs were expressed in year 2006 Euros (€1 = US$1.27095; 29 September 2006).
Fig. 1.
Sequential regimens of highly active antiretroviral therapy (HAART) used in each modelled patient in the clinical judgement alone and genotype resistance arms. LPV, lopinavir; r, ritonavir.
Model structure
In the model, health states are defined according to current and maximum HIV RNA levels and CD4 cell counts and history of clinical events [13–15]. In the absence of acute illness, patients reside in a chronic health state and are subject to the risks governing the progression of HIV disease and deterioration of immune function. In the event of an acute clinical illness (e.g. opportunistic disease), patients enter a temporary acute health state. While in a temporary acute health state, patients may advance to a new chronic state which incorporates the history of the specific clinical event that occurred. Deaths occur in patients residing in either a chronic or an acute state.
HIV disease progression is linked to both CD4 cell count and HIV RNA level. In the absence of ART, the rate of CD4 decline over time is determined by each patient’s initial HIV RNA level or setpoint [18]. HIV-related morbidity and mortality are determined by the CD4 cell count [13,19]. Effective opportunistic disease prophylaxis results in a reduction in the incidence of the opportunistic disease against which it is instituted [20,21]. Effective ART results in HIV RNA suppression and a CD4 cell count increase at rates reported in the literature. ART, whether or not effective in suppressing HIV RNA, also decreases the probability of opportunistic diseases and AIDS-related death [10,22,23]. If ART fails, HIV RNA increases. Once HIV RNA returns to the setpoint, CD4 cell counts begin to decrease 12 months later [18].
Input data
Clinical data
Mean age, sex, initial median CD4 cell count and HIV RNA level were obtained from the NARVAL trial, details of which have been published elsewhere [10]. Briefly, patients failing ART with previous exposure to at least one protease inhibitor (PI) for at least 3 months were randomly assigned to one of three treatment arms: (1) treatment guided by phenotype testing, (2) treatment guided by genotype testing, or (3) treatment guided by clinical judgement alone. From April to October 1999, 541 patients were randomized, with 192 entering the genotype testing arm and 159 entering the clinical judgement alone arm. In this study, resistance assays did not demonstrate clinical benefit over clinical judgement alone in the short term.
French estimates of the monthly incidence of opportunistic diseases and death as a function of CD4 cell count in the absence of ART and opportunistic disease prophylaxis were derived using data obtained from two French clinical cohorts (Table 1) [24]. We used opportunistic disease prophylaxis strategies recommended in French national guidelines [25]. For each prophylaxis regimen, the efficacy in preventing opportunistic diseases and rates of toxic events were derived from published RCTs (Table 1) [20,21].
Table 1.
Base case values for model variables
| Opportunistic infection incidence rate (per 100 person-months) [24]
| ||||||
|---|---|---|---|---|---|---|
| CD4 cell count/mm3 | PCP | Toxo | CMV | MAC | Fungal | Other OD |
| Primary | ||||||
| >500 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.2 |
| 301–500 | 0.1 | 0.0 | 0.1 | 0.1 | 0.1 | 0.3 |
| 201–300 | 0.2 | 0.0 | 0.1 | 0.0 | 0.1 | 0.7 |
| 101–200 | 0.4 | 0.2 | 0.3 | 0.2 | 0.2 | 0.9 |
| 51–100 | 0.4 | 0.3 | 0.1 | 0.4 | 0.2 | 1.5 |
| 0–50 | 1.2 | 0.5 | 0.7 | 0.9 | 0.9 | 2.4 |
| Relapse | 0.9 | 2.0 | 3.4 | 3.0 | 0.7 | 2.0 |
| Efficacy of prophylaxis | Decrease in incidence of infection (%) |
|---|---|
| Pneumocystis carinii pneumonia (trimethoprim-sulfamethoxazole) [20] | 97.8 |
| Toxoplasmic encephalitis (trimethoprim-sulfamethoxazole) [20] | 65.0 |
| Mycobacterium avium complex bacteraemia (azithromycin) [21] | 63.4 |
| Efficacy of HAART | HIV RNA suppression (%) | Increase in CD4† cell count/mm3 |
|---|---|---|
| Initial regimen [10] | 38.5 at week 12 | 27 at week 12 |
| 1st subsequent regimen | ||
| Clinical judgement arm (LPV/r-enfuvirtide-containing regimen) | ||
| LPV/r susceptible [26] | 45.0 at week 48 | 127 at week 48 |
| LPV/r resistant [26] | 8.0 at week 48 | 57 at week 48 |
| Genotype resistance testing arm | ||
| LPV/r-enfuvirtide-containing regimen [26] | 45.0 at week 48 | 127 at week 48 |
| Darunavir/r-enfuvirtide-containing regimen [27] | 63.0 at week 48 | 102 at week 48 |
| 2nd subsequent regimen (Darunavir/r-containing regimen without enfuvirtide) | ||
| Clinical judgement arm | ||
| LPV/r susceptible after the initial regimen failure [26,30] | 26.0 at week 24 | 31 at week 48 |
| LPV/r resistant after the initial regimen failure (assumption) | 13.0 at week 24 | 31 at week 48 |
| Genotype resistance testing arm | ||
| LPV/r susceptible after the initial regimen failure [26,30] | 26.0 at week 24 | 31 at week 48 |
| LPV/r resistant after the initial regimen failure | No virological or immunological efficacy but clinical benefit | |
| Cost of care for patients with no history of AIDS [24] (per person-month) CD4 cell count/mm3 | Cost (2006 €)†† |
|---|---|
| >500 | 369 |
| 301–500 | 448 |
| 201–300 | 576 |
| 101–200 | 615 |
| 51–100 | 578 |
| 0–50 | 790 |
| Cost of care for patients with specific AIDS-defining events and history of AIDS-defining events (per person-month) (2006 €)†† | ||||||
|---|---|---|---|---|---|---|
| PCP | Toxo | CMV | MAC | Fungal | Other OD | |
| Specific events | 3851 | 4751 | 4872 | 3853 | 1631 | 2635 |
| History of events | 702 | 2555 | 1808 | 419 | 735 | 870 |
| Cost of care for patients during the one month prior to death | Cost (2006 €)†† |
|---|---|
| No history of an AIDS-defining event | 3942 |
| >30 days after an AIDS-defining event | 6772 |
| ≤ 30 days after an AIDS-defining event | 8571 |
| Cost per month of opportunistic infection prophylaxis, antiretroviral drugs, and cost of tests | Cost (2006 €) |
|---|---|
| Trimethoprim-sulfamethoxazole | 3 |
| Azithromycin | 33 |
| Initial antiretroviral regimen in genotype testing arm | 880 |
| Initial antiretroviral regimen in clinical judgement arm | 1040 |
| First subsequent antiretroviral regimen: LPV/r-enfuvirtide-containing♦ | 2534 |
| First subsequent antiretroviral regimen: Darunavir/r-enfuvirtide-containing♦ | 3108 |
| Second subsequent antiretroviral regimen | 1532 |
| CD4 cell count (per test) [51] | 11 |
| HIV RNA level (per test) [51] | 81 |
| Genotype test (per test) [51] | 297 |
| Drug level monitoring test (per test) [51] | 32 |
| Health-related quality adjustment scores in patients with no history of AIDS♣ | |
|---|---|
| CD4 cell count/mm3 | Score |
| >500 | 0.87 |
| 301–500 | 0.86 |
| 201–300 | 0.86 |
| 101–200 | 0.85 |
| 51–100 | 0.85 |
| 0–50 | 0.83 |
| Health-related quality of life adjustment scores in patients with AIDS♣ | Acute opportunistic infections | History of opportunistic infections |
|---|---|---|
| PCP | 0.74 | 0.78 |
| Toxo | 0.69 | 0.74 |
| MAC | 0.69 | 0.73 |
| Fungal infections | 0.78 | 0.76 |
| CMV | 0.78 | 0.74 |
| Other AIDS-defining events | 0.69 | 0.77 |
The CD4 increase was assumed to occur during the first 10 months of antiretroviral therapy with a rapid increase during the first 2 months (80% of the overall increase), and a slower increase during months 3–10 (20% of the overall increase). Details of these methods have been described elsewhere [14,15].
Excludes opportunistic infection prophylaxis, antiretroviral regimens, CD4 cell count and HIV RNA level test costs.
In the base case analysis, a CD4 cell count and an HIV RNA test were performed every 3 months. For the initial antiretroviral regimen, drugs dispensed in each trial arm were estimated from NARVAL. For the subsequent antiretroviral regimen, the cost of drugs dispensed was conservatively considered to be the same in the genotype testing and clinical judgement alone arms and equal to a weighted average of the NARVAL trial estimates. Data on the unit cost of opportunistic infection prophylaxis and antiretroviral drugs were derived from the Tourcoing Hospital pharmacy records.
Lopinavir/r-, enfuvirtide-; darunavir/r-, enfuvirtide-; and darunavir/r-containing regimens costs were estimated assuming that in addition to these drugs patients have a backbone of two nucleoside reverse transcriptase inhibitors. Darunavir/r does not yet have a cost in Europe; we therefore assumed the same cost as tipranavir, the last approved protease inhibitor in Europe (€34 per day).
The value of 1.0 is assigned to the optimal level of health-related quality of life and the value of 0.0 to death.
CMV, cytomegalovirus infection; Fungal, fungal infections (mainly Candida esophagitis); LPV/r, lopinavir/ritonavir; MAC, Mycobacterium avium complex bacteraemia; Other OD, including bacterial infections, tuberculosis, Kaposi sarcoma, and other AIDS-defining illnesses; PCP, Pneumocystis carinii pneumonia; Toxo, toxoplasmic encephalitis.
The initial ART regimen after model entry was assumed to be the regimen used in the NARVAL trial. In the trial, this regimen was based on a genotype resistance assay in patients enrolled in the genotype resistance testing arm, and not based on a resistance assay in those enrolled in the clinical judgement alone arm (Fig. 1) [10]. Virological success, CD4 cell count increase, and rates of severe toxic events (i.e. events requiring in-patient admission) for patients on ART were derived from the NARVAL trial. In the clinical judgement alone arm, the subsequent ART regimen (i.e. ‘second regimen’) was assumed to be a lopinavir/ritonavir- and enfuvirtide-containing regimen. This regimen was not based on a genotype resistance assay and was chosen because, upon trial enrolment, all patients in NARVAL were lopinavir/ritonavir and enfuvirtide naïve.
In the genotype resistance testing arm, we assumed that a second genotype resistance test was performed after the initial regimen failure. In this arm, the second ART regimen was assumed to be: (1) a lopinavir/ritonavir- and enfuvirtide-containing regimen in patients with strains susceptible to lopinavir/ritonavir; or (2) a darunavir/ritonavir- and enfuvirtide-containing regimen in patients with strains resistant to lopinavir/ritonavir. To estimate the efficacy of this regimen, we first determined the prevalence of lopinavir/ritonavir resistance at week 12 in patients enrolled in the NARVAL trial. Strains were assumed to be resistant to lopinavir/ritonavir if at least six of the following 13 protease mutations were present: L10F/I/R/V, K20M/R, L241, L33F, M46I/L, 150V, F53L, I54M/L/T/V, L63P, A71I/L/V/T, V82A/F/S/T, I84V and L90M (according to the French National Agency for AIDS Research genotype-resistance guidelines) [23]. The proportion of strains resistant to lopinavir/ritonavir was higher in the clinical judgement arm (50.7%) than in the genotype testing arm (41.9%) as a result of the accumulation of protease mutations in the clinical judgement arm, where patients resistant to PIs continued to receive these drugs. We then used data from the medical literature to estimate the virological and immunological success of a PI-boosted enfuvirtide regimen with respect to the susceptibility of strains for lopinavir/ritonavir, and overall for darunavir (Table 1) [26,27].
In both the clinical judgement alone and genotype resistance testing arms, the remaining subsequent ART regimen (‘third regimen’) was assumed to be a darunavir/ritonavir-containing regimen without enfuvirtide. For patients in both arms who were sensitive to lopinavir/ritonavir after the initial ART regimen failure and who had never received darunavir, we considered that probabilities of virological and immunological success for this third regimen were identical (Table 1). In patients in the clinical judgement alone arm who were resistant to lopinavir/ritonavir after the initial ART regimen failure but who had never received darunavir/ritonavir, we assumed that the probabilities of virological and immunological success for this third regimen were lower than the probabilities of success in previous patients. This assumption was based on the hypothesis that resistance mutations to PIs would accumulate because these patients were started on a regimen to which they were resistant (i.e. patients received lopinavir/ritonavir although resistant to lopinavir/ritonavir). In patients in the genotype testing arm who were resistant to lopinavir/ritonavir after initial ART regimen failure and who had already received darunavir/ritonavir, we assumed that the antiretroviral regimen had a potential clinical benefit, but not a virological or immunological benefit [28,29]. In general, because of the potential clinical benefit of remaining on ART despite virological rebound, we assumed continuation of the third regimen even after virological rebound occurred [28]. The efficacy of the third regimen was estimated using data from the medical literature or was based on assumptions when data were not available (Table 1) [26,30].
For enfuvirtide-containing regimens, toxic event rates were from the TORO studies, which reported higher probabilities of minor toxicity with enfuvirtide-containing regimens than with other ART regimens as a consequence of enfuvirtide injection site reactions [31]. For other subsequent regimens, toxic event rates were estimated to be equal to the weighted average of the rates of toxic events in both arms of the NARVAL trial. For the initial and subsequent regimens, the duration of ART benefit beyond the clinical trial endpoint was extrapolated from a matrix derived from trial-based efficacy data [13–15]. We assumed that all patients receiving ART would experience failure of their current regimen after 120 months [32–34].
Cost and health-related quality-of-life data
Direct costs of treatment for opportunistic diseases and routine medical care in the absence of an opportunistic disease were based on data from a previously described French clinical cohort (Table 1) [24]. For the initial ART regimen after study enrolment, the costs of drugs, drug level monitoring tests and toxic events in each arm were estimated from the NARVAL trial. These costs demonstrate a higher mean cost per patient per month in the clinical judgement alone arm than in the genotype testing arm. This was attributable primarily to higher expenditures on antiretroviral medications in the clinical judgement alone arm (€1040 vs €880 per month; P = 0.0001; Table 1) [35]. For subsequent ART regimens, drug costs were from the pharmacy records of Tourcoing Hospital in France. The cost of toxic events was conservatively considered to be the same in the genotype testing and clinical judgement alone arms, and equal to a weighted average of estimates from the NARVAL trial.
Morbidity was incorporated in a single outcome measure which adjusted life expectancy for quality of life [14,15,17]. Health-related quality weights for different HIV-related health states were obtained from the HIV Cost and Services Utilization Study as previously described [36–38].
Sensitivity analysis
We specifically evaluated the implications of alternative assumptions in areas where we lacked primary data. We explored the impact of varying the prevalence of resistance to lopinavir/ritonavir between the genotype testing and clinical judgement alone arms after initial ART regimen failure. In addition, in patients with strains resistant to lopinavir/ritonavir, we varied the efficacy of the subsequent ART regimens consisting of darunavir/ritonavir with and without enfuvirtide.
We also evaluated the implication of several pessimistic scenarios regarding genotype resistance testing. For example, we considered the possibility that information from earlier genotype tests performed may be available for patients in the clinical judgement only arm, allowing a proportion of these patients with strains resistant to lopinavir/ritonavir to receive a darunavir/ritonavir- and enfuvirtide-containing regimen, rather than a lopinavir/ritonavir- and enfuvirtide-containing regimen. In another sensitivity analysis, we also evaluated the impact of the availability of a new class of HIV antiretroviral drugs, the integrase inhibitors (i.e. MK-0518), on the results [39].
In addition, we performed sensitivity analyses on other model input parameters, including genotype testing costs, darunavir costs, enfuvirtide costs, costs of subsequent antiretroviral regimens in the clinical judgement alone arm, health-related quality-of-life weights, and the discount rate.
Results
Long-term clinical impact of genotype testing
In the long term, mean projected undiscounted life expectancy increased from 132.2 months (108.7 months discounted) with clinical judgement alone to 147.9 months with genotype testing (119.1 months discounted) (Fig. 2). The survival curve highlights the increased proportion of patients surviving in the genotype resistance testing arm 10–15 years after enrolment. Median undiscounted survival was estimated at 125.0 months in the clinical judgement alone arm and 143.0 months with genotype testing.
Fig. 2.
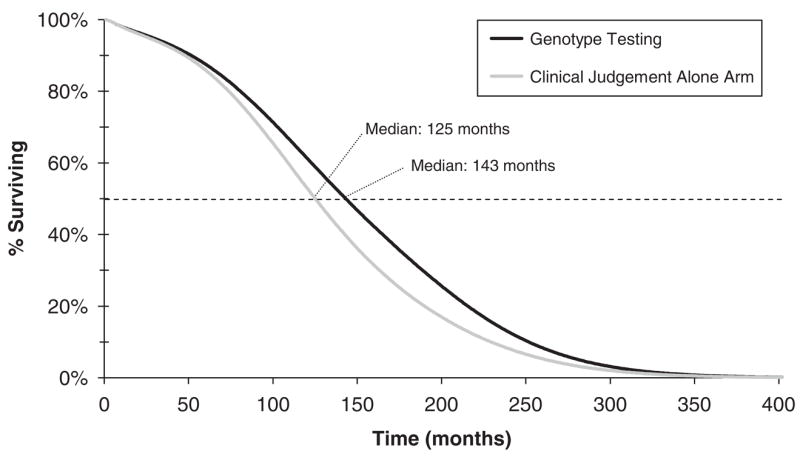
Model-based survival curves for the simulated patient cohort in the clinical judgement alone and genotype resistance testing arms.
In sensitivity analysis, when we removed the benefit of genotype testing in reducing the occurrence of lopinavir/ritonavir resistance in subsequent ART regimens (Table 2), we still found that genotype resistance testing increased discounted life expectancy by 10.3 months (15.4 months undiscounted). When we considered that up to 50% of patients in the clinical judgement alone arm with strains resistant to lopinavir/ritonavir received a darunavir/ritonavir- and enfuvirtide-containing regimen after the first virological failure, genotype resistance testing still increased discounted life expectancy by 3.5 months (Table 2). When we increased by 50% the virological efficacy of the second-line darunavir/ritonavir- and enfuvirtide-containing regimen in patients in the genotype testing arm who were resistant to lopinavir/ritonavir after the initial ART regimen, discounted gains in life expectancy attributable to genotype testing increased by 18.3 months. Availability of the integrase inhibitors during follow-up increased discounted gains in life expectancy in both the clinical judgement alone arm (from 108.7 to 134.5 months) and the genotype testing arm (from 119.1 to 142.6 months). The relative benefit of genotype resistance testing vs clinical judgement alone was not sensitive to the availability of these drugs.
Table 2.
Sensitivity analysis of potentially important model variables on the long-term effectiveness of genotype testing vs clinical judgement alone*
| Increase in Life Expectancy (months) | |
|---|---|
| Resistance to LPV/r after the initial regimen failure in genotype testing arm (vs 49.3% in the clinical judgement only arm) | |
| 49.3% | 10.3 |
| 41.9% (base case) | 10.5 |
| Efficacy of darunavir/r-containing regimen without enfuvirtide in patients with resistant strains to LPV/r in the clinical judgement alone arm | |
| 13% suppression, week 24 (base case) | 10.5 |
| 16% suppression, week 24 | 10.3 |
| 20% suppression, week 24 | 9.9 |
| Efficacy of darunavir/r- and enfuvirtide-containing regimen used in patients in genotype testing arm | |
| 63% suppression at week 48 (base case) | 10.5 |
| 78% suppression at week 48 | 14.2 |
| 95% suppression at week 48 | 18.3 |
| Proportion of patients with resistant strains to LPV/r in the clinical judgement alone arm who received a darunavir/r – and enfuvirtide-containing regimen after the first virological failure† | |
| 0% (base case) | 10.5 |
| 30% | 6.3 |
| 50% | 3.5 |
| A new class of HIV antiviral drugs available†† | |
| No (base case) | 10.5 |
| Yes, MK-0518 | 8.1 |
Life expectancies reported in this table are discounted.
Based on information from genotype resistance tests performed during earlier failures.
For this analysis, we hypothesized that MK-0518 would be available after the second antiretroviral regimen failure. In the clinical judgement alone arm and in patients in the genotype resistance testing arm who where sensitive to lopinavir/ritonavir (LPV/r) after the initial antiretroviral therapy (ART) regimen failure, the third ART regimen was assumed to be a darunavir/ritonavir MK-0518-containing regimen. In patients in the genotype resistance testing arm who were resistant to lopinavir/ritonavir after the initial ART regimen failure, the third ART regimen was assumed to be a MK-0518-containing regimen without darunavir. Efficacy data on a MK-0518-containing regimen were from the interim study results of a phase 2b, multicentre, randomized, double-blind, dose-ranging, placebo-controlled study that compared MK-0518 plus optimized background therapy (OBT) to placebo plus OBT in experienced patients [39]. The cost of MK-0518 was considered to be the same as that of darunavir.
Cost-effectiveness of genotype testing
In the base case analysis, discounted lifetime costs increased from €263100 with clinical judgement alone to €313 900 with genotype testing. After discounting costs and health effects and adjusting for health-related quality of life, the incremental cost-effectiveness of genotype testing was €69 600 per quality-adjusted life-year (QALY) gained compared with clinical judgement alone.
In the sensitivity analyses, the cost-effectiveness of genotype resistance testing compared with clinical judgement alone was not sensitive to: (1) the prevalence of resistance to lopinavir/ritonavir after initial ART regimen failure in the genotype testing and clinical judgement alone arms; (2) the virological efficacy of the second-line darunavir/r- and enfuvirtide-containing regimen; or (3) the proportion of patients with strains resistant to lopinavir/ritonavir in the clinical judgement alone arm who received a darunavir/ritonavir- and enfuvirtide-containing regimen (Table 3). Although the life expectancy and total medical costs were dependent on these variables, the cost-effectiveness ratios were not.
Table 3.
Sensitivity analysis on the cost, effectiveness and cost-effectiveness of genotype resistance testing compared with clinical judgement alone*
| Increase in Lifetime Costs (€) | Increase in QALE (months) | C/E ratio (€/QALY) | |
|---|---|---|---|
| Resistance to LPV/r after the initial regimen failure in the genotype testing arm (vs 49.3% in the clinical judgement only arm) | |||
| 49.3% | 53 530 | 8.6 | 74 700 |
| 41.9% (base case) | 50 802 | 8.8 | 69 600 |
| Efficacy of darunavir/r-containing regimen without enfuvirtide in patients with resistant strains to LPV/r in the clinical judgement alone arm. | |||
| 13% suppression, week 24 (base case) | 50 802 | 8.8 | 69 600 |
| 16% suppression, week 24 | 50 399 | 8.6 | 70 300 |
| 20% suppression, week 24 | 49 669 | 8.3 | 71 600 |
| Efficacy of darunavir/r- and enfuvirtide-containing regimen used in patients in genotype testing arm | |||
| 63% suppression at week 48 (base case) | 50 802 | 8.8 | 69 600 |
| 78% suppression at week 48 | 68 020 | 11.9 | 68 800 |
| 95% suppression at week 48 | 86 938 | 15.3 | 68 300 |
| Proportion of patients with resistant strains to LPV/r in the clinical judgement alone arm who received a darunavir/r- and enfuvirtide-containing regimen after the first virological failure† | |||
| 0% (base case) | 50 802 | 8.8 | 69 600 |
| 30% | 32 144 | 5.3 | 73 000 |
| 50% | 19 706 | 3.0 | 80 000 |
| A new class of HIV antiviral drugs available†† | |||
| No (base case) | 50 802 | 8.8 | 69 600 |
| Yes, MK-0518 | 45 783 | 6.7 | 81 500 |
| Genotype test cost | |||
| 297 € (base case) | 50 802 | 8.8 | 69 600 |
| 200 € | 50 571 | 8.8 | 69 200 |
| 100 € | 50 333 | 8.8 | 68 900 |
| Darunavir/r cost | |||
| 34 € per day (base case) | 50 802 | 8.8 | 69 600 |
| 21 € per day | 45 504 | 8.8 | 62 300 |
| 15 € per day ( = LPV/r cost) | 43 500 | 8.8 | 59 600 |
| Enfuvirtide cost | |||
| 52 € per day (base case) | 50 802 | 8.8 | 69 600 |
| 31 € per day | 50 713 | 8.8 | 69 400 |
| 21 € per day | 45 016 | 8.8 | 61 600 |
| Costs of subsequent antiretroviral regimens in the clinical judgement alone arm | |||
| = costs of that regimen in the genotype resistance testing arm (base case) | 50 802 | 8.8 | 69 600 |
| A 15% increase in costs of that regimen in the genotype resistance testing arm§ | 25 766 | 8.8 | 35 300 |
Cost and QALE reported in this table are discounted.
Based on information from genotype resistance tests performed during earlier failures.
For this analysis, we hypothesized that MK-0518 would be available after the second antiretroviral regimen failure. In the clinical judgement alone arm and in patients in the genotype resistance testing arm who where sensitive to lopinavir/ritonavir (LPV/r) after the initial antiretroviral therapy (ART) regimen failure, the third ART regimen was assumed to be a darunavir/ritonavir MK-0518-containing regimen. In patients in the genotype resistance testing arm who were resistant to lopinavir/ritonavir after the initial ART regimen failure, the third ART regimen was assumed to be a MK-0518-containing regimen without darunavir. Efficacy data on a MK-0518-containing regimen were from the interim study results of a phase 2b, multicentre, randomized, double-blind, dose-ranging, placebo-controlled study that compared MK-0518 plus optimized background therapy (OBT) to placebo plus OBT in experienced patients [39]. The cost of MK-0518 was considered to be the same as that of darunavir.
The 15% increase in costs for the antiretroviral regimen in the clinical judgement alone arm compared with the genotype resistance arm was proposed based on the observed higher expenditures on antiretroviral medications in the clinical judgement alone arm in NARVAL.
C/E, cost-effectiveness; LPV/r, lopinavir/ritonavir; QALE, quality-adjusted life expectancy; QALY, quality-adjusted life year.
Results were also not sensitive to genotype testing costs (Table 3). When the darunavir/ritonavir daily cost was reduced from €34 to €15 (i.e. the daily cost of lopinavir/ritonavir), the cost-effectiveness of genotype testing vs clinical judgement alone decreased to €59 600/QALY gained. However, the results were highly sensitive to drug costs in the clinical judgement alone arm. In the NARVAL trial, the cost of drugs and of toxic events were approximately 15% higher in the clinical judgement alone arm than in the genotype testing arm. In this analysis, we conservatively considered that both toxic event costs and the costs of the backbone regimen associated with lopinavir/ritonavir, darunavir/ritonavir and enfuvirtide would be the same in the genotype testing and clinical judgement alone arms, and equal to the weighted average of estimates from the NARVAL trial. In sensitivity analysis, a 15% increase in the cost of drugs for the second- and third-line regimens in the clinical judgement arm yielded an incremental cost-effectiveness ratio of €35 300/QALY gained for genotype testing compared with clinical judgement alone.
Discussion
Most studies of genotype testing have examined short-term efficacy and resistance patterns after the test [3–6,10]. We sought to understand the likely long-term outcomes related to genotype testing in patients with advanced HIV disease, basing our analysis on the NARVAL trial [10]. We used a published simulation model and found that genotype testing is likely to increase discounted life expectancy by approximately 11 months in patients with extensive prior antiretroviral exposure in the long term, a finding that the short-term trial was not designed to evaluate (16 months undiscounted).
The long-term benefits of genotype testing may be attributable to a number of factors. First, patients in the genotype testing arm were more likely than those in the clinical judgement alone arm to receive two active new drugs in their treatment regimens. Among those enrolled in NARVAL, a high proportion of patients were resistant to lopinavir/ritonavir after the first antiretroviral regimen failure despite not having received this drug previously. As a result, in our analysis, when genotype resistance testing was not performed, resistance to lopinavir/ritonavir was not detected and these patients were given a regimen containing lopinavir/ritonavir plus only one active drug (i.e. enfuvirtide).
Previous studies have shown that adding new drugs to salvage regimens in antiretroviral-experienced patients can improve and sustain HIV RNA suppression, emphasizing that the use of two or more active drugs in treatment regimens is more likely to achieve and maintain a virological response [40–42]. The results of this analysis are consistent with those reports. Even when we considered that some resistance information from previous failures may be available in the clinical judgement alone arm, genotype resistance testing at late failure still increased life expectancy. In this analysis, we also demonstrated that the upfront use of two active drugs in a treatment regimen is associated with a better long-term efficacy than use of a single active drug sequentially in two subsequent regimens.
Our results on the long-term benefit of genotype testing are also related to the HIV-resistance ‘cost’ associated with ART. In the NARVAL trial, lopinavir/ritonavir-resistant strains were less frequent in the genotype testing arm at week 12 compared with the clinical judgement alone arm, despite patients’ inexperience with this drug. Development of drug resistance mutations in failing regimens has been shown to be time dependent, especially for nucleoside reverse transcriptase inhibitors and PIs [43,44]. Even in patients with extensive prior antiretroviral exposure with a lack of fully active agents, genotype resistance testing may be used for choosing drugs in subsequent regimens to avoid additional accumulation of resistance mutations and thus prevent the development of high-level class resistance. This is particularly important because of the ongoing risk of accumulating additional resistance mutations [43,44]. Increases in the risk of cross-resistance may decrease the effectiveness of experimental drugs under development, therefore jeopardizing future treatment options [45,46].
In this study, we found incremental cost-effectiveness ratios for genotype testing (€69 600/QALY gained) that were higher than those previously reported in both the USA and Europe [14,47]. Studies using data from other RCTs have reported incremental cost-effectiveness ratios for genotype testing after failure of ART to be US$22 800/QALY gained (year 2006 US$; €17 900 year 2006 euros) in the USA [14], and €25 000/year of life saved in Germany (year 2006 euros) [47]. However, unlike these studies, the current study was conducted in patients with extensive prior antiretroviral exposure and advanced HIV disease for whom the background cost of care, most notably drug costs, contributes to the higher cost-effectiveness ratios. The impact of high drug costs is illustrated in the sensitivity analysis, which found that the cost-effectiveness of genotype testing is not sensitive to genotype test costs, but is more sensitive to drug costs. The cost-effectiveness ratios decrease when drug costs, in particular those of enfuvirtide and darunavir/ritonavir, decrease. The cost-effectiveness ratio for genotype testing in this study was similar to the incremental cost-effectiveness ratio reported for enfuvirtide use in treatment-experienced patients (€62 800/QALY gained; US$79 800 in 2006, compared with an optimized background regimen), a recommended treatment strategy in France and the USA for patients with advanced HIV disease [31].
There are several limitations to this analysis. First, to estimate the long-term benefit of genotype testing, we used a simulation model of HIV disease that combines input data from multiple sources and relies on several assumptions. For example, when modelling ART efficacy for initial and subsequent regimens, long-term outcomes were extrapolated from short-term studies. Data on the efficacy of subsequent regimens were from subanalyses of RCTs with large confidence intervals surrounding the point estimates [22,23]. Uncertainties regarding the modelling of ART efficacy were, however, considered in sensitivity analyses. Even with pessimistic assumptions regarding the benefits of genotype testing, the results were stable with respect to the long-term clinical benefits of genotype testing.
Salvage ART regimens in HIV-infected patients with prior antiretroviral exposure have lower success rates and are more expensive than early regimens [48–50]. In this analysis, we demonstrated that, in treatment-experienced patients, genotype testing is likely to effectively guide the choice of subsequent therapy in the long term. Substantial gains in life expectancy as a result of genotype testing relate to the fact that patients with extensive prior ART exposure were more likely to receive two active new drugs in their treatment regimens. In addition, the cost-effectiveness of genotype testing compared with the use of clinical judgement alone is commensurate with other accepted strategies in the care of patients with advanced HIV disease. In heavily experienced HIV-infected patients, the inclusion of genotype testing in HIV management guidelines should be strongly encouraged.
Acknowledgments
We acknowledge the assistance of Francoise Brun-Vezinet, MD, PhD and Dominique Costagliola, PhD for suggestions on the analysis. We are also indebted to April D. Kimmel, MS, A. David Paltiel, PhD and George R. Seage III, ScD for their valuable suggestions during the development of the model and for helpful comments on the analysis. This study was supported by grants from the French Agence National de Recherches sur le SIDA (ANRS 088), the US National Institute of Allergy and Infectious Diseases (NIAID AI42006, K23 AI0794, K24 AI062476, K25 AI50436 and CFAR P30 AI42851), the US Centers for Disease Control and Prevention (Cooperative Agreements U64/CCU 114927 and U64/CCU 119525), and the Doris Duke Charitable Foundation Clinical Scientist Development Award to RPW.
References
- 1.Lucas GM, Chaisson RE, Moore RD. Highly active antiretroviral therapy in a large urban clinic: risk factors for virologic failure and adverse drug reactions. Ann Intern Med. 1999;131:81–87. doi: 10.7326/0003-4819-131-2-199907200-00002. [DOI] [PubMed] [Google Scholar]
- 2.Ledergerber B, Egger M, Opravil M, et al. Clinical progression and virological failure on highly active antiretroviral therapy in HIV-1 patients: a prospective cohort study. Swiss HIV Cohort Study. Lancet. 1999;353:863–868. doi: 10.1016/s0140-6736(99)01122-8. [DOI] [PubMed] [Google Scholar]
- 3.Baxter JD, Mayers DL, Wentworth DN, et al. A randomized study of antiretroviral management based on plasma genotypic antiretroviral resistance testing in patients failing therapy. CPCRA 046 Study Team for the Terry Beirn Community Programs for Clinical Research on AIDS. AIDS. 2000;14:F83–F93. doi: 10.1097/00002030-200006160-00001. [DOI] [PubMed] [Google Scholar]
- 4.Durant J, Clevenbergh P, Halfon P, et al. Drug-resistance genotyping in HIV-1 therapy: the VIRADAPT randomised controlled trial. Lancet. 1999;353:2195–2199. doi: 10.1016/s0140-6736(98)12291-2. [DOI] [PubMed] [Google Scholar]
- 5.Tural C, Ruiz L, Holtzer C, et al. Clinical utility of HIV-1 genotyping and expert advice: the Havana trial. AIDS. 2002;16:209–218. doi: 10.1097/00002030-200201250-00010. [DOI] [PubMed] [Google Scholar]
- 6.Cingolani A, Antinori A, Rizzo MG, et al. Usefulness of monitoring HIV drug resistance and adherence in individuals failing highly active antiretroviral therapy: a randomized study (ARGENTA) AIDS. 2002;16:369–379. doi: 10.1097/00002030-200202150-00008. [DOI] [PubMed] [Google Scholar]
- 7.Hirsch MS, Brun-Vezinet F, Clotet B, et al. Antiretroviral drug resistance testing in adults infected with human immunodeficiency virus type 1: 2003 recommendations of an International AIDS Society-USA Panel. Clin Infect Dis. 2003;37:113–128. doi: 10.1086/375597. [DOI] [PubMed] [Google Scholar]
- 8.Yeni PG, Hammer SM, Carpenter CC, et al. Antiretroviral treatment for adult HIV infection in 2002: updated recommendations of the International AIDS Society-USA Panel. J Am Med Assoc. 2002;288:222–235. doi: 10.1001/jama.288.2.222. [DOI] [PubMed] [Google Scholar]
- 9.Panidou ET, Trikalinos TA, Ioannidis JP. Limited benefit of antiretroviral resistance testing in treatment-experienced patients: a meta-analysis. AIDS. 2004;18:2153–2161. doi: 10.1097/00002030-200411050-00007. [DOI] [PubMed] [Google Scholar]
- 10.Meynard JL, Vray M, Morand-Joubert L, et al. Phenotypic or genotypic resistance testing for choosing antiretroviral therapy after treatment failure: a randomized trial. AIDS. 2002;16:727–736. doi: 10.1097/00002030-200203290-00008. [DOI] [PubMed] [Google Scholar]
- 11.Badri SM, Adeyemi OM, Max BE, Barker DE. Response to ‘limited benefit of antiretroviral resistance testing in treatment-experienced patients: a meta-analysis’. AIDS. 2005;19:1241–1242. doi: 10.1097/01.aids.0000176232.24652.d9. [DOI] [PubMed] [Google Scholar]
- 12.Chaix C, Grenier-Sennelier C, Clevenbergh P, et al. Economic evaluation of drug resistance genotyping for the adaptation of treatment in HIV-infected patients in the VIRADAPT study. J Acquir Immune Defic Syndr. 2000;24:227–231. doi: 10.1097/00126334-200007010-00005. [DOI] [PubMed] [Google Scholar]
- 13.Yazdanpanah Y, Goldie SJ, Losina E, et al. Lifetime cost of HIV care in France during the era of highly active antiretroviral therapy. Antiviral Ther. 2002;7:257–266. [PubMed] [Google Scholar]
- 14.Weinstein MC, Goldie SJ, Losina E, et al. Use of genotypic resistance testing to guide HIV therapy: clinical impact and cost-effectiveness. Ann Intern Med. 2001;134:440–450. doi: 10.7326/0003-4819-134-6-200103200-00008. [DOI] [PubMed] [Google Scholar]
- 15.Freedberg KA, Losina E, Weinstein MC, et al. The cost effectiveness of combination antiretroviral therapy for HIV disease. N Engl J Med. 2001;344:824–831. doi: 10.1056/NEJM200103153441108. [DOI] [PubMed] [Google Scholar]
- 16.Doubilet P, Begg CB, Weinstein MC, Braun P, McNeil BJ. Probabilistic sensitivity analysis using Monte Carlo simulation. A practical approach. Med Decis Making. 1985;5:157–177. doi: 10.1177/0272989X8500500205. [DOI] [PubMed] [Google Scholar]
- 17.Gold R, Siegel J, Russell L, Weinstein M. Cost-Effectiveness in Health and Medicine. New York, NY: Oxford University Press; 1996. [Google Scholar]
- 18.Mellors JW, Munoz A, Giorgi JV, et al. Plasma viral load and CD4 + lymphocytes as prognostic markers of HIV-1 infection. Ann Intern Med. 1997;126:946–954. doi: 10.7326/0003-4819-126-12-199706150-00003. [DOI] [PubMed] [Google Scholar]
- 19.Yazdanpanah Y, Chene G, Losina E, et al. Incidence of primary opportunistic infections in two human immunodeficiency virus-infected French clinical cohorts. Int J Epidemiol. 2001;30:864–871. doi: 10.1093/ije/30.4.864. [DOI] [PubMed] [Google Scholar]
- 20.Ioannidis JP, Cappelleri JC, Skolnik PR, Lau J, Sacks HS. A meta-analysis of the relative efficacy and toxicity of Pneumocystis carinii prophylactic regimens. Arch Intern Med. 1996;156:177–188. [PubMed] [Google Scholar]
- 21.Havlir DV, Dube MP, Sattler FR, et al. Prophylaxis against disseminated Mycobacterium avium complex with weekly azithromycin, daily rifabutin, or both. California Collaborative Treatment Group. N Engl J Med. 1996;335:392–398. doi: 10.1056/NEJM199608083350604. [DOI] [PubMed] [Google Scholar]
- 22.Kempf DJ, Isaacson JD, King MS, et al. Analysis of the virological response with respect to baseline viral phenotype and genotype in protease inhibitor-experienced HIV-1-infected patients receiving lopinavir/ritonavir therapy. Antiviral Ther. 2002;7:165–174. [PubMed] [Google Scholar]
- 23.Masquelier B, Breilh D, Neau D, et al. Human immunodeficiency virus type 1 genotypic and pharmacokinetic determinants of the virological response to lopinavir-ritonavir-containing therapy in protease inhibitor-experienced patients. Antimicrob Agents Chemother. 2002;46:2926–2932. doi: 10.1128/AAC.46.9.2926-2932.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yazdanpanah Y, Goldie SJ, Paltiel AD, et al. Prevention of human immunodeficiency virus-related opportunistic infections in France: a cost-effectiveness analysis. Clin Infect Dis. 2003;36:86–96. doi: 10.1086/344902. [DOI] [PubMed] [Google Scholar]
- 25.Delfraissy JF, editor. Prise en Charge Thérapeutique des Personnes Infectées par le VIH. Rapport 2000. Paris, France: Flammarion, Médecine-Sciences, Ministère des Affaires Sociales, Secrétariat d’Etat à la Santé et à la Sécurité Sociale, 2000.; [Google Scholar]
- 26.Nelson M, Arasteh K, Clotet B, et al. Durable efficacy of enfuvirtide over 48 weeks in heavily treatment-experienced HIV-1-infected patients in the T-20 versus optimized background regimen only 1 and 2 clinical trials. J Acquir Immune Defic Syndr. 2005;40:404–412. doi: 10.1097/01.qai.0000185314.56556.c3. [DOI] [PubMed] [Google Scholar]
- 27.Lazzarin A, Queiroz-Telles F, Frank I, et al. TMC 114 provides durable viral load suppression in treatment-experienced patients: POWER 1 and 2 combined week 48 analysis. 16th International AIDS Conference; Toronto, Canada. August 2006; Abstract TUAB0104. [Google Scholar]
- 28.Miller V, Sabin CA, Phillips AN, et al. The impact of protease inhibitor-containing highly active antiretroviral therapy on progression of HIV disease and its relationship to CD4 and viral load. AIDS. 2000;14:2129–2136. doi: 10.1097/00002030-200009290-00009. [DOI] [PubMed] [Google Scholar]
- 29.Cole SR, Hernan MA, Robins JM, et al. Effect of highly active antiretroviral therapy on time to acquired immunodeficiency syndrome or death using marginal structural models. Am J Epidemiol. 2003;158:687–694. doi: 10.1093/aje/kwg206. [DOI] [PubMed] [Google Scholar]
- 30.Pozniak A, Saag MS, Bellos N, et al. Efficacy of TMC114/r in treatment-experienced HIV patients: factors influencing outcome in the pooled 24-week analysis of POWER 1, 2 and 3. 12th Annual Conference of the British HIV Association; Brighton, UK. March–April 2006; Abstract P3. [Google Scholar]
- 31.Sax PE, Losina E, Weinstein MC, et al. Cost-effectiveness of enfuvirtide in treatment-experienced patients with advanced HIV disease. J Acquir Immune Defic Syndr. 2005;39:69–77. doi: 10.1097/01.qai.0000160406.08924.a2. [DOI] [PubMed] [Google Scholar]
- 32.Gras L, Van Sighem A, Fraser C, et al. Predictors for changes in CD4 cell count 7 years after starting HAART. 13th Conference on Retroviruses and Opportunistic Infections; Denver, CO. February 2006; Abstract S30. [Google Scholar]
- 33.Keruly J, Moore R. Increases in CD4 cell count to 5 years in persons with sustained virologic suppression. 13th Conference on Retroviruses and Opportunistic Infections; Denver, CO. November 2005; Abstract 529. [Google Scholar]
- 34.Murphy R, daSilva B, McMillan F, et al. Seven year follow-up of a lopinavir/ritonavir (LPV/r)-based regimen in antiretroviral (ARV)-naïve subjects. 10th European AIDS Conference; Dublin, Ireland. November 2005; Abstract PE7.9/3. [Google Scholar]
- 35.Yazdanpanah Y, Vray M, Meynard J, et al. Cost-effectiveness of genotypic resistance testing in patients with extensive prior antiretroviral exposure: modeling results from NARVAL trial. Antiviral Ther Suppl. 2003;1:217. [Google Scholar]
- 36.Brazier J, Usherwood T, Harper R, Thomas K. Deriving a preference-based single index from the UK SF-36 Health Survey. J Clin Epidemiol. 1998;51:1115–1128. doi: 10.1016/s0895-4356(98)00103-6. [DOI] [PubMed] [Google Scholar]
- 37.Bozzette SA, Berry SH, Duan N, et al. The care of HIV-infected adults in the United States. HIV Cost Services Utilization Study Consortium. N Engl J Med. 1998;339:1897–1904. doi: 10.1056/NEJM199812243392606. [DOI] [PubMed] [Google Scholar]
- 38.Schackman BR, Goldie SJ, Freedberg KA, Losina E, Brazier J, Weinstein MC. Comparison of health state utilities using community and patient preference weights derived from a survey of patients with HIV/AIDS. Med Decis Making. 2002;22:27–38. doi: 10.1177/0272989X0202200103. [DOI] [PubMed] [Google Scholar]
- 39.Grinsztejn B, Nguyen B-Y, Katlama C, et al. Potent antiretroviral effect of MK-0518, a novel HIV-1 integrase inhibitor, in patients with triple class-resistant virus. 46th Interscience Conference on Antimicrobial Agents and Chemotherapy; San Francisco, CA. September 2006; Oral Presentation #H-1670b. [Google Scholar]
- 40.Hicks CB, Cahn P, Cooper DA, et al. Durable efficacy of tipranavir-ritonavir in combination with an optimised background regimen of antiretroviral drugs for treatment-experienced HIV-1-infected patients at 48 weeks in the Randomized Evaluation of Strategic Intervention in multi-drug reSistant patients with Tipranavir (RESIST) studies: an analysis of combined data from two randomised open-label trials. Lancet. 2006;368:466–475. doi: 10.1016/S0140-6736(06)69154-X. [DOI] [PubMed] [Google Scholar]
- 41.Lalezari JP, Henry K, O’Hearn M, et al. Enfuvirtide, an HIV-1 fusion inhibitor, for drug-resistant HIV infection in North and South America. N Engl J Med. 2003;348:2175–2185. doi: 10.1056/NEJMoa035026. [DOI] [PubMed] [Google Scholar]
- 42.Lazzarin A, Clotet B, Cooper D, et al. Efficacy of enfuvirtide in patients infected with drug-resistant HIV-1 in Europe and Australia. N Engl J Med. 2003;348:2186–2195. doi: 10.1056/NEJMoa035211. [DOI] [PubMed] [Google Scholar]
- 43.Kantor R, Shafer RW, Follansbee S, et al. Evolution of resistance to drugs in HIV-1-infected patients failing antiretroviral therapy. AIDS. 2004;18:1503–1511. doi: 10.1097/01.aids.0000131358.29586.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Napravnik S, Edwards D, Stewart P, Stalzer B, Matteson E, Eron JJ., Jr HIV-1 drug resistance evolution among patients on potent combination antiretroviral therapy with detectable viremia. J Acquir Immune Defic Syndr. 2005;40:34–40. doi: 10.1097/01.qai.0000174929.87015.d6. [DOI] [PubMed] [Google Scholar]
- 45.De Meyer S, Hill A, De Baere I, et al. Effect of baseline susceptibility and on-treatment mutations on TMC114 and control PI efficacy: preliminary analysis of data from PI-experienced patients from POWER 1 and POWER 2. 13th Conference on Retroviruses and Opportunistic Infections; Denver, CO. February 2006; Abstract 157. [Google Scholar]
- 46.Vingerhoets J. Impact of baseline resistance on the virologic response to a novel NNRTI, TMC125, in patients with extensive NNRTI and PI resistance: analysis of Study TMC125-C223. 13th Conference on Retroviruses and Opportunistic Infections; Denver, CO. February 2006; Abstract 154. [Google Scholar]
- 47.Corzillius M, Muhlberger N, Sroczynski G, Jaeger H, Wasem J, Siebert U. Cost effectiveness analysis of routine use of genotypic antiretroviral resistance testing after failure of antiretroviral treatment for HIV. Antiviral Ther. 2004;9:27–36. [PubMed] [Google Scholar]
- 48.Stansell J, Barrett J, Holtzer C, Lapins D. Incremental costs of HIV suppression in HIV therapeutic failure. 7th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA. January–February 2000; Abstract 761. [Google Scholar]
- 49.Carpenter CC, Cooper DA, Fischl MA, et al. Antiretroviral therapy in adults: updated recommendations of the International AIDS Society–USA Panel. J Am Med Assoc. 2000;283:381–390. doi: 10.1001/jama.283.3.381. [DOI] [PubMed] [Google Scholar]
- 50.Hammer SM, Squires KE, Hughes MD, et al. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. AIDS Clinical Trials Group 320 Study Team. N Engl J Med. 1997;337:725–733. doi: 10.1056/NEJM199709113371101. [DOI] [PubMed] [Google Scholar]
- 51.Anonymous. Nomenclature des Actes de Biologie Médicale. Paris, France: Union des Caisses Nationales de Sécurité Sociale; 1997. [Google Scholar]