Summary
Background
Cough may be a manifestation of gastro-esophageal reflux disease (GERD). The utility of acid suppression in GERD-related cough is uncertain.
Aim
To assess the impact of high-dose acid suppression with proton pump inhibitors (PPI) on chronic cough in subjects with rare or no heartburn.
Methods
Subjects were non-smokers without history of asthma, with chronic cough for > 8 weeks. All subjects underwent a baseline 24 hr pH/impedance study, methacholine challenge test (MCT), and laryngoscopy. Subjects were randomized to either 40 mg of esomeprazole twice daily or placebo for 12 weeks. The primary outcome measure was the Cough-Specific Quality of Life Questionnaire (CQLQ). Secondary outcomes were response on Fisman Cough Severity/Frequency scores, and change in laryngeal findings.
Results
40 subjects were randomized (22 PPI, 18 placebo) and completed the study. There was no difference between PPI and placebo in CQLQ (mean improvement 9.8, vs. 5.9 in placebo, p = 0.3), or Fisman Cough Severity/Frequency scores. The proportion of patients who improved by >1 standard deviation on the CQLQ was 27.8% (5/18) and 31.8% (7/22) in the placebo and PPI groups respectively.
Conclusions
In subjects with chronic cough and rare or no heartburn, high-dose PPI did not improve cough-related quality of life or symptoms in this randomized controlled trial.
Keywords: Cough, Gastroesophageal Reflux Disease, Proton Pump Inhibitors, Randomized Controlled Trial, GERD-related cough
Introduction
Chronic cough is a common symptom that affects 8–12% of adults (1–5). Patients with chronic cough have impaired quality of life and increased healthcare utilization (6, 7). Gastroesophageal reflux disease (GERD) is thought to be a common cause of chronic cough, which may be responsible for 20–40% of cases (8–13).
The most efficacious treatment for GERD-related cough is unclear. Because double-standard dose proton pump inhibitor (PPI) therapy has been used to treat asthma related to GERD (14–18), it has been suggested that similar treatment may be needed for GERD-related cough (17). Although the relationship between GERD and cough is widely accepted, the evidence to substantiate the effectiveness of PPI therapy for GERD-related cough is weak.
Previous prospective studies of the role of PPIs in GERD-related cough have yielded equivocal results. Two small randomized controlled trials (n=17 and 29) suggested that PPI therapy may improve cough symptoms, but validated outcome measures were not used, and treatment effects were modest and lacked precision (19, 20). Furthermore, because positive pH tests were required for entry into these trials and pH impedance testing was not performed, the role of PPI therapy in persons with negative pH testing (who may have weakly acidic or non-acid reflux as a cause of cough) is uncertain.
Despite the paucity of confirmatory clinical trial data, societal guidelines recommend an empiric PPI trial for suspected GERD-related cough (21, 22). However, a recent Cochrane analysis found “insufficient evidence” to conclude that PPI therapy is universally beneficial for GERD-related cough(23), and a second meta-analysis on this topic concluded that randomized controlled trials using valid cough outcomes were needed to justify international guidelines.(24) Because of these uncertainties, we sought to assess the efficacy of empiric PPI therapy in persons with chronic cough.
Methods
Study Design and Overview
We conducted a randomized, double-blind, placebo-controlled trial of subjects with chronic cough of unknown etiology and minimal or no heartburn symptoms. After initial eligibility screening, all consenting participants entered a 7-day run-in period, during which all subjects took a placebo pill twice a day, for the purpose of ensuring compliance with study medication and assessment of cough severity under baseline conditions. Participants who took >90% of medication during the run-in period and met cough severity criteria (see below) completed 3 baseline cough-specific instruments (the Cough-Specific Quality of Life Questionnaire (CQLQ) and the Fisman Cough Severity and Frequency Scores), and an assessment of gastrointestinal symptom severity and frequency (the Gastroesophageal Reflux Disease Symptom Assessment Score (GSAS)). Subjects also underwent a methacholine challenge test, a 24-hour esophageal pH/impedance study, and indirect laryngoscopy. Participants were then randomized in a blinded fashion to either treatment with esomeprazole 40 mg twice daily or placebo, stratified by pH study results (high vs. low esophageal acid exposure). After 12 weeks of treatment, subjects repeated the CQLQ, Fisman Cough Severity and Frequency Scores, and indirect laryngoscopy.
Study Subjects
Potential subjects were males and females between the ages of 18 and 70 who were able to speak English, had chronic cough of > 8 weeks in duration, with symptom severity criteria of 2 or greater on the Fisman Cough Severity Questionnaire and 3 or greater or the Fisman Cough Frequency Questionnaire. Because post-nasal drip is a common cause of chronic cough, subjects were required to have failed a trial of post-nasal drip therapy (e.g. intranasal steroids). Subjects were excluded from the study if they had an abnormal chest x-ray, reported heartburn symptoms greater than two times per month, had failed to respond to past PPI therapy of ≥12 weeks duration, had previous surgical or endoscopic antireflux procedure, had a previous aerodigestive malignancy or Barrett’s esophagus, were current smokers or ex-smokers (defined as those who quit smoking < 3 months prior to study enrollment or those who have quit, but have a ≥20 pack year smoking history), had an upper respiratory infection within 8 weeks of study enrollment, or who were currently using a PPI, H2 blocker, beta-blocker, angiotensin-converting enzyme inhibitor, corticosteroid, methylxanthine, inhaled beta-agonist, anti-inflammatory agent, or anticholinesterase drug at time of enrollment.
All subjects provided informed consent for participation. This study protocol was approved by the Institutional Review Board at the University of North Carolina. This trial was registered in the ClinicalTrials.gov registry (number NCT00287339).
Study Setting
Potential subjects were recruited from the Otolaryngology/Head and Neck Surgery, Pulmonary Medicine, and Gastroenterology outpatient clinics at the University of North Carolina Hospitals in Chapel Hill, North Carolina, as well as through recruitment advertisements. Methacholine challenge tests (MCT) were performed in the Pulmonary Medicine Clinic, indirect fiberoptic laryngoscopy procedures were performed in the Otolaryngology/Head and Neck Surgery clinic, and the ambulatory 24 hour pH/impedance studies were performed by the Gastroenterology Motility laboratory, all at the University of North Carolina.
Study Intervention
Upon successful completion of the run-in period, participants were randomized to receive either esomeprazole at a dose of 40 mg or placebo twice daily for 12 weeks. All participants were instructed to take their study medication 30 minutes before breakfast and 30 minutes before dinner. Esomeprazole was supplied by the investigational pharmacy at the University of North Carolina as blue capsules without identifying features. Placebo was identically packaged and supplied.
Study Measures and Procedures
Cough-Specific Quality of Life Questionnaire
The CQLQ is a validated, 28-item assessment tool designed specifically to evaluate decrements in disease-targeted quality of life due to chronic cough. This questionnaire measures cough-related symptoms, as well as the social implications and psychological impact of chronic cough. Examples of items on the CQLQ include, “I cannot sleep at night” and “I cough and it makes me retch.” The final score is obtained by summing the responses to 28 cough-specific questions, each scored on a 1–4 scale, where 1 is “strongly disagree,” and 4 is “strongly agree.” The minimum and maximum CQLQ scores are 28 and 112 respectively, with increasing score indicating more severe impairment.
Fisman Cough Severity/Cough Frequency Scores
The Fisman Cough Severity Score is a validated scale that is scored from 0–4, where 0 is no cough, 4 is strenuous cough with chest discomfort. The Fisman Cough Frequency Score is a validated visual analog scale, scored from 1–10, where 1 = “I never cough,” and 10 = “I cough all day long.” Both measures were designed for use in clinical trials of cough (25).
Gastroesophageal Reflux Disease Symptom Assessment Scale
The Gastroesophageal Reflux Disease Symptom Assessment Scale (GSAS) is a validated symptom scale for use in patients with GERD (26). The GSAS has been used previously in other clinical trials of GERD treatment (27, 28). The GSAS includes 4 scales: number of symptoms, frequency of symptoms, severity and bother/distress. The GSAS score ranges from 0 to 4 with higher values associated with greater frequency or severity. As the severity and distress scores are highly correlated (r = 0.95)(26), only the distress score was used for this trial.
Methacholine Challenge Test
In order to evaluate for the presence of reactive airways disease, MCT were performed. Tests were performed using disposable single-use Provocholine® kits with methacholine chloride powder for inhalation, according to standard protocol (29). Results of MCT were classified as either positive or negative using established definitions (30).
Esophageal pH/impedance testing
Each participant underwent 24 hour ambulatory esophageal pH/impedance monitoring off all acid-suppressing medications using the Sleuth® monitoring system (Sandhill Scientific, Highlands Ranch, CO) and standard technique (31–34). Results of pH testing were scored based on the DeMeester scoring system (35). A score of greater than 14.7 was categorized as “high distal esophageal acid” and a score of 14.7 or less was classified as “low distal esophageal acid”. These categories were used to stratify participants prior to randomization. Other results of pH/impedance testing included 1) number of proximal and distal reflux episodes (both acidic and non-acidic), 2) patient-reported coughs during study, 3) number of coughs with corresponding acid or non-acid reflux events, and 4) cough symptom index. The cough symptom index was calculated as: (number of reflux-related coughs/total coughs) × 100%, and both acid and non-acid reflux-related coughs were included in the numerator. The cough symptom index was considered positive if it was ≥ 50%. Non-acid reflux was defined as an impedance reflux episode with pH >4
Indirect laryngoscopy and the Reflux Finding Score
Fiberoptic indirect laryngoscopy was performed at baseline and at the end of study by an experienced otolaryngologist masked to patient assignment. Each laryngoscopy was scored using the Reflux Finding Score (RFS). The RFS is a validated 8-item measure to assess laryngoscopy findings associated with reflux-related cough (36). This scoring system is designed to detect findings of laryngopharyngeal reflux (37). A score of 7 or more on the RFS is consistent with laryngopharyngeal reflux (36).
Study Outcomes
The primary outcome of this trial was change in CQLQ, comparing PPI-treated subjects to placebo. Secondary outcomes were change in Fisman Cough Severity and Frequency Scores, and change in RFS, comparing PPI-treated subjects to placebo.
Sub-analyses were planned a priori to assess whether there was a treatment effect in 1) participants with high esophageal acid exposure; 2) participants reporting any heartburn on GSAS at study enrollment; 3) participants with a negative MCT (indicating absence of detectable airway reactivity) and 4) participants with a RFS greater than 7, suggesting laryngopharyngeal reflux.
Randomization and blinding
Randomization was performed using a computerized random number generator with a permuted blocks scheme. We used a stratified randomization technique, based on results of pH testing (DeMeester score <14.7 vs. ≥14.7). Independent pharmacists within the investigational drug pharmacy at the University of North Carolina dispensed either esomeprazole or placebo according to the randomization code. All study personnel and participants were blinded to treatment allocation for the duration of the study. Interpreters of study tests (MCT, pH-impedance testing, indirect laryngoscopy) and the study statistician were also blinded to treatment assignment.
Statistical analysis
Sample size calculations were based on the primary outcome measure, the CQLQ. This questionnaire has a reported mean score of 69.4 and a standard deviation of 12.9 in persons with chronic cough from a variety of causes. Thirty-two subjects were necessary to achieve 80% power (at α=0.05) to detect a change of one standard deviation in CQLQ score. A total sample size of 40 was planned in order to accommodate potential dropouts. This enrollment was expected to give the study power to detect even modest improvements in disease-specific quality of life.
To compare the two treatment groups with respect to the primary outcome (CQLQ score difference from baseline to end of study), 2 sample t-tests of differences were used. To compare the two treatment groups with respect to the secondary outcomes of Fisman Cough Severity Score, Fisman Cough Frequency Score, and Reflux Finding Score, 2 sample t-tests of differences were used. To evaluate treatment response within groups (i.e. change from baseline to end of study), paired t-tests were used. Use of Wilcoxon Rank-Sum tests and Wilcoxon Signed-Rank tests were performed in the case of non-normally distributed data. All analyses were intention to treat. Statistical analyses were performed using SAS (Cary, NC) and STATA version 10.0 (College Station, TX) by 2 of the authors (SDC and JAG).
Results
Flow of participants
One hundred and sixty-eight subjects were assessed for eligibility. Forty subjects were randomized (22 PPI, 18 placebo), and all 40 completed the trial. One subject completed study treatment and all study questionnaires, but did not complete the final laryngoscopy (Figure 1). The first participant was randomized on 10/24/2006, and the last participant completed the study on 10/7/2008. Overall adherence to study therapy as measured by pill counts throughout the study was 93%.
Figure 1.
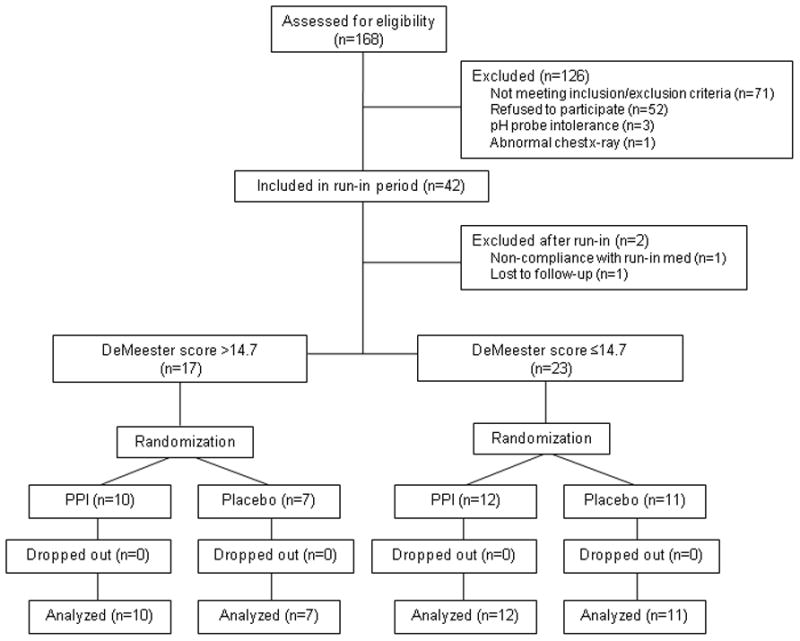
CONSORT diagram of participants in a single center double blind, randomized controlled trial of twice daily esomeprazole vs. placebo for the treatment of chronic cough
Baseline demographics and scores
The mean age of participants was 50 years (SD 11.7) and 78% were female. Positive MCT were present in 33% of subjects (Table 1). Baseline scores on the CQLQ, Fisman Cough Severity and Cough Frequency, RFS, and GSAS were similar between groups.
Table 1.
Baseline characteristics of the study population
| Variable | PPI (n=22) | Placebo (n=18) | p value* |
|---|---|---|---|
| Age (mean, SD) | 49.5 (12.0) | 51.0 (11.6) | 0.7 |
| Sex (n, %) | 0.4 | ||
| Female | 16 (73) | 15 (83) | |
| Male | 6 (27) | 3 (17) | |
| Race (n, %) | 0.7 | ||
| Asian | 1 (5) | 0 (0) | |
| African American | 4 (18) | 3 (17) | |
| White | 17 (77) | 15 (83) | |
| Positive MCT (n, %) | 8 (36) | 5 (28) | 0.6 |
| Positive pH study (n, %) | 10 (45) | 7 (39) | 0.7 |
| GSAS frequency score (median, IQR) | 16.2 (7.5, 30.4) | 14.3 (6.7, 52.5) | 1.0 |
| GSAS distress score (median, IQR) | 1.3 (1.2, 2.0) | 1.9 (1.4, 2.5) | 0.07 |
| CQLQ (mean, SD) | 52.9 (12.0) | 58.2 (11.8) | 0.2 |
| Cough Severity Score (mean, SD) | 2.9 (0.8) | 2.8 (0.7) | 0.8 |
| Cough Frequency Score (mean, SD) | 6.2 (1.8) | 6.8 (2.0) | 0.3 |
| Reflux Finding Score (mean, SD) | 7.6 (2.2) | 8.3 (2.6) | 0.4 |
MCT: Methacholine challenge test; GSAS: Gastroesophageal reflux Severity Assessment Score; CQLQ: Cough Specific Quality of Life Questionnaire
p values obtained via Student’s t-tests or rank sum tests for continuous variables, or chi-squared tests for categorical variables
Esophageal pH/impedance testing
The mean DeMeester score was 15.0 (SD 17.1), and 17/40 (43%) of subjects had a positive pH test as defined by a DeMeester score of 14.7 or greater. There were an average of 26 patient-reported coughs during each 24 hour pH study, and the mean number of coughs related to acid and non-acid reflux events were 4.3 and 1.9 respectively (Table 2). A total of 23 participants had at least 1 non-acid reflux event related to cough. The mean cough symptom index was 26%, and 7 subjects had a cough symptom index ≥ 50% (indicating that at least 50% of coughs were preceded by an impedance-detected reflux event).
Table 2.
24 hour esophageal pH testing and impedance pH testing results
| Variable | Mean (SD)(range) |
|---|---|
| DeMeester score* | 15.0 (17.1) (1.2 – 69.1) |
| Number of proximal reflux episodes | 13.5 (11.0) (1 – 41) |
| Number of distal reflux episodes | 35.6 (19.5) (9 – 97) |
| Number of patient-reported coughs during study | 26.3 (25.2) (1 – 115) |
| Number of acid-related coughs | 4.3 (4.7) (0 – 17) |
| Number of non-acid related coughs | 1.9 (2.5) (0 – 10) |
| Cough symptom index (%) | 26.3 (20.4) (0 – 83) |
Normal DeMeester score: <14.7
Primary and secondary outcomes
Although subjects in both the PPI and placebo arm experienced significant improvement in CQLQ over baseline scores, subjects in the PPI arm did not show a greater improvement in CQLQ scores compared to placebo (mean improvement 9.8 in the PPI group, vs. 5.9 in the placebo group, p = 0.3) (Figure 2, Table 3). Using the benchmark of a change of 1 SD in the CQOL as a clinically-relevant change, there were 12 subjects who had a CQOL change ≥12.9 in the study including 5/18 (27.8%) subjects in the placebo arm, and 7/22 (31.8%) subjects in the PPI arm. Similarly, the mean changes in Fisman Cough Severity or Frequency scores were not significantly different between PPI and placebo (Severity: 1.0 vs. 0.8, p = 0.7; Frequency: 3.2 vs. 2.3, p = 0.3). The change in RFS was also similar for PPI and placebo groups (0.6 vs. 0.1, p = 0.5, table 3).
Figure 2.
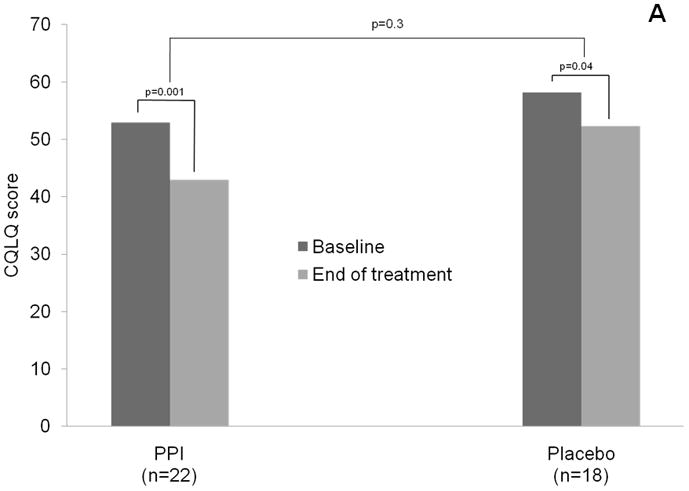
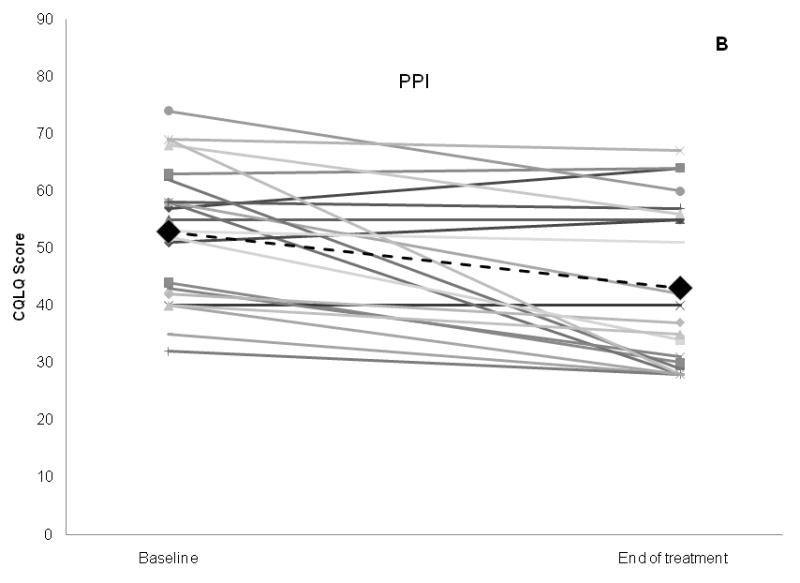
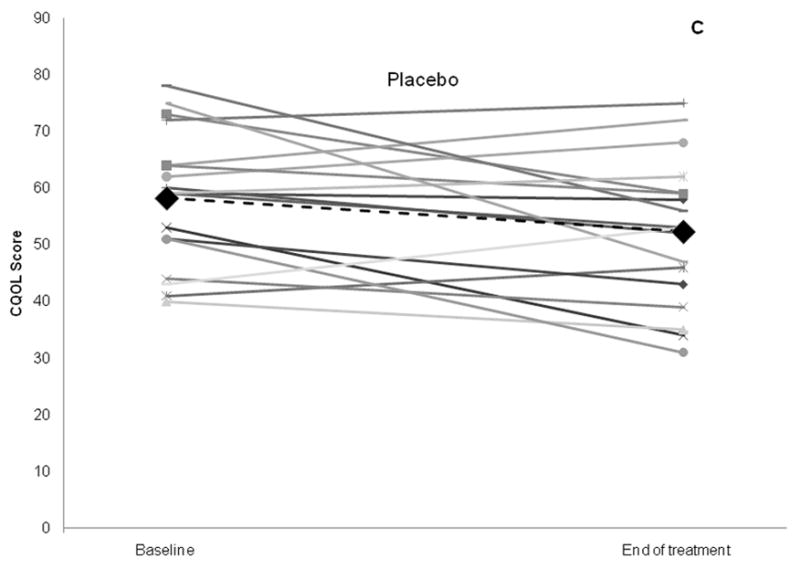
Change in Cough Specific Quality of Life Questionnaire score, the primary outcome, for PPI and placebo groups. Bar graph of aggregate results is shown in (A), and scatter plot of results for individual subjects in each study arm shown in (B) and (C). Large black diamonds and black dotted lines represent mean for each group.
p values comparing baseline vs. end of treatment scores within each treatment group obtained via paired t-tests, whereas p values obtained comparing mean change between treatment groups obtained via 2 sample t-test of differences.
CQOL: Cough Specific Quality of Life score; PPI: proton pump inhibitor group (intervention arm)
Table 3.
Comparison of primary and secondary outcomes between treatment groups
| Outcome | PPI (n=22) |
Placebo (n=18) |
p† |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline mean (SD) | End mean (SD) | Change mean (SD) | p* | Baseline mean (SD) | End mean (SD) | Change mean (SD) | p* | ||
| CQLQ | 52.9 (12.0) | 43.0 (14.3) | 9.8 (12.2) | 0.001 | 58.2 (11.8) | 52.3 (12.8) | 5.9 (11.1) | 0.04 | 0.3 |
| Cough Severity Score | 2.9 (0.8) | 1.9 (0.9) | 1.0 (1.3) | 0.002 | 2.8 (0.7) | 2.0 (1.0) | 0.8 (1.2) | 0.009 | 0.7 |
| Cough Frequency Score | 6.2 (1.8) | 3.0 (1.8) | 3.2 (2.4) | <0.0001 | 6.8 (2.0) | 4.5 (2.5) | 2.3 (2.7) | 0.002 | 0.3 |
| Reflux Finding Score | 7.6 (2.2) | 6.9 (2.2) | 0.6 (2.0) | 0.2 | 8.3 (2.6) | 8.2 (3.6) | 0.1 (2.9) | 0.9 | 0.5 |
p values obtained via paired t-tests, comparing baseline vs. end of treatment scores within each treatment group
p values obtained via 2 sample t-test of differences, comparing mean change between treatment groups. Nonparametric testing also performed with Wilcoxon Rank-sum tests: p=>0.05 for all comparisons in this column. CQLQ: Cough-specific Quality of Life Questionnaire: a validated 27 item disease-targeted quality of life measure
Subgroup analyses
When only subjects with a positive pH study were considered, scores on the CQLQ were not significantly improved on PPI therapy (mean change of 11.1 for PPI vs. 4.7 for placebo, p = 0.2), nor were changes in Cough Severity or Frequency scores (Severity: 1.1 vs. 0.6, p = 0.4; Frequency: 3.4 vs. 3.7, p = 0.8) (Table 4). Similarly, change in CQLQ scores or Cough Severity and Cough Frequency scores did not differ significantly between treatment groups when stratified by MCT results, RFS results, or by presence or absence of heartburn at study baseline (data not shown).
Table 4.
Comparison of primary and secondary outcomes between treatment groups stratified by pH study results.
| Outcome | PPI |
Placebo |
p† |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline mean (SD) | End mean (SD) | Change mean (SD) | p* | Baseline mean (SD) | End mean (SD) | Change mean (SD) | p* | ||
| High acid | n=10 | n=7 | |||||||
| CQLQ | 49.8 (12.7) | 38.7 (13.6) | 11.1 (9.5) | 0.005 | 60.0 (12.3) | 55.3 (10.7) | 4.7 (9.4) | 0.2 | 0.2 |
| Cough Severity Score | 2.9 (0.7) | 1.8 (0.9) | 1.1 (1.4) | 0.03 | 2.9 (0.7) | 2.3 (1.1) | 0.6 (0.8) | 0.1 | 0.4 |
| Cough Frequency Score | 5.6 (1.9) | 2.2 (1.0) | 3.4 (2.0) | 0.0004 | 8.0 (2.2) | 4.3 (2.8) | 3.7 (2.1) | 0.003 | 0.8 |
| Reflux Finding Score | 7.3 (2.9) | 7.3 (2.5) | 0 (2.4) | 1.0 | 9.7 (2.0) | 9.3 (3.3) | 0.4 (3.1) | 0.7 | 0.8 |
| Low acid | n=12 | n=11 | |||||||
| CQLQ | 55.4 (11.3) | 46.7 (14.4) | 8.8 (14.5) | 0.06 | 57.1 (11.9) | 50.5 (14.2) | 6.6 (12.4) | 0.1 | 0.7 |
| Cough Severity Score | 2.9 (0.9) | 2.0 (1.0) | 0.9 (1.3) | 0.03 | 2.8 (0.8) | 1.8 (1.0) | 1.0 (1.3) | 0.04 | 0.9 |
| Cough Frequency Score | 6.7 (1.7) | 3.7 (2.0) | 3.0 (2.7) | 0.003 | 6.1 (1.5) | 4.7 (2.5) | 1.5 (2.8) | 0.1 | 0.2 |
| Reflux Finding Score | 7.8 (1.4) | 6.6 (2.0) | 1.1 (1.5) | 0.02 | 7.4 (2.6) | 7.5 (3.7) | − 0.1 (2.8) | 0.9 | 0.2 |
High and low esophageal acid defined as DeMeester score of > or ≤ 14.7 respectively on baseline pH impedance testing
p values obtained via paired t-tests, comparing baseline vs. end of treatment scores within each treatment group
p values obtained via 2 sample t-test of differences, comparing mean change between treatment groups
CQLQ: Cough-specific Quality of Life Questionnaire: a validated 27 item disease-targeted quality of life measure
Adverse events
There were no serious adverse events, and no one was withdrawn from the study for safety.
Discussion
In this study, high dose PPI therapy for 12 weeks in persons with chronic cough and minimal or no reflux symptoms did not improve symptoms or health-related quality of life. Similarly, amongst those with positive pH tests, and those with a negative methacholine challenge test (indicating an absence of airway reactivity) there was also no difference between active treatment and placebo with respect to quality of life or cough severity or frequency.
The question we sought to answer was whether it was worthwhile to consider empiric therapy with high dose acid suppression for a subject presenting with chronic cough and no obvious etiologic source. While subjects with frequent heartburn would likely merit an empirical trial of PPI therapy regardless of cough, it is less clear whether PPIs should be used to address subjects in whom cough is the predominant complaint, and heartburn rare or absent. Although we rigorously characterized our patients with laryngoscopy, MCT, and pH studies, we used none of these tests as study exclusions, because we wished to replicate the situation in the generalists’ office, where the results of such tests would not commonly be available to the physician on initial evaluation. Additionally, we did not want to exclude subjects who might benefit from PPI therapy with any one of the above tests, since there is no “gold standard” for the diagnosis of GERD-induced cough. Because pH studies were originally normalized to detect disease in those with classic symptoms of GERD, subjects with cough and a pH study showing low esophageal acid exposures might still have adequate acid exposures to develop GERD-induced cough, and therefore could potentially benefit from treatment.
Our findings are of particular interest in light of the recent trial demonstrating a lack of efficacy of esomeprazole for the treatment of poorly controlled asthma, another possible extra-esophageal manifestation of GERD (38). Our study had an analogous design to this trial, and similarly did not demonstrate effectiveness of PPI therapy for a putative extra-esophageal manifestation of reflux disease.
We included an evaluation of larygopharyngeal reflux via indirect laryngoscopy. Although laryngoscopy has been reported to accurately detect extra-esophageal GERD, the responsiveness of laryngoscopic reflux findings to GERD therapy is uncertain and previous trials have reported conflicting results (39, 40). We found that results of indirect laryngoscopy did not change significantly despite PPI therapy, and that the RFS score did not predict response to therapy, even in those subjects with an RFS score consistent with probable laryngopharyngeal reflux.
The observed lack of effect of acid suppressive therapy to ameliorate symptoms of cough or improve cough-specific quality of life may be due to several reasons. First, the role of GERD in the generation of cough symptoms may be over-stated. It is well known that chronic cough has multiple causes, and GERD-related cough may not be as common as previously reported (13, 41). Secondly, in those with multi-factorial cough, the relative contribution of GERD may be less important, such that the overall symptom complex does not change substantially in response to acid suppression. Thirdly, the lack of response may be due to inadequate efficacy of the treatment; it may be that only small amounts of reflux (or even non-acidic reflux) may be adequate to perpetuate chronic cough in some patients. Finally, it may also point to the need for longer duration of therapy, as has been suggested by other investigators (42). Regardless of the pathogenesis of the persistent symptoms, these findings cast considerable doubt on the utility of an empiric trial of acid suppression in chronic cough as is recommended by several treatment guidelines.
This study had several strengths. Rigorous outcome measurement was performed. We used previously-derived and validated measures of cough-specific quality of life, and cough severity and frequency. Patients were well-characterized in their pathophysiology. In addition to combined pH/impedance testing on study entry, we also performed methacholine challenge tests and pre- and post-treatment indirect laryngoscopy on each participant. The intervention was also robust: 40 mg of esomeprazole twice daily. Given that <5% of subjects with extra-esophageal GERD symptoms have abnormal pH studies when on twice daily PPI (43), lack of efficacy is unlikely due to under-dosing of acid suppressive medication. Patients were treated for 3 months, a period during which response to therapy has been demonstrated for other extra-esophageal manifestations of GERD (15, 18). Patient follow-up was complete and compulsive. We observed excellent (>90%) compliance with study medication throughout the trial.
This study was performed at a single center, and participants were mostly female and white, which may limit generalizability, though African Americans represented nearly 18% of participants. The study was planned with a power to detect a difference of 1 standard deviation between groups with respect to the primary outcome, the CQLQ. Indeed, when there were differences between groups, they were all <1 SD, a level not demonstrated to be clinically meaningful (44). Furthermore, the SD of the CQLQ in this study (11.9) was similar to previous reports (6, 44, 45). However, because of the limited trial size, the number of subjects in some patient subgroups was small, and the lack of statistically significant differences between some subgroups may have been due to inadequate statistical power.
In conclusion, in subjects with chronic cough and minimal heartburn symptoms, high dose PPI therapy for 12 weeks did not improve cough symptoms or cough-related quality of life. This randomized double blind placebo-controlled trial adds to the growing body of evidence that suggests that acid suppressive therapy may not substantially improve extra-esophageal symptoms presumed secondary to reflux disease, and questions the common practice of an empiric trial of acid-suppressive therapy in patients with chronic cough.
Footnotes
Trial registration: Clinicaltrials.gov registration number NCT00287339
Statement of interests:
1. Authors’ declaration of personal interests:
i. Dr. Shaheen has served as a consultant to Astra Zeneca and CSA Medical, and has received research support from Astra Zeneca, Takeda, Procter & Gamble, BARRX Medical, CSA Medical and Oncoscope.
ii. Dr. Madanick is on the speakers bureau and has received honoraria from Astra Zeneca
iii. Dr. Dellon has received research support from Astra Zeneca.
iv. Dr. Morgan has received research support from Astra Zeneca.
v. Dr. Henke is on the speakers bureau for Astra Zeneca.
vi. Other co-authors have no competing interests to declare
2. Declaration of funding interests:
i. This research was conducted with support from the Investigator-Sponsored Study Program of AstraZeneca, LLP.
ii. This research was also supported, in part, by funding from the National Institutes of Health for the Digestive Disease Epidemiology Training Program (T32 DK007634), and the Center for Gastrointestinal Biology and Disease (P30 DK3497).
iii. The study sponsor had no role in the study design or the collection, analysis, or interpretation of data.
References
- 1.Wynder EL, Lemon FR, Mantel N. Epidemiology of Persistent Cough. Am Rev Respir Dis. 1965 May;91:679–700. doi: 10.1164/arrd.1965.91.5.679. [DOI] [PubMed] [Google Scholar]
- 2.Woodwell DA, Cherry DK. National Ambulatory Medical Care Survey: 2002 summary. Adv Data. 2004 Aug;26(346):1–44. [PubMed] [Google Scholar]
- 3.Di Pede C, Viegi G, Quackenboss JJ, Boyer-Pfersdorf P, Lebowitz MD. Respiratory symptoms and risk factors in an Arizona population sample of Anglo and Mexican-American whites. Chest. 1991 Apr;99(4):916–22. doi: 10.1378/chest.99.4.916. [DOI] [PubMed] [Google Scholar]
- 4.Enright PL, Kronmal RA, Higgins MW, Schenker MB, Haponik EF. Prevalence and correlates of respiratory symptoms and disease in the elderly. Cardiovascular Health Study. Chest. 1994 Sep;106(3):827–34. doi: 10.1378/chest.106.3.827. [DOI] [PubMed] [Google Scholar]
- 5.Ford AC, Forman D, Moayyedi P, Morice AH. Cough in the community: a cross sectional survey and the relationship to gastrointestinal symptoms. Thorax. 2006 Nov;61(11):975–9. doi: 10.1136/thx.2006.060087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polley L, Yaman N, Heaney L, Cardwell C, Murtagh E, Ramsey J, et al. Impact of cough across different chronic respiratory diseases: comparison of two cough-specific health-related quality of life questionnaires. Chest. 2008 Aug;134(2):295–302. doi: 10.1378/chest.07-0141. [DOI] [PubMed] [Google Scholar]
- 7.Metlay JP, Stafford RS, Singer DE. National trends in the use of antibiotics by primary care physicians for adult patients with cough. Arch Intern Med. 1998 Sep 14;158(16):1813–8. doi: 10.1001/archinte.158.16.1813. [DOI] [PubMed] [Google Scholar]
- 8.Jaspersen D, Kulig M, Labenz J, Leodolter A, Lind T, Meyer-Sabellek W, et al. Prevalence of extra-oesophageal manifestations in gastro-oesophageal reflux disease: an analysis based on the ProGERD Study. Aliment Pharmacol Ther. 2003 Jun 15;17(12):1515–20. doi: 10.1046/j.1365-2036.2003.01606.x. [DOI] [PubMed] [Google Scholar]
- 9.Poe RH, Kallay MC. Chronic cough and gastroesophageal reflux disease: experience with specific therapy for diagnosis and treatment. Chest. 2003 Mar;123(3):679–84. doi: 10.1378/chest.123.3.679. [DOI] [PubMed] [Google Scholar]
- 10.Pratter MR, Bartter T, Akers S, DuBois J. An algorithmic approach to chronic cough. Ann Intern Med. 1993 Nov 15;119(10):977–83. doi: 10.7326/0003-4819-119-10-199311150-00003. [DOI] [PubMed] [Google Scholar]
- 11.Smyrnios NA, Irwin RS, Curley FJ. Chronic cough with a history of excessive sputum production. The spectrum and frequency of causes, key components of the diagnostic evaluation, and outcome of specific therapy. Chest. 1995 Oct;108(4):991–7. doi: 10.1378/chest.108.4.991. [DOI] [PubMed] [Google Scholar]
- 12.Smyrnios NA, Irwin RS, Curley FJ, French CL. From a prospective study of chronic cough: diagnostic and therapeutic aspects in older adults. Arch Intern Med. 1998 Jun 8;158(11):1222–8. doi: 10.1001/archinte.158.11.1222. [DOI] [PubMed] [Google Scholar]
- 13.Irwin RS, Curley FJ, French CL. Chronic cough. The spectrum and frequency of causes, key components of the diagnostic evaluation, and outcome of specific therapy. Am Rev Respir Dis. 1990 Mar;141(3):640–7. doi: 10.1164/ajrccm/141.3.640. [DOI] [PubMed] [Google Scholar]
- 14.Harding SM. Gastroesophageal reflux and asthma: insight into the association. J Allergy Clin Immunol. 1999 Aug;104(2 Pt 1):251–9. doi: 10.1016/s0091-6749(99)70360-x. [DOI] [PubMed] [Google Scholar]
- 15.Harding SM, Richter JE, Guzzo MR, Schan CA, Alexander RW, Bradley LA. Asthma and gastroesophageal reflux: acid suppressive therapy improves asthma outcome. Am J Med. 1996 Apr;100(4):395–405. doi: 10.1016/S0002-9343(97)89514-9. [DOI] [PubMed] [Google Scholar]
- 16.Harding SM, Sontag SJ. Asthma and gastroesophageal reflux. Am J Gastroenterol. 2000 Aug;95(8 Suppl):S23–32. doi: 10.1016/s0002-9270(00)01075-3. [DOI] [PubMed] [Google Scholar]
- 17.Kiljander TO. The role of proton pump inhibitors in the management of gastroesophageal reflux disease-related asthma and chronic cough. Am J Med. 2003 Aug 18;115(Suppl 3A):65S–71S. doi: 10.1016/s0002-9343(03)00196-7. [DOI] [PubMed] [Google Scholar]
- 18.Kiljander TO, Salomaa ER, Hietanen EK, Terho EO. Gastroesophageal reflux in asthmatics: A double-blind, placebo-controlled crossover study with omeprazole. Chest. 1999 Nov;116(5):1257–64. doi: 10.1378/chest.116.5.1257. [DOI] [PubMed] [Google Scholar]
- 19.Ours TM, Kavuru MS, Schilz RJ, Richter JE. A prospective evaluation of esophageal testing and a double-blind, randomized study of omeprazole in a diagnostic and therapeutic algorithm for chronic cough. Am J Gastroenterol. 1999 Nov;94(11):3131–8. doi: 10.1111/j.1572-0241.1999.01504.x. [DOI] [PubMed] [Google Scholar]
- 20.Kiljander TO, Salomaa ER, Hietanen EK, Terho EO. Chronic cough and gastro-oesophageal reflux: a double-blind placebo-controlled study with omeprazole. Eur Respir J. 2000 Oct;16(4):633–8. doi: 10.1034/j.1399-3003.2000.16d11.x. [DOI] [PubMed] [Google Scholar]
- 21.Irwin RS, Boulet LP, Cloutier MM, Fuller R, Gold PM, Hoffstein V, et al. Managing cough as a defense mechanism and as a symptom. A consensus panel report of the American College of Chest Physicians. Chest. 1998 Aug;114(2 Suppl Managing):133S–81S. doi: 10.1378/chest.114.2_supplement.133s. [DOI] [PubMed] [Google Scholar]
- 22.Morice AH, Fontana GA, Sovijarvi AR, Pistolesi M, Chung KF, Widdicombe J, et al. The diagnosis and management of chronic cough. Eur Respir J. 2004 Sep;24(3):481–92. doi: 10.1183/09031936.04.00027804. [DOI] [PubMed] [Google Scholar]
- 23.Chang AB, Lasserson TJ, Gaffney J, Connor FL, Garske LA. Gastro-oesophageal reflux treatment for prolonged non-specific cough in children and adults. Cochrane Database Syst Rev. 2006;(4):CD004823. doi: 10.1002/14651858.CD004823.pub3. [DOI] [PubMed] [Google Scholar]
- 24.Chang AB, Lasserson TJ, Kiljander TO, Connor FL, Gaffney JT, Garske LA. Systematic review and meta-analysis of randomised controlled trials of gastro-oesophageal reflux interventions for chronic cough associated with gastro-oesophageal reflux. BMJ. 2006 Jan 7;332(7532):11–7. doi: 10.1136/bmj.38677.559005.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fisman EZ, Shapira I, Motro M, Pines A, Tenenbaum A. The combined cough frequency/severity scoring: a new approach to cough evaluation in clinical settings. J Med. 2001;32(3–4):181–7. [PubMed] [Google Scholar]
- 26.Rothman M, Farup C, Stewart W, Helbers L, Zeldis J. Symptoms associated with gastroesophageal reflux disease: development of a questionnaire for use in clinical trials. Dig Dis Sci. 2001 Jul;46(7):1540–9. doi: 10.1023/a:1010660425522. [DOI] [PubMed] [Google Scholar]
- 27.Damiano A, Handley K, Adler E, Siddique R, Bhattacharyja A. Measuring symptom distress and health-related quality of life in clinical trials of gastroesophageal reflux disease treatment: further validation of the Gastroesophageal Reflux Disease Symptom Assessment Scale (GSAS) Dig Dis Sci. 2002 Jul;47(7):1530–7. doi: 10.1023/a:1015815102175. [DOI] [PubMed] [Google Scholar]
- 28.Damiano A, Siddique R, Xu X, Johanson J, Sloan S. Reductions in symptom distress reported by patients with moderately severe, nonerosive gastroesophageal reflux disease treated with rabeprazole. Dig Dis Sci. 2003 Apr;48(4):657–62. doi: 10.1023/a:1022812103923. [DOI] [PubMed] [Google Scholar]
- 29.Popa V. ATS guidelines for methacholine and exercise challenge testing. Am J Respir Crit Care Med. 2001 Jan;163(1):292–3. doi: 10.1164/ajrccm.163.1.16310b. [DOI] [PubMed] [Google Scholar]
- 30.Crapo RO, Casaburi R, Coates AL, Enright PL, Hankinson JL, Irvin CG, et al. Guidelines for methacholine and exercise challenge testing-1999. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. Am J Respir Crit Care Med. 2000 Jan;161(1):309–29. doi: 10.1164/ajrccm.161.1.ats11-99. [DOI] [PubMed] [Google Scholar]
- 31.Fass R, Eidelman L, Garcia-Calderon W. Ambulatory 24-Hour Esophageal pH Monitoring. In: Drossman DASN, Grimm IS, editors. Handbook of Gastroenterologic Procedures. 4. Baltimore: Lippincott Williams & Wilkins; 2005. pp. 299–305. [Google Scholar]
- 32.Shay S, Tutuian R, Sifrim D, Vela M, Wise J, Balaji N, et al. Twenty-four hour ambulatory simultaneous impedance and pH monitoring: a multicenter report of normal values from 60 healthy volunteers. Am J Gastroenterol. 2004 Jun;99(6):1037–43. doi: 10.1111/j.1572-0241.2004.04172.x. [DOI] [PubMed] [Google Scholar]
- 33.Bredenoord AJ, Tutuian R, Smout AJ, Castell DO. Technology review: Esophageal impedance monitoring. Am J Gastroenterol. 2007 Jan;102(1):187–94. doi: 10.1111/j.1572-0241.2006.00966.x. [DOI] [PubMed] [Google Scholar]
- 34.Tutuian R, Castell DO. Multichannel intraluminal impedance: general principles and technical issues. Gastrointest Endosc Clin N Am. 2005 Apr;15(2):257–64. doi: 10.1016/j.giec.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 35.Johnson LF, DeMeester TR. Development of the 24-hour intraesophageal pH monitoring composite scoring system. J Clin Gastroenterol. 1986;8( Suppl 1):52–8. doi: 10.1097/00004836-198606001-00008. [DOI] [PubMed] [Google Scholar]
- 36.Belafsky PC, Postma GN, Koufman JA. The validity and reliability of the reflux finding score (RFS) Laryngoscope. 2001 Aug;111(8):1313–7. doi: 10.1097/00005537-200108000-00001. [DOI] [PubMed] [Google Scholar]
- 37.Belafsky PC, Rees CJ, Rodriguez K, Pryor JS, Katz PO. Esophagopharyngeal reflux. Otolaryngol Head Neck Surg. 2008 Jan;138(1):57–61. doi: 10.1016/j.otohns.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 38.Mastronarde JG, Anthonisen NR, Castro M, Holbrook JT, Leone FT, Teague WG, et al. Efficacy of esomeprazole for treatment of poorly controlled asthma. N Engl J Med. 2009 Apr 9;360(15):1487–99. doi: 10.1056/NEJMoa0806290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reichel O, Dressel H, Wiederanders K, Issing WJ. Double-blind, placebo-controlled trial with esomeprazole for symptoms and signs associated with laryngopharyngeal reflux. Otolaryngol Head Neck Surg. 2008 Sep;139(3):414–20. doi: 10.1016/j.otohns.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 40.Vaezi MF, Richter JE, Stasney CR, Spiegel JR, Iannuzzi RA, Crawley JA, et al. Treatment of chronic posterior laryngitis with esomeprazole. Laryngoscope. 2006 Feb;116(2):254–60. doi: 10.1097/01.mlg.0000192173.00498.ba. [DOI] [PubMed] [Google Scholar]
- 41.Farrokhi F, Vaezi MF. Extra-esophageal manifestations of gastroesophageal reflux. Oral Dis. 2007 Jul;13(4):349–59. doi: 10.1111/j.1601-0825.2007.01380.x. [DOI] [PubMed] [Google Scholar]
- 42.Oridate N, Takeda H, Asaka M, Nishizawa N, Mesuda Y, Mori M, et al. Acid-suppression therapy offers varied laryngopharyngeal and esophageal symptom relief in laryngopharyngeal reflux patients. Dig Dis Sci. 2008 Aug;53(8):2033–8. doi: 10.1007/s10620-007-0114-9. [DOI] [PubMed] [Google Scholar]
- 43.Charbel S, Khandwala F, Vaezi MF. The role of esophageal pH monitoring in symptomatic patients on PPI therapy. Am J Gastroenterol. 2005 Feb;100(2):283–9. doi: 10.1111/j.1572-0241.2005.41210.x. [DOI] [PubMed] [Google Scholar]
- 44.French CT, Irwin RS, Fletcher KE, Adams TM. Evaluation of a cough-specific quality-of-life questionnaire. Chest. 2002 Apr;121(4):1123–31. doi: 10.1378/chest.121.4.1123. [DOI] [PubMed] [Google Scholar]
- 45.Field SK, Conley DP, Thawer AM, Leigh R, Cowie RL. Effect of the management of patients with chronic cough by pulmonologists and certified respiratory educators on quality of life: a randomized trial. Chest. 2009 Oct;136(4):1021–8. doi: 10.1378/chest.08-2399. [DOI] [PubMed] [Google Scholar]


