SUMMARY
A variety of immune cell therapies proposed for use in the treatment of cancer, including both autologus cells (Lymphokine Activated Killer, Cytokine Induced Killer) or cell lines (TALL-104, NK-92), rely on recognition of NKG2D ligands on malignant cells for targeting. These ligands, such as MICA and MICB in humans are stress response ligands and are commonly, but not ubiquitously expressed within tumors. Several tumor escape mechanisms have been reported, including ligand down regulation and internalization, or proteolytic cleavage and shedding of their exposed portions (releasing soluble MICA and MICB; sMICA, sMICB). Therefore, an ability to pre-screen patients for the level of tumor cell surface expression and shedding of these ligands would prevent needless treatment of patients that are unable to respond, while targeted pre-treatment of patients to increase surface expression and/ or block shedding would enhance the subsequent effectiveness of these therapies. Here we report that serum tests of sMICA and sMICB in conjunction with tumor measurements might be used to determine rates of shedding from a tumor and that treatment with a selected combination of histone deacetylase inhibitors (to upregulate cell surface MICA/B in some tumors), and metalloproteinase inhibitors (to block MICA/B shedding in others) can be incorporated to regulate cell surface MICA/B levels prior to immune cell therapy, significantly enhancing their effectiveness (either used alone or as carrier vehicles for oncolytic viruses). Ultimately pre-screening patients undergoing such immune cell therapies might be used to personalize cancer treatment regimens based on the NKG2D-ligand status of the tumor.
Keywords: Immunotherapy, cancer, NKG2D, MICA, MICB, histone deacetylase, metalloproteinase
INTRODUCTION
It is likely that successful cancer therapies will require some interaction with the host’s immune response to assist in the clearance of residual cancer cells and in the reduction or prevention of relapse. As such, multiple approaches, ranging from recombinant cytokines 1,2, bone marrow transplants to monoclonal antibodies 3-5 are routinely used in the clinic, while many others, including cancer vaccines 6-9 and adoptive transfer of tumor infiltrating lymphocytes 10,11 or chimeric antigen receptor expressing T-cells 12-14 are being developed. One further group of immune cells that have demonstrated some success during early clinical evaluation are more associated with the innate arm of the immune response, and express NK-cell markers, such as NKG2D on their surface. These include lymphokine activated killer (LAK) 15,16 and cytokine induced killer (CIK) cells 17-19, that are expanded ex vivo from the peripheral blood of patients or matched siblings, or cultured cell lines, such as TALL-104 20 or NK-92 cells 21. These therapeutics share a common mechanism of action as they all recognize tumor targets through their expression of NKG2D ligands, such as MICA and MICB in humans 22. These ligands are typically induced in response to stress, such as produced by the hypoxic or nutrient limited environment commonly found within cancers. However, some cancers are known to develop escape mechanisms that reduce the levels of these ligands on their surface, including down regulation of expression or internalization of the ligands 23; or shedding (proteolytic cleavage) of their soluble extracellular domains (sMICA, sMICB) 24,25.
Pre-screening of patients for the level of cell-surface MICA or MICB on tumor cells and the rate of shedding of sMICA and sMICB would therefore provide a valuable predictive readout for the likelihood of response to immune cell therapies, such as the use of CIK cells, as well as providing a mechanism for NKG2D-recognition avoidance within those tumors unlikely to respond. Further, because serum levels of MICA/B domains shed in this way have been proposed as biomarkers for tumor burden 28,29 sensitive ELISA’s exist to measure these peptides in the serum 30. Here we propose that a combination of measurements of circulating MICA/B domains and tumor volume can be combined to determine the rate of shedding, and that this data, possibly coupled with a non-invasive or biopsy related determination of MICA/B surface expression in the tumor, could be used to determine likely response to CIK (or related) cell therapy.
Furthermore, several standard or experimental cancer therapies are known to reverse or block some of these NKG2D-avoidance mechanisms, including the use of some histone deacetylase inhibitors (HDACi) to upregulate MICA/B surface expression 26,27 or the use of some arsenic compounds (such as phenylarsine oxide, PAO) or metalloproteinase inhibitors (MMPI) to block the cleavage and shedding of sMICA or sMICB from the cell surface 24. We therefore also demonstrate that screening for MICA/B status within the tumor can be used to determine regimens to pre-treat cancer patients prior to immune cell therapy with NKG2D expressing cells, and that this can stabilize MICA/MICB expression, and so significantly enhancing the overall anti-tumor benefits.
MATERIALS and METHODS
Chemicals and Reagents
Phenylarsine oxide (PAO) and Histone Deacetylase Inhibitors: TSA ( Trichostatin A), sBUT (sodium butyrate) and VPA( Valproic acid sodium salt) were all purchased from Sigma-Aldrich (St.Louis, MO); Unless otherwise stated TSA was used at 0.375uM in vitro for 24h or for in vivo expts, 5ug of TSA was delivered per mouse in100ul PBS by intraperitoneal injection. PAO was used at 0.1uM in vitro. The MMP inhibitor III was from Calbiochem (Merck, Darmstadt, Germany) and used at 5uM in vitro or 100ul of 50uM was delivered via intraperitoneal injection in vivo
Cells and viruses
Human ovarian cancer cell line UCI-101 was a kind gift from Drs. P. DiSaia and A. Manetta (University of California, San Diego), SKOV-33, Ovcar-3 and HeLa cells were obtained from ATCC. Stable transductants of all cell lines were produced by retroviral transfection in order to produce cells expressing luciferase. Vaccinia virus strain vvB18R strain is a version of vaccinia virus strain WR expressing luciferase from the site of the viral thymidine kinase gene and contains additional deletion of the viral B18R gene (encoding a secreted IFN binding protein) and has been reported previously 31.
Flow Cytometry and Immunofluorescence
For flow cytometry, cells were stained with PE-conjugated anti-human MICA/MICB (eBioscience, San Diego, CA USA) for 30mins on ice or stained by PE-conjugated mouse anti-hMICA and APC-conjugated mouse anti-hMICB separately (R&D systems, Minneapolis, MN USA). PE-conjugated mouse IgG2ak isotype was used as control (eBioscience, San Diego, CA USA). Samples were run on a Cyan (DAKO), and analysis was by FloJo. Immunofluorescence was performed on frozen sections of tumors using primary antibody rabbit anti-human MICA/B polyclonal IgG (Santa Cruz biotechnology Inc., Santa Cruz, CA USA) and secondary staining with fluorescence labeled anti-rabbit antibody. Additional anti-CD31 antibody (Santa Cruz biotechnology Inc., Santa Cruz, CA USA) and Hoescht 33342 staining was also performed. Images taken on a Leica TCS NT confocal microscope.
ELISA
ELISA measurement of sMICA and sMICB levels in cell culture medium or mouse serum were determined by Human MICA Duoset ELISA Development kit and Human MICB Duoset ELISA Development kit (R&D Systems, Inc. Minneapolis, USA). The procedures are in accordance with the protocols supplied with the kit.
Cellular cytotoxicity assay
Human cytokine-induced killer (hCIK) cells were prepared from de-identified human peripheral blood (Buffy coat) obtained from the Pittsburgh Blood Bank according to established protocols 17. Tumor cell lysis by effector cells (hCIK cells) was assessed by measurement of the luciferase activity of surviving target cells determined 4 hours after mixing effector to target cells at a ration of 5:1 (unless otherwise stated) in black-walled 96-well plates. Luciferin was then added to each well [2 μl/well of 30 mg/ml luciferin (Caliper Life Science)] and light output (photons·second-1/well) measured on an IVIS 200 imaging system (Xenogen part of Caliper Life Science). Percent cytotoxicity was then determined relative to control wells (target only or target only pretreated with 70% ethanol).
MICA/B siRNA
Cells expressing luciferase were either treated with human MICA/B siRNA or control siRNA (MICA/B siRNA kit, Santa Cruz Biotechnology, Inc. Santa Cruz, CA USA). The experimental procedure was in accordance with the protocol supplied with the kit. After treatment and continued incubation the cells were collected for flow cytometry analysis or for CIK cellular cytotoxicity assay.
Mouse models
Athymic nu-/nu- mice (female, 6-8 weeks) obtained from Taconic Corporation (Germantown, NY USA) were used for xenograft studies. Mice received subcutaneous injections of 1 × 107 UCI-101 or HeLa tumor cells subcutaneously. One palpable tumors formed (50-100mm) treatment was begun. Mice were treated with TSA (5ug in100ul PBS by I.P. injection), MMPi III (50uM delivered via IP injection in 100ul), hCIK cells (2×107 cells delivered via intravenous tail vein injection), or hCIK pre-mixed for 1h with 2×107 PFU of vvB18R. Treatment regimens are outlined in the figure legends. Mice were bled by sub-mandibular bleeding for serum sMICA analysis.
Tumor volumes were determined by caliper measurement, or mice were sacrificed and tumors collected and either frozen for sectioning or dissociated for flow cytometry by grinding through a 100uM cell strainer. In one experiment, the single cells were collected and stained with PE-labeled mouse anti-human CD56 (BD Pharmingen, San Diego, CA USA) and run on flow cytometry to quantify the CIK cells in tumor. All experiments were run with University of Pittsburgh IACUC approval.
Statistics
Parametric, two-tailed T-tests were run unless otherwise stated, for significance p<0.05.
RESULTS
Ovarian Cancer cells treated with HDACi display increased levels of cell surface MICA/MICB
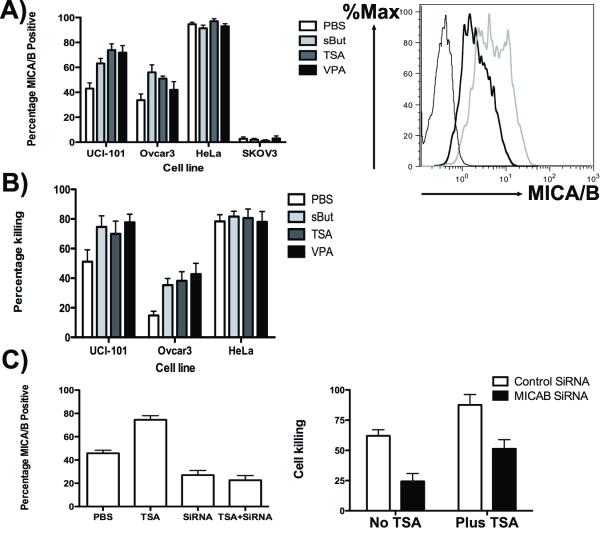
Initially we tested several human ovarian and cervical cancer cell lines, including UCI-101, HeLa, Ovcar-3 and SKOV-3 with several different off the shelf histone deacetylase inhibitors, including TSA (Trichostatin A), sBUT (sodium butyrate) and VPA (Valproic acid sodium salt). It was found (Fig 1A) that sub-lethal doses of these treatments increased the levels of MICA/B on the cell surface of several of these cell lines (as determined by flow cytometry), notably cells that initially displayed partial MICA/B cell surface expression (UCI-101 and Ovcar3). In both these cell lines, all HDACi tested resulted in significantly increased (p<0.05) percentages of the cells expressing MICA and /or MICB, with the exception of VPA treatment in Ovcar-3 cells, where the effects did not reach significance (p=0.15). Almost 95% of HeLa cells initially expressed MICA/B, and so any further increase was limited, while SKOV3 initially expressed no cell surface MICA/B (less than 3% cells positive) and HDACi treatment was found not to be capable of altering MICA/B surface expression in this cell line. Therefore, this approach is not universally applicable, and tumors with no MICA/B expression may be considered as not amenable to therapy with NKG2D-expressing immune cells. All HDACi tested produced similar effects in each cell line, but VPA treatment was typically the least effective.
Fig 1.
Histone deactylase inhibitors enhance sensitivity of some cancer cells to CIK mediated killing through NKG2D ligand expression. (A) Different human ovarian and cervical cancer cell lines were treated with different histone deactylase inhibitors for 24h as indicated. At the end of this time, cells were stained with a pan anti-MICAS/MICB antibody and percentage positive cells determined by flow cytometry (a typical flow cytometry plot is shown for UCI-101 cells with (grey) or without (black) TSA treatment. Isotype is thin black plot). (B) Enhanced MICA/MICB expression correlates with enhanced sensitivity to CIK-mediated killing. Cells treated as before (and expressing luciferase) were mixed with human CIK cells at an effector to target ration of 5:1 for 4h before luciferase signal was determined following luciferin addition. Luciferase signal (relative to target cells only (0%) or ethanol treated target cells (100%)) was used to determine percentage killing. (C) This enhanced killing is mostly mediated through MICA/MICB expression levels. Cells (UCI-101) were treated with TSA or anti-MICA/MICB siRNA, or both, and levels of MICA/B surface expression by antibody staining and flow cytometry (non-targeting siRNA was used as a control)(left panel); CIK cell killing was determined as before for cells treated with TSA with or without MICA/MICB siRNA (right panel).
HDACi treatment enhances CIK targeting of ovarian cancer cells through upregulation of the NKG2D-ligands MICA/MICB
In order to determine if immune cells expressing NKG2D were capable of enhanced targeting of HDACi treated target cells, UCI-101, HeLa and Ovcar3 cells were again treated with sub-lethal doses of sBUT, TSA and VPA and cell killing following exposure to human Cytokine Induced Killer (CIK) cells (at a ratio of 5 effector to 1 target cell) was determined in a cell survival assay (Fig 1B). This confirmed that HDACi treatment that resulted in enhanced MICA/MICB surface expression also sensitized the tumor cells to killing by the innate immune cells expressing NKG2D. All HDACi treatments led to significantly enhanced (p<0.05, n=6) cell killing when used against UCI-101 or Ovcar-3 cells, however no difference was seen in HeLa cells, where PBS treated cells already displayed >80% killing after exposure to CIK cells. Although tumor cells surviving after CIK treatment displayed a reduction in surface MICA/MICB expression (indicating that these ligands were being targeted), some expression remained, indicating that longer exposure to CIK cells, or greater numbers of CIK cells would result in further target cell killing.
However, because HDACi treatment is known to affect multiple gene products and processes within the cell, we looked to determine if factors other than MICA/B expression might be mediating the enhanced sensitivity. For example, it was noted that VPA treatment resulted in the greatest increase in sensitivity to CIK cell killing in Ovcar-3 cells, but the lowest increase in MICA/B surface expression. To examine this, siRNA targeting MICA and MICB was employed along with HDACi treatment (Fig 1C). The siRNA significantly reduced levels of MICA/MICB on the cell surface of UCI-101 cells (p=0.041), and also completely abrogated the effects of TSA on MICA/MICB expression levels (p=0.0001). The siRNA also significantly reduced the CIK-mediated cell killing of control and TSA treated UCI-101 cells (p=0.0014 and 0.0052 respectively) confirming that MICA/B surface expression level was a key mediator in the increased sensitivity of the target cells to CIK treatment. However, siRNA and TSA combination treated cells still displayed enhanced sensitivity to CIK cell killing relative to siRNA alone treated cells (p=0.0093), indicating that other mechanisms may also be contributing to HDACi-mediated sensitization to CIK killing.
CIK cell therapy anti-tumor effects are enhanced in vivo after HDACi treatment
Because UCI-101 cells displayed significant increases in the numbers of MICA/B positive cells after HDACi treatment, we used these as a model for in vivo studies, and because TSA gave the most significant up-regulation of MICA/B levels in this cell line, we focused on this HDACi. Athymic nu-/nu- mice were therefore implanted subcutaneously with UCI-101 tumor cells and once tumors were palpable (50-100 mm3) they were treated intravenously with TSA and the levels of MICA/B in the tumor were determined post mortem both by immunofluoresence microscopy, using pan MICA/B antibody staining in tumor sections and with MICA and MICB specific antibodies via flow cytometry of dissociated tumor cells (Fig 2A). This confirmed that both MICA and MICB levels were upregulated in vivo following systemic HDACi treatment, and that both upregulation occurred throughout the tumor environment. In order to examine the effects and safety of CIK and HDACi combinations in vivo, athymic nu-/nu- mice implanted subcutaneously with UCI-101 cells were treated with single or combination therapies once tumors became palpable. Initial studies demonstrated that significantly more CIK cells could be recovered from tumors pretreated with TSA (Fig 2B)(p=0.013), either due to increased infiltration of the tumors or enhanced proliferation of the CIK cells within the tumor.
Fig 2.
Histone deactylase inhibitor treatment increases MICA/B level and CIK anti-tumor effects in vivo in mouse tumor models. (A) Anti-MICA/B staining reveals up-regulation of surface expression of these ligands. UCI-101 tumors implanted subcutaneously into athymic nu-/nu- mice were treated with TSA (IP injection) and mice sacrificed after 24h. Sections were stained with anti-MICA/MICB antibody (green), anti-CD31 antibody (red) to reveal vasculature, and with Hoescht 3323 (blue) (magnification 200×), while flow cytomrety of dissociated UCI-101 cells from the same tumors stained with antibodies specific for MICA or MICB reveals that both ligands are upregulated (right panels; red is no TSA; blue is with TSA). (B) TSA treatment also increases the numbers of CIK cells within tumors. Athymic nu-/nu- mice implanted with UCI-101 cells as before and treated with TSA (IP, day 0) and CIK cells IV, day 1), were sacrificed on day 4 and tumors dissociated. Numbers of CIK cells in the tumor were determined after staining with an anti-human CD-56 antibody. (C) Anti-tumor effects are enhanced when CIK cells and TSA are combined. Athymic nu-/nu- mice implanted with UCI-101 tumors as before were treated with PBS, TSA (IP, day 0), CIK cells (day 1, IV) or both. The combined effect is significantly greater than any therapy used as a single agent (p=0.046)(n=8 per group). (D) Combining TSA with CIK cells pre-infected with the oncolytic vaccinia virus vvB18R (in the same tumor model) also leads to significantly enhanced anti-tumor effects relative to the same therapy without TSA (p=0.014). In this case 8 out of 10 mice had complete responses (n=10 per group).
HDACi treatment (TSA) was also shown to enhance CIK anti-tumor effects in vivo. Athymic nu-/nu- mice implanted subcutenaously with UCI-101 cells as before were treated with either (i) PBS, (ii) TSA IP (day 0,3 and 5), (iii) CIK cells IV (day 1) or (iv) both CIK cells and TSA (n=8 per group). Tumor volumes were measured over time via caliper measurement (Fig 2C). It was seen that TSA treatment significantly enhanced the anti-tumor effects of CIK cells (p=0.046), despite the fact TSA alone had little benefit in this model. However no complete responses were seen, even with the CIK and TSA combination therapy.
We have recently described a novel therapy combining CIK cells with an oncolytic virus (vvB18R)32, such that the CIK cells can act as a delivery vehicle to carry the viral therapy to the tumor target, and the oncolytic virus can enhance the tumor killing potential of the CIK cells. We therefore looked to see if CIK-vvB18R therapy was also capable of being enhanced through combination with TSA. This is especially pertinent as we recently reported that HDAC inhibitors could enhance the in vitro and in vivo replication and antitumor effects of vvB18R alone (In Press). In this way, the HDACi may act to improve the therapeutic effects of both components of the CIK-vvB18R therapy.
Indeed, it was found that the CIK-vvB18R therapy in mouse models of UCI-101 tumors was significantly enhanced by pre-treatment with TSA (Fig 2D)(p=0.014), and that the addition of TSA led to 8 of 10 animals displaying a complete response with no relapse for at least 90 days.
Shedding of sMICA and sMICB through proteolytic cleavage of MICA/B extracellular domains can be reduced by PAO or MMPi treatment, so enhancing CIK therapy
We next examined several human cancer cell lines for the level of sMICA and sMICB that were shed into the media, as determined by ELISA. It was seen that, in agreement with previously published reports 24, HeLa cells shed high levels of NKG2D ligands, notably sMICA, this was a log greater than for UCI-101 (Fig 3A). We therefore looked to determine if shedding could be reduced through different treatments, and to see if this effected the action of CIK cells. We initially focused on several treatments that had previously been implicated in blocking of sMICA and sMICB shedding 24. As such, phenylarsine oxide (PAO) and a general metalloproteinase inhibitor (MMPi), MMPI III, were both found to significantly reduce shedding of sMICA from HeLa cells, so reducing the levels of sMICA (and sMICB – data not shown) in the media of cultured cells (p=0.033 and 0.022 respectively) (Fig 3B). We also found that UCI-101 cells displayed increased levels of shedding following HDACi treatment (Fig 3C) (p=0.011). This could potentially reduce the effectiveness of the HDACi treatment in enhancing sensitivity of some tumors to CIK therapy, and so we examined whether MMPi treatment could block this effect. It was seen (Fig 3C) that although MMPi had no significant effect on UCI-101 shedding when used alone (which is unsurprising as UCI-101 cells displayed only low levels of shedding), it reduced the levels of shedding in HDACi (TSA) treated cells to background levels (p=0.040). It is not known if all tumor cells capable of shedding sMIC/sMICB would respond to these treatments in the same way however.
Fig 3.
Shedding of soluble MICA or MICB can be blocked by PAO or MMPi treatment, leading to enhanced anti-tumor effects. (A) HeLa and UCI-101 cells were grown for 24h in culture, before the media was sampled and assayed for sMICA and sMICB by ELISA. HeLa displayed greatest shedding of soluble MICA/MICB domains, with sMICA being the major shed domain. (B) Use of either PAO or MMPi can significantly reduce accumulation of sMICA in the media. HeLa cells were cultured as before, only in some wells PAO or MMPi III were added. ELISA was used to assay sMICA levels in the media after 24h. (C) The same experiment was repeated for UCI-101 cells with or without TSA treatment. TSA leads to upregulation of sMICA shedding that can be reduced with combination with MMPi. (D) CIK cells were mixed with media from HeLa cells (cultured for 24h) or media from HeLa cells treated with MMPi before being added to UCI-101-luciferase target cells (at effector:target of 5:1). After 4h, UCI-101 cell survival was determined by imaging of luciferase signal.
The importance of shedding of MICA/B extracellular domains on the effectiveness of CIK therapy was highlighted by the fact that spent media from HeLa cells, pre-mixed with CIK cells could significantly reduce their ability to destroy tumor targets, even when the target cells themselves did not shed sMICA/sMICB (Fig 3D)(p=0.007). It is assumed that the sMICA and/ or sMICB acts to bind and block the NKG2D receptor on the CIK cells. However, spent media from HeLa cells treated with MMPI (or PAO) displayed significantly less ability to inhibit or block the CIK cell killing (p=0.009). The spent media alone had no effect on the target cells.
In vivo experiments, using HeLa cells implanted subcutaneously into athymic nu-/nu- mice demonstrated that there was a direct correlation between tumor volume and sMICA ELISA measurement (Fig 4A), indeed it was even possible to normalize the sMICA ELISA to tumor volume to produce a ‘rate of shedding’ value (pg/ml per mm3). This value was found to decrease after systemic MMPi treatment (p=0.019)(Fig 4B), demonstrating that the reduction in sMICA shedding from this tumor cell after MMPi treatment was also seen in vivo. (N.B. PAO was found to show some toxicity at the required concentrations in vivo). Post mortem analysis of MICA and MICB surface expression on dissociated HeLa tumor cells from animals treated with MMPi or not found that systemic MMPi treatment could actually increase the levels of MICA on the cell surface within the tumor (while MICB levels were unaffected in this model)(Fig 4C). When sMICA ELISAs were run on serum samples taken from athymic nu-/nu- mice implanted with UCI-101 cells following different treatments, and normalized for tumor burden (Fig 4D), it was seen that (as for the in vitro expt, Fig 3C), TSA treatment significantly increased the levels of sMICA in the serum (p=0.001), while combination of TSA with MMPi reduced the serum levels back to background. Again, the direct correlation between ‘rates of shedding’ in vivo and media determinations of shedding in vitro indicates that sMICA ELISA on serum normalized to tumor volume can be a reliable measurement of the relative amount of release of sMICA from a tumor. It is possible that this approach might be used as a means to pre-screen patients for the need to pre-treat with MMPi prior to CIK therapy.
Fig 4.
MMPi treatment in vivo leads to increased MICA expression in tumors, reduced serum levels of sMICA and enhanced anti-tumor effects of CIK cells. (A) serum was taken from athymic nu-nu- mice at different times after subcutaneous implantation with HeLa cells and sMICA levels determined by ELISA. At the same time tumor measurement was determined by caliper measurement, and the correlation between these two plotted. (B) In HeLa tumor models as before, serum levels of sMICA (determined by ELISA) were adjusted for tumor volume (caliper measurement) for mice 24h after IP MMPi or PBS treatment (n=5 per group) (C) Cell surface expression of MICA and MICB from cells dissociated from HeLa tumors in athymic nu-/nu- mice formed by subcutaneous implantation. Single cell suspensions were stained with anti-MICA or anti-MICB antibodies and levels determined by flow cytometry. (D) Athymic nu/nu mice were implanted subcutaneously with UCI-101 cells and treated with TSA, MMPi or TSA and MMPi once palpable tumors had formed. Serum was drawn after 24h and sMICA levels determined by ELISA. Serum levels were normalized for tumor volume as before. (E) Anti-tumor effect of athymic nu-nu- mice bearing subcutaneous HeLa tumors and treated with CIK cells alone (day 1), or in combination with MMPi (day 0, and CIK cells at day 1). Combination therapy led to significantly enhanced therapeutic benefit relative to CIK therapy alone (P=0.015 at day 21)(n=8 per group).
CIK anti-tumor effects can be enhanced in vivo following MMPi pretreatment
Finally we examined the ability of systemic MMPi treatment of athymic nu-/nu- mice implanted with palpable, subcutaneous HeLa tumors (known to shed MICA/B) to enhance the therapeutic benefit of subsequent CIK treatment. As with TSA treatment of tumors that down regulate MICA/B expression, MMPi treatment leading to stabilization of cell surface MICA/MICB expression was capable of enhancing the CIK therapy (p=0.015) (Fig 4E).
Discussion
A variety of immune cells that possess the ability to recognize and target tumors through innate signaling pathways have been tested in the clinic. These primarily involve expression of NKG2D and related receptors on the immune cell therapeutics binding to MICA and MICB on their tumor targets. Of these, Cytokine Induced Killer (CIK) cells have displayed safety and promising anti-tumor effects in early clinical testing in several trials in the US 19, and are routinely used in China 33. However, a potential limitation for the clinical application of these cells is the development of mechanisms by cancer cells to reduce their surface expression of NKG2D ligands during progression of some tumors. The two pathways described to date that lead to reduced NKG2D responsiveness involve either (i) down regulation of the surface expression of the ligands on the tumor, or (ii) proteolytic cleavage and shedding of their extracellular domains. Here we have demonstrated that it is feasible to identify tumors that have developed these avoidance mechanisms and pre-treat them if necessary, depending on the route of innate immune avoidance incorporated, and so enhance the therapeutic benefits of subsequent CIK-mediated therapy.
We initially demonstrated that ‘rates’ or levels of shedding can be easily determined through ELISA measurement of concentrations of sMICA and/ or sMICB in the serum coupled with tumor volume determination through non-invasive structural imaging (such as CT, US or X-ray). We anticipate that it would be feasible to determine a threshold value above which the level of shedding would impede CIK cell therapy, and so pre-treatment with drugs capable of blocking this shedding (e.g. MMPi, PAO) would be used. We further predict that non-invasive molecular imaging of binding of NKG2D sequence domains fused to radiolabeled PET imaging tracers could be used to similarly predict levels of NKG2D receptor surface expression within the tumor, or else immunohistochemical analysis of tumor biopsy specimens could be used to determine cell surface levels of MICA/MICB. Low surface expression of MICA/MICB would indicate a need for pre-treatment with HDACi prior to CIK therapy. In this way, it is conceivable that a picture of NKG2D susceptibility or route of avoidance within a tumor could be used to predict response to CIK therapy, such that, if deemed necessary, pre-treatment with different clinically relevant drugs (such as histone deacetylase inhibitors or MMPI’s, such as marimastat or possibly doxycyclin 34) could personalize the treatment approach and allow for enhanced anti-tumor effects when CIK, or related therapies are applied. Alternatively, a combination of histone deacetylase inhibitor and MMPi could be routinely provided prior to CIK therapy as a potential means to stabilize cell surface MICA/MICB levels. However, more study is needed to determine if pathways others than those examined here are involved.
Down regulation of MICA and MICB, the best studied human NKG2D receptors, can be reversed through treatment with histone deacetylase inhibitors 24. Here we show this treatment leads to enhanced sensitivity of some tumor cells to CIK therapy, through a mechanism found to be primarily, although not entirely, mediated through the up-regulation of NKG2D receptors on the cell surface by the HDACi. This was demonstrated for all but one of the cell lines tested that displayed reduced MICA/MICB surface expression and occurred both in vitro and in vivo, leading to significantly enhanced anti-tumor effects. However, SKOV3 cells, the one cell line that displayed no MICA or MICB background surface expression, could not be induced to express these proteins under any of the conditions examined, indicating that this approach would not work under all circumstances.
Interestingly we have also recently reported that CIK cells can be effectively combined with oncolytic viral therapy, whereby the CIK cells are pre-infected with the viral vectors and used as delivery vehicles to traffic the viruses to their tumor targets 32,35. Furthermore, we have found that histone deacetylase inhibitors can act to enhance the replication of some of these viral therapies within the tumor, so additionally enhancing their therapeutic potential (In Press). We therefore looked to examine the effects of combining the CIK-oncolytic vaccinia dual therapy with histone deactylase treatment, and found that this represented a highly effective therapy, with 8 of 10 mice demonstrating durable complete responses in the model used. This is an attractive approach as the activity and the specificity of both the CIK and the oncolytic viral therapy were enhanced by the HDACi, while a variety of safe HDACi are available for clinical use.
Shedding of MICA or MICB extracellular soluble domains can be disrupted through addition of inhibitors of protein disulphide isomerase, such as phenylarsine oxide (PAO), and we demonstrated that PAO treatment can indeed reduce sMICA or sMICB from inhibiting CIK-mediated killing of tumor target cells. However this chemical has pleiotropic effects and is toxic in vivo at the concentration required for effectiveness. Therefore we examined the specific effects of matrix metalloproteinase inhibitors. A variety of matrix metalloproteinases are upregulated in cancer, and have been implicated in the proteolytic cleavage and shedding of sMICA and sMICB. It was found that an MMPI was effective at blocking the shedding of sMICA/sMICB from HeLa cells, and also HDACi treated UCI-101 cells (where the upregulation of MICA/MICB correlated with an additional upregulation in sMICA/sMICB shedding). This resulted in more effective CIK-mediated killing in vitro and greater anti-tumor effects in vivo. We utilized a broad spectrum, cell permeable MMP inhibitor, capable of inhibiting MMPI-1, MMPI-2, MMPI-3, MMPI-7 and MMPI-13. However it is unclear how other MMP upregulation may be affected by this treatment or whether alternative mechanisms of shedding exist that might not be affected by this approach to stabilizing NKG2D ligand cell surface expression within the tumor. As such, it will be necessary to examine further tumor cells known to release sMICA/sMICB before a more global treatment approach is determined.
We have therefore proposed an approach to significantly enhancing CIK, or related therapies (e.g. LAK, NK-92, TALL-104 etc) either by pre-screening cancer patients prior to therapy to determine the levels of cell surface and shed MICA/MICB or by pre-treating all patients prior to therapy with a mix of HDACi and MMPi to stabilize MICA/MICB surface expression in the tumor. We have determined that these pre-treatment methods could be utilized either to personalize the therapy or in a more general fashion so that NKG2D ligands can be most effectively stably expressed on the tumor cell surface.
Acknowledgments
This work was supported by NIH/NCI award R01CA140215 to SHT
Footnotes
Financial Disclosure: All authors have declared there are no financial conflicts of interest in regards to this work.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Kirkwood JM. Adjuvant IFN alpha2 therapy of melanoma. Lancet. 1998;351:1901–1903. doi: 10.1016/S0140-6736(05)78608-6. [DOI] [PubMed] [Google Scholar]
- 2.Chang AE, Rosenberg SA. Overview of interleukin-2 as an immunotherapeutic agent. Semin Surg Oncol. 1989;5:385–390. doi: 10.1002/ssu.2980050604. [DOI] [PubMed] [Google Scholar]
- 3.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 4.Bokemeyer C, Bondarenko I, Makhson A, Hartmann JT, Aparicio J, de Braud F, et al. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2009;27:663–671. doi: 10.1200/JCO.2008.20.8397. [DOI] [PubMed] [Google Scholar]
- 5.Hudis CA. Trastuzumab--mechanism of action and use in clinical practice. N Engl J Med. 2007;357:39–51. doi: 10.1056/NEJMra043186. [DOI] [PubMed] [Google Scholar]
- 6.Pejawar-Gaddy S, Finn OJ. Cancer vaccines: accomplishments and challenges. Crit Rev Oncol Hematol. 2008;67:93–102. doi: 10.1016/j.critrevonc.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 7.Moingeon P. Cancer vaccines. Vaccine. 2001;19:1305–1326. doi: 10.1016/s0264-410x(00)00372-8. [DOI] [PubMed] [Google Scholar]
- 8.Wang E, Panelli MC, Monsurro V, Marincola FM. A global approach to tumor immunology. Cell Mol Immunol. 2004;1:256–265. [PubMed] [Google Scholar]
- 9.Yannelli JR, Wroblewski JM. On the road to a tumor cell vaccine: 20 years of cellular immunotherapy. Vaccine. 2004;23:97–113. doi: 10.1016/j.vaccine.2003.12.036. [DOI] [PubMed] [Google Scholar]
- 10.Dudley ME, Rosenberg SA. Adoptive cell transfer therapy. Semin Oncol. 2007;34:524–531. doi: 10.1053/j.seminoncol.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morgan RA, Dudley ME, Rosenberg SA. Adoptive cell therapy: genetic modification to redirect effector cell specificity. Cancer J. 16:336–341. doi: 10.1097/PPO.0b013e3181eb3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bridgeman JS, Hawkins RE, Hombach AA, Abken H, Gilham DE. Building better chimeric antigen receptors for adoptive T cell therapy. Curr Gene Ther. 10:77–90. doi: 10.2174/156652310791111001. [DOI] [PubMed] [Google Scholar]
- 14.June CH, Blazar BR, Riley JL. Engineering lymphocyte subsets: tools, trials and tribulations. Nat Rev Immunol. 2009;9:704–716. doi: 10.1038/nri2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Melder RJ, Whiteside TL, Vujanovic NL, Hiserodt JC, Herberman RB. A new approach to generating antitumor effectors for adoptive immunotherapy using human adherent lymphokine-activated killer cells. Cancer Res. 1988;48:3461–3469. [PubMed] [Google Scholar]
- 16.Sasaki A, Melder RJ, Whiteside TL, Herberman RB, Jain RK. Preferential localization of human adherent lymphokine-activated killer cells in tumor microcirculation. J Natl Cancer Inst. 1991;83:433–437. doi: 10.1093/jnci/83.6.433. [DOI] [PubMed] [Google Scholar]
- 17.Lu PH, Negrin RS. A novel population of expanded human CD3+CD56+ cells derived from T cells with potent in vivo antitumor activity in mice with severe combined immunodeficiency. J Immunol. 1994;153:1687–1696. [PubMed] [Google Scholar]
- 18.Verneris MR, Karami M, Baker J, Jayaswal A, Negrin RS. Role of NKG2D signaling in the cytotoxicity of activated and expanded CD8+ T cells. Blood. 2004;103:3065–3072. doi: 10.1182/blood-2003-06-2125. [DOI] [PubMed] [Google Scholar]
- 19.Leemhuis T, Wells S, Scheffold C, Edinger M, Negrin RS. A phase I trial of autologous cytokine-induced killer cells for the treatment of relapsed Hodgkin disease and non-Hodgkin lymphoma. Biol Blood Marrow Transplant. 2005;11:181–187. doi: 10.1016/j.bbmt.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 20.Visonneau S, Cesano A, Porter DL, Luger SL, Schuchter L, Kamoun M, et al. Phase I trial of TALL-104 cells in patients with refractory metastatic breast cancer. Clin Cancer Res. 2000;6:1744–1754. [PubMed] [Google Scholar]
- 21.Tonn T, Becker S, Esser R, Schwabe D, Seifried E. Cellular immunotherapy of malignancies using the clonal natural killer cell line NK-92. J Hematother Stem Cell Res. 2001;10:535–544. doi: 10.1089/15258160152509145. [DOI] [PubMed] [Google Scholar]
- 22.Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, et al. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285:727–729. doi: 10.1126/science.285.5428.727. [DOI] [PubMed] [Google Scholar]
- 23.Long EO. Tumor cell recognition by natural killer cells. Semin Cancer Biol. 2002;12:57–61. doi: 10.1006/scbi.2001.0398. [DOI] [PubMed] [Google Scholar]
- 24.Kaiser BK, Yim D, Chow IT, Gonzalez S, Dai Z, Mann HH, et al. Disulphide-isomerase-enabled shedding of tumour-associated NKG2D ligands. Nature. 2007;447:482–486. doi: 10.1038/nature05768. [DOI] [PubMed] [Google Scholar]
- 25.Groh V, Wu J, Yee C, Spies T. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature. 2002;419:734–738. doi: 10.1038/nature01112. [DOI] [PubMed] [Google Scholar]
- 26.Armeanu S, Bitzer M, Lauer UM, Venturelli S, Pathil A, Krusch M, et al. Natural killer cell-mediated lysis of hepatoma cells via specific induction of NKG2D ligands by the histone deacetylase inhibitor sodium valproate. Cancer Res. 2005;65:6321–6329. doi: 10.1158/0008-5472.CAN-04-4252. [DOI] [PubMed] [Google Scholar]
- 27.Skov S, Pedersen MT, Andresen L, Straten PT, Woetmann A, Odum N. Cancer cells become susceptible to natural killer cell killing after exposure to histone deacetylase inhibitors due to glycogen synthase kinase-3-dependent expression of MHC class I-related chain A and B. Cancer Res. 2005;65:11136–11145. doi: 10.1158/0008-5472.CAN-05-0599. [DOI] [PubMed] [Google Scholar]
- 28.Holdenrieder S, Stieber P, Peterfi A, Nagel D, Steinle A, Salih HR. Soluble MICB in malignant diseases: analysis of diagnostic significance and correlation with soluble MICA. Cancer Immunol Immunother. 2006;55:1584–1589. doi: 10.1007/s00262-006-0167-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holdenrieder S, Stieber P, Peterfi A, Nagel D, Steinle A, Salih HR. Soluble MICA in malignant diseases. Int J Cancer. 2006;118:684–687. doi: 10.1002/ijc.21382. [DOI] [PubMed] [Google Scholar]
- 30.Nuckel H, Switala M, Sellmann L, Horn PA, Durig J, Duhrsen U, et al. The prognostic significance of soluble NKG2D ligands in B-cell chronic lymphocytic leukemia. Leukemia. 24:1152–1159. doi: 10.1038/leu.2010.74. [DOI] [PubMed] [Google Scholar]
- 31.Kirn DH, Wang Y, Le Boeuf F, Bell J, Thorne SH. Targeting of interferon-beta to produce a specific, multi-mechanistic oncolytic vaccinia virus. PLoS Med. 2007;4:e353. doi: 10.1371/journal.pmed.0040353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thorne SH, Negrin RS, Contag CH. Synergistic antitumor effects of immune cell-viral biotherapy. Science. 2006;311:1780–1784. doi: 10.1126/science.1121411. [DOI] [PubMed] [Google Scholar]
- 33.Linn YC, Hui KM. Cytokine-induced NK-like T cells: from bench to bedside. J Biomed Biotechnol. 2010:435745. doi: 10.1155/2010/435745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramnath N, Creaven PJ. Matrix metalloproteinase inhibitors. Curr Oncol Rep. 2004;6:96–102. doi: 10.1007/s11912-004-0020-7. [DOI] [PubMed] [Google Scholar]
- 35.Thorne SH, Liang W, Sampath P, Schmidt T, Sikorski R, Beilhack A, et al. Targeting Localized Immune Suppression Within the Tumor Through Repeat Cycles of Immune Cell-oncolytic Virus Combination Therapy. Mol Ther. doi: 10.1038/mt.2010.140. [DOI] [PMC free article] [PubMed] [Google Scholar]